- 1Max Planck Partner Group, School of International Chinese Language Education, Beijing Normal University, Beijing, China
- 2Lise Meitner Research Group Cognition and Plasticity, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany
Background: The causal relationships between neural substrates and human language have been investigated by transcranial magnetic stimulation (TMS). However, the robustness of TMS neuromodulatory effects is still largely unspecified. This study aims to systematically examine the efficacy of TMS on healthy participants’ language performance.
Methods: For this meta-analysis, we searched PubMed, Web of Science, PsycINFO, Scopus, and Google Scholar from database inception until October 15, 2022 for eligible TMS studies on language comprehension and production in healthy adults published in English. The quality of the included studies was assessed with the Cochrane risk of bias tool. Potential publication biases were assessed by funnel plots and the Egger Test. We conducted overall as well as moderator meta-analyses. Effect sizes were estimated using Hedges’g (g) and entered into a three-level random effects model.
Results: Thirty-seven studies (797 participants) with 77 effect sizes were included. The three-level random effects model revealed significant overall TMS effects on language performance in healthy participants (RT: g = 0.16, 95% CI: 0.04–0.29; ACC: g = 0.14, 95% CI: 0.04–0.24). Further moderator analyses indicated that (a) for language tasks, TMS induced significant neuromodulatory effects on semantic and phonological tasks, but didn’t show significance for syntactic tasks; (b) for cortical targets, TMS effects were not significant in left frontal, temporal or parietal regions, but were marginally significant in the inferior frontal gyrus in a finer-scale analysis; (c) for stimulation parameters, stimulation sites extracted from previous studies, rTMS, and intensities calibrated to the individual resting motor threshold are more prone to induce robust TMS effects. As for stimulation frequencies and timing, both high and low frequencies, online and offline stimulation elicited significant effects; (d) for experimental designs, studies adopting sham TMS or no TMS as the control condition and within-subject design obtained more significant effects.
Discussion: Overall, the results show that TMS may robustly modulate healthy adults’ language performance and scrutinize the brain-and-language relation in a profound fashion. However, due to limited sample size and constraints in the current meta-analysis approach, analyses at a more comprehensive level were not conducted and results need to be confirmed by future studies.
Systematic review registration: [https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=366481], identifier [CRD42022366481].
Introduction
Human language performance, including both comprehension and production abilities, is proposed to be a milestone of human evolution, distinct from any other animals (e.g., Friederici et al., 2017; Tattersall, 2017; Fishbein et al., 2020; Torday, 2021). Numerous studies have intensively investigated the neurobiology of human language performance through neuroimaging techniques such as electroencephalography (EEG), functional magnetic resonance imaging (fMRI), functional near-infrared spectroscopy (fNIRS), and magnetoencephalography (MEG) (e.g., Soltanlou et al., 2018; Tian et al., 2020; Wang et al., 2020; see also Friederici et al., 2017 for a systematic review). Nevertheless, these correlative neuroimaging approaches seem to be insufficient to interpret causal relationships between neural correlates and language functions (Devlin and Watkins, 2007; Sandrini et al., 2011; Hartwigsen, 2015). Clinical studies on post-stroke aphasia have evidenced such causality via mapping specific deficits after circumscribed lesions to language areas (Fridriksson et al., 2015; Wilson and Hula, 2019). However, patients’ language performance might be confounded by the long-term reorganization of brain networks, and broad lesions also brings difficulties to precise localization of language regions (Krieger-Redwood et al., 2013; Klaus et al., 2020). To overcome these problems, transcranial magnetic stimulation (TMS) was introduced as a focal method for cognitive neuroscience to probe the causal neural mechanisms for language in a non-invasive fashion (Flöel, 2012; Papeo et al., 2013; Hartwigsen, 2015; Uddén et al., 2017; Kennedy-Higgins et al., 2020; Kuhnke et al., 2020). In brief, the current in the TMS coil induces a perpendicular magnetic field which penetrates the scalp without attenuation to reach the stimulated brain region, and this magnetic field in turn induces a short-lived current that leads to stimulation of the neurons within the target region, resulting in either a facilitation or an inhibition effect as reflected by behavioral performance changes (Klomjai et al., 2015; Pitcher et al., 2020). Notably, these effects are collectively referred to as “neuromodulatory effects” in the current study.
Initially introduced for the stimulation of human motor cortex [Barker et al., 1985, as cited in Hartwigsen (2015)], TMS has been widely applied in clinical studies on language performance in recent years, including the identification of language lateralization as well as the localization of language-related brain regions before surgical resection in tumor patients (Han et al., 2021; Nettekoven et al., 2021), and the rehabilitation or treatment of language impairments such as post-stroke aphasia (Murdoch and Barwood, 2013). To guide such clinical applications and provide insight into the functional relevance of the language network, TMS studies in the healthy population are essential. The impact of TMS studies in healthy volunteers is twofold: On the one hand, causal mapping of structure-function relationships via TMS might deepen our understanding of the neurobiological mechanisms underlying normal language performance (Devlin and Watkins, 2007; Flöel, 2012); on the other hand, healthy participants with smaller variance might serve as an ideal model for the assessment of comparatively stable brain plasticity and compensatory effects (Hartwigsen et al., 2013; Jung and Lambon Ralph, 2016).
However, TMS effects are prone to be moderated by various factors such as tasks, target brain regions, brain state before and during stimulation, and stimulation parameters (Vallar and Bolognini, 2011; Hartwigsen, 2015; Hartwigsen and Silvanto, 2022), rendering the between-study heterogeneity inflated. For instance, with regard to specific language functions, TMS could reduce the accuracy (ACC) and prolong the reaction time (RT) for semantic tasks like picture naming and synonym judgment tasks (Whitney et al., 2012; Krieger-Redwood and Jefferies, 2014; Papeo et al., 2015; Klaus et al., 2020), whereas certain studies did not find any modulatory effects (Hartwigsen et al., 2010, 2016), or instead discovered reversed effects (Bonnì et al., 2015; Piai et al., 2020). This was also the case for syntactic and phonological tasks, in which the results were still disputed (Sakai et al., 2002; Uddén et al., 2008, 2017; Hartwigsen et al., 2010, 2016; Sliwinska et al., 2012; Acheson and Hagoort, 2013; Klaus and Hartwigsen, 2019; Deschamps et al., 2020; Ishkhanyan et al., 2020). Moreover, TMS effects also varied for different cortical regions (Devlin et al., 2003; Gough et al., 2005; Bonnì et al., 2015; Klaus and Hartwigsen, 2019). Since language areas are widely distributed across the frontal, temporal and parietal lobes in the left hemisphere, and within each lobe, a functional gradient for phonological, syntactic and semantic processes has been assumed (Xiang et al., 2010; Hagoort, 2013; Klaus and Hartwigsen, 2019), it becomes necessary to evaluate the efficacy of TMS on these three relatively large language-related lobes as well as more specific brain regions such as the inferior frontal gyrus (IFG), the superior temporal gyrus (STG), and the middle temporal gyrus (MTG). Therefore, the present study conducted moderator analyses on language tasks (of different language functions, including semantic, syntactic, and phonological tasks) as well as different cortical targets (i.e., brain regions of interest, including frontal, temporal and parietal lobes and IFG, STG and MTG).
In terms of TMS methodology, different TMS protocols were reported to be critical for influencing TMS effects (Klomjai et al., 2015; Sollmann et al., 2015; Sondergaard et al., 2021; Hartwigsen and Silvanto, 2022). Such parameters mainly involve (but are not limited to):
(a) Methods of localization (i.e., to choose an appropriate stimulation site for a target region through referring to previous studies or conducting localization by the current study per se) (Sparing et al., 2008; Sack et al., 2009; Flöel, 2012; Papeo et al., 2015; Jost et al., 2020);
(b) Stimulation types [differentiated according to the frequency and duration of magnetic pulses. Please note that in this study, TMS serves as a broad technical term for different specific stimulation types, containing: (i) repetitive TMS, consisting of trains of regularly repeated pulses of very short duration (milliseconds), including navigated rTMS, a combination of MR-based neuro-navigation systems and rTMS; (ii) theta burst stimulation (TBS), also known as patterned rTMS, characterized by the application of patterned bursts with short intervals of no stimulation, including intermittent TBS (iTBS) and continuous TBS (cTBS)] (Franzmeier et al., 2012; Schuhmann et al., 2012; Hartwigsen et al., 2013; Murdoch and Barwood, 2013; Jung and Lambon Ralph, 2016; León Ruiz et al., 2018; Deschamps et al., 2020);
(c) Timing (online and offline stimulation) (Hartwigsen, 2015; Sliwinska et al., 2017);
(d) Frequencies [high (>1 Hz) and low (≤1 Hz) frequency] (Bailey et al., 2001; Fitzgerald et al., 2006; Murdoch and Barwood, 2013; Hartwigsen, 2015; Beynel et al., 2019);
(e) Intensities [usually calibrated according to the resting motor threshold (RMT) or active motor threshold (AMT)] (Bailey et al., 2001; Sparing et al., 2001; Siebner et al., 2009).
Studies with various settings regarding these parameters have yielded mixed and unstable neuromodulatory effects on language performance. For example, studies investigating phonological processing found that high or low frequencies could lead to either enhancement or impairment of task performance (Andoh et al., 2006; Romero et al., 2006; Klaus and Hartwigsen, 2019). Similarly, stimulation intensities above or below the RMT might modulate the semantic response speed, but might also leave it unaffected (Hartwigsen et al., 2010; Krieger-Redwood and Jefferies, 2014; Bonnì et al., 2015). Therefore, for each parameter, the TMS effect was evaluated to identify optimal protocols for future TMS studies on language processing in healthy volunteers.
The specific experimental design is another potential factor contributing to the inconsistency among the related TMS studies’ findings. For example, regarding the control conditions, effective (active) stimulation of control sites, that is, unrelated brain regions (such as the vertex and other brain regions considered as irrelevant to the language functions under investigation), was claimed to be better than merely using sham stimulation (e.g., changing the angle of the stimulation coil with the target region un-stimulated) (Hartwigsen, 2015; Beynel et al., 2019). Nevertheless, the possibility that effective stimulation on the control sites could also interfere with task performance cannot be completely ruled out (Sandrini et al., 2011). Some studies, for instance, found that TMS perturbation of the occipital control sites affected semantic processing (Pobric et al., 2010; Papeo et al., 2015). There are also studies utilizing the comparison between different experimental tasks or language processing phases as the control condition to observe TMS effects (referred to as “others” in the current study), of which the effectiveness still awaits confirmation. As for the group designs, relative to between-subject designs, within-subject designs may reduce the individual variance for different conditions, but might suffer from repetition effects (i.e., improved task performance through repetitive presentation of certain materials), carry-over effects (i.e., effects carried over from one experimental treatment to another), or practice effects (i.e., improved task performance simply due to practice), and have to be implemented with a sufficiently long inter-session interval (usually about 1 week) for respective conditions (Hartwigsen, 2015). Therefore, it is still uncertain which control conditions or designs could elicit more robust TMS effects on language performance.
The latest search shows there have been only three meta-analyses addressing TMS effects on healthy participants’ language performance (Klaus and Schutter, 2018; Beynel et al., 2019; Johnson, 2021), but none of them took TMS or language comprehension as well as production abilities as their primary focus. Therefore, this study aims to systematically evaluate the efficacy of neuromodulatory TMS effects on language performance in healthy participants by meta-analyzing (a) the overall TMS effects on healthy participants’ language task performance, and (b) zooming in on the main moderators of TMS effects in previous TMS language performance-related studies — language tasks, cortical targets, stimulation parameters, and experimental designs. We expect the findings of this meta-analysis to (a) enrich the results found by previous TMS meta-analyses on aphasics (Ren et al., 2014; Bucur and Papagno, 2019; Hong et al., 2021; Zhang et al., 2021), (b) clarify the efficacy of TMS effects on healthy participants’ language performance, including both comprehension and production abilities (cf. Klaus and Schutter, 2018; Beynel et al., 2019), and (c) inform future TMS studies in the neurolinguistic field with respect to optimized designs and parameters.
Materials and methods
This study followed the PRISMA (The Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines (Page et al., 2021) and has been registered in the international prospective register of systematic reviews (PROSPERO) under the registration number: CRD42022366481.
Study search and selection
We conducted a literature search in PubMed, Web of Science, PsycINFO, Scopus, and Google Scholar from the inception of the databases to October 15th, 2022 to identify eligible studies by querying all TMS studies (including original studies, reviews, and meta-analyses) on language comprehension and production in healthy participants, published in English. The search terms were any combination of (“Language, Syntax, Grammar, Semantics, Meaning, Phonetics, OR Phonology”) AND (“TMS” OR “transcranial magnetic stimulation”). The reference lists of previous reviews and meta-analyses (Vigneau et al., 2006; Flöel, 2012; Ren et al., 2014; Otal et al., 2015; Klaus and Schutter, 2018; Beynel et al., 2019; Bucur and Papagno, 2019; Hartwigsen and Saur, 2019; Hong et al., 2021; Zhang et al., 2021) were also screened in case any related studies were overlooked. The eligible study selection criteria are listed as following:
(a) Participants were healthy adults (aged between 18 and 60 years old) (Gingras et al., 2021), right-handed, and for each experiment, the sample size was ≥5 (participants). Since children’s and teenagers’ brains are still developing, and aging brains (>60 years old) may confront both structural and functional decrease, only healthy adults within the specified age range were deemed an ideal and comparatively steady target population for this study. Therefore, studies reporting juvenile or aging participants’ data were excluded (Taylor and Burke, 2002; Wierenga et al., 2014; Jernigan et al., 2016).
(b) Transcranial magnetic stimulation was applied to the cerebral cortex of the participants. Given that the relationship between the cerebellum and language functions is still largely unclear, studies applying TMS over the cerebellum were tentatively excluded (e.g., Argyropoulos and Muggleton, 2013; de Smet et al., 2013). Moreover, several navigated TMS (nTMS) studies mapping language functions (esp., word production via picture-naming) were also excluded because there was no explicit baseline. Also, since such nTMS studies aimed to map all possible brain regions involved in language processing (i.e., brain-language mapping), they focused on the relative rather than the absolute task sensitivity differences among the brain regions (e.g., Krieg et al., 2016; Tussis et al., 2017).
(c) Research contents included language comprehension and/or production tasks. Note that certain studies using language materials to investigate general cognitive abilities such as memory, attention, and reasoning (e.g., Innocenti et al., 2013; Möttönen et al., 2014), and studies focusing on sign languages (e.g., Banaszkiewicz et al., 2021), low-level orthographic processing (e.g., Lavidor and Walsh, 2003; Pattamadilok et al., 2010) as well as extended meaning comprehension and discourse reading engaging advanced language processing (e.g., Pobric et al., 2008; Tomasino et al., 2008; Cacciari et al., 2011; Gough et al., 2013) were excluded.
