
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Hum. Neurosci., 16 February 2022
Sec. Brain Imaging and Stimulation
Volume 15 - 2021 | https://doi.org/10.3389/fnhum.2021.743846
This article is part of the Research TopicNeural Bases of Neurological and Psychiatric Disorders and Their Neuromodulation TreatmentsView all 36 articles
Background and Objective: There is vast published literature proposing repetitive transcranial magnetic stimulation (rTMS) technology on the motor cortex (M1) for the treatment of neuropathic pain (NP). Systematic reviews (SRs) focus on a specific problem and do not provide a comprehensive overview of a research area. This study aimed to summarize and analyze the evidence of rTMS on the M1 for NP treatment through a new synthesis method called evidence mapping.
Methods: Searches were conducted in PubMed, EMBASE, Epistemonikos, and The Cochrane Library to identify the studies that summarized the effectiveness of rTMS for NP. The study type was restricted to SRs with or without meta-analysis. All literature published before January 23, 2021, was included. Two reviewers independently screened the literature, assessed the methodological quality, and extracted the data. The methodological quality of the included SRs was assessed by using the A Measurement Tool to Assess Systematic Reviews (AMSTAR-2). Data were extracted following a defined population, intervention, comparison, and outcome (PICO) framework from primary studies that included SRs. The same PICO was categorized into PICOs according to interventions [frequency, number of sessions (short: 1–5 sessions, medium: 5–10 sessions, and long: >10 sessions)] and compared. The evidence map was presented in tables and a bubble plot.
Results: A total of 38 SRs met the eligibility criteria. After duplicate primary studies were removed, these reviews included 70 primary studies that met the scope of evidence mapping. According to the AMSTAR-2 assessment, the quality of the included SRs was critically low. Of these studies, 34 SRs scored “critically low” in terms of methodological quality, 2 SR scored “low,” 1 SR scored “moderate,” and 1 SR scored “high.”
Conclusion: Evidence mapping is a useful methodology to provide a comprehensive and reliable overview of studies on rTMS for NP. Evidence mapping also shows that further investigations are necessary to highlight the optimal stimulation protocols and standardize all parameters to fill the evidence gaps of rTMS. Given that the methodological quality of most included SRs was “critically low,” further investigations are advised to improve the methodological quality and the reporting process of SRs.
Neuropathic pain (NP) is an ongoing and challenging condition due to its high morbidity rate of 7–10% in the general population; it usually results from lesions in the somatosensory nervous system, including the peripheral or central nervous system (Colloca et al., 2017). NP negatively impacts patients’ quality of life by reducing functional mobility, activities of daily living, and participation in social roles, which may lead to psychological problems (Kalia and O’Connor, 2005; Gromisch et al., 2020). The initial treatment applied to NP is generally pharmacotherapy, such as use of antidepressants, anticonvulsants, and opioids (Finnerup et al., 2015). However, even with complex treatment regimens, the results of pharmacological approaches remain unsatisfactory, and some may lead to adverse events, such as toxicity, gastrointestinal events, or increased risk of addiction or drug abuse (Papanas and Ziegler, 2016; Urits et al., 2019). Therefore, non-pharmacological interventions, which are considered safe and effective, have been used to treat NP. Repetitive transcranial magnetic stimulation (rTMS) technology is widely accepted at present as a non-pharmacological intervention for treating NP.
The rTMS technique uses a transient high-intensity magnetic field acting on the cerebral cortex to generate induced currents. It alters the action potential of cortical nerve cells, depolarizes neurons in the targeted brain region, and ultimately leads to neuroplastic changes (Paulus et al., 2013). Stimulation target, frequency, and number of sessions are considered critical variables for analgesic efficacy. In terms of stimulation target, the primary motor cortex (M1) is the most commonly used target of stimulation for clinical treatment and has been the most extensively studied. In the 2020 guidelines for rTMS (Lefaucheur et al., 2020), the M1 was recommended as Level A evidence (definitive efficacy) for the treatment of NP. However, clinical promotion is still limited to some extent due to the heterogeneity of treatment protocols, such as frequency and sessions, and effectiveness among various studies. The European Society of Neurology encourages studies to collect and summarize evidence on the factors affecting these techniques (Cruccu et al., 2016). SRs are a common method for synthesizing research evidence. Nonetheless, SRs tend to address more specific research and practice questions and cannot provide a comprehensive overview of rTMS for NP. For example, the following research gaps are unknown: (1) SRs focus on a specific stimulation type or specific pain type (such as pain after spinal cord injury, post-stroke, and diabetic neuropathy), while research on other pain types needs to be developed. (2) The frequency, duration, and other parameters of interventions collected by the SRs varied, and the large amount of evidence with a lack of summarization and classification may lead to clinicians’ confusion. (3) Differences in the quality between individual trials and SRs contributed to the heterogeneity of the evidence. The same primary study may be included in different SRs, which may yield various conclusions due to varying inclusion criteria.
A novel approach to evidence synthesis research called evidence mapping (Grant and Booth, 2009; Haddaway et al., 2016; Miake-Lye et al., 2016) has been developed. Evidence mapping is designed to provide an overview of a research area by including published SRs. Evidence mapping uses published SRs as units of analysis. In the population, intervention, control, and outcome (PICO) framework, evidence mapping extracts and categorizes these data from primary studies, which are included in the SRs. On the basis of the classification criteria, the obtained PICO is integrated into different PICOs, and the contribution of the number of primary studies related to that classification is also calculated to summarize the current interventions (Petersen et al., 2015). The characteristic of the evidence mapping method is to overcome the limitations of primary studies by using the selection of studies, effect size analysis, and bias evaluation of SRs. Considering that the quality of SRs affects the credibility of the evidence, the same primary studies may be included in SRs of different quality. Various conclusions may be drawn due to different inclusion criteria, such as random and double-blind bias. Therefore, evidence mapping uses AMSTAR-2 to evaluate the quality of SRs and the credibility of the results of SRs (Ballesteros et al., 2017; Madera Anaya et al., 2019). Evidence mapping can be translated into two visual products, namely, tables (general information tables and study-specific characteristic tables) and bubble plots (multidimensional composite presentation of classification criteria, quantity, and quality of evidence), which also provide a descriptive narrative summary of the results (Bragge et al., 2011; Haddaway et al., 2016).
Evidence mapping aims to summarize, identify, and analyze the current available evidence in SRs regarding rTMS on M1 for NP. Collecting and integrating data from primary studies on the basis of SRs provide breadth of evidence. Assessing the quality of SRs provides strength of evidence. This information is provided in a user-friendly manner that helps identify research gaps and assist evidence users in the decision-making process.
Evidence was mapped on the basis of the methodology proposed by Global Evidence Mapping (Bragge et al., 2011). The study process was divided into four phases (Figure 1: Core tasks performed to map evidence).
Studies and guidelines related to NP were referred, and an expert with research background in NP was consulted to frame the evidence map. With the help of the expert, the specific terminology of the search strategy was confirmed and the possible evidence users (pain, neurology, psychiatry, anesthesiology, and rehabilitation) involved were discussed. On the basis of the above information, the eligibility criteria have been established for inclusion in the study. Studies containing rTMS for NP were considered eligible. Studies on patients with NP were included, whereas experimental subjects that were animals or healthy people were excluded. The intervention should be rTMS, and the comparison could be rTMS, sham rTMS, other treatments of relieving pain, or no treatment. The outcome should be pain measured with various clinically validated tools [e.g., Visual Analog Scale (VAS), Numerical Rating Scale (NRS), Short-Form McGill Pain Questionnaire, and Brief Pain Inventory]. Studies that did not address intervention outcomes, such as those that aimed to explore NP-related pathophysiology and focus on cost-effectiveness, were excluded. Studies that reported other outcomes (e.g., fatigue, motor function, spasticity, sensory function, and cognition) but pain were also excluded. Only SRs (with or without meta-analysis) were included as they provided reliable evidence.
We conducted searches of systematic literature on PubMed, EMBASE, Epistemonikos, and The Cochrane Library Published before January 23, 2021. Medical subject headings (mesh terms), free-text terms, and synonymous terms available for NP and transcranial magnetic stimulation, such as “neuralgia,” “neurodynia,” “atypical neuralgia,” “nerve pain,” and “stump neuralgia,” were combined. Literature published in non-English languages was excluded. References of the relevant studies that met the inclusion criteria were added to potential additional reviews. The details of the search strategies are reported in Supplementary Material 1. EndNote (version X9) was applied to manage the search results. Duplicate SRs were removed, and two reviewers (YZa and XL) independently screened the titles and abstracts to exclude irrelevant studies. Full-text studies were obtained and reviewed to make a terminal decision. Any disagreements in the decision-making process were resolved through negotiation or by discussion with a third reviewer (YoZ).
