- 1Department of Nephrology, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
- 2Department of Neurosurgery, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
Background: Cognitive impairment (CI) is a common complication of end-stage renal disease (ESRD). Many resting-state functional magnetic resonance imaging (rs-fMRI) studies have identified abnormal spontaneous low-frequency brain activity in ESRD dialysis patients. However, these studies have reported inconsistent results. So far, no meta-analyses on this topic have been published. This meta-analysis aimed to identify the more consistently vulnerable brain regions in ESRD patients at rest and to reveal its possible neuropathophysiological mechanisms.
Methods: We systematically searched PubMed, Cochrane Library, Web of Science, Medline, and EMBASE databases up to July 20, 2022 based on the amplitude of low-frequency fluctuation (ALFF) or fractional amplitude of low-frequency fluctuation (fALFF). Brain regions with abnormal spontaneous neural activity in ESRD compared to healthy controls (HCs) from previous studies were integrated and analyzed using an activation likelihood estimation (ALE) method. Jackknife sensitivity analysis was carried out to assess the reproducibility of the results.
Results: In total, 11 studies (380 patients and 351 HCs) were included in the final analysis. According to the results of the meta-analysis, compared with HCs, ESRD patients had decreased ALFF/fALFF in the right precuneus, right cuneus, and left superior temporal gyrus (STG), while no brain regions with increased brain activity were identified. Jackknife sensitivity analysis showed that our results were highly reliable.
Conclusion: Compared to HCs, ESRD dialysis patients exhibit significant abnormalities in spontaneous neural activity associated with CI, occurring primarily in the default mode network, visual recognition network (VRN), and executive control network (ECN). This contributes to the understanding of its pathophysiological mechanisms.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/], identifier [CRD42022348694].
Introduction
A common comorbidity of end-stage renal disease (ESRD) is cognitive impairment (CI). CI is a deterioration of cognitive function beyond that expected with normal aging and is usually acquired and progressive (Busse et al., 2006). Poorer cognitive function may reduce quality of survival and treatment adherence, increase health care costs, morbidity, and mortality (van Zwieten et al., 2019). Many studies have shown that the prevalence of CI is higher in patients with chronic kidney disease (CKD) than in the general population and is more common in patients with ESRD, including varying degrees of impairment in perception, memory, attention, and motor function (O’Lone et al., 2016; Viggiano et al., 2020). According to O’Lone et al. (2016), up to 70% of patients on dialysis have moderate to severe CI at no less than 55 years of age, three times more than controls of the same age (Griva et al., 2010). Currently, the pathogenesis of CI in ESRD patients is unclear. Moreover, its diagnosis is mainly based on clinical symptoms, neuropsychological assessments and neuroimaging examinations, and there is a lack of reliable and objective biological markers. Therefore, it is particularly important to explore the exact pathophysiological mechanisms.
In previous studies, ESRD patients had poorer cognitive test scores compared to the general population (Luo et al., 2016). Neuroimaging studies had found alterations in brain structure and local neurological function in ESRD. For example, previous magnetic resonance imaging (MRI) studies had found that ESRD patients presented with more severe brain atrophy (Drew et al., 2013), significantly reduced brain gray matter volume (Qiu et al., 2014), cortical thinning (Chiu et al., 2019; Richerson et al., 2021), and decreased white matter integrity (Hsieh et al., 2009; Chiu et al., 2019) compared to healthy controls (HCs), all of which are associated with CI (Zhang et al., 2013). In addition, different analyses such as positron emission tomography (PET) (Kuwabara et al., 2002) and arterial spin-labeled (ASL) imaging (Jiang et al., 2016) had also identified hemodynamic, metabolic, and functional alterations in specific brain regions in ESRD patients.
In recent years, functional MRI has developed rapidly and has become an important aid in diagnosing brain function. Task-state functional MRI has differences in experimental design, patient cooperation, and the task itself, which can easily lead to heterogeneity (Bennett and Miller, 2010; Pan et al., 2017a). Resting-state functional magnetic resonance imaging (rs-fMRI) is widely used to explore brain function in patients with neuropsychiatric disorders due to its non-invasive and task-free features, providing a new perspective on the pathophysiological mechanisms of CI in ESRD patients (Wang et al., 2020). The amplitude of low-frequency fluctuation (ALFF) or fractional amplitude of low-frequency fluctuation (fALFF) is a common data analysis method for rs-fMRI and is commonly used to detect spontaneous brain activity when subjects are not performing a task. The ALFF is a measure of blood oxygen level-dependent (BOLD) signal fluctuations in a specific low frequency range (0.01–0.08 Hz) based on voxel levels, which can respond to the strength of local neuronal activity (Yang et al., 2007). The fALFF serves as a standardized index of ALFF, which reduces the effect of physiological noise and has higher sensitivity and specificity in detecting spontaneous brain activity on low-frequency amplitude signals of neurons, but is less reliable than ALFF (Zou et al., 2008). Nowadays, rs-fMRI is used in diseases such as Alzheimer’s disease (Ibrahim et al., 2021; Zhang et al., 2021), depression (Gong et al., 2020), Parkinson’s disease (Jia et al., 2018), and epilepsy (Zhu et al., 2021). Several previous studies have used ALFF/fALFF to explore the pathophysiological mechanisms of ESRD and have found abnormal spontaneous brain activity in a wide range of brain regions. These include precuneus (Luo et al., 2016; Li et al., 2018; Chen et al., 2020; Peng et al., 2021; Su et al., 2021), parietal lobe (Luo et al., 2016; Chen et al., 2020; Peng et al., 2021), posterior cingulate cortex (Luo et al., 2016; Peng et al., 2021), medial prefrontal cortex (Luo et al., 2016; Li et al., 2018), occipital lobe (Luo et al., 2016; Peng et al., 2021), angular gyrus (Chen et al., 2020; Peng et al., 2021; Su et al., 2021), cuneus (Chen et al., 2020; Peng et al., 2021; Su et al., 2021), and others. However, these findings report a wide variety of brain regions.
Considering the above findings of ALFF/fALFF and the inconsistent results of the studies, we conducted a study using the most common meta-analysis method in the field of brain imaging, ALE meta-analysis, aiming to identify reliable neuroimaging markers and possible pathophysiological mechanisms by performing a comprehensive analysis of abnormal brain regions in ESRD patients identified in previous studies using ALFF/fALFF methods.
Materials and methods
Our study followed the meta-analysis of observational studies in epidemiological (MOOSE) guideline (Stroup et al., 2000) and had been pre-registered in the PROSPERO database (CRD42022348694).
Literature search and study selection
A comprehensive search of MEDLINE, Cochrane Library, PubMed, Web of Science, and EMBASE databases for studies from inception until July 2022 that reported altered brain activation in patients with ESRD using the ALFF/fALFF method was conducted. The following keywords were used: (“resting-state functional magnetic resonance imaging” OR “rs-fMRI” OR “amplitude of low-frequency fluctuation” OR “ALFF” OR “fractional amplitude of low-frequency fluctuation” OR “fALFF”); AND “cognitive impairment” or “CI”; AND “end-stage renal disease” OR “ESRD” OR “dialysis”. To prevent omissions, the citations of review articles and included studies were manually searched to identify additional articles.
