- 1Aging, Mobility, and Cognitive Neuroscience Laboratory, The University of British Columbia, Vancouver, BC, Canada
- 2Djavad Mowafaghian Centre for Brain Health, The University of British Columbia, Vancouver, BC, Canada
- 3Centre for Hip Health and Mobility, Vancouver Coastal Health Research Institute, Vancouver, BC, Canada
- 4Department of Radiology, The University of British Columbia, Vancouver, BC, Canada
- 5Hinda and Arthur Marcus Institute for Aging Research, Hebrew SeniorLife, Boston, MA, United States
- 6Harvard Medical School, Harvard University, Boston, MA, United States
- 7School of Biomedical Engineering, The University of British Columbia, Vancouver, BC, Canada
- 8Division of Geriatrics, Department of Medicine, The University of British Columbia, Vancouver, BC, Canada
- 9College of Medicine, King Saud University, Riyadh, Saudi Arabia
Background: Falls in older adults are a major public health problem. White matter hyperintensities (WMHs) are highly prevalent in older adults and are a risk factor for falls. In the absence of a cure for WMHs, identifying potential strategies to counteract the risk of WMHs on falls are of great importance. Physical activity (PA) is a promising countermeasure to reduce both WMHs and falls risk. However, no study has yet investigated whether PA attenuates the association of WMHs with falls risk. We hypothesized that PA moderates the association between WMHs and falls risk.
Methods: Seventy-six community-dwelling older adults aged 70–80 years old were included in this cross-sectional study. We indexed PA using the Physical Activity Score for the Elderly (PASE) Questionnaire. Falls risk was assessed using the Physiological Profile Assessment (PPA), and WMH volume (mm3) was determined by an experienced radiologist on T2-weighted and PD-weighted MRI scans. We first examined the independent associations of WMH volume and PASE score with PPA. Subsequently, we examined whether PASE moderated the relationship between WMH volume and PPA. We plotted simple slopes to interpret the interaction effects. Age, sex, and Montreal Cognitive Assessment (MoCA) score were included as covariates in all models.
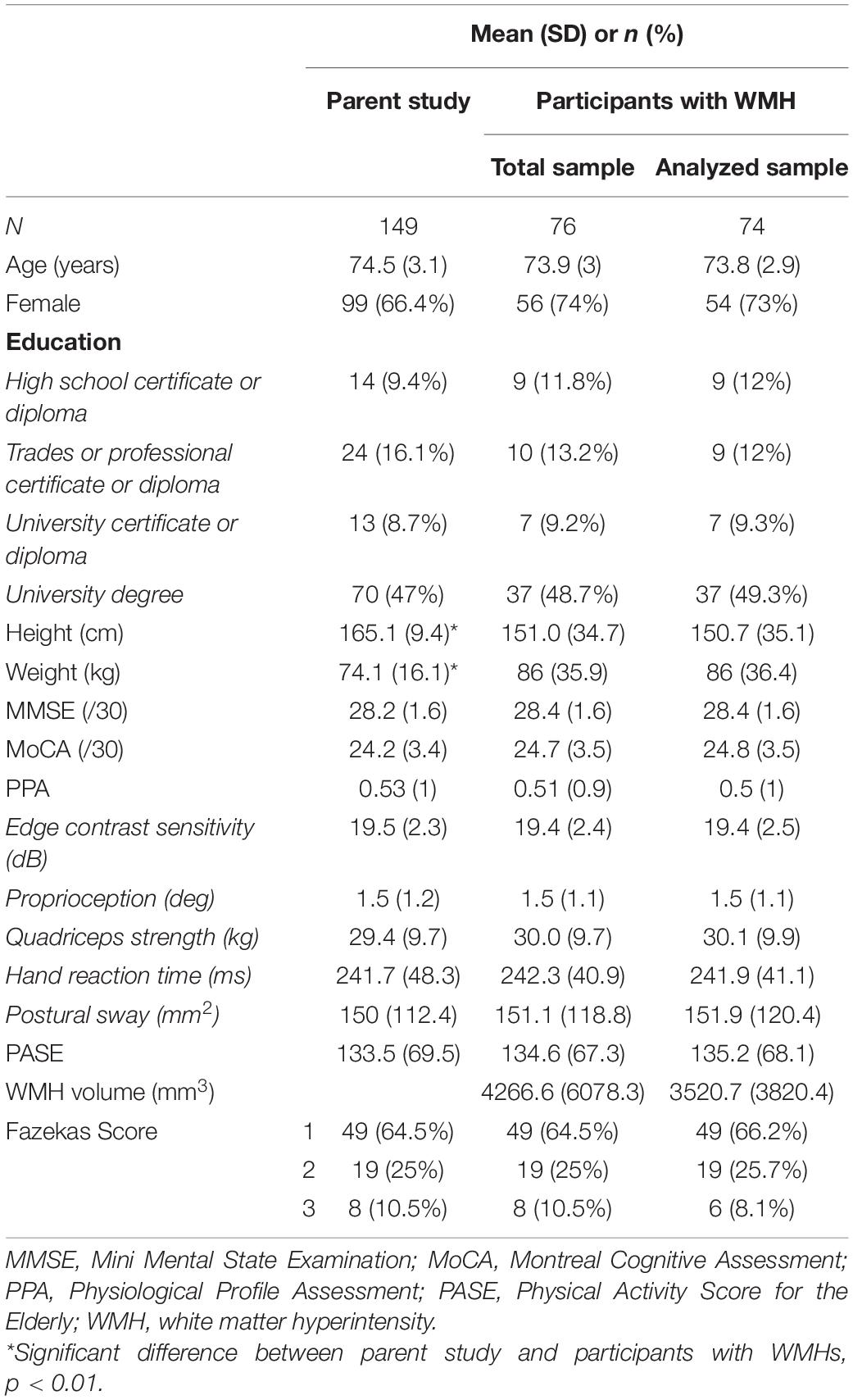
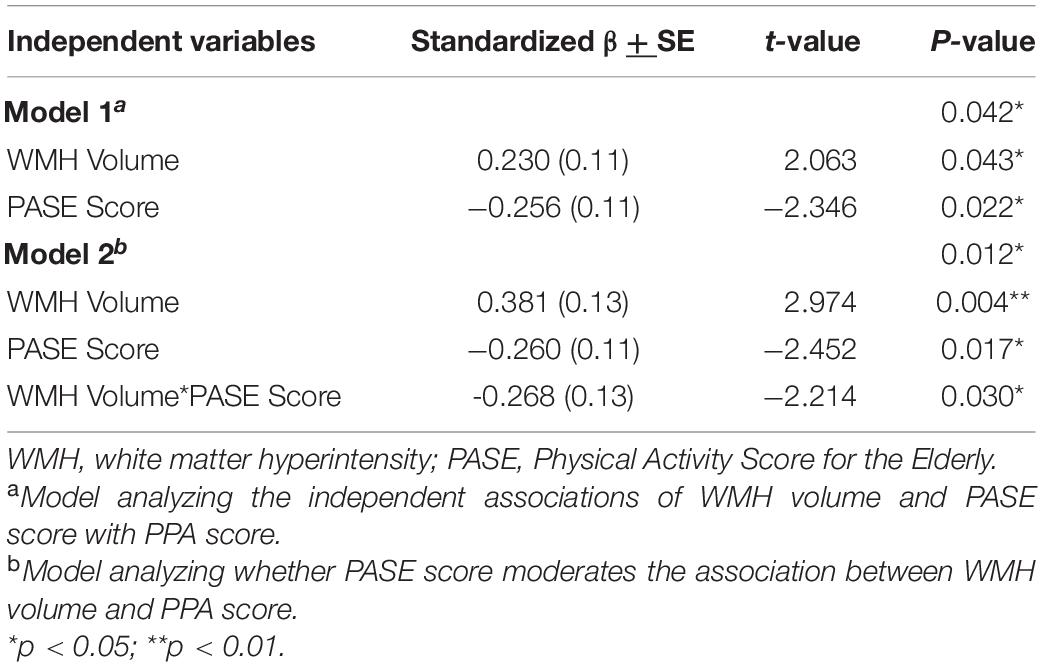
Results: Participants had a mean age of 74 years (SD = 3 years) and 54 (74%) were female. Forty-nine participants (66%) had a Fazekas score of 1, 19 (26%) had a score of 2, and 6 (8%) a score of 3. Both PASE (β = −0.26 ± 0.11; p = 0.022) and WMH volume (β = 0.23 ± 0.11; p = 0.043) were each independently associated with PPA score. The interaction model indicated that PASE score moderated the association between WMH volume and PPA (β = −0.27 ± 0.12; p = 0.030), whereby higher PASE score attenuated the association between WMHs and falls risk.
Conclusion: PA is an important moderator of falls risk. Importantly, older adults with WMH can reduce their risk of falls by increasing their PA.
Introduction
Falls are a major global public health problem (World Health Organization, 2008; Ageing and Life Course Unit), with over a third of older adults experiencing at least one fall each year (Tinetti et al., 1994). White matter hyperintensities (WMH) are a prominent feature of cerebrovascular disease and are prevalent in older adults (de Leeuw et al., 2001). Importantly, WMHs are a major risk factor for falls (Zheng et al., 2011). As there is currently no cure for WMHs (Alber et al., 2019), strategies which can mitigate the risks of WMH on falls are needed. Physical activity (PA) is an important modifiable lifestyle factor associated with both lower WMH volumes (Burzynska et al., 2014; Dao et al., 2018; Alber et al., 2019) and reduced risk of falling (Khan et al., 2001). However, whether PA moderates the burden of WMH volume on falls risk is not well established.
