- 1College of Medical Information and Engineering, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Chongqing Hospital of Traditional Chinese Medicine, Chongqing, China
Background: The neural activity of irritable bowel syndrome (IBS) patients in the resting state without any intervention has not been systematically studied. The purpose of this study was to compare the resting-state brain functions of IBS patients with healthy controls (HCs).
Methods: The published neuroimage studies were obtained from electronic databases including PubMed, EMBASE, PsycINFO, Web of Science Core, CNKI Database, Wanfang Database, VIP Database, and CBMdisc. Search dates were from inception to March 14th, 2022. The studies were identified by the preidentified inclusion and exclusion criteria. Two independent reviewers compiled the studies and evaluated them for quality and bias.
Results: Altogether 22 fMRI studies were included in this review. The risk of bias of the included studies was generally low. The findings indicated that in IBS patients, increased or decreased brain areas were mostly associated with visceral sensations, emotional processing, and pain processing. According to brain network research, IBS may exhibit anomalies in the DMN, CEN, and emotional arousal networks. The fluctuations in emotion (anxiety, sadness) and symptoms in IBS patients were associated with alterations in the relevant brain regions.
Conclusion: This study draws a preliminary conclusion that there are insufficient data to accurately distinguish the different neurological features of IBS in the resting state. Additional high-quality research undertaken by diverse geographic regions and teams is required to reach reliable results regarding resting-state changed brain regions in IBS.
Introduction
Irritable bowel syndrome (IBS), one of the most prevalent chronic pain conditions, is defined by persistent stomach pain, as well as changes in stool shape and frequency (Ford and Talley, 2012; Holtmann et al., 2016; Ford et al., 2020). The global estimated prevalence of IBS ranged from 5 to 15% (Lovell and Ford, 2012; Quigley et al., 2012; Ford et al., 2020). Moreover, IBS significantly damages patients’ mental health and quality of life (Chang et al., 2010; Wong et al., 2013; Ford et al., 2017; Aziz et al., 2021), that IBS is more prevalent in patients with psychological co-morbidities (Ford et al., 2020). The incidence of IBS in combination with anxiety or depression has been estimated to be between 40 and 60% or higher (Dekel et al., 2013). Nevertheless, the pathophysiology of IBS is not fully understood, which has hampered IBS diagnosis and treatment.
Changes in the signals between the brain and the gut have also been linked to visceral hypersensitivity and central nervous alternation in IBS patients (Mayer, 2011; Kano et al., 2018). This brain-gut link is a complex integrated circuit that transmits information from the emotional and cognitive centers of the brain to the gastrointestinal tract via neurotransmitters (Gaman and Kuo, 2008). This explains why stress and psychological factors have a substantial association with IBS. Thus, understanding the brain activity related to IBS is critical for elucidating the pathophysiology.
Neuroimaging developments have contributed to the elucidation of the brain-gut relationship. In IBS, rectal stimulation revealed brain activation patterns consistent with visceral pain (Tillisch et al., 2011). It has been shown that the altered brain regions in IBS mainly engaged in processing affective and cognitive aspects of visceral sensitivity/pain (Skrobisz et al., 2019). These findings are comparable to those observed in repeatable reactions to pain in a variety of clinical conditions (Apkarian et al., 2005). The frontal orbital cortex integrates sensory signals including feeding behavior associated with the digestive tract, visceral pain, and other sensory signals that feed into the insula and anterior cingulate cortex (ACC). Ringel et al. (2006) observed that ACC activity (non-marginal ACC) was lower in IBS patients with drug abuse history than in IBS patients without drug abuse history and healthy controls (HCs). However, earlier studies using positron emission tomography (PET) showed that IBS patients had lower functional connectivity (FC) between ACC and anterior middle cingulate cortex (aMCC) activity when rectal dilatation was attempted (Ringel et al., 2003). Regardless of the distinctions, neuro-digestive brain imaging investigations enable a more precise assessment of the relationship between brain and peripheral processes, particularly the mechanisms through which psychosocial factors affect visceral brain sensitivity.
Resting-state imaging techniques have also been utilized to investigate abnormal spontaneous brain activity in IBS (O’Connor and Zeffiro, 2019), with the purpose of elucidating the brain’s inherent activities. Resting-state functional magnetic resonance imaging (rs-fMRI) is the most frequently utilized neuroimaging tool for examining pathological abnormalities in brain function associated with IBS. Consistent findings from several existing resting-state functional MRI studies indicated that patients with IBS have abnormal brain functions associated with versal sensation, psychological emotions (Aizawa et al., 2012; Hubbard et al., 2015; Ma et al., 2015). The exact neural substrate of IBS remains to be clarified due to the heterogeneity of the study methods, which makes it difficult to generalize the findings.
The present review aimed to identify the difference in brain functions between IBS patients and HCs without any intervention and provided a reference for further applications in neuroimage on IBS pathogenies.
Materials and Methods
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 statement (Page et al., 2021) and recommendations for neuroimaging meta-analysis (Nichols et al., 2017). This study evaluated available journal articles without regard for ethical considerations.
Eligibility Criteria
Studies met the following inclusion or exclusion criteria were involved in the present review. We included studies: (1) peer-reviewed journal papers; (2) IBS patients as study population (no limitation of diagnosis); (3) a patient group with IBS and a HCs group were contrasts, there was no limitation of age in both groups; (4) Imaging technology for brain screening was not limited. The following studies were excluded: (1) reviews, systematic reviews, medical cases, research protocols, conference papers, animal studies, letter; (2) duplicate literature, including duplicates in English and Chinese, or studies using the same registration number/ethics number; (3) studies of brain structure (gray matter, white matter, and cortical thickness); or functional studies involving brain structures, such as FC of gray matter volumes; (4) small sample size (<10 IBS patients/HCs); (5) task-state studies.
Search Strategies
Journal article from electronic databases included PubMed, EMBASE, PsycINFO, Web of Science Core, CNKI Database, Wanfang Database, VIP Database, and CBMdisc. Search dates from inception to March 14th, 2022. The reference published in English and Chinese were included. The search strategies were based on various combinations of the search terms, including “Irritable Bowel Syndrome,” “Magnetic Resonance Imaging,” “Functional MRI,” “Computed Tomography,” “Positron-Emission Tomography,” etc. Specific search strategies were appended in the Supplementary Material.
Data Selection and Collection
L-YL searched the literature in the appropriate search library based on the search formula. Two reviewers (Y-YL and LY) independently screened the included records to complete the literature selection. Next, L-YL determined the disagreement between individual judgments. Purpose, imaging methods, diagnoses, subject characteristics, brain regions, and clinical outcomes were extracted from the study documents. The extraction operation was performed independently by two reviewers (Y-YL and LY). L-YL determined the disagreement between individual judgments. Researchers were contacted by L-YL via email for unreported data or other details. All the above operations were done in Excel.
Risk of Bias Assessment
We assessed the study quality and risk of bias based on Nichol et al.’s (2017) study. The estimation items included: (1) research objective; (2) recruitment; (3) eligible criteria; (4) population demographics; (5) imaging methodology; (6) comparison group. Full reporting of the above six items represents a low risk of bias in this study; studies meeting the five items are at medium risk of bias; studies meeting four or lower above items are at high risk of bias.
