
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci. , 11 June 2021
Sec. Motor Neuroscience
Volume 15 - 2021 | https://doi.org/10.3389/fnhum.2021.684895
This article is part of the Research Topic Technological Advances in Neuromodulation Therapy for Movement Disorders View all 7 articles
 Shuo Xu1,2
Shuo Xu1,2 Wenfei Wang3
Wenfei Wang3 Si Chen1,2
Si Chen1,2 Qianqian Wu1,2
Qianqian Wu1,2 Chao Li1,2
Chao Li1,2 Xiangyu Ma1,2
Xiangyu Ma1,2 Teng Chen1,2
Teng Chen1,2 Weiguo Li1,2
Weiguo Li1,2 Shujun Xu1,2*
Shujun Xu1,2*Background: As a complication-prone operation, deep brain stimulation (DBS) has become the first-line surgical approach for patients with advanced Parkinson’s disease (PD). This study aimed to evaluate the incidence and risk factors of DBS-associated complications.
Methods: We have reviewed a consecutive series of patients with PD undergoing DBS procedures to describe the type, severity, management, and outcome of postoperative complications from January 2011 to December 2018. Both univariate and multivariate analyses were performed to identify statistically significant risk factors. We also described our surgical strategies to minimize the adverse events.
Results: A total of 225 patients underwent 229 DBS implantation procedures (440 electrodes), of whom 20 patients experienced 23 DBS-associated complications, including ten operation-related complications and 13 hardware-related ones. Univariate analysis elucidated that comorbid medical conditions (P = 0.024), hypertension (P = 0.003), early-stage operation (P < 0.001), and unilateral electrode implantation (P = 0.029) as risk factors for overall complications, or more specifically, operation-related complications demonstrated in the stratified analysis. In contrast, no risk factor for hardware-related complications was identified. Statistical significances of hypertension (OR = 3.33, 95% CI: 1.14–9.71, P = 0.027) and early-stage (OR = 11.04, 95% CI: 2.42–50.45, P = 0.002) were further validated via multivariate analysis. As the annual number of DBS procedures increased, the incidence of complications gradually decreased (R = −0.699, P < 0.01). Additionally, there was a strong correlation between surgical complications and unplanned readmission (R = 0.730, P < 0.01).
Conclusion: The importance of cumulative experience and relevant technique modifications should be addressed to prevent DBS-associated complications and unplanned readmission.
Deep brain stimulation (DBS) is the therapeutic approach of intracranial electrical stimulation, which uses a four-contact stimulating electrode stereotactically implanted in the target and connected via a subcutaneous wire to an implantable pulse generator (IPG) that is placed on the chest wall underneath the collarbone. Most targets of DBS are deep brain structures, including deep nucleus and white matter tracts (Herrington et al., 2016). By preventing the transmission of pathologic bursting and improving the processing of sensorimotor information, DBS results in a considerable reduction in various symptoms of movement disorders, including tremor, bradykinesia, and stiffness (Miocinovic et al., 2013).
As a minimally invasive, effective, reversible, and controllable approach, DBS has gradually replaced conventional destructive surgery and has become the most common surgical option for advanced Parkinson’s disease (PD)(Okun, 2012; Ramirez-Zamora and Ostrem, 2018). In most cases, the overall benefits of DBS surgery greatly outweigh its risks. Despite the good tolerability and safety of this therapeutic approach, a broad range of DBS-related complications have arisen, which marks it a complication-prone operation (Boviatsis et al., 2010; Rughani et al., 2013; Kantzanou et al., 2021).
In this retrospective analysis, we reviewed the demographic and clinical features of 225 patients who underwent DBS surgery in our center over an 8-year period (2011–2018) to analyze the incidence and risk factors for DBS-related complications and summarize certain surgical strategies to minimize such adverse events.
The patients with PD who had undergone the DBS procedure in our center from January 2011 to December 2018 were retrospectively analyzed. Of note, a DBS procedure was defined as any stereotactic surgery that involved implantation of the new intracranial electrode(s) as well as IPG. A total of 229 DBS procedures of 225 patients were enrolled, and data was cross-checked with the manufacturers’ records (PINS Medical, Beijing, China and Medtronic, MN, United States). All DBS operations were performed by two primary surgeons (SjX and WL). Follow-up was done at the outpatient service and telephone interview by December 31, 2020. Readmission referred to the unplanned admission to our center for any clinical situation related to DBS procedure after the primary discharge. Appointed readmissions for IPG exchange and contralateral electrode implantation were excluded. Ethical approval was obtained from the Medical Ethical Committee, Qilu Hospital of Shandong University (KYLL-2019-1-066).
Surgery was performed following our standard procedure protocol (Ma et al., 2017). In short, we performed a positioning MRI scan with Gadolinium-based contrast prior to surgery. These images are then fused with the Leksell Model G stereotactic frame (Elekta AB, Stockholm, Sweden) based on CT images acquired on the day of surgery. Microelectrode recording (MER) was conducted for intraoperative electrophysiological localization of subthalamic nucleus (STN) or globus pallidus internus (GPi) without sedative agents. The electrode placement was further confirmed via macro-stimulation. The IPG was then implanted into the subclavian pouch under general anesthesia. The electrodes were connected to the IPG through corresponding extension wires. The position of the electrodes was examined again in a postoperative CT review within 24 h.
Based on the literature review and our practical experience, we have gradually adopted several surgical modifications and summarize them as follows: (1) Special drapes for DBS surgery. The one-piece design with an integrated observation window was easy to use and facilitated patient-surgeon communication. The impermeable non-woven textile met the recommendation of WHO Guidelines for the prevention of surgical site infection (World Health Organization (WHO), 2018) (Figures 1A–C). (2) Frontal incisions. Bilateral C-shaped frontal incisions were performed about 1 inch ahead of the burr holes on the coronal suture so that the incisions would not overlie the electrode anchoring devices. This modification effectively prevented cable damaging and lowered the local scalp tension, which might cause delayed healing and infection (Figure 1D). (3) Extension wire fixation. The incision for the extension wire connection was moved upwards from the posterior to the pinna to the parietal eminence to shorten the distance to the frontal incisions. Scalp skin near the parietal eminence was thick and lacking lymph nodes, which reduced skin erosion and infection incidence. Also, the connectors were constrained with a remodeled 2-hole titanium microplates to prevent the migration (Figures 1E–I). (4) IPG fixation. The prepectoral subfascial pocket was created for the IPG implantation to minimize the local exudate and skin erosion observed in the subcutaneous pocket. The IPG was then fixed to the pectoralis muscle or the clavicle to avoid device migration.