A data extraction table was designed to record the main characteristics and compare the methodological differences of the included SRs. Two authors (YZa and XL) assessed the methodological quality and extracted data independently. Any difference of opinions was discussed with the third author (YoZ). The original authors were contacted for missing information when necessary. Data were grouped into three categories:
(a) The Assessment Methodological Quality for Systematic Reviews (AMSTAR-2) was used to assess the methodological quality of SRs (Shea et al., 2017). AMSTAR-2 is a practical tool used to assess the quality of SRs that include randomized or non-randomized studies of healthcare interventions or both. It has 16 items, with an overall rating based on weaknesses in critical domains (items: 2, 4, 7, 9, 11, 13, and 15). In brief, the evaluation results of the SRs are generally divided into the following four categories: “High,” no critical weakness and no more than one non-critical weakness; “Moderate,” no critical weakness and more than one non-critical weakness; “Low,” one critical flaw with or without non-critical weaknesses; and “Critically low,” more than one critical flaw with or without non-critical weaknesses.
(b) The following characteristics of SRs were extracted: authors, years of publication, types of SR (with or without meta-analysis), objectives, dates of search, sample sizes, designs, and numbers of included studies.
(c) The PICO framework was used to extract data from primary studies included in the SRs. The four key components are populations, intervention, comparison, and outcomes. Details including population characteristics (e.g., NP related to spinal cord injury, post-stroke symptoms, and complex regional pain syndrome), interventions (e.g., target area, frequency, and intensity of transcranial magnetic stimulation), comparative measures (e.g., placebo and sham stimulation), and outcomes were extracted. The obtained data of PICO from primary studies in SRs were classified into different groups based on similar characteristics (population; interventions: frequency, session, and intensity; control group; and outcome scale).
For descriptive purposes, the effect of rTMS on NP reported by the SR authors was grouped into the following five categories on the basis of the previously reported criteria: “Potentially better,” the conclusions reported rTMS as more beneficial than the control group; “Mixed results,” the same primary study had different findings among different studies (e.g., some studies found no difference in rTMS compared with the control group in the same population, whereas others found potential benefits of transcranial magnetic stimulation over the control group); “Unclear,” insufficient evidence to draw definitive conclusions about the effectiveness of rTMS on pain; “No difference,” the conclusions provided evidence of no difference between intervention and control; and “Potentially worse,” the conclusions reported TMS as less beneficial than the control group. The same primary study may be included in multiple SRs. If the primary study has multiple consistent results, it would be added to the appropriate group, and multiple conflicting findings were included in the “Mixed Results” group (Miake-Lye et al., 2019).
Each clinical question addressed in each included review was adapted into a PICO format that specified the types of participants, interventions (or comparison), and outcomes. Evidence mapping allows the reader to visualize any gaps in the literature base, with results presented in the form of tables and graphs.
(a) The basic characteristics, quality assessment of the included SRs, and characteristics of all integrated PICOs were described in tables.
(b) A heat map was used to present the quality of the included SRs.
(c) Graphical display was provided through bubble plots. The bubble plot displayed information in four dimensions: (1) bubble size (number of articles), the size of each bubble is proportional to the number of individual trials included in the SRs; (2) bubble color (research characteristics), bubbles labeled with different colors indicate different PICOs; (3) X-axis (effect of TMS on NP), the classification of authors’ conclusions represented on the X-axis (“potentially better,” “mixed results,” “unclear,” “no difference,” and “worse”); and (4) Y-axis (AMSTAR-2 evaluation results), four different colors were used to indicate study quality, with red indicating critically low, orange indicating low, yellow indicating moderate, and green indicating high quality.
This retrieval yielded 125 records. Another 11 articles were added from the references that met the inclusion criteria. After duplicates were removed, 97 articles remained for screening of the titles and abstracts. Subsequently, 44 articles were excluded after the screening. In the remaining 53 articles, 13 were excluded after full-text reviews. Finally, 38 articles met the eligibility criteria (Figure 2). The list of excluded studies along with exclusion rationales can be found in Supplementary Material 2.
According to the AMSTAR-2 criteria, 34 SRs scored “critically low,” 2 SR scored “low,” 1 SR scored “moderate,” and 1 SR scored “high” in terms of methodological quality (Figure 3). The most frequent drawbacks were as follows: no mention of the protocol in the systematic overview, no description of the rationale for the study designs included in the review, no report of excluded studies or reasons for exclusion, and no statement of funding for the included studies. The detailed assessment process is provided in Supplementary Material 3.
Table 1 shows the characteristics of the included SRs. All SRs were published between 2009 and 2020. Among the 38 included SRs, 17 SRs conducted a meta-analysis. The number of included studies ranged from 3 to 131, and they were conducted between 2001 and 2020. Each SR included patients ranging from 97 to 15,776. Six SRs did not report or incompletely reported the designs of the included individual studies. All studies reported study designs, and a total of 678 randomized controlled trials (RCTs) accounted for 86% of the included studies in all SRs. Of all SRs, 18 SRs included only RCTs, 15 SRs included patients with NP with different underlying causes, and 25 were exclusively conducted on NP with specific etiologies or due to a single disease. Six SRs included pain after spinal cord injury (SCI), another 5 SRs included central post-stroke pain (P), 3 SRs included phantom limb pain (PLP), 2 SRs included migraine, 2 SRs included complex regional pain syndrome (CRPS), 2 SRs included headache, 1 SR included diabetic peripheral neuropathy (DPN), 1 SR included multiple sclerosis (MS), and 1 SR included orofacial pain (OFP). As for the intervention, 13 SRs only assessed rTMS, 12 SRs assessed other non-invasive stimulations, 3 SRs assessed neuromodulation techniques, 4 SRs assessed non-pharmacological interventions, 5 SRs assessed pharmacological and non-pharmacological management of NP, and 1 SR assessed non-invasive brain stimulation combined with exercise.
After merging the duplicate primary studies included in the 38 SRs, 70 primary studies were integrated into 19 PICO groups based on the PICO characteristics. Studies that did not provide the mandatory parameter information were not included in the PICOs. Among the included SRs, populations with NP were from various diseases and etiologies, and the treatment protocols adopted various parameters, including frequency, sessions, and pulses. Sham stimulation or placebo was the most common intervention in the control group of rTMS on the M1. The primary outcome of the included studies was self-reported subjective nociception. VAS and NRS were the most commonly validated pain assessment scales. The details of the characteristics are enumerated in Supplementary Material 4.
As a result of unavoidable heterogeneity of the rTMS protocol among studies, classifying and categorizing all parameters can be difficult. Thus, the classification of PICO focused on interventions and comparison, as well as the population involved and outcome assessments, as presented in Table 2. We use Figure 4 to explain the connection between the bubble polt and Table 2. In terms of interventions, we classified them to frequency and session of rTMS, as they have been shown to influence the analgesic effects and are identified as clinically significant factors (Ahmed et al., 2012; O’Connell et al., 2018; Gatzinsky et al., 2020; Pacheco-Barrios et al., 2020). High and low frequency of rTMS can induce transient excitatory and inhibitory effects, respectively (Klein et al., 2015). Sessions of rTMS are considered an important factor in maintaining the effects. The characteristics of the interventions were categorized based on frequency (low or high frequency) and number of sessions (short: 1–5 sessions, medium: 5–10 sessions, and long: >10 sessions).
The key characteristics of PICOs are listed in Supplementary Material 5. A total of 19 PICOs were categorized based on stimulation target, frequency, and session (short, medium, and long). On the basis of the stimulation target, 17 PICOs used high-frequency rTMS (>1 Hz), and 2 used low-frequency rTMS (<1 Hz). In terms of the number of sessions, 1–5 sessions were considered short sessions, 6–10 were medium sessions, and more than 10 were regarded as long sessions. Twelve PICOs had short sessions, three had medium sessions, and four had long sessions. In addition, 12 PICOs used the same sessions of sham stimulation or placebo as a control to study the effectiveness of rTMS in patients with NP. Two PICOs studied the effects of different sessions of rTMS, while other PICOs involved different stimulation areas: M1 unilateral stimulation versus bilateral stimulation, rTMS compared with botulinum toxin injection, rTMS compared with transcranial direct current stimulation (tDCS), or rTMS combined with theta-burst stimulation. The PICOs were concentrated in the following characteristics: 20 Hz, short-term sessions versus sham stimulation (11 PICOs), and 10 Hz, short-term sessions versus sham stimulation (18 PICOs).
