- 1NPSY-Lab.VR, Department of Human Sciences, University of Verona, Verona, Italy
- 2Department of Human Sciences, University of Verona, Verona, Italy
- 3Department of Surgery, Dentistry, Paediatrics and Gynaecology, University of Verona, Verona, Italy
Fibromyalgia (FM) is characterised by chronic, continuous, widespread pain, often associated with a sense of fatigue, non-restorative sleep and physical exhaustion. Due to the nature of this condition and the absence of other neurological issues potentially able to induce disorders in body representations per se, it represents a perfect model since it provides an opportunity to study the relationship between pain and the bodily self. Corporeal illusions were investigated in 60 participants with or without a diagnosis of FM by means of an ad hoc devised interview. In addition, motor imagery was investigated and illusions relating to body part movements and changes in body size, feelings of alienness, and sensations of body parts not belonging to one’s own body (disownership and somatoparaphrenic-like sensations) were found. Crucially, these symptoms do not correlate with any of the clinical measures of pain or functional deficits. The results showed that motor imagery was also impaired, and the severity of the deficits found correlated with the functional impairment of the participant. This indicates that disorders in body representations and motor imagery are part of the clinical expression of FM. However, while motor imagery seems to be linked to reduced autonomy and functional deficits, bodily illusions are independent and potentially represent a concurrent symptom.
Introduction
Although perceived by individuals as unique and consistent, the sense of self is a complex construct which is built over the course of a person’s life on the basis of affective and social experiences, cognition (e.g., memories) and sensory-motor activity. A component of the sense of self is the bodily self, that is, the contemporaneous experiences of the presence of a body, having that body and also being the same body. In other words, we sense that the body we inhabit is our own body and that we are that body. This experience is taken for granted in daily life and is not something which we pay attention to unless something particular or unexpected happens (e.g., when your arm falls asleep due to the compression of peripheral nerves).
In spite of this apparent stability, the sense of body is extremely plastic and fragile, as shown in several studies on neurological patients with body representation disorders as a consequence of brain damage (Pacella et al., 2019; Fornia et al., 2020; Moro et al., 2021b). Furthermore, experiments with healthy participants indicate that even in the absence of a brain lesion, information coming from the body may be delusive and induce corporeal illusions (Botvinick and Cohen, 1998; Pavani et al., 2000; Maravita et al., 2003; Tsakiris and Haggard, 2005; Pavani and Zampini, 2007; Tsakiris et al., 2010; Tieri et al., 2015a,b; Golaszewski et al., 2021). These experiments used multisensory integration in order to mislead the brain in its attempt to interpret sensory information which is processed as coming from one’s own body although in fact they come from non-bodily sources. Two examples of this type of experiment are the rubber hand illusion (Botvinick and Cohen, 1998) and the enfacement illusion (Porciello et al., 2018) in which people have reported the sensation that a rubber hand or another person’s face have become their own hand or face when they received a tactile stimulation that was synchronous (and spatially congruent) with another stimulus which appeared to be administered to the rubber hand or the other person’s face. In particular, temporal synchrony is crucial in both rubber hand (Costantini et al., 2016; Motyka and Litwin, 2019) and enfacement illusions (Tajadura-Jiménez and Tsakiris, 2014; Apps et al., 2015).
This supports the idea that sensory information plays a crucial role in bodily self, as shown by studies on deafferented people, in particular individuals suffering from spinal cord injury (SCI; Scandola et al., 2014, 2017a,2019b; Moro et al., 2021a) or locked-in-syndrome (Nizzi et al., 2012), and recently confirmed by the effects of interoceptive modulations on the sense of body (Salvato et al., 2017; Jenkinson et al., 2020; Monti et al., 2020; Todd et al., 2021).Thus, representations underlying bodily self are the result of a continuous integration of multiple sources of information. This integration process provides immediate feedback on the current state of the body and is also integrated with higher-order cognitive functions (e.g., spatial perception and memory) in order to obtain a detailed map of the body and its relationship with the environment in terms of action planning and motor imagery. Although authors on the subject agree on this point (namely, that body representations are the complex result of multiple components, see Berlucchi and Aglioti, 2010), the contribution of the individual components still needs to be disambiguated. For example, the potential role of pain involved in the sense of body remains for the most part unknown (see Haggard et al., 2013 for a review).
From a naïve perspective, one might be led to think that pain has a detrimental effect on body representations. Indeed, painful conditions alter the threshold of sensitivity (Le Bars et al., 1979) and reduce the ability to localise other sensory stimuli (Haggard et al., 2013; Osumi et al., 2015). Nevertheless, if chronic pain has been found to be commonly associated with a reduced tactile sensitivity of the affected part (Moriwaki and Yuge, 1999; Pleger et al., 2006), acute pain seems to facilitate the perception of touch (Ploner et al., 2004). In addition, in chronic syndromes (i.e., complex regional pain syndrome), pain impacts on the mental imagery of movement, but only when the actions relate to the painful body parts (Schwoebel et al., 2001; Schwoebel and Coslett, 2005; Martínez et al., 2018). The results of this research indicate a link between pain, body representations and motor imagery.
Furthermore, the results of experimentation with deafferented people suffering from spinal cord injury support the presence of such a link and indicate the possibility of differentiated effects of pain on body representations and motor imagery (Gustin et al., 2008; Soler et al., 2010, 2021; Kumru et al., 2013; Scandola et al., 2017a,b). In particular, musculoskeletal, neuropathic, and visceral pain all seem to contribute to misperceptions of body parts (e.g., the feeling of having some body parts in a position which is different to the actual position), while the presence of neuropathic pain might have a “protective” effect on body representations, reducing the feeling of illusory movements of body parts (i.e., sensations of motion that are not voluntarily controlled and muscular fatigue after illusiory movements). However, it is worth noting that in spinal cord injured people, pain is not the main, distinctive symptom, since the massive deafferentation and deefferentation which these people suffer from may cause body representation disorders per se.
Fibromyalgia (FM) is a clinical condition that provides an opportunity to study the specific impact of pain on body representations. It is a chronic musculoskeletal condition characterised by widespread pain and evoked pain at tender points (Wolfe et al., 2016). Other typical symptoms are a sense of fatigue, sleep disorders and non-restorative sleep. Physical exhaustion and cognitive difficulties, in particular involving the individual’s memory, have also been reported. In addition, most patients report a wide range of additional somatic and psychological symptoms, such as rheumatic disorders, irritable bowel syndrome, as well as anxiety and depression (Häuser et al., 2015). As a consequence, FM negatively impacts psychological wellbeing, social relationships and work life (Chinn et al., 2016; Andrade et al., 2020). In industrially developed countries, up to 5.8% of the population present with a diagnosis of FM (Branco et al., 2010; Ablin et al., 2012; Wolfe et al., 2013), with a much higher prevalence in the case of women (8–10:1, female to male ratio). The aetiology is to date unknown but appears to be centred on genetic, environmental and personality contributors (Furness et al., 2018).
There are a number of reasons why FM represents a good model for a study of the effects of pain on body representations. Firstly, it is a chronic condition with symptoms that last over time with a certain degree of stability. Patients typically report continuous pain that they remember accompanying them from childhood or youth (although the diagnosis is more frequently made when the patient is between 40 and 60 years of age; Häuser and Fitzcharles, 2018) and it becomes a constant presence in their life. In terms of the connection discussed above, it appears that this condition extends its effects onto the bodily self, as indicated by previous results of research into chronic pain (Moseley, 2004; Moseley et al., 2012; Akkaya et al., 2013; Tsay et al., 2015). Another crucial feature is that FM is not associated with neurological deficits or specific lesions in the central nervous system that might cause direct modifications in cognitive representations of the body or alterations to sensory-motor systems. This makes it possible to investigate directly whether potential disorders in body and motor representations are a consequence of pain (without other causes) or if they may represent specific, independent aspects of the syndrome. Finally, FM tends to affect young people (in particular women) who are attentive to signals coming from their body and are able to describe these accurately giving detailed reports.
In this study, we capitalised on previous results coming from a specific battery of tests which investigated various different components of body representation (Scandola et al., 2017a). These were administered to a sample of women, with or without a diagnosis of FM, in order to determine the potential effects of chronic pain on the bodily self. In addition, we analysed the potential relationships between clinical symptoms, body representations and the ability to represent actions (i.e., motor imagery). The effects of personality traits and clinical variables such as the severity of the symptoms, the various different typologies of pain (musculoskeletal, neuropathic, and visceral pain) and the time interval since the onset of symptoms were also considered. The results are potentially useful not only in terms of the comprehension of the various facets of the bodily self, but also with regard to the clinical impact on diagnosis and interventions for FM.