(d) Means and standard deviations of reaction time (RT) and/or accuracy (ACC) were reported or obtained upon authors’ requests.
(e) The trials were controlled and randomized.
(f) Studies were formally published in international peer-reviewed English journals and were officially approved by medical ethical committees or review boards.
Each study underwent three screening steps for final inclusion: (1) Removal of duplicates (this was done by XQ, ZW, YC, QX, ZL, and LL); (2) Screening of titles and abstracts (XQ and ZW first independently evaluated all the studies and then conducted a check together. Studies met with disagreement were entered into the third step); (3) Full-text review (by XQ and ZW). Disagreement was resolved through group discussions among all authors. In addition, we also calculated Cohen’s kappa coefficient to assess inter-rater reliability (k = 0.803, p < 0.001), obtaining strong consistency (McHugh, 2012). The procedures of the study search and selection are depicted in Figure 1.
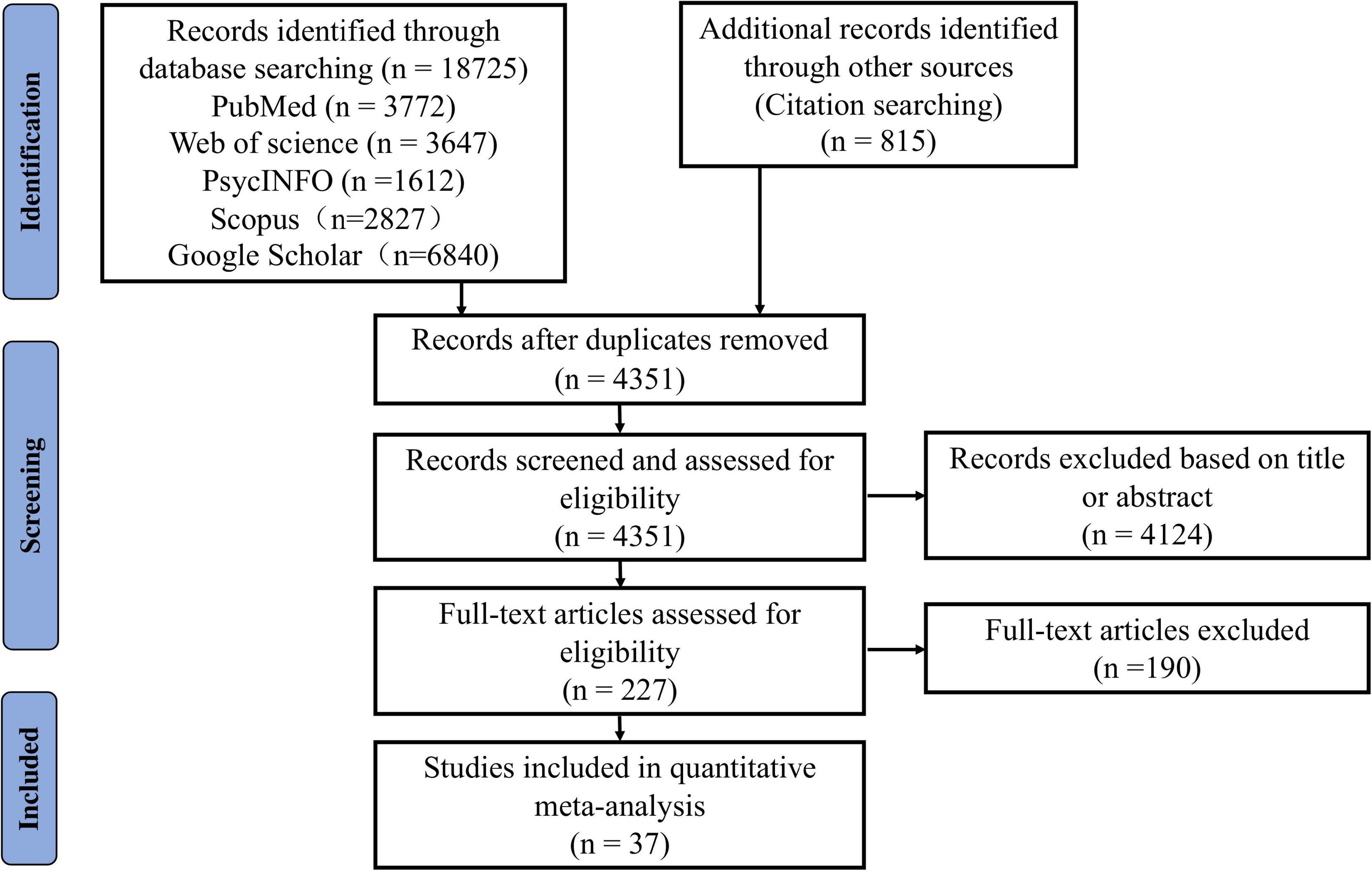
Figure 1. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram of literature search and selection for the meta-analysis.
Data extraction
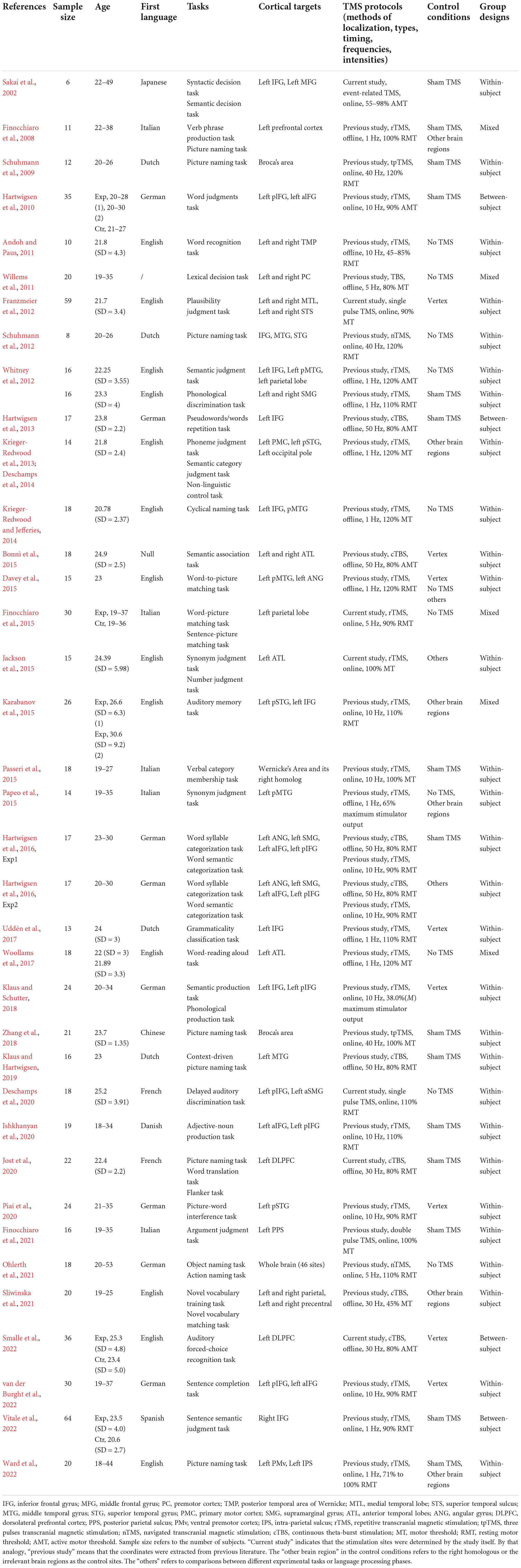
Thirty-seven eligible studies were identified. The means and standard deviations of RT and ACC, and the sample sizes were extracted. For each study, the following information was collected: literature information (authors and publication year), participant characteristics (sample size, gender, age, and native language), tasks, cortical targets, TMS protocols (methods of localization, stimulation types, timing, frequencies, and intensities), and study designs (control conditions and group designs). Table 1 provides an overview of the included studies.
XQ, ZW, YC, QX, ZL, and LL independently extracted data from each study. XQ and ZW further checked the extracted data together. Missing data pertinent to the current study were obtained and authorized by e-mailing the authors. The extracted data were recorded in excel spreadsheets. Data extraction and management were conducted manually.
Data analyses
We used mean and standard deviation to calculate Hedges’g (abbreviated as “g” hereafter) to estimate effect size, which provides a less biased estimate of the true effect than Cohens’d, especially for studies with small sample sizes (Hedges and Olkin, 1985). In line with Klaus and Schutter (2018), this meta-analysis mainly focused on the absolute effect sizes, that is, the magnitude of the neuromodulatory TMS effects regardless of the effect directions (i.e., improvement of behavioral performance as facilitation, and disruption of behavioral performance as inhibition). We performed data aggregation to avoid multiple similar data points from a single experiment entering the analysis, ensuring that each experiment provided only one measured value (Borenstein et al., 2009). Nevertheless, if several conditions were tested for more elaborate comparisons of the experimental variables within one experiment, the effect sizes would be calculated separately for each comparison (see also Klaus and Schutter, 2018). For example, if a study compared the conditions within various brain regions, for each brain region, the effect sizes were aggregated as one and included in the meta-analysis.
Since the included studies encompassed within-subject designs, it was difficult to assume statistical independence in the meta-analysis, and the issue of effect size dependency should be considered (Hunter and Schmidt, 2004). In previous studies, there were five commonly used methods to deal with correlated effect size: (a) Ignore dependencies; (b) Average dependent effect sizes across studies; (c) Extract only one effect size from each study; (d) Introduce the correlation coefficient r. However, these methods are suggested to be confronted with problems such as the exaggeration of relevant significance tests (Cheung, 2014), the lowering of the statistical power of meta-analysis and the precision of parameter estimation (Van den Noortgate et al., 2013) and too conservative estimation of coefficient value (Hedges and Pigott, 2001; Cheung, 2014). The fifth method (e) adopts modeling, such as the frequently used multilevel random effects model. Compared to the aforementioned four methods, using multilevel random effects model to estimate the effect sizes in meta-analysis is more accurate, effective and flexible. The model can incorporate multiple predictors to account for heterogeneity between studies or add additional random effects to address the various dependencies of effect sizes within and between studies (Fernández-Castilla et al., 2020). Therefore, to solve the problem of effect size dependency especially for within-subject designs, a three-level random effects model was adopted for the meta-analysis in this study (see Supplementary material for more details regarding the five methods and related rationales).
We also conducted the likelihood ratio test (LRT) to explore whether the three-level random effects model was more suitable for this meta-analysis compared to the traditional two-level random effects model. There was a significant difference between the traditional random effects model and the three-level random effects model in ACC (LRT = 9.99, p = 0.007), and a trend toward significance in RT (LRT = 2.80, p = 0.09). This indicated that compared with the traditional random effects model, the three-level model provided a better model fit.
Study heterogeneity was estimated by Cochran’s Q and p-value (Huedo-Medina et al., 2006), and all effect sizes were entered into the three-level random effects model. We also conducted sensitivity analysis to verify the robustness of the results.
According to the Cochrane guideline (Higgins et al., 2019), we used the Cochrane risk of bias tool (RoB 2.0, Sterne et al., 2019) to evaluate the quality of the included studies in the following aspects: (a) randomization process; (b) deviations from intended interventions; (c) missing outcome data; (d) measurement of the outcome; (e) selection of the reported result. Two researchers assessed the quality of each study. Studies met with disagreement were evaluated through pairing with a third party for group assessment.
Moreover, to account for the fact that the overall effect size might be overestimated because of publication bias (Rothstein and Bushman, 2012), we tested for a potential publication bias by constructing a “funnel plot”. If there was no bias, the graph would present an inverted funnel shape, and the distribution of the points (i.e., the included studies) would be roughly symmetrical. In case of a publication bias, the funnel would be skewed (Van den Bussche et al., 2009). However, concerns about the subjectivity of funnel plots have been raised. To ensure reliability, we further quantified publication bias by the Egger Test (Egger et al., 1997).
Moderator analyses were performed, since experimental parameters such as the language tasks (especially for different language functions, including semantic, syntactic, and phonological tasks), cortical targets [i.e., brain regions of interest, containing frontal, temporal, and parietal regions, as well as more specific regions including IFG, STG, and MTG. Please note that owing to the lack of adequate sample sizes, we did not perform meta-analysis on other specific regions such as the premotor cortex (PC), the anterior temporal lobe (ATL) and the angular gyrus (AG)], stimulation parameters of the TMS protocols [i.e., methods of brain region localization, stimulation types (further analysis on cTBS but not iTBS (only one study) was conducted considering the inadequate sample sizes), timing, frequencies, and intensities], and experimental designs (i.e., control conditions and group design types) are crucial for the examination of TMS effects on language performance in healthy participants. Effect sizes with an associated 95% confidence intervals (CI) were calculated when at least two studies were available for a particular estimate (see also Valentine et al., 2010). The analysis-structure of both the overall and the moderator analyses are summarized in Figure 2.