White matter hyperintensities are evident in over 90% of older adults (de Leeuw et al., 2001). They are small lesions that can be identified as bright, hyperintense regions on T2-weighted, proton density-weighted, and fluid attenuated inversion recovery magnetic resonance imaging (Wardlaw et al., 2015). They are most commonly caused by damage to the connecting blood vessels, resulting in reduced blood flow and oxygen to the brain cells (Sharma et al., 2020). Damage to brain white matter in the form of WMHs may lead to a reduction in the number and quality of important neural connections (Langen et al., 2017).
A meta-analysis by Kloppenborg et al. (2014), identified both cross-sectional and longitudinal associations between WMHs and multiple cognitive domains, including attention, processing speed, and executive functions. Deficits in these cognitive domains were identified as significant predictors of future falls risk in older adults (Mirelman et al., 2012). Further, impaired gait speed, balance and functional mobility are also associated with WMHs (Zheng et al., 2011) and have been identified as significant risk factors for falls (Lord et al., 1994; Ganz et al., 2007; Quach et al., 2011). Consequently, WMHs are associated with deficits in cognition and mobility, both of which contribute to an increased risk of falling. Several studies support the notion that older adults with a history of falls are more likely to have WMHs than non-fallers (Callisaya et al., 2015; Torres et al., 2015). In addition, Srikanth et al. (2009) identified that older adults in the top quintile for total WMH volume had double the risk of falling compared with those in the bottom quintile.
Lifestyle factors, such as PA, are a possible avenue to prevent and/or reverse WMH progression (Torres et al., 2015; Dao et al., 2018). PA is defined as any bodily movement produced by skeletal muscles that requires energy expenditure (Caspersen et al., 1985). Older adults with greater WMH volume are more likely to be inactive (Saczynski et al., 2008), and lower levels of PA are predictive of greater progression of WMH volume 3 years later (Gow et al., 2012). Twelve months of PA in the form of exercise training reduces WMH volumes (Bolandzadeh et al., 2015; Suo et al., 2016) indicating that increasing PA may help prevent WMH progression.
Being more physically active is also associated with reduced falls risk. Klenk et al. (2015) showed that older adults who walked for less than 1 h per day experienced more falls compared with their more active peers. Importantly, the most successful falls prevention programs center on increasing PA (Khan et al., 2001). A meta-analysis of 116 randomized controlled trials found that PA in the form of exercise training reduced the rate of falls by 23–42% (Sherrington et al., 2020).
While PA may thus counteract WMH progression and reduces fall risk, it is still unclear whether PA moderates the association of WMHs with falls risk in older adults. Identifying whether PA reduces the association of WMH with falls risk will provide important insight for the development of falls prevention programs targeting this population. The aim of this study is to determine whether level of PA moderates the association between WMH volume and falls risk. It is hypothesized that greater levels of PA will attenuate the association of WMH with falls risk.
Materials and Methods
Participants
This study was a secondary cross-sectional analysis of a longitudinal study (N = 149) aimed at investigating the relationship between mobility, functional connectivity, and cognition (Hsu et al., 2014). For the present analysis, we only included a subset of participants (n = 76) with WMH on baseline MRI.