Studies were included in this meta-analysis if they (1) met the diagnostic criteria for ESRD (Levey et al., 2005); (2) maintained on dialysis for ≥3 months; (3) compared the ALFF/fALFF between ESRD and HCs; (4) reported three-dimensional coordinates [Montreal Neurological Institute (MNI) or Talairach] of the whole brain. This meta-analysis excluded studies with the following conditions: (1) age < 18 years; (2) research based on other disorders such as depression, schizophrenia, etc., and (3) review articles, case reports, letters, conference abstracts, and editorials. Two reviewers (HC and FL) independently completed the literature search and screening process to determine the final inclusion in the meta-analysis. Where there were disagreements between reviewers, these were resolved by a consensus reviewer (XF).
Date extraction
The two reviewers (HC and FL) independently extracted and summarized the required information from the included studies. Data collected include study and subject characteristics [name of the first author, year of publication, the sample size of subjects, mean age, male/female ratio, duration of dialysis, education level, and Montreal Cognitive Assessment (MoCA) scores], imaging characteristics [method of analysis, peak coordinates of activated brain regions (Foci), three-dimensional coordinates (MNI/Talairach), scanner field strength and analysis software].
Assessment of methodological quality
Independent reviewers (HC and FL) used the Newcastle-Ottawa scale (NOS) (Stang, 2010) to assess the quality and risk of bias of the included literature. The NOS has three dimensions (choice, comparability, and exposure), with eight entries and a total score of nine. If there were differences between reviewers, consensus reviewers (XF) would discuss them together to resolve these differences.
Data analysis
A total of 100 foci were reported in 11 trials involving 671 participants in this meta-analysis. The results of comparing ESRD and HCs were divided into two groups based on increased and decreased ALFF/fALFF: (a) ESRD: increased ALFF/fALFF (5 experiments; 10 foci); (b) ESRD: decreased ALFF/fALFF (11 experiments; 90 foci). This study followed the latest recommendations for ALE meta-analysis using GingerALE version 3.0.21 (Müller et al., 2018). The ALE method used activation likelihood as an indicator to calculate the likelihood of activation across experiments for each voxel and to test hypotheses on these likelihoods to obtain consistency of brain activation across multiple experiments. First, the stereotactic coordinates (X, Y, and Z) of the studies included in the meta-analysis were extracted, and the extracted coordinates were the brain coordinates that underwent changes in dialysis ESRD compared to HCs. To ensure that all coordinates were in the same coordinate system when analyzed, all Talairach coordinates were converted to MNI coordinates using the GingerALE converter foci tool (Lancaster et al., 2007). Then, all the foci and basic information were sorted into two text files. These files were imported into the software to read the foci. According to the recommendations of Müller et al. (2018), cluster-level corrected family-wise error (FWE) at p < 0.05, cluster-forming threshold at p < 0.001, and 5,000 permutations were used to calculate the ALE diagram. To better present the results of the result, we used the Mango software2 to present the results into a standard template (Colin27_T1_seg_MNI) (Lancaster et al., 2010).
In addition, Jackknife sensitive analysis was used to assess the robustness of the results of the meta-analysis, excluding one dataset at a time (Lyles and Lin, 2010; Pan et al., 2017b). After excluding one study at a time, the data from the remaining studies were subjected to a repeated ALE meta-analysis using GingerALE 3.0.2 software. In general, we considered the result to be highly replicable if a brain region remained significant in most (>50%) of the study combinations (Eickhoff et al., 2016; Pan et al., 2017b).
Results
Search results
Figure 1 showed the literature screening process and results. In total, 456 studies were identified by the search strategy described, 153 studies were excluded because of duplication, and 257 studies were excluded for titles and abstracts. We screened the remaining 46 studies and further excluded 35 studies for the following reasons: not in the area of interest (n = 15), age < 18 years (n = 1), coordinates unavailable (n = 1), based on local or specific brain regions (n = 1), other data analysis methods, such as regional homogeneity (ReHo) (n = 3) and functional connectivity (FC) (n = 14). Finally, 11 studies including 671 subjects were included in the meta-analysis.
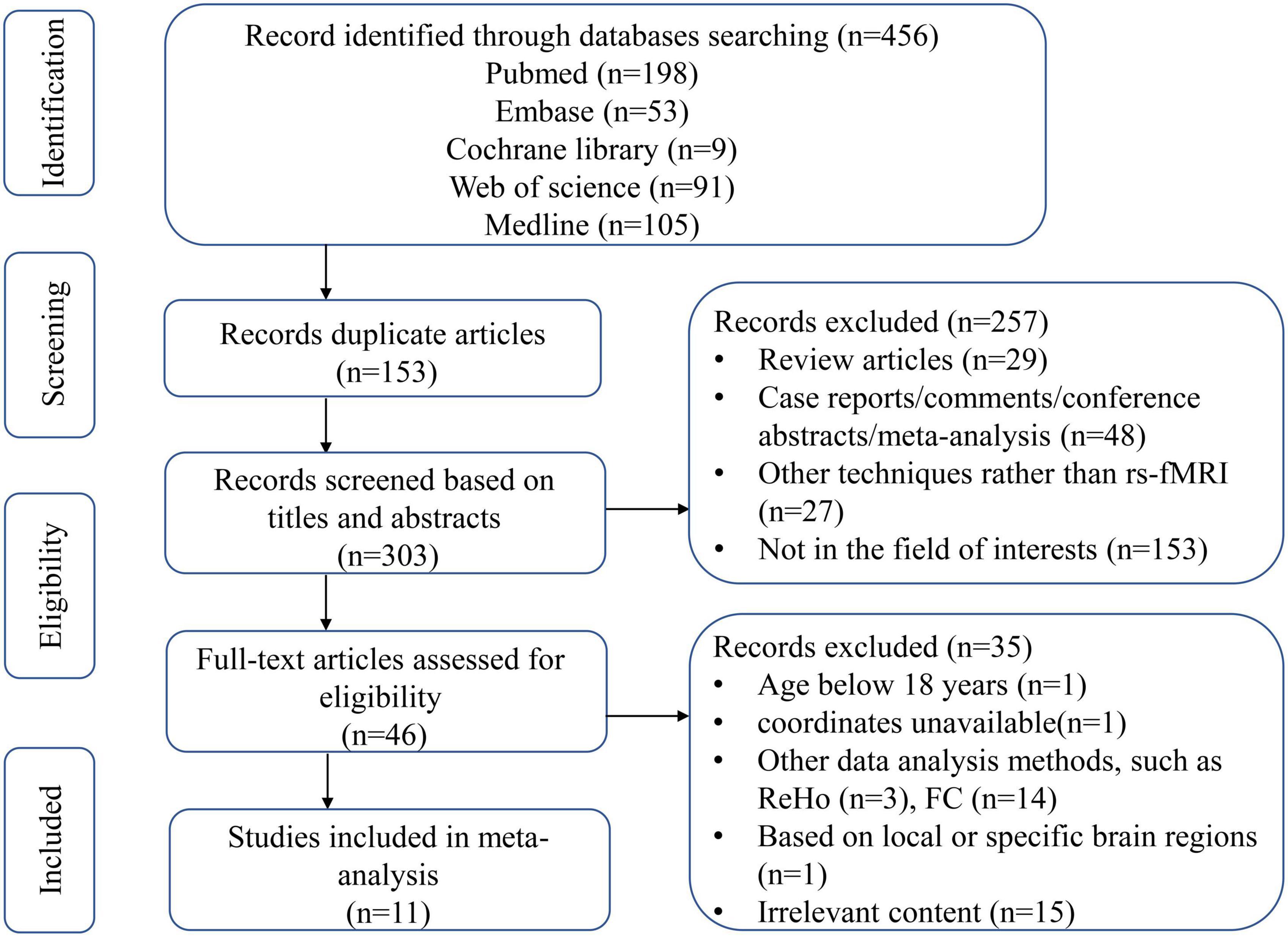
Figure 1. Flow diagram of article selection. ReHo, regional homogeneity; FC, functional connectivity.