Materials and Methods
Participants
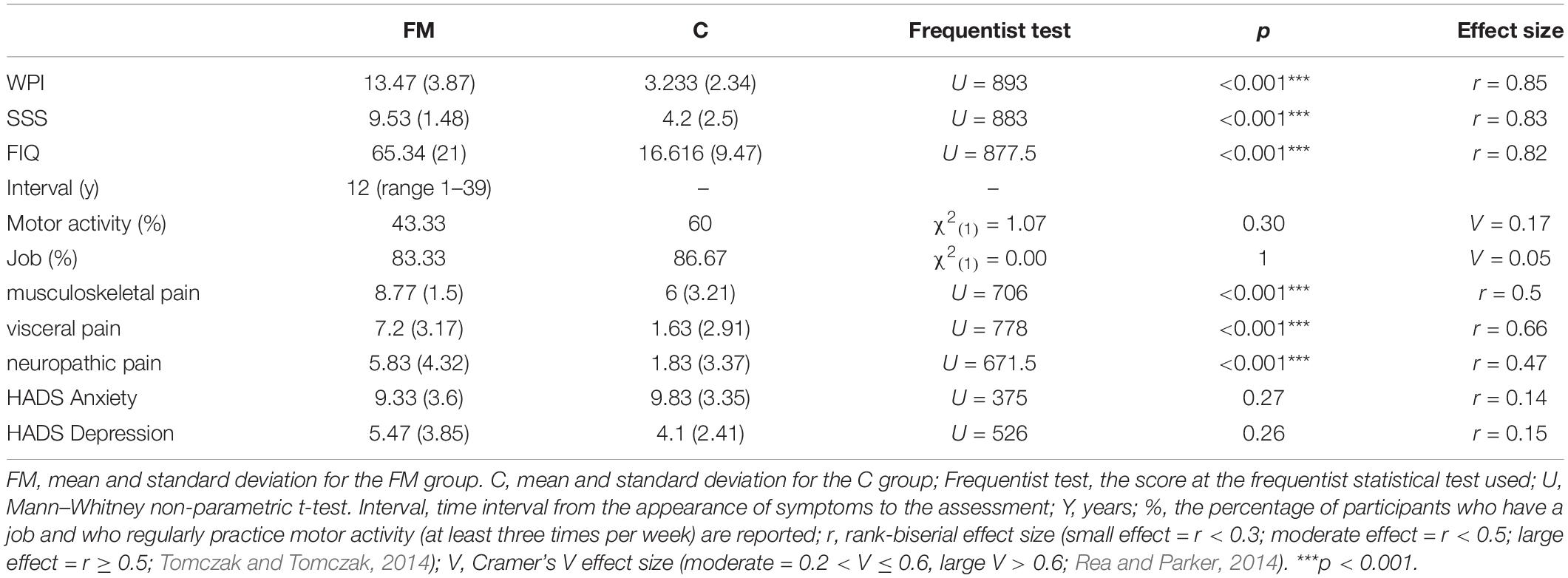
Sixty women participated in the study over a period of one year, 30 with a diagnosis of FM, who were recruited at the Analgesic Therapy Unit, Borgo Roma Hospital, Verona during their periodic medical visits, and 30 healthy controls (C) who were recruited through the friends and contacts of the experimenters. No men were recruited because of the low prevalence of FM in males. The two groups were matched for age [FM: M (SD) = 48.45 (10.92), C: M (SD) = 49.9 (10.22), Mann–Whitney U = 397.5, p = 0.441, r = 0.1) but not standard of education [FM: M (SD) = 12.3 (3.505), C: M (SD) = 14.77 (2.582), U = 269.5, p = 0.005, r = 0.36]. The clinical diagnosis of FM was confirmed or excluded by means of combining the individuals’ scores on the Widespread Pain Index (WPI) and the Symptom Severity Scale (SSS; Wolfe et al., 2016, 2018). The WPI is a self-reported pain index resulting from the summary count of the number of painful regions out of the 19 regions considered in the Regional Pain Scale (Wolfe, 2003) (score range = 0–19). The SSS is the sum (range 0–12) of the severity scores of three symptoms (i.e., fatigue, waking unrefreshed, and cognitive symptoms, score range for each item = 0–3), along with the sum of the number of other symptoms that might have co-occurred during the previous 6 months (i.e. headaches, pain or cramps in the lower abdomen, and depression, score 0–3) (Wolfe et al., 2018). Combinations of WPI ≥ 7 and SSS ≥ 5 or WPI ≥ 4 and SSS ≥ 9 were considered as two valid cut-offs for a diagnosis of FM. This meant in fact that, in the FM group, pain was present in at least 4 or 5 regions (with jaw, chest, and abdominal pain not included in the list) and symptoms had been present for at least 3 months. It is to be noted that, following these diagnostical criteria (Wolfe et al., 2018), the diagnosis of FM is considered to be valid irrespective of other diagnoses and does not exclude the presence of other serious clinical illnesses (Wolfe et al., 2016, 2018).
The participants in the control group were recruited through experimenters’ personal contacts and each FM patient was matched with a control of the same age (±5 years). In this group, the presence of FM was excluded by means of the WPI and SSS scores.
Preliminary Clinical Assessment
To investigate in depth the nature of the pain that had been reported by the FM participants, and in particular to ascertain whether they reported musculoskeletal, visceral, or neuropathic pain differently from each other, we analysed the participants’ responses (both FM and C) to a questionnaire with a scale previously devised in our laboratory (Scandola et al., 2017b). The validation of this scale (higher scores, more severe symptoms) is currently in progress, but preliminary results confirm a high correlation with both the Brief Pain inventory (BFI; Caraceni et al., 1996) and the Douleur Neuropathique 4 question scale (Bouhassira et al., 2005; Scandola et al., 2017b). For the purposes of the analyses carried out in this case, we have only considered the scores indicated by the participants as reflecting the degree of each typology of pain in the worst moment of pain that they could remember. Details of the characteristics of the various typologies of pain and the frequency of each of these features in the two groups are shown in the Supplementary Material (Supplementary Table 1).
A check for the presence of potential disorders in mood was also carried out by means of the Hospital Anxiety and Depression Scale (Bjelland et al., 2002), a self-administered scale that gives scores separately for anxiety and depression. 14 multiple-choice questions are asked that investigate the frequency (1 = never, 5 = always) with which a specific mood occurs (e.g., “I feel agitated and tense” “I feel in a good mood”).
Finally, in order to consider the impact of pain on daily life activities, the Italian version of the Fibromyalgia Impact Questionnaire (FIQ; Sarzi-Puttini et al., 2003) was administered. This questionnaire is divided into three parts which focus on the condition of the patient as perceived subjectively during the week before the assessment. The questions refer to: (i) the patient’s ability to perform daily tasks involving the large muscles (e.g., shopping, housework, gardening); (ii) the number of days in the past week that they felt good and the number of days that they missed work because of pain; and (iii) the intensity of symptoms such as pain, fatigue, morning tiredness, stiffness, anxiety, depression, and the ability to do one’s job. The scores for the three parts are then normalised to give a total score ranging from 0 to 100, with higher scores indicating worse conditions (for details, see Sarzi-Puttini et al., 2003).
The scores of the two groups on these scales and other clinical data are reported in Table 1 and confirm the differences between the two groups in all of the measures relating to FM (WPI, SSS, and FIQ) and in all of the three typologies of pain (musculoskeletal, visceral, and neuropathic pain).
Other information, such as the patient’s work situation (FM = 25 out of 30, C = 26 out of 30; χ2(1) = 0; p = 1; Cramer’s V = 0.05) and whether they regularly do motor activity (at least three times per week, FM = 13 out of 30, C = 18 out of 30; χ2(1) = 1.07; p = 0.30; Cramer’s V = 0.17) were recorded, with no differences in frequency between the groups. Finally, it is noteworthy that the groups did not show any differences relating to anxiety and depression. The presence of correlations between these clinical variables and any symptoms of corporeal illusions or motor imagery deficits was also assessed (see below).
The Evaluation of Body Representation Disorders
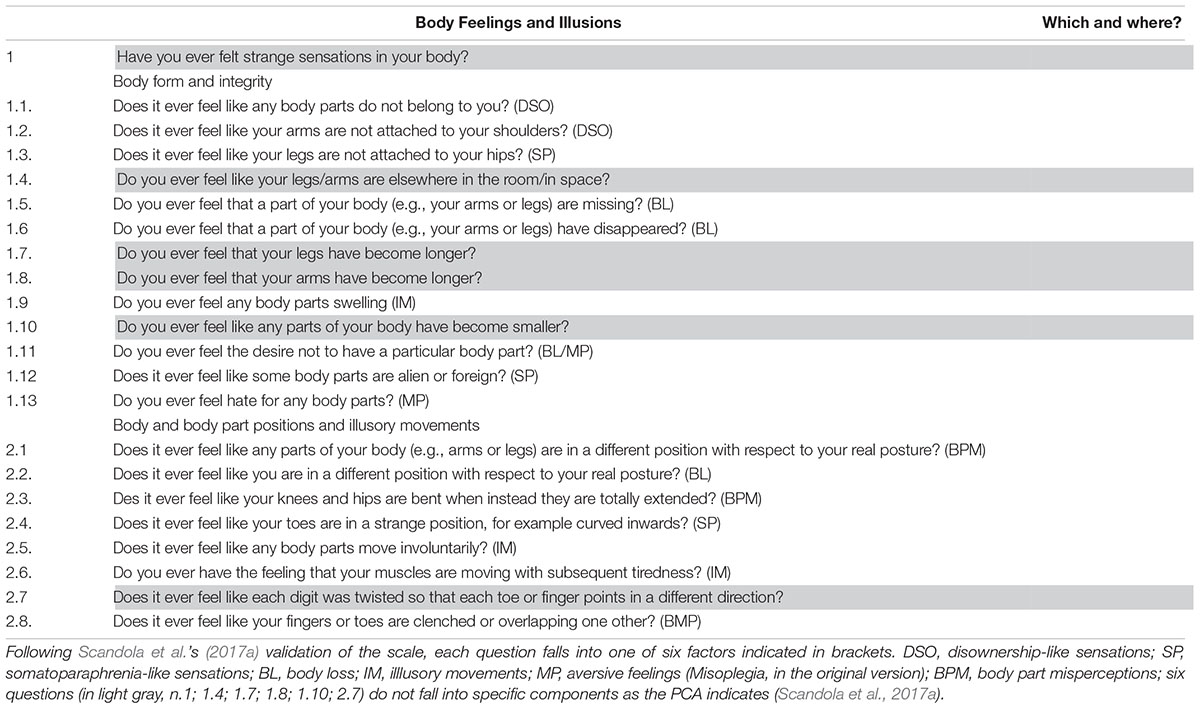
The Body Feelings and Illusions questionnaire (BoFI; Scandola et al., 2017a) was administered to the two groups of participants. The questionnaire is comprised of 22 questions each of which investigate the presence or absence (score 1–0) of any symptoms related to Body form and integrity (Q1.1–1.13) or any feelings relating to Body part positions or illusory movements (Q2.1–2.8). When a symptom was reported, further questions were asked about the situations in which the feeling occurred and its qualitative characteristics. Based on a Principal Component Analysis carried out in a previous validation of the questionnaire (Scandola et al., 2017a), the majority of the questions fall into specific components (see Table 2) such as: feelings of Body loss (i.e., sensations of body parts disappearing or missing); Illusory motion (i.e., sensations of motion that are not voluntarily controlled with muscular fatigue after illusionary movements); Body part misperception (i.e., the feeling of having some body parts in a position which is different to the actual position); Aversive feelings (i.e., negative feelings toward a given body part; in the original study this was called Misoplegia to describe symptoms reported by spinal cord injured people who are paralysed in the lower lesioned body parts); Disownership-like feelings (i.e., the feeling that body parts do not belong to the person) and Somatoparaphrenia-like sensations (i.e., the feeling that body parts are “alien” or detached from the body).