We recruited community dwelling older adults from Greater Vancouver. Participants were: (1) aged 70 to 80 years old; (2) scored >24/30 on the Mini-Mental State Examination (MMSE) (Folstein et al., 1975); (3) right hand dominant as measured by the Edinburgh Handedness Inventory (Oldfield, 1971); (4) living independently in their own homes; (5) had a visual acuity of at least 20/40, with or without corrective lenses; and (6) provided informed consent. Ethics approval (H07-00160) was obtained from the Vancouver Coastal Health Research Institute and University of British Columbia’s Clinical Research Ethics Board.
Measures
For descriptive purposes, age in years, height in centimeters, and weight in kilograms were measured. General cognition was also assessed using the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005) tool.
Falls Risk
Falls risk was assessed using the Physiological Profile Assessment© (PPA) [Prince of Wales Medical Research Institute, Randwick, Sydney, NSW, Australia (Lord et al., 2003)]. The PPA is a validated measure for quantifying falls risk in older adults (Lord et al., 2003) with a 75% predictive accuracy for falls (Lord et al., 1994). This five component assessment battery creates a composite falls risk score from: (1) hand reaction time; (2) knee extension strength; (3) visual contrast sensitivity; (4) balance; and (5) proprioception. A higher PPA score is indicative of greater falls risk.
Physical Activity
The Physical Activity Scale for the Elderly (PASE) is a questionnaire designed to determine PA levels in older adults over the age of 65 (Washburn et al., 1993). The PASE combines information from household, leisure, and occupational activities typical of this population. It includes measures of frequency, duration, and level of intensity of the activity. Scores range from 0 to 793 with higher scores indicative of greater PA levels.
White Matter Hyperintensity Quantification
Magnetic resonance imaging (MRI) scans were conducted at the UBC MRI Research Center on a 3T Philips Achieva Scanner with an 8-channel SENSE neurovascular coil. T2-weighted and proton density (PD)-weighted structural MRI scans were acquired for each subject. The T2-weighted scan used a repetition time (TR) of 2,500 ms, echo time (TE) of 382 ms, and a 312 × 312 acquisition matrix. The PD-weighted scan, used a 3,000 ms TR, 30 ms TE, and 252 × 250 acquisition matrix.
The MRI scans were preprocessed using standard neuroimaging tools including: (1) a structure-preserving noise removal filter (SUSAN) (Smith and Brady, 1997); (2) a brain extraction tool (BET) to remove all non-brain tissue (Smith, 2002); and (3) a multiscale version of the non-parametric non-uniform intensity normalization method (N3) for MR intensity inhomogeneity correction (Sled et al., 1998). White matter hyperintensities were determined by an experienced radiologist (McAusland et al., 2010). The seeding procedure guidelines were to: (1) mark all distinct WMHs regardless of size; (2) use additional points if more than one point would help define the extent of the lesion; (3) place at least one point near the center of each lesion (McAusland et al., 2010). The WMHs were then automatically segmented by computing the extent of each lesion using a customized Parzen windows classifier (McAusland et al., 2010). The average total WMH volume across both hemispheres was used to calculate a single WMH volume value per participant (Liu-Ambrose et al., 2006).
Data Analysis
All statistical analyses were completed in R version 4.0.3 (see Supplementary Materials 1, 2). Descriptive statistics for all participants were calculated using tableone 0.12.0. We conducted a linear regression model, which examined: (1) the association of WMH volume with falls risk independent of PA; and (2) the association of PA and falls risk independent of WMH volume. WMH volume and PASE score were included in the linear regression model as the independent variables of interest; PPA score was the dependent variable. Baseline age, sex and baseline MoCA score were included as covariates of no interest.
To examine whether PA moderated the association of WMH volume with falls risk, we performed a second linear regression model, which included as independent variables: (1) WMH volume, (2) PASE score, and (3) the interaction of WMH volume with PASE score. PPA score was the dependent variable of interest; age, sex, and MoCA were included as covariates. The interaction (i.e., WMH volume X PASE score) was then decomposed using model-based estimates of simple slopes, in which the relation between WMH volume and PPA score was estimated separately for low PASE score (i.e., 1 SD below the mean; ∼67/703) and high PASE score (1 SD above the mean; ∼203/793). Standardized beta estimates, standard errors, and p-values are presented. All significant relationships were plotted with ggplot2 3.3.2.
In the event that PA was found to significantly moderate the association between WMH and PPA. We conducted exploratory analyses to identify if PA moderated the association between WMH and any of the five individual components of the PPA specifically.
In accordance with our ethical guidelines, data is available upon review of specific requests.
Results
Participant Characteristics
Of the 76 participants included in the study, two were considered outliers (i.e., >3 SD above mean WMH volume) and were excluded. Thus, a total of 74 participants were included in the final analyses (see Table 1); mean (SD) age of 73.8 (2.9) years, 54 female (73%). Over half of the participants (66%) had a Fazekas score of 1, indicating low WMH load, while 36% had a score of 2 or 3, meeting the criteria for moderate to high WMH load, respectively (Fazekas et al., 1987). Mean PPA score was 0.5 (0.1), which is considered a mild risk for falling (Lord et al., 2003). Overall, participants were slightly more physically active than the average for this age group (Washburn et al., 1999), with a mean PASE score of 135.2 (68.1). Of note, 50% of participants met the criteria for mild cognitive impairment, scoring <26/30 on the MoCA (Nasreddine et al., 2005). There was a significant difference in height (p = 0.001) and weight (p = 0.007) between the analyzed sample and the parent study sample. There were no other significant differences between the two samples.
Independent Associations of WMH Volume and PASE Score With Falls Risk
The independent associations of WMH volume and PASE score with PPA are illustrated in Figure 1. Greater WMH volume was significantly associated with greater falls risk (β = 0.23 ± 0.11; p = 0.043), independent of PA. We also found that higher PASE score was significantly associated with lower falls risk (β = −0.26 ± 0.11; p = 0.022), independent of WMH volume (see Table 2, model 1).
