
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci., 03 June 2022
Sec. Motor Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnhum.2022.846205
This article is part of the Research TopicUse of Computerized Gait Analysis in Neurological PathologiesView all 14 articles
Background: Altered motor control is common in cerebral palsy (CP). Understanding how altered motor control affects movement and treatment outcomes is important but challenging due to complex interactions with other neuromuscular impairments. While regression can be used to examine associations between impairments and movement, causal modeling provides a mathematical framework to specify assumed causal relationships, identify covariates that may introduce bias, and test model plausibility. The goal of this research was to quantify the causal effects of altered motor control and other impairments on gait, before and after single-event multi-level orthopedic surgery (SEMLS).
Methods: We evaluated the impact of SEMLS on change in Gait Deviation Index (ΔGDI) between gait analyses. We constructed our causal model with a Directed Acyclic Graph that included the assumed causal relationships between SEMLS, ΔGDI, baseline GDI (GDIpre), baseline neurologic and orthopedic impairments (Imppre), age, and surgical history. We identified the adjustment set to evaluate the causal effect of SEMLS on ΔGDI and the impact of Imppre on ΔGDI and GDIpre. We used Bayesian Additive Regression Trees (BART) and accumulated local effects to assess relative effects.
Results: We prospectively recruited a cohort of children with bilateral CP undergoing SEMLS (N = 55, 35 males, age: 10.5 ± 3.1 years) and identified a control cohort with bilateral CP who did not undergo SEMLS (N = 55, 30 males, age: 10.0 ± 3.4 years). There was a small positive causal effect of SEMLS on ΔGDI (1.70 GDI points). Altered motor control (i.e., dynamic and static motor control) and strength had strong effects on GDIpre, but minimal effects on ΔGDI. Spasticity and orthopedic impairments had minimal effects on GDIpre or ΔGDI.
Conclusion: Altered motor control did have a strong effect on GDIpre, indicating that these impairments do have a causal effect on a child’s gait pattern, but minimal effect on expected changes in GDI after SEMLS. Heterogeneity in outcomes suggests there are other factors contributing to changes in gait. Identifying these factors and employing causal methods to examine the complex relationships between impairments and movement will be required to advance our understanding and care of children with CP.
Children diagnosed with cerebral palsy (CP) exhibit altered motor control due to an injury to the brain at or near the time of birth (Desloovere, 2005; Handsfield et al., 2016; O’Brien et al., 2021). Altered motor control can be observed in CP in many ways, such as increased co-contraction, decreased capacity to selectively move individual joints, spasticity, dystonia, and altered movement patterns. Prior research has suggested that quantifying motor control is important to understand function and inform treatment planning (Fowler et al., 2010; Cahill-Rowley and Rose, 2014; Schwartz et al., 2016; Shuman et al., 2018; Bekius et al., 2020). However, altered motor control occurs and interacts with many other impairments in CP, which makes quantifying and isolating the effects of altered motor control challenging. In addition to altered motor control, orthopedic impairments can also develop, including muscle contractures and altered bone morphology (Crane, 1959; Fabry et al., 1973; O’Dwyer et al., 1989; Lee et al., 2009; Mathewson and Lieber, 2015). Together, these neurologic and orthopedic impairments are associated with limitations in movement and impact the capacity of children with CP to participate in daily activities (Rose et al., 1989; Johnston et al., 2004; Bjornson et al., 2014; Kamp et al., 2014; Gross et al., 2018).
The complexity of CP makes it challenging to objectively determine the causal effects of specific impairments on gait. As a result, many children with CP undergo clinical gait analysis (CGA) (Gage et al., 2009), which provides quantitative measures of a child’s gait pattern that can be tracked over time and used to inform treatment decisions (Miller et al., 1996; Steinwender et al., 2000; Gough and Shortland, 2008). In particular, CGA was historically developed to support decision making for orthopedic surgery (Gage et al., 1984; Lee et al., 1992; Sullivan et al., 1995; Gage and Novacheck, 2001). Many children’s hospitals now have CGA laboratories used for pre-operative and post-operative assessments.
While CGA has been used for treatment planning for over 30 years, deciphering causal effects of impairments on gait has remained elusive. Data from CGA is traditionally used to evaluate associations between a specific impairment and an outcome measure, typically using bivariate or multivariate regression analyses applied to retrospective data (Kramer and Ann MacPhail, 1994; Damiano et al., 2000; Ross and Engsberg, 2007; Shin et al., 2015; MacWilliams et al., 2020). In cases where multivariate regression has been used, the choice of variables for inclusion has often not had a clear causal basis. Our prior work to evaluate the impact of motor control on gait and treatment outcomes have relied on these methods (Steele et al., 2015; Schwartz et al., 2016; Shuman et al., 2018). Using multivariate regression with retrospective data from multiple hospitals, we have repeatedly demonstrated that Dynamic Motor Control (DMC) during walking is associated with outcomes (i.e., Gross Motor Functional Classification System Levels, Gait Deviation Index, Walking Speed, Pediatric Outcomes Data Collection Instrument) after orthopedic surgery, rhizotomy, or botulinum toxin injections (Steele et al., 2015; Schwartz et al., 2016). Similar analyses have demonstrated that other impairments—such as strength, hamstring length, or torsional deformities—are also correlated with treatment outcomes (Chambers et al., 1998; Hicks et al., 2011; Shore et al., 2012; Galarraga et al., 2017; Rajagopal et al., 2018).