Characteristics and quality assessment
Table 1 summarized the demographic, imaging characteristics and quality scores of all studies included in the meta-analysis. ESRD patients and HCs in each of the included studies were matched by age, gender, and education. In this included studies, 345 patients with ESRD (131 females and 214 males, mean age 42.4 years) and 326 HCs (142 females and 184 males, mean age 41.56 years) were reported. All studies underwent whole brain-based ALFF/fALFF analysis (ESRD > HCs: five experiments, 10 Fico; ESRD < HCs: 11 experiments, 90 Foci). In total, 9 of the 11 studies used a 3.0 T scanner for data collection and the remaining two studies were conducted on a 1.5 T scanner. All studies included in the meta-analysis had a quality score of ≥8, indicating that the overall quality of these studies was high. A specific assessment of the risk of bias for each of the included studies is shown in Figure 2.
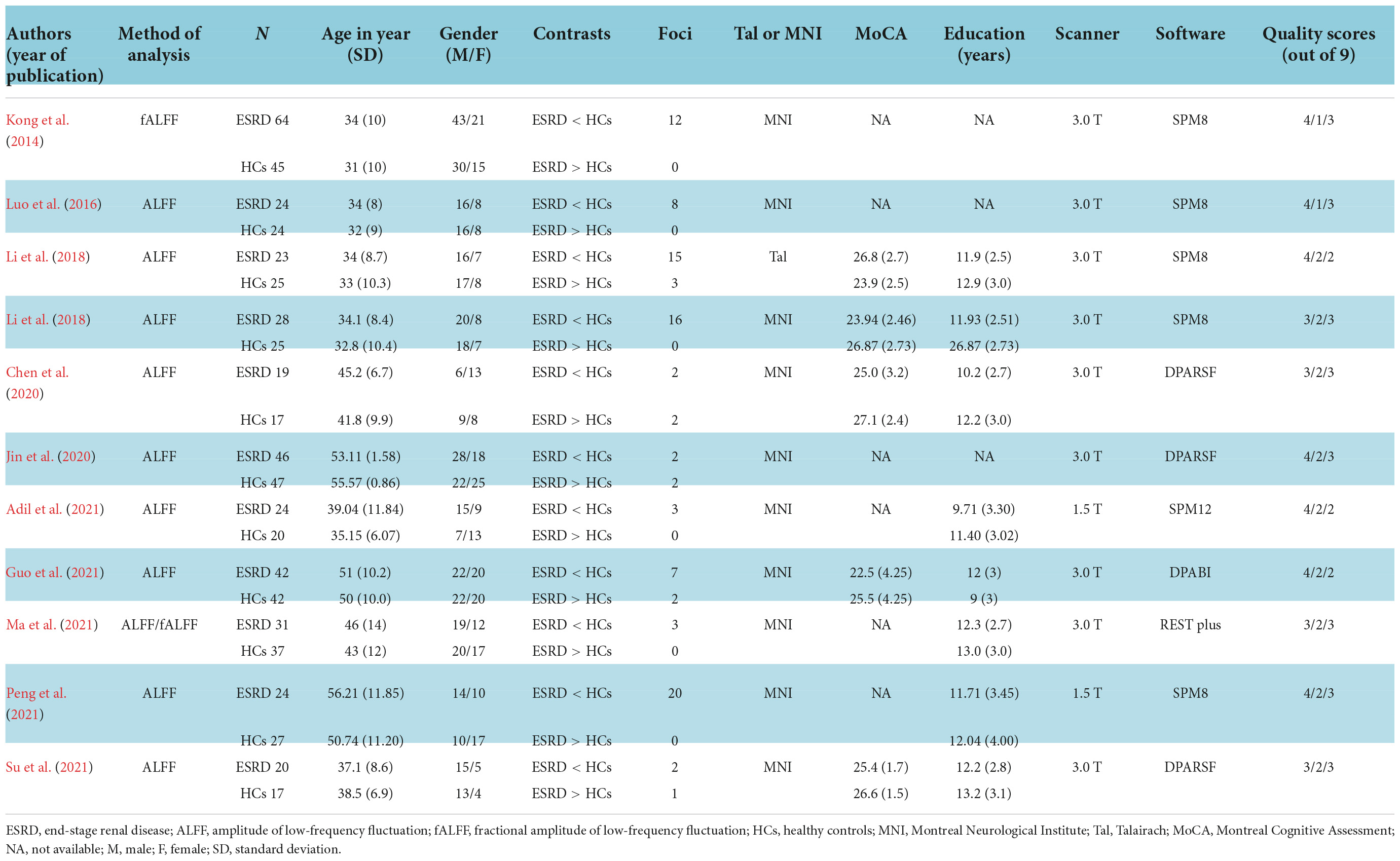
Table 1. Demographic and imaging characteristics and quality scores of all studies included in the meta-analysis.
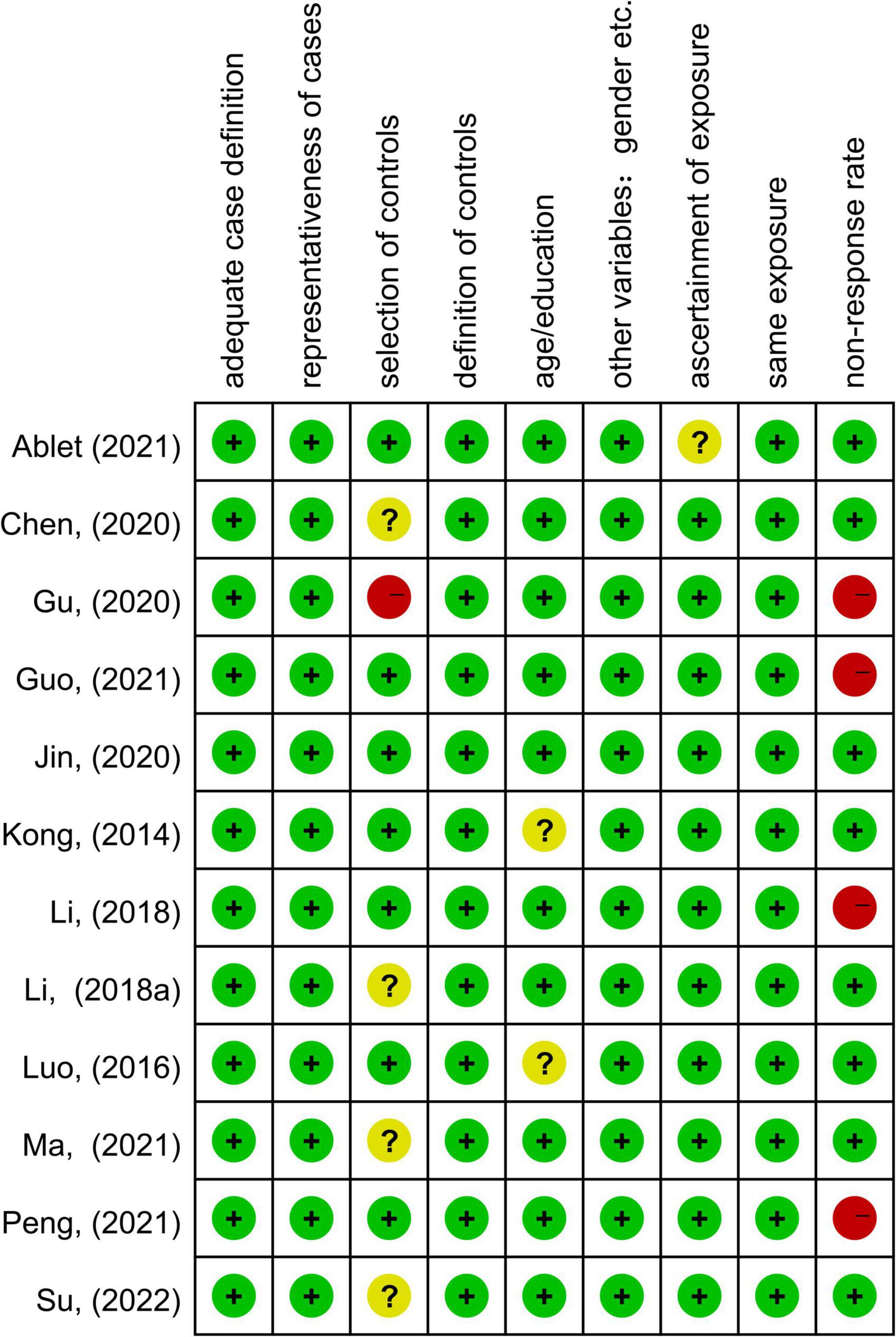
Figure 2. Summary of risk of bias for included studies according to the Newcastle-Ottawa scale (NOS).
