- Psychology Department, Dominican University, River Forest, IL, United States
Bilingualism is of great interest to the neuroscience of language, and understanding the anatomical changes associated with second language learning help inform theories of bilingual advantage across the lifespan. While the literature on structural differences between bilinguals and monolinguals is robust, relatively few studies of gray matter (GM) have directly compared bilinguals with monolinguals in a whole-brain analysis. Overall, this and heterogeneity of study samples and methodology have led to a lack of clear anatomical support for major theories. Here, we engage in an activation likelihood estimate (ALE) meta-analysis of voxel-based morphometry (VBM) studies of GM for cases that directly compare bilingual and monolingual subjects in a whole-brain analysis. The analysis (sixteen foci, from ten contrasts across eight studies) resulted in one cluster located primarily within the anterior lobe of the right cerebellum. However, when the one pediatric study was removed, the analysis revealed no consistent results across the studies included in this meta-analysis. This suggests that for VBM studies of bilingual and monolingual adults there is considerable heterogeneity of results that complicate the understanding of the bilingual brain. Future studies will need to include larger, more well-defined samples and interrogate more fine-grained anatomical features such as cortical thickness and surface area in order to more fully examine the anatomical changes associated with bilingualism across the lifespan.
Introduction
Bilingualism continues to be a topic of intense interest, providing a unique lens into the study of the neuroscience of language. However, it also holds relevance more broadly in the realm of understanding how the brain is able to acquire a skill, taking advantage of language learning being a very common task. Benefits of learning multiple languages have been discussed and debated at length (Bialystok, 2017; Antoniou, 2019), and recent reviews have discussed topics including general cognitive benefits, enhanced neuroplasticity, and protection against aging (Baum and Titone, 2014; Li et al., 2014; Bialystok et al., 2016; Grundy et al., 2017). While there are different theories on how these benefits develop and manifest themselves (Green and Abutalebi, 2013; Abutalebi and Green, 2016; Grundy et al., 2017), the aforementioned reviews detail the evidence that suggest benefits to acquiring multiple languages exist. The proposed gains of learning a second language do come with some caveats, for example, age of acquisition appears to play a strong role in cognitive and brain changes associated with bilingualism (Berken et al., 2017) and immersion in the language being learned may also impact structural changes observed in the brain (Stein et al., 2014). Along with the importance of understanding the potential benefits of multi-language learning comes the concern of heterogeneity of samples in studies of bilingualism and the brain due to the roles of factors such as age of acquisition and how the second language is learned. As such Garcia-Penton et al. (2016), has outlined how the variability in sample selection and methodology have led to a lack of generalizability of results across studies, and relatively little neuroanatomical support for major theories of bilingualism. It is therefore important to understand where consistencies and inconsistencies exist across the literature of bilingualism and the brain, both for the current understanding of the neuroscience of language and learning and in planning future studies to adequately address the gaps in the existing knowledge base.
In terms of the impact of bilingualism on brain structure, a common tool for investigating differences in gray matter (GM) is voxel-based morphometry (VBM; Ashburner and Friston, 2000, 2005; Mechelli et al., 2005; Ashburner, 2007; Douaud et al., 2007). While there are concerns over whether this is the best measure for probing brain differences and relationships with neurocognitive measures (e.g., cortical thickness and surface area may provide more unique information; Panizzon et al., 2009; Winkler et al., 2010), it has been a very common tool used in neuroimaging studies of bilingualism. Overall, studies utilizing VBM in bilingualism have found increased GM in frontal, parietal, and cingulate regions associated with second language ability and acquisition, with motor system involvement including aspects of the basal ganglia (Grundy et al., 2017). However, while many studies utilize this technique, relatively few studies have directly compared bilingual and monolingual individuals in whole-brain analyses. For example, there have been investigations into simultaneous vs. sequential bilinguals (Kaiser et al., 2015; Berken et al., 2016), early vs. late bilinguals (Wei et al., 2015), high vs. low proficiency in second language (Reiterer et al., 2011), bilinguals vs. multilinguals (Grogan et al., 2012), correlational studies of gray matter volume with neurocognitive measures (e.g., Abutalebi et al., 2012; Martínez-Horta et al., 2019), and longitudinal studies of adults learning a new language (Osterhout et al., 2008; Stein et al., 2012; Deluca et al., 2018). Additionally, of the VBM studies that do directly compare monolinguals and bilinguals, several are region of interest (ROI) based and do not examine effects across the entire brain (Zou et al., 2012; Abutalebi et al., 2013, 2015; Del Maschio et al., 2018). While the approaches across these studies have provided important information in understanding the bilingual brain, in order to fully understand how the bilingual brain differs from the monolingual brain it is necessary to utilize whole-brain direct comparisons of the two groups.