Results
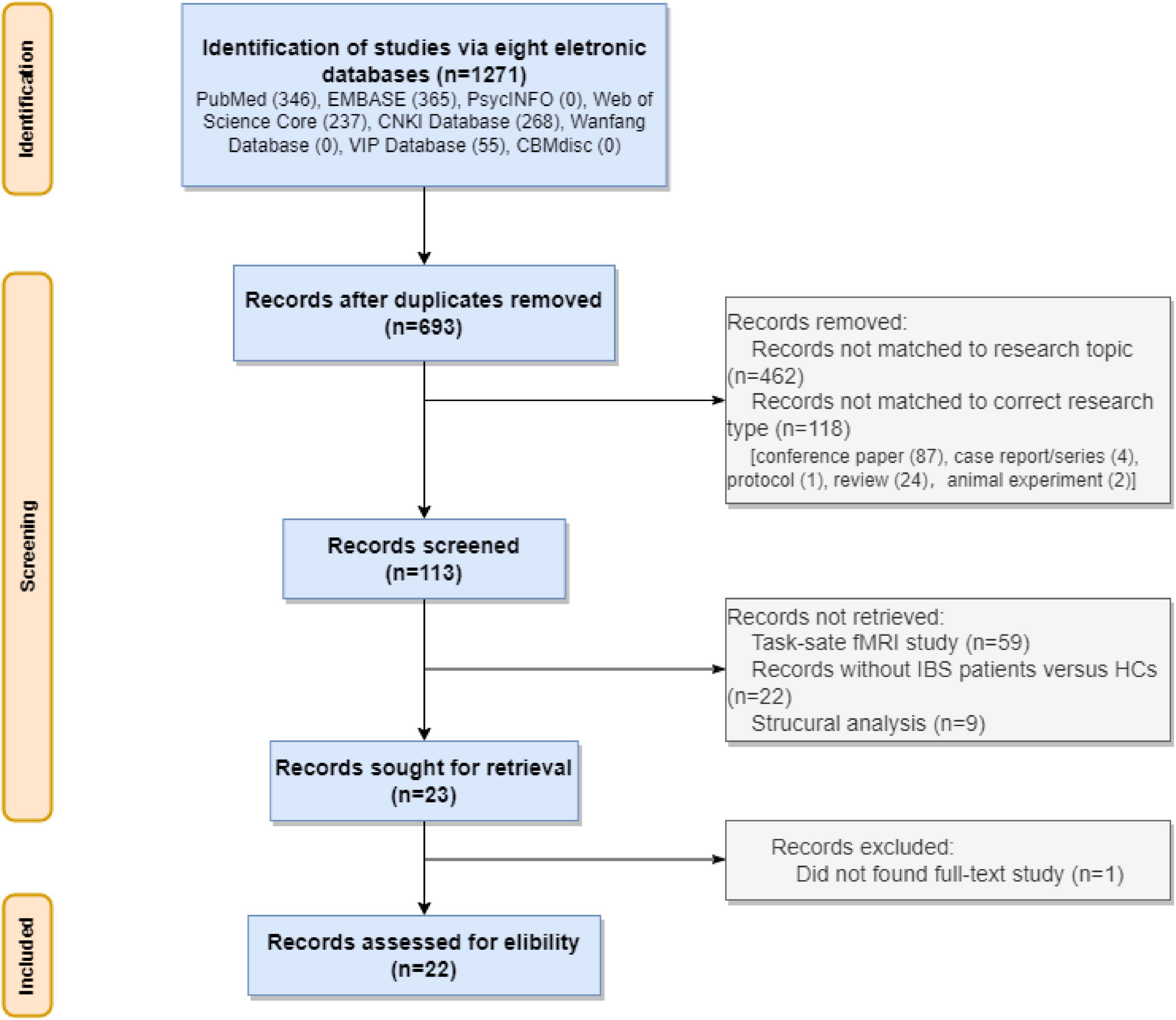
A total of 1271 studies were obtained throughout the electronic databases. Of this, 578 duplicated papers were removed. After examining the research titles, we removed 462 irrelevant studies and 118 studies with inappropriate study types (87 conference papers, 4 case reports/series, 1 protocol, 24 reviews, and 2 animal studies). Of the remaining 113 studies, we removed 59 task-state fMRI studies, 21 studies with the wrong comparison, 9 studies for brain structural analysis or functional analysis based on brain structure. Of the 23 potential studies, the full text of one study (Bernstein et al., 1999) was not available. We tried to contact the corresponding author for full text but did not receive any answer. Finally, we involved 22 rs-fMRI studies. See Figure 1.
Study Characteristics
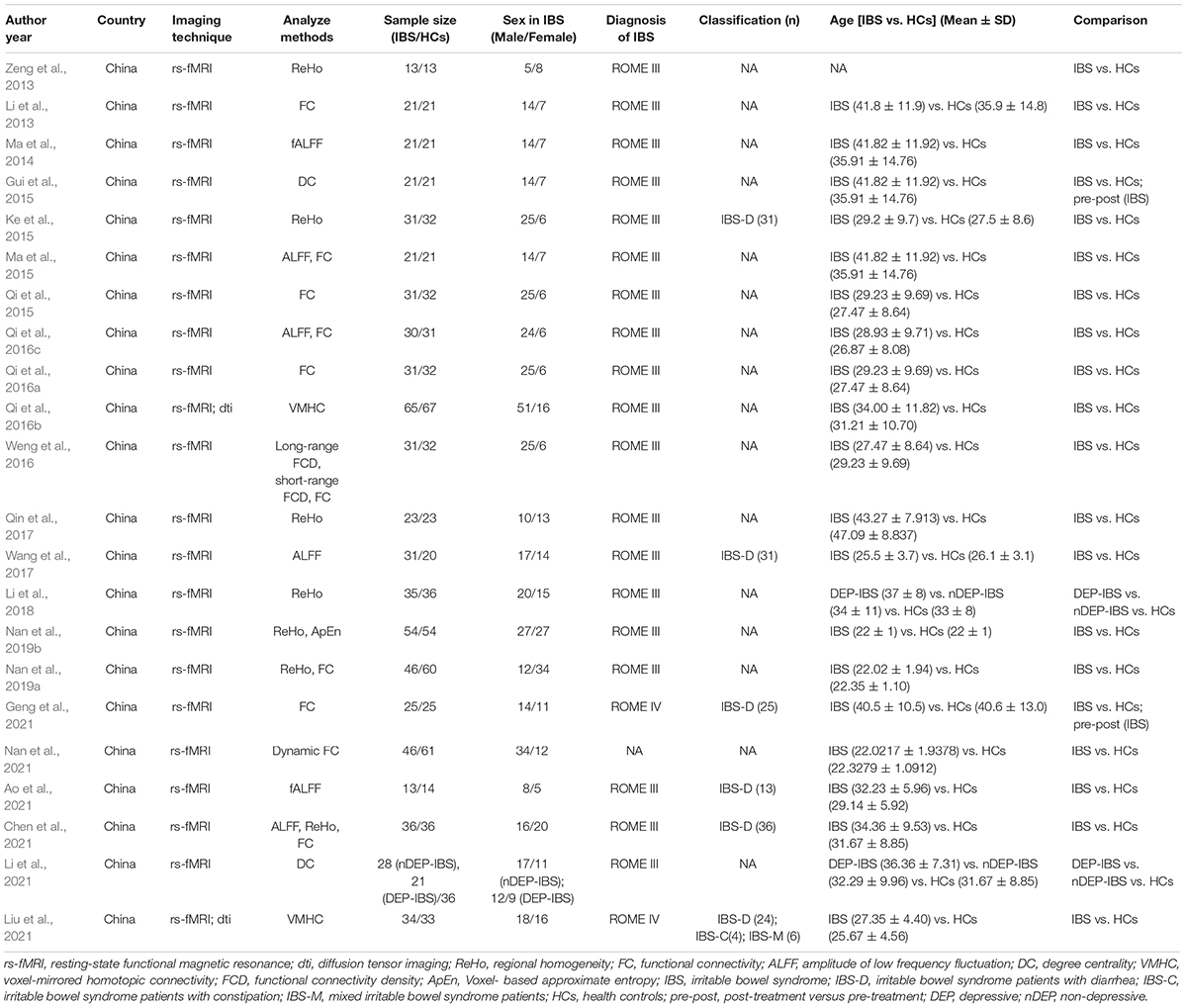
This systematic review comprised 22 rs-fMRI studies aimed at characterizing brain function (Li et al., 2013, 2018, 2021; Zeng et al., 2013; Ma et al., 2014, 2015; Gui et al., 2015; Ke et al., 2015; Qi et al., 2015, 2016a,b; Weng et al., 2016; Qin et al., 2017; Wang et al., 2017; Nan et al., 2019a,b, 2021; Ao et al., 2021; Chen et al., 2021; Geng et al., 2021; Liu et al., 2021). These studies were conducted at a variety of research sites around China and contained a sample size of at least 10 participants. The diagnostic criteria for IBS in the included studies were respectively ROME III (Longstreth et al., 2006) in 19 studies (Li et al., 2013, 2018, 2021; Zeng et al., 2013; Ma et al., 2014, 2015; Gui et al., 2015; Ke et al., 2015; Qi et al., 2015, 2016a,b; Weng et al., 2016; Qin et al., 2017; Wang et al., 2017; Nan et al., 2019a,b; Ao et al., 2021; Chen et al., 2021), ROME IV (Drossman, 2016) in one study (Geng et al., 2021), and undefined criteria in one study (Nan et