Understanding whether altered motor control and other impairments cause altered gait or treatment outcomes is nearly impossible with non-causal regression alone. Given the complexity and heterogeneity of CP, this “implied cause by association” approach, without regard to possible confounding, is likely to lead to confusing and even erroneous conclusions. For example, researchers may observe that strength is associated with walking speed. However, strength is also affected by other primary neurologic deficits, like poor motor control, which may have an independent causal impact on speed. Understanding causal effects is impossible without considering these causal pathways and adjusting for relevant factors.
In recent years, there has been remarkable growth in the development and successful applications of causal inference methods (Pearl, 2009; Imbens and Rubin, 2015). From a conceptual perspective, causal methods allow researchers to explicitly share assumed causal relationships and mathematically define covariates necessary for estimating causal effects (Pearl, 1995). From a computational perspective, numerous algorithms have been developed for modeling causal outcomes. Among the most successful of these are Bayesian Additive Regression Trees (BART), which have been shown to produce estimates of causal effects with low levels of bias and variance and realistic confidence intervals (Chipman et al., 2010; Hill, 2011; Dorie et al., 2019; Hahn et al., 2020). Williams et al. (2018) have highlighted the potential of causal inference for pediatrics. However, these methods have had limited application in CP or biomechanics research.
The goal of this research was to quantify the causal effects of motor control and other impairments on gait, before and after orthopedic surgery. Specifically, we prospectively recruited children with CP who were undergoing single-event multilevel orthopedic surgery (SEMLS). We also identified a cohort of controls from the same time period who were not undergoing SEMLS between gait analyses. We developed a causal model and used BART to quantify the effects of motor control and other impairments on changes in gait kinematics after SEMLS. These methods provide a foundation for understanding the complex and interactive effects of impairments on gait for children with CP.
We recruited children with bilateral CP who were between 6 and 18 years old at the time of baseline gait analyses and scheduled for SEMLS. The goal of our prospective recruitment was to follow a representative cohort of patients at Gillette Children’s Specialty Healthcare from their baseline gait analysis through two follow-up assessments at six-months and one-year after SEMLS. The one-year analysis was our primary outcome; however, for nine participants we used the six-month follow-up visit due to pandemic and other scheduling related disruptions. We included patients whose baseline gait analysis was no more than six months before their scheduled surgery date. We defined SEMLS as surgery consisting of two or more major orthopedic procedures on a single side. One participant was scheduled for SEMLS, but only received a single procedure, bilateral femoral derotation osteotomy. We included this participant in the analysis. We also identified a cohort of controls with CP who did not undergo SEMLS. We identified children with bilateral CP who underwent multiple gait analyses with kinematic and electromyographic (EMG) recordings, with a maximum time of 2.5 years between visits during the same time period. We excluded participants who underwent prior or current rectus femoris transfer, since we were evaluating motor control from EMG recordings. This research was conducted with approval from the University of Minnesota Institutional Review Board.
For this analysis we focused on evaluating the impact of SEMLS on gait kinematics. We a priori specified our outcome measure as the Gait Deviation Index (GDI, ClinicalTrials.gov NCT02699554) as a common summary measure of walking kinematics that has been used extensively in prior studies to evaluate and predict treatment outcomes.
We constructed our causal model with a Directed Acyclic Graph (DAG) (Verma and Pearl, 1991; Shrier and Platt, 2008; Brewer et al., 2017). The logic behind our DAG is as follows (Figure 1):
(1) Our objective was to determine the impact of SEMLS on change in GDI (ΔGDI). Thus, SEMLS is our exposure and ΔGDI is our outcome. SEMLS induces a change in impairment (ΔImp) that causes the observed ΔGDI.
(2) The covariates we identified as common causes of both SEMLS (i.e., variables that impact the choice to undergo SEMLS) and ΔGDI included: Age and baseline impairment (Imppre). Baseline impairments represent a set of variables collected during CGA to evaluate neurologic and orthopedic impairments (Table 1).
(3) Baseline GDI (GDIpre) and ΔGDI are related by measurement methods (i.e., noise, errors, regression to the mean) and other, unmeasured factors.
(4) Surgical treatment history (Hx) is a common cause of baseline impairment (Imppre) and whether or not SEMLS is recommended.
(5) We included a general severity (Sev) measure as an unmeasured factor that impacts baseline impairment (Imppre) and surgical treatment history (Hx).

Figure 1. DAG describing the assumed causal relationships between SEMLS (exposure) and ΔGDI (outcome). The causal relationship between SEMLS and ΔGDI is mediated by changes in impairments (ΔImp). Baseline GDI (GDIpre) and ΔGDI are related by measurement methods and other, unmeasured factors. Baseline impairment (Imppre), surgical history (Hx), and Age are also included as causal factors. The DAG also includes unmeasured factors related to general CP severity, which impact baseline impairment and surgical history. The step-by-step process and rationale for this DAG are available in the Supplementary Material and an interactive version is available on dagitty (http://dagitty.net/mUCSPWo).
Note that similar DAGs could be constructed for other outcome measures such as walking speed or energy cost. Similarly, other factors could be added to the DAG, if there were rational arguments that they were common causes of one of the variables in the DAG and ΔGDI. The step-by-step process we used to construct our DAG is illustrated in the Supplementary Material.