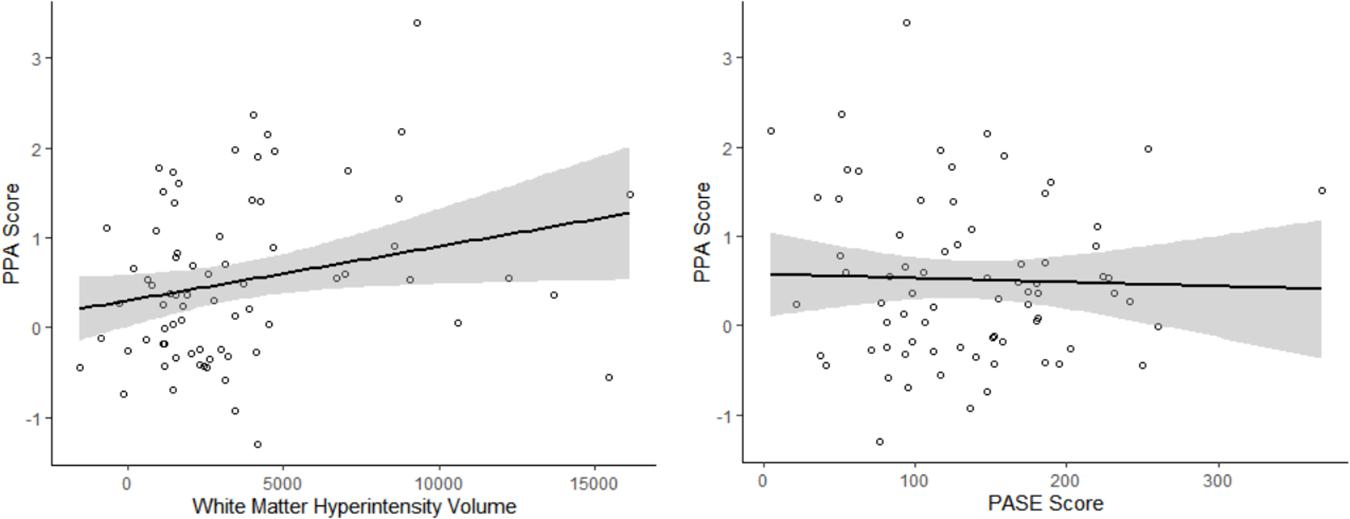
Figure 1. The independent associations of white matter hyperintensity (WMH) volume and Physical Activity Score for the Elderly (PASE) with Physiological Profile Assessment (PPA) score. WMH volume was associated with greater falls risk, independent of PASE score (Adjusted R2 = 5.3%). Higher PASE score was associated with lower falls risk, independent of WMH volume (Adjusted R2 = 6.9%). Age, sex, and MoCA score were included as covariates of no interest.
PASE Score Moderates the Association Between WMH Volume and Falls Risk
Our analyses examining if PASE score moderated the association between WMH volume and PPA score are described in Figure 2. We found that PASE score significantly moderated the association between WMH volume and falls risk (β = −0.27 ± 0.12; p = 0.030), whereby higher PASE score attenuated the association between WMH volume and PPA score (see Table 2, model 2). Importantly, our simple slopes analyses indicated that for participants with high PASE score, there was no longer an association between WMH and PPA score (β = 0.11 ± 0.12; p = 0.351).
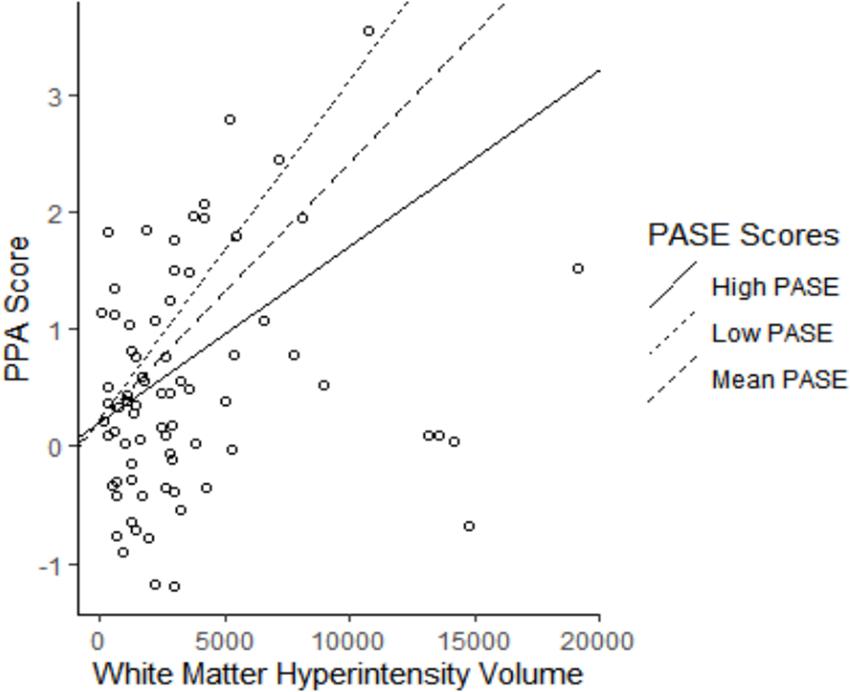
Figure 2. The association of white matter hyperintensity (WMH) volume with falls risk moderated by physical activity level. Higher Physical Activity Score for the Elderly (PASE) attenuated the association between WMH volume and Physiological Profile Assessment (PPA) score (Adjusted R2 = 5.8%). High PASE = 1 SD above mean; Low PASE = 1 SD below mean.