al., 2021). For IBS categories, five studies included IBS-Diarrhea (IBS-D) patients (Ke et al., 2015; Wang et al., 2017; Ao et al., 2021; Chen et al., 2021; Geng et al., 2021), and one study (Liu et al., 2021) included patients with different IBS categories, including IBS-constipation (IBS-C), IBS-mix (IBS-M) and IBS-D. Two studies by Li J’s team explored IBS with or without depression versus HCs (Li et al., 2018, 2021). Table 1 summarized the study’s characteristics.
Study Quality and Risk of Bias
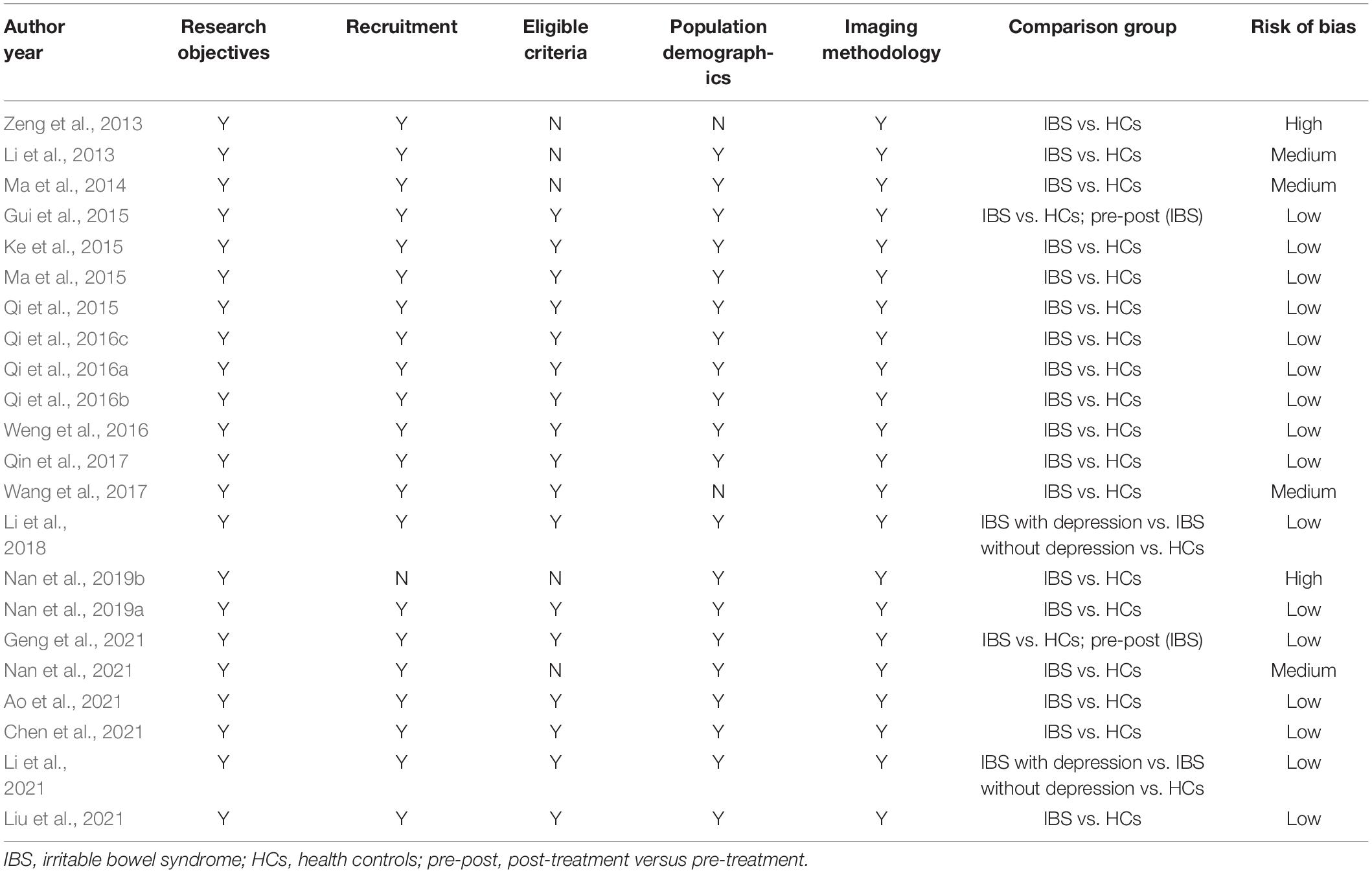
Two reviewers (Y-YL and LY) independently evaluated the risk of bias through six items and eventually reached a consensus. Among all, 16 studies were low risk of bias (Gui et al., 2015; Ke et al., 2015; Ma et al., 2015; Qi et al., 2015, 2016a,b; Weng et al., 2016; Qin et al., 2017; Li et al., 2018, 2021; Nan et al., 2019a; Ao et al., 2021; Chen et al., 2021; Geng et al., 2021; Liu et al., 2021). Four studies reduced to medium risk of bias, for undetailed exclusion criteria (Li et al., 2013; Ma et al., 2014; Nan et al., 2021), or insufficient baseline (Wang et al., 2017). In addition, two studies were considered high risk of bias (Zeng et al., 2013; Nan et al., 2019b). One study lacked the descriptions of exclusion criteria and population demographics (Zeng et al., 2013). Another study made no reference to specific recruitment or eligibility criteria (Nan et al., 2019b). See Table 2.
fMRI Findings
Regional homogeneity (ReHo), amplitude of low frequency fluctuations (ALFF)/fractional ALFF (fALFF), FC, dynamic FC, density connectivity (DC), functional connectivity density (FCD), voxel-mirrored homotopic connectivity (VMHC) were applied in altogether 22 studies. We did not assess meta-analysis due to the high heterogeneity between studies. Likewise, we cautioned to interpretate the outcomes. See Figure 2.