Meta-analysis results
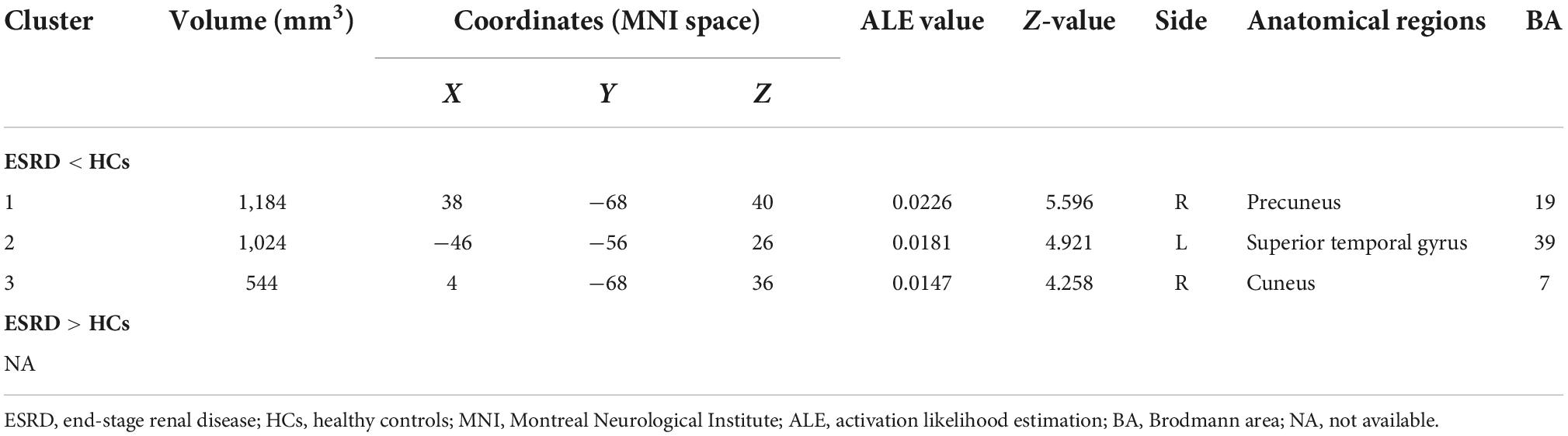
As shown in Figure 3, according to the results of the ALE meta-analysis, ESRD patients had decreased ALFF/fALFF in the right precuneus (Brodmann area 19, BA 19), right cuneus (BA 7), and left superior temporal gyrus (STG) (BA 39) compared to HCs, while no brain regions with increased ALFF/fALFF were identified. Table 2 showed the coordinates of the cluster maximums.
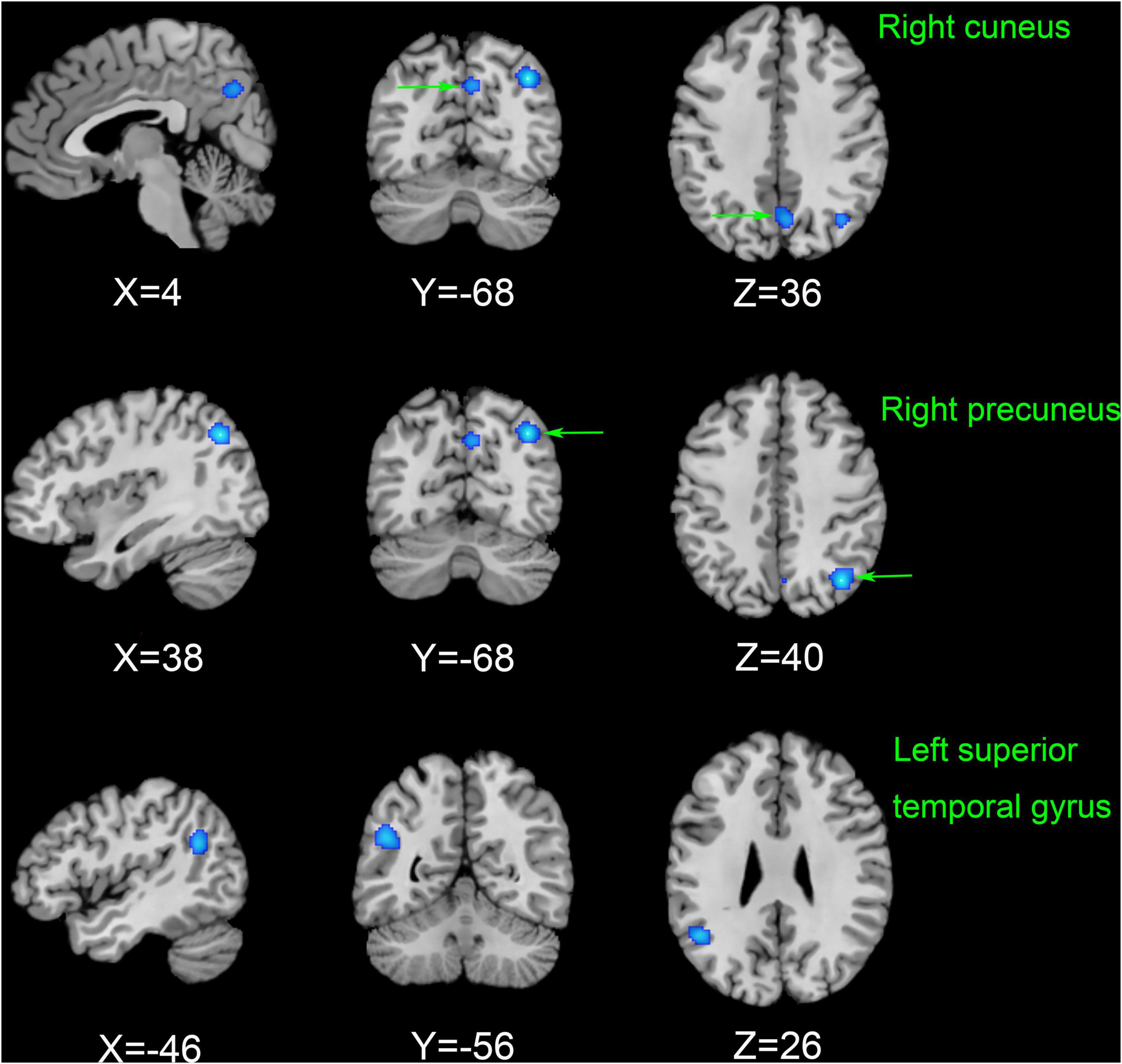
Figure 3. The results of the meta-analysis of all 11 datasets compared the differences between ESRD dialysis patients and HCs. Areas of reduced ALFF/fALFF are shown in blue relative to HCs. The results reached the threshold at p < 0.001 cluster-corrected and p < 0.05 FWE corrected. ESRD, end-stage renal disease; HCs, healthy controls; ALFF, amplitude of low-frequency fluctuation; fALFF, fractional amplitude of low-frequency fluctuation; FWE, family-wise error.