From the DAG we determined the variables that needed to be included in any model (e.g., regression, BART) to evaluate the total causal effect of SEMLS on ΔGDI. These variables are called the adjustment set, representing the confounding covariates that could produce bias if not included in an analysis. For this DAG, the minimal sufficient adjustment set to estimate the total causal effect of SEMLS on ΔGDI was: Age, GDIpre, and Imppre. We also determined the adjustment set to evaluate the total causal effect of baseline impairment (Imppre) on ΔGDI and GDIpre. The minimal sufficient adjustments sets were Age and Hx for ΔGDI and Age for GDIpre. The plausibility of a DAG can be evaluated by identifying conditional independencies, variables that should be independent given the causal relationships defined in the DAG. We identified the adjustment sets and independencies with dagitty (Textor et al., 2016) and all analyses were conducted in R (version 4.1.0) (R Core Team, 2021).
To assess the total causal effects of SEMLS and baseline impairment (Imppre) on change in GDI (ΔGDI) we used Bayesian Additive Regression Trees (BART), a machine learning method that uses a boosted ensemble of regression trees for non-parametric function estimation relying on a Bayesian probability model (Chipman et al., 2010). Like other tree-based regression methods, an advantage of BART is that it can handle non-linear effects and interactions (Tan and Roy, 2019). For causal modeling, recent work has demonstrated that BART-based models achieve accurate and precise causal predictions (Hill, 2011; Dorie et al., 2019).
For this analysis, we used BART models to estimate ΔGDI using the adjustment sets identified by the DAG. Thus, to identify the impact of SEMLS on ΔGDI, we included the covariates Age, GDIpre, and Imppre. Baseline impairments were not available for all participants. Missing data in Imppre were imputed using multivariate imputation by chained equations (MICE) (van Buuren and Groothuis-Oudshoorn, 2011). We used the bartMachine package to implement the analysis (Kapelner and Bleich, 2016). We optimized the hyperparameters for each BART model using 10-fold cross-validation. We report the pseudo-R2 (1 – SSE/SST) for each BART model and used k-fold cross-validation (k = 10) to determine the out-of-sample root mean square error (RMSE).
To assess the relative effects of individual variables from BART, we used accumulated local effect (ALE) analysis (Molnar et al., 2018). The ALE analysis is similar to a partial dependence plot, but the averaging is done locally to avoid including observations that are unlikely to ever be realized (e.g., someone walking three standard deviations slower than average but with a normal cadence). The ALE plots illustrate the impact of each variable over the range of values for that variable, conditioned on the other covariates in the model. Thus, ALE plots can be useful for examining non-linear effects identified by BART. For example, the ALE plot can highlight non-linear effects such as when a variable impacts GDI with a deviation from average (i.e., a U-shaped plot) or when a variable only impacts GDI above or below a certain cut-off (i.e., a step function or discontinuity).