The scores of each component were computed as the sum of the responses to the questions with positive loadings to the component, minus the responses to the questions with negative loadings. Some questions do not fall into any of the components but were, however, administered, even though they were not considered in the statistical analyses (Table 2).
The Assessment of Motor Imagery
Two subscales of the Visual Motor Imagery Questionnaire-2 (Isaac et al., 1986; Roberts et al., 2008) were used to assess motor imagery abilities from first and third person perspectives. More specifically, the subscale of External Visual Imagery (VMIQ-EVI) necessitates imaging oneself performing actions from a third person perspective (“as if you were watching yourself from an external position”). In contrast, the first person perspective imagery was assessed by means of the Kinesthetic Imagery scale (VMIQ-KIN) with the participants being asked to “imagine feeling themselves performing the movement.” Thus, the two subscales involve different cognitive processes, specifically visual imagery in the case of the former and simulation of bodily sensations for the latter (Ionta et al., 2010; Scandola et al., 2017b; Moro et al., 2021a). The vividness of each action that they imagined (12 identical actions in the two subscales) was rated by the participant on a 5-point Likert scale (with 1 = perfectly vivid imagined action and 5 = not imagined at all). The sums of the scores were considered as the final scores for the two subscales (maximum score = 60) (Table 3).
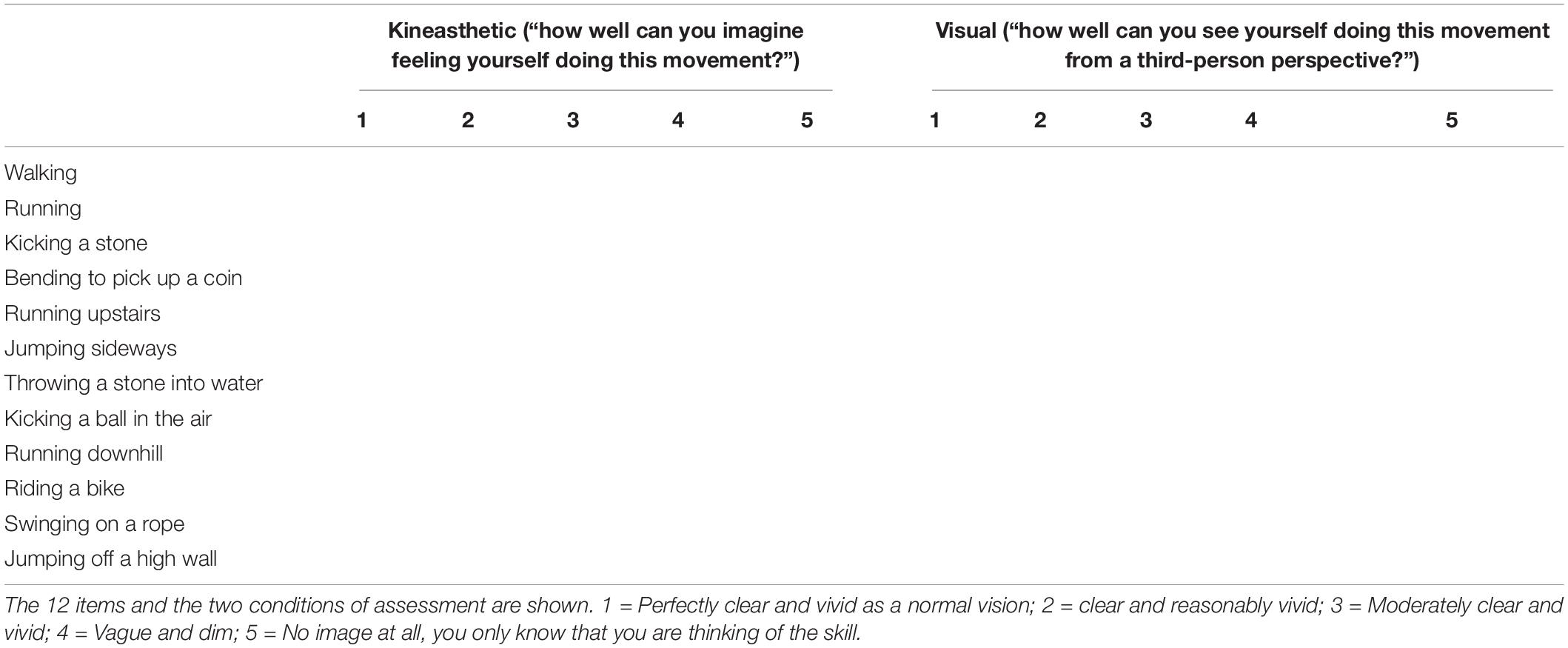
Table 3. The Visual Motor Imagery Questionnaire (Roberts et al., 2008).
Procedure
The participants were recruited during their follow-up visits which included the administration of WPI, SSS, and FIQ. They were informed of the aims of the study and were requested to sign the informed consent form. They were then interviewed in a quiet room in the Anthalgic Therapy Unit (Department of Surgery, Dentistry, Paediatrics and Gynaecology) by two examiners (GP and VM or MS), with one asking the questions and the other transcribing the answers. First, a clinical assessment was carried out using the Verona Pain Scale, HADS and a number of questions regarding motor activity and the participant’s employment situation. These tasks were administered in a counterbalanced order. Subsequently, the BoFI and VMIQ (VMIQ-EVI and VMIQ-KIN) were executed in a counterbalanced order. The whole session lasted from 30 to 45 min, depending on the dialogue that was created between the participant and the examiners and the number of symptoms and details to be recorded.
Analysis of Data
The experimental variables were not parametrically distributed (all Shapiro–Wilk tests p < 0.05), thus, in order to compare the FM and C groups, non-parametric tests were used. When a variable was continuous, the Mann–Whitney test for independent samples was used, using the r rank-biserial correlation as the measure of the effect size. This ranged between 0 and 1 (small effect = r < 0.3; moderate effect = r < 0.5; large effect = r ≥ 0.5; Tomczak and Tomczak, 2014). When a variable was scored as a frequency, a χ2 test was used with Cramer’s V as effect size (moderate = 0.2 < V ≤ 0.6, large V > 0.6; Rea and Parker, 2014). Furthermore, in order to assess any potential association between bodily illusions and clinical variables, a series of correlations were executed separately for the two groups. All of the statistical analyses were executed by means of the R statistical framework (R Core Team, 2020).
Results
Body Representation Disorders
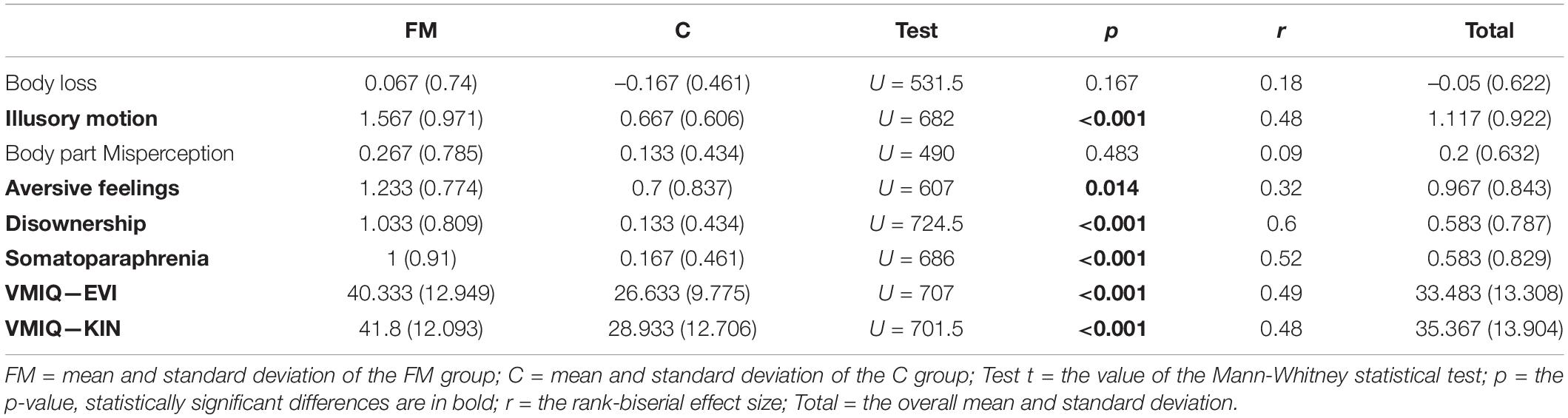
The results of the comparison between the FM and C groups are reported in Table 4. They indicate that in the case of the FM group, feelings of Illusory Motion, Disownership-like sensations and Somatoparaphrenia-like sensations, as well as Aversive feelings are more frequent than in the C group. No differences were recorded for feelings of Body Loss and Body part Misperception. The FM group did not perform as well as the C group in terms of motor imagery, both in the VMIQ-KIN and VMIQ-EVI subscales.