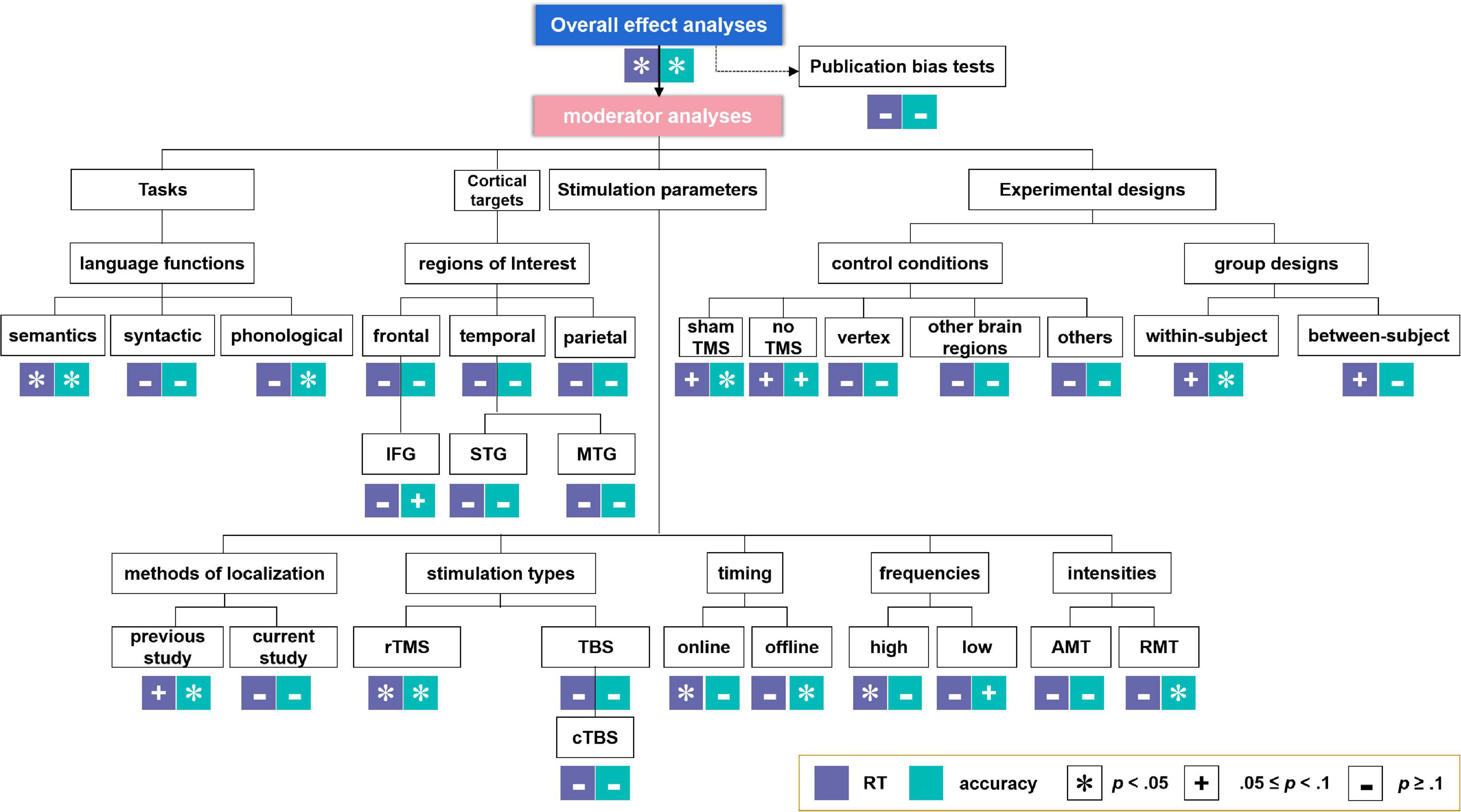
Figure 2. Analysis-structure and results for overall effect analyses and moderator analyses. *Indicates significant effects. +Indicates marginally significant effects. –Indicates non-significant effects.
All effect size computations, summary analyses, sensitivity analysis, risk of bias tests and the publication bias tests were conducted by using the “metafor 3.0-2” (Viechtbauer, 2010) implemented in R (version 4.0.4, R Core Team, 2021). The results of all effect analyses of RT and ACC were graphically synthesized in forest plots.
Results
Overall transcranial magnetic stimulation effects
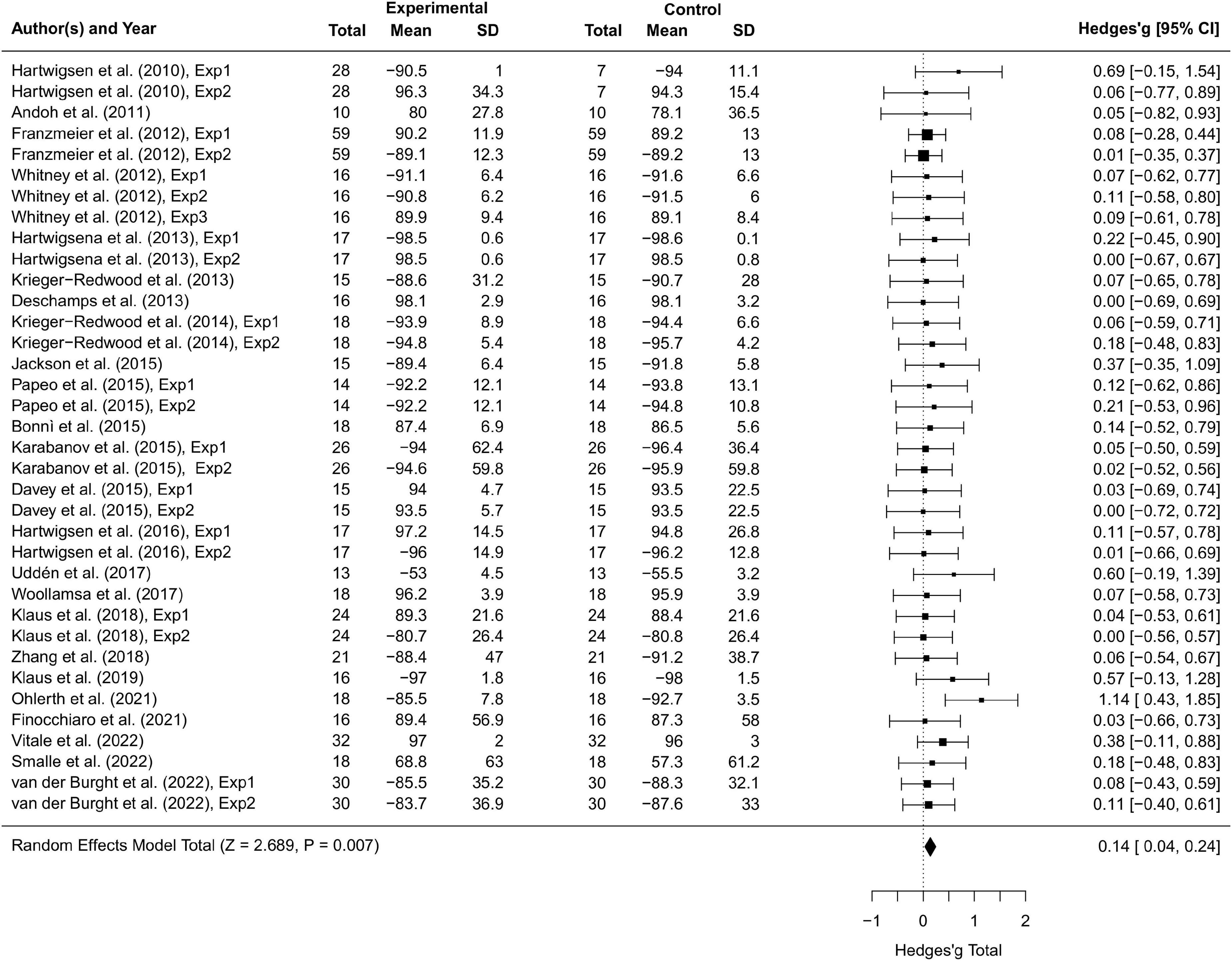
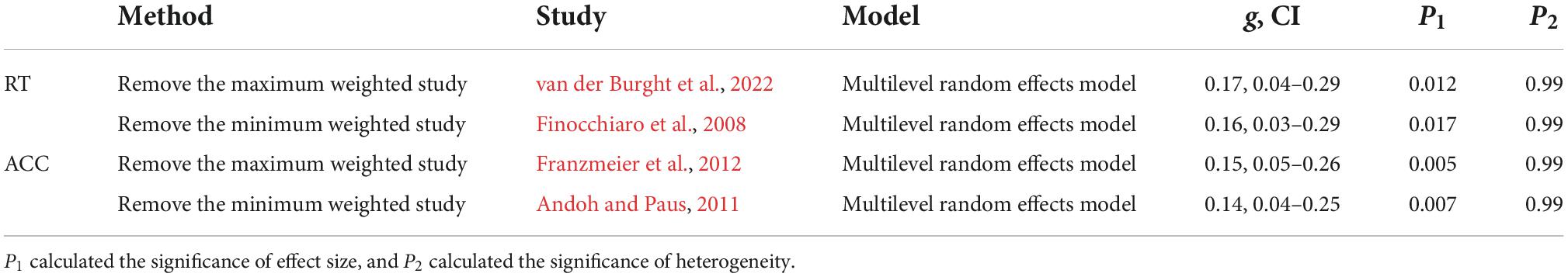
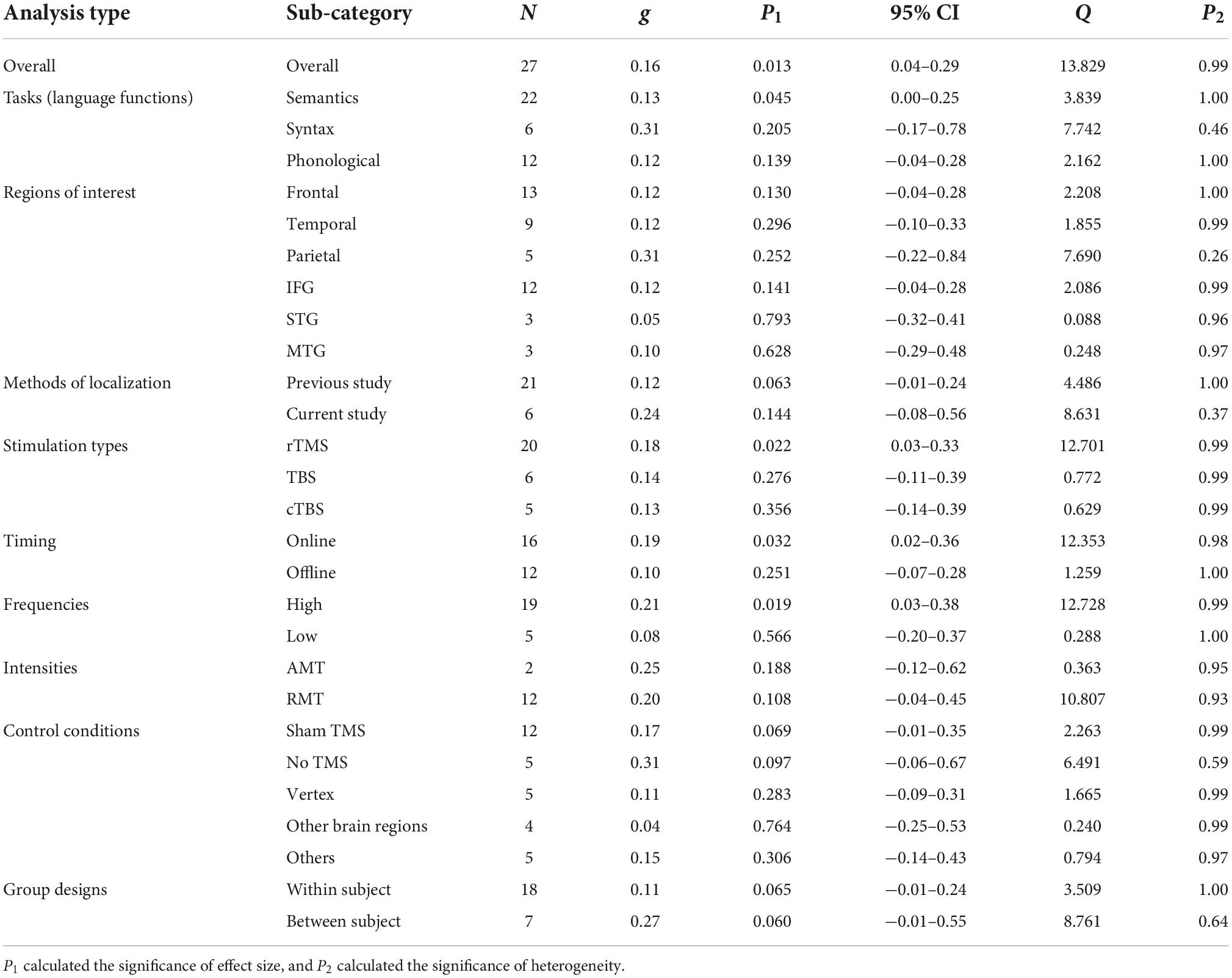
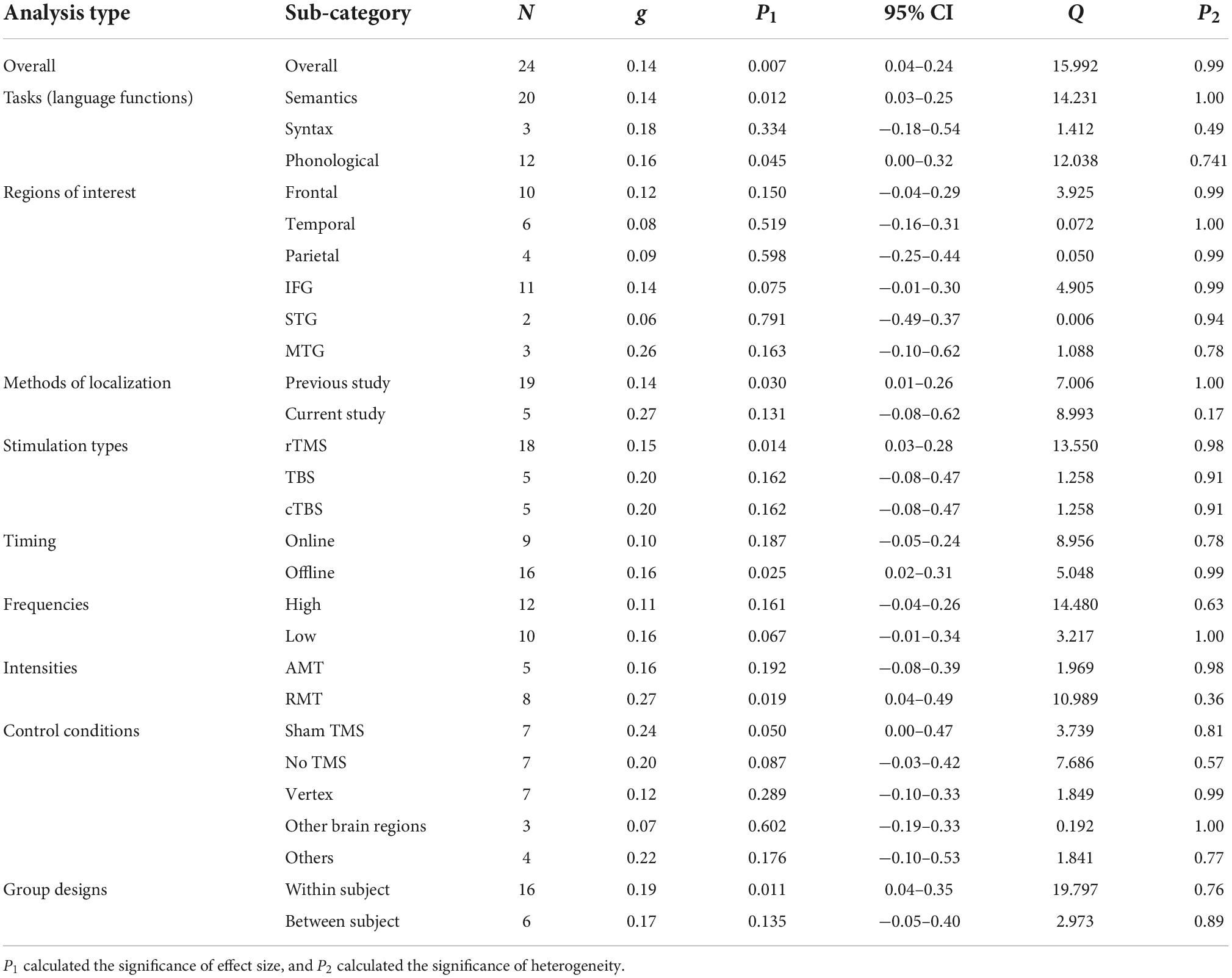
Thirty-seven studies including 797 participants and 77 effect sizes were computed for the overall effect analysis. The results (see also Figures 3, 4) showed that for both RT and ACC, TMS could exert significant neuromodulatory effects on language performance in healthy participants (RT: g = 0.16, 95% CI: 0.04–0.29, Z = 2.496, p = 0.013; ACC: g = 0.14, 95% CI: 0.04–0.24, Z = 2.689, p = 0.007). The sensitivity analysis revealed that the results were still significant after removing the studies with the maximum and the minimum weight, which indicated the stability of RT and ACC in the overall analysis (see Table 2 for details).