Jackknife sensitivity analysis
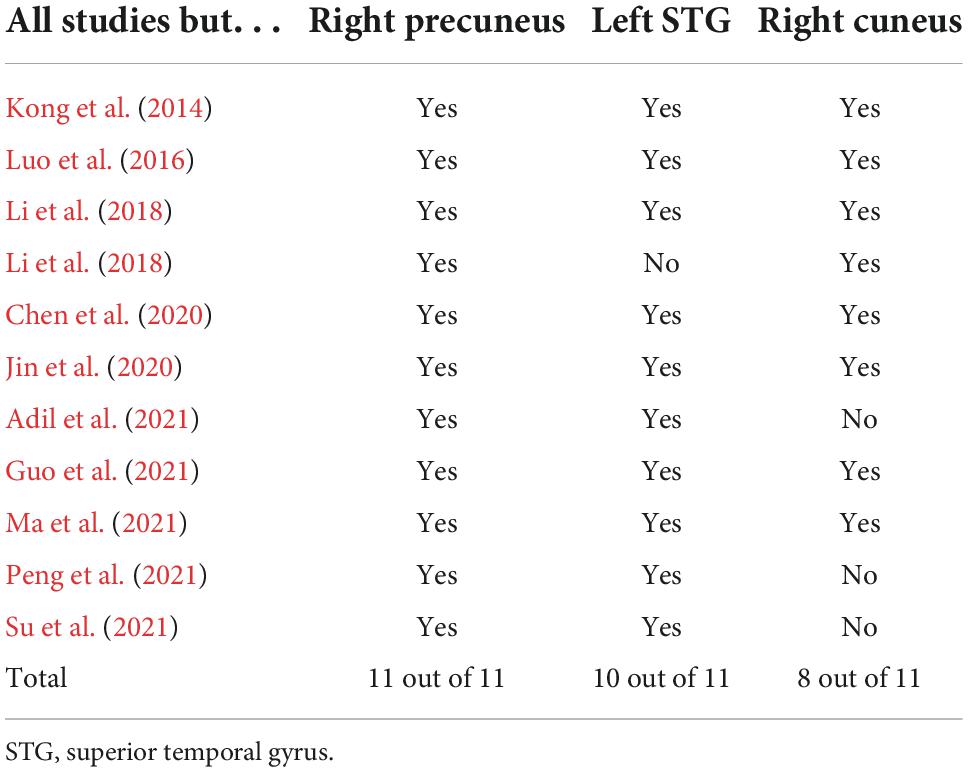
In Table 3, jackknife sensitivity analysis showed that the brain regions identified by ALE meta-analysis were all highly reliable. Of these, the decreased ALFF/fALFF in the right precuneus was the most reliable and replicable in all 11 datasets. The right cuneus and left STG were also highly replicable, as they were significant in 10 and 8 datasets, respectively.
Discussion
In this study, we used an ALE meta-analysis to explore changes in abnormal neuronal activity in ESRD compared to HCs. To our knowledge, no meta-analysis on such topics has been published. The abnormal brain neuronal activity identified in the meta-analysis mainly involved the default mode network (DMN) (right precuneus), the visual recognition network (VRN) (right cuneus), and the executive control network (ECN) (left STG). Jackknife sensitivity analyses found these results to be highly reliable and not driven by individual studies. Overall, the changes in specific brain regions analyzed in this study are valuable in helping us to uncover possible neural mechanisms of CI in ESRD dialysis patients.
Our meta-analysis showed that ALFF/fALFF was decreased in the right precuneus, right cuneus, and left STG in the patient group compared with HCs. However, no brain regions with increased ALFF/fALFF were found. This is consistent with previous findings from studies of ESRD patients (Luo et al., 2016; Peng et al., 2021). Decreased ALFF/fALFF reflects diminished spontaneous activity of brain neurons and impaired brain function, while increased ALFF/fALFF may be a compensatory mechanism (Yang et al., 2007). A growing number of studies suggested that CI in ESRD patients may be associated with disrupted connectivity in the DMN. The DMN is associated with spontaneous cognitive functions, external environmental monitoring functions, and internal mental activity and is the most widely studied resting-state subnetwork in ESRD patients, including the posterior cingulate cortex, medial prefrontal cortex, precuneus, hippocampus, inferior parietal lobule, and lateral temporal cortex (Raichle and Snyder, 2007; Ni et al., 2014). These regions are usually more active when the brain is in resting state but will deactivate when performing tasks, corresponding to brain regions showing negative activation.
The precuneus, located on the medial aspect of the parietal lobe, is a key node in the DMN and plays a crucial role in various cognitive processes. Functionally, it is closely associated mainly with memory, emotion, and visuospatial executive functions (Bullmore and Sporns, 2009). A graph theory-based analysis pointed out that impaired function of the DMN may underlie CI in ESRD, and further correlation analysis showed that function of the precuneus in ESRD patients correlated with cognitive performance (Yue et al., 2021). This finding was confirmed in the present study. An ASL imaging performed by Cheng et al. (2019) demonstrated a widespread decrease in cerebral blood flow (CBF) in dialysis patients compared to non-dialysis patients. To our knowledge, atrophy of the gray matter cortex of the brain is associated with lower CBF, which can lead to memory deficits and CI (Drew et al., 2013). The study also found a consistent reduction in precuneus ReHo values in ESRD patients and a positive correlation with digit symbol test scores (Liang et al., 2013). This resonates with our study and suggests that decreased ALFF/fALFF in the precuneus may be a potentially promising biomarker for predicting CI in ESRD patients.
The cuneus is located in the occipital lobe of the brain and is an important part of the VRN, with the main functions involving the processing of visual information, facial perception, emotion, and working memory (Parker et al., 2014; He et al., 2021). Abnormal FC in the cuneus has been shown to be associated with altered brain activity in ESRD patients (Su et al., 2021). Previous studies have also shown widespread impairment of the VRN in ESRD dialysis patients (Rozeman et al., 1992). In addition, Peng et al. (2021) showed decreased ALFF in the bilateral cuneus based on rs-fMRI analysis. All of these suggest that dysfunction in the cuneus may cause slowed integration of visual information processing and working memory. However, it is noteworthy that studies using nodal centrality analysis found significant activation of the cuneus in ESRD patients (Wu et al., 2020). These inconsistent results may be related to the severity of the patients’ disease, small sample sizes, and different study methods.