A statistical analysis of the responses (details of the frequencies of the responses to individual items are shown in Supplementary Table 2) indicates that, among the questions falling into the category of Illusory motion components, the feeling of illusory muscular work, with a consequent sensation of fatigue (Q2.6) is present in 26.67% of the FM participants, while in the C group, this was only reported by one participant (χ2(1) = 4.71, p = 0.03, Cramer’s V = 0.33). Some FM patients reported this sensation involving the whole body and other that it was localised to their limbs and hands. In the same component category, the feeling that some body parts involuntarily move (Q2.5, legs and arms in particular) was present in 43.33% of the FM participants, while in the C group this was reported by 20% of the participants, who, however, reported feelings of muscular fasciculation rather than limb movements. Even if the results relating to this sensation are not statistically different between the two groups (χ2(1) = 2.77, p = 0.10, Cramer’s V = 0.25), there is a qualitative difference. Indeed, if we remove the feeling of muscular fasciculation from both groups and consider only actual sensations of body part movement, the difference becomes statistically significant (χ2(1) = 7.55, p = 0.006, Cramer’s V = 0.39).
Finally, a sensation of body part swelling (Q1.9) was more frequent in the FM group (87%) than in the C group (43%) (χ2(1) = 10.55, p < 0.001, Cramer’s V = 0.45). This sensation specifically involves the hands and feet and sometimes an entire limb. In five patients, sensations of swelling were reported in the abdomen and four participants reported the sensation involving their face. Sensations of swelling were also present in the C group with a number of them claiming that they had experienced this, but not so frequently.
With regard to Disownership-like sensations, the FM participants reported both sensations that certain body parts did not belong to their body (Q1.1, 56.67%) and that their arms were not attached (Q1.2, 46.67%), feelings which were rare or absent in the C group (10 and 3.33%, respectively). In both cases, the differences were statistically significant (χ2(1) = 12.67, p < 0.001, Cramer’s V = 0.5; χ2(1) = 12.8, p < 0.001, Cramer’s V = 0.5, respectively). These sensations commonly involve the limbs, but one of the FM participants reported this sensation in the head, and two in their whole body. In particular, these seem to occur during stressful or anxious moments, but also when they are half-asleep, tired or at the moment when the pain is exacerbated.
The feeling of one’s legs not being attached (Q1.3, in the Somatoparaphrenia-like sensations component category) was frequent in the FM group (43.33%) but not in the C group (10%; χ2(1) = 12.67, p < 0.001, Cramer’s V = 0.5). Specific sensations of alienness of body parts (Q1.12) were present in around a quarter of the FM participants (23%), but were not reported at all by the C participants (0%; χ2(1) = 5.82, p = 0.016, Cramer’s V = 0.36). In this component category, there were also reports of sensations of the toes being in strange positions (Q2.4), as claimed by one third of the FM participants (33%) but only in a few cases in the C group (6.67%; χ2(1) = 5.1, p = 0.024, Cramer’s V = 0.33).
Other frequent symptoms in the FM group referred to a desire not to have a particular body part (Q1.11; FM = 63.33%; C = 40%; χ2(1) = 5.42, p = 0.001, Cramer’s V = 0.32) and a feeling of hate toward certain body parts Q1.13; FN = 60%; C = 30%; χ2(1) = 4.31, p = 0.038, Cramer’s V = 0.3). These sensations are mostly felt toward painful body parts and, while in the C group they were limited in number and extremely specific, but the most relevant characteristic was that these painful sensations were constant or almost constant. (the pain sensations felt from people of the C group reporting hate toward the bodily parts were: toothache (three people), pain in the stomach, intestine and the pelvic floor (one person), pain in a buttock and a calf (one person), pain at shoulders and head (one person), pain in the right heel and hip (one person), pain in the left eye (one person); one person also associated pain with the feeling of restless legs during night), in the FM group they involved large areas of the body and sometimes the whole body. It is to be noted that, in the PCA (Scandola et al., 2017a), Q1.11 (regarding the desire not to have a particular body part) loads negatively in the Body Loss component and positively in the Aversive feeling component (see Table 2).
Within the BoFi-FM, the various components are partially correlated in the FM group, for which Illusory movements correlate with Body Loss (r = 0.43, p < 0.05), Disownership-like sensations (0.46, p < 0.05) and Somatoparaphrenia-like sensations (0.62, p < 0.001). These latter two components also correlate with each other (0.66, p < 0.001) and with Aversive feelings toward body parts (0.49, p < 0.01). In the C group, only Somatoparaphrenic and Disownership-like sensations were correlated (0.57, p < 0.001). The complete results are reported in Supplementary Tables 3A,B.
Effects of the Clinical Variables on Body Representation Disorders
In contrast to our expectations, we did not find any specific correlations between clinical variables (WPI, SSS, pain severity, interval from symptoms onset and diagnosis, and functional deficits at the FIQ) and corporeal illusions in the FM group, but there was an inverse correlation between age and Body loss: –0.5, p < 0.01. In the C group, the severity of pain symptoms (as measured by the SSS) correlated with some components such as Body loss (–0.39, p < 0.05), Illusory movement (0.39, p < 0.05), and Aversive feelings (0.49, p < 0.01). In this latter group, a correlation between standard of education and somatoparaphrenic-like sensations was also found (0.57, p < 0.001).
Motor Imagery
Patients with FM show disorders in motor imagery in both the visual and kinaesthetic conditions as compared to the matched controls. In the FM group, the two subscales correlated with each other and also with the severity of the symptoms as measured by the FIQ (VMIQ-EVI: 0.38, p < 0.05; VMIQ-KIN: 0.42, p < 0.05). A negative correlation was found between the VMIQ-EVI and the duration of the illness (i.e., the interval of time between the first symptom and the assessment, –0.39, p < 0.01), indicating that this capacity deteriorates over time. In the C group, the VMIQ KIN correlated with all the measures of pain and functional deficits (FIQ: 0.44, p < 0.05, SSS: 0.52, p < 0.52), while the VMIQ EVI correlated with WPI (0.36, p < 0.05) and SSS (0.67, p < 0.001).
Correlations Between Clinical Variables
In the FM group, anxiety and depression did not correlate with other clinical variables or with bodily misperceptions and motor imagery, while in the C group anxiety was associated with WPI (0.37, p < 0.05).
Discussion
In the present study, the BoFI questionnaire was used to investigate the presence of spontaneous sensations and misperceptions relating to the body. The main result regards the evidence that was found that bodily self is compromised in FM, although with symptoms that are more specific than in other clinical conditions such as strokes (Romano and Maravita, 2019) or deafferentation and deefferentation due to spinal cord injuries (Scandola et al., 2017a). Indeed, while in these conditions corporeal illusions involve the feeling of the presence of the body itself (Jenkinson et al., 2018), the FM patients did not report sensations of Body loss more frequently than the controls (i.e., sensations of body parts disappearing or missing) or misperception of body parts (i.e., the feeling of having some body parts in a position which is different to the actual position). Corporeal illusions seem to be specifically associated with a sense of strangeness and alienness of the body, in other words, giving a sense of being in their body and having their body. In fact, they reported the impression of illusory motion and disownership or somatoparaphrenic-like sensations, that is, the feelings that body parts do not belong to them or are “alien”. The presence of Aversive feelings (Misoplegia) is consistent with previous data indicating lower levels of acceptance regarding painful body parts (Martínez et al., 2018).
A second interesting, unexpected result is the lack of correlations between these bodily sensations and pain. This suggests the possibility that alterations in the bodily self represent a specific feature of the syndrome which is not secondary to other clinical aspects. Although Aversive feelings toward the body were reported, pain and body sensations were independent from mood, in particular from anxiety and depression in the FM group, unlike in the case of the controls for whom the extent of the pain they experienced (as measured by WPI) correlated with the degree of anxiety.
Finally, a reduced capacity with regard to motor imagery was found in the FM group, both when the task was executed from a first person perspective (with an internal simulation of the movement—VMIQ-KIN) and a third person perspective (with the participant imaging as seing themselves in a mirror while performing the action—VMIQ-EV). Motor imagery disorders correlated with functional deficits (FIQ) in both groups. Furthermore, an effect of mood was reported, since in the FM group both of the results of the motor imagery tasks correlated with depression.
Disorders in Body Representations in Fibromyalgia
Body representation may be investigated by means of various methodologies, and in FM a number of different approaches have been used, ranging from interviews and illness narratives (Valenzuela-Moguillansky et al., 2013), to scales and questionnaires (Akkaya et al., 2013; Rost et al., 2017) and experimental procedures (Valenzuela-Moguillansky et al., 2017; Martínez et al., 2018, 2019). In the present study, a specific questionnaire was used to investigate the presence of spontaneous sensations and misperceptions relating to the body. This allowed us to distinguish various different aspects involving corporeal illusions. The hypothesis that these six components are independent of each other was supported by the results from the control group which indicate that they do not correlate with each other, with the exception of Disownership and Somatoparaphrenic-like sensations. In contrast, in the FM group, correlations between Illusory movements and feelings of Body loss, Disownership and Somatoparaphrenic-like sensations were found, and these latter correlated with Aversive responses (Misoplegia).
Phenomena related to sensations of illusory motion, with involuntary motion and consequent muscular fatigue, such as those recorded with the Illusory Motion subscale, previously been reported in patients suffering from neurological disorders (Jenkinson et al., 2015; Scandola et al., 2017a). In cases of FM, the most frequent symptom involves sensations of swelling in certain body parts, which is also associated with body size perception. This result confirms previous studies in these patients, showing difficulties in estimating their own body size, with enlarged body size perception and shrinkage of the surrounding space, in particular when pain is exacerbated (Valenzuela-Moguillansky et al., 2013). In an experimental study, a body-scaled action anticipation task was used, in which the participants were asked to estimate whether they would be able to pass through apertures of varying widths that simulated doors. The “passability ratio” (i.e., the aperture size for a 50% positive response rate divided by the participant’s shoulder width) was higher for the FM participants, indicating an overestimation of their body size. This correlated positively with pain and functionality, but, as in our results, not with pain intensity (Valenzuela-Moguillansky et al., 2017).