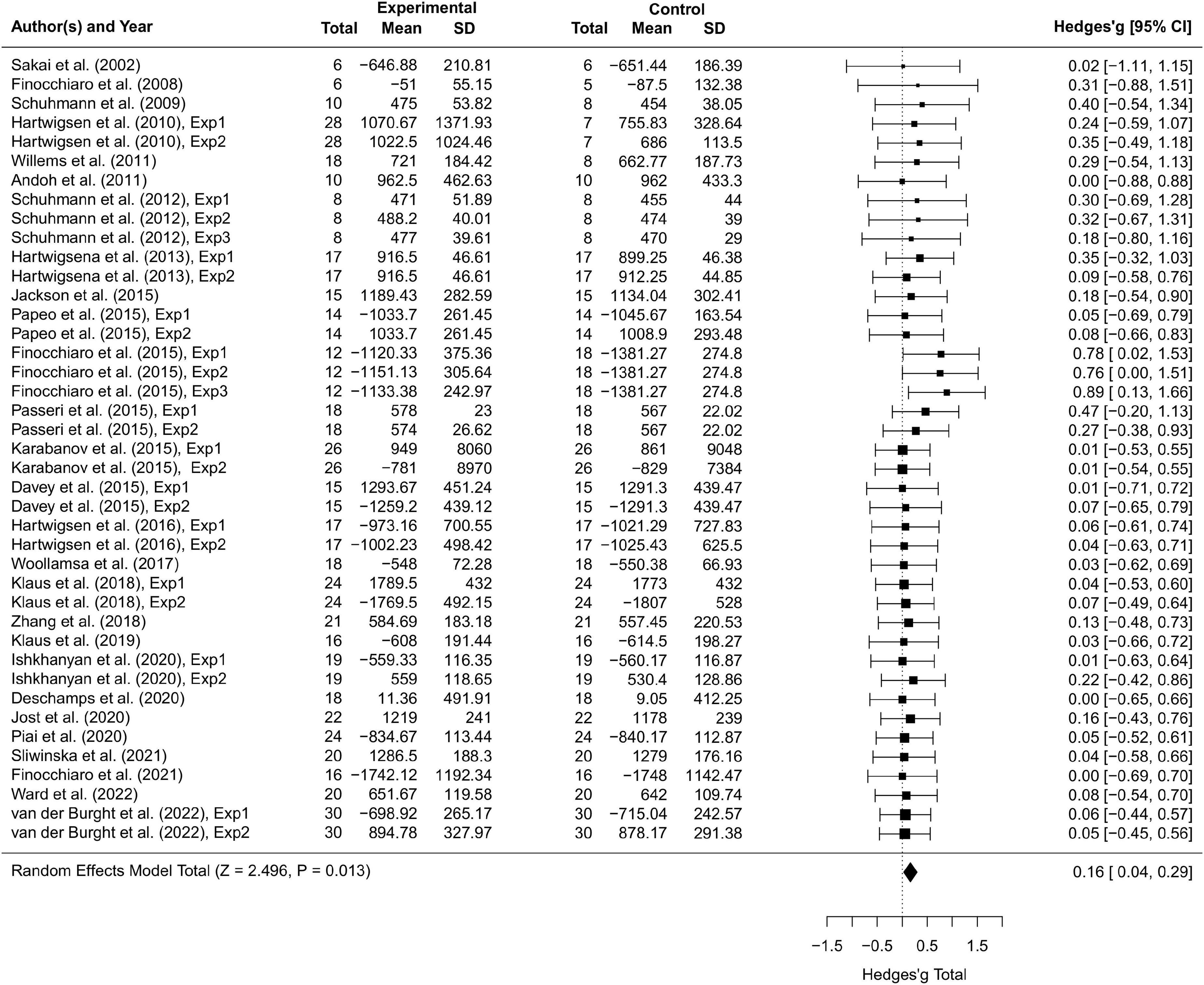
Figure 3. Forest plot of reaction times (RTs) of overall effect analysis. The discrepancy in sample size between the experimental group and the control group in some studies is due to the between-subject experimental design.
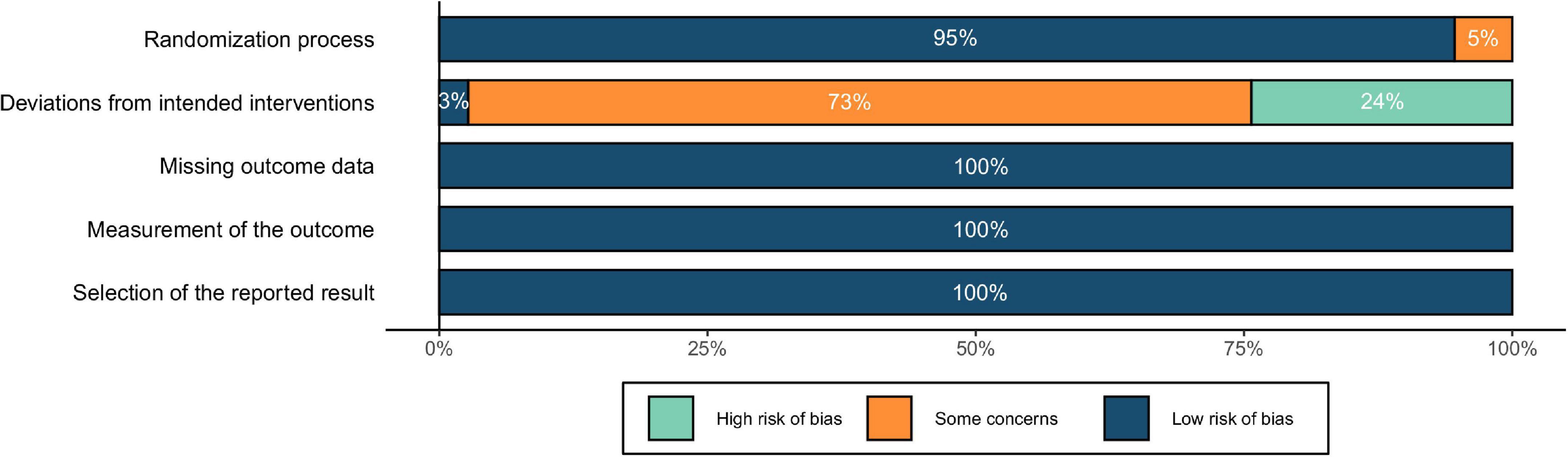
The risk of bias was depicted in Figure 5. The results showed a low risk of bias in terms of randomization process. As for the risk of deviations from intended interventions (i.e., effects of blindness), all studies were single blind except for one double-blind study. Regarding the blinding of participants, studies using “vertex,” “other brain regions,” and “others” as the control conditions simulated the auditory and somatosensory sensations caused by the active stimulation, resulting in a low risk of bias. With “sham TMS,” although the auditory sensations could be similar to that of active stimulation, the somatosensory sensations were different, which might lead to mild concerns of blindness. In comparison, the control condition of “no TMS” could be easily differentiated from the active stimulation by the participants, resulting in potential high risk of bias. Finally, for the risk of missing the outcome data, inaccurate measurement of the outcomes, and reporting selected results, no evidence indicating an unclear or high risk of bias was found in the included studies. To summarize, although there might be a few risks of blinding, the overall quality could still be acceptable, and no study was eliminated because of low quality.
The publication bias tests showed no significant results in RT or ACC (RT: Egger: p = 0.69; ACC: Egger: p = 0.23), indicating that the overall effect sizes should not be enhanced by publication biases (see also Figure 6).
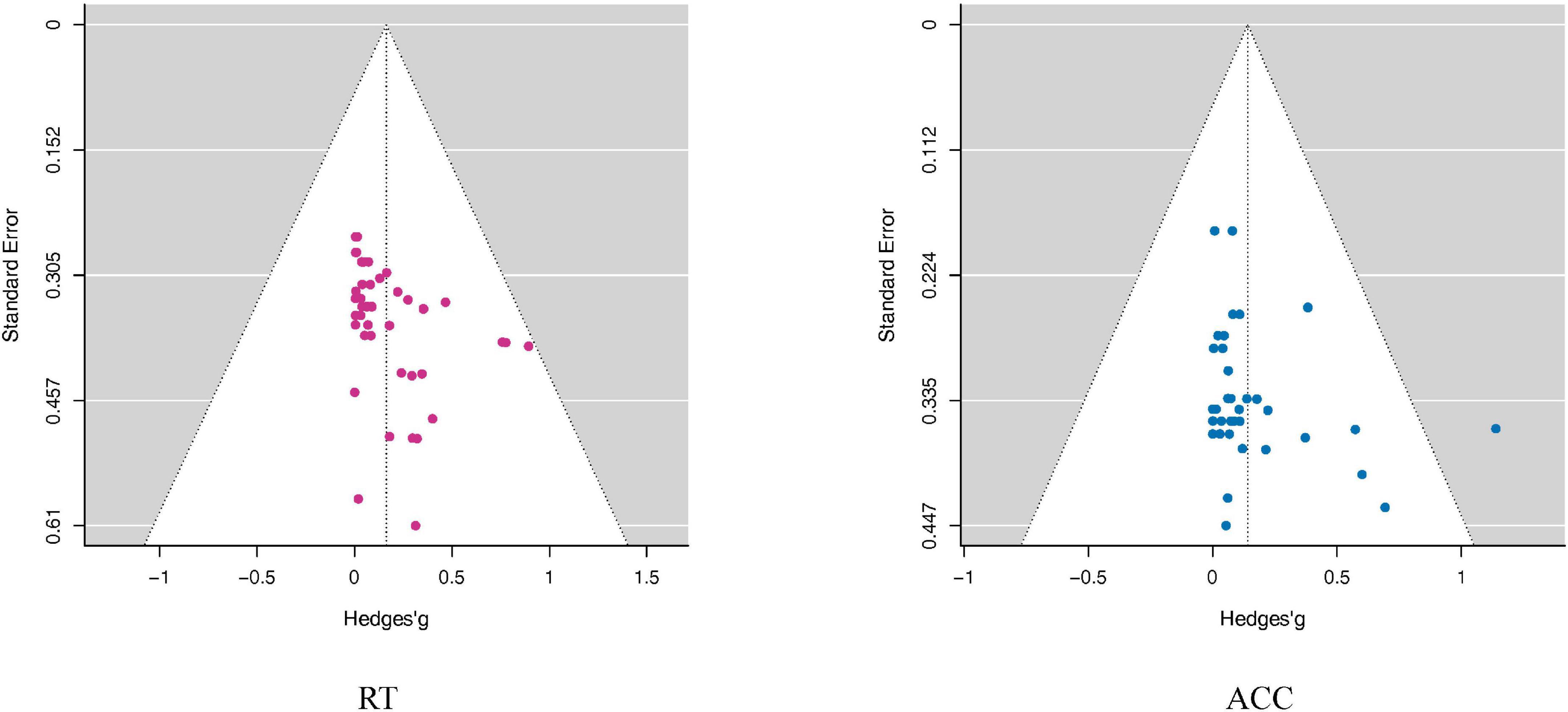
Figure 6. Funnel plots assess publication bias in the RT and ACC outcomes. The Funnel plots took the Mean difference as the abscissa and Standard Error as the ordinate. The small dots in the figure represent the included studies. In the funnel plot, the dotted line perpendicular to the horizontal axis represents the overall effect, and the dotted line on both sides represents the 95% confidence interval (CI). As shown in this figure, the distribution of all studies in the funnel plot was roughly symmetric, suggesting that there was no publication bias. Although the funnel plot can visually observe publication bias, it is subjective, and it can be seen that individual studies deviate from 95% confidence interval (CI). Therefore, we adopted Egger’s test to quantify the publication bias. Note that the point outside the white area in the ACC funnel plot does not represent an outlier, because the significance of the further quantitative Egger Test was not affected by including or excluding this study.
Moderator analyses results
The moderator analyses results were summarized in Tables 3, 4 for RT and ACC, respectively, and displayed in Figure 2 (see Supplementary material for the forest plots, including moderator analyses and additional analyses).
Language tasks
For tasks concerning different language functions, TMS induced significant neuromodulatory effects for semantic tasks on both RT and ACC (RT: g = 0.13, 95% CI: 0.00–0.25, p = 0.045; ACC: g = 0.14, 95% CI: 0.03–0.25, p = 0.012). However, TMS showed no significant influence on syntactic tasks. For phonological tasks, TMS significantly affected ACC (g = 0.16, 95% CI: 0.00–0.32, p = 0.045).
Cortical targets
Transcranial magnetic stimulation did not show significant effects in larger frontal, temporal, or parietal regions. Further analysis on specific brain regions indicated that TMS had marginal significant effects on the IFG in ACC (g = 0.14, 95% CI: −0.01–0.30, p = 0.075).
Parameters of the stimulation protocols
The moderator analysis on the methods of localization indicated that TMS on the coordinates extracted from previous studies could exert significant effects on ACC (g = 0.14, 95% CI: 0.01–0.26, p = 0.030) and presented a trend for significance in RT (g = 0.12, 95% CI: −0.01–0.24, p = 0.063), whereas TMS on the sites detected by the studies per se elicited non-significant effects.
As for the stimulation types, rTMS had robust neuromodulatory effects on both RT and ACC (RT: g = 0.18, 95% CI: 0.03–0.33, p = 0.022; ACC: g = 0.15, 95% CI: 0.03–0.28, p = 0.014). Neither TBS nor cTBS showed significant effects on RT or ACC.