The evidence map of rTMS for NP is presented in Figure 5. The bubble diagram is a visual display of data represented in Supplementary Material 5. We integrated similar intervention characteristics from primary studies into PICOs. In the bubble chart, different colors indicate varying PICOs. Each bubble was plotted in accordance with the conclusion of the effect of rTMS on NP (X-axis) and the quality of the related SRs (Y-axis), while the size of bubbles represented the number of primary studies included in PICOs. The evidence tables (Supplementary Material 5) provided details of the included SRs (Supplementary Material 4). Some primary studies may be included in multiple SRs. If SRs synthesized different conclusions for the same primary study, the same PICOs would appear in different classifications on the X-axis. If the same primary study was included by SRs of different quality, the same PICOs would appear in different classifications on the Y-axis. Evidence mapping showed that 5–20 Hz, high-frequency rTMS of M1 with short (1–5), medium (6–10), or long (>10) sessions usually lead to “potentially better” treatment effects compared with sham stimulation, although some had transient effects. By contrast, the synthesis results for the lower frequencies (1 and 0.5 Hz) showed either no difference or mixed effects. Thirteen PICOs included 52 primary studies rated as “potentially better,” and four of these PICOs involved 13 primary studies that were also included in a high-quality meta-analysis. In accordance with the AMSTAR-2 quality assessment, the interventions in these four PICOs were considered beneficial in most cases. Nine PICOs included 18 primary studies with different findings in different SRs and were rated as “mixed,” implying that the interventions in these eight PICOs had limited confidence in the effect estimates, and the true effect may be different from the study reports (Miake-Lye et al., 2019). One PICO conclusion was rated as “unclear” because its effect was not reported in the SR (Yang and Chang, 2020) with a critically low quality. Eight PICOs included 17 primary studies that concluded that rTMS showed no difference compared with controls. Of these, six studies showed a potentially better effect of rTMS in short-term follow-up but no difference during long-term follow up (Supplementary Material 4 and Supplementary Material 5). After studies that were ineffective during follow-up were excluded, 8 of 11 primary studies were also included by a high-quality meta-analysis. This finding indicated less effectiveness of these intervention protocols or inapplicability to a particular NP, and the treatment effects could be uncertain. Two PICOs included two primary studies that showed a “potentially worse” conclusion.
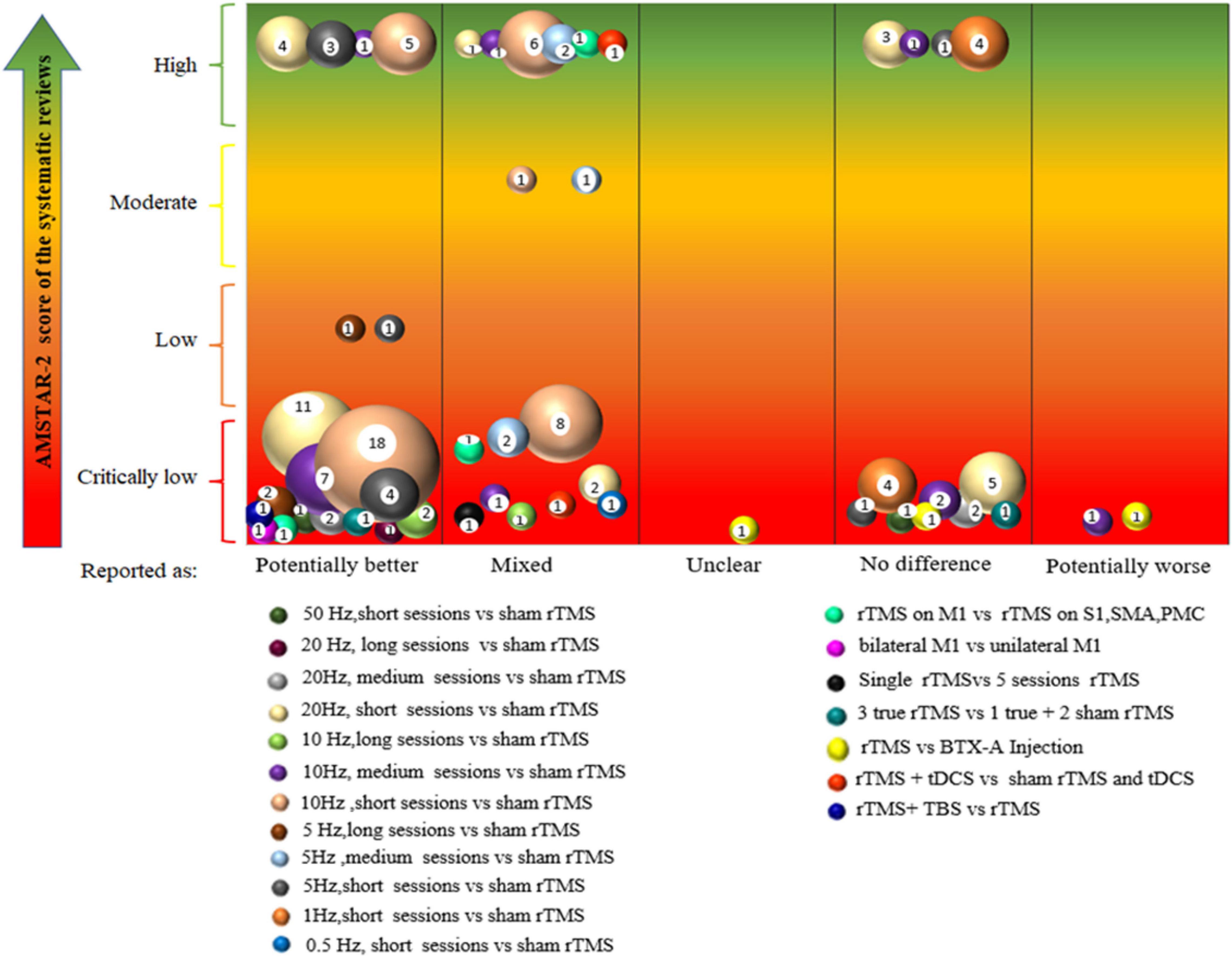
Figure 5. Evidence mapping of the rTMS on neuropathic pain (NP). short, 1–5 sessions, medium, 5–10 sessions, long, >10 sessions. rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; TBS, theta-burst stimulation; M1,motor cortex, S1, primary somatosensory cortex; SMA, supplementary motor cortex; PMC, premotor cortex; BTX-A, botulinum toxin type A.
This evidence map included 40 SRs, and the majority of the primary studies included were RCTs, which is the best study design to assess the effectiveness of interventions (Sylvester et al., 2017). Evidence mapping provided a broad overview of the available evidence of rTMS on NP, showing the focus and counting contributions of available studies by categorizing and generalizing them to help interpret the published SRs. (1) Research gaps: The included SRs covered most types of NP, including SCI, CPSP, CRPS, PLP, DNP, and headache; however, this left an evidence gap of the rTMS for some specific types of NP, such as postherpetic pain, radiculopathy pain, trigeminal neuralgia, post-traumatic brain injury pain, and cancer-related NP. In addition, the control groups were mostly given sham stimulation. Open questions about the effectiveness of rTMS associated with other therapies (such as pharmacotherapy, neurorehabilitation, and psychotherapy) are recommended. Future SRs are needed to analyze immediate, short-term, and long-term effects, which may help clarify the sessions of rTMS. Stimulation parameters, namely, frequency and intensity variable time, are also the direction for further research. (2) Summarization and classification of evidence: Evidence mapping showed that 5–20 Hz, high-frequency rTMS of M1 with short (1–5), medium (6–10), or long (>10) sessions usually lead to a “potentially better” conclusion compared with sham stimulation, suggesting that these interventions are beneficial in most cases. By contrast, the synthesis results for the low frequencies (1 and 0.5 Hz) showed no difference or were mixed, meaning these intervention protocols may be less effective or inappropriate for some specific NPs. (3) The impact of the quality of the SRs on the strength of evidence: Some PICOs from high-quality SRs drew a potentially better conclusion, suggesting that these interventions were beneficial in most cases. Similarly, some PICOs from high-quality SRs did not show any difference in the conclusion, indicating that these interventions may be less effective or inappropriate for some specific NPs. This evidence map is not intended to replace any clinical protocol or guidelines, nor is it intended to provide a standardized protocol. Therefore, the clinical diagnosis of each patient, the existing alternatives, cost-effectiveness, available resources, and other factors must be carefully considered before offering any recommendation (Nussbaumer-Streit et al., 2018).