A novelty in the present study regards evidence of feelings of detachment, alienness, and a reduced sense of ownership of body parts, such as the feeling that arms or legs are detached from the shoulders or hips or that these do not belong to one’s own body (Body loss, Disownership, and Somatoparaphrenic-like sensations subscales). Sometimes these feelings are present when there is pain, but they often occur in relaxed moments or when patients are resting in bed. Thus, a direct explanation based on the presence of pain cannot be drawn from our data.
Sensory information processing may contribute to these sensations. Distorted localisation of tactile stimulation (administered to painful sites) has been found in FM patients (Martínez et al., 2019) who report anomalous sensations (e.g., tingling, pins and needles, a feeling of heaviness, and cramps) as if they were emanating from somewhere away from the site being stimulated, sometimes within the same dermatome but often remotely or in another limb. Interoception (i.e., the processing of information coming from inside one’s own body) has also been investigated, with some experimental data indicating a perception of reduced heartbeat in FM patients (Borg et al., 2018) and other reports showing no difference with healthy subjects (Rost et al., 2017; Valenzuela-Moguillansky et al., 2017). It is thus plausible that altered information coming from the body contributes to corporeal illusions as an indirect result of neuroplastic processes. Interoception might also have a role in the lack of connection between chronic pain and bodily illusions. Indeed, interoception is an important source for body representations (Craig, 2003), that is altered in chronic pain conditions (Di Lernia et al., 2016), in particular in chronic visceral pain (Bonaz et al., 2021).
However, experimental studies suggest that other cognitive elements contribute, and these are associated more with high order, body representations. For example, FM patients performed significantly worse with respect to controls in a task involving specifying the laterality of a hand (Martínez et al., 2019). In this task, the participants look at images of hands (or feet) which are rotated to different degrees (e.g., 0°–90°–180°–270°), and they are asked to decide if the image corresponds with the right or left body part. When the participant activates body representation, the response times increase with the increase in the degree of rotation in the image (i.e., 180°). This indicates that the task is executed by means of a bodily simulation of the position of the body part. When the task is executed with a visual, rather than corporeal, strategy, the response times do not change with the degree of rotation. This is considered to represent an index of disorders in body representation and was recorded in cases of FM, as well as in other clinical conditions (Parsons, 1994; Ionta and Blanke, 2009; Conson et al., 2013; Scandola et al., 2019a). In line with this, FM patients seem to be more sensitive to the effects of the rubber hand illusion (Botvinick and Cohen, 1998). When their own hand and a rubber hand are synchronously stroked, the patients’ response is accentuated with respect to the controls, both in terms of the proprioceptive drift and the sense of ownership and motor control over the rubber hand (Martínez et al., 2018). Taken together, these results suggest that a feature of the FM syndrome regards the fragility of one’s own body, characterised by instability regarding self-body representations and a greater sensitivity to illusions. Rather than being a consequence of pain, disorders relating to the bodily self might represent a concurrent symptom (Martínez et al., 2018).
Neuroimaging studies support this hypothesis. Although in fMRI brain activity during touch is similar in FM patients and controls, patients show differences when they are asked to rate pleasure and pain with deactivation and hyperactivation in the posterior insular contralateral to the stimulated arm, respectively (Boehme et al., 2020). Voxel-brain-morphometry analyses also reveal reduced gray matter density in the anterior insula and hippocampus (Boehme et al., 2020). Finally, alterations in the connectivity of the insula have been reported in cases of FM, with higher levels of strength with reference to the connectivity between the right inferior parietal sulcus and the insula cortex (van Ettinger-Veenstra et al., 2020). The functional role of the insula and its connections to sensations relating to the body are well known (Karnath et al., 2005; Moro et al., 2016; Pacella et al., 2019) and seem to concern both its fundamental role in the central processing of interoceptive signals from the body (Craig, 2009) and its contribution to the salience network of the brain (Menon and Uddin, 2010). Indeed, over-focalisation on bodily sensations (Rost et al., 2017) and a perceptual style involving the amplification of interoceptive stimulation (Borg et al., 2018; Martínez et al., 2018) have been reported in FM patients, but these are not associated with greater interoceptive accuracy (Borg et al., 2018).
Corporeal Illusion and Clinical Variables
No correlations were found between the severity of symptoms or their impact on autonomy (as measured by the SSS, the WPI, and the FIQ) and the various types of corporeal illusions, or between these latter and the intensity of neuromuscular, neuropathic or visceral pain. In the FM group, the only correlations found were between age and feelings of body loss and between age and neuropathic pain.
This was an unexpected result considering that, in the C group, a correlation with the severity of pain was recorded. Furthermore, distortions in body size, weight and localisation have been found to be associated with pain in previous studies (Valenzuela-Moguillansky et al., 2013; Teodoro et al., 2018 for a review). It is possible that the setting or the timing of the interview have an effect and that the link between clinical variables only becomes evident when the patients are interviewed in conjunction with an episode of pain (as for example in Valenzuela-Moguillansky et al., 2013, in which interviews involving the elicitation of pain were used). At the same, it might be worth considering whether the absence of any impact relating to anxiety and depression on corporeal illusions may depend on the specific sample of patients who took part in this study, since none of them were undergoing a painful episode. Anxiety and depressive symptoms are reported as being relatively high among FM patients (Malt et al., 2002; Hadlandsmyth et al., 2019), but no differences were recorded in our sample in comparison to the healthy controls.
Interestingly, somatoparaphrenic-like sensations directly correlate with education in the C group. Relationships between education and bodily illusions have been rarely investigated, probably because the standards of education in experimental studies are usually matched between groups. However, recent studies with the Rubber Hand Illusion are showing that interindividual differences such as education might have an impact on bodily illusions (Haans et al., 2012; Kállai et al., 2015; Burin et al., 2019; Lush et al., 2020, 2021; Romano et al., 2021). However, these interindividual characteristics are not sufficient to explain the individual responses to the induced illusion (David et al., 2013; Scandola et al., 2014; Ehrsson et al., 2021). A possible explanation for this effect is that more educated people show a greater “Openness to Experience” trait of personality, that also involves a leaning toward non-traditional values and experiences (Schretlen et al., 2010). This might lead to the tendency to acceptance of unusual bodily sensations. This is indeed only a speculative hypothesis, as in this study these data on personality traits were not collected
Motor Imagery
Another result of this study regards the evidence found of specific disorders in motor imagery in FM patients. These involve both the capacity to visually imagine one’s own body while performing an action and the ability to imagine the kinematic sensations produced by that action. In particular, they showed a reduced motor imagery both in the kinaesthetic and third-person perspective imagery. In the FM group, these capacities were reduced in correlation with the loss of functional abilities (FIQ), while no correlations were found with the severity of symptoms and pain intensity, as occurred in the case of the controls. In addition, the FM patient’s history of illness (in terms of the time interval between the appearance of the first symptoms and the degree of depression) correlated with their visual motor imagery disorders.
Motor imagery is closely connected to action execution, as demonstrated by neuroimaging results showing that MI involves neural structures largely overlapping with those involved in actually performing the imagined movements, in particular the pre-motor areas, the left intraparietal sulcus, and subcortical structures such as basal ganglia and cerebellum (Bonda et al., 1995; Decety, 1996; Gerardin et al., 2000; Corradi-Dell’Acqua et al., 2009). The inherent link between motor imagery and action execution has been confirmed in studies showing that MI is altered in a number of pathological conditions characterised by an impairment of the ability to actually perform actions such as locked-in syndrome (Conson et al., 2008), spinal cord injury (Scandola et al., 2017a) amyotrophic lateral sclerosis (Fiori et al., 2013), and chronic pain conditions (Schwoebel et al., 2001; Coslett et al., 2010). Our results suggest that rather than the pain itself, it is the functional deficits which induce motor imagery deficits in FM patients. This would be also consistent with results relating to spinal cord injured patients, which indicate that disorders in motor imagery specifically refer to the actions that have become impossible to execute due to paralysis (Pernigo et al., 2012; Scandola et al., 2017b). The direction of this relationship has yet to be fully understood, however, since although it is possible that functional deficits mediate a reduction in motor imagery, the opposite is also plausible, that is, that motor imagery disorders influence functional abilities.
Conclusion
The main limitation of the study concerns the number of participants which was not very large in terms of the methodology used which was based on a verbal interview (BoFI-FM) and a self-judgment task (VMIQ). However, the sample seems to be sufficiently representative of the clinical population if one considers the strict inclusion and exclusion criteria for the two groups and the in-depth clinical assessment administered that included functional and emotional measures. In addition, only women were recruited for the study, due to the rarity of the syndrome among males. This did not allow testing for gender differences. Further studies are needed to confirm these preliminary results and compare subjective reports with objective measures of body representations and somatosensory and interoceptive sensitivity. It was not possible to identify the potential presence of a topography relating to corporeal illusions and motor imagery deficits. In other words, it was not established whether the body representation and motor imagery disorders were specific to the painful body parts or were generalised to the whole body. This limitation is inherent to the specific clinical condition, since FM is characterised by widespread pain that involves various different body parts, without a specific topographic organisation—something which is, in contrast, observable, for example, in focal dystonia, or chronic regional pain. It is thus extremely difficult to trace a topography of body representation disorders. Finally, no measure of interoception was collected.
In conclusion, the results indicate that bodily self and motor imagery disorders are two features of FM and that these may be investigated in clinical settings using specific instrumentation. This may, at least in part, explain the higher frequency of falls in these patients which has previously been associated with an altered body schema (Jones et al., 2009; Meireles et al., 2014). From a more general, theoretical perspective, our results demonstrate that FM is a complex, multifaceted syndrome and that body representation disorders, although concurrent, are, however, independent from pain.