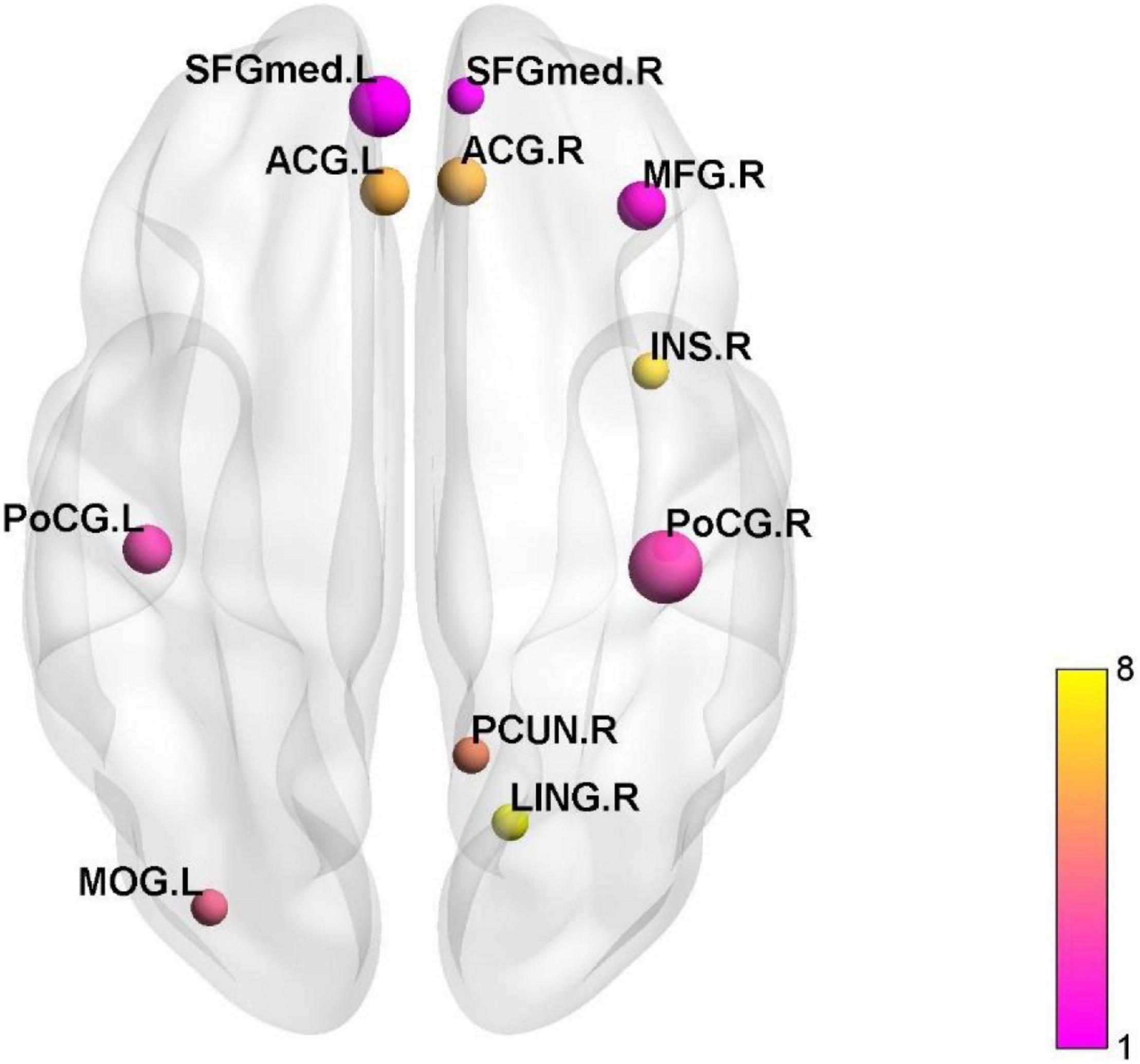
Figure 2. The active and inactive brain regions in the cluster analysis. SFGmed, superior frontal gyrus, medial; ACG, anterior cingulate and paracingulate gyri; MFG, middle frontal gyrus; INS, insula; PoCG, postcentral gyrus; PCUN, precuneus; LING lingual gyrus; MOG, middle occipital gyrus.
Amplitude of Low Frequency Fluctuations/fractional ALFF/Regional Homogeneity
We summarized 12 studies adopted ALFF (Ma et al., 2015; Qi et al., 2016c; Wang et al., 2017; Chen et al., 2021), fALFF (Ma et al., 2014; Ao et al., 2021), ReHo (Zeng et al., 2013; Ke et al., 2015; Qin et al., 2017; Li et al., 2018; Nan et al., 2019a,b), and performed clustering analysis of activated or inactivated brain regions. Nevertheless, we discovered a wide distribution of active or inactive brain regions in the 12 studies. The cluster analysis revealed that postcentral gyrus (PoCG), superior frontal gyrus (SFG), middle frontal gyrus (MFG), ACC, middle occipital gyrus (MOG), precuneus (PCUN), insula (INS), and lingual gyrus (LING) were the most frequently reported (see Figure 1). In IBS patients, activated or inactive brain areas were primarily associated with visceral sensation (PoCG, ACC, INS), emotional processing [hippocampus (HIP)], and pain processing [frontal lobe, parietal lobe, supplementary motor area (SMA), hypothalamus, HIP, cerebellum, caudate nucleus, and PCUN]. See Supplementary Table 1.
Functional Connectivity Density/Density Connectivity
Three studies assessed brain function in IBS patients using a data-driven FCD/DC algorithm (Gui et al., 2015; Weng et al., 2016; Li et al., 2021). Reduced DC was observed in regions of the brain associated with higher cognitive functions (prefrontal gyrus, PCUN), DMN [right orbitofrontal cortex], and middle temporal gyrus (MTG) in Gui et al.’s (2015) study. Weng et al.’s (2016) study showed reduced long-range FCD and short-range FCD in anterior MCC and inferior parietal cortex (IPC), and increased long-range FCD and short-range FCD in primary motor cortex (PoCG and PreCG). Li et al. (2021) found that differential brain activities between IBS patients and HCs was predominantly in the left medial PFC.