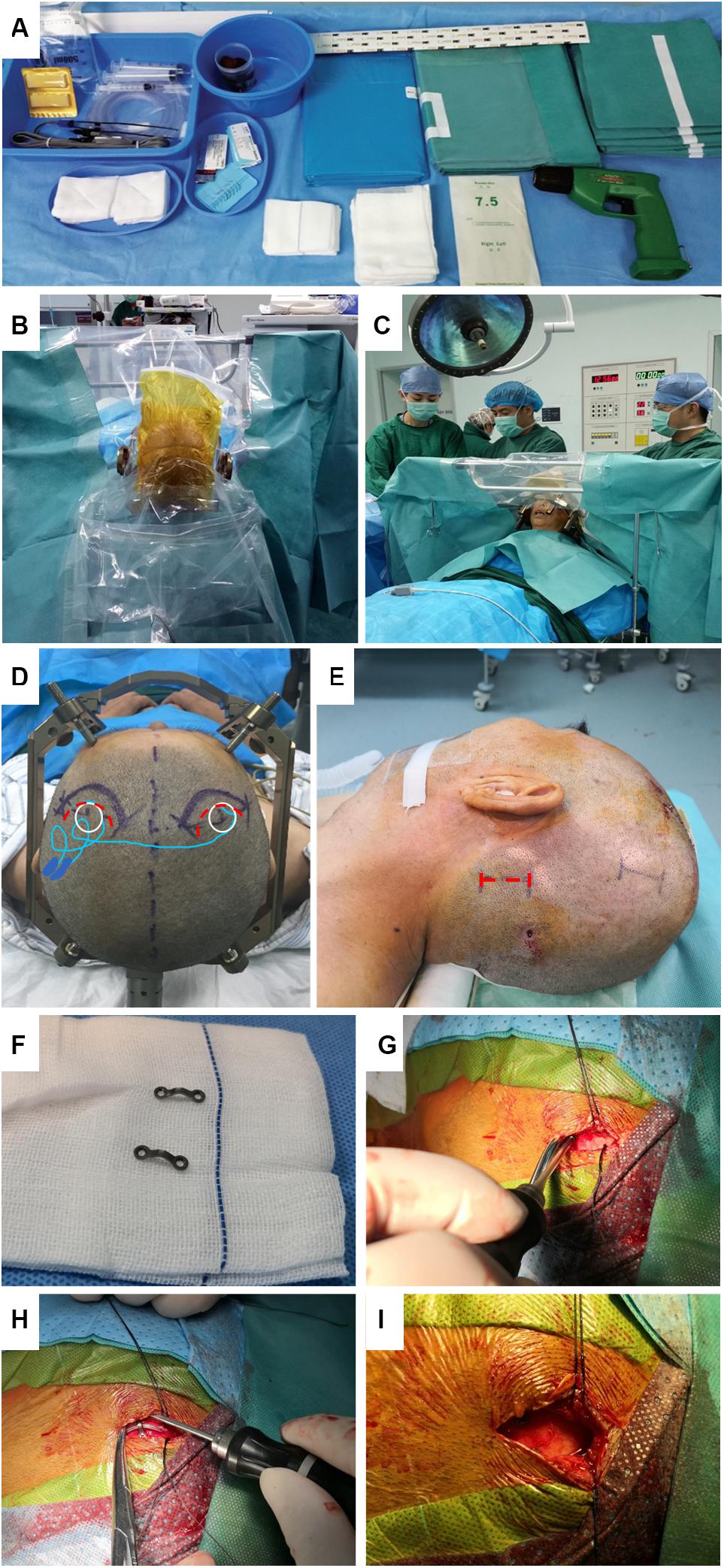
Figure 1. DBS Surgical modificationsin our center. (A) Demonstration of the non-woven drape exclusive for DBS surgery. (B,C) Transparent polyethylene film was easy to use and facilitated patient-surgeon communication during surgery. (D) Compared to the original incision on the coronal suture (red dotted curve), the distance between our modified forward-moving incision (purple solid curves) and burr hole and incision was increased to avoid placing hardware directly under the suture line. (E) The incision for the extension wire was moved upwards from occipital/mastoid region (red dotted line) to parietal eminence region (purple solid line) with thick skin and no lymph nodes. (F) Titanium microplate was bent to constrain the connector. (G,H) The skin was retracted aside so that the microplate was easily fixed to the skull. (I) Direct extension wire implantation under the suture line should be meticulously avoided.
Statistical analysis was performed using IBM SPSS Statistics for Windows (Version 22.0. IBM Corp., Armonk, NY, United States). The significance level was set at P < 0.05. Intergroup analysis was performed by either Student’s t-test or a combination of the chi-square test and Fisher exact test. Intergroup correlation was analyzed by the Spearman test. The risk factors were analyzed by multivariate logistic regression analysis using selected factors with a statistically significant difference in the univariate analysis. Graphs were drawn using Prism6 (GraphPad, United States). All the data was carefully reviewed by an experienced statistician (WW).
From January 2011 to December 2018, 229 DBS procedures were performed for 225 patients with PD in our center. A total of 440 electrodes and 238 IPGs were implanted. At the same observation window, nine patients with dystonia, essential tremor, Meige’s syndrome, Tourette’s syndrome, and chorea underwent DBS surgery, and 39 patients with PD underwent 42 procedures of IPG replacement surgery in our center. Considering the limited sample size, these cases were then excluded from the following study aimed to analyze the DBS-associated complications for patients with PD.