Data Availability Statement
The original contributions presented in the study are publicly available. This data can be found here: osf.io/vxgqj.
Ethics Statement
The studies involving human participants were reviewed and approved by Comitato etico per la Sperimentazione Clinica (CESC) delle Province di Verona e Rovigo. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MS: study ideation and planning, data collection, statistical analyses, and writing. GP: study planning, control recruitment, and data collection. GL: data collection. EP: patient recruitment and clinical assessment. VS: patient recruitment, clinical assessment, and writing. VM: study ideation and planning, data collection, writing, and supervision of all phases of the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Italian University Ministry (VM; PRIN 2017–2017N7WCLP) and by the Cariverona Foundation (VM & MS; project Eccellenza Cariverona 2018 (ROL10782-COD.SIME 2018.0898).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the participants in the study. Many thanks also to Erica Secchettin for her help in the procedures for Ethical Committee application and Cristina Lonardi and Roberta Fraccaroli who aided the efforts of the authors during data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2021.798912/full#supplementary-material
References
Ablin, J. N., Oren, A., Cohen, S., Aloush, V., Buskila, D., Elkayam, O., et al. (2012). Prevalence of fibromyalgia in the Israeli population. Clin. Exp. Rheumatol. 30, 39–43.
Akkaya, N., Atalay, N. S., Selcuk, S. T., Alkan, H., Catalbas, N., and Sahin, F. (2013). Frequency of fibromyalgia syndrome in breast cancer patients. Int. J. Clin. Oncol. 18, 285–292. doi: 10.1007/s10147-012-0377-9
Andrade, A., Vilarino, G. T., Sieczkowska, S. M., Coimbra, D. R., Bevilacqua, G. G., and Steffens, R. D. A. K. (2020). The relationship between sleep quality and fibromyalgia symptoms. J. Health Psychol. 25, 1176–1186. doi: 10.1177/1359105317751615
Apps, M. A. J., Tajadura-Jiménez, A., Sereno, M., Blanke, O., and Tsakiris, M. (2015). Plasticity in unimodal and multimodal brain areas reflects multisensory changes in self-face identification. Cereb. Cortex 25, 46–55. doi: 10.1093/cercor/bht199
Berlucchi, G., and Aglioti, S. M. (2010). The body in the brain revisited. Exp. Brain Res. 200, 25–35. doi: 10.1007/s00221-009-1970-7
Bjelland, I., Dahl, A. A., Haug, T. T., and Neckelmann, D. (2002). The validity of the hospital anxiety and depression scale. J. Psychosom. Res. 52, 69–77. doi: 10.1016/S0022-3999(01)00296-3
Boehme, R., Van Ettinger-Veenstra, H., Olausson, H., Gerdle, B., and Nagi, S. S. (2020). Anhedonia to gentle touch in fibromyalgia: normal sensory processing but abnormal evaluation. Brain Sci. 10:306. doi: 10.3390/brainsci10050306
Bonaz, B., Lane, R. D., Oshinsky, M. L., Kenny, P. J., Sinha, R., Mayer, E. A., et al. (2021). Diseases, disorders, and comorbidities of interoception. Trends Neurosci. 44, 39–51. doi: 10.1016/j.tins.2020.09.009
Bonda, E., Petrides, M., Frey, S., and Evans, A. (1995). Neural correlates of mental transformations of the body-in-space. Proc. Natl. Acad. Sci. U.S.A. 92, 11180–11184. doi: 10.1073/pnas.92.24.11180
Borg, C., Chouchou, F., Dayot-Gorlero, J., Zimmermann, P., Maudoux, D., Laurent, B., et al. (2018). Pain and emotion as predictive factors of interoception in fibromyalgia. J. Pain Res. 11, 823–835. doi: 10.2147/JPR.S152012
Botvinick, M., and Cohen, J. (1998). Rubber hands “feel” touch that eyes see. Nature 391:756. doi: 10.1038/35784
Bouhassira, D., Attal, N., Alchaar, H., Boureau, F., Brochet, B., Bruxelle, J., et al. (2005). Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 114, 29–36. doi: 10.1016/j.pain.2004.12.010
Branco, J. C., Bannwarth, B., Failde, I., Abello Carbonell, J., Blotman, F., Spaeth, M., et al. (2010). Prevalence of fibromyalgia: a survey in five European countries. Semin. Arthritis Rheum. 39, 448–453. doi: 10.1016/j.semarthrit.2008.12.003
Burin, D., Pignolo, C., Ales, F., Giromini, L., Pyasik, M., Ghirardello, D., et al. (2019). Relationships between personality features and the rubber hand illusion: an exploratory study. Front. Psychol. 10:2762. doi: 10.3389/fpsyg.2019.02762
Caraceni, A., Mendoza, T. R., Mencaglia, E., Baratella, C., Edwards, K., Forjaz, M. J., et al. (1996). A validation study of an Italian version of the brief pain inventory (Breve Questionario per la Valutazione del Dolore). Pain 65, 87–92. doi: 10.1016/0304-3959(95)00156-5
Chinn, S., Caldwell, W., and Gritsenko, K. (2016). Fibromyalgia pathogenesis and treatment options update. Curr. Pain Headache Rep. 20, 1–10. doi: 10.1007/s11916-016-0556-x
Conson, M., Mazzarella, E., and Trojano, L. (2013). Developmental changes of the biomechanical effect in motor imagery. Exp. Brain Res. 226, 441–449. doi: 10.1007/s00221-013-3456-x
Conson, M., Sacco, S., Sarà, M., Pistoia, F., Grossi, D., and Trojano, L. (2008). Selective motor imagery defect in patients with locked-in syndrome. Neuropsychologia 46, 2622–2628. doi: 10.1016/j.neuropsychologia.2008.04.015
Corradi-Dell’Acqua, C., Tomasino, B., and Fink, G. R. (2009). What is the position of an arm relative to the body? Neural correlates of body schema and body structural description. J. Neurosci. 29, 4162–4171. doi: 10.1523/JNEUROSCI.4861-08.2009
Coslett, H. B., Medina, J., Kliot, D., and Burkey, A. R. (2010). Mental motor imagery indexes pain: the hand laterality task. Eur. J. Pain 14, 1007–1013. doi: 10.1016/j.ejpain.2010.04.001
Costantini, M., Robinson, J., Migliorati, D., Donno, B., Ferri, F., and Northoff, G. (2016). Temporal limits on rubber hand illusion reflect individuals’ temporal resolution in multisensory perception. Cognition 157, 39–48. doi: 10.1016/j.cognition.2016.08.010
Craig, A. D. (2003). Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 13, 500–505. doi: 10.1016/S0959-4388(03)00090-4
Craig, A. D. (2009). How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70. doi: 10.1038/nrn2555
David, N., Fiori, F., and Aglioti, S. M. (2013). Susceptibility to the rubber hand illusion does not tell the whole body-awareness story. Cogn. Affect. Behav. Neurosci. 14, 297–306. doi: 10.3758/s13415-013-0190-6
Decety, J. (1996). Neural representations for action. Rev. Neurosci. 7, 285–297. doi: 10.1515/REVNEURO.1996.7.4.285
Di Lernia, D., Serino, S., and Riva, G. (2016). Pain in the body. Altered interoception in chronic pain conditions: a systematic review. Neurosci. Biobehav. Rev. 71, 328–341. doi: 10.1016/j.neubiorev.2016.09.015
Ehrsson, H. H., Fotopoulou, A., Radziun, D., Longo, M., and Tsakiris, M. (2021). No specific relationship between hypnotic suggestibility and the rubber hand illusion?: a commentary with new and verification analyses. psyArxiv [Preprint] doi: 10.31234/osf.io/pmzgc
Fiori, F., Sedda, A., Ferrè, E. R., Toraldo, A., Querzola, M., Pasotti, F., et al. (2013). Exploring motor and visual imagery in amyotrophic lateral sclerosis. Exp. brain Res. 226, 537–547. doi: 10.1007/s00221-013-3465-9
Fornia, L., Puglisi, G., Leonetti, A., Bello, L., Berti, A., Cerri, G., et al. (2020). Direct electrical stimulation of the premotor cortex shuts down awareness of voluntary actions. Nat. Commun. 11:705. doi: 10.1038/s41467-020-14517-4
Furness, P. J., Vogt, K., Ashe, S., Taylor, S., Haywood-Small, S., and Lawson, K. (2018). What causes fibromyalgia? An online survey of patient perspectives. Heal. Psychol. Open 5:2055102918802683. doi: 10.1177/2055102918802683
Gerardin, E., Sirigu, A., Lehéricy, S., Poline, J. B., Gaymard, B., Marsault, C., et al. (2000). Partially overlapping neural networks for real and imagined hand movements. Cereb. Cortex 10, 1093–1104. doi: 10.1093/cercor/10.11.1093
Golaszewski, S., Frey, V., Thomschewski, A., Sebastianelli, L., Versace, V., Saltuari, L., et al. (2021). Neural mechanisms underlying the rubber hand illusion: a systematic review of related neurophysiological studies. Brain Behav. 11, 1–13. doi: 10.1002/brb3.2124
Gustin, S. M., Wrigley, P. J., Gandevia, S. C., Middleton, J. W., Henderson, L. A., and Siddall, P. J. (2008). Movement imagery increases pain in people with neuropathic pain following complete thoracic spinal cord injury. Pain 137, 237–244. doi: 10.1016/j.pain.2007.08.032
Haans, A., Kaiser, F. G., Bouwhuis, D. G., and Ijsselsteijn, W. A. (2012). Individual differences in the rubber-hand illusion: predicting self-reports of people’s personal experiences. Acta Psychol. 141, 169–177. doi: 10.1016/j.actpsy.2012.07.016
Hadlandsmyth, K., Dailey, D. L., Rakel, B. A., Zimmerman, M. B., Vance, C. G. T., Merriwether, E. N., et al. (2019). Somatic symptom presentations in women with fibromyalgia are differentially associated with elevated depression and anxiety. J. Health Psychol. 25:135910531773657. doi: 10.1177/1359105317736577