Functional Connectivity
Altogether, nine studies examined the brain regions that differed between IBS and HCs (Li et al., 2013; Ma et al., 2015; Qi et al., 2015, 2016a; Weng et al., 2016; Nan et al., 2019b; Chen et al., 2021; Geng et al., 2021). Seven studies performed voxel-wise FC analysis (Li et al., 2013; Ma et al., 2015; Qi et al., 2016a; Weng et al., 2016; Nan et al., 2019b; Chen et al., 2021; Geng et al., 2021), while two studies conducted ROI-wise analysis (Qi et al., 2015, 2016c). Regarding ROI selection, we found that the ROI was primarily located in primary sensory cortex including PoCG, emotional arousal network including amygdala (AMYG) and default mode network (DMN) including HIP. Alternatively, four studies explored the FC between regions activated or inactivated in the IBS brain (Ma et al., 2015; Qi et al., 2016c; Weng et al., 2016; Chen et al., 2021).
Nan et al. (2019a) revealed that brain activities of PoCG in IBS patients showed closely correlated with visceral pain. When classifying the IBS and the HCs, PoCG demonstrated greater sensitivity than INS. Qi et al. (2016b) found that IBS patients had higher positive resting-state FC between the AMYG and INS, midbrain, parahippocampal gyrus, precentral gyrus (PreCG), PoCG and SMA. Three studies identified HIP as the region of interest (ROI), but the FC between HIP and other brain regions varied significantly (Li et al., 2013; Qi et al., 2016c; Geng et al., 2021). The FC between HIP and brain regions associated with advanced cognition [left MFG, left superior parietal gyrus (SPG), and right PCUN] was found to be increased (Li et al., 2013). The findings of Geng et al.’s (2021) study showed reduced FC between emotion-related regions [bilateral inferior temporal gyrus (ITG), bilateral cingulate gyrus] and HIP of IBS-D patients with mood disturbances. However, in the Ma et al.’s (2015) study, no FC were identified between HIP and other brain regions. Furthermore, Qi et al. (2016c) demonstrated that IBS patients had lower FC across DMN subregions, most notably between the PCUN and ACC, and prefrontal cortex (PFC).
Voxel-Mirrored Homotopic Connectivity
Two studies (Qi et al., 2016b; Liu et al., 2021) found increased VMHC in the cuneus, occipital, and posterior cingulate gyrus in patients with IBS, suggesting increased strength of inter-regional temporal correlations or functional connectivity between the cerebral hemispheres.
Brain Activity Associated With Clinical Characteristics
Associations between clinical characteristics and the brain activity were explored in 12 studies (Zeng et al., 2013; Ke et al., 2015; Qi et al., 2015, 2016a,b; Weng et al., 2016; Li et al., 2018, 2021; Ao et al., 2021; Chen et al., 2021; Liu et al., 2021). The following rating scales were used to assess clinical symptoms for IBS patients: anxiety (Hamilton Anxiety Scale, HAMA scale), depression (Hamilton Depression Scale, HAMD scale), IBS symptom severity (IBS -symptom severity system, IBS-SSS), pain intensity of IBS (visual analog scale, VAS), IBS duration, gastrointestinal symptoms (gastrointestinal symptom rating scale, GSRS), quality of life (IBS-quality of life, IBS-QOL). The result showed that brain activities in SFG (Zeng et al., 2013), SMA (Zeng et al., 2013), midbrain (Chen et al., 2021) were positively connected with anxiety of IBS, and that in PCC (posterior cingulate cortex) (Li et al., 2018), midbrain (Chen et al., 2021), INS (Li et al., 2021) were positively connected with depression of IBS. The IBS-SSS scores positively or negatively correlated with FCD/FC/ReHo values in certain brain regions. Qi et al.’s (2016a) and Weng et al.’s (2016) study showed that the functional activities of INS were positively correlated with IBS symptoms. Qi et al. (2015) showed that the average DMN FC was negatively correlated with IBS-SSS in IBS patients. Otherwise, Ke et al. (2015) found that the IBS-SSS scores positively correlated with ReHo values in the left thalamus (THA) and negatively correlated with those in the right ventral medial PFC and MFG. Two studies showed that GSRS scores were positively correlated activities of midbrain and PoCG (Li et al., 2018; Chen et al., 2021).
Ke et al. (2015) found that the ReHo values of the PFC was found to be negatively correlated with the pain intensity. Qi et al. (2016c) also found the negative FC between medial PFC and cuneus was negatively correlated with the patients’ pain intensity. Three studies demonstrated that the disease duration and brain activity in brain regions associated with pain processing and perceptions (caudate, PCUN, SMA, INS, MCC) were positively connected (Ke et al., 2015; Weng et al., 2016; Ao et al., 2021).
Discussion
Summary of the Present Study
This study was a descriptive analysis of the 22 rs-fMRI studies examining the difference in brain activity between IBS patients and healthy controls. The included studies were scored in line with the guidance for neuroimaging meta-analysis. The majority (16/22) of imaging studies included in this review were completely stated and included all pertinent entries. In comparison to a prior high-quality systematic review (Tillisch et al., 2011), we eliminated task-state studies including rectal distension, which necessitates a different interpretation of our findings. Clustering of activated or inactive brain regions revealed that brain regions related with IBS anomalies were mostly associated with visceral feeling, emotional processing, and pain processing. The DMN, central execution network (CEN), and emotional arousal network are the study’s focal areas.
The Increased-Decreased Brain Regions in Irritable Bowel Syndrome
The results of this study suggest decreased or increased of extensive frontal and parietal brain regions in patients with IBS, but there are no uniform conclusions. The altered brain regions in IBS patients are associated with visceral pain during resting state. When visceral pain pathways become sensitive, painful sensations persist, making this persistent chronic pain difficult to treat with classical medications (Lelic et al., 2014). Previous studies suggest that abnormal brain area changes caused by visceral pain are also present in patients with long-term IBS in remission, and not only during the rectal distension (Weaver, 2016; Saps, 2017). The insula (INS), hypothalamus (HIP), amygdala (AMYG), cingulate cortex, and prefrontal cortex (PFC) were the primary brain regions associated with pain processing (Liu et al., 2016). The results of three studies showed the inactivation of INS (Weng et al., 2016; Qin et al., 2017; Wang et al., 2017), which revealed the deterioration of pain inhibitory pathways in IBS patients (Seminowicz et al., 2010). Comparatively, the ACC and prefrontal cortex (PFC) are part of the medial pain system, which mediates pain experience, emotional and cognitive components (Bliss et al., 2016; Smith et al., 2021). The included studies also showed inactivation of ACC (Ke et al., 2015; Qi et al., 2016c) and PFC (Ke et al., 2015; Qi et al., 2016c; Weng et al., 2016; Li et al., 2021), suggesting that pain experience and cognition in IBS patients abnormalities.