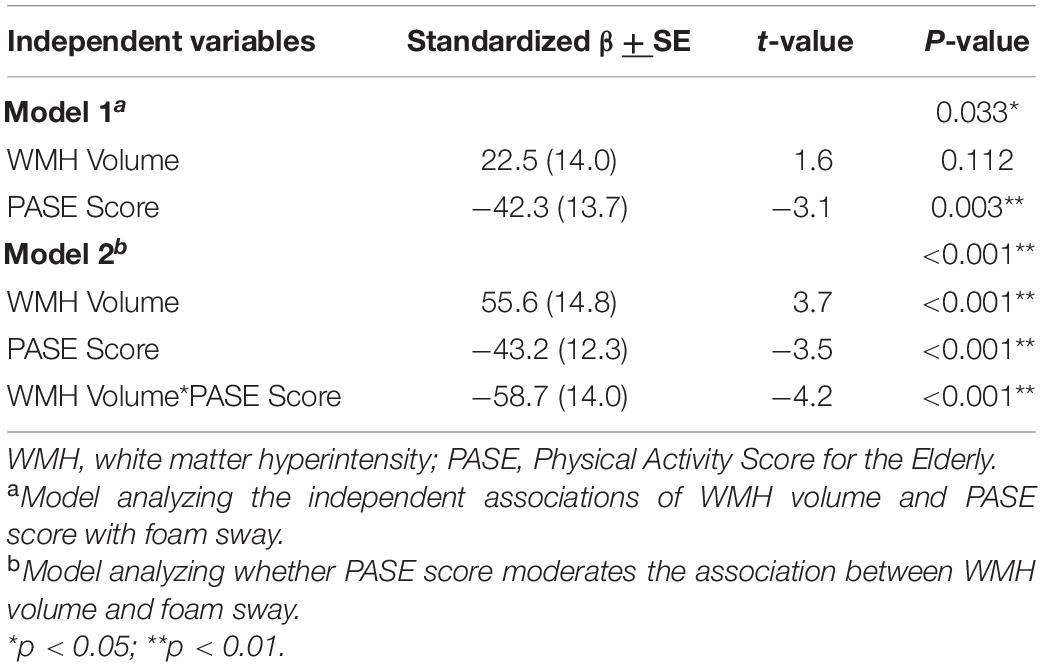
As a result of the main findings being significant, we conducted exploratory analyses investigating whether PASE score moderated the association between WMH volume and each individual component of the PPA. We identified that PASE score significantly moderated the association between WMH volume and postural sway (β = −58.7 ± 14; p < 0.001), whereby higher PASE score attenuated the association between WMH and postural sway (see Table 3, model 2). The results of the simple slope analyses can be seen in Figure 3. Consistent with the PPA findings, there was no longer an association between WMH and foam sway in participants with high PASE scores (β < 0.001 ± 0.004; p = 0.83).
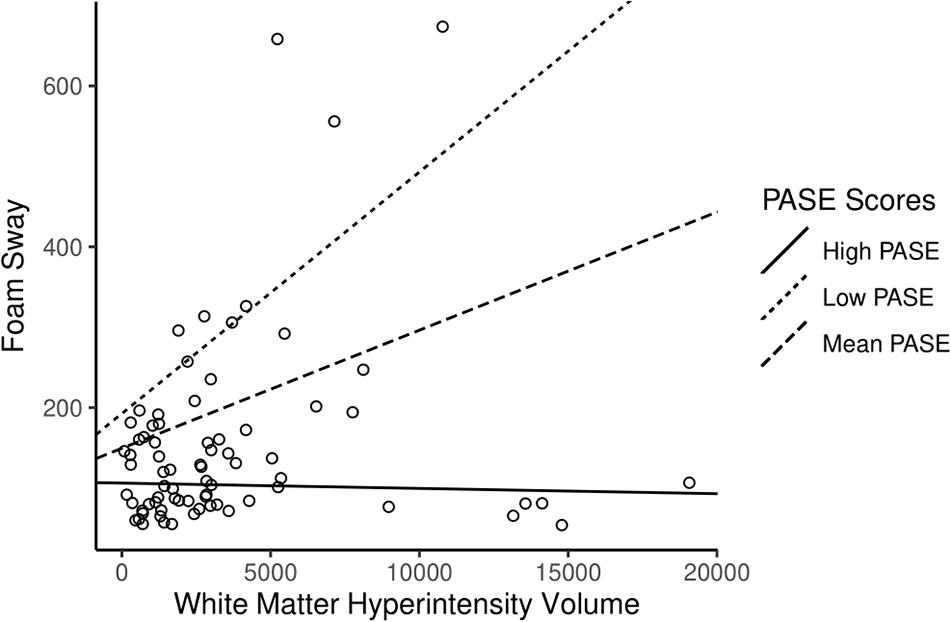
Figure 3. The association of white matter hyperintensity (WMH) volume with foam sway moderated by physical activity level. Higher Physical Activity Score for the Elderly (PASE) attenuated the association between WMH volume and Foam Sway (Adjusted R2 = 17.4%). High PASE = 1 SD above mean; Low PASE = 1 SD below mean.
All other analyses were not significant (see Supplementary Material 2).
Discussion
Our findings suggest PA significantly reduces the association between WMHs with falls risk after accounting for age, sex and global cognition. Thus, PA may be an important component of falls-prevention for older adults with WMHs. High volumes of WMHs substantially increase falls risk (Srikanth et al., 2009). However, our results suggest PA can reduce the association of WMHs with falls risk in older adults with low, moderate, as well as high WMH volumes. Our simple slope analysis indicates that higher PASE score attenuates the association between WMHs and falls risk; indeed, WMHs were not associated with falls risk for participants with high PASE score (>203/793). The results of our exploratory analyses identified that PA significantly moderated the association between WMH volume and the postural sway component of the PPA.
We theorize two possible underlying mechanisms by which PA may moderate the association between WMHs and falls. Disruptions to crucial neural connections as a result of WMHs has been suggested as a potential means by which WMH may lead to increased risk of falling (Zheng et al., 2011). WMHs are found to disrupt the neural connectivity of networks involved in both cognition and mobility (Murray et al., 2010; Sun et al., 2011; Ding et al., 2018), which are both associated with an increased risk of falling (Zheng et al., 2011; Nagamatsu et al., 2013). PA may counteract this deficit through neural compensation (Dao et al., 2018; Hsu et al., 2018). This pertains to the concept of less wiring more firing (Daselaar et al., 2015), whereby older adults with reduced structural connectivity are able to maintain task performance through greater activation of the remaining connections. However, this increase in neural activation requires greater resources, such as an increased oxygen supply (Lassen, 1959; Ozugur et al., 2020). PA increases blood flow, leading to greater supply of oxygen and vital nutrients to the brain (Scheinberg et al., 1954; Kleinloog et al., 2019). Thus, despite there being fewer and/or weaker neural connections in older adults with greater WMH volume, PA may support neural compensation by enabling greater activation of the remaining connections. This is highly relevant in the context of this study, as aberrant functional connectivity within and between major networks has been seen in older adults with a history of falling (Hsu et al., 2014) and is associated with poorer postural stability using the foam sway task (Crockett et al., 2017, 2019). In addition, changes in functional connectivity was significantly associated with improved mobility in older adults after 6 months of aerobic training (Hsu et al., 2017). Therefore, despite the presence of WMHs, PA may aid brain function, reducing the disruption to important networks required for falls prevention.