By comparing the results of the SRs, the same PICOs obtained from SRs were presented with different conclusions in the evidence map. The possible reasons were as follows: (1) some studies that reported different sessions had varied effects of rTMS. For example, a good outcome could be found in the short term, but not in the long term, that led to a mixed conclusion. Future SRs should focus on follow-up and explore the long-term effectiveness of the intervention. (2) Some SRs conducted meta-analysis but some did not. Qualitative studies may arrive at conclusions different from those in quantitative studies. For example, a primary study by Onesti et al. (2013) and meta-analysis of Zeng et al. (2020) had a “no difference” conclusion, while the review of Yang and Chang (2020), which only mentioned pain relief, showed a “potentially better” conclusion. The final conclusion in the evidence map of Onesti et al.’s study was “mixed.” (3) Populations with different diseases that cause NP could cause heterogeneity. Previous studies have indicated that patients with orofacial pain have a better analgesic response than those with CPSP, SCI, or BPL (Lefaucheur et al., 2004). For example, a primary study on patients with CRPS (Picarelli et al., 2010) by Chang et al. (2020) and another one involving chronic pain by O’Connell et al. (2018) did not reach the same conclusion. This gap could also be a caveat to provide more reliable and reproducible data. Future studies must consider including more homogeneous groups of participants or stratifying patients in accordance with clinical characteristics and underlying pathogenesis.
If different SRs with varied conclusions included some primary studies that were overlapping and some unique studies, future investigations could synthesize studies that were included in these reviews and find new outcomes including all potential evidence. From the “mixed” results, future investigations could focus on comparing different stimulation protocols (doses, sessions, variable time, and intervals among sessions) of rTMS on NP.
We evaluated the quality of SRs and the credibility of the results of SRs with a new version of AMSTAR. Compared with the previous version, AMSTAR-2 was adapted to include SRs on the basis of RCTs or non-randomized intervention studies or both and provide more refined and rigorous evaluation item criteria. Assessment in this field suggested room for improving SRs’ quality. Future SRs should place more emphasis on the following domains to improve the quality of studies and the validity of the results: reporting an explicit statement about the description of the methodology prior to conducting the review; any significant deviations from the protocol should be justified; explaining the selection of the study designs for inclusion in the review; providing a list of excluded studies and justifying the exclusions; indicating the sources of funding or support for the individual studies included in the SRs; and interpreting or discussing the effect of the risk of bias in individual studies on the total effect.
This evidence map may be the first on rTMS for NP. Evidence mapping is a relatively new tool for the synthesis of available evidence, so we explain the methodology and results in more detail compared with published evidence maps and provide all the data of our study process to facilitate the reader’s understanding and use. To avoid selection and data extraction bias, we constructed a rich search string for retrieving from four different databases. In addition, the reference lists of the selected studies were manually scanned for the detection of additional relevant studies, minimizing the risk of missing relevant studies. Study selection and data extraction were made via a double confirmation. Two authors conducted the process of selecting and extracting separately from one another, and any disagreements were then discussed with a third researcher until a final agreement was reached. Furthermore, results were mapped using various graphs, such as bubble plots, heat maps, and tables, which helped improve traceability between the extracted data and the conclusion.
Certain limitations in this evidence map should be considered. First, the map only chose studies published in English, which limited the scope of evidence mapping. Second, given that only SRs were included as a source of evidence, some studies, such as newly published or studies included in these SRs, could have been missed. Third, the methodologies of some SRs had limitations. Furthermore, several different types of studies in SRs comparing therapeutic interventions for NP were included. Although most trials were RCTs, some case reports and observational, open-label, and cohort studies were also available. Finally, evidence mapping synthesized the SRs as a unit rather than individual studies, which could lead to some primary studies being repeated.
Neuropathic pain is a complex and refractory group of diseases. Evidence mapping showed that rTMS, as a compliant and safety neuromodulation treatment, is promising for the treatment of NP. Evidence mapping could encourage clinicians and professionals involved in related areas, such as pain, neurology, psychiatry, and anesthesiology, to pay more attention to non-pharmacological treatments on patients with NPs, especially those with drug resistance. Evidence mapping is a useful and reliable method to identify the currently available evidence on therapeutic interventions and pinpoint gaps to suggest future research. In the future, when designing treatment protocols, rehabilitation practitioners are recommended to consider the duration and sessions of rTMS. More research efforts are needed to highlight the optimal stimulation protocols and standardize all parameters to fill evidence gaps, and more homogeneous groups of participants should be considered. Meanwhile, as the methodological quality of most included SRs scored “critically low,” further efforts are needed to improve the methodological quality and reporting process of SRs.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
YZa, YoZ, and YiZ were designed the study. YZa and XL were collected the screening studies, extraction data and charted under the guidance of YoZ. YZa and YoZ analyzed the data and drafted the manuscript. YiZ reviewed the results. SG, YY, JG, and YiZ revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
This work was supported by National Natural Science Foundation of China Project (No. 81860875).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2021.743846/full#supplementary-material
Aamir, A., Girach, A., Sarrigiannis, P. G., Hadjivassiliou, M., Paladini, A., Varrassi, G., et al. (2020). Repetitive magnetic stimulation for the management of peripheral neuropathic pain: a systematic review. Adv. Ther. 37, 998–1012. doi: 10.1007/s12325-020-01231-2
Ahmed, M. A., Darwish, E. S., Khedr, E. M., El Serogy, Y. M., and Ali, A. M. (2012). Effects of low versus high frequencies of repetitive transcranial magnetic stimulation on cognitive function and cortical excitability in Alzheimer’s dementia. J. Neurol. 259, 83–92. doi: 10.1007/s00415-011-6128-4
Ahmed, M. A., Mohamed, S. A., and Sayed, D. (2011). Long-term antalgic effects of repetitive transcranial magnetic stimulation of motor cortex and serum beta-endorphin in patients with phantom pain. Neurol. Res. 33, 953–958. doi: 10.1179/1743132811Y.0000000045
Akyuz, G., and Giray, E. (2019). Noninvasive neuromodulation techniques for the management of phantom limb pain: a systematic review of randomized controlled trials. Int. J. Rehabil. Res. 42, 1–10. doi: 10.1097/mrr.0000000000000317
Ambriz-Tututi, M., Alvarado-Reynoso, B., and Drucker-Colin, R. (2016). Analgesic effect of repetitive transcranial magnetic stimulation (rTMS) in patients with chronic low back pain. Bioelectromagnetics 37, 527–535. doi: 10.1002/bem.22001
Andre-Obadia, N., Magnin, M., and Garcia-Larrea, L. (2011). On the importance of placebo timing in rTMS studies for pain relief. Pain 152, 1233–1237. doi: 10.1016/j.pain.2010.12.027
Andre-Obadia, N., Magnin, M., Simon, E., and Garcia-Larrea, L. (2018). Somatotopic effects of rTMS in neuropathic pain? A comparison between stimulation over hand and face motor areas. Eur. J. Pain 22, 707–715. doi: 10.1002/ejp.1156
Andre-Obadia, N., Mertens, P., Gueguen, A., Peyron, R., and Garcia-Larrea, L. (2008). Pain relief by rTMS: differential effect of current flow but no specific action on pain subtypes. Neurology 71, 833–840. doi: 10.1212/01.wnl.0000325481.61471.f0
Andre-Obadia, N., Mertens, P., Lelekov-Boissard, T., Afif, A., Magnin, M., Garcia-Larrea, L., et al. (2014). Is Life better after motor cortex stimulation for pain control?Results at long-term and their prediction by preoperative rTMS. Pain Phys. 17, 53–62. doi: 10.36076/ppj.2014/17/53