In addition, our meta-analysis found decreased low-frequency brain activity in the left STG. The STG is part of the auditory language center, as well as the ECN. This network is involved in several higher cognitive tasks and has a significant role in attention allocation, goal-directed behavior, and control of emotions (Song et al., 2021). It has been shown that FC in the ECN is associated with substance-dependent approach behavior and that approach behavior is stronger in the left-sided ECN in the resting state (Krmpotich et al., 2013). This is consistent with our findings. In an F-18-fluorodeoxyglucose PET study, Song et al. (2008) found reduced perfusion in the STG and significantly reduced cerebral metabolism in ESRD patients. Using diffusion tensor imaging (DTI), Hsieh et al. (2009) observed lower fractional anisotropy in depression-related regions (STG) in older adults and ESRD dialysis patients than in HCs. To our knowledge, the prevalence of depression among ESRD patients is as high as 12–52% (Cohen et al., 2016). Previous studies have also shown that disruption of FC in the STG is associated with multiple psychological disorders, including depression (Gutman et al., 2009). As we hypothesized, each of these findings could partially explain cognitive dysfunction in ESRD dialysis patients, for example, memory, balance, and emotional processing. Therefore, we presume that a decreased ALFF/fALFF in the STG may be a sign of mental impairment in ESRD dialysis patients.
In this study, we only found positive results for decreased ALFF/fALFF and did not find positive results for increased ALFF/fALFF. However, many single studies have been performed showing that ESRD patients present with common brain abnormalities, such as the increased ALFF/fALFF in the right precentral gyrus (Chen et al., 2020; Su et al., 2021). Yet, no positive results for it were found in our meta-analysis. This may be due to the limitations of the ALE meta-analysis. ALE meta-analysis as a probabilistic analysis can effectively eliminate false positive results and avoid the problem of low statistical test power in individual imaging studies, but it is difficult to avoid false negative results (Radua et al., 2012). In addition, only five trials and 10 foci from our meta-analysis were included in the analysis of increased ALFF/fALFF. A small number of coordinates may not have reached the threshold. These may lead to a decreased accuracy of the results. On the other hand, this may also be related to the influence of confounding factors such as gender, age, and education level of the study.
In the present study, we only analyzed ALFF/fALFF studies in ESRD patients, excluding other imaging studies such as ReHo, DTI, and FC, which to some extent reduces the bias of the results due to the combination of rs-fMRI with other analysis methods. Nevertheless, this study still has some limitations. First, our dataset was limited in number (11 studies) and some results should still be interpreted with caution. Second, our meta-analysis was unable to determine the causal relationship between ESRD and abnormal brain activity. This was due to the fact that the studies we included were cross-sectional studies. Third, the studies included in our meta-analysis were all from Asian countries and may be limited by the application of other national populations. Fourth, owing to insufficient data from relevant studies, we only analyzed ESRD patients on dialysis and did not perform a meta-analysis of dialysis modalities. Finally, because the ALE meta-analysis method did not consider the intensity of activation, it was possible that some brain regions with low activation levels were ignored.
Conclusion
In summary, using the ALE method, the current meta-analysis shows that in the resting state, ESRD dialysis patients have abnormal spontaneous low-frequency brain activity compared to HCs, mainly involving the DMN, VRN, and ECN. These findings may be potential imaging biomarkers of CI in ESRD dialysis patients and could be considered as a focus of attention for follow-up studies. Meanwhile, more in-depth studies are needed in the future to validate our results and explore more specific biomarkers of early stage of CI in ESRD dialysis patients.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
HC and FL drafted and revised the manuscript critically for important intellectual content, contributed to the search of the literature, the collection of relevant information, and the data analysis. XF and EZ agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. BK, JS, and YX contributed to the concept and design of the study. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 82160143 and 81860133), the Hospital Science and Technology Program of the Second Affiliated Hospital of Nanchang University (No. 2014YNLC12002), the Natural Science Foundation of Jiangxi Province (No. 20192BAB205046), and the Jiangxi Key Technology Research and Development Program (No. 20202BBG73030).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ESRD, end-stage renal disease; CI, cognitive impairment; rs-fMRI, resting-state functional magnetic resonance imaging; ALFF, amplitude of low-frequency fluctuation; fALFF, fractional amplitude of low-frequency fluctuation; HCs, healthy controls; ALE, activation likelihood estimation; ASL, arterial spin-labeled; PET, positron emission tomography; BOLD, blood oxygen level-dependent; MNI, Montreal Neurological Institute; FEW, family-wise error; MoCA, Montreal cognitive assessment; ReHo, regional homogeneity; BA, Brodmann area; DMN, default mode network; VRN, visual recognition network; ECN, executive control network; STG, superior temporal gyrus; CBF, cerebral blood flow; FC, functional connectivity; DTI, diffusion tensor imaging.
Footnotes
References
Adil, A., Eskeljiang, H., Zou, K., and Tian, X. (2021). Aberrant static and dynamic spontaneous brain activities in patients with end-stage renal disease: A resting-state fMRI imaging study. Int. J.Clin.Exp. Med. 14, 2509–2519.
Bennett, C. M., and Miller, M. B. (2010). How reliable are the results from functional magnetic resonance imaging? Ann N Y Acad Sci. 1191, 133–155. doi: 10.1111/j.1749-6632.2010.05446.x
Bullmore, E., and Sporns, O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 10, 186–198. doi: 10.1038/nrn2575
Busse, A., Hensel, A., Gühne, U., Angermeyer, M. C., and Riedel-Heller, S. G. (2006). Mild cognitive impairment: long-term course of four clinical subtypes. Neurology 67, 2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1
Chen, H. J., Qiu, J., Fu, Q., and Chen, F. (2020). Alterations of Spontaneous Brain Activity in Hemodialysis Patients. Front Hum Neurosci. 14:278. doi: 10.3389/fnhum.2020.00278