At the time of writing, to our knowledge there have been ten experiments across eight studies that have employed a whole-brain comparison of gray matter between bilinguals and monolinguals using VBM (Mechelli et al., 2004; Ressel et al., 2012; Gold et al., 2013; Abutalebi et al., 2014, 2015; Pliatsikas et al., 2014; Olulade et al., 2016; García-Pentón et al., 2019). These studies vary considerably in several aspects of the sample characteristics, including first (L1) and second (L2) language, age of acquisition of L2 for the bilingual group, language proficiency, sample size, covariates of no interest included in the analyses, and in overall reporting of the sample details. There is also considerable variation in the methodology, such as multiple comparison correction and software package used (i.e., FSL1, Douaud et al., 2007; or SPM2, Ashburner and Friston, 2000). As previously discussed (Garcia-Penton et al., 2016), this variation also has likely contributed to an overall inconsistent literature and lack of clear evidence to back up theory.
The present study presents an activation likelihood estimate (ALE) meta-analysis of VBM studies of GM with direct whole-brain comparisons of bilinguals and monolinguals, in an effort to test the consistency of brain differences across these studies. Considering the variability in samples and details of the methodology (as described by Garcia-Penton et al., 2016) it was expected that there would be little consistency in the results, highlighting the need for larger and more well-defined studies to better understand the relationship between bilingualism and the brain.
Methods
Selection of Studies
We searched for articles on PubMed3 and Google Scholar (google.com/scholar) using the search terms: “voxel-based morphometry,” “gray matter volume,” “bilingualism,” “differences,” and “brain.” Additionally, reference lists from publications were inspected to discover additional relevant articles, and articles that cited eligible studies were reviewed using Google Scholar to maximize inclusion of relevant articles. For this meta-analysis, we only selected studies that used whole-brain VBM analyses to compare GM between bilingual and monolingual participants. Our inclusion criteria were as follows: (1) the study used VBM analysis (FSL-Douaud et al., 2007 or SPM-Ashburner and Friston, 2000); (2) both monolingual and bilingual subjects were included; (3) subjects were healthy and did not report neurological/psychiatric disorders; and (4) foci were generated from a whole-brain analysis. Our exclusion criteria were as follows: (1) data generated from ROI analyses; (2) subjects from patient populations that may impact neurological status; (3) non-VBM studies of volume; (4) group comparisons other than bilingual vs. monolingual (e.g., simultaneous vs. sequential bilinguals, bilinguals vs. multi-linguals); and (5) studies of bimodal bilinguals (e.g., English/American Sign-Language).
The last exclusion criteria for bimodal bilinguals is based on the different experience of these bilingual individuals (simultaneous vs. independent usage), and evidence that suggests cognitive control and brain related differences may not exist compared to monolingual individuals (Emmorey et al., 2008; Olulade et al., 2016). It is important to note that we did not exclude studies for age range of participants or L1/L2 language (other than bimodal bilinguals as noted above), as there are not enough studies fitting the criteria to investigate consistency within specific age ranges (see below). One of the studies identified using the inclusion criteria above used the non-modulated version of VBM which reports gray matter density (GMD) as opposed to gray matter volume (GMV) (Mechelli et al., 2004). As it is common to include both modulated and unmodulated samples in VBM meta-analyses (e.g., Linkersdörfer et al., 2012; Barron et al., 2013; Richlan et al., 2013), and it was not an exclusion criteria for our search, we have included it the present analyses. No other whole-brain VBM comparisons in our search reported density as opposed to volume. It is also worth stating that we included García-Pentón et al. (2019), which at the time was a preprint available on bioRxiv4. The methodology was determined to be of the quality of the other studies found, and as it met all of the inclusion criteria it was determined appropriate to include in the final meta-analyses conducted here.