Tests on the timing parameters revealed that online stimulation could induce significant neuromodulatory effects on RT (g = 0.19, 95% CI: 0.02–0.36, p = 0.032), whereas offline TMS manifested significance in ACC (g = 0.16, 95% CI: 0.02–0.31, p = 0.025).
Regarding the stimulation frequencies, the moderator analysis with absolute effect sizes supported the notion that high-frequency TMS would significantly influence RT (g = 0.21, 95% CI: 0.03–0.38, p = 0.019), while low-frequency TMS showed a trend toward significantly affecting ACC (g = 0.16, 95% CI: −0.01–0.34, p = 0.067). Given that different frequency types were proposed to be related to different effect directions (at least in the motor system), that is, high-frequency TMS tends to have a facilitatory effect, whereas low-frequency TMS tends to have an inhibitory effect (Sandrini et al., 2011; Hartwigsen, 2015; Beynel et al., 2019), we ran an additional analysis with the original effect sizes. Although no significant effect was found, the results indicated that high-frequency TMS tended to reduce ACC instead of facilitating it (see Supplementary Figure 50).
Finally, the moderator analysis of TMS intensities revealed that RMT rather than AMT induced significant effects on ACC (g = 0.27, 95% CI: 0.04–0.49, p = 0.019).
Experimental designs
The moderator analysis on control conditions revealed that sham TMS displayed significance in ACC and was marginally significant in RT, suggesting that compared to other control conditions, this condition could serve as a promising baseline for detecting TMS effects (RT: g = 0.17, 95% CI: −0.01–0.35, p = 0.069; ACC: g = 0.24, 95% CI: 0.00–0.47, p = 0.050). Also, the “no TMS” control condition showed a trend of significance in RT and ACC (RT: g = 0.31, 95% CI: −0.06–0.67, p = 0.097; ACC: g = 0.20, 95% CI: −0.03–0.42, p = 0.087). The other conditions did not show any significant effects.
When taking the individual variance into consideration, within-subject designs seemed to be optimal for identifying significant TMS effects on ACC and showed marginally significant effects on RT (RT: g = 0.11, 95% CI: −0.01–0.24, p = 0.065; ACC: g = 0.19, 95% CI: 0.04–0.35, p = 0.011). Between-subject designs only exhibited TMS effects on RT in an approaching-significance way (g = 0.27, 95% CI: −0.01–0.55, p = 0.060).
Discussion
By meta-analyzing the currently available data, this study aimed at evaluating neuromodulatory TMS effects on language performance in healthy adult volunteers. The overall effect analyses revealed that TMS significantly affected language task performance (as reflected by changes in RT and ACC), which was in line with the findings of previous studies (e.g., Klaus and Schutter, 2018; Beynel et al., 2019).
Although TMS seems to be a promising non-invasive technique for investigating causal structure-function relationship in language domain, both the stability and reliability still await to be assessed (Walsh and Cowey, 2000). Therefore, our subsequent moderator analyses further specified the efficacy of TMS on language performance regarding moderators in four critical aspects—language tasks, cortical targets, stimulation parameters, and experimental designs.
Language tasks
Transcranial magnetic stimulation significantly modulated task performance for both semantic and phonological tasks, contrasting its non-significant effects on syntactic tasks.
For semantic tasks, TMS effects were manifested both on RT and ACC. These robust modulatory effects might be attributed to two reasons. First, semantic processing recruits broadly distributed but highly interactive regions in the left hemisphere such as the inferior frontal, and posterior temporo-parietal cortices (Hartwigsen et al., 2010, 2016; Papeo et al., 2015; Passeri et al., 2015). TMS studies, utilizing “condition-and-perturb” or “perturb-and-measure” paradigms on semantic processing revealed that TMS effects on the stimulation site might spread to other regions that are structurally or functionally connected to the stimulated area (Hartwigsen et al., 2016; Vitale et al., 2021). This suggests that the broad semantic network might be affected as a whole when targeting a semantic key region, leading to a significant change in semantic task performance. Second, potential compensatory effects within larger networks seem to be strictly constrained by the experimental factors. As pinpointed by Klaus et al. (2020), the compensatory effects of the semantic network might only be observed when TMS intensity and executive control components of the task are relatively low. As a result, semantic task performance can not be maintained to the original level by compensatory effects.
Phonological processing also involves a distributed neural network, but previous work suggests that each region might make relatively independent and unique contributions to phonological processing (Hartwigsen et al., 2010), thus potentially reducing effective compensatory effects among regions and leading to significant effects on task performance. Another finding worth noting is that only ACC but not RT was affected by TMS. This could be due to the fact that phonological processing relies on a larger domain-general verbal working memory system (Deschamps et al., 2014, 2020), and previous studies have shown that TMS effects on working memory tasks tend to be manifested especially on ACC (Mottaghy et al., 2003; Nixon et al., 2004; Romero et al., 2006; Osaka et al., 2007; Acheson et al., 2011). As pointed out by Deschamps et al. (2014), most of the tasks adopted by studies investigating phonological processing actually recruited verbal working memory, such as the same/different judgment task (Deschamps et al., 2014), the delayed auditory discrimination task (Deschamps et al., 2020), and phonological decision tasks (Hartwigsen et al., 2016), all involving sub-vocal rehearsal and the maintenance of phonological information in working memory. Another potential explanation for the observed effects is that compared to the retrieval and decision phase, perturbation of the encoding phase tends to cause changes in ACC as opposed to RT (Karabanov et al., 2015). Most of the included phonological studies in our meta-analysis focused on the posterior inferior frontal gyrus (pIFG), supramarginal gyrus (SMG), and posterior superior temporal gyrus (pSTG), which have been proven to play a role in the rehearsal and encoding phase, but not in the retrieval or decision phase of phonological working memory tasks (Nixon et al., 2004; Kahn et al., 2005; Kirschen et al., 2006).
Compared to semantic and phonological studies, the number of syntactic studies was relatively small, hampering the possibility to obtain a stable significant result and a conclusive interpretation. However, some studies did show a significant TMS effect on syntactic tasks (Uddén et al., 2017; Ishkhanyan et al., 2020; van der Burght et al., 2022). It is also possible that the overall insignificant results are related to the degree to which sub-regions are differentiated. Taking Finocchiaro et al. (2015) as an example, this study probed the function of anterior, middle and posterior parietal sites in thematic role assignment and only found a significant TMS effect for the posterior site. Therefore, for more comprehensive and deeper understanding of TMS neuromodulatory effects on syntactic processing, future studies should adopt different syntactic tasks and focus on specialized sub-regions (such as IFG and pSTG).
Cortical targets
With regard to stimulation sites, none of the three broad regions (including frontal, temporal, and parietal regions) showed significant TMS effects on RT or ACC. Further analyses at a finer-scale only revealed marginally significant TMS effects on ACC in IFG, but not in STG and MTG.
These results might be interpreted from four directions. First, the basic rationale of TMS studies is to explore, or to be more specific, to verify the potential causal relationship between cognitive functions and certain brain regions based on previous neuroimaging and clinical studies (Flöel, 2012; Papeo et al., 2013; Hartwigsen, 2015). Therefore, the results can be both verification as well as falsification. When analyzing the brain regions alone regardless of the effects of other moderators such as language functions, it seems equally possible to obtain significant as well as non-significant effect. For example, the findings of Bonnì et al. (2015) contradicted the existing clinical studies, showing that TMS on left ATL had no behavioral effects on written word processing. Krieger-Redwood et al. (2013) found that, despite positive evidence from neuroimaging results, TMS over the primary motor cortex (PMC) did not disrupt the mapping of speech sounds onto semantic categories. As a result, future meta-analyses should investigate interactions and try to separate the relationships between different moderators.
Second, it has become increasingly evident that some key language-related brain regions such as IFG and STG can be divided into finer anatomical structures specialized for different functions (Whitney et al., 2012; Klaus and Hartwigsen, 2019; Deschamps et al., 2020; Piai et al., 2020). This brought new challenges for studies to localize the precise stimulation site for the target brain regions underlying certain functions, along with the already existing barriers regarding the limited spatial resolution of TMS (between 0.5 and 1 cm, Sliwinska et al., 2015) and the variance in precision of different methods of localization, leading to the failure to capture significant modulatory effects of TMS. Moreover, it has also been demonstrated that these sub-regions are quite sensitive to task difficulty. For example, in Whitney et al. (2012), stimulation to the anterior inferior frontal gyrus (aIFG) only affected semantic tasks with higher executive control demands, while leaving more automatic tasks unaffected. Future TMS studies are therefore recommended to differentiate not only between specific task types for each language function (i.e., semantic, syntactic and phonological) but also between different degrees of task difficulty within each task.
Third, unlike long-term effects of recovery in patients with brain lesions (Walsh and Cowey, 2000; Krieger-Redwood et al., 2013), the “virtual lesion” caused by TMS may be compensated by rapid functional reorganization within the distributed neural network for language in a rather short time (Hartwigsen et al., 2013, 2016; Klaus and Hartwigsen, 2019), making the transient TMS effects harder to detect. Combined with the finer division of regions and corresponding functions, the implication for future studies is to aim for the network instead of single nodes and target key/hub nodes within the networks for different functions.
Finally, for the marginally significant TMS effects on IFG, we reason that this may reflect the relatively large number of studies investigating this region. Since IFG is the language hub where the classic language region, Broca’s region resides, studies probing semantic, syntactic, or phonological processing could all take IFG into account and have indeed proven its involvement in these three language functions (Krieger-Redwood and Jefferies, 2014; Zhang et al., 2018; Ishkhanyan et al., 2020). On the other hand, the modulatory effects of TMS on STG and MTG are still rather unstable owing to the lack of adequate studies and need to be confirmed by future meta-analysis including more studies.
Stimulation parameters
The present results support the coordinates of focal stimulation sites (brain regions) extracted from previous studies over the localization determined by the researchers themselves. Taking a closer look at the specific approaches for targeting, we found that studies determining stimulation sites by themselves mostly utilized coarse-grained targeting such as scalp measurement in reference to certain landmarks on the skull (e.g., the inion) (Finocchiaro et al., 2008; Jackson et al., 2015) or standard electrode cap from the EEG 10-20 system (Franzmeier et al., 2012; Jost et al., 2020). These approaches neither account for inter-individual differences in the anatomical structures beneath the scalp nor for the differences in the functional organization of the brain (Sandrini et al., 2011; Beynel et al., 2019). Some studies also combined anatomical magnetic resonance imaging (MRI) scans with the use of frameless stereotaxic neuro-navigation systems to realize more accurate “online” localization of the target site (Zhang et al., 2018; Deschamps et al., 2020), but still, this approach lacks the precision regarding inter-individual differences in structure-to-function relationships. By contrast, relying on anatomical coordinates from previous fMRI studies or meta-analyses with the same task paradigm or tasks probing similar language processing under investigation (Krieger-Redwood and Jefferies, 2014; Passeri et al., 2015) seems to be more promising. This function-guided approach has been proven to be the optimal localization approach with higher experimental power, especially when individual fMRI localizers within the same participants are used (Sack et al., 2009; Sandrini et al., 2011; Beynel et al., 2019).
When considering the stimulation types, we found that rTMS could exert significant neuromodulatory effects on language task performance. This may be because rTMS could prolong the stimulation time, thus accumulating and enhancing the effect sizes (Sandrini et al., 2011). As for TBS, this protocol may be more susceptible to inter-individual differences in focal neuronal states, neural compensation mechanisms, and the specific location within the structurally complex brain regions (Silvanto and Pascual-Leone, 2008; Thut and Pascual-Leone, 2010; Hamada et al., 2013; Vernet et al., 2013; Jost et al., 2020), and thus elicited variable modulatory effects in the present meta-analysis (e.g., Hartwigsen et al., 2013; Bonnì et al., 2015; Jung and Lambon Ralph, 2016).
Our moderator analysis on the timing parameter (i.e., when to apply TMS) demonstrated that online TMS could exert significant neuromodulatory effects especially on RT while offline TMS elicited significant effects on ACC. During online TMS, stimulation is administered immediately before or during the task to transiently disrupt the ongoing neural processing (sometimes referred to as “virtual lesion”). However, it has also been proposed that such online disruption, unlike an actual lesion that would terminate the ongoing process, may rather induce neuronal noise in the targeted area (Devlin and Watkins, 2007; Sandrini et al., 2011). Consequently, online TMS might mainly result in a quantitative change of response efficiency (i.e., as reflected in the response speed), with the quality of response (i.e., the accuracy rates) being spared. Furthermore, according to the “state-dependency” concept (Silvanto and Pascual-Leone, 2008; Miniussi et al., 2013), the induced noise may not be completely random but dependent on the brain state induced by the task, and could turn into part of the signal if it synchronizes with the ongoing neural activity. For example, some studies discovered that TMS given immediately prior to the task could pre-activate related neuron populations and facilitate picture naming speed (Töpper et al., 1998; Mottaghy et al., 1999). Collectively, these findings support the notion that the transient online TMS effect is more likely to affect response efficiency but may not be detrimental enough to disrupt response quality. On the contrary, offline TMS is given before a task, with the aftereffects typically lasting for up to 30 min after the stimulation (Hartwigsen, 2015; Beynel et al., 2019). The accumulated rTMS effects are not restricted to the stimulated sites but may spread to other connected brain regions within a network. Such long-lasting remote effects may modulate the whole network and disrupt or facilitate processing, leading to a perturbation or enhancement in task accuracy.
The current results confirmed that both high and low TMS frequencies could affect healthy participants’ language performance, with high frequencies exerting more stable effects. This accords with a series of studies focusing on the influence of specific stimulation parameters (including frequency) on TMS effects (Sparing et al., 2001; Sollmann et al., 2015, 2018; Nettekoven et al., 2021), which support the idea that higher frequencies may induce more reliable disruption of language functions. There are two possible explanations for this finding. The first explanation is related to potential side effects, especially discomfort or pain during stimulation. The distraction caused by physical discomfort (e.g., twitching and contractions of face muscles) or more severe side effects (e.g., dysarthria resulting from stimulation-induced contraction of cranial muscles, Sollmann et al., 2018) are non-specific TMS effects and are very likely to confound the interpretation of the results. Therefore, it has been proposed that higher frequencies correlated with lower pain levels and were therefore more optimal for obtaining reliable TMS effects (Nettekoven et al., 2021). Secondly, it is likely that using TMS frequencies matching with the natural frequency band of endogenous brain oscillations increases the probability of TMS pulses to interfere with cortical processing at the appropriate timing (Thut and Miniussi, 2009; Miniussi et al., 2013; Nettekoven et al., 2021). Indeed, evidence from MEG studies has associated language-related processing with brain oscillations in higher frequency bands, such as the beta (17–25 Hz) and the low gamma band (26–50 Hz) (Hirata et al., 2010; Hinkley et al., 2020; Youssofzadeh et al., 2020). It is also worth noting that different language brain regions may be sensitive to different stimulation frequencies. However, studies exploring optimal frequencies for distinct regions are still lacking, leaving room for further progress.