Cheng, B. C., Chen, P. C., Chen, P. C., Lu, C. H., Huang, Y. C., Chou, K. H., et al. (2019). Decreased cerebral blood flow and improved cognitive function in patients with end-stage renal disease after peritoneal dialysis: An arterial spin-labelling study. Eur Radiol. 29, 1415–1424. doi: 10.1007/s00330-018-5675-9
Chiu, Y.-L., Tsai, H.-H., Lai, Y.-J., Tseng, H.-Y., Wu, Y.-W., Peng, Y.-S., et al. (2019). Cognitive impairment in patients with end-stage renal disease: Accelerated brain aging? J Formos Med Assoc. 118, 867–875. doi: 10.1016/j.jfma.2019.01.011
Cohen, S. D., Cukor, D., and Kimmel, P. L. (2016). Anxiety in Patients Treated with Hemodialysis. Clin J Am Soc Nephrol. 11, 2250–2255. doi: 10.2215/CJN.02590316
Drew, D. A., Bhadelia, R., Tighiouart, H., Novak, V., Scott, T. M., Lou, K. V., et al. (2013). Anatomic brain disease in hemodialysis patients: a cross-sectional study. Am J Kidney Dis. 61, 271–278. doi: 10.1053/j.ajkd.2012.08.035
Eickhoff, S. B., Nichols, T. E., Laird, A. R., Hoffstaedter, F., Amunts, K., Fox, P. T., et al. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 137, 70–85. doi: 10.1016/j.neuroimage.2016.04.072
Gong, J., Wang, J., Qiu, S., Chen, P., Luo, Z., Wang, J., et al. (2020). Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: voxel-based meta-analysis. Transl Psychiatry. 10, 353. doi: 10.1038/s41398-020-01036-5
Griva, K., Stygall, J., Hankins, M., Davenport, A., Harrison, M., and Newman, S. P. (2010). Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis. 56, 693–703. doi: 10.1053/j.ajkd.2010.07.003
Guo, H., Liu, W., Li, H., and Yang, J. (2021). Structural and functional brain changes in hemodialysis patients with end-stage renal disease: DTI analysis results and ALFF analysis results. Int. J. Nephrol. Renovasc. Dis. 14, 77–86. doi: 10.2147/IJNRD.S295025
Gutman, D. A., Holtzheimer, P. E., Behrens, T. E. J., Johansen-Berg, H., and Mayberg, H. S. A. (2009). tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry 65, 276–282. doi: 10.1016/j.biopsych.2008.09.021
He, X., Li, X., Fu, J., Xu, J., Liu, H., Zhang, P., et al. (2021). The morphometry of left cuneus mediating the genetic regulation on working memory. Hum Brain Mapp. 42, 3470–3480. doi: 10.1002/hbm.25446
Hsieh, T.-J., Chang, J.-M., Chuang, H.-Y., Ko, C.-H., Hsieh, M.-L., Liu, G.-C., et al. (2009). End-stage renal disease: in vivo diffusion-tensor imaging of silent white matter damage. Radiology 252, 518–525. doi: 10.1148/radiol.2523080484
Ibrahim, B., Suppiah, S., Ibrahim, N., Mohamad, M., Hassan, H. A., Nasser, N. S., et al. (2021). Diagnostic power of resting-state fMRI for detection of network connectivity in Alzheimer’s disease and mild cognitive impairment: A systematic review. Hum Brain Mapp. 42, 2941–2968. doi: 10.1002/hbm.25369
Jia, X., Li, Y., Li, K., Liang, P., and Fu, X. (2018). Precuneus Dysfunction in Parkinson’s Disease With Mild Cognitive Impairment. Front Aging Neurosci. 10:427. doi: 10.3389/fnagi.2018.00427
Jiang, X. L., Wen, J. Q., Zhang, L. J., Zheng, G., Li, X., Zhang, Z., et al. (2016). Cerebral blood flow changes in hemodialysis and peritoneal dialysis patients: an arterial-spin labeling MR imaging. Metab Brain Dis. 31, 929–936. doi: 10.1007/s11011-016-9829-7
Jin, M., Wang, L., Wang, H., Han, X., Diao, Z., Guo, W., (2020). Disturbed neurovascular coupling in hemodialysis patients. PeerJ. 8:e8989. doi: 10.7717/peerj.8989
Kong, X., Ji, X., Qi, R., Liang, X., Wen, J., Luo, S., et al. (2014). Study of brain activity on hemodialysis patients with end-stage renal disease by using resting-state functional MRI with amplitude of low frequency fluctuation algorithm. Chin. J. Radiol. 48, 827–831. doi: 10.3760/cma.j.issn.1005-1201.2014.10.009
Krmpotich, T. D., Tregellas, J. R., Thompson, L. L., Banich, M. T., Klenk, A. M., and Tanabe, J. L. (2013). Resting-state activity in the left executive control network is associated with behavioral approach and is increased in substance dependence. Drug Alcohol Depend. 129, 1–7. doi: 10.1016/j.drugalcdep.2013.01.021
Kuwabara, Y., Sasaki, M., Hirakata, H., Koga, H., Nakagawa, M., Chen, T., et al. (2002). Cerebral blood flow and vasodilatory capacity in anemia secondary to chronic renal failure. Kidney Int. 61, 564–569. doi: 10.1046/j.1523-1755.2002.00142.x
Lancaster, J. L., Cykowski, M. D., McKay, D. R., Kochunov, P. V., Fox, P. T., Rogers, W., et al. (2010). Anatomical global spatial normalization. Neuroinformatics 8, 171–182. doi: 10.1007/s12021-010-9074-x
Lancaster, J. L., Tordesillas-Gutiérrez, D., Martinez, M., Salinas, F., Evans, A., Zilles, K., et al. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 28, 1194–1205. doi: 10.1002/hbm.20345
Levey, A. S., Eckardt, K.-U., Tsukamoto, Y., Levin, A., Coresh, J., Rossert, J., et al. (2005). Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 67, 2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x
Li, P., Ding, D., Ma, X., Zhang, H., Liu, J., and Zhang, M. (2018). Altered intrinsic brain activity and memory performance improvement in patients with end-stage renal disease during a single dialysis session. Brain Imaging Behav. 12, 1640–1649. doi: 10.1007/s11682-018-9828-x
Liang, X., Wen, J., Ni, L., Zhong, J., Qi, R., Zhang, L. J., et al. (2013). Altered pattern of spontaneous brain activity in the patients with end-stage renal disease: a resting-state functional MRI study with regional homogeneity analysis. PLoS One 8:e71507. doi: 10.1371/journal.pone.0071507
Luo, S., Qi, R. F., Wen, J. Q., Zhong, J. H., Kong, X., Liang, X., et al. (2016). Abnormal Intrinsic Brain Activity Patterns in Patients with End-Stage Renal Disease Undergoing Peritoneal Dialysis: A Resting-State Functional MR Imaging Study. Radiology 278, 181–189. doi: 10.1148/radiol.2015141913
Lyles, R. H., and Lin, J. (2010). Sensitivity analysis for misclassification in logistic regression via likelihood methods and predictive value weighting. Stat Med. 29, 2297–2309. doi: 10.1002/sim.3971
Ma, C., Gao, S., Li, W., Yu, L., Fu, S., and Ren, Y. (2021). Study on the changes of spontaneous brain activity in maintenance hemodialysis patients with end-stage renal disease based on three different resting state-functional magnetic resonance low-frequency amplitude algorithms. Nat. Med. J. China 101, 265–270. doi: 10.3760/cma.j.cn112137-20200513-01524
Müller, V. I., Cieslik, E. C., Laird, A. R., Fox, P. T., Radua, J., Mataix-Cols, D., et al. (2018). Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev. 84, 151–161. doi: 10.1016/j.neubiorev.2017.11.012
Ni, L., Wen, J., Zhang, L. J., Zhu, T., Qi, R., Xu, Q., et al. (2014). Aberrant default-mode functional connectivity in patients with end-stage renal disease: a resting-state functional MR imaging study. Radiology 271, 543–552. doi: 10.1148/radiol.13130816