The Altered Brain Networks in Irritable Bowel Syndrome
The DMN is critical for maintaining resting brain function and is thought to be preferentially affected by chronic pain (Farmer et al., 2012). The DMN consists of highly interconnected medial PFC, ACC/PCC and bilateral inferior parietal cortex (IPC), HIP and other brain regions (Raichle, 2015). Qi et al. (2015) demonstrated that reduced FC in DMN subregions between the ACC and PCUN in IBS patients compared to healthy controls, partially explaining the dysregulation of visceral sensation (Naliboff et al., 2006; Albert et al., 2012). There is now a general consensus that HIP dysfunction in IBS patients has been presented in studies (Aizawa et al., 2012). In the present study, three studies explored FC with the hippocampus as the ROI and reported disparate results (Li et al., 2013; Ma et al., 2015; Geng et al., 2021). Li et al. (2013) found enhanced FCs between the right HIP and brain regions associated with higher cognitions (left MFG, left SPG, and right PCUN) in IBS patients. However, Ma et al. (2015) did not find significant FCS between hippocampus and ACC, and MCC. Geng et al. (2021) identified reduced right HIP-left insula FC in IBS-D patients compared to HCs, possibly due to abnormal activation in this brain region and decreased neural activity in the brain, resulting in reduced emotional regulation.
The DMN showed activation during resting waking states, while the CEN showed activation during cognitively and emotionally challenging activities. CEN maintains and manipulates information in working memory and is also responsible for decision making and problem solving in the pursuit of goal-oriented behavior. In this study, IBS patients had abnormalities in the extensive frontoparietal area (DLPFC, ACC, orbitofrontal cortex, parietal cortex). The excessive concern of IBS patients about their gastrointestinal symptoms further exacerbates visceral hypersensitivity and causes abnormalities in CEN. Emotional arousal networks play an important role in determining the magnitude and duration of autonomic regulation of various intestinal functions. The AMYG is an important link in the emotional arousal network in visceral pain. Qi et al. (2016a) found disturbed resting-state functional connectivity of the amygdala with cortical limbic regions in patients with IBS, which may partially explain the enhanced emotional arousal and visceral information processing associated with IBS.
Psychological Factors in Irritable Bowel Syndrome
The psychiatric co-morbidity of IBS often contribute to a vicious cycle of gastrointestinal symptoms. Li et al. (2018, 2021) showed that depressed IBS patients have impaired brain function in the sensorimotor network, limbic network, suggesting anomalies in nociceptive perception in depressed individuals. From these two studies, whether brain alterations are specific to chronic visceral pain in IBS could not be determined. Therefore, studies on psychological aspects in IBS should be expanded to focus on trait factors (anxiety, depression) or state factors (poor mood) to discover whether brain abnormalities are specific to chronic visceral pain in IBS (Mayer et al., 2019).
Summary and Conclusion
In this retrospective review, rs-fMRI studies with different analytical methods were summarized. These findings pave the way for further investigation of the brain-gut axis pathways in IBS. However, the widespread increased/decreased brain regions in IBS remains unexplained. There is still a need for: more directed, high-quality studies to explore (1) objective brain biomarkers of IBS. (2) central-peripheral functional links in IBS (3) psychological modulation of central pain processing in IBS.
Author Contributions
ZY and L-YL: concept and design. L-YL, Y-YL, and LY: acquisition of data. ZY, L-YL, and Z-LT: drafting of the manuscript. QZ, F-RL, Q-HZ, and S-YY: critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (no. CI2021A04013), the National Natural Science Fund of China Youth (no. 81804207), the Chengdu University of Traditional Chinese Medicine Special Fund for Young Talents of “Xinglin Talent” (no. QJRC2021014), the National Natural Science Foundation of China (no. 82105032), and the Natural Science Foundation of Chongqing (no. cstc2021jcyj-bsh0062).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2022.851586/full#supplementary-material
Abbreviations
ACC, anterior cingulate cortex; ALFF, amplitude of low frequency fluctuations; AMYG, amygdala; CEN, central execution network; DC, density connectivity; DMN, default mode network; FC, functional connectivity; FCD, functional connectivity density; GSRS, gastrointestinal symptom rating scale; HAMA scale, Hamilton Anxiety Scale; HAMD scale, Hamilton Depression Scale; HCs, healthy controls; HIP, hippocampus; IBS, irritable bowel syndrome; IBS-D, IBS-Diarrhea; IBS-C, IBS-constipation (IBS-C); IBS-M, IBS-mix; IBS-QOL, irritable bowel syndrome-quality of life; IBS-SSS, irritable bowel syndrome-symptom severity system; INS, insula; IPC, inferior parietal cortex; ITG, inferior temporal gyrus; LING, lingual gyrus; MCC, middle cingulate cortex; MFG, middle frontal gyrus; MOG, middle occipital gyrus; PCC, posterior cingulate cortex; PCUN, precuneus; PET, positron emission tomography; PFC, prefrontal cortex; PoCG, postcentral gyrus; PreCG, precentral gyrus; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; ReHo, regional homogeneity; ROI, region of interest; rs-fMRI, resting-state functional magnetic resonance imaging; SFG, superior frontal gyrus; SMA, supplementary motor area; SPG, superior parietal gyrus; THA, thalamus; VAS, visual analog scale; VMHC, voxel-mirrored homotopic connectivity.
References
Aizawa, E., Sato, Y., Kochiyama, T., Saito, N., Izumiyama, M., Morishita, J., et al. (2012). Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on FMRI and dynamic causal modeling. Gastroenterology 143, 1188–1198. doi: 10.1053/j.gastro.2012.07.104
Albert, J., Lopez-Martin, S., Tapia, M., Montoya, D., and Carretie, L. (2012). The role of the anterior cingulate cortex in emotional response inhibition. Hum. Brain Mapp. 33, 2147–2160. doi: 10.1002/hbm.21347
Ao, W., Cheng, Y., Chen, M., Wei, F., Yang, G., An, Y., et al. (2021). Intrinsic brain abnormalities of irritable bowel syndrome with diarrhea: a preliminary resting-state functional magnetic resonance imaging study. BMC Med. Imaging 21:4. doi: 10.1186/s12880-020-00541-9
Apkarian, A. V., Bushnell, M. C., Treede, R. D., and Zubieta, J. K. (2005). Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 9, 463–484. doi: 10.1016/j.ejpain.2004.11.001
Aziz, M. N. M., Kumar, J., Muhammad Nawawi, K. N., Raja Ali, R. A., and Mokhtar, N. M. (2021). Irritable bowel syndrome, depression, and neurodegeneration: a bidirectional communication from gut to brain. Nutrients 13:3061. doi: 10.3390/nu13093061
Bernstein, C., Frankenstein, U. N., Rawsthorne, P., Sweetland, C., Meier, C., and McIntyre, M. C. (1999). Activation of the anterior cingulate and dorsolateral prefrontal cortex in normals, IBD and IBS by functional MRI (fMRI). Gastroenterology.