As shown in Table 1, male patients accounted for 59.6% (134/225). The average age at surgery was 61.6 ± 7.9 years (range from 30 to 82 years), with the disease duration of 9.9 ± 4.8 years. Most of the patients (222/225) who underwent DBS procedures were Han Chinese. Among the 73 patients (32.4%) who presented with at least one comorbid condition, 48 (21.3%) had high blood pressure. 38 patients (16.9%) were with smoking history. Nearly two-thirds of PD patients (63.6%) received the surgical procedures in 2017-2018. Bilateral STNs were the dominant implantation targets (96.4%). About three-quarters majority (75.6%) of the newly implanted IPGs were manufactured by PINS Medical Inc., and the rest by Medtronic. Reasons for the second-time implantation procedures included the aborted procedures (2 cases), contralateral electrode implantation (1 case), and electrode misplace (1 case). At the end of December 2020, all patients were followed up for at least 24 months, with an average follow-up duration of 45.2 ± 17.7 months.
Generally, operation-related complications are defined as those that could potentially be prevented by a change in DBS surgical technique and hardware-related complications as they are more difficult to relate to surgical technique (Ramayya et al., 2017). In our series, 23 complications were observed in 20 patients, including 10 operation-related complications in nine patients and 13 hardware-related complications in 13 patients (shown in Table 2).
The observed operation-related complication included epileptic seizure combined with intracranial hematoma (Patient #7, Figure 2A), intraoperative respiratory distress (Patient #21, 59), severe peri-electrode edema (Patient #2, 12), electrode misplace (Patient #1, 17), acute heart failure (Patient #217) and hydrocephalus (Patient #61).
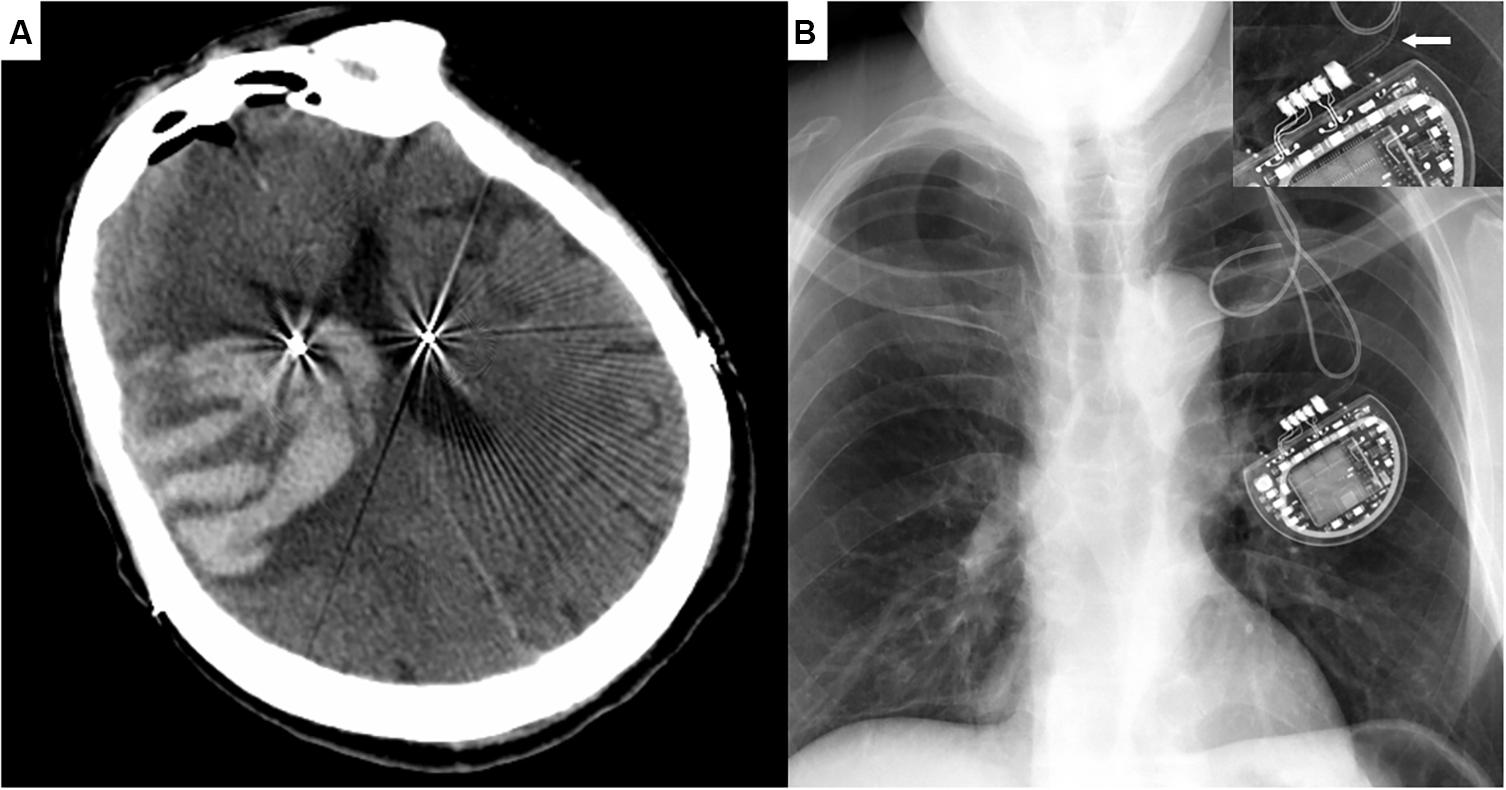
Figure 2. Representative Cases. (A) Cranial CT image of Patient #7 demonstrating the massive intracranial hematoma three days after the surgery, with symptoms of a generalized seizure. (B) Chest X-ray image of Patient #36 showing the fracture of extension wire near the IPG. Enlarged damaged wire in the right upper corner.