Description of Eligible Studies
A total of ten eligible comparisons across eight studies met our inclusion criteria, with a total of sixteen foci for results of bilingual > monolingual contrasts (see Tables 1, 2 for more study characteristics). The number of studies included here is similar to several previous ALE meta-analyses of gray matter morphometry (for example: Rotge et al., 2010; Linkersdörfer et al., 2012; Titova et al., 2013). Foci from monolingual > bilingual contrasts were only reported for one of the eight studies (Olulade et al., 2016), so only the bilingual > monolingual contrast analyses were run and reported here. Out of the ten eligible comparisons, two included separate age groups with unique contrasts eligible for inclusion: older and younger adults (Gold et al., 2013), and adults and children (García-Pentón et al., 2019). The latter study was the only study included that contained children. Of the remaining contrasts, two more consisted of older adults (Abutalebi et al., 2014, 2015), and four in young adults (Mechelli et al., 2004; Ressel et al., 2012; Pliatsikas et al., 2014; Olulade et al., 2016).
Methodological variability included software package, where two studies used FSL (Pliatsikas et al., 2014; García-Pentón et al., 2019), and the rest used SPM (Mechelli et al., 2004; Ressel et al., 2012; Gold et al., 2013; Abutalebi et al., 2014, 2015; Olulade et al., 2016). While FSL and VBM are different packages they are both voxel-based morphometric comparisons of gray matter, and studies have been included in the same meta-analysis (for example: Fusar-Poli et al., 2011). Multiple comparison corrections also varied, with FSL-based studies using threshold free cluster enhancement (TFCE; Pliatsikas et al., 2014; García-Pentón et al., 2019), and SPM-based analyses using variable corrections including family wise error (FWE) correction at the voxel level (Mechelli et al., 2004; Ressel et al., 2012; Gold et al., 2013), FWE correction at the cluster level (Abutalebi et al., 2014, 2015), non-stationary cluster correction (Olulade et al., 2016), and some studies additionally reported coordinates for clusters not corrected for multiple comparisons (Mechelli et al., 2004; Ressel et al., 2012). We include all the reported foci in the first analysis (to get a full picture from the limited overall number of eligible studies), and subsequently included only foci corrected for multiple comparisons for a more stringent analysis (described below). It is important to note that Gold et al. (2013) reported no foci for their bilingual vs. monolingual contrast, and Ressel et al. (2012) only reported uncorrected foci. Each of these studies were still included in our foci files for the meta-analyses described below. All eligible contrasts reported their coordinates in MNI space.
ALE Analysis Methods
All ALE analyses were run using GingerALE 3.0.25 (Eickhoff et al., 2009, 2012; Turkeltaub et al., 2012) using the most recent users’ manual as a guide6. The first analysis included all sixteen foci from the ten eligible contrasts, and the second analysis included only foci that had been corrected for multiple comparisons in the original study (thirteen foci from the ten contrasts, subtracting two from Ressel et al., 2012, and one from Mechelli et al., 2004). Next, we ran the same two analyses with the difference of not including the one contrast in children (one foci from García-Pentón et al., 2019). In this case, the first analysis consisted of fifteen foci from the nine total contrasts, and the second analysis (only foci corrected for multiple comparisons) consisting of twelve foci from the nine contrasts (again with two foci removed from Ressel et al., 2012, and one from Mechelli et al., 2004).