We prospectively recruited 55 children with bilateral CP who underwent SEMLS (Table 2). During this same time period, we identified 55 children with bilateral CP who visited the gait laboratory for repeat visits and no intervening surgical procedures. The participants who underwent SEMLS were older and had more femoral anteversion, more tibial torsion, and lower GDI scores at the initial gait analysis than the participants who did not undergo SEMLS. The SEMLS participants received, on average, five procedures (Figure 2).
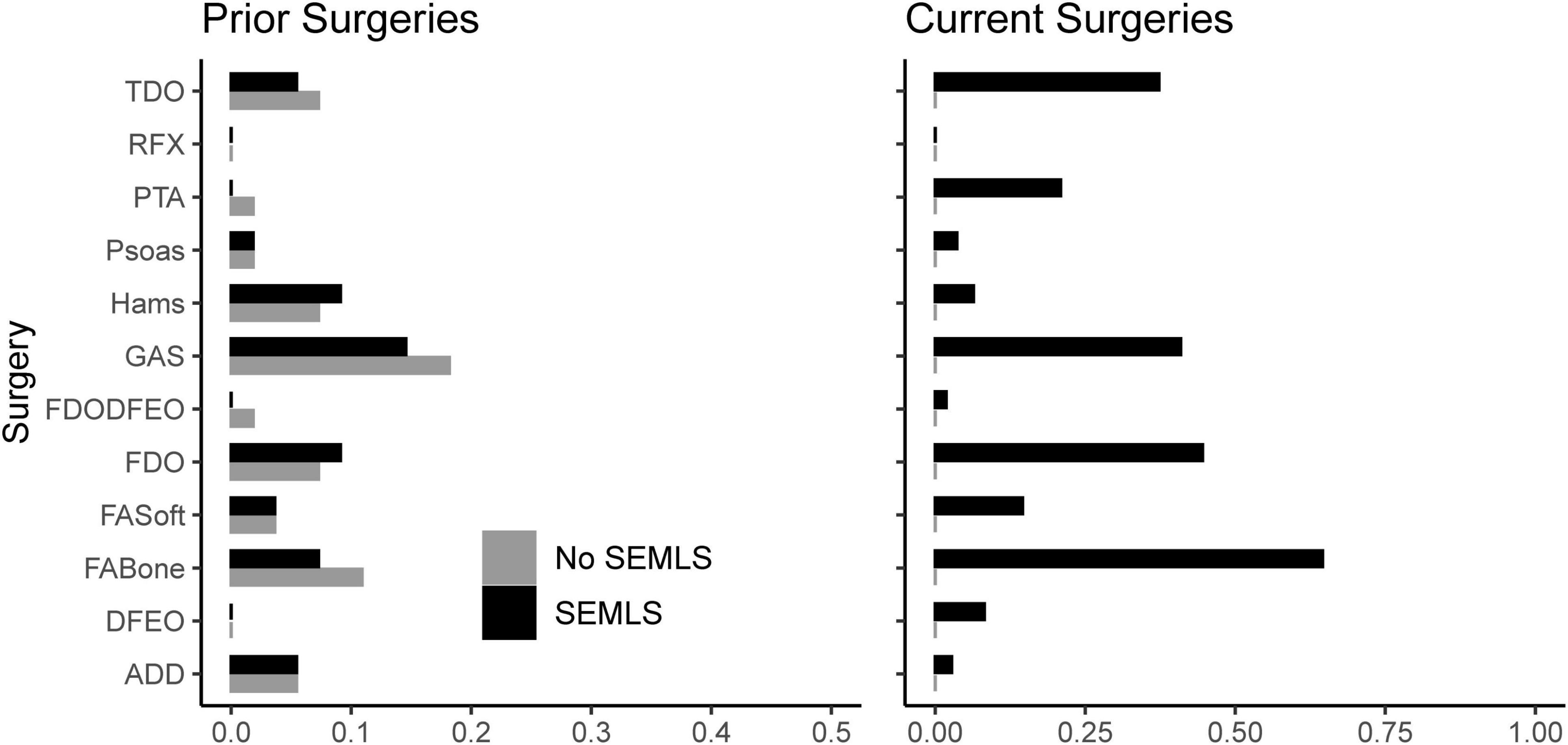
Figure 2. Prior and current surgeries of participants in both cohorts. Note that we excluded potential participants who underwent rectus femors transfer. TDO, tibial derotation osteotomy; RFX, rectus femoris transfer; PTA, patellar tendon advancement; Psoas, psoas lengthening or release; Hams, hamstring lengthening; GAS, plantarflexor lengthening; FDODFEO, distal femoral derotation and extension osteotomy; FDO, femoral derotation osteotomy; FAsoft, foot/ankle soft tissue procedure; FABone, foot/ankle boney procedure; DFEO, distal femoral extension osteotomy; ADD, adductor lengthening or release.
There was a small positive causal effect of SEMLS on ΔGDI. The estimated total causal effect of SEMLS on ΔGDI was 1.70 GDI points, representing the difference between the SEMLS (+0.85 GDI points) and control (–0.85 GDI points) cohorts. While the average change in GDI between visits was 2.74 ± 8.08 for the SEMLS cohort and –0.26 ± 7.44 for the control cohort, the total causal effects represents the estimated effect of SEMLS after adjusting for differences in Age, GDIpre, and Imppre. The BART model explained 18% of the variance in ΔGDI, with an out-of-sample root mean square error of 7.77. The implied conditional independencies of the DAG were also evaluated and all partial correlations were less than 0.3, supporting model plausibility (Supplementary Material).
Baseline values of neurologic and orthopedic impairments (Imppre) had minimal effects on ΔGDI (Figure 3). SMC, DMC, and strength had moderate effects on GDIpre, but not ΔGDI. Greater SMC or DMC resulted in higher GDIpre scores, while muscle weakness had a negative impact on GDIpre scores. Orthopedic impairments had smaller effects on GDIpre. Knee extension range of motion and tibial torsion (i.e., bimalleolar angle) had the largest effect among orthopedic impairments on GDI. Participants who had excessive knee range of motion (i.e., hyperextension) had worse baseline GDI scores. Contracture of the plantarflexors, hamstrings, or iliopsoas, as well as femoral anteversion had minimal impact on GDIpre or ΔGDI. The BART models evaluating the effects of impairments explained 63% of the variance in GDIpre and 9% of the variance in ΔGDI. The out-of-sample performance of the BART models were RMSE = 8.57 for GDIpre and RMSE = 8.04 for ΔGDI.