Haggard, P., Iannetti, G. D., and Longo, M. R. (2013). Spatial sensory organization and body representation in pain perception. Curr. Biol. 23, R164–R176. doi: 10.1016/j.cub.2013.01.047
Häuser, W., Ablin, J., Fitzcharles, M. A., Littlejohn, G., Luciano, J. V., Usui, C., et al. (2015). Fibromyalgia. Nat. Rev. Dis. Prim. 1, 1–16. doi: 10.1038/nrdp.2015.22
Häuser, W., and Fitzcharles, M. A. (2018). Facts and myths pertaining to fibromyalgia. Dialogues Clin. Neurosci. 20, 53–62. doi: 10.31887/DCNS.2018.20.1/whauser
Ionta, S., and Blanke, O. (2009). Differential influence of hands posture on mental rotation of hands and feet in left and right handers. Exp. Brain Res. 195, 207–217. doi: 10.1007/s00221-009-1770-0
Ionta, S., Fourkas, A. D., and Aglioti, S. M. (2010). Egocentric and object-based transformations in the laterality judgement of human and animal faces and of non-corporeal objects. Behav. Brain Res. 207, 452–457. doi: 10.1016/j.bbr.2009.10.037
Isaac, A., Marks, D. F., and Russell, D. G. (1986). An instrument for assessing imagery of movement: the vividness of movement imagery questionnaire (VMIQ). J. Ment. Imag. 10, 23–30. doi: 10.1037/t07980-000
Jenkinson, P. M., Edelstyn, N. M. J., Preston, C., and Ellis, S. J. (2015). Anarchic hand with abnormal agency following right inferior parietal lobe damage: a case report. Neurocase 21, 471–478. doi: 10.1080/13554794.2014.925936
Jenkinson, P. M., Moro, V., and Fotopoulou, A. (2018). Definition: asomatognosia. Cortex 101, 300–301. doi: 10.1016/j.cortex.2018.02.001
Jenkinson, P. M., Papadaki, C., Besharati, S., Moro, V., Gobbetto, V., Crucianelli, L., et al. (2020). Welcoming back my arm: affective touch increases body ownership following right-hemisphere stroke. Brain Commun. 2:fcaa034. doi: 10.1093/braincomms/fcaa034
Jones, K. D., Horak, F. B., Winters-Stone, K., Irvine, J. M., and Bennett, R. M. (2009). Fibromyalgia is associated with impaired balance and falls. J. Clin. Rheumatol. 15, 16–21. doi: 10.1097/RHU.0b013e318190f991
Kállai, J., Hegedüs, G., Feldmann, Á, Rózsa, S., Darnai, G., Herold, R., et al. (2015). Temperament and psychopathological syndromes specific susceptibility for rubber hand illusion. Psychiatry Res. 229, 410–419. doi: 10.1016/j.psychres.2015.05.109
Karnath, H.-O., Baier, B., and Nägele, T. (2005). Awareness of the functioning of one’s own limbs mediated by the insular cortex? J. Neurosci. 25, 7134–7138. doi: 10.1523/JNEUROSCI.1590-05.2005
Kumru, H., Soler, D., Vidal, J., Navarro, X., Tormos, J. M., Pascual-Leone, A., et al. (2013). The effects of transcranial direct current stimulation with visual illusion in neuropathic pain due to spinal cord injury: an evoked potentials and quantitative thermal testing study. Eur. J. Pain 17, 55–66. doi: 10.1002/j.1532-2149.2012.00167.x
Le Bars, D., Dickenson, A. H., and Besson, J. (1979). Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain 6, 305–327. doi: 10.1016/0304-3959(79)90050-2
Lush, P., Botan, V., Scott, R. B., Seth, A. K., Ward, J., and Dienes, Z. (2020). Trait phenomenological control predicts experience of mirror synaesthesia and the rubber hand illusion. Nat. Commun. 11:4853. doi: 10.1038/s41467-020-18591-6
Lush, P., Seth, A. K., and Dienes, Z. (2021). Hypothesis awareness confounds asynchronous control conditions in indirect measures of the rubber hand illusion. R. Soc. Open Sci. 8:210911. doi: 10.1098/rsos.210911
Malt, E. A., Olafsson, S., Lund, A., and Ursin, H. (2002). Factors explaining variance in perceived pain in women with fibromyalgia. BMC Musculoskelet. Disord. 3:12. doi: 10.1186/1471-2474-3-12
Maravita, A., Spence, C., and Driver, J. (2003). Multisensory integration and the body schema: close to hand and within reach. Curr. Biol. 13, R531–R539. doi: 10.1016/S0960-9822(03)00449-4
Martínez, E., Aira, Z., Buesa, I., Aizpurua, I., Rada, D., and Azkue, J. J. (2018). Embodied pain in fibromyalgia: disturbed somatorepresentations and increased plasticity of the body schema. PLoS One 13:e0194534. doi: 10.1371/journal.pone.0194534
Martínez, E., Guillen, V., Buesa, I., and Azkue, J. J. (2019). A distorted body schema and susceptibility to experiencing anomalous somatosensory sensations in fibromyalgia syndrome. Clin. J. Pain 35, 887–893. doi: 10.1097/AJP.0000000000000754
Meireles, S. A., Antero, D. C., Kulczycki, M. M., and Skare, T. L. (2014). Prevalence of falls in fibromyalgia patients. Acta Ortop. Bras. 22, 163–166. doi: 10.1590/1413-78522014220300386
Menon, V., and Uddin, L. Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667. doi: 10.1007/s00429-010-0262-0
Monti, A., Porciello, G., Tieri, G., and Aglioti, S. M. (2020). The “embreathment” illusion highlights the role of breathing in corporeal awareness. J. Neurophysiol. 123, 420–427. doi: 10.1152/JN.00617.2019
Moriwaki, K., and Yuge, O. (1999). Topographical features of cutaneous tactile hypoesthetic and hyperesthetic abnormalities in chronic pain. Pain 81, 1–6. doi: 10.1016/S0304-3959(98)00257-7
Moro, V., Pacella, V., Scandola, M., Besharati, S., Rossato, E., Jenkinson, P., et al. (2021b). A fronto-insular-parietal network for the sense of body ownership. Research Square [Preprint] doi: 10.21203/rs.3.rs-428666/v1
Moro, V., Corbella, M., Ionta, S., Ferrari, F., and Scandola, M. (2021a). Cognitive training improves disconnected limbs’ mental representation and peripersonal space after spinal cord injury. Int. J. Environ. Res. Public Health 18:9589. doi: 10.3390/ijerph18189589
Moro, V., Pernigo, S., Tsakiris, M., Avesani, R., Edelstyn, N. M. J., Jenkinson, P. M., et al. (2016). Motor versus body awareness: voxel-based lesion analysis in anosognosia for hemiplegia and somatoparaphrenia following right hemisphere stroke. Cortex 83, 62–77. doi: 10.1016/j.cortex.2016.07.001
Moseley, G. L. (2004). Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial. Pain 108, 192–198. doi: 10.1016/j.pain.2004.01.006
Moseley, G. L., Gallace, A., and Spence, C. (2012). Bodily illusions in health and disease: physiological and clinical perspectives and the concept of a cortical “body matrix”. Neurosci. Biobehav. Rev. 36, 34–46. doi: 10.1016/j.neubiorev.2011.03.013
Motyka, P., and Litwin, P. (2019). Proprioceptive precision and degree of visuo-proprioceptive discrepancy do not influence the strength of the rubber hand illusion. Perception 48, 882–891. doi: 10.1177/0301006619865189
Nizzi, M. C., Demertzi, A., Gosseries, O., Bruno, M. A., Jouen, F., and Laureys, S. (2012). From armchair to wheelchair: how patients with a locked-in syndrome integrate bodily changes in experienced identity. Conscious. Cogn. 21, 431–437. doi: 10.1016/j.concog.2011.10.010
Osumi, M., Okuno, H., Nishigami, T., Ueta, K., and Morioka, S. (2015). Tactile localization training for pain, sensory disturbance, and distorted body image: a case study of complex regional pain syndrome. Neurocase 21, 628–634. doi: 10.1080/13554794.2014.961482
Pacella, V., Foulon, C., Jenkinson, P. M., Scandola, M., Bertagnoli, S., Avesani, R., et al. (2019). Anosognosia for hemiplegia as a tripartite disconnection syndrome. Elife 8:e46075. doi: 10.7554/eLife.46075
Parsons, L. M. (1994). Temporal and kinematic properties of motor behavior reflected in mentally simulated action. J. Exp. Psychol. Hum. Percept. Perform. 20, 709–730.
Pavani, F., and Zampini, M. (2007). The role of hand size in the fake-hand illusion paradigm. Perception 36, 1547–1554. doi: 10.1068/p5853
Pavani, F., Spence, C., and Driver, J. (2000). Visual capture of touch: out-of-the-body experiences with rubber gloves. Psychol. Sci. 11, 353–359. doi: 10.1111/1467-9280.00270
Pernigo, S., Moro, V., Avesani, R., Miatello, C., Urgesi, C., and Aglioti, S. M. (2012). Massive somatic deafferentation and motor deefferentation of the lower part of the body impair its visual recognition: a psychophysical study of patients with spinal cord injury. Eur. J. Neurosci. 36, 3509–3518. doi: 10.1111/j.1460-9568.2012.08266.x
Pleger, B., Ragert, P., Schwenkreis, P., Förster, A.-F., Wilimzig, C., Dinse, H., et al. (2006). Patterns of cortical reorganization parallel impaired tactile discrimination and pain intensity in complex regional pain syndrome. Neuroimage 32, 503–510. doi: 10.1016/j.neuroimage.2006.03.045
Ploner, M., Pollok, B., and Schnitzler, A. (2004). Pain facilitates tactile processing in human somatosensory cortices. J. Neurophysiol. 92, 1825–1829. doi: 10.1152/jn.00260.2004
Porciello, G., Bufalari, I., Minio-Paluello, I., Di Pace, E., and Aglioti, S. M. (2018). The ‘Enfacement’ illusion: a window on the plasticity of the self. Cortex 104, 261–275. doi: 10.1016/j.cortex.2018.01.007
R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rea, L. M., and Parker, R. A. (2014). Designing and Conducting Survey Research: A Comprehensive Guide. San Francisco, CA: John Wiley & Sons.