Physical activity has also been shown to improve muscular strength, functional mobility and balance in older adults (Langhammer et al., 2018). Reduced muscle strength, and poor functional mobility and balance are associated with greater risk of falling (Tinetti et al., 1988; Campbell et al., 1989; Wang et al., 2016). It is possible that the effect of PA on these peripheral factors may outweigh the deficit caused by WMHs, resulting in a net reduction in falls risk. This is further highlighted by our finding that, in addition to overall falls risk, PA was able to significantly moderate the association between WMHs and postural sway specifically. Therefore, it is possible that higher levels of PA in areas that improve balance, strength, and mobility may underlie the association with lower falls risk in spite of the presence of WMHs. However, PA has previously also been shown to preserve age-related decline in proprioception, which is another key risk factor for falls (Ribeiro and Oliveira, 2007). Thus, it is important to acknowledge that although the results of this study highlight the significant association between PA and postural stability, PA likely targets multiple fall risk factors simultaneously. Further research is required to greater understand the underlying mechanisms by which physical activity moderates the association between WMHs and falls risk.
Limitations
Due to its cross-sectional design, the extent to which PA moderates the association of WMHs with falls risk cannot be generalizable to longitudinal or causal predictions of PA. Further research investigating how this relationship manifests over time would be highly beneficial. We used a self-report measure of PA. This is subject to bias and memory recall (Falck et al., 2016). Further research using an objective measure of PA is needed to support these findings. The aim of this study was to understand the role of overall PA level in moderating the relationship between WMH and falls risk. Thus, the PASE is a validated measure for this purpose (Washburn et al., 1993; Washburn and Ficker, 1999). However, using this measure, it is not possible to identify the role of each PA component on this relationship. Future research should consider identifying which, if any, components of PA may be driving this moderation. We assessed falls risk as opposed to history of falling and did not include measures of other fall risk factors, such as medication (Woolcott et al., 2009), and depression (Iaboni and Flint, 2013). While the PPA is a validated measure for assessing falls risk (Lord et al., 2003), replicating these findings with additional data of falls incidence, and other risk factors for falls, would be appropriate. Finally, the study sample was above average in PA level, and had on average a mild risk of falling. Thus, our findings may not be generalizable to older adults who are more sedentary and/or frail.
Conclusion
Older adults with greater WMH volume are at an increased risk of falling. However, PA attenuates the association of WMHs with falls risk whereby there is no longer a statistically significant association of WMH volume with falls risk in individuals with high PA. Future work should determine the longitudinal impact of PA on WMHs and falls risk in older adults.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Vancouver Coastal Health Research Institute, and the University of British Columbia’s Clinical Research Ethics Board (H07-00160). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RC, CH, RT, WA, and TL-A involved in the designing and performing of the study. RC, RF, and TL-A contributed to the data analysis and were involved in the interpretation of results. RC wrote the first draft of the manuscript. RF, CH, ED, and TL-A wrote portions of the manuscript and critically reviewed the manuscript. All authors have read and approved the manuscript.
Funding
Funding was provided by the Canadian Institutes of Health Research (MOB-93373) to TL-A. RC is a recipient of the UBC Rehabilitation Sciences Doctoral Award, and CH and ED are Canadian Institutes of Health Research Post-Doctoral Fellows. TL-A is a Canada Research Chair (Tier 2) in Physical Activity, Mobility, and Cognitive Neuroscience.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2021.671464/full#supplementary-material
References
Alber, J., Alladi, S., Bae, H. J., Barton, D. A., Beckett, L. A., Bell, J. M., et al. (2019). White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): knowledge gaps and opportunities. Alzheimers Dement. 5, 107–117.
Bolandzadeh, N., Tam, R., Handy, T. C., Nagamatsu, L. S., Hsu, C. L., Davis, J. C., et al. (2015). Resistance training and white matter lesion progression in older women: exploratory analysis of a 12-month randomized controlled trial. J. Am. Geriatr. Soc. 63, 2052–2060. doi: 10.1111/jgs.13644
Burzynska, A. Z., Chaddock-Heyman, L., Voss, M. W., Wong, C. N., Gothe, N. P., Olson, E. A., et al. (2014). Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PLoS One 9:e107413. doi: 10.1371/journal.pone.0107413
Callisaya, M. L., Beare, R., Phan, T., Blizzard, L., Thrift, A. G., Chen, J., et al. (2015). Progression of white matter hyperintensities of presumed vascular origin increases the risk of falls in older people. J. Gerontol. A Biol. Sci. Med. Sci. 70, 360–366. doi: 10.1093/gerona/glu148
Campbell, A. J., Borrie, M. J., and Spears, G. F. (1989). Risk factors for falls in a community-based prospective study of people 70 years and older. J. Gerontol. 44, M112–M117.
Caspersen, C. J., Powell, K. E., and Christenson, G. M. (1985). Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 100, 126–131.
Crockett, R. A., Hsu, C. L., Best, J. R., Beauchet, O., and Liu-Ambrose, T. (2019). Head over heels but i forget why: disruptive functional connectivity in older adult fallers with mild cognitive impairment. Behav. Brain Res. 376:112104. doi: 10.1016/j.bbr.2019.112104
Crockett, R. A., Hsu, C. L., Best, J. R., and Liu-Ambrose, T. (2017). Resting state default mode network connectivity, dual task performance, gait speed, and postural sway in older adults with mild cognitive impairment. Front. Aging Neurosci. 9:423. doi: 10.3389/fnagi.2017.00423
Dao, E., Hsiung, G. R., and Liu-Ambrose, T. (2018). The role of exercise in mitigating subcortical ischemic vascular cognitive impairment. J. Neurochem. 144, 582–594. doi: 10.1111/jnc.14153
Daselaar, S. M., Iyengar, V., Davis, S. W., Eklund, K., Hayes, S. M., and Cabeza, R. E. (2015). Less wiring, more firing: low-performing older adults compensate for impaired white matter with greater neural activity. Cereb. Cortex 25, 983–990. doi: 10.1093/cercor/bht289
de Leeuw, F. E., de Groot, J. C., Achten, E., Oudkerk, M., Ramos, L. M., Heijboer, R., et al. (2001). Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam scan study. J. Neurol. Neurosurg. Psychiatry 70, 9–14. doi: 10.1136/jnnp.70.1.9
Ding, J. R., Ding, X., Hua, B., Xiong, X., Wen, Y., Ding, Z., et al. (2018). Altered connectivity patterns among resting state networks in patients with ischemic white matter lesions. Brain Imaging Behav. 12, 1239–1250. doi: 10.1007/s11682-017-9793-9
Falck, R. S., McDonald, S. M., Beets, M. W., Brazendale, K., and Liu-Ambrose, T. (2016). Measurement of physical activity in older adult interventions: a systematic review. Br. J. Sports Med. 50, 464–470. doi: 10.1136/bjsports-2014-094413
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., and Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 149, 351–356. doi: 10.2214/ajr.149.2.351
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198.