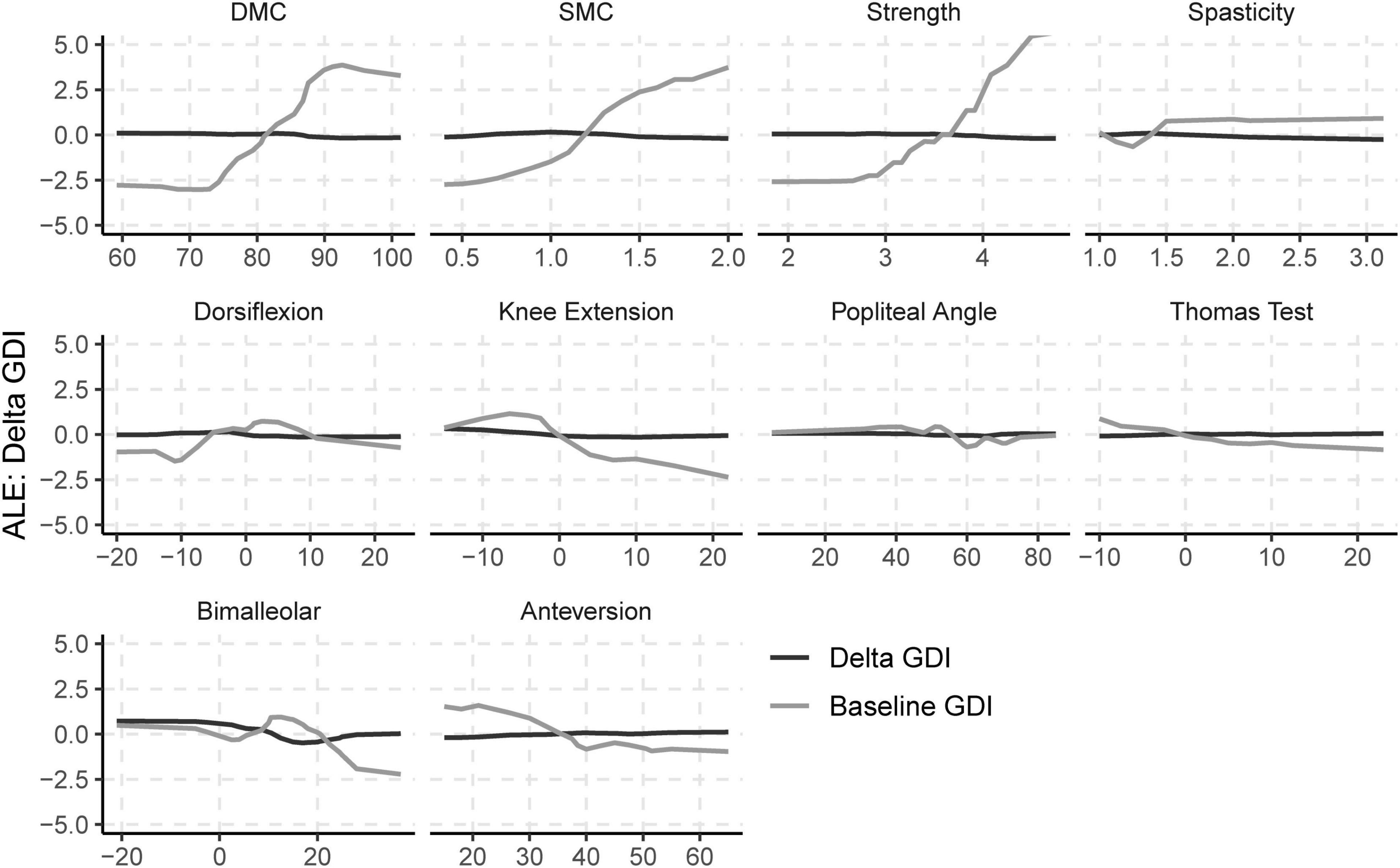
Figure 3. Accumulated local effects (ALE) of baseline neurologic and orthopedic impairments on GDIpre and ΔGDI.
This study showed that SEMLS has a small positive causal effect on change in GDI for children with bilateral CP. A 10-point change in GDI is generally considered a clinically significant improvement in walking function (Massaad et al., 2014). The observed change in GDI and total causal effect were far below this threshold. However, the cohorts who did not undergo SEMLS experienced a reduction in GDI between visits, resulting in a net effect of SEMLS around 1.70 GDI points. While average changes in GDI were modest, there was significant variation in outcome between participants, which could not be predicted by the model that included baseline age, impairment level, or surgical history. We found that SEMLS produced an increase in GDI larger than five points for 35% of participants, but also a decrease of more than five points in 20% of the participants. Such heterogeneous responses to SEMLS have motivated our team’s investigations into patient-specific factors that can improve outcomes for children with CP. We ultimately want to be able to determine why an individual walks the way they do and anticipate their responses to treatment. We had previously hypothesized that motor control could be one such factor.
Our prior retrospective regression analyses demonstrated that DMC was associated with GDI after treatment across analyses at multiple clinical centers (Schwartz et al., 2016; Shuman et al., 2018). In this study, we used a causal model to control for and evaluate the relative effects of various impairments on change in GDI. Importantly, DMC and other impairments did have a strong effect on baseline GDI, indicating that these impairments do have a causal effect on a child’s gait pattern. However, these impairments had minimal effect on ΔGDI. In other words, a child who had greater DMC at baseline was likely to have a higher GDI than a child with lower DMC, but better motor control had minimal effect on expected changes in GDI. An important point in these analyses is that the overall causal effect of SEMLS was small, which contributes to the small observed effects of impairments on ΔGDI. Despite these small treatment effects, the wide heterogeneity in outcomes suggests that there are still causal factors contributing to treatment outcomes that we are missing. These may include post-operative rehabilitation, surgeon skill, or other measures of neurologic impairment. Identifying patient-specific factors that can help us understand the causal pathways that impact gait and treatment outcomes continues to be an important area for future research.
Causal modeling provides a framework to evaluate the complex relationships between impairments and outcomes in CP. We created a DAG to identify the assumed relationships between SEMLS and GDI. The DAG used in this research could be expanded to include more detail about the assumed causal relationships between specific neurologic and orthopedic impairments or to evaluate other outcome measures. Similarly, our goal in this research was not to make outcome predictions for individual patients. Rather, we wanted to understand the impact of SEMLS and impairments on GDI. This led us toward more coarse modeling choices. As an example, we ignored details of surgical procedures and did not attempt to define the causal relationships between various neurologic and orthopedic impairments, although this is an area for future study.