Roberts, R., Callow, N., Hardy, L., Markland, D., and Bringer, J. (2008). Movement imagery ability: development and assessment of a revised version of the vividness of movement imagery questionnaire. J. Sport Exerc. Psychol. 30, 200–21. doi: 10.1123/jsep.30.2.200
Romano, D., and Maravita, A. (2019). The dynamic nature of the sense of ownership after brain injury. Clues from asomatognosia and somatoparaphrenia. Neuropsychologia 132:107119. doi: 10.1016/j.neuropsychologia.2019.107119
Romano, D., Maravita, A., and Perugini, M. (2021). Psychometric properties of the embodiment scale for the rubber hand illusion and its relation with individual differences. Sci. Rep. 11:5029. doi: 10.1038/s41598-021-84595-x
Rost, S., Van Ryckeghem, D. M. L., Schulz, A., Crombez, G., and Vögele, C. (2017). Generalized hypervigilance in fibromyalgia: normal interoceptive accuracy, but reduced self-regulatory capacity. J. Psychosom. Res. 93, 48–54. doi: 10.1016/j.jpsychores.2016.12.003
Salvato, G., Peviani, V., Scarano, E., Scarpa, P., Leo, A., Redaelli, T., et al. (2017). Dissociation between preserved body structural description and impaired body image following a pediatric spinal trauma. Neurocase 23, 149–153. doi: 10.1080/13554794.2017.1332227
Sarzi-Puttini, P., Atzeni, F., Fiorini, T., Panni, B., Randisi, G., Turiel, M., et al. (2003). Validation of an Italian version of the fibromyalgia impact questionnaire (FIQ-I). Clin. Exp. Rheumatol. 21, 459–464.
Scandola, M., Aglioti, S. M., Avesani, R., Bertagnoni, G., Marangoni, A., and Moro, V. (2017a). Corporeal illusions in chronic spinal cord injuries. Conscious. Cogn. 49, 278–290. doi: 10.1016/j.concog.2017.01.010
Scandola, M., Aglioti, S. M., Pozeg, P., Avesani, R., and Moro, V. (2017b). Motor imagery in spinal cord injured people is modulated by somatotopic coding, perspective taking, and post-lesional chronic pain. J. Neuropsychol. 11, 305–326. doi: 10.1111/jnp.12098
Scandola, M., Tidoni, E., Avesani, R., Brunelli, G., Aglioti, S. M., and Moro, V. (2014). Rubber hand illusion induced by touching the face ipsilaterally to a deprived hand: evidence for plastic “somatotopic” remapping in tetraplegics. Front. Hum. Neurosci. 8:404. doi: 10.3389/fnhum.2014.00404
Scandola, M., Togni, R., Tieri, G., Avesani, R., Brambilla, M., Aglioti, S. M., et al. (2019b). Embodying their own wheelchair modifies extrapersonal space perception in people with spinal cord injury. Exp. Brain Res. 237, 2621–2632. doi: 10.1007/s00221-019-05618-8
Scandola, M., Dodoni, L., Lazzeri, G., Arcangeli, C. A., Avesani, R., Moro, V., et al. (2019a). Neurocognitive benefits of physiotherapy for spinal cord injury. J. Neurotrauma 36, 2028–2035. doi: 10.1089/neu.2018.6123
Schretlen, D. J., van der Hulst, E.-J., Pearlson, G. D., and Gordon, B. (2010). A neuropsychological study of personality: trait openness in relation to intelligence, fluency, and executive functioning. J. Clin. Exp. Neuropsychol. 32, 1068–1073. doi: 10.1080/13803391003689770
Schwoebel, J., and Coslett, H. B. (2005). Evidence for multiple, distinct representations of the human body. J. Cogn. Neurosci. 17, 543–553. doi: 10.1162/0898929053467587
Schwoebel, J., Friedman, R., Duda, N., and Coslett, H. B. (2001). Pain and the body schema: evidence for peripheral effects on mental representations of movement. Brain 124(Pt 10) 2098–2104. doi: 10.1093/brain/124.10.2098
Soler, D., Moriña, D., Kumru, H., Vidal, J., and Navarro, X. (2021). Transcranial direct current stimulation and visual illusion effect according to sensory phenotypes in patients with spinal cord injury and neuropathic pain. J. Pain 22, 86–96. doi: 10.1016/j.jpain.2020.06.004
Soler, M. D., Kumru, H., Pelayo, R., Vidal, J., Tormos, J. M., Fregni, F., et al. (2010). Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain 133, 2565–2577. doi: 10.1093/brain/awq184
Tajadura-Jiménez, A., and Tsakiris, M. (2014). Balancing the “inner” and the “outer” self: interoceptive sensitivity modulates self-other boundaries. J. Exp. Psychol. Gen. 143, 736–744. doi: 10.1037/a0033171
Teodoro, T., Edwards, M. J., and Isaacs, J. D. (2018). A unifying theory for cognitive abnormalities in functional neurological disorders, fibromyalgia and chronic fatigue syndrome: systematic review. J. Neurol. Neurosurg. Psychiatry 89, 1308–1319. doi: 10.1136/jnnp-2017-317823
Tieri, G., Tidoni, E., Pavone, E. F., and Aglioti, S. M. (2015b). Mere observation of body discontinuity affects perceived ownership and vicarious agency over a virtual hand. Exp. brain Res. 233, 1247–1259. doi: 10.1007/s00221-015-4202-3
Tieri, G., Tidoni, E., Pavone, E. F., and Aglioti, S. M. (2015a). Body visual discontinuity affects feeling of ownership and skin conductance responses. Sci. Rep. 5:17139. doi: 10.1038/srep17139
Todd, J., Cardellicchio, P., Swami, V., Cardini, F., and Aspell, J. E. (2021). Weaker implicit interoception is associated with more negative body image: evidence from gastric-alpha phase amplitude coupling and the heartbeat evoked potential. Cortex 143, 254–266. doi: 10.1016/j.cortex.2021.07.006
Tomczak, M., and Tomczak, E. (2014). The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 1, 19–25.
Tsakiris, M., and Haggard, P. (2005). The rubber hand illusion revisited: visuotactile integration and self-attribution. J. Exp. Psychol. Hum. Percept. Perform. 31, 80–91. doi: 10.1037/0096-1523.31.1.80
Tsakiris, M., Longo, M. R., and Haggard, P. (2010). Having a body versus moving your body: neural signatures of agency and body-ownership. Neuropsychologia 48, 2740–2749. doi: 10.1016/j.neuropsychologia.2010.05.021
Tsay, A., Allen, T. J., Proske, U., and Giummarra, M. J. (2015). Sensing the body in chronic pain: a review of psychophysical studies implicating altered body representation. Neurosci. Biobehav. Rev. 52, 221–232. doi: 10.1016/j.neubiorev.2015.03.004
Valenzuela-Moguillansky, C., O’Regan, J. K., and Petitmengin, C. (2013). Exploring the subjective experience of the “rubber hand” illusion. Front. Hum. Neurosci. 7:659. doi: 10.3389/fnhum.2013.00659
Valenzuela-Moguillansky, C., Reyes-Reyes, A., and Gaete, M. I. (2017). Exteroceptive and interoceptive body-self awareness in fibromyalgia patients. Front. Hum. Neurosci. 11:117. doi: 10.3389/fnhum.2017.00117
van Ettinger-Veenstra, H., Boehme, R., Ghafouri, B., Olausson, H., Wicksell, R. K., and Gerdle, B. (2020). Exploration of functional connectivity changes previously reported in fibromyalgia and their relation to psychological distress and pain measures. J. Clin. Med. 9:3560. doi: 10.3390/jcm9113560
Wolfe, F. (2003). Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J. Rheumatol. 30, 369–378.
Wolfe, F., Brähler, E., Hinz, A., and Häuser, W. (2013). Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis Care Res. 65, 777–785. doi: 10.1002/acr.21931
Wolfe, F., Clauw, D. J., Fitzcharles, M. A., Goldenberg, D. L., Häuser, W., Katz, R. L., et al. (2016). 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 46, 319–329. doi: 10.1016/j.semarthrit.2016.08.012
Keywords: body representations, chronic pain, chronic pain and fibromyalgia, disturbed sense of ownership, action representation, bodily self, anxiety and depression
Citation: Scandola M, Pietroni G, Landuzzi G, Polati E, Schweiger V and Moro V (2022) Bodily Illusions and Motor Imagery in Fibromyalgia. Front. Hum. Neurosci. 15:798912. doi: 10.3389/fnhum.2021.798912
Received: 20 October 2021; Accepted: 13 December 2021;
Published: 20 January 2022.
Edited by:
Silvia Serino, Catholic University of the Sacred Heart, ItalyReviewed by:
Enrica Laura Santarcangelo, University of Pisa, ItalyDomna Banakou, University of Barcelona, Spain
Copyright © 2022 Scandola, Pietroni, Landuzzi, Polati, Schweiger and Moro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michele Scandola, bWljaGVsZS5zY2FuZG9sYUB1bml2ci5pdA==; Valentina Moro, dmFsZW50aW5hLm1vcm9AdW5pdnIuaXQ=
 Michele Scandola
Michele Scandola Giorgia Pietroni1
Giorgia Pietroni1 Valentina Moro
Valentina Moro