Ganz, D. A., Bao, Y., Shekelle, P. G., and Rubenstein, L. Z. (2007). Will my patient fall? JAMA 297, 77–86. doi: 10.1001/jama.297.1.77
Gow, A. J., Bastin, M. E., Munoz Maniega, S., Valdes Hernandez, M. C., Morris, Z., Murray, C., et al. (2012). Neuroprotective lifestyles and the aging brain: activity, atrophy, and white matter integrity. Neurology 79, 1802–1808. doi: 10.1212/wnl.0b013e3182703fd2
Hsu, C. L., Best, J. R., Davis, J. C., Nagamatsu, L. S., Wang, S., Boyd, L. A., et al. (2018). Aerobic exercise promotes executive functions and impacts functional neural activity among older adults with vascular cognitive impairment. Br. J. Sports Med. 52, 184–191. doi: 10.1136/bjsports-2016-096846
Hsu, C. L., Best, J. R., Wang, S., Voss, M. W., Hsiung, R. G. Y., Munkacsy, M., et al. (2017). The impact of aerobic exercise on fronto-parietal network connectivity and its relation to mobility: an exploratory analysis of a 6-month randomized controlled trial. Front. Hum. Neurosci. 11:344. doi: 10.3389/fnhum.2017.00344
Hsu, C. L., Voss, M. W., Handy, T. C., Davis, J. C., Nagamatsu, L. S., Chan, A., et al. (2014). Disruptions in brain networks of older fallers are associated with subsequent cognitive decline: a 12-month prospective exploratory study. PLoS One 9:e93673. doi: 10.1371/journal.pone.0093673
Iaboni, A., and Flint, A. J. (2013). The complex interplay of depression and falls in older adults: a clinical review. Am. J. Geriatr. Psychiatry 21, 484–492. doi: 10.1016/j.jagp.2013.01.008
Khan, K. M., Liu-Ambrose, T., Donaldson, M. G., and McKay, H. A. (2001). Physical activity to prevent falls in older people: time to intervene in high risk groups using falls as an outcome. Br. J. Sports Med. 35, 144–145. doi: 10.1136/bjsm.35.3.144
Kleinloog, J. P. D., Mensink, R. P., Ivanov, D., Adam, J. J., Uludag, K., and Joris, P. J. (2019). Aerobic exercise training improves cerebral blood flow and executive function: a randomized, controlled cross-over trial in sedentary older men. Front. Aging Neurosci. 11:333. doi: 10.3389/fnagi.2019.00333
Klenk, J., Kerse, N., Rapp, K., Nikolaus, T., Becker, C., Rothenbacher, D., et al. (2015). Physical activity and different concepts of fall risk estimation in older people–results of the ActiFE-Ulm study. PLoS One 10:e0129098. doi: 10.1371/journal.pone.0129098
Kloppenborg, R. P., Nederkoorn, P. J., Geerlings, M. I., and van den Berg, E. (2014). Presence and progression of white matter hyperintensities and cognition: a meta-analysis. Neurology 82, 2127–2138. doi: 10.1212/wnl.0000000000000505
Langen, C. D., Zonneveld, H. I., White, T., Huizinga, W., Cremers, L. G. M., de Groot, M., et al. (2017). White matter lesions relate to tract-specific reductions in functional connectivity. Neurobiol. Aging 51, 97–103. doi: 10.1016/j.neurobiolaging.2016.12.004
Langhammer, B., Bergland, A., and Rydwik, E. (2018). The importance of physical activity exercise among older people. Biomed Res. Int. 2018:7856823.
Lassen, N. A. (1959). Cerebral blood flow and oxygen consumption in man. Physiol. Rev. 39, 183–238. doi: 10.1152/physrev.1959.39.2.183
Liu-Ambrose, T., Khan, K. M., Donaldson, M. G., Eng, J. J., Lord, S. R., and McKay, H. A. (2006). Falls-related self-efficacy is independently associated with balance and mobility in older women with low bone mass. J. Gerontol. A Biol. Sci. Med. Sci. 61, 832–838. doi: 10.1093/gerona/61.8.832
Lord, S. R., Menz, H. B., and Tiedemann, A. (2003). A physiological profile approach to falls risk assessment and prevention. Phys. Ther. 83, 237–252. doi: 10.1093/ptj/83.3.237
Lord, S. R., Ward, J. A., Williams, P., and Anstey, K. J. (1994). Physiological factors associated with falls in older community-dwelling women. J. Am. Geriatr. Soc. 42, 1110–1117. doi: 10.1111/j.1532-5415.1994.tb06218.x
McAusland, J., Tam, R. C., Wong, E., Riddehough, A., and Li, D. K. (2010). Optimizing the use of radiologist seed points for improved multiple sclerosis lesion segmentation. IEEE Trans. Biomed. Eng. 57, 2689–2698. doi: 10.1109/tbme.2010.2055865
Mirelman, A., Herman, T., Brozgol, M., Dorfman, M., Sprecher, E., Schweiger, A., et al. (2012). Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS One 7:e40297. doi: 10.1371/journal.pone.0040297
Murray, M. E., Senjem, M. L., Petersen, R. C., Hollman, J. H., Preboske, G. M., Weigand, S. D., et al. (2010). Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch. Neurol. 67, 1379–1385.