Bliss, T. V., Collingridge, G. L., Kaang, B. K., and Zhuo, M. (2016). Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat. Rev. Neurosci. 17, 485–496. doi: 10.1038/nrn.2016.68
Chang, J. Y., Locke, G. R. III, McNally, M. A., Halder, S. L., Schleck, C. D., Zinsmeister, A. R., et al. (2010). Impact of functional gastrointestinal disorders on survival in the community. Am. J. Gastroenterol. 105, 822–832. doi: 10.1038/ajg.2010.40
Chen, X. F., Guo, Y., Lu, X. Q., Qi, L., Xu, K. H., Chen, Y., et al. (2021). Aberrant Intraregional Brain Activity and Functional Connectivity in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Front. Neurosci. 15:721822. doi: 10.3389/fnins.2021.721822
Dekel, R., Drossman, D. A., and Sperber, A. D. (2013). The use of psychotropic drugs in irritable bowel syndrome. Expert Opin. Investig. Drugs 22, 329–339. doi: 10.1517/13543784.2013.761205
Drossman, D. A. (2016). Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterol. [Online ahead of print]. doi: 10.1053/j.gastro.2016.02.032
Farmer, M. A., Baliki, M. N., and Apkarian, A. V. (2012). A dynamic network perspective of chronic pain. Neurosci. Lett. 520, 197–203. doi: 10.1016/j.neulet.2012.05.001
Ford, A. C., Lacy, B. E., and Talley, N. J. (2017). Irritable bowel syndrome. N. Engl. J. Med. 376, 2566–2578.
Ford, A. C., Sperber, A. D., Corsetti, M., and Camilleri, M. (2020). Irritable bowel syndrome. Lancet 396, 1675–1688.
Gaman, A., and Kuo, B. (2008). Neuromodulatory processes of the brain-gut axis. Neuromodulation 11, 249–259. doi: 10.1111/j.1525-1403.2008.00172.x
Geng, H., Feng, S. J., Zhao, T. T., Chen, L., Wu, X. L., Zhou, J. L., et al. (2021). [Mind-regulating and spleen-strengthening needling technique improves abdominal hypersensitivity and emotion by enhancing functional connectivity between hippocampus and brain regions in diarrhea-predominant irritable bowel syndrome patients]. Zhen Ci Yan Jiu 46, 318–325. doi: 10.13702/j.1000-0607.200569
Gui, R. H., Li, S. X., Zheng, X. P., Wang, X. Y., Zhang, D. X., and Wu, Y. X. (2015). [Functional network changes by evaluating degree centrality in patients with irritable bowel syndrome]. Chin. J. Neuromed. 14, 1148–1151.
Holtmann, G. J., Ford, A. C., and Talley, N. J. (2016). Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 1, 133–146.
Hubbard, C. S., Hong, J., Jiang, Z., Ebrat, B., Suyenobu, B., Smith, S., et al. (2015). Increased attentional network functioning related to symptom severity measures in females with irritable bowel syndrome. Neurogastroenterol. Motil. 27, 1282–1294. doi: 10.1111/nmo.12622
Kano, M., Dupont, P., Aziz, Q., and Fukudo, S. (2018). Understanding neurogastroenterology from neuroimaging perspective: a comprehensive review of functional and structural brain imaging in functional gastrointestinal disorders. J. Neurogastroenterol. Motil. 24, 512–527. doi: 10.5056/jnm18072
Ke, J., Qi, R., Liu, C., Xu, Q., Wang, F., Zhang, L., et al. (2015). Abnormal regional homogeneity in patients with irritable bowel syndrome: a resting-state functional MRI study. Neurogastroenterol. Motil. 27, 1796–1803. doi: 10.1111/nmo.12692
Lelic, D., Brock, C., Simren, M., Frokjaer, J. B., Softeland, E., Dimcevski, G., et al. (2014). The brain networks encoding visceral sensation in patients with gastrointestinal symptoms due to diabetic neuropathy. Neurogastroenterol. Motil. 26, 46–58. doi: 10.1111/nmo.12222
Li, J., Li, G. X., Guo, Y., Lu, X. Q., Li, L., and Ding, J. P. (2018). [Regional homogeneity in the patients of irritable bowel syndrome complicated with depression: a resting-state functional magnetic resonance imaging study]. Zhonghua Yi Xue Za Zhi 98, 196–201. doi: 10.3760/cma.j.issn.0376-2491.2018.03.008
Li, J., Lu, X., Liu, M., Ding, J., He, P., Guo, Y., et al. (2021). A resting-state functional magnetic resonance imaging study of whole-brain functional connectivity of voxel levels in patients with irritable bowel syndrome with depressive symptoms. J. Neurogastroenterol. Motil. 27, 248–256. doi: 10.5056/jnm20209
Li, S. M., Jiang, G. H., Ma, X. F., Su, H. H., Zhan, W. F., Tian, J. Z., et al. (2013). [Functional Connectivity of Right Hippocampus in Irritable Bowel Syndrome during Resting State]. J. Sun Yat-sen Univ. 34, 932–937.
Liu, G., Li, S., Chen, N., Zhao, Z., Guo, M., Liu, H., et al. (2021). Inter-hemispheric Functional Connections Are More Vulnerable to Attack Than Structural Connection in Patients With Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 27, 426–435. doi: 10.5056/jnm20134
Liu, X., Silverman, A., Kern, M., Ward, B. D., Li, S. J., Shaker, R., et al. (2016). Excessive coupling of the salience network with intrinsic neurocognitive brain networks during rectal distension in adolescents with irritable bowel syndrome: a preliminary report. Neurogastroenterol. Motil. 28, 43–53. doi: 10.1111/nmo.12695
Longstreth, G. F., Thompson, W. G., Chey, W. D., Houghton, L. A., Mearin, F., and Spiller, R. C. (2006). Functional bowel disorders. Gastroenterology 130, 1480–1491.
Lovell, R. M., and Ford, A. C. (2012). Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 10, 712–721.e4. doi: 10.1016/j.cgh.2012.02.029
Ma, X., Wang, T., Xu, Y., Li, S., Tian, J., Jiang, G., et al. (2015). Altered brain spontaneous activity and connectivity network in irritable bowel syndrome patients: a resting-state fMRI study. Clin. Neurophysiol. 126, 1190– 1197.
Ma, X. F., Jiang, G. H., Li, S. M., Fang, J., Zhan, W. F., Tian, J. Z., et al. (2014). [Fraction amplitude of low-frequency fluctuation in irritable bowel syndrome patients: a resting-state fMRI study]. Chin. J Neuromed. 13, 292–295.
Mayer, E. A. (2011). Gut feelings: the emerging biology of gut-brain communication. Nat. Rev. Neurosci. 12, 453–466. doi: 10.1038/nrn3071
Mayer, E. A., Labus, J., Aziz, Q., Tracey, I., Kilpatrick, L., Elsenbruch, S., et al. (2019). Role of brain imaging in disorders of brain-gut interaction: a Rome Working Team Report. Gut 68, 1701–1715. doi: 10.1136/gutjnl-2019-318308
Naliboff, B. D., Berman, S., Suyenobu, B., Labus, J. S., Chang, L., Stains, J., et al. (2006). Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology 131, 352–365. doi: 10.1053/j.gastro.2006.05.014
Nan, J., Yang, W., Meng, P., Huang, W., Zheng, Q., Xia, Y., et al. (2019a). Changes of the postcentral cortex in irritable bowel syndrome patients. Brain Imaging Behav. 14, 1566–1576. doi: 10.1007/s11682-019-00087-7
Nan, J., Zhang, L. L., Zheng, Q., Zhang, M. H., and Lu, Z. T. (2019b). [Comparison between approximate entropy and regional homogeneity for identification of irritable bowel syndrome based on functional magnetic resonance imaging]. Nan Fang Yi Ke Da Xue Xue Bao 39, 1023–1029. doi: 10.12122/j.issn.1673-4254.2019.09.04
Nan, J. F., Meng, P. T., Zong, N. N., and Zhang, J. C. (2021). [Dynamic characteristics of brain networks in patients with irritable bowel syndrome based on functional magnetic resonance imaging]. Acta Physiol. Sin. 73, 355–368.