Wire fracture/high resistance was the most common hardware-related adverse event (Patient #17, 36, 38, 44, 160, as shown in Figure 2B). The others included electrode migration (Patient #67, 81), subcutaneous exudate/infection (Patient #59, 132), IPG migration (Patient #171, 207) and neck stricture formation (Patient #29, 66). Of note, two patients with subcutaneous exudate were categized into the minor infection, whom both recovered after local pressure and antibiotics administration. No etiological agent was diagnosed from the exudate laboratory examination.
As shown in Table 2, the outcomes were favorable under the appropriate interventions. No mortality or permanent morbidity was observed.
The univariate analysis revealed that patients with hypertension encountered complications more frequently (P = 0.003, Table 1). Given the high association with hypertension (R = 0.751, P < 0.01), comorbid medical conditions were also demonstrated as a risk factor (P = 0.024). Also, the complication rate of operation performed in 2011-2016 (hereinafter referred to as early-stage) was significantly higher than that in 2017-2018 (hereinafter referred to as late-stage, P < 0.001). Unexpectedly, the complication rate was higher in patients who received unilateral electrode implantation (P = 0.029), probably because of a weak but statistically significant association between unilateral electrode implantation and early-stage operation (R = 0.248, P < 0.01). No other variables were identified as risk factors. Of note, neither the primary diagnosis (PD or non-PD) nor surgical procedure (DBS or IPG replacement) appeared to affect the complication rates (P > 0.99 and P = 0.304, respectively).
Furthermore, a stratified analysis was conducted to elucidate the specific risk factors for operation- versus hardware-related complications. Notably, all these variables, including comorbid conditions, hypertension (P = 0.003), early stage (P = 0.003) and unilateral implantation (P = 0.006) were demonstrated as risk factors for operation-related complications. On the contrary, no potential predictors for hardware-related complications were identified (Table 3).
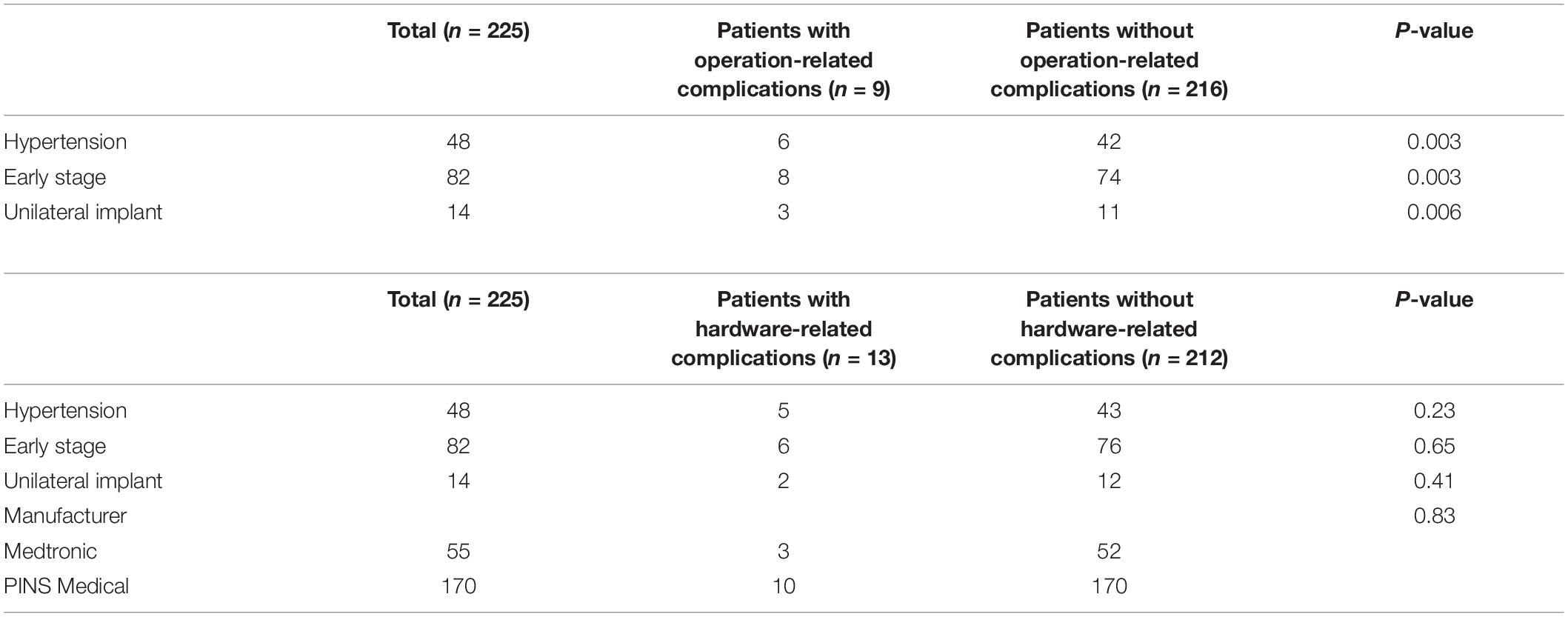
Table 3. Univariate Analysis of risk factors in complication subcategories with Chi-square Test with Continuity Correction.
The variable comorbid conditions was excluded from the multivariate analysis because of its strong association with hypertension. The other three variables were then entered into the multivariate logistic regression analysis, which demonstrated hypertension (OR = 3.33, 95% CI: 1.14-9.71, P = 0.027) and early stage (OR = 11.04, 95% CI: 2.42-50.45, P = 0.002) as independent risk factors for postoperative complications. Although unilateral implantation was established as a univariate indicator of risk, its statistical significance was not demonstrated in the multivariate analysis (P = 0.074).