O’Lone, E., Connors, M., Masson, P., Wu, S., Kelly, P. J., Gillespie, D., et al. (2016). Cognition in People With End-Stage Kidney Disease Treated With Hemodialysis: A Systematic Review and Meta-analysis. Am J Kidney Dis. 67, 925–935. doi: 10.1053/j.ajkd.2015.12.028
Pan, P., Zhan, H., Xia, M., Zhang, Y., Guan, D., and Xu, Y. (2017a). Aberrant regional homogeneity in Parkinson’s disease: A voxel-wise meta-analysis of resting-state functional magnetic resonance imaging studies. Neurosci Biobehav Rev. 72, 223–231. doi: 10.1016/j.neubiorev.2016.11.018
Pan, P., Zhu, L., Yu, T., Shi, H., Zhang, B., Qin, R., et al. (2017b). Aberrant spontaneous low-frequency brain activity in amnestic mild cognitive impairment: A meta-analysis of resting-state fMRI studies. Ageing Res Rev. 35, 12–21. doi: 10.1016/j.arr.2016.12.001
Parker, J. G., Zalusky, E. J., and Kirbas, C. (2014). Functional MRI mapping of visual function and selective attention for performance assessment and presurgical planning using conjunctive visual search. Brain Behav. 4, 227–237. doi: 10.1002/brb3.213
Peng, C., Yang, H., Ran, Q., Zhang, L., Liu, C., Fang, Y., et al. (2021). Immediate Abnormal Intrinsic Brain Activity Patterns in Patients with End-stage Renal Disease During a Single Dialysis Session: Resting-state Functional MRI Study. Clin Neuroradiol. 31, 373–381. doi: 10.1007/s00062-020-00915-0
Qiu, Y., Lv, X., Su, H., Jiang, G., Li, C., and Tian, J. (2014). Structural and functional brain alterations in end stage renal disease patients on routine hemodialysis: a voxel-based morphometry and resting state functional connectivity study. PLoS One 9:e98346. doi: 10.1371/journal.pone.0098346
Radua, J., Mataix-Cols, D., Phillips, M. L., El-Hage, W., Kronhaus, D. M., Cardoner, N., et al. (2012). A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry 27, 605–611. doi: 10.1016/j.eurpsy.2011.04.001
Raichle, M. E., and Snyder, A. Z. A. (2007). default mode of brain function: a brief history of an evolving idea. Neuroimage* 37, doi: 10.1016/j.neuroimage.2007.02.041
Richerson, W. T., Umfleet, L. G., Schmit, B. D., and Wolfgram, D. F. (2021). Changes in Cerebral Volume and White Matter Integrity in Adults on Hemodialysis and Relationship to Cognitive Function. Nephron 145, 35–43. doi: 10.1159/000510614
Rozeman, C. A., Jonkman, E. J., Poortvliet, D. C., Emmen, H. H., de Weerd, A. W., van der Maas, A. P., et al. (1992). Encephalopathy in patients on continuous ambulatory peritoneal dialysis and patients on chronic haemodialysis. Nephrol Dial Transplant. 7, 1213–1218. doi: 10.1093/ndt/7.12.1213
Song, S. H., Kim, I. J., Kim, S.-J., Kwak, I. S., and Kim, Y.-K. (2008). Cerebral glucose metabolism abnormalities in patients with major depressive symptoms in pre-dialytic chronic kidney disease: statistical parametric mapping analysis of F-18-FDG PET, a preliminary study. Psychiatry Clin Neurosci. 62, 554–561. doi: 10.1111/j.1440-1819.2008.01849.x
Song, Y., Xu, W., Chen, S., Hu, G., Ge, H., Xue, C., et al. (2021). Functional MRI-Specific Alterations in Salience Network in Mild Cognitive Impairment: An ALE Meta-Analysis. Front Aging Neurosci. 13:695210. doi: 10.3389/fnagi.2021.695210
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012. doi: 10.1001/jama.283.15.2008
Su, H., Fu, S., Liu, M., Yin, Y., Hua, K., Meng, S., et al. (2021). Altered spontaneous brain activity and functional integration in hemodialysis patients with end-stage renal disease. Front. Neurol. 12:801336. doi: 10.3389/fneur.2021.801336
van Zwieten, A., Wong, G., Ruospo, M., Palmer, S. C., Teixeira-Pinto, A., Barulli, M. R., et al. (2019). Associations of Cognitive Function and Education Level With All-Cause Mortality in Adults on Hemodialysis: Findings From the COGNITIVE-HD Study. Am J Kidney Dis. 74, 452–462. doi: 10.1053/j.ajkd.2019.03.424
Viggiano, D., Wagner, C. A., Martino, G., Nedergaard, M., Zoccali, C., Unwin, R., et al. (2020). Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol. 16, 452–469. doi: 10.1038/s41581-020-0266-9
Wang, R., Liu, N., Tao, Y.-Y., Gong, X.-Q., Zheng, J., Yang, C., et al. (2020). The Application of rs-fMRI in Vascular Cognitive Impairment. Front Neurol. 11:951. doi: 10.3389/fneur.2020.00951
Wu, B., Li, X., Zhang, M., Zhang, F., Long, X., Gong, Q., et al. (2020). Disrupted brain functional networks in patients with end-stage renal disease undergoing hemodialysis. J Neurosci Res. 98, 2566–2578. doi: 10.1002/jnr.24725
Yang, H., Long, X.-Y., Yang, Y., Yan, H., Zhu, C.-Z., Zhou, X.-P., et al. (2007). Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage 36, 144–152. doi: 10.1016/j.neuroimage.2007.01.054
Yue, Z., Wang, P., Li, X., Ren, J., and Wu, B. (2021). Abnormal brain functional networks in end-stage renal disease patients with cognitive impairment. Brain Behav. 11, e02076. doi: 10.1002/brb3.2076
Zhang, L. J., Wen, J., Ni, L., Zhong, J., Liang, X., Zheng, G., et al. (2013). Predominant gray matter volume loss in patients with end-stage renal disease: a voxel-based morphometry study. Metab Brain Dis. 28, 647–654. doi: 10.1007/s11011-013-9438-7
Zhang, Q., Wang, Q., He, C., Fan, D., Zhu, Y., Zang, F., et al. (2021). Altered Regional Cerebral Blood Flow and Brain Function Across the Alzheimer’s Disease Spectrum: A Potential Biomarker. Front Aging Neurosci. 13:630382. doi: 10.3389/fnagi.2021.630382
Zhu, J., Xu, C., Zhang, X., Qiao, L., Wang, X., Zhang, X., et al. (2021). Altered amplitude of low-frequency fluctuations and regional homogeneity in drug-resistant epilepsy patients with vagal nerve stimulators under different current intensity. CNS Neurosci Ther. 27, 320–329. doi: 10.1111/cns.13449
Keywords: end-stage renal disease, dialysis, amplitude of low-frequency fluctuation, fractional amplitude of low-frequency fluctuation, activation likelihood estimation
Citation: Cao H, Lin F, Ke B, Song J, Xue Y, Fang X and Zeng E (2022) Alterations of amplitude of low-frequency fluctuations and fractional amplitude of low-frequency fluctuations in end-stage renal disease on maintenance dialysis: An activation likelihood estimation meta-analysis. Front. Hum. Neurosci. 16:1040553. doi: 10.3389/fnhum.2022.1040553
Received: 02 October 2022; Accepted: 16 November 2022;
Published: 01 December 2022.
Edited by:
Ruben Gur, University of Pennsylvania, United StatesReviewed by:
Chuanlong Cao, Chengdu No.4 People’s Hospital, ChinaHai-Feng Shi, Changzhou No.2 People’s Hospital, China
Copyright © 2022 Cao, Lin, Ke, Song, Xue, Fang and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangdong Fang, eGlhbmdkb25nZmFuZzgxOEBzaW5hLmNvbQ==; Erming Zeng, ZXJtaW5nemVuZ0BhbGl5dW4uY29t
†These authors have contributed equally to this work and share first authorship
 Huiling Cao
Huiling Cao Feng Lin
Feng Lin Ben Ke
Ben Ke Jianling Song1
Jianling Song1 Xiangdong Fang
Xiangdong Fang Erming Zeng
Erming Zeng