Nichols, T. E., Das, S., Eickhoff, S. B., Evans, A. C., Glatard, T., Hanke, M., et al. (2017). Best practices in data analysis and sharing in neuroimaging using MRI. Nat. Neurosci. 20, 299–303. doi: 10.1038/nn.4500
O’Connor, E. E., and Zeffiro, T. A. (2019). Why is clinical fMRI in a resting state? Front. Neurol. 10:420. doi: 10.3389/fneur.2019.00420
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71.
Qi, R., Ke, J., Xu, Q., Zhang, L. J., Lu, G. M., Liu, C., et al. (2016c). Intrinsic brain abnormalities in irritable bowel syndrome and effect of anxiety and depression. Brain Imaging Behav. 10, 1127–1134. doi: 10.1007/s11682-015-9478-1
Qi, R., Ke, J., Xu, Q., Zhang, L. J., Lu, G. M., Schoepf, U. J., et al. (2015). Topological reorganization of the default mode network in irritable bowel syndrome. Mol. Neurobiol. 53, 6585–6593. doi: 10.1007/s12035-015-9558-7
Qi, R., Liu, C., Ke, J., Xu, Q., Ye, Y., Jia, L., et al. (2016a). Abnormal amygdala resting-state functional connectivity in irritable bowel syndrome. AJNR Am. J. Neuroradiol. 37, 1139–1145. doi: 10.3174/ajnr.A4655
Qi, R., Weng, Y., Xu, Q., Zhang, L. J., Lu, G. M., Liu, C., et al. (2016b). Disturbed interhemispheric functional connectivity rather than structural connectivity in irritable bowel syndrome. Front. Mol. Neurosci. 9:141. doi: 10.3389/fnmol.2016.00141
Qin, M., Zhang, Q., and Huang, J. B. (2017). [Regional homogeneity of brain in irritable bowel syndrome patient: a resting state fMRI study]. J. Pract. Med. 33, 3455–3458.
Quigley, E. M., Abdel-Hamid, H., Barbara, G., Bhatia, S. J., Boeckxstaens, G., De Giorgio, R., et al. (2012). A global perspective on irritable bowel syndrome: a consensus statement of the World Gastroenterology Organisation Summit Task Force on irritable bowel syndrome. J. Clin. Gastroenterol. 46, 356–366. doi: 10.1097/MCG.0b013e318247157c
Ringel, Y., Drossman, D. A., Leserman, J. N., Lin, W., Wilber, K., Suyenobu, B. Y., et al. (2006). Association between central activation and pain reports in women with IBS. Gastroenterology 130, A77–A78.
Ringel, Y., Drossman, D. A., Turkington, T. G., Bradshaw, B., Hawk, T. C., Bangdiwala, S., et al. (2003). Regional brain activation in response to rectal distension in patients with irritable bowel syndrome and the effect of a history of abuse. Dig. Dis. Sci. 48, 1774–1781. doi: 10.1023/a:1025455330704
Saps, M. (2017). Functional neuroimaging in irritable bowel syndrome: a brain twister. J. Pediatr. Gastroenterol. Nutr. 65, 489–490. doi: 10.1097/MPG.0000000000001654
Seminowicz, D. A., Labus, J. S., Bueller, J. A., Tillisch, K., Naliboff, B. D., Bushnell, M. C., et al. (2010). Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology 139:e42. doi: 10.1053/j.gastro.2010.03.049
Skrobisz, K., Piotrowicz, G., Drozdowska, A., Markiet, K., Sabisz, A., Szurowska, E., et al. (2019). Use of functional magnetic resonance imaging in patients with irritable bowel syndrome and functional dyspepsia. Prz. Gastroenterol. 14, 163–167. doi: 10.5114/pg.2019.88163
Smith, M. L., Asada, N., and Malenka, R. C. (2021). Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science 371, 153–159. doi: 10.1126/science.abe3040
Tillisch, K., Mayer, E. A., and Labus, J. S. (2011). Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 140, 91–100. doi: 10.1053/j.gastro.2010.07.053
Wang, L. J., Mao, G. Q., Cheng, Y. G., Wei, F. Q., and Tu, G. F. (2017). [Changes of low frequency fluctuation amplitude of resting state brain functional MRI in irritable bowel syndrome]. J. Med. Imaging 27, 2220–2223.
Weaver, K. R. (2016). Neuroimaging the brain-gut axis in patients with irritable bowel syndrome. World J. Gastrointest. Pharmacol. Ther. 7, 320–333. doi: 10.4292/wjgpt.v7.i2.320
Weng, Y., Qi, R., Ke, J., Xu, Q., Zhang, L. J., Lu, G. M., et al. (2016). Disrupted functional connectivity density in irritable bowel syndrome patients. Brain Imaging Behav. 11, 1812–1822. doi: 10.1007/s11682-016-9653-z
Wong, R. K., Drossman, D. A., Weinland, S. R., Morris, C. B., Leserman, J., Hu, Y., et al. (2013). Partner burden in irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 11, 151–155. doi: 10.1016/j.cgh.2012.07.019
Zeng, S. Q., Jiang, G. H., Ma, X. F., Tian, J. Z., Xu, M., Fang, J., et al. (2013). [Relationship between spontaneous neural activities of the local brain regions and Hamilton anxiety scale scores in patients with irritable bowel syndrome: a resting-state fMRI study]. Chin. J. Neuromed. 12, 719–722.
Keywords: irritable bowel syndrome, neuroimaging, brain activity, fMRI, systematic review
Citation: Yu Z, Liu LY, Lai YY, Tian ZL, Yang L, Zhang Q, Liang FR, Yu SY and Zheng QH (2022) Altered Resting Brain Functions in Patients With Irritable Bowel Syndrome: A Systematic Review. Front. Hum. Neurosci. 16:851586. doi: 10.3389/fnhum.2022.851586
Received: 10 January 2022; Accepted: 21 March 2022;
Published: 29 April 2022.
Edited by:
Vilfredo De Pascalis, Sapienza University of Rome, ItalyReviewed by:
Dan Lucian Dumitrascu, Iuliu Hat̨ieganu University of Medicine and Pharmacy, RomaniaEmeran A. Mayer, University of California, Los Angeles, United States
Copyright © 2022 Yu, Liu, Lai, Tian, Yang, Zhang, Liang, Yu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian-Hua Zheng, emhlbmdxaWFuaHVhQGNkdXRjbS5lZHUuY24=; Si-Yi Yu, Y2R1dGNteXN5QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Zheng Yu
Zheng Yu Li-Ying Liu
Li-Ying Liu Yuan-Yuan Lai2†
Yuan-Yuan Lai2† Zi-Lei Tian
Zi-Lei Tian Fan-Rong Liang
Fan-Rong Liang Si-Yi Yu
Si-Yi Yu