In our cohort, an upward trend in the annual number of DBS surgeries was evident (Figure 3A). Meanwhile, the overall complication rate experienced a correspondingly dramatic fall (Figure 3B, R = −0.699, P < 0.01), which highlighted the importance of surgical experience in line with findings of some other studies (Falowski et al., 2015; Sorar et al., 2018). As shown in Figure 3C, the surgical experience was significantly related to the incidence of operation-related complications (R = −0.676, P < 0.01), rather than that of hardware-related complications (Figure 3D, R = 0.041, P = 0.538). Consequently, of all the complications, the proportion of hardware-related ones gradually increased (Figure 3E).
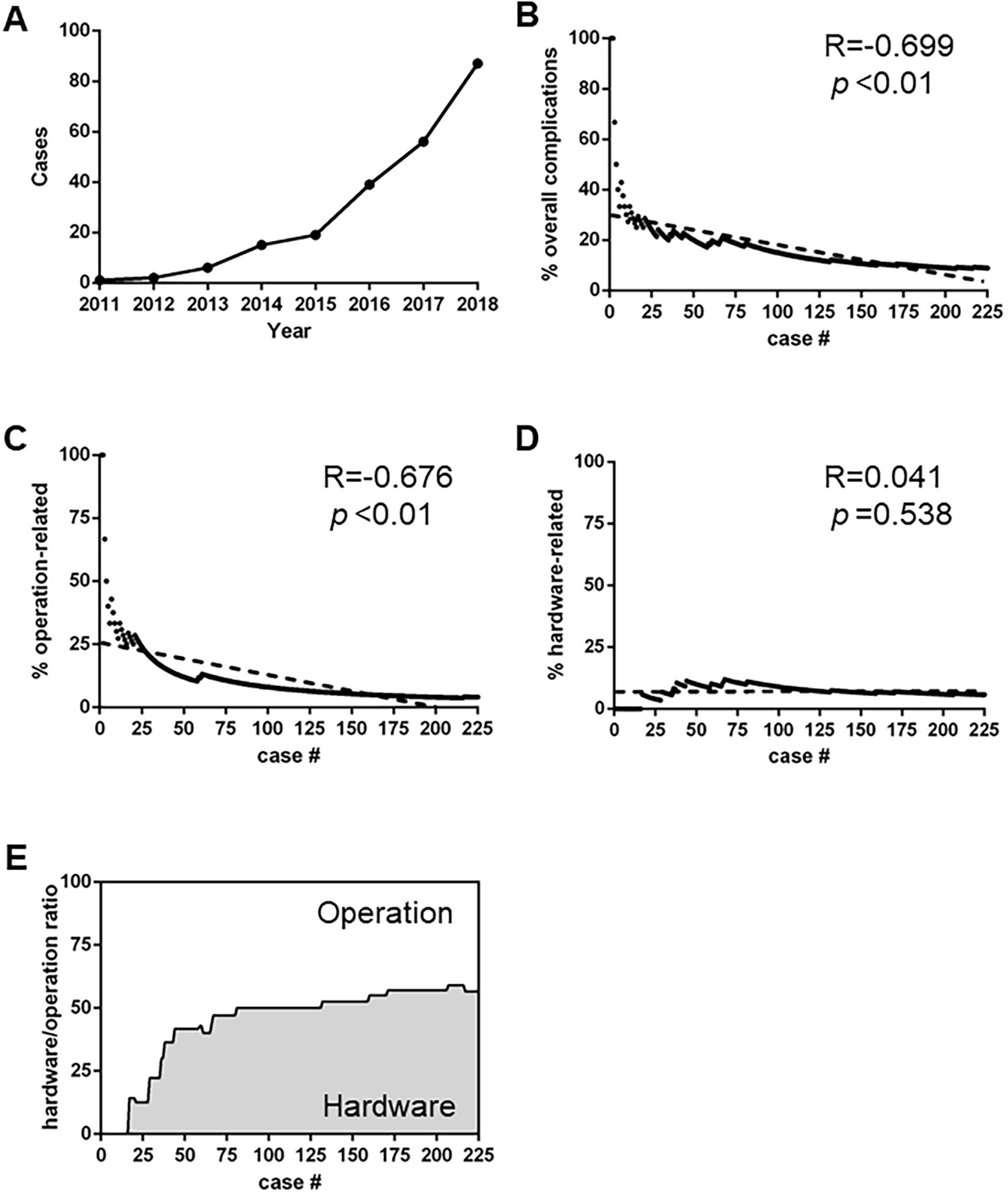
Figure 3. Secular trend of postoperative complications. (A): The annual number of DBS surgical procedures in our center. (B) The incidence of overall complications with increased number of Deep brain stimulation (DBS) volumes (R = –0.699, p < 0.01) (C,D) Different trends of operation-related (C) and hardware-related (D) complications (R = –0.676, p < 0.01 and R = 0.041, p = 0.538, respectively). (E) The ratio of hardware-related complications versus operation-related complications.
Among 225 patients, 15 patients experienced unplanned readmissions (6.2%, Table 4). The average interval between primary surgery and readmission was 18.0 ± 19.2 months. Of these patients, 13 cases were admitted to the hospital due to complications; therefore, the complications were closely related to patient readmission (R = 0.730, P < 0.01). 12 out of 15 patients underwent surgery, including device reimplantation (n = 3), electrode revision (n = 2), extension wire replacement (n = 4), relief of stricture (n = 1), device removal (n = 1), and ventriculoperitoneal shunt (n = 1). Unsurprisingly, patients with complications were more likely to experience readmissions (P < 0.001).
Deep brain stimulation surgery has drawn extensive attention regarding its safety issue (Rughani et al., 2013; Fenoy and Simpson, 2014; Fernandez-Pajarin et al., 2017; Rumalla et al., 2018). To date, several retrospective studies which investigated the incidence and risk factors for DBS-related complications have shown conflicting results, mainly due to differences in population characteristics, study design (PD alone or together with other movement disorders), cohort size, surgical experiences, and follow-up duration (Goodman et al., 2006; Baizabal Carvallo et al., 2012; Hu et al., 2017; Ramayya et al., 2017). Herein, we have reviewed a consecutive series of patients with advanced PD who underwent DBS surgery in a single center to eliminate most interference factors and reveal the incidence and risk factors of DBS-related complications in the Chinese population.