Andre-Obadia, N., Peyron, R., Mertens, P., Mauguière, F., Laurent, B., and Garcia-Larrea, L. (2006). Transcranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin. Neurophysiol. 117, 1536–1544. doi: 10.1016/j.clinph.2006.03.025
Attal, N., Ayache, S. S., De Andrade, D. C., Mhalla, A., Baudic, S., and Jazat, F. (2016). Repetitive transcranial magnetic stimulation and transcranial direct-current stimulation in neuropathic pain due to radiculopathy: a randomized sham-controlled comparative study. Pain 157, 1224–1231. doi: 10.1097/j.pain.0000000000000510
Ayache, S. S., Ahdab, R., Chalah, M. A., Farhat, W. H., Mylius, V., Goujon, C., et al. (2016). Analgesic effects of navigated motor cortex rTMS in patients with chronic neuropathic pain. Eur. J. Pain 20, 1413–1422. doi: 10.1002/ejp.864
Ballesteros, M., Montero, N., López-Pousa, A., Urrútia, G., Solà, I., Rada, G., et al. (2017). Evidence mapping based on systematic reviews of therapeutic interventions for gastrointestinal stromal tumors (GIST). BMC Med. Res. Methodol. 17:135. doi: 10.1186/s12874-017-0402-9
Boldt, I., Eriks-Hoogland, I., Brinkhof, M. W. G., de Bie, R., Joggi, D., and von Elm, E. (2014). Non-pharmacological interventions for chronic pain in people with spinal cord injury. Cochrane Database of Syst. Rev. 28:CD009177. doi: 10.1002/14651858.CD009177.pub2
Bragge, P., Clavisi, O., Turner, T., Tavender, E., Collie, A., and Gruen, R. L. (2011). The Global Evidence Mapping Initiative: scoping research in broad topic areas. BMC Med. Res. Methodol. 11:92. doi: 10.1186/1471-2288-11-92
Cardenas-Rojas, A., Pacheco-Barrios, K., Giannoni-Luza, S., Rivera-Torrejon, O., and Fregni, F. (2020). Noninvasive brain stimulation combined with exercise in chronic pain: a systematic review and meta-analysis. Exp. Rev. Neurother. 20, 401–412. doi: 10.1080/14737175.2020.1738927
Cervigni, M., Onesti, E., Ceccanti, M., Gori, M. C., Tartaglia, G., Campagna, G., et al. (2018). Repetitive transcranial magnetic stimulation for chronic neuropathic pain in patients with bladder pain syndrome/interstitial cystitis. Neurourol. Urodyn. 37, 2678–2687. doi: 10.1002/nau.23718
Chang, M. C., Kwak, S. G., and Park, D. (2020). The effect of rTMS in the management of pain associated with CRPS. Transl. Neurosci. 11, 363–370. doi: 10.1515/tnsci-2020-0120
Chen, C. C., Chuang, Y. F., Huang, A. C., Chen, C. K., and Chang, Y. J. (2016). The antalgic effects of non-invasive physical modalities on central post-stroke pain: a systematic review. J. Phys. Ther. Sci. 28, 1368–1373. doi: 10.1589/jpts.28.1368
Choi, G. S., and Chang, M. C. (2018). Effects of high-frequency repetitive transcranial magnetic stimulation on reducing hemiplegic shoulder pain in patients with chronic stoke: a randomized controlled trial. Int. J. Neurosci. 128, 110–116. doi: 10.1080/00207454.2017.1367682
Choi, G. S., Kwak, S. G., Lee, H. D., and Chang, M. C. (2018). Effect of high-frequency repetitive transcranial magnetic stimulation on chronic central pain after mild traumatic brain injury: a pilot study. J. Rehabil. Med. 50, 246–252. doi: 10.2340/16501977-2321
Colloca, L., Ludman, T., Bouhassira, D., Baron, R., Dickenson, A. H., Yarnitsky, D., et al. (2017). Neuropathic pain. Nat. Rev. Dis. Primers. 3:17002. doi: 10.1038/nrdp.2017.2
Cossins, L., Okell, R. W., Cameron, H., Simpson, B., Poole, H. M., and Goebel, A. (2013). Treatment of complex regional pain syndrome in adults: a systematic review of randomized controlled trials published from June 2000 to February 2012. Eur. J. Pain 17, 158–173. doi: 10.1002/j.1532-2149.2012.00217.x
Cragg, J. J., Warner, F. M., Finnerup, N. B., Jensen, M. P., Mercier, C., Richards, J. S., et al. (2016). Meta-analysis of placebo responses in central neuropathic pain: impact of subject, study, and pain characteristics. Pain 157, 530–540. doi: 10.1097/j.pain.0000000000000431
Cruccu, G., Garcia-Larrea, L., Hansson, P., Keindl, M., Lefaucheur, J. P., Paulus, W., et al. (2016). EAN guidelines on central neurostimulation therapy in chronic pain conditions. Eur. J. Neurol. 23, 1489–1499. doi: 10.1111/ene.13103
Defrin, R., Grunhaus, L., Zamir, D., and Zeilig, G. (2007). The effect of a series of repetitive transcranial magnetic stimulations of the motor cortex on central pain after spinal cord injury. Arch. Phys. Med. Rehabil. 88, 1574–1580. doi: 10.1016/j.apmr.2007.07.025
Feng, Y., Zhang, B., Zhang, J., and Yin, Y. (2019). Effects of non-invasive brain stimulation on headache intensity and frequency of headache attacks in patients with migraine: a systematic review and meta-analysis. Headache 59, 1436–1447. doi: 10.1111/head.13645
Finnerup, N. B., Attal, N., Haroutounian, S., McNicol, E., Baron, R., Dworkin, R. H., et al. (2015). Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 14, 162–173. doi: 10.1016/s1474-4422(14)70251-0
Fricova, J., Klirova, M., Masopust, V., Novak, T., Verebova, K., and Rokyta, R. (2013). Repetitive transcranial magnetic stimulation in the treatment of chronic orofacial pain. Physiol. Res. 62, S125–S134.
Gaertner, M., Kong, J. T., Scherrer, K. H., Foote, A., Mackey, S., and Johnson, K. A. (2018). Advancing transcranial magnetic stimulation methods for complex regional pain syndrome: an open-label study of paired theta burst and high-frequency stimulation. Neuromodulation 21, 409–416. doi: 10.1111/ner.12760
Galhardoni, R., Correia, G. S., Araujo, H., Yeng, L. T., Fernandes, D. T., Kaziyama, H. H., et al. (2015). Repetitive transcranial magnetic stimulation in chronic pain: a review of the literature. Arch. Phys. Med. Rehabil. 96, S156–S172. doi: 10.1016/j.apmr.2014.11.010
Gao, F., Chu, H., Li, J., Yang, M., Du, L., Li, J., et al. (2017). Repetitive transcranial magnetic stimulation for pain after spinal cord injury: a systematic review and meta-analysis. J. Neurosurg. Sci. 61, 514–522. doi: 10.23736/s0390-5616.16.03809-1
Gatzinsky, K., Bergh, C., Liljegren, A., Silander, H., Samuelsson, J., Svanberg, T., et al. (2020). Repetitive transcranial magnetic stimulation of the primary motor cortex in management of chronic neuropathic pain: a systematic review. Scand. J. Pain 21, 8–21. doi: 10.1515/sjpain-2020-0054
Goudra, B., Shah, D., Balu, G., Gouda, G., Balu, A., Borle, A., et al. (2017). Repetitive transcranial magnetic stimulation in chronic pain: a meta-analysis. Anesth. Essays Res. 11, 751–757. doi: 10.4103/aer.AER_10_17
Grant, M. J., and Booth, A. (2009). A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr. J. 26, 91–108. doi: 10.1111/j.1471-1842.2009.00848.x
Gromisch, E. S., Kerns, R. D., and Beauvais, J. (2020). Pain-related illness intrusiveness is associated with lower activity engagement among persons with multiple sclerosis. Mult. Scler. Relat. Disord. 38, 101882. doi: 10.1016/j.msard.2019.101882
Haddaway, N. R., Bernes, C., Jonsson, B. G., and Hedlund, K. (2016). The benefits of systematic mapping to evidence-based environmental management. Ambio 45, 613–620. doi: 10.1007/s13280-016-0773-x
Hamid, P., Malik, B. H., and Hussain, M. L. (2019). Noninvasive transcranial magnetic stimulation (TMS) in chronic refractory pain: a systematic review. Cureus 11:e6019. doi: 10.7759/cureus.6019
Henssen, D. J. H. A., Hoefsloot, W., Groenen, P. S. M., Van Cappellen van Walsum, A. M., Kurt, E., and Kozicz, T. (2019). Bilateral vs. unilateral repetitive transcranial magnetic stimulation to treat neuropathic orofacial pain: a pilot study. Brain Stimul. 12, 803–805. doi: 10.1016/j.brs.2019.02.001
Herrero Babiloni, A., Guay, S., Nixdorf, D. R., de Beaumont, L., and Lavigne, G. (2018). Non-invasive brain stimulation in chronic orofacial pain: a systematic review. J. Pain Res. 11, 1445–1457. doi: 10.2147/jpr.S168705
Hirayama, A., Saitoh, Y., Kishima, H., Shimokawa, T., Oshino, S., Hirata, M., et al. (2006). Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain 122, 22–27. doi: 10.1016/j.pain.2005.12.001
Hosomi, K., Shimokawa, T., Ikoma, K., Nakamura, Y., Sugiyama, K., Ugawa, Y., et al. (2013). Daily repetitive transcranial magnetic stimulation of primary motor cortex for neuropathic pain: a randomized, multicenter, double-blind, crossover, sham-controlled trial. Pain 154, 1065–1072. doi: 10.1016/j.pain.2013.03.016
Irlbacher, K., Kuhnert, J., Ro richt, S., Meyer, B. U., and Brandt, S. A. (2006). Central and peripheral deafferent pain: therapy with repetitive transcranial magnetic stimulation. Nervenarzt 77, 1196–1203. doi: 10.1007/s00115-006-2148-1
Jetté, F., Cote, I., Meziane, H. B., and Mercier, C. (2013). Effect of single-session repetitive transcranial magnetic stimulation applied over the hand versus leg motor area on pain after spinal cord injury. Neurorehabil. Neural. Repair 27, 636–643. doi: 10.1177/1545968313484810
Jin, Y., Xing, G., Li, G., Wang, A., Feng, S., Tang, Q., et al. (2015). High frequency repetitive transcranial magnetic stimulation therapy for chronic neuropathic pain: a meta-analysis. Pain Phys. 18, E1029–E1046.