GingerALE analyses were carried out in MNI space using the more conservative mask size, and the non-additive ALE method to avoid bias from multiple small clusters that are close together from a single study dominating the results (Turkeltaub et al., 2012). Results thresholds were set using the suggested most conservative and appropriate levels in the GingerALE manual5, p < 0.001 voxel-wise threshold and p < 0.05 FWE cluster corrected threshold with 1,000 permutations. Resulting ALE maps were visualized using Mango software7 with the Colin brain template in MNI space5.
Data Availability Statement
In line with transparency of meta-analysis results as suggested in a recent discussion of best practices (Müller et al., 2018), we have provided the datasets for this study including all of our foci files (the input for GingerALE) and all of the GingerALE output files (thresholded maps, maps of foci locations, full descriptions of anatomical locations of resulting clusters, etc.) on our Open Science Framework8 project page9. These files can be used to rerun our analyses or new analyses if desired.
Results
Analysis 1: All Foci, All Contrasts
For the ALE analysis containing all sixteen foci across ten contrasts, one cluster where bilinguals showed greater GM than monolinguals was identified with two peaks: (18, –44, –20) and (12, –58, –8). This cluster was located primarily in right culmen within the anterior lobe of the cerebellum, extending slightly into the posterior cerebellum and lingual gyrus (BA 19). Foci from Pliatsikas et al. (2014) and García-Pentón et al. (2019) contributed to this cluster. Table 3 contains the peak coordinate information and Figure 1 (left) provides visualization of the cluster.
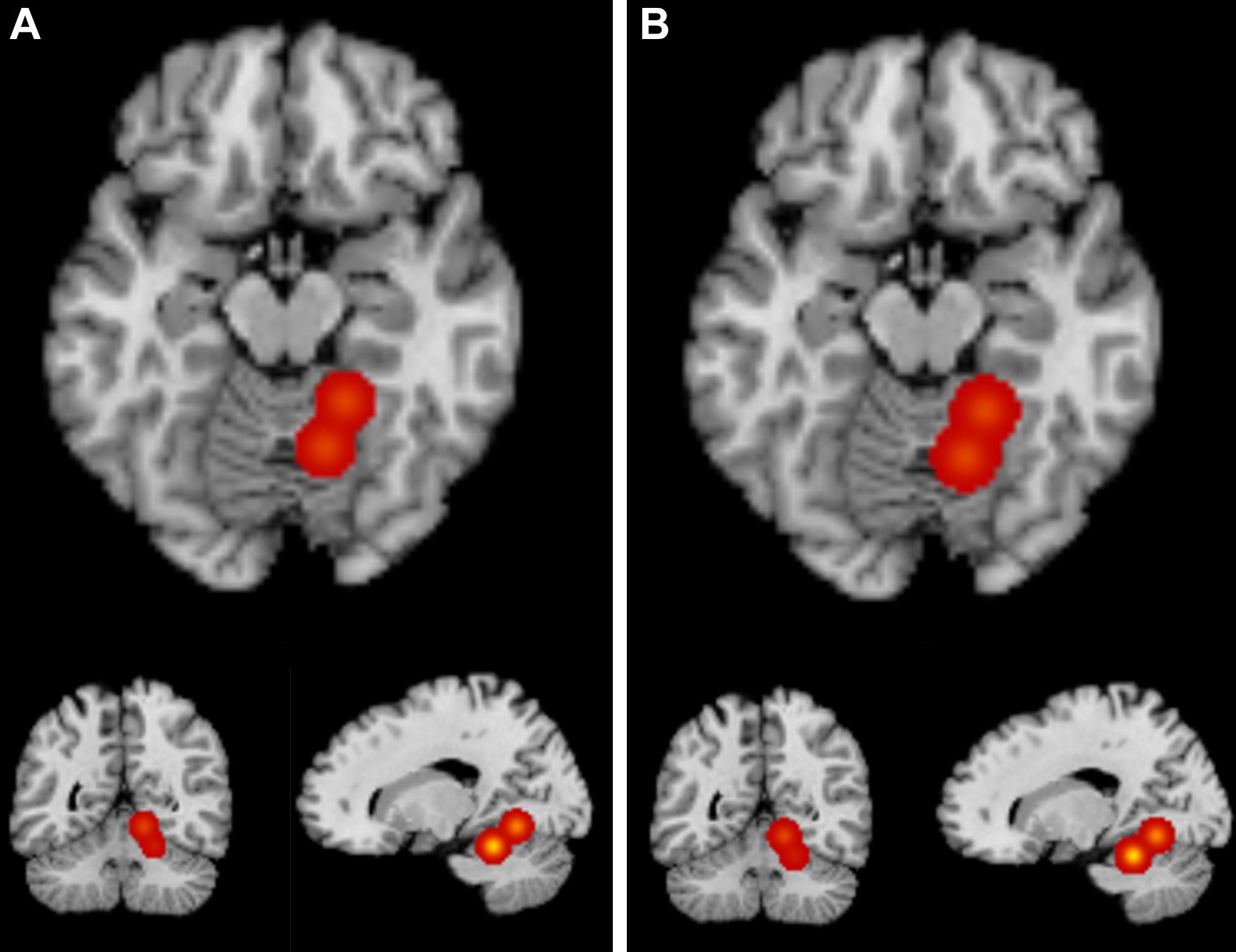
Figure 1. ALE Results. ALE maps from the bilinguals > monolinguals analyses for Analysis 1 (A) and Analysis 2 (B) at a voxel-wise threshold of p < 0.001 and an FWE cluster correction at p < 0.05 with 1,000 permutations. Both analyses identified one cluster primarily located in the right culmen within the anterior cerebellum. See Table 3 for cluster information.