In this study, the statistical significance of early stage operation was demonstrated for operation-related complications. Practice makes perfect. Although it was reported that no DBS-related complication had be yet observed for the very first operations in a newly established neurosurgical center (Razmkon et al., 2019), our data confirmed the well-known merits of surgical experience on preventing postoperative complications, or more specifically, operation-related ones. We also observed a tendency that patients with one complication were more likely to present with another one at a later stage (Patient #7, 17, 59), which had been reported by Paluzzi et al. (2006). It is noteworthy that hardware-related complications were more common and problematic in our cohort, including wire fracture/high resistance (2.2%), electrode migration (0.89%), IPG migration (0.89%), and neck stricture formation (0.89%). There might be several reasons. First, the average follow-up duration was 45.2 months in this series, and a longer follow-up increased the cumulative probability of hardware-related complications (Oh et al., 2002). Second, although the ratio of hardware-related complications was higher, its incidence (13 out of 225 patients) still represented a lower rate than that in the most published studies. Third, the dualistic definitions of the operation- and hardware-related complications can be oversimplified, which causes ambiguity in categorizing certain types of adverse events. Nevertheless, the low incidence of the overall DBS-related complications shown in this study was quite encouraging.
Hypertension, as illustrated, is not only the most common comorbidity for patients with PD, but also a tremendous risk factor for operation-related complications in our cohort. Since it remains controversial whether hypertension increases the risk of postoperative complications (Yang et al., 2020), we have reviewed the complication details associated with hypertension. Interestingly, both patients (Patient #2, 12) who encountered severe peri-electrode edema were with hypertension (P = 0.0448, Fisher’s exact test). Severe peri-electrode edema is a rare, self-limited operation-related complication (Whiting et al., 2018), with the main symptoms of psychosis. Notwithstanding that the etiology of edema remains unknown (Deogaonkar et al., 2011; Nazzaro et al., 2017), we hence proposed that hypertension might mediate the symptomatic edema by the mechanisms of either CT-negative micro-hematoma or local cerebral perfusion pressure dysregulation. Also, we had a 79-year old patient with hypertension encountering acute heart failure and pneumonia soon after surgery (Patient #217). Therefore, the association between hypertension and postoperative complications could be more complex and extensive than previously assumed (Adogwa et al., 2017). Further studies in the larger cohorts should be conducted to determine whether hypertension is related to DBS-related complications.
Intracranial hemorrhage is a devastating operation-related complication. Retrospective studies reported the incidence of intracerebral hemorrhage between 0.6–6.0% (Binder et al., 2005; Park et al., 2011; Wang et al., 2017). In our series, one patient (0.44%) encountered intracranial hematoma who recovered without surgical intervention. Careful planning on preoperative contrast-enhanced MRI images and MER recording with single microelectrode are essential for avoiding vascular injury. Invasive arterial blood pressure should be controlled below 140/90mmhg during the entire course in our center.
Wound complications, especially infection, are among the most frequent and concerning hardware-related complications. While the incidence of infection ranged from 0 to 15.2% (Sixel-Doring et al., 2010; Bjerknes et al., 2014; Kim et al., 2017; Hardaway et al., 2018; Kantzanou et al., 2021), merely two patients (0.89%) experienced subcutaneous exudate in this cohort and were soon cured after local compression dressing and antibiotic administration. Regrading wound complications, our rationale is that the probability of such events can be minimized by the long-term efforts of a specialized operation team with strict enforcement of sterility, and reductant surgical modifications. Direct hardware implantation under the suture line should be meticulously avoided (Falowski et al., 2015). Alcohol-based solution was used for gross skin cleaning, and then povidone-iodine for formal incision preparation. Intraoperative irrigation with povidone-iodine and saline solutions before layer by layer closure with triclosan-coated absorbable sutures should be also noted.
There is sufficient discussion in the literature regarding the surgical technique issue (Fontaine et al., 2013; Linhares et al., 2013; Falowski et al., 2015; Rasouli and Kopell, 2016; White-Dzuro et al., 2016; Zhou et al., 2018); however, the relatively low incidence for postoperative complications in this cohort, especially infection, was attributed primarily to the reductant preventive approaches as we described, no matter how subtle or ordinary they were. For every complication requiring further surgery, there could be many more adverse events that were neglected. Therefore, it is reasonable and necessary to apply multiple surgical modifications. Meanwhile, device improvements, for instance, extension wire with high elasticity and corrosion resistance, and firmer electrode anchoring device, might also help lower the hardware-related risk.
There are some limitations to our study. First, this study was retrospective, non-randomized, and monocentric. For instance, most patients were Han Chinese in our study cohort, a population who were seldomly studied. Therefore, the results should be interpreted with caution. Second, the surgical modifications described above were gradually adopted during our practice, making it difficult to demonstrate the correlation between these modifications and the decrease of complication incidence. Third, with 23 adverse events in 20 cases, statistical power could be limited due to the low number of events per variable for logistic regression analysis (Peduzzi et al., 1996). Larger scale studies are necessary to solve this issue. Fourth, the association of DBS-related complications with quality of life (QoL) was not investigated in this study. Although the variable whether post-operation complications existed was not evaluated in a prognostic model to predict improvement in QoL following DBS surgery in patients with PD (Frizon et al., 2019), it would be helpful to understand the influence of DBS-related complications on QoL in patients with PD in the future.
In summary, we demonstrated that hypertension was the most significant individual risk factor for DBS surgery, in contrast to surgical experience as a major iatrogenic factor. Stratified analysis validated these risk factors for operation-related complications. In contrast, no risk factor was identified for the hardware-related complications. These findings provide a new perspective to understanding the DBS-related complications for patients with PD, and highlight the importance of device improvement in lowering hardware-related adverse events.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Medical Ethical Committee, Qilu Hospital of Shandong University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
SX performed the acquisition, analysis and interpretation of data, and wrote the manuscript. WW performed the statistical analysis. SC and QW participated in the acquisition of data. CL and XM provided with valuable feedback on the manuscript. WL and TC provided with valuable feedback about the technique modifications. SjX designed and supervised the study, and conducted the final approval of the manuscript. All authors reviewed the manuscript.