Johnson, S., Summers, J., and Pridmore, S. (2006). Changes to somatosensory detection and pain thresholds following high frequency repetitive TMS of the motor cortex in individuals suffering from chronic pain. Pain 123, 187–192. doi: 10.1016/j.pain.2006.02.030
Kalia, L. V., and O’Connor, P. W. (2005). Severity of chronic pain and its relationship to quality of life in multiple sclerosis. Mult. Scler. 11, 322–327. doi: 10.1191/1352458505ms1168oa
Kalita, J., Bhoi, S. K., and Misra, U. K. (2017). Effect of high rate rTMS on somatosensory evoked potential in migraine. Cephalalgia 37, 1222–1230. doi: 10.1177/0333102416675619
Kalita, J., Laskar, S., Bhoi, S. K., and Misra, U. K. (2016). Efficacy of single versus three sessions of high rate repetitive transcranial magnetic stimulation in chronic migraine and tension-type headache. J. Neurol. 263, 2238–2246. doi: 10.1007/s00415-016-8257-2
Kang, B. S., Shin, H. I., and Bang, M. S. (2009). Effect of repetitive transcranial magnetic stimulation over the hand motor cortical area on central pain after spinal cord injury. Arch. Phys. Med. Rehabil. 90, 1766–1771. doi: 10.1016/j.apmr.2009.04.008
Khedr, E. M., Kotb, H. I., Mostafa, M. G., Mohamad, M. F., Amr, S. A., Ahmed, M. A., et al. (2015). Repetitive transcranial magnetic stimulation in neuropathic pain secondary to malignancy: a randomized clinical trial. Eur. J. Pain 19, 519–527. doi: 10.1002/ejp.576
Khedr, E. M., Kotb, H., Kamel, N. F., Ahmed, M. A., Sadek, R., and Rothwell, J. C. (2005). Long-lasting analgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J. Neurol. Neurosurg. Psychiatry 76, 833–838. doi: 10.1136/jnnp.2004.055806
Klein, M. M., Treister, R., Raij, T., Pascual-Leone, A., Park, L., Nurmikko, T., et al. (2015). Transcranial magnetic stimulation of the brain: guidelines for pain treatment research. Pain 156, 1601–1614. doi: 10.1097/j.pain.0000000000000210
Kobayashi, M., Fujimaki, T., Mihara, B., and Ohira, T. (2015). Repetitive transcranial magnetic stimulation once a week induces sustainable long-term relief of central poststroke pain. Neuromodulation 18, 249–254. doi: 10.1111/ner.12301
Kohútová, B., Fricová, J., Klírová, M., Novák, T., and Rokyta, R. (2017). Theta burst stimulation in the treatment of chronic orofacial pain: a randomized controlled trial. Physiol. Res. 66, 1041–1047. doi: 10.33549/physiolres.933474
Kumar, B., Kalita, J., Kumar, G., and Misra, U. K. (2009). Central poststroke pain: a review of pathophysiology and treatment. Anesthesia Anal. 108, 1645–1657. doi: 10.1213/ane.0b013e31819d644c
Kumru, H., Albu, S., Vidal, J., and Tormos, J. M. (2017). Effectiveness of repetitive trancranial or peripheral magnetic stimulation in neuropathic pain. Disabil. Rehabil. 39, 856–866. doi: 10.3109/09638288.2016.1170213
Lan, L., Zhang, X., Li, X., Rong, X., and Peng, Y. (2017). The efficacy of transcranial magnetic stimulation on migraine: a meta-analysis of randomized controlled trails. J. Headache Pain 18, 86. doi: 10.1186/s10194-017-0792-4
Lang, M., Treister, R., Klein, M. M., and Oaklander, A. L. (2014). Repetitive transcranial magnetic stimulation (rTMS) of the primary motor cortex for treating facial neuropathic pain-preliminary results of arandomized, sham-controlled, cross-over study. Mol. Pain 10:6. doi: 10.1111/j.1526-4637.2009.00657.x
Lawson, M. A., Frank, S., Zafar, N., Waschke, A., Kalff, R., and Reichart, R. (2018). Time course of the response to navigated repetitive transcranial magnetic stimulation at 10 Hz in chronic neuropathic pain. Neurol. Res. 40, 564–572. doi: 10.1080/01616412.2018.1453636
Lefaucheur, J. P. (2003). Evaluation électrophysiologique des boucles réflexes spinales potentiellement impliquées dans la spasticité [Electrophysiological assessment of reflex pathways involved in spasticity]. Neurochirurgie 49, 205–214.