Our additional analysis examining the direction of TMS effects revealed no significant results. Yet, we did find that high-frequency rTMS was prone toward inhibition as manifested by the ACC decrease. This supports the notion that a simple transfer of the relationship between frequency type and effect direction (i.e., high frequency for facilitation, and low frequency for inhibition, see Bailey et al., 2001; Fitzgerald et al., 2006; Murdoch and Barwood, 2013) from the motor to the language system does not hold. Rather, this relationship may be influenced by multiple factors such as task types, stimulation intensities, and target brain regions (Vallar and Bolognini, 2011; Hartwigsen, 2015; Beynel et al., 2019). Nevertheless, no strong conclusion could be made given the non-significant results.
The moderator analysis also showed that compared to the intensities calibrated according to the AMT, RMT could exert more significant TMS effects on language performance. This result is not surprising and directly relates to higher stimulation intensities. RMT is typically defined as the lowest amount of stimulator output (intensity) necessary to produce a motor evoked potential (MEP) in the resting muscle exceeding 50 μV in at least 50% of the total trials. In contrast, AMT is assessed under voluntary pre-contraction of the target muscle, requiring MEP sizes of at least 150 μV (Sandrini et al., 2011). A a result, individual RMTs are usually considerably higher (approximately 15%) than AMTs and consequently leading to a higher TMS intensity, which was proposed to introduce more severe perturbations, rendering functional compensation more difficult (Klaus et al., 2020), and exert stronger remote, long-distance effects spreading across specialized networks (Hartwigsen, 2015). Also, researches (although rare) distinguishing between RMT and AMT (e.g., Wassermann, 2002) argued that experimental error and other unstable determinants of threshold may account for about 36% of the cross-subject variability at rest and about 50% during voluntary contraction, which suggests that compared to RMT, AMT is more vulnerable to factors such as coil placement, stimulation frequency and other unknown physiological sources of individual variability and is therefore a less stable reference to determine the stimulation intensity.
Experimental designs
Regarding control conditions, sham TMS outperformed other solutions. It is noteworthy that TMS on presumably unrelated control sites might elicit unwanted effects due to their connections with target sites, thus potentially confounding experimental and control conditions (Franzmeier et al., 2012; Papeo et al., 2015; Klaus and Hartwigsen, 2019; Vitale et al., 2021). Therefore, researchers should be very cautious when selecting control sites. It should also be emphasized that the inclusion of sham TMS alone does not control for potential side effects of the stimulation such as muscle twitches and pain. Consequently, such conditions are not sufficient and studies without active control sites are more prone to false positives (Jost et al., 2020; Vitale et al., 2022). The optimal TMS study on language should include both an active control site and sham stimulation.
As for the group design types, within-subject designs were less affected by the individual variance which might submerge the TMS effects in between-subject designs (see also Passeri et al., 2015; Zhang et al., 2018). For example, Zhang et al. (2018) adopted a between-subject design and considered this a major limitation due to the large individual variance. Passeri et al. (2015) further emphasized that the neuromodulatory TMS effects are largely affected by the different degrees of language-related brain region lateralization of individuals. Therefore, the individual brain’s structural and functional variance might be critical for identifying TMS effects.
Limitations and outlook
The present systematic meta-analysis provides first insights into TMS neuromodulatory effects on language performance in healthy adults, elucidating both overall as well as specific effects regarding the moderators of language tasks, cortical targets, stimulation parameters, and experimental designs, and therefore identifies conditions more prone to elicit robust TMS effects. However, it is premature to draw strong conclusions about a “perfect TMS study design or protocol” in neurolinguistics, as TMS effects are moderated by the various factors stated above.
Besides, due to the limitation of the sample sizes (i.e., the number of studies and participants per study), the classification of these factors at a finer-grained level seems to remain challenging. For instance, sample sizes of the specific task types for each language function (such as different tasks for semantic processing), and some specific brain regions within each lobe (e.g., the PC within the frontal lobe, the ATL within the temporal lobe and the AG within the parietal lobe) were comparatively small, making the TMS effects at a more specific level challenging to evaluate. Moreover, regarding the meta-analysis approach, the readers should be cautious that we mainly focused on the absolute effect sizes in the current meta-analyses, and that the findings concerning the effect directions were relatively limited, thus awaiting to be explored in future studies. We did not perform a multiple-factor analysis either, that is, analyzing the TMS effects when considering the influences from several factors simultaneously owing to the more demanding analysis technique. Future studies may address these issues in a more profound fashion, and complement our current results and assumptions with more evidence and specific designs.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XQ, ZW, and LC came up with the idea of this study and conducted the meta-analysis. XQ, ZW, YC, QX, ZL, and LL conducted the searching, screening, and coding of the included studies. XQ, ZW, LC, and GH completed the first draft of this manuscript, which was further revised by YC, QX, ZL, LL, and LF. All authors participated in the discussion of the results and prepared the final version of the manuscript for submission.
Funding
This work was funded by the National Social Science Fund of China (22CYY017).
Acknowledgments
We thank the reviewers for their insightful comments. Special thanks are extended to Xinming Wang for his assistance and helpful inputs in R programming for this meta-analysis. We also thank Liaoyuan Zhang and Junjie Wu for their helpful comments and constructive inputs. We sincerely feel grateful for Zhongshan Li for the arrangement of English polishing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2022.1027446/full#supplementary-material
References
Acheson, D. J., and Hagoort, P. (2013). Stimulating the brain’s language network: Syntactic ambiguity resolution after TMS to the inferior frontal gyrus and middle temporal gyrus. J. Cogn. Neurosci. 25, 1664–1677. doi: 10.1162/jocn_a_00430
Acheson, D. J., Hamidi, M., Binder, J. R., and Postle, B. R. (2011). A common neural substrate for language production and verbal working memory. J. Cogn. Neurosci. 23, 1358–1367. doi: 10.1162/jocn.2010.21519
Andoh, J., Artiges, E., Pallier, C., Rivière, D., Mangin, J. F., Cachia, A., et al. (2006). Modulation of language areas with functional MR image-guided magnetic stimulation. Neuroimage 29, 619–627. doi: 10.1016/j.neuroimage.2005.07.029
Andoh, J., and Paus, T. (2011). Combining functional neuroimaging with off-line brain stimulation: Modulation of task-related activity in language areas. J. Cogn. Neurosci. 23, 349–361. doi: 10.1162/jocn.2010.21449
Argyropoulos, G. P., and Muggleton, N. G. (2013). Effects of cerebellar stimulation on processing semantic associations. Cerebellum 12, 83–96. doi: 10.1007/s12311-012-0398-y
Bailey, C. J., Karhu, J., and Ilmoniemi, R. J. (2001). Transcranial magnetic stimulation as a tool for cognitive studies. Scand. J. Psychol. 42, 297–306.
Banaszkiewicz, A., Bola, Ł., Matuszewski, J., Szczepanik, M., Kossowski, B., Mostowski, P., et al. (2021). The role of the superior parietal lobule in lexical processing of sign language: Insights from fMRI and TMS. Cortex 135, 240–254. doi: 10.1016/j.cortex.2020.10.025
Beynel, L., Appelbaum, L. G., Luber, B., Crowell, C. A., Hilbig, S. A., Lim, W., et al. (2019). Effects of online repetitive transcranial magnetic stimulation (rTMS) on cognitive processing: A meta-analysis and recommendations for future studies. Neurosci. Biobehav. Rev. 107, 47–58. doi: 10.1016/j.neubiorev.2019.08.018
Bonnì, S., Koch, G., Miniussi, C., Bassi, M. S., Caltagirone, C., and Gainotti, G. (2015). Role of the anterior temporal lobes in semantic representations: Paradoxical results of a cTBS study. Neuropsychologia 76, 163–169. doi: 10.1016/j.neuropsychologia.2014.11.002
Borenstein, M., Hedges, L. V., Higgins, J. P. T., and Rothstein, H. (2009). Introduction to meta-analysis. Cornwall: John Wiley and Sons Press.
Bucur, M., and Papagno, C. (2019). Are transcranial brain stimulation effects long-lasting in post-stroke aphasia? A comparative systematic review and meta-analysis on naming performance. Neurosci. Biobehav. Rev. 102, 264–289. doi: 10.1016/j.neubiorev.2019.04.019
Cacciari, C., Bolognini, N., Senna, I., Pellicciari, M. C., Miniussi, C., and Papagno, C. (2011). Literal, fictive and metaphorical motion sentences preserve the motion component of the verb: A TMS study. Brain Lang. 119, 149–157. doi: 10.1016/j.bandl.2011.05.004
Cheung, M. W. (2014). Modeling dependent effect sizes with three-level meta-analyses: A structural equation modeling approach. Psychol. Methods 19, 211–229. doi: 10.1037/a0032968
Davey, J., Cornelissen, P. L., Thompson, H. E., Sonkusare, S., Hallam, G., Smallwood, J., et al. (2015). Automatic and controlled semantic retrieval: TMS reveals distinct contributions of posterior middle temporal gyrus and angular gyrus. J. Neurosci. 35, 15230–15239. doi: 10.1523/JNEUROSCI.4705-14.2015
de Smet, H. J., Paquier, P., Verhoeven, J., and Mariën, P. (2013). The cerebellum: Its role in language and related cognitive and affective functions. Brain Lang. 127, 334–342. doi: 10.1016/j.bandl.2012.11.001
Deschamps, I., Baum, S. R., and Gracco, V. L. (2014). On the role of the supramarginal gyrus in phonological processing and verbal working memory: Evidence from rTMS studies. Neuropsychologia 53, 39–46. doi: 10.1016/j.neuropsychologia.2013.10.015
Deschamps, I., Courson, M., Dick, A. S., and Tremblay, P. (2020). The phonological loop: Is speech special? Exp. Brain Res. 238, 2307–2321. doi: 10.1007/s00221-020-05886-9
Devlin, J. T., Matthews, P. M., and Rushworth, M. F. (2003). Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J. Cogn. Neurosci. 15, 71–84. doi: 10.1162/089892903321107837
Devlin, J. T., and Watkins, K. E. (2007). Stimulating language: Insights from TMS. Brain 130(Pt 3), 610–622. doi: 10.1093/brain/awl331
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Fernández-Castilla, B., Jamshidi, L., Declercq, L., Beretvas, S. N., Onghena, P., and Van den Noortgate, W. (2020). The application of meta-analytic (multi-level) models with multiple random effects: A systematic review. Behav. Res. Methods 52, 2031–2052. doi: 10.3758/s13428-020-01373-9
Finocchiaro, C., Capasso, R., Cattaneo, L., Zuanazzi, A., and Miceli, G. (2015). Thematic role assignment in the posterior parietal cortex: A TMS study. Neuropsychologia 77, 223–232. doi: 10.1016/j.neuropsychologia.2015.08.025
Finocchiaro, C., Cattaneo, L., Lega, C., and Miceli, G. (2021). Thematic reanalysis in the left posterior parietal sulcus: A TMS study. Neurobiol. Lang. 2, 416–432.
Finocchiaro, C., Fierro, B., Brighina, F., Giglia, G., Francolini, M., and Caramazza, A. (2008). When nominal features are marked on verbs: A transcranial magnetic stimulation study. Brain Lang. 104, 113–121. doi: 10.1016/j.bandl.2007.09.002
Fishbein, A. R., Fritz, J. B., Idsardi, W. J., and Wilkinson, G. S. (2020). What can animal communication teach us about human language? Philos. Trans. R. Soc. Lond. B Biol. Sci. 375:20190042. doi: 10.1098/rstb.2019.0042
Fitzgerald, P. B., Fountain, S., and Daskalakis, Z. J. (2006). A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin. Neurophysiol. 117, 2584–2596. doi: 10.1016/j.clinph.2006.06.712
Flöel, A. (2012). Non-invasive brain stimulation and language processing in the healthy brain. Aphasiology 26, 1082–1102. doi: 10.1080/02687038.2011.589892
Franzmeier, I., Hutton, S. B., and Ferstl, E. C. (2012). The role of the temporal lobe in contextual sentence integration: A single-pulse transcranial magnetic stimulation study. Cogn. Neurosci. 3, 1–7. doi: 10.1080/17588928.2011.556248
Fridriksson, J., Fillmore, P., Guo, D., and Rorden, C. (2015). Chronic Broca’s aphasia is caused by damage to Broca’s and wernicke’s areas. Cereb. Cortex 25, 4689–4696. doi: 10.1093/cercor/bhu152
Friederici, A. D., Chomsky, N., Berwick, R. C., Moro, A., and Bolhuis, J. J. (2017). Language, mind and brain. Nat. Hum. Behav. 1, 713–722. doi: 10.1038/s41562-017-0184-4
Gingras, C., Coll, M. P., Tessier, M. H., Tremblay, P., and Jackson, P. L. (2021). Pain evaluation and prosocial behaviour are affected by age and sex. Eur. J. Pain 25, 1925–1937. doi: 10.1002/ejp.1809
Gough, P. M., Campione, G. C., and Buccino, G. (2013). Fine tuned modulation of the motor system by adjectives expressing positive and negative properties. Brain Lang. 125, 54–59. doi: 10.1016/j.bandl.2013.01.012
Gough, P. M., Nobre, A. C., and Devlin, J. T. (2005). Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J. Neurosci. 25, 8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005
Hagoort, P. (2013). MUC (memory, unification, control) and beyond. Front. Psychol. 4:416. doi: 10.3389/fpsyg.2013.00416
Hamada, M., Murase, N., Hasan, A., Balaratnam, M., and Rothwell, J. C. (2013). The role of interneuron networks in driving human motor cortical plasticity. Cereb. Cortex 23, 1593–1605. doi: 10.1093/cercor/bhs147
Han, Y., Tong, X., Wang, X., Teng, F., Deng, Q., Zhou, J., et al. (2021). A concordance study determining language dominance between navigated transcranial magnetic stimulation and the Wada test in patients with drug-resistant epilepsy. Epilepsy Behav. 117:107711. doi: 10.1016/j.yebeh.2020.107711
Hartwigsen, G. (2015). The neurophysiology of language: Insights from non-invasive brain stimulation in the healthy human brain. Brain Lang. 148, 81–94. doi: 10.1016/j.bandl.2014.10.007
Hartwigsen, G., Price, C. J., Baumgaertner, A., Geiss, G., Koehnke, M., Ulmer, S., et al. (2010). The right posterior inferior frontal gyrus contributes to phonological word decisions in the healthy brain: Evidence from dual-site TMS. Neuropsychologia 48, 3155–3163. doi: 10.1016/j.neuropsychologia.2010.06.032
Hartwigsen, G., and Saur, D. (2019). Neuroimaging of stroke recovery from aphasia - Insights into plasticity of the human language network. Neuroimage 190, 14–31. doi: 10.1016/j.neuroimage.2017.11.056
Hartwigsen, G., Saur, D., Price, C. J., Ulmer, S., Baumgaertner, A., and Siebner, H. R. (2013). Perturbation of the left inferior frontal gyrus triggers adaptive plasticity in the right homologous area during speech production. Proc. Natl. Acad. Sci. U.S.A. 110, 16402–16407. doi: 10.1073/pnas.1310190110
Hartwigsen, G., and Silvanto, J. (2022). Noninvasive brain stimulation: Multiple effects on cognition. Neuroscientist [Epub ahead of print]. doi: 10.1177/10738584221113806
Hartwigsen, G., Weigel, A., Schuschan, P., Siebner, H. R., Weise, D., Classen, J., et al. (2016). Dissociating parieto-frontal networks for phonological and semantic word decisions: A condition-and-perturb TMS study. Cereb. Cortex 26, 2590–2601. doi: 10.1093/cercor/bhv092
Hedges, L. V., and Olkin, I. (1985). Statistical methods for meta-analysis. New York, NY: Academic Press.