Analysis 2: All Foci From Contrasts Corrected for Multiple Comparisons
Next, the same analysis was repeated, but removing foci from contrasts that were not corrected for multiple comparisons (as described in section Methods), which included thirteen foci from the ten contrasts. For this ALE analysis, again one cluster where bilinguals showed greater GM than monolinguals was identified with two peaks: (18, –44, –20) and (12, –58, –8). While slightly larger in volume, this cluster is largely identical to the cluster identified in Analysis 1 (primarily in the right culmen within the anterior lobe of the cerebellum, extending into the posterior cerebellum and lingual gyrus-BA 19). Foci from Pliatsikas et al. (2014) and García-Pentón et al. (2019) contributed to this cluster. Table 3 contains the peak coordinate information and Figure 1 (right) provides visualization of the cluster.
Analysis 3: All Foci, All Adult Contrasts
The results of Analyses 1 and 2 indicated that the right cerebellum showed significant consistency across studies, though only two contrasts contributed to the cluster (Pliatsikas et al., 2014; García-Pentón et al., 2019). As one of the contributing studies represented the only pediatric sample of the included contrasts, it was decided to run the same two analyses without the foci from García-Pentón et al. (2019). The ALE analysis (including fifteen foci across nine contrasts) revealed no significant results at the voxel-wise p < 0.001, cluster level FWE corrected p < 0.05 threshold. This suggests no consistency across adult VBM studies of GM for bilinguals > monolinguals.
Analysis 4: All Foci From Contrasts Corrected for Multiple Comparisons for Adult Contrasts
Finally, repeating Analysis 2 for the foci only from adult studies that were corrected for multiple comparisons (twelve foci across nine contrasts), the ALE analysis revealed no significant results at the voxel-wise p < 0.001, cluster level FWE corrected p < 0.05 threshold. Again, this suggests no consistency across adult VBM studies of GM for bilinguals > monolinguals.