The DAG we created for this research gave rise to the adjustment sets necessary to evaluate the impact of SEMLS and impairments on GDI. The DAG indicates which variables should not be included in the adjustment set. For example, changes in impairments (ΔImp) are mediators in the DAG; including these variables in the adjustment set would introduce bias. SEMLS did lead to changes in femoral anteversion and ankle contracture (see Supplementary Material). These adjustment sets can be used with any modeling method, including linear regression or other machine learning methods. We selected BART rather than linear regression or other models because we do not expect the impact of many impairments on gait to be linear. For example, we expect impairments like tibial torsion to reduce GDI scores with excessive internal or external rotation, producing a “U-shaped” response. Similarly, for some impairments like spasticity, there may be a threshold above or below which the impairment has an effect on gait. BART also provides a Bayesian framework that gives posterior distributions for each parameter.
A limitation in this research was that we did not recruit a prospective control group. Rather, we identified participants who were evaluated at multiple CGAs without any intervening surgical procedures. This cohort may also be subject to sample bias, but randomization is not feasible for this population. Since we were interested in evaluating DMC measured from EMG recordings, we also excluded children who underwent rectus femoris transfer, since the impact of moving the insertion of this muscle on recruitment and synergies remains unclear. Thus, this sample may not capture the impact of impairments that influence stiff-knee gait in children with CP.
The overall causal effect of SEMLS on change in GDI is modest. While motor control and strength do influence an individual’s gait pattern, their effect on expected changes in GDI after SEMLS were small. It is important to consider causal frameworks when analyzing observational data to avoid bias arising from confounding. Critically evaluating current CGA practices and integrating measures such as postoperative care, surgical details, or neuroimaging into treatment planning may enhance our ability to perform casual analyses aimed at understanding and improving movement for children with CP.
The prospective data used for this analysis will be made available by the authors upon request.
The studies involving human participants were reviewed and approved by University of Minnesota. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
MS organized the database. KS performed the statistical analysis and wrote the first draft of the manuscript. Both authors contributed to conception and design of the study and contributed to manuscript revision, read, and approved the submitted version.
Research reported in this publication was supported by the National Institute of Neurological Disorders & Stroke of the National Institutes of Health under award number R01NS091056.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the staff at the James R. Gage Center for Gait & Motion Analysis at Gillette Children’s Specialty Healthcare for their collaboration and insight. The authors also thank Benjamin Shuman, Alyssa Spomer, Andy Ries, and Andy Georgiadis for their valuable feedback and discussions.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2022.846205/full#supplementary-material
Bekius, A., Bach, M. M., van der Krogt, M. M., de Vries, R., Buizer, A. I., and Dominici, N. (2020). Muscle synergies during walking in children with cerebral palsy: a systematic review. Front. Physiol. 11:632. doi: 10.3389/fphys.2020.00632
Bjornson, K. F., Zhou, C., Stevenson, R. D., and Christakis, D. (2014). Relation of stride activity and participation in mobility-based life habits among children with cerebral palsy. Arch. Phys. Med. Rehabil. 95, 360–368. doi: 10.1016/j.apmr.2013.10.022
Brewer, L. E., Wright, J. M., Rice, G., Neas, L., and Teuschler, L. (2017). Causal inference in cumulative risk assessment: the roles of directed acyclic graphs. Environ. Int. 102, 30–41. doi: 10.1016/j.envint.2016.12.005
Cahill-Rowley, K., and Rose, J. (2014). Etiology of impaired selective motor control: emerging evidence and its implications for research and treatment in cerebral palsy. Dev. Med. Child Neurol. 56, 522–528. doi: 10.1111/dmcn.12355
Chambers, H., Lauer, A., Kaufman, K., Cardelia, J. M., and Sutherland, D. (1998). Prediction of Outcome After Rectus Femoris Surgery in Cerebral Palsy: the role of cocontraction of the rectus femoris and vastus lateralis. J. Pediatr. Orthop. 18, 703–711.
Chipman, H. A., George, E. I., and McCulloch, R. E. B. A. R. T. (2010). Bayesian additive regression trees. Ann. Appl. Stat. Insti. Math. Stat. 4, 266–298.
Damiano, D. L., Martellotta, T. L., Sullivan, D. J., Granata, K. P., and Abel, M. F. (2000). Muscle force production and functional performance in spastic cerebral palsy: relationship of cocontraction. Arch. Phys. Med. Rhabil. 81, 895–900. doi: 10.1053/apmr.2000.5579
Desloovere, K. (2005). Pathophysiology, measurement, and treatment of spasticity in children. Neuromodulation 8, 187–189. doi: 10.1111/j.1525-1403.2005.05237-5.x
Dorie, V., Hill, J., Shalit, U., Scott, M., and Cervone, D. (2019). Automated versus do-it-yourself methods for causal inference: lessons learned from a data analysis competition. Stat. Sci. Inst. Math. Stat. 34, 43–68.
Fabry, G., Macewen, G. D., and Shands, A. Jr. (1973). Torsion of the Femur: a follow-up study in normal and abnormal conditions. JBJS 55, 1726–1738.
Fowler, E. G., Staudt, L. A., and Greenberg, M. B. (2010). Lower−extremity selective voluntary motor control in patients with spastic cerebral palsy: increased distal motor impairment. Dev. Med. Child Neurol. 52, 264–269. doi: 10.1111/j.1469-8749.2009.03586.x
Gage, J. R., Fabian, D., Hicks, R., and Tashman, S. (1984). Pre- and postoperative gait analysis in patients with spastic diplegia: a preliminary report. J. Pediatr. Orthop. 4, 715–725. doi: 10.1097/01241398-198411000-00012
Gage, J. R., and Novacheck, T. F. (2001). An update on the treatment of gait problems in cerebral palsy. J. Pediatr. Orthop. B. 10, 265–274.