Hedges, L. V., and Pigott, T. D. (2001). The power of statistical tests in meta-analysis. Psychol. Methods 6, 203–217. doi: 10.1037/1082-989X.6.3.203
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (eds). (2019). Cochrane handbook for systematic reviews of interventions, 2nd Edn. Chichester: John Wiley and Sons.
Hinkley, L. B. N., De Witte, E., Cahill-Thompson, M., Mizuiri, D., Garrett, C., Honma, S., et al. (2020). Optimizing magnetoencephalographic imaging estimation of language lateralization for simpler language tasks. Front. Hum. Neurosci. 14:105. doi: 10.3389/fnhum.2020.00105
Hirata, M., Goto, T., Barnes, G., Umekawa, Y., Yanagisawa, T., Kato, A., et al. (2010). Language dominance and mapping based on neuromagnetic oscillatory changes: Comparison with invasive procedures. J. Neurosurg. 112, 528–538. doi: 10.3171/2009.7.JNS09239
Hong, Z., Zheng, H., Luo, J., Yin, M., Ai, Y., Deng, B., et al. (2021). Effects of low-frequency repetitive transcranial magnetic stimulation on language recovery in poststroke survivors with aphasia: An updated meta-analysis. Neurorehabil. Neural Repair 35, 680–691. doi: 10.1177/15459683211011230
Huedo-Medina, T. B., Sánchez-Meca, J., Marín-Martínez, F., and Botella, J. (2006). Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 11, 193–206. doi: 10.1037/1082-989X.11.2.193
Hunter, J. E., and Schmidt, F. L. (2004). “Methods of meta-analysis,” in Correcting error and bias in research findings, 2nd Edn, eds J. E. Hunter and F. L. Schmidt (Thousand Oaks, CA: SAGE Publications).
Innocenti, I., Cappa, S. F., Feurra, M., Giovannelli, F., Santarnecchi, E., Bianco, G., et al. (2013). TMS interference with primacy and recency mechanisms reveals bimodal episodic encoding in the human brain. J. Cogn. Neurosci. 25, 109–116. doi: 10.1162/jocn_a_00304
Ishkhanyan, B., Michel Lange, V., Boye, K., Mogensen, J., Karabanov, A., Hartwigsen, G., et al. (2020). Anterior and posterior left inferior frontal gyrus contribute to the implementation of grammatical determiners during language production. Front. Psychol. 11:685. doi: 10.3389/fpsyg.2020.00685
Jackson, R. L., Lambon Ralph, M. A., and Pobric, G. (2015). The timing of anterior temporal lobe involvement in semantic processing. J. Cogn. Neurosci. 27, 1388–1396. doi: 10.1162/jocn_a_00788
Jernigan, T. L., Brown, T. T., Bartsch, H., and Dale, A. M. (2016). Toward an integrative science of the developing human mind and brain: Focus on the developing cortex. Dev. Cogn. Neurosci. 18, 2–11. doi: 10.1016/j.dcn.2015.07.008
Johnson, M. A. (2021). Neurocognitive relations between language and thought. doctor’s dissertation. Los Angeles, CA: University of California.
Jost, L. B., Pestalozzi, M. I., Cazzoli, D., Mouthon, M., Müri, R. M., and Annoni, J. M. (2020). Effects of continuous theta burst stimulation over the left Dlpfc on mother tongue and second language production in late bilinguals: A behavioral and ERP study. Brain Topogr. 33, 504–518. doi: 10.1007/s10548-020-00779-0
Jung, J. Y., and Lambon Ralph, M. A. (2016). Mapping the dynamic network interactions underpinning cognition: A cTBS-fMRI study of the flexible adaptive neural system for semantics. Cereb. Cortex 26, 3580–3590. doi: 10.1093/cercor/bhw149
Kahn, I., Pascual-Leone, A., Theoret, H., Fregni, F., Clark, D., and Wagner, A. D. (2005). Transient disruption of ventrolateral prefrontal cortex during verbal encoding affects subsequent memory performance. J. Neurophysiol. 94, 688–698. doi: 10.1152/jn.01335.2004
Karabanov, A. N., Paine, R., Chao, C. C., Schulze, K., Scott, B., Hallett, M., et al. (2015). Participation of the classical speech areas in auditory long-term memory. PLoS One 10:e0119472. doi: 10.1371/journal.pone.0119472
Kennedy-Higgins, D., Devlin, J. T., Nuttall, H. E., and Adank, P. (2020). The causal role of left and right superior temporal GYRI in speech perception in noise: A transcranial magnetic stimulation study. J. Cogn. Neurosci. 32, 1092–1103. doi: 10.1162/jocn_a_01521
Kirschen, M. P., Davis-Ratner, M. S., Jerde, T. E., Schraedley-Desmond, P., and Desmond, J. E. (2006). Enhancement of phonological memory following transcranial magnetic stimulation (TMS). Behav. Neurol. 17, 187–194. doi: 10.1155/2006/469132
Klaus, J., and Hartwigsen, G. (2019). Dissociating semantic and phonological contributions of the left inferior frontal gyrus to language production. Hum. Brain Mapp. 40, 3279–3287. doi: 10.1002/hbm.24597
Klaus, J., and Schutter, D. J. L. G. (2018). Non-invasive brain stimulation to investigate language production in healthy speakers: A meta-analysis. Brain Cogn. 123, 10–22. doi: 10.1016/j.bandc.2018.02.007
Klaus, J., Schutter, D. J. L. G., and Piai, V. (2020). Transient perturbation of the left temporal cortex evokes plasticity-related reconfiguration of the lexical network. Hum. Brain Mapp. 41, 1061–1071. doi: 10.1002/hbm.24860
Klomjai, W., Katz, R., and Lackmy-Vallée, A. (2015). Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 58, 208–213. doi: 10.1016/j.rehab.2015.05.005
Krieg, S. M., Sollmann, N., Tanigawa, N., Foerschler, A., Meyer, B., and Ringel, F. (2016). Cortical distribution of speech and language errors investigated by visual object naming and navigated transcranial magnetic stimulation. Brain Struct. Funct. 221, 2259–2286. doi: 10.1007/s00429-015-1042-7
Krieger-Redwood, K., Gareth Gaskell, M., Lindsay, S., and Jefferies, E. (2013). The selective role of premotor cortex in speech perception: A contribution to phoneme judgements but not speech comprehension. J. Cogn. Neurosci. 25, 2179–2188. doi: 10.1162/jocn_a_00463
Krieger-Redwood, K., and Jefferies, E. (2014). TMS interferes with lexical-semantic retrieval in left inferior frontal gyrus and posterior middle temporal gyrus: Evidence from cyclical picture naming. Neuropsychologia 64, 24–32. doi: 10.1016/j.neuropsychologia.2014.09.014
Kuhnke, P., Beaupain, M. C., Cheung, V. K. M., Weise, K., Kiefer, M., and Hartwigsen, G. (2020). Left posterior inferior parietal cortex causally supports the retrieval of action knowledge. Neuroimage 219:117041. doi: 10.1016/j.neuroimage.2020.117041
Lavidor, M., and Walsh, V. (2003). A magnetic stimulation examination of orthographic neighborhood effects in visual word recognition. J. Cogn. Neurosci. 15, 354–363. doi: 10.1162/089892903321593081
León Ruiz, M., Sospedra, M., Arce Arce, S., Tejeiro-Martínez, J., and Benito-León, J. (2018). Current evidence on the potential therapeutic applications of transcranial magnetic stimulation in multiple sclerosis: A systematic review of the literature. Neurologia [Epub ahead of print]. doi: 10.1016/j.nrl.2018.03.023
Miniussi, C., Harris, J. A., and Ruzzoli, M. (2013). Modelling non-invasive brain stimulation in cognitive neuroscience. Neurosci. Biobehav. Rev. 37, 1702–1712. doi: 10.1016/j.neubiorev.2013.06.014
Mottaghy, F. M., Gangitano, M., Krause, B. J., and Pascual-Leone, A. (2003). Chronometry of parietal and prefrontal activations in verbal working memory revealed by transcranial magnetic stimulation. Neuroimage 18, 565–575. doi: 10.1016/s1053-8119(03)00010-7
Mottaghy, F. M., Hungs, M., Brügmann, M., Sparing, R., Boroojerdi, B., Foltys, H., et al. (1999). Facilitation of picture naming after repetitive transcranial magnetic stimulation. Neurology 53, 1806–1812. doi: 10.1212/wnl.53.8.1806
Möttönen, R., van de Ven, G. M., and Watkins, K. E. (2014). Attention fine-tunes auditory-motor processing of speech sounds. J. Neurosci. 34, 4064–4069. doi: 10.1523/JNEUROSCI.2214-13.2014
Murdoch, B. E., and Barwood, C. H. (2013). Non-invasive brain stimulation: A new frontier in the treatment of neurogenic speech-language disorders. Int. J. Speech Lang. Pathol. 15, 234–244. doi: 10.3109/17549507.2012.745605
Nettekoven, C., Pieczewski, J., Neuschmelting, V., Jonas, K., Goldbrunner, R., Grefkes, C., et al. (2021). Improving the efficacy and reliability of rTMS language mapping by increasing the stimulation frequency. Hum. Brain Mapp. 42, 5309–5321. doi: 10.1002/hbm.25619
Nixon, P., Lazarova, J., Hodinott-Hill, I., Gough, P., and Passingham, R. (2004). The inferior frontal gyrus and phonological processing: An investigation using rTMS. J. Cogn. Neurosci. 16, 289–300. doi: 10.1162/089892904322984571
Ohlerth, A. K., Bastiaanse, R., Negwer, C., Sollmann, N., Schramm, S., Schröder, A., et al. (2021). Benefit of action naming over object naming for visualization of subcortical language pathways in navigated transcranial magnetic stimulation-based diffusion tensor imaging-fiber tracking. Front. Hum. Neurosci. 15:748274. doi: 10.3389/fnhum.2021.748274
Osaka, N., Otsuka, Y., Hirose, N., Ikeda, T., Mima, T., Fukuyama, H., et al. (2007). Transcranial magnetic stimulation (TMS) applied to left dorsolateral prefrontal cortex disrupts verbal working memory performance in humans. Neurosci. Lett. 418, 232–235. doi: 10.1016/j.neulet.2007.01.087
Otal, B., Olma, M. C., Flöel, A., and Wellwood, I. (2015). Inhibitory non-invasive brain stimulation to homologous language regions as an adjunct to speech and language therapy in post-stroke aphasia: A meta-analysis. Front. Hum. Neurosci. 9:236. doi: 10.3389/fnhum.2015.00236
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 372:n71. doi: 10.1136/bmj.n71
Papeo, L., Lingnau, A., Agosta, S., Pascual-Leone, A., Battelli, L., and Caramazza, A. (2015). The origin of word-related motor activity. Cereb. Cortex 25, 1668–1675. doi: 10.1093/cercor/bht423
Papeo, L., Pascual-Leone, A., and Caramazza, A. (2013). Disrupting the brain to validate hypotheses on the neurobiology of language. Front. Hum. Neurosci. 7:148. doi: 10.3389/fnhum.2013.00148
Passeri, A., Capotosto, P., and di Matteo, R. (2015). The right hemisphere contribution to semantic categorization: A TMS study. Cortex 64, 318–326. doi: 10.1016/j.cortex.2014.11.014
Pattamadilok, C., Knierim, I. N., Kawabata Duncan, K. J., and Devlin, J. T. (2010). How does learning to read affect speech perception? J. Neurosci. 30, 8435–8444. doi: 10.1523/JNEUROSCI.5791-09.2010
Piai, V., Nieberlein, L., and Hartwigsen, G. (2020). Effects of transcranial magnetic stimulation over the left posterior superior temporal gyrus on picture-word interference. PLoS One 15:e0242941. doi: 10.1371/journal.pone.0242941
Pitcher, D., Parkin, B., and Walsh, V. (2020). Transcranial magnetic stimulation and the understanding of behavior. Annu. Rev. Psychol. 72, 97–121. doi: 10.1146/annurev-psych-081120
Pobric, G., Jefferies, E., and Ralph, M. A. (2010). Amodal semantic representations depend on both anterior temporal lobes: Evidence from repetitive transcranial magnetic stimulation. Neuropsychologia 48, 1336–1342. doi: 10.1016/j.neuropsychologia.2009.12.036
Pobric, G., Mashal, N., Faust, M., and Lavidor, M. (2008). The role of the right cerebral hemisphere in processing novel metaphoric expressions: A transcranial magnetic stimulation study. J. Cogn. Neurosci. 20, 170–181. doi: 10.1162/jocn.2008.20005
Ren, C. L., Zhang, G. F., Xia, N., Jin, C. H., Zhang, X. H., Hao, J. F., et al. (2014). Effect of low-frequency rTMS on aphasia in stroke patients: A meta-analysis of randomized controlled trials. PLoS One 9:e102557. doi: 10.1371/journal.pone.0102557
Romero, L., Walsh, V., and Papagno, C. (2006). The neural correlates of phonological short-term memory: A repetitive transcranial magnetic stimulation study. J. Cogn. Neurosci. 18, 1147–1155. doi: 10.1162/jocn.2006.18.7.1147
Rothstein, H. R., and Bushman, B. J. (2012). Publication bias in psychological science: Comment on Ferguson and Brannick (2012). Psychol. Methods 17, 129–136. doi: 10.1037/a0027128
Sack, A. T., Cohen Kadosh, R., Schuhmann, T., Moerel, M., Walsh, V., and Goebel, R. (2009). Optimizing functional accuracy of TMS in cognitive studies: A comparison of methods. J. Cogn. Neurosci. 21, 207–221. doi: 10.1162/jocn.2009.21126
Sakai, K. L., Noguchi, Y., Takeuchi, T., and Watanabe, E. (2002). Selective priming of syntactic processing by event-related transcranial magnetic stimulation of Broca’s area. Neuron 35, 1177–1182. doi: 10.1016/s0896-6273(02)00873-5