Discussion
The neuroanatomy of bilingualism continues to be of great interest for the neuroscience of language and in understanding the potential cognitive and brain advantages of learning multiple languages (Baum and Titone, 2014; Li et al., 2014; Bialystok et al., 2016; Grundy et al., 2017). VBM has been a common tool used in studies investigating the neuroanatomy of bilingualism, however, methodology has been quite variable and there is considerable heterogeneity across results. While VBM studies of bilingualism overall have implicated frontal, parietal, and cingulate cortex, along with motor system involvement (e.g., basal ganglia) in aspects of L2 learning and ability (Grundy et al., 2017), very few have directly compared bilingual and monolingual subjects in whole-brain analyses. While correlational and ROI analyses can give important insights into the neuroanatomy of bilingualism, understanding what changes in the brain with acquisition of a second language is a crucial piece of the puzzle that also requires studies using direct comparisons of these groups. Here, we searched the literature for voxel-based morphometry studies that included contrasts that directly compared bilingual and monolingual individuals in a whole-brain analysis. We then ran an ALE meta-analysis on the foci extracted from these studies to probe whether there are consistencies in increased gray matter volume in the brains of bilinguals compared with monolinguals. The initial analysis revealed one cluster primarily in the right anterior cerebellum that was also revealed when only foci from the original studies that were corrected for multiple comparisons were included. However, only two of the ten contrasts contributed to this cluster, including the only study with a pediatric sample (García-Pentón et al., 2019). When the foci from this study were removed, it was revealed that there were no consistencies across the adult VBM studies of bilingualism in GM.
As discussed by Garcia-Penton et al. (2016), the heterogeneity of samples and methodology in the neuroimaging studies of bilingualism has led to a lack of generalization across studies and an overall lack of anatomical support for theories of bilingualism. The studies included in the current meta-analysis highlight these particular sources of heterogeneity in the literature (see Tables 1, 2). While all of these studies include whole-brain VBM comparisons of GM between bilinguals and monolinguals, the samples are all very unique. The languages for the monolingual groups are contained to English, Spanish, and Italian, but the languages of the bilingual groups are unique to each individual study, and in the case of Gold et al. (2013) there are several unique bilingual combinations included. While it is reasonable to expect there will be some anatomical consistencies of bilingualism regardless of the specific languages learned, there may also be unique aspects for certain combinations of languages. For example, bilinguals of two different writing systems (e.g., English and Chinese) may require different levels of cognitive control than bilinguals whose two languages are relatively similar. Antoniou and Wright (2017) discuss how languages that are typologically different may require greater effort to learn, while those that are more typologically similar may require more inhibitory control of overlapping features, thus both leading to increased cognitive reserve via different mechanisms. While there has been little direct testing of this, studies across many bilingual language pairs (such as those discussed here) suggest benefits of various typological similarity. However, more studies aimed at testing this notion directly are needed to further understand the relationship between typological distance and behavior/brain benefits of bilingualism (Antoniou and Wright, 2017).
Other areas of concern for heterogeneity in the current sample of studies is the variability in age of acquisition and proficiency of the bilingual subjects. Not all of the studies included here describe these characteristics fully in their samples. For those that do, how they determined early acquisition is not consistently defined. Age of acquisition can have a significant effect on the anatomy of bilingualism (Berken et al., 2017), which gives caution to the interpretation of the ALE results presented here. Ideally, there would be larger numbers of studies that included lifelong bilinguals and others that include late bilinguals which would allow for parallel analyses. From a methodological point of view, while FSL and SPM versions of VBM both allow for similar analysis there is evidence that suggests results can differ based on the software package used (Rajagopalan et al., 2014; Rajagopalan and Pioro, 2015). Additionally, thresholds varied across studies (as discussed in section Methods) which leaves open the possibility that changing this parameter could lead to different results. With this in mind, the studies included here have relatively similar sample sizes and methodological rigor, making it impertinent to hold one individual study’s results above another. Truly, additional studies across these criteria are needed to fully understand the complex phenomena at play.