Lefaucheur, J. P., Aleman, A., Baeken, C., Benninger, D. H., Brunelin, J., Di Lazzaro, V., et al. (2020). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018). Clin. Neurophysiol. 131, 474–528. doi: 10.1016/j.clinph.2019.11.002
Lefaucheur, J. P., Ayache, S. S., Sorel, M., Farhat, W. H., Zouari, H. G., and Ciampi de Andrade, D. (2012). Analgesic effects of repetitive transcranial magnetic stimulation of the motor cortex in neuropathic pain: influence of theta burst stimulation priming. Eur. J. Pain 16, 1403–1413. doi: 10.1002/j.1532-2149.2012.00150.x
Lefaucheur, J. P., Drouot, X., Keravel, Y., and Nguyen, J. P. (2001a). Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport 12, 2963–2965. doi: 10.1097/00001756-200109170-00041
Lefaucheur, J. P., Drouot, X., and Nguyen, J. P. (2001b). Interventional neurophysiology for pain control: duration of pain relief following repetitive transcranial magnetic stimulation of the motor cortex. Neurophysiol. Clin. 31, 247–252. doi: 10.1016/s0987-7053(01)00260-x
Lefaucheur, J. P., Drouot, X., Menard-Lefaucheur, I., Keravel, Y., and Nguyen, J. P. (2008). Motor cortex rTMS in chronic neuropathic pain: pain relief is associated with thermal sensory perception improvement. J. Neurol. Neurosurg. Psychiatry 79, 1044–1049. doi: 10.1136/jnnp.2007.135327
Lefaucheur, J. P., Drouot, X., Menard-Lefaucheur, I., Zerah, F., Bendib, B., Cesaro, P., et al. (2004). Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J. Neurol. Neurosurg. Psychiatry 75, 612–616. doi: 10.1136/jnnp.2003.022236
Lefaucheur, J. P., Hatem, S., Nineb, A., Ménard-Lefaucheur, I., Wendling, S., Keravel, Y., et al. (2006). Somatotopic organization of the analgesic effects of motor cortex rTMS in neuropathic pain. Neurology 67, 1998–2004. doi: 10.1212/01.wnl.0000247138.85330.88
Lefaucheur, J. P., Menard-Lefaucheur, I., Goujon, C., Keravel, Y., and Nguyen, J. P. (2011). Predictive value of rTMS in the identification of responders to epidural motor cortex stimulation therapy for pain. J. Pain 12, 1102–1111. doi: 10.1016/j.jpain.2011.05.004
Leung, A., Donohue, M., Xu, R., Lee, R., Lefaucheur, J. P., Khedr, E. M., et al. (2009). rTMS for suppressing neuropathic pain: a meta-analysis. J. Pain 10, 1205–1216. doi: 10.1016/j.jpain.2009.03.010
Leung, A., Shukla, S., Fallah, A., Song, D., Lin, L., Golshan, S., et al. (2016). Repetitive transcranial magnetic stimulation in managing mild traumatic brain injury-related headaches. Neuromodulation 19, 133–141. doi: 10.1111/ner.12364
Liampas, A., Rekatsina, M., Vadalouca, A., Paladini, A., Varrassi, G., and Zis, P. (2020b). Non-pharmacological management of painful peripheral neuropathies: a systematic review. Adv. Ther. 37, 4096–4106. doi: 10.1007/s12325-020-01462-3
Liampas, A., Velidakis, N., Georgiou, T., Vadalouca, A., Varrassi, G., Hadjigeorgiou, G. M., et al. (2020a). Prevalence and management challenges in central post-stroke neuropathic pain: a systematic review and meta-analysis. Adv. Ther. 37, 3278–3291. doi: 10.1007/s12325-020-01388-w
Lin, H., Li, W., Ni, J., and Wang, Y. (2018). Clinical study of repetitive transcranial magnetic stimulation of the motor cortex for thalamic pain. Medicine 97:e11235. doi: 10.1097/md.0000000000011235
Ma, S. M., Ni, J. X., Li, X. Y., Yang, L. Q., Guo, Y. N., and Tang, Y. Z. (2015). High-frequency repetitive transcranial magnetic stimulation reduces pain in postherpetic neuralgia. Pain Med. 16, 2162–2170. doi: 10.1111/pme.12832
Madera Anaya, M., Franco, J. V. A., Ballesteros, M., Solà, I., Urrútia Cuchí, G., and Bonfill Cosp, X. (2019). Evidence mapping and quality assessment of systematic reviews on therapeutic interventions for oral cancer. Cancer Manag. Res. 11, 117–130. doi: 10.2147/cmar.S186700
Malavera, A., Silva, F. A., Fregni, F., Carrillo, S., and Garcia, R. G. (2016). Repetitive transcranial magnetic stimulation for phantom limb pain in land mine victims: a double-blinded, randomized, sham-controlled trial. J. Pain. 17, 911–918. doi: 10.1016/j.jpain.2016.05.003
Malavera, M., Silva, F., Garcia, R., Quiros, J., Dallos, M., and Pinzon, A. (2013). Effects of transcranial magnetic stimulation in the treatment of phantom limb pain in landmine victims: a randomized clinical trial. J. Neurol. Sci. 333:e534. doi: 10.1016/j.jns.2013.07.1880
Matsumura, Y., Hirayama, T., and Yamamoto, T. (2013). Comparison between pharmacologic evaluation and repetitive transcranial magnetic stimulation-induced analgesia in poststroke pain patients. Neuromodulation 16, 349–354. doi: 10.1111/ner.12019
Mehta, S., Orenczuk, K., McIntyre, A., Willems, G., Wolfe, D. L., Hsieh, J. T., et al. (2013). Neuropathic pain post spinal cord injury part 1: systematic review of physical and behavioral treatment. Top. Spinal. Cord. Inj. Rehabil. 19, 61–77. doi: 10.1310/sci1901-61
Melchior, C., Gourcerol, G., Chastan, N., Verin, E., Menard, J. F., Ducrotte, P., et al. (2014). Effect of transcranial magnetic stimulation on rectal sensitivity in irritable bowel syndrome: a randomized, placebo-controlled pilot study. Colorectal. Dis. 16, O104–O111. doi: 10.1111/codi.12450
Melchior, M., Chastang, J. F., Head, J., Goldberg, M., Zins, M., Nabi, H., et al. (2013). Socioeconomic position predicts long-term depression trajectory: a 13-year follow-up of the GAZEL cohort study. Mol. Psychiatry 18, 112–121. doi: 10.1038/mp.2011.116
Miake-Lye, I. M., Hempel, S., Shanman, R., and Shekelle, P. G. (2016). What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst Rev. 5, 28. doi: 10.1186/s13643-016-0204-x
Miake-Lye, I. M., Mak, S., Lee, J., Luger, T., Taylor, S. L., Shanman, R., et al. (2019). Massage for pain: an evidence map. J. Alter. Comple. Med. 25, 475–502. doi: 10.1089/acm.2018.0282
Misra, U. K., Kalita, J., Tripathi, G. M., and Bhoi, S. K. (2013). Is beta endorphin related to migraine headache and its relief? Cephalalgia 33, 316–322. doi: 10.1177/0333102412473372
Misra, U. K., Kalita, J., Tripathi, G., and Bhoi, S. K. (2017). Role of beta endorphin in pain relief following high rate repetitive transcranial magnetic stimulation in migraine. Brain Stimul. 10, 618–623. doi: 10.1016/j.brs.2017.02.006
Moisset, X., Bouhassira, D., Avez Couturier, J., Alchaar, H., Conradi, S., Delmotte, M. H., et al. (2020b). Pharmacological and non-pharmacological treatments for neuropathic pain: systematic review and French recommendations. Rev. Neurol. 176, 325–352. doi: 10.1016/j.neurol.2020.01.361
Moisset, X., Pereira, B., de Andrade, D., Fontaine, D., Lantéri-Minet, M., and Mawet, J. (2020a). Neuromodulation techniques for acute and preventive migraine treatment: a systematic review and meta-analysis of randomized controlled trials. J. Headache Pain 21:142. doi: 10.1186/s10194-020-01204-4
Moreno-Duarte, I., Morse, L. R., Alam, M., Bikson, M., Zafonte, R., and Fregni, F. (2014). Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. NeuroImage 85, 1003–1013. doi: 10.1016/j.neuroimage.2013.05.097
Mulla, S. M., Wang, L., Khokhar, R., Izhar, Z., Agarwal, A., Couban, R., et al. (2015). Management of central poststroke pain: systematic review of randomized controlled trials. Stroke 46, 2853–2860. doi: 10.1161/strokeaha.115.010259
Nardone, R., Versace, V., Sebastianelli, L., Brigo, F., Christova, M., Scarano, G. I., et al. (2019). Transcranial magnetic stimulation in subjects with phantom pain and non- painful phantom sensations: a systematic review. Brain Res. Bull. 148, 1–9. doi: 10.1016/j.brainresbull.2019.03.001
Nurmikko, T., MacIver, K., Bresnahan, R., Hird, E., Nelson, A., and Sacco, P. (2016). Motor cortex reorganization and repetitive transcranial magnetic stimulation for pain-a methodological study. Neuromodulation 19, 669–678. doi: 10.1111/ner.12444
Nussbaumer-Streit, B., Grillich, L., Glechner, A., Affengruber, L., Gartlehner, G., Morche, J., et al. (2018). [GRADE: evidence to Decision (EtD) frameworks - a systematic and transparent approach to making well informed healthcare choices. 1: Introduction]. Z. Evid. Fortbild. Qual. Gesundhwes. 134, 57–66. doi: 10.1016/j.zefq.2018.05.004
O’Connell, N. E., Marston, L., Spencer, S., Desouza, L. H., and Wand, B. M. (2018). Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst. Rev. 4:CD008208. doi: 10.1002/14651858.CD008208.pub5
Onesti, E., Gabriele, M., Cambieri, C., Ceccanti, M., Raccah, R., Di Stefano, G., et al. (2013). H-coil repetitive transcranial magnetic stimulation for pain relief in patients with diabetic neuropathy. Eur. J. Pain 17, 1347–1356. doi: 10.1002/j.1532-2149.2013.00320.x
Pacheco-Barrios, K., Meng, X., and Fregni, F. (2020). Neuromodulation techniques in phantom limb pain: a systematic review and meta-analysis. Pain Med. 21, 2310–2322. doi: 10.1093/pm/pnaa039
Papanas, N., and Ziegler, D. (2016). Emerging drugs for diabetic peripheral neuropathy and neuropathic pain. Exp. Opin. Emerg. Drugs 21, 393–407. doi: 10.1080/14728214.2016.1257605
Paulus, W., Peterchev, A. V., and Ridding, M. (2013). Transcranial electric and magnetic stimulation: technique and paradigms. Handb. Clin. Neurol. 116, 329–342. doi: 10.1016/b978-0-444-53497-2.00027-9
Pei, Q., Wu, B., Tang, Y., Yang, X., Song, L., Wang, N., et al. (2019). Repetitive transcranial magnetic stimulation at different frequencies for postherpetic neuralgia: a double-blind, sham-controlled,randomized trial. Pain Phys. 22, E303–E313.