Sandrini, M., Umiltà, C., and Rusconi, E. (2011). The use of transcranial magnetic stimulation in cognitive neuroscience: A new synthesis of methodological issues. Neurosci. Biobehav. Rev. 35, 516–536. doi: 10.1016/j.neubiorev.2010.06.005
Schuhmann, T., Schiller, N. O., Goebel, R., and Sack, A. T. (2009). The temporal characteristics of functional activation in Broca’s area during overt picture naming. Cortex 45, 1111–1116. doi: 10.1016/j.cortex.2008.10.013
Schuhmann, T., Schiller, N. O., Goebel, R., and Sack, A. T. (2012). Speaking of which: Dissecting the neurocognitive network of language production in picture naming. Cereb. Cortex 22, 701–709. doi: 10.1093/cercor/bhr155
Siebner, H. R., Hartwigsen, G., Kassuba, T., and Rothwell, J. C. (2009). How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition. Cortex 45, 1035–1042. doi: 10.1016/j.cortex.2009.02.007
Silvanto, J., and Pascual-Leone, A. (2008). State-dependency of transcranial magnetic stimulation. Brain Topogr. 21, 1–10. doi: 10.1007/s10548-008-0067-0
Sliwinska, M. W., Elson, R., and Pitcher, D. (2021). Stimulating parietal regions of the multiple-demand cortex impairs novel vocabulary learning. Neuropsychologia 162:108047. doi: 10.1016/j.neuropsychologia.2021.108047
Sliwinska, M. W., James, A., and Devlin, J. T. (2015). Inferior parietal lobule contributions to visual word recognition. J. Cogn. Neurosci. 27, 593–604. doi: 10.1162/jocn_a_00721
Sliwinska, M. W., Khadilkar, M., Campbell-Ratcliffe, J., Quevenco, F., and Devlin, J. T. (2012). Early and sustained supramarginal gyrus contributions to phonological processing. Front. Psychol. 3:161. doi: 10.3389/fpsyg.2012.00161
Sliwinska, M. W., Violante, I. R., Wise, R. J. S., Leech, R., Devlin, J. T., Geranmayeh, F., et al. (2017). Stimulating multiple-demand cortex enhances vocabulary learning. J. Neurosci. 37, 7606–7618. doi: 10.1523/JNEUROSCI.3857-16.2017
Smalle, E., Daikoku, T., Szmalec, A., Duyck, W., and Möttönen, R. (2022). Unlocking adults’ implicit statistical learning by cognitive depletion. Proc. Natl. Acad. Sci. U.S.A. 119:e2026011119. doi: 10.1073/pnas.2026011119
Sollmann, N., Fuss-Ruppenthal, S., Zimmer, C., Meyer, B., and Krieg, S. M. (2018). Investigating stimulation protocols for language mapping by repetitive navigated transcranial magnetic stimulation. Front. Behav. Neurosci. 12:197. doi: 10.3389/fnbeh.2018.00197
Sollmann, N., Ille, S., Obermueller, T., Negwer, C., Ringel, F., Meyer, B., et al. (2015). The impact of repetitive navigated transcranial magnetic stimulation coil positioning and stimulation parameters on human language function. Eur. J. Med. Res. 20:47. doi: 10.1186/s40001-015-0138-0
Soltanlou, M., Sitnikova, M. A., Nuerk, H. C., and Dresler, T. (2018). Applications of functional near-infrared spectroscopy (fNIRS) in studying cognitive development: The case of mathematics and language. Front. Psychol. 9:277. doi: 10.3389/fpsyg.2018.00277
Sondergaard, R. E., Martino, D., Kiss, Z., and Condliffe, E. G. (2021). TMS motor mapping methodology and reliability: A structured review. Front. Neurosci. 15:709368. doi: 10.3389/fnins.2021.709368
Sparing, R., Buelte, D., Meister, I. G., Pauš, T., and Fink, G. R. (2008). Transcranial magnetic stimulation and the challenge of coil placement: A comparison of conventional and stereotaxic neuronavigational strategies. Hum. Brain Mapp. 29, 82–96. doi: 10.1002/hbm.20360
Sparing, R., Mottaghy, F. M., Hungs, M., Brügmann, M., Foltys, H., Huber, W., et al. (2001). Repetitive transcranial magnetic stimulation effects on language function depend on the stimulation parameters. J. Clin. Neurophysiol. 18, 326–330. doi: 10.1097/00004691-200107000-00004
Sterne, J., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. doi: 10.1136/bmj.l4898
Tattersall, I. (2017). The material record and the antiquity of language. Neurosci. Biobehav. Rev. 81(Pt B), 247–254. doi: 10.1016/j.neubiorev.2017.01.043
Taylor, J. K., and Burke, D. M. (2002). Asymmetric aging effects on semantic and phonological processes: Naming in the picture-word interference task. Psychol. Aging 17, 662–676. doi: 10.1037/0882-7974.17.4.662
Thut, G., and Miniussi, C. (2009). New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn. Sci. 13, 182–189. doi: 10.1016/j.tics.2009.01.004
Thut, G., and Pascual-Leone, A. (2010). Integrating TMS with EEG: How and what for? Brain Topogr. 22, 215–218. doi: 10.1007/s10548-009-0128-z
Tian, L., Chen, H., Zhao, W., Wu, J., Zhang, Q., De, A., et al. (2020). The role of motor system in action-related language comprehension in L1 and L2: An fMRI study. Brain Lang. 201:104714. doi: 10.1016/j.bandl.2019.104714
Tomasino, B., Fink, G. R., Sparing, R., Dafotakis, M., and Weiss, P. H. (2008). Action verbs and the primary motor cortex: A comparative TMS study of silent reading, frequency judgments, and motor imagery. Neuropsychologia 46, 1915–1926. doi: 10.1016/j.neuropsychologia.2008.01.015
Töpper, R., Mottaghy, F. M., Brügmann, M., Noth, J., and Huber, W. (1998). Facilitation of picture naming by focal transcranial magnetic stimulation of Wernicke’s area. Exp. Brain Res. 121, 371–378. doi: 10.1007/s002210050471
Torday, J. S. (2021). Cellular evolution of language. Prog. Biophys. Mol. Biol. 167, 140–146. doi: 10.1016/j.pbiomolbio.2021.05.009
Tussis, L., Sollmann, N., Boeckh-Behrens, T., Meyer, B., and Krieg, S. M. (2017). Identifying cortical first and second language sites via navigated transcranial magnetic stimulation of the left hemisphere in bilinguals. Brain Lang. 168, 106–116. doi: 10.1016/j.bandl.2017.01.011
Uddén, J., Folia, V., Forkstam, C., Ingvar, M., Fernandez, G., Overeem, S., et al. (2008). The inferior frontal cortex in artificial syntax processing: An rTMS study. Brain Res. 1224, 69–78. doi: 10.1016/j.brainres.2008.05.070
Uddén, J., Ingvar, M., Hagoort, P., and Petersson, K. M. (2017). Broca’s region: A causal role in implicit processing of grammars with crossed non-adjacent dependencies. Cognition 164, 188–198. doi: 10.1016/j.cognition.2017.03.010
Valentine, W. J., Pollock, R. F., Plun-Favreau, J., and White, J. (2010). Systematic review of the cost-effectiveness of biphasic insulin aspart 30 in type 2 diabetes. Curr. Med. Res. Opin. 26, 1399–1412. doi: 10.1185/03007991003689381
Vallar, G., and Bolognini, N. (2011). Behavioural facilitation following brain stimulation: Implications for neurorehabilitation. Neuropsychol. Rehabil. 21, 618–649. doi: 10.1080/09602011.2011.574050
Van den Bussche, E., Van den Noortgate, W., and Reynvoet, B. (2009). Mechanisms of masked priming: A meta-analysis. Psychol. Bull. 135, 452–477. doi: 10.1037/a0015329
Van den Noortgate, W., López-López, J. A., Marín-Martínez, F., and Sánchez-Meca, J. (2013). Three-level meta-analysis of dependent effect sizes. Behav. Res. Methods 45, 576–594. doi: 10.3758/s13428-012-0261-6
van der Burght, C. L., Numssen, O., Schlaak, B., Goucha, T., and Hartwigsen, G. (2022). Differential contributions of inferior frontal gyrus subregions to sentence processing guided by intonation. Hum. Brain Mapp. [Epub ahead of print]. doi: 10.1002/hbm.26086
Vernet, M., Bashir, S., Yoo, W. K., Perez, J. M., Najib, U., and Pascual-Leone, A. (2013). Insights on the neural basis of motor plasticity induced by theta burst stimulation from TMS-EEG. Eur. J. Neurosci. 37, 598–606. doi: 10.1111/ejn.12069
Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. doi: 10.18637/jss.v036.i03
Vigneau, M., Beaucousin, V., Hervé, P. Y., Duffau, H., Crivello, F., Houdé, O., et al. (2006). Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage 30, 1414–1432. doi: 10.1016/j.neuroimage.2005.11.002
Vitale, F., Monti, I., Padrón, I., Avenanti, A., and de Vega, M. (2022). The neural inhibition network is causally involved in the disembodiment effect of linguistic negation. Cortex 147, 72–82. doi: 10.1016/j.cortex.2021.11.015
Vitale, F., Padrón, I., Avenanti, A., and de Vega, M. (2021). Enhancing motor brain activity improves memory for action language: A tDCS study. Cereb. Cortex 31, 1569–1581. doi: 10.1093/cercor/bhaa309
Walsh, V., and Cowey, A. (2000). Transcranial magnetic stimulation and cognitive neuroscience. Nat. Rev. Neurosci. 1, 73–79. doi: 10.1038/35036239
Wang, L., Wlotko, E., Alexander, E., Schoot, L., Kim, M., Warnke, L., et al. (2020). Neural evidence for the prediction of animacy features during language comprehension: Evidence from MEG and EEG representational similarity analysis. J. Neurosci. 40, 3278–3291. doi: 10.1523/JNEUROSCI.1733-19.2020
Ward, E., Brownsett, S., McMahon, K. L., Hartwigsen, G., Mascelloni, M., and de Zubicaray, G. I. (2022). Online transcranial magnetic stimulation reveals differential effects of transitivity in left inferior parietal cortex but not premotor cortex during action naming. Neuropsychologia 174:108339. doi: 10.1016/j.neuropsychologia.2022.108339
Wassermann, E. M. (2002). Variation in the response to transcranial magnetic brain stimulation in the general population. Clin. Neurophysiol. 113, 1165–1171. doi: 10.1016/s1388-2457(02)00144-x
Whitney, C., Kirk, M., O’Sullivan, J., Lambon Ralph, M. A., and Jefferies, E. (2012). Executive semantic processing is underpinned by a large-scale neural network: Revealing the contribution of left prefrontal, posterior temporal, and parietal cortex to controlled retrieval and selection using TMS. J. Cogn. Neurosci. 24, 133–147. doi: 10.1162/jocn_a_00123
Wierenga, L. M., Langen, M., Oranje, B., and Durston, S. (2014). Unique developmental trajectories of cortical thickness and surface area. Neuroimage 87, 120–126. doi: 10.1016/j.neuroimage.2013.11.010
Willems, R. M., Labruna, L., D’Esposito, M., Ivry, R., and Casasanto, D. (2011). A functional role for the motor system in language understanding: Evidence from theta-burst transcranial magnetic stimulation. Psychol. Sci. 22, 849–854. doi: 10.1177/0956797611412387
Wilson, S. M., and Hula, W. D. (2019). Multivariate approaches to understanding aphasia and its neural substrates. Curr. Neurol. Neurosci. Rep. 19:53. doi: 10.1007/s11910-019-0971-6
Woollams, A. M., Madrid, G., and Lambon Ralph, M. A. (2017). Using neurostimulation to understand the impact of pre-morbid individual differences on post-lesion outcomes. Proc. Natl. Acad. Sci. U.S.A. 114, 12279–12284. doi: 10.1073/pnas.1707162114
Xiang, H. D., Fonteijn, H. M., Norris, D. G., and Hagoort, P. (2010). Topographical functional connectivity pattern in the perisylvian language networks. Cereb. Cortex 20, 549–560. doi: 10.1093/cercor/bhp119
Youssofzadeh, V., Stout, J., Ustine, C., Gross, W. L., Conant, L. L., Humphries, C. J., et al. (2020). Mapping language from MEG beta power modulations during auditory and visual naming. Neuroimage 220:117090. doi: 10.1016/j.neuroimage.2020.117090
Zhang, J., Zhong, D., Xiao, X., Yuan, L., Li, Y., Zheng, Y., et al. (2021). Effects of repetitive transcranial magnetic stimulation (rTMS) on aphasia in stroke patients: A systematic review and meta-analysis. Clin. Rehabil. 35, 1103–1116. doi: 10.1177/0269215521999554
Keywords: language, healthy participants, TMS, neuromodulatory effect, meta-analysis, non-invasive brain stimulation
Citation: Qu X, Wang Z, Cheng Y, Xue Q, Li Z, Li L, Feng L, Hartwigsen G and Chen L (2022) Neuromodulatory effects of transcranial magnetic stimulation on language performance in healthy participants: Systematic review and meta-analysis. Front. Hum. Neurosci. 16:1027446. doi: 10.3389/fnhum.2022.1027446
Received: 25 August 2022; Accepted: 17 November 2022;
Published: 05 December 2022.
Edited by:
Ferdinand Binkofski, RWTH Aachen University, GermanyReviewed by:
Cheng-Chang Yang, Taipei Medical University, TaiwanJeYoung Jung, University of Nottingham, United Kingdom
Renhong He, Southern Medical University, China
Copyright © 2022 Qu, Wang, Cheng, Xue, Li, Li, Feng, Hartwigsen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luyao Chen, bHV5YW9jaGVuQGJudS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
‡These authors share senior authorship
 Xingfang Qu
Xingfang Qu Zichao Wang
Zichao Wang Yao Cheng
Yao Cheng Qingwei Xue
Qingwei Xue Zimu Li1
Zimu Li1 Lu Li
Lu Li Liping Feng
Liping Feng Gesa Hartwigsen
Gesa Hartwigsen Luyao Chen
Luyao Chen