It is worth discussing the potential role of the cerebellum, as this is the only consistent result from the ALE analysis. The structure and function of the cerebellum has been linked with verbal language fluency in the general population (Desmond and Fiez, 1998; Richardson and Price, 2009). Clinically, cerebellum lesions have been linked with aphasia (Marien et al., 2000), reduced GMV in the cerebellum has been implicated in developmental dyslexia (Brambati et al., 2004; Eckert et al., 2005), and increased GMV in right cerebellum following reading intervention may be linked with reading improvement (Krafnick et al., 2011). As such, its potential role in second language learning would not be surprising. Pliatsikas et al. (2014) discusses the role of the cerebellum in second language learning within the context of procedural memory and grammatical processing, and Grogan et al. (2009) found a correlation between cerebellum gray matter and fluency across L1 and L2. The average age of acquisition for subjects in these two studies was after the start of formal schooling (Grogan et al., 2009; Pliatsikas et al., 2014), and it is possible that age of acquisition could impact the cerebellum’s role in L2 acquisition or proficiency. Regardless, while the role of the cerebellum in bilingualism is potentially interesting, the results of this meta-analysis in support of this should be taken with caution due to the concerns of heterogeneity described above and the loss of this result when the pediatric study is removed from the analysis.
On a related note, it should be mentioned that the lack of consistency here could suggest a lack of evidence for a “bilingual advantage” (at least related to structural changes in the brain), however, we would caution against this interpretation due to the considerations discussed previously and again below. Instead, we echo the call of Garcia-Penton et al. (2016) for the need of studies with increased sample sizes and better subject characteristic and methodological descriptions so that the intricacies of the bilingual brain can be better understood. While correlational and ROI studies can provide valuable information, whole-brain comparisons in studies with large sample sizes can provide better estimates of group differences between bilinguals and monolinguals. With clear and motivated definitions of early vs. late age of acquisition and fluency in L1 and L2, future meta-analyses of this literature that can utilize a larger literature in more defined areas will provide more specific knowledge related to a “bilingual advantage” for brain structure. Another important consideration for studying the neuroanatomy of bilingualism is utilizing more fine-grained anatomical analyses than VBM. Volume (the most commonly reported metric of the VBM studies included in the present meta-analysis) is the combination of cortical thickness and surface area, and as these measures have unique genetic influence (Panizzon et al., 2009; Winkler et al., 2010), studying these measures may provide more useful information than volume or density alone. Recent studies have begun to investigate bilingualism using cortical thickness (Klein et al., 2014; Archila-Suerte et al., 2018) and surface area (Archila-Suerte et al., 2018; Hämäläinen et al., 2018) providing evidence that these techniques can be employed within the bilingualism field with success. A final consideration for the field as a whole is to engage in more pediatric samples that directly compare bilingual and monolingual children, preferably with a longitudinal component. To fully understand the changes in the brain in acquiring a second language, looking across the lifespan and using longitudinal approaches have tremendous potential to provide valuable information.
Overall, the results of the ALE meta-analyses described here suggest a general lack of consistency in the VBM literature of bilingualism, and highlight the need for larger and more well-defined studies in order to determine the changes that occur in the brain with acquisition of a second language. While these results should be taken with caution due to the overall small number of studies that have employed a whole-brain comparison of bilinguals and monolinguals, this only further indicates the need to design and implement studies that have greater potential to inform the theories and models of bilingual advantage in a meaningful way.
Ethics Statement
The studies involving human participants were reviewed and approved by Dominican University IRB. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
AK and AD developed the idea for the meta-analysis, executed the literature search, and wrote the manuscript. AD ran the analyses in GingerALE.
Funding
AD received funding in the form of a summer stipend from the Neuroscience program at Dominican University to support this work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank fellow attendees of the Faculty for Undergraduate Neuroscience undergraduate poster session and Society for Neuroscience annual meeting for helpful and supportive comments regarding this work.