Gage, J. R., Schwartz, M. H., Koop, S. E., and Novacheck, T. F. (2009). The Identification and Treatment of Gait Problems in Cerebral Palsy. Hoboken, NJ: John Wiley & Sons,
Galarraga, C. O. A., Vigneron, V., Dorizzi, B., Khouri, N., and Desailly, E. (2017). Predicting postoperative gait in cerebral palsy. Gait Posture 52, 45–51.
Gough, M., and Shortland, A. P. (2008). Can clinical gait analysis guide the management of ambulant children with bilateral spastic cerebral palsy? J. Pediatr. Orthop. 28, 879–883. doi: 10.1097/BPO.0b013e31818e197c
Gross, P. H., Bailes, A. F., Horn, S. D., Hurvitz, E. A., Kean, J., Shusterman, M., et al. (2018). Setting a patient−centered research agenda for cerebral palsy: a participatory action research initiative. Dev. Med. Child Neurol. 60, 1278–1284. doi: 10.1111/dmcn.13984
Hahn, P. R., Murray, J. S., and Carvalho, C. M. (2020). Bayesian regression tree models for causal inference: regularization, confounding, and heterogeneous effects (with Discussion). Bayesian Anal. 15, 965–1056.
Handsfield, G. G., Meyer, C. H., Abel, M. F., and Blemker, S. S. (2016). Heterogeneity of muscle sizes in the lower limbs of children with cerebral palsy. Muscle Nerve Wiley Online Lib. 53, 933–945. doi: 10.1002/mus.24972
Hicks, J. L., Delp, S. L., and Schwartz, M. H. (2011). Can biomechanical variables predict improvement in crouch gait? Gait Posture 34, 197–201. doi: 10.1016/j.gaitpost.2011.04.009
Hill, J. L. (2011). Bayesian nonparametric modeling for causal inference. J. Comput. Graph. Stat. Taylor Francis 20, 217–240.
Imbens, G. W., and Rubin, D. B. (2015). Causal Inference in Statistics, Social, and Biomedical Sciences: an Introduction. New York, NY: Cambridge University Press.
Johnston, T. E., Moore, S. E., Quinn, L. T., and Smith, B. T. (2004). Energy cost of walking in children with cerebral palsy: relation to the gross motor function classification system. Dev. Med. Child Neurol. 46, 34–38.
Kamp, F., Lennon, N., Holmes, L., Dallmeijer, A., Henley, J., and Miller, F. (2014). Energy cost of walking in children with spastic cerebral palsy: relationship with age, body composition and mobility capacity. Gait Posture 40, 209–214. doi: 10.1016/j.gaitpost.2014.03.187
Kapelner, A., and Bleich, J. (2016). bartMachine: Machine Learning with Bayesian Additive Regression Trees. J. Stat. Softw. 70, 1–40.
Kramer, J. F., and Ann MacPhail, H. E. (1994). Relationships among measures of walking efficiency, gross motor ability, and isokinetic strength in adolescents with cerebral palsy. Pediatr. Phys. Ther. 6, 3–9.
Lee, E. H., Goh, J. C., and Bose, K. (1992). Value of gait analysis in the assessment of surgery in cerebral palsy. Arch Phys Med Rehabil. Elsevier 73, 642–646.
Lee, S. H., Chung, C. Y., Park, M. S., Choi, I. H., and Cho, T.-J. (2009). Tibial torsion in cerebral palsy: validity and reliability of measurement. Clin. Orthop. Relat. Res. 467, 2098–2104. doi: 10.1007/s11999-009-0705-1
MacWilliams, B. A., Prasad, S., Shuckra, A. L., and Schwartz, M. H. (2020). Causal factors affecting gross motor function in children diagnosed with cerebral palsy. medRxiv. [Preprint] doi: 10.1101/2020.10.26.20217232
Massaad, A., Assi, A., Skalli, W., and Ghanem, I. (2014). Repeatability and validation of gait deviation index in children: typically developing and cerebral palsy. Gait Posture. 39, 354–358. doi: 10.1016/j.gaitpost.2013.08.001
Mathewson, M. A., and Lieber, R. L. (2015). Pathophysiology of muscle contractures in cerebral palsy. Phys. Med. Rehabil. Clin. 26, 57–67.
Miller, F., Castagno, P., Richards, J., Lennon, N., Quigley, E., and Niiler, T. (1996). Reliability of kinematics during clinical gait analysis: a comparison between normal and children with cerebral palsy. Gait Posture 4, 169–170.
Molnar, C., Bischl, B., and Casalicchio, G. (2018). iml: an R package for interpretable machine learning. JOSS J. Open Source Softw. 3, 786.
O’Brien, S. M., Carroll, T. J., Barber, L. A., and Lichtwark, G. A. (2021). Plantar flexor voluntary activation capacity, strength and function in cerebral palsy. Eur. J. Appl. Physiol. 121, 1733–1741. doi: 10.1007/s00421-021-04638-z
O’Dwyer, N. J., Neilson, P. D., and Nash, J. (1989). Mechanisms of muscle growth related to muscle contracture in cerebral palsy. Dev. Med. Child Neurol. 31, 543–547. doi: 10.1111/j.1469-8749.1989.tb04034.x
R Core Team (2021). R: A Language and Environment for Statistical Computing [Internet]. Vienna: R Foundation for Statistical Computing.