This study was funded by National Natural Science Foundation of China (81502164), Taishan Scholarship Young Expert Program (tsqn201909174), and Department of Science and Technology of Shandong Province (2016GSF201055).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Adogwa, O., Elsamadicy, A. A., Vuong, V. D., Moreno, J., Cheng, J., Karikari, I. O., et al. (2017). Geriatric comanagement reduces perioperative complications and shortens duration of hospital stay after lumbar spine surgery: a prospective single-institution experience. J. Neurosurg. Spine 27, 670–675. doi: 10.3171/2017.5.spine17199
Baizabal Carvallo, J. F., Mostile, G., Almaguer, M., Davidson, A., Simpson, R., and Jankovic, J. (2012). Deep brain stimulation hardware complications in patients with movement disorders: risk factors and clinical correlations. Stereotact. Funct. Neurosurg. 90, 300–306. doi: 10.1159/000338222
Binder, D. K., Rau, G. M., and Starr, P. A. (2005). Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery 56, 722–732. doi: 10.1227/01.neu.0000156473.57196.7e
Bjerknes, S., Skogseid, I. M., Saehle, T., Dietrichs, E., and Toft, M. (2014). Surgical site infections after deep brain stimulation surgery: frequency, characteristics and management in a 10-year period. PLoS One 9:e105288. doi: 10.1371/journal.pone.0105288O
Boviatsis, E. J., Stavrinou, L. C., Themistocleous, M., Kouyialis, A. T., and Sakas, D. E. (2010). Surgical and hardware complications of deep brain stimulation. A seven-year experience and review of the literature. Acta Neurochir. (Wien) 152, 2053–2062. doi: 10.1007/s00701-010-0749-8
Deogaonkar, M., Nazzaro, J. M., Machado, A., and Rezai, A. (2011). Transient, symptomatic, post-operative, non-infectious hypodensity around the deep brain stimulation (DBS) electrode. J. Clin. Neurosci. 18, 910–915. doi: 10.1016/j.jocn.2010.11.020
Falowski, S. M., Ooi, Y. C., and Bakay, R. A. (2015). Long-term evaluation of changes in operative technique and hardware-related complications with deep brain stimulation. Neuromodulation 18, 670–677. doi: 10.1111/ner.12335
Fenoy, A. J., and Simpson, R. K. Jr. (2014). Risks of common complications in deep brain stimulation surgery: management and avoidance. J. Neurosurg. 120, 132–139. doi: 10.3171/2013.10.jns131225
Fernandez-Pajarin, G., Sesar, A., Ares, B., Relova, J. L., Aran, E., Gelabert-Gonzalez, M., et al. (2017). Delayed complications of deep brain stimulation: 16-year experience in 249 patients. Acta Neurochir. (Wien.) 159, 1713–1719. doi: 10.1007/s00701-017-3252-7
Fontaine, D., Vandersteen, C., Saleh, C., Von Langsdorff, D., and Poissonnet, G. (2013). Two-step tunneling technique of deep brain stimulation extension wires-a description. Acta Neurochir. (Wien.) 155, 2399–2402. doi: 10.1007/s00701-013-1870-2
Frizon, L. A., Hogue, O., Achey, R., Floden, D. P., Nagel, S., Machado, A. G., et al. (2019). Quality of Life improvement following deep brain stimulation for parkinson disease: development of a prognostic model. Neurosurgery 85, 343–349. doi: 10.1093/neuros/nyy287
Goodman, R. R., Kim, B., Mcclelland, S. III, Senatus, P. B., Winfield, L. M., Pullman, S. L., et al. (2006). Operative techniques and morbidity with subthalamic nucleus deep brain stimulation in 100 consecutive patients with advanced Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 77, 12–17. doi: 10.1136/jnnp.2005.069161
Hardaway, F. A., Raslan, A. M., and Burchiel, K. J. (2018). Deep brain stimulation-related infections: analysis of rates, timing, and seasonality. Neurosurgery 83, 540–547. doi: 10.1093/neuros/nyx505
Herrington, T. M., Cheng, J. J., and Eskandar, E. N. (2016). Mechanisms of deep brain stimulation. J. Neurophysiol. 115, 19–38.
Hu, K., Moses, Z. B., Hutter, M. M., and Williams, Z. (2017). Short-term adverse outcomes after deep brain stimulation treatment in patients with parkinson disease. World Neurosurg. 98, 365–374. doi: 10.1016/j.wneu.2016.10.138
Kantzanou, M., Korfias, S., Panourias, I., Sakas, D. E., and Karalexi, M. A. (2021). Deep brain stimulation-related surgical site infections: a systematic review and meta-analysis. Neuromodulation 24, 197–211. doi: 10.1111/ner.13354
Kim, M. S., Jeong, J. S., Ryu, H. S., Choi, S. H., and Chung, S. J. (2017). Infection related to deep brain stimulation in patients with Parkinson disease: clinical characteristics and risk factors. J. Neurol. Sci. 383, 135–141. doi: 10.1016/j.jns.2017.10.031
Linhares, P., Carvalho, B., and Vaz, R. (2013). One-step tunneling of DBS extensions–a technical note. Acta Neurochir. (Wien.) 155, 837–840. doi: 10.1007/s00701-013-1667-3
Ma, X., Li, W., Chen, S., Chen, T., Li, C., Xu, S., et al. (2017). Standard operation procedure and checklist of deep brain stimulation in department of neurosurgery, Qilu hospital of Shandong University. J. Shandong Univ. 55:4.