Nagamatsu, L. S., Boyd, L. A., Hsu, C. L., Handy, T. C., and Liu-Ambrose, T. (2013). Overall reductions in functional brain activation are associated with falls in older adults: an fMRI study. Front. Aging Neurosci. 5:91. doi: 10.3389/fnagi.2013.00091
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. doi: 10.1016/0028-3932(71)90067-4
Ozugur, S., Kunz, L., and Straka, H. (2020). Relationship between oxygen consumption and neuronal activity in a defined neural circuit. BMC Biol. 18:76. doi: 10.1186/s12915-020-00811-6
Quach, L., Galica, A. M., Jones, R. N., Procter-Gray, E., Manor, B., Hannan, M. T., et al. (2011). The nonlinear relationship between gait speed and falls: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston Study. J. Am. Geriatr. Soc. 59, 1069–1073. doi: 10.1111/j.1532-5415.2011.03408.x
Ribeiro, F., and Oliveira, J. (2007). Aging effects on joint proprioception: the role of physical activity in proprioception preservation. Eur. Rev. Aging Phys. A 4, 71–76. doi: 10.1007/s11556-007-0026-x
Saczynski, J. S., Jonsdottir, M. K., Sigurdsson, S., Eiriksdottir, G., Jonsson, P. V., Garcia, M. E., et al. (2008). White matter lesions and cognitive performance: the role of cognitively complex leisure activity. J. Gerontol. A Biol. Sci. Med. Sci. 63, 848–854. doi: 10.1093/gerona/63.8.848
Scheinberg, P., Blackburn, L. I., Rich, M., and Saslaw, M. (1954). Effects of vigorous physical exercise on cerebral circulation and metabolism. Am. J. Med. 16, 549–554. doi: 10.1016/0002-9343(54)90371-x
Sharma, R., Sekhon, S., and Cascella, M. (2020). White Matter Lesions. Treasure Island, FL: StatPearls.
Sherrington, C., Fairhall, N., Kwok, W., Wallbank, G., Tiedemann, A., Michaleff, Z. A., et al. (2020). Evidence on physical activity and falls prevention for people aged 65+ years: systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 17:144.
Sled, J. G., Zijdenbos, A. P., and Evans, A. C. (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging 17, 87–97. doi: 10.1109/42.668698
Smith, S. M. (2002). Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155. doi: 10.1002/hbm.10062
Smith, S. M., and Brady, J. M. (1997). SUSAN – a new approach to low level image processing. Int. J. Comp. Vis. 23, 45–78.
Srikanth, V., Beare, R., Blizzard, L., Phan, T., Stapleton, J., Chen, J., et al. (2009). Cerebral white matter lesions, gait, and the risk of incident falls: a prospective population-based study. Stroke 40, 175–180. doi: 10.1161/strokeaha.108.524355
Sun, Y. W., Qin, L. D., Zhou, Y., Xu, Q., Qian, L. J., Tao, J., et al. (2011). Abnormal functional connectivity in patients with vascular cognitive impairment, no dementia: a resting-state functional magnetic resonance imaging study. Behav. Brain Res. 223, 388–394. doi: 10.1016/j.bbr.2011.05.006
Suo, C., Singh, M. F., Gates, N., Wen, W., Sachdev, P., Brodaty, H., et al. (2016). Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry 21, 1633–1642. doi: 10.1038/mp.2016.19
Tinetti, M. E., Baker, D. I., McAvay, G., Claus, E. B., Garrett, P., Gottschalk, M., et al. (1994). A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N. Engl. J. Med. 331, 821–827. doi: 10.1056/nejm199409293311301
Tinetti, M. E., Speechley, M., and Ginter, S. F. (1988). Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 319, 1701–1707. doi: 10.1056/nejm198812293192604
Torres, E. R., Strack, E. F., Fernandez, C. E., Tumey, T. A., and Hitchcock, M. E. (2015). Physical activity and white matter hyperintensities: a systematic review of quantitative studies. Prev. Med. Rep. 2, 319–325. doi: 10.1016/j.pmedr.2015.04.013
Wang, X., Ma, Y., Wang, J., Han, P., Dong, R., Kang, L., et al. (2016). Mobility and muscle strength together are more strongly correlated with falls in suburb-dwelling older Chinese. Sci. Rep. 6:25420.
Wardlaw, J. M., Valdes Hernandez, M. C., and Munoz-Maniega, S. (2015). What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc. 4:001140.
Washburn, R. A., and Ficker, J. L. (1999). Physical activity scale for the elderly (PASE): the relationship with activity measured by a portable accelerometer. J. Sports Med. Phys. Fitness 39, 336–340.
Washburn, R. A., McAuley, E., Katula, J., Mihalko, S. L., and Boileau, R. A. (1999). The physical activity scale for the elderly (PASE): evidence for validity. J. Clin. Epidemiol. 52, 643–651.
Washburn, R. A., Smith, K. W., Jette, A. M., and Janney, C. A. (1993). The physical activity scale for the elderly (PASE): development and evaluation. J. Clin. Epidemiol. 46, 153–162. doi: 10.1016/0895-4356(93)90053-4
Woolcott, J. C., Richardson, K. J., Wiens, M. O., Patel, B., Marin, J., Khan, K. M., et al. (2009). Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch. Intern. Med. 169, 1952–1960. doi: 10.1001/archinternmed.2009.357
World Health Organization (2008). Ageing and Life Course Unit. WHO Global Report on Falls Prevention in Older Age. Geneva: World Health Organization.
Keywords: white matter hyperintensities, physical activity, falls risk, aging, cerebrovascular disease
Citation: Crockett RA, Falck RS, Dao E, Hsu CL, Tam R, Alkeridy W and Liu-Ambrose T (2021) Sweat the Fall Stuff: Physical Activity Moderates the Association of White Matter Hyperintensities With Falls Risk in Older Adults. Front. Hum. Neurosci. 15:671464. doi: 10.3389/fnhum.2021.671464
Received: 24 February 2021; Accepted: 29 April 2021;
Published: 21 May 2021.
Edited by:
Henriette van Praag, Florida Atlantic University, United StatesReviewed by:
Tiffany E. Shubert, University of North Carolina at Chapel Hill, United StatesJulia C. Basso, Virginia Tech, United States
Copyright © 2021 Crockett, Falck, Dao, Hsu, Tam, Alkeridy and Liu-Ambrose. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teresa Liu-Ambrose, dGVyZXNhLmFtYnJvc2VAdWJjLmNh
 Rachel A. Crockett
Rachel A. Crockett Ryan. S. Falck
Ryan. S. Falck Elizabeth Dao
Elizabeth Dao Chun Liang Hsu
Chun Liang Hsu Roger Tam4,7
Roger Tam4,7 Teresa Liu-Ambrose
Teresa Liu-Ambrose