Rajagopal, A., Kidziński, Ł, McGlaughlin, A. S., Hicks, J. L., Delp, S. L., and Schwartz, M. H. (2018). Estimating the effect size of surgery to improve walking in children with cerebral palsy from retrospective observational clinical data. Sci. Rep. 8:16344. doi: 10.1038/s41598-018-33962-2
Rose, J., Gamble, J. G., Medeiros, J., Burgos, A., and Haskell, W. L. (1989). Energy cost of walking in normal children and in those with cerebral palsy: comparison of heart rate and oxygen uptake. J. Pediatr. Orthop. 9, 276–279.
Ross, S. A., and Engsberg, J. R. (2007). Relationships between spasticity, strength, gait, and the GMFM-66 in persons with spastic diplegia cerebral palsy. Arch. Phys. Med. Rehabil. 88, 1114–1120. doi: 10.1016/j.apmr.2007.06.011
Schwartz, M. H., and Rozumalski, A. (2008). The gait deviation index: a new comprehensive index of gait pathology. Gait Posture 28, 351–357. doi: 10.1016/j.gaitpost.2008.05.001
Schwartz, M. H., Rozumalski, A., and Steele, K. M. (2016). Dynamic motor control is associated with treatment outcomes for children with cerebral palsy. Dev. Med. Child Neurol. 58, 1139–1145. doi: 10.1111/dmcn.13126
Shin, H.-I., Sung, K. H., Chung, C. Y., Lee, K. M., Lee, S. Y., Lee, I. H., et al. (2015). Relationships between isometric muscle strength, gait parameters, and gross motor function measure in patients with cerebral palsy. Yonsei. Med. J. 57, 217–224.
Shore, B. J., Yu, X., Desai, S., Selber, P., Wolfe, R., and Graham, H. K. (2012). Adductor surgery to prevent hip displacement in children with cerebral palsy: the predictive role of the gross motor function classification system. JBJS 94, 326–334.
Shrier, I., and Platt, R. W. (2008). Reducing bias through directed acyclic graphs. BMC Med. Res. Methodol. 8:70. doi: 10.1186/1471-2288-8-70
Shuman, B. R., Goudriaan, M., Desloovere, K., Schwartz, M. H., and Steele, K. M. (2018). Associations between muscle synergies and treatment outcomes in cerebral palsy are robust across clinical centers. Arch. Phys. Med. Rehabil. 99, 2175–2182. doi: 10.1016/j.apmr.2018.03.006
Shuman, B. R., Schwartz, M. H., and Steele, K. M. (2017). Electromyography data processing impacts muscle synergies during gait for unimpaired children and children with cerebral palsy. Front. Comput. Neurosci. 11:50. doi: 10.3389/fncom.2017.00050
Steele, K. M., Rozumalski, A., and Schwartz, M. H. (2015). Muscle synergies and complexity of neuromuscular control during gait in cerebral palsy. Dev. Med. Child Neurol. 57, 1176–1182. doi: 10.1111/dmcn.12826
Steinwender, G., Saraph, V., Scheiber, S., Zwick, E. B., Uitz, C., and Hackl, K. (2000). Intrasubject repeatability of gait analysis data in normal and spastic children. Clin. Biomech. 15, 134–139. doi: 10.1016/s0268-0033(99)00057-1
Sullivan, K., Richards, J., Miller, F., Castagno, P., and Lennon, N. (1995). Predicting the outcome of surgery for children with cerebral palsy using pre-operative gait analysis. Gait Posture 2:92.
Tan, Y. V., and Roy, J. (2019). Bayesian additive regression trees and the General BART model. ArXiv190107504 Stat [Preprint] Available online at: http://arxiv.org/abs/1901.07504 (accessed July 27, 2021), doi: 10.1002/sim.8347
Textor, J., van der Zander, B., Gilthorpe, M. S., Liśkiewicz, M., and Ellison, G. T. (2016). Robust causal inference using directed acyclic graphs: the R package ‘dagitty.’. Int. J. Epidemiol. 45, 1887–1894. doi: 10.1093/ije/dyw341
van Buuren, S., and Groothuis-Oudshoorn, C. G. M. (2011). mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67. doi: 10.3978/j.issn.2305-5839.2015.12.63
Verma, T., and Pearl, J. (1991). Equivalence and Synthesis of Causal Models. Los Angeles, CA: UCLA, Computer Science Department.
Keywords: cerebral palsy, machine learning, motor control, electromyography (EMG), gait, orthopedic surgery, weakness, spasticity
Citation: Steele KM and Schwartz MH (2022) Causal Effects of Motor Control on Gait Kinematics After Orthopedic Surgery in Cerebral Palsy: A Machine-Learning Approach. Front. Hum. Neurosci. 16:846205. doi: 10.3389/fnhum.2022.846205
Received: 30 December 2021; Accepted: 09 May 2022;
Published: 03 June 2022.
Edited by:
Marco Iosa, Sapienza University of Rome, ItalyReviewed by:
Kristan Leech, University of Southern California, United StatesCopyright © 2022 Steele and Schwartz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katherine M. Steele, a21zdGVlbGVAdXcuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.