Petersen, K., Vakkalanka, S., and Kuzniarz, L. (2015). Guidelines for conducting systematic mapping studies in software engineering: an update. Inform. Softw. Technol. 64, 1–18. doi: 10.1016/j.infsof.2015.03.007
Picarelli, H., Teixeira, M. J., de Andrade, D. C., Myczkowski, M. L., Luvisotto, T. B., Yeng, L. T., et al. (2010). Repetitive transcranial magnetic stimulation is efficacious as an add-on to pharmacological therapy in complex regional pain syndrome (CRPS) type I. J. Pain. 11, 1203–1210. doi: 10.1016/j.jpain.2010.02.006
Pinot-Monange A, Moisset X, Chauvet P, Gremeau AS, Comptour A, Canis M, et al. (2019). Repetitive transcranial magnetic stimulation therapy. (rTMS) for endometriosis patients with refractory pelvic chronic pain: a pilot study. J. Clin. Med. 8:508. doi: 10.3390/jcm8040508
Pleger, B., Janssen, F., Schwenkreis, P., Volker, B., Maier, C., and Tegenthoff, M. (2004). Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neurosci. Lett. 356, 87–90. doi: 10.1016/j.neulet.2003.11.037
Pommier, B., Creac’h, C., Beauvieux, V., Nuti, C., Vassal, F., and Peyron, R. (2016). Robot-guided neuronavigated rTMS as an alternative therapy for central. (neuropathic) pain: clinical experience and long-term follow-up. Eur. J. Pain 20, 907–916. doi: 10.1002/ejp.815
Quesada, C., Pommier, B., Fauchon, C., Bradley, C., Créac’h, C., Vassal, F., et al. (2018). Robot-guided neuronavigated repetitive transcranial magnetic stimulation. (rTMS) in Central neuropathic pain. Arch. Phys. Med. Rehabil. 99, 2203–2215. doi: 10.1016/j.apmr.2018.04.013
Ramger, B. C., Bader, K. A., Davies, S. P., Stewart, D. A., Ledbetter, L. S., Simon, C. B., et al. (2019). Effects of non-invasive brain stimulation on clinical pain intensity and experimental pain sensitivity among individuals with central post-stroke pain: a systematic review. J. Pain Res. 12, 3319–3329. doi: 10.2147/JPR.S216081
Rollnik, J. D., Wustefeld, S., Dauper, J., Karst, M., Fink, M., Kossev, A., et al. (2002). Repetitive transcranial magnetic stimulation for the treatment of chronic pain – a pilot study. Eur. Neurol. 48, 6–10.
Saitoh, Y., Hirayama, A., Kishima, H., Oshino, S., Hirata, M., Kato, A., et al. (2006). Stimulation of primary motor cortex for intractable deafferentation pain. Acta Neurochir. Suppl. 99, 57–59. doi: 10.1007/978-3-211-35205-2_11
Saitoh, Y., Hirayama, A., Kishima, H., Shimokawa, T., Oshino, S., Hirata, M., et al. (2007). Reduction of intractable deafferentation pain due to spinal cord or peripheral lesion by high-frequency repetitive transcranial magnetic stimulation of the primary motor cortex. J. Neurosurg. 107, 555–559. doi: 10.3171/jns-07/09/0555
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358, j4008. doi: 10.1136/bmj.j4008
Shehata, H. S., Esmail, E. H., Abdelalim, A., El-Jaafary, S., Elmazny, A., Sabbah, A., et al. (2016). Repetitive transcranial magnetic stimulation versus botulinum toxin injection in chronic migraine prophylaxis: a pilot randomized trial. J. Pain Res. 9, 771–777. doi: 10.2147/JPR.S116671
Shen, Z., Li, Z., Ke, J., He, C., Liu, Z., Zhang, D., et al. (2020). Effect of non-invasive brain stimulation on neuropathic pain following spinal cord injury: a systematic review and meta-analysis. Medicine 99:e21507. doi: 10.1097/md.0000000000021507
Shimizu, T., Hosomi, K., Maruo, T., Goto, Y., Yokoe, M., Kageyama, Y., et al. (2017). Efficacy of deep rTMS for neuropathic pain in the lower limb: a randomized, double-blind crossover trial of an H-coil and figure-8 coil. J. Neurosurg. 127, 1172–1180. doi: 10.3171/2016.9.JNS16815
Stilling, J. M., Monchi, O., Amoozegar, F., and Debert, C. T. (2019). Transcranial magnetic and direct current stimulation (TMS/tDCS) for the treatment of headache: a systematic review. Headache 59, 339–357. doi: 10.1111/head.13479
Sylvester, R. J., Canfield, S. E., Lam, T. B., Marconi, L., MacLennan, S., Yuan, Y., et al. (2017). Conflict of evidence: resolving discrepancies when findings from randomized controlled trials and meta-analyses disagree. Eur. Urol. 71, 811–819. doi: 10.1016/j.eururo.2016.11.023
Teo, W. P., Kannan, A., Loh, P. K., Chew, E., Sharma, V. K., and Chan, Y. C. (2014). Tolerance of motor cortex rTMS in chronic migraine. J. Clin. Diagn. Res. 8, MM01–MM02.
Urits, I., Adamian, L., Fiocchi, J., Hoyt, D., Ernst, C., Kaye, A. D., et al. (2019). Advances in the understanding and management of chronic pain in multiple sclerosis: a comprehensive review. Curr. Pain Headache Rep. 23:59. doi: 10.1007/s11916-019-0800-2
Yang, S., and Chang, M. C. (2020). Effect of repetitive transcranial magnetic stimulation on pain management: a systematic narrative review. Front. Neurol. 11:114. doi: 10.3389/fneur.2020.00114
Yilmaz, B., Kesikburun, S., Yasar, E., and Tan, A. K. (2014). The effect of repetitive transcranial magnetic stimulation on refractory neuropathic pain in spinal cord injury. J. Spinal. Cord. Med. 37, 397–400.
Yu, B., Qiu, H., Li, J., Zhong, C., and Li, J. (2020). Noninvasive brain stimulation does not improve neuropathic pain in individuals with spinal cord injury: evidence from a meta-analysis of 11 randomized controlled trials. Am. J. Phys. Med. Rehabil. 99, 811–820. doi: 10.1097/phm.0000000000001421
Zaghi, S., Thiele, B., Pimentel, D., Pimentel, T., and Fregni, F. (2011). Assessment and treatment of pain with non-invasive cortical stimulation. Restorat. Neurol. Neurosci. 29, 439–451. doi: 10.3233/RNN-2011-0615
Zeng, H., Pacheco-Barrios, K., Cao, Y., Li, Y., Zhang, J., Yang, C., et al. (2020). Non-invasive neuromodulation effects on painful diabetic peripheral neuropathy: a systematic review and meta-analysis. Sci Rep. 10:19184. doi: 10.1038/s41598-020-75922-9
Keywords: neuropathic pain, non-pharmacological, repetitive transcranial magnetic stimulation, evidence mapping, evidence synthesis, motor cortex
Citation: Zang Y, Zhang Y, Lai X, Yang Y, Guo J, Gu S and Zhu Y (2022) Evidence Mapping Based on Systematic Reviews of Repetitive Transcranial Magnetic Stimulation on the Motor Cortex for Neuropathic Pain. Front. Hum. Neurosci. 15:743846. doi: 10.3389/fnhum.2021.743846
Received: 02 August 2021; Accepted: 15 November 2021;
Published: 16 February 2022.
Edited by:
Kai Wang, Anhui Medical University, ChinaReviewed by:
Joaquim Pereira Brazil-Neto, Unieuro, BrazilCopyright © 2022 Zang, Zhang, Lai, Yang, Guo, Gu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Zhu, emh1eWkxMDEwQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.