Miocinovic, S., Somayajula, S., Chitnis, S., and Vitek, J. L. (2013). History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 70, 163–171. doi: 10.1001/2013.jamaneurol.45
Nazzaro, J. M., Pahwa, R., and Lyons, K. E. (2017). Symptomatic, non-infectious, non-hemorrhagic edema after subthalamic nucleus deep brain stimulation surgery for Parkinson’s disease. J. Neurol. Sci. 383, 42–46. doi: 10.1016/j.jns.2017.10.003
Oh, M. Y., Abosch, A., Kim, S. H., Lang, A. E., and Lozano, A. M. (2002). Long-term hardware-related complications of deep brain stimulation. Neurosurgery 50, 1268–1274. 1268-1274; discussion, doi: 10.1097/00006123-200206000-00017
Okun, M. S. (2012). Deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 367, 1529–1538.
Paluzzi, A., Belli, A., Bain, P., Liu, X., and Aziz, T. M. (2006). Operative and hardware complications of deep brain stimulation for movement disorders. Br. J. Neurosurg. 20, 290–295. doi: 10.1080/02688690601012175
Park, J. H., Chung, S. J., Lee, C. S., and Jeon, S. R. (2011). Analysis of hemorrhagic risk factors during deep brain stimulation surgery for movement disorders: comparison of the circumferential paired and multiple electrode insertion methods. Acta Neurochir. (Wien.) 153, 1573–1578. doi: 10.1007/s00701-011-0997-2
Peduzzi, P., Concato, J., Kemper, E., Holford, T. R., and Feinstein, A. R. (1996). A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 49, 1373–1379. doi: 10.1016/s0895-4356(96)00236-3
Ramayya, A. G., Abdullah, K. G., Mallela, A. N., Pierce, J. T., Thawani, J., Petrov, D., et al. (2017). Thirty-day readmission rates following deep brain stimulation surgery. Neurosurgery 81, 259–267. doi: 10.1093/neuros/nyx019
Ramirez-Zamora, A., and Ostrem, J. L. (2018). Globus pallidus interna or subthalamic nucleus deep brain stimulation for parkinson disease: a review. JAMA Neurol. 75, 367–372. doi: 10.1001/jamaneurol.2017.4321
Rasouli, J. J., and Kopell, B. H. (2016). The adjunctive use of vancomycin powder appears safe and may reduce the incidence of surgical-site infections after deep brain stimulation surgery. World Neurosurg. 95, 9–13. doi: 10.1016/j.wneu.2016.07.063
Razmkon, A., Yousefi, O., Rezaei, R., Salehi, S., Petramfar, P., Mani, A., et al. (2019). Initial results of bilateral subthalamic nucleus stimulation for parkinson disease in a newly established center in a developing country: Shiraz, Southern Iran. World Neurosurg. 121, e129–e135.
Rughani, A. I., Hodaie, M., and Lozano, A. M. (2013). Acute complications of movement disorders surgery: effects of age and comorbidities. Mov. Disord. 28, 1661–1667. doi: 10.1002/mds.25610
Rumalla, K., Smith, K. A., Follett, K. A., Nazzaro, J. M., and Arnold, P. M. (2018). Rates, causes, risk factors, and outcomes of readmission following deep brain stimulation for movement disorders: analysis of the U.S. Nationwide Readmissions Database. Clin. Neurol. Neurosurg. 171, 129–134. doi: 10.1016/j.clineuro.2018.06.013
Sixel-Doring, F., Trenkwalder, C., Kappus, C., and Hellwig, D. (2010). Skin complications in deep brain stimulation for Parkinson’s disease: frequency, time course, and risk factors. Acta Neurochir. (Wien.) 152, 195–200. doi: 10.1007/s00701-009-0490-3
Sorar, M., Hanalioglu, S., Kocer, B., Eser, M. T., Comoglu, S. S., and Kertmen, H. (2018). Experience reduces surgical and hardware-related complications of deep brain stimulation surgery: a single-center study of 181 patients operated in six years. Parkinsons Dis. 2018:3056018.
Wang, X., Wang, J., Zhao, H., Li, N., Ge, S., Chen, L., et al. (2017). Clinical analysis and treatment of symptomatic intracranial hemorrhage after deep brain stimulation surgery. Br. J. Neurosurg. 31, 217–222. doi: 10.1080/02688697.2016.1244252
World Health Organization (WHO). (2018). Global Guidelines for the Prevention of Surgical Site Infection. Geneva: World Health Organization.
White-Dzuro, G. A., Lake, W., Eli, I. M., and Neimat, J. S. (2016). Novel approach to securing deep brain stimulation leads: technique and analysis of lead migration, breakage, and surgical infection. Stereotact. Funct. Neurosurg. 94, 18–23. doi: 10.1159/000442893
Whiting, A. C., Catapano, J. S., Walker, C. T., Godzik, J., Lambert, M., and Ponce, F. A. (2018). Peri-lead edema after deep brain stimulation surgery: a poorly understood but frequent complication. World Neurosurg. S1878-8750, 32915–2.
Yang, C., Qiu, Y., Wang, J., Wu, Y., Hu, X., and Wu, X. (2020). Intracranial hemorrhage risk factors of deep brain stimulation for Parkinson’s disease: a 2-year follow-up study. J. Int. Med. Res. 48, 300060519856747.
Keywords: deep brain stimulation, Parkinson’s disease, complications, operation, hardware, surgical modifications
Citation: Xu S, Wang W, Chen S, Wu Q, Li C, Ma X, Chen T, Li W and Xu S (2021) Deep Brain Stimulation Complications in Patients With Parkinson’s Disease and Surgical Modifications: A Single-Center Retrospective Analysis. Front. Hum. Neurosci. 15:684895. doi: 10.3389/fnhum.2021.684895
Received: 24 March 2021; Accepted: 17 May 2021;
Published: 11 June 2021.
Edited by:
Fangang Meng, Capital Medical University, ChinaReviewed by:
Carmen Terranova, University of Messina, ItalyCopyright © 2021 Xu, Wang, Chen, Wu, Li, Ma, Chen, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shujun Xu, eHUtc2h1anVucWxAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.