Footnotes
- ^ https://fsl.fmrib.ox.ac.uk/fsl/fslwiki
- ^ https://www.fil.ion.ucl.ac.uk/spm/
- ^ www.ncbi.nlm.nih.gov/pubmed/
- ^ https://www.biorxiv.org/
- ^ http://brainmap.org/ale/
- ^ http://brainmap.org/ale/manual.pdf
- ^ http://rii.uthscsa.edu/mango/
- ^ http://brainmap.org/ale
- ^ https://osf.io/evs4f/
References
Abutalebi, J., Guidi, L., Borsa, V., Canini, M., Della Rosa, P. A., Parris, B. A., et al. (2015). Bilingualism provides a neural reserve for aging populations. Neuropsychologia 69, 201–210. doi: 10.1016/j.neuropsychologia.2015.01.040
Abutalebi, J., Canini, M., Della Rosa, P. A., Sheung, L. P., Green, D. W., and Weekes, B. S. (2014). Bilingualism protects anterior temporal lobe integrity in aging. Neurobiol. Aging 35, 2126–2133. doi: 10.1016/j.neurobiolaging.2014.03.010
Abutalebi, J., Della Rosa, P. A., Green, D. W., Hernandez, M., Scifo, P., Keim, R., et al. (2012). Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cereb. Cortex 22, 2076–2086. doi: 10.1093/cercor/bhr287
Abutalebi, J., and Green, D. W. (2016). Neuroimaging of language control in bilinguals: neural adaptation and reserve. Biling. Lang. Cogn. 19, 689–698. doi: 10.1017/S1366728916000225
Abutalebi, J., Rosa, P. A. D., Castro Gonzaga, A. K., Keim, R., Costa, A., and Perani, D. (2013). The role of the left putamen in multilingual language production. Brain Lang. 125, 307–315. doi: 10.1016/j.bandl.2012.03.009
Antoniou, M. (2019). The advantages of bilingualism debate. Annu. Rev. Linguist. 5, 395–415. doi: 10.1146/annurev-linguistics-011718-011820
Antoniou, M., and Wright, S. M. (2017). Uncovering the mechanisms responsible for why language learning may promote healthy cognitive aging. Front. Psychol. 8:2217. doi: 10.3389/fpsyg.2017.02217
Archila-Suerte, P., Woods, E. A., Chiarello, C., and Hernandez, A. E. (2018). Neuroanatomical profiles of bilingual children. Dev. Sci. 21, e12654. doi: 10.1111/desc.12654
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage 38, 95–113. doi: 10.1016/j.neuroimage.2007.07.007
Ashburner, J., and Friston, K. J. (2000). Voxel-Based Morphometry—The Methods. NeuroImage 11, 805–821. doi: 10.1006/nimg.2000.0582
Ashburner, J., and Friston, K. J. (2005). Unified segmentation. NeuroImage 26, 839–851. doi: 10.1016/j.neuroimage.2005.02.018
Barron, D. S., Fox, P. M., Laird, A. R., Robinson, J. L., and Fox, P. T. (2013). Thalamic medial dorsal nucleus atrophy in medial temporal lobe epilepsy: a VBM meta-analysis. NeuroImage Clin. 2, 25–32. doi: 10.1016/j.nicl.2012.11.004
Baum, S., and Titone, D. (2014). Moving toward a neuroplasticity view of bilingualism, executive control, and aging. Appl. Psychol. 35, 857–894. doi: 10.1017/S0142716414000174
Berken, J. A., Gracco, V. L., Chen, J.-K., and Klein, D. (2016). The timing of language learning shapes brain structure associated with articulation. Brain Struct. Funct. 221, 3591–3600. doi: 10.1007/s00429-015-1121-9
Berken, J. A., Gracco, V. L., and Klein, D. (2017). Early bilingualism, language attainment, and brain development. Neuropsychologia 98, 220–227. doi: 10.1016/j.neuropsychologia.2016.08.031
Bialystok, E. (2017). The bilingual adaptation: how minds accommodate experience. Psychol. Bull. 143, 233–262. doi: 10.1037/bul0000099
Bialystok, E., Abutalebi, J., Bak, T. H., Burke, D. M., and Kroll, J. F. (2016). Aging in two languages: implications for public health. Ageing Res. Rev. 27, 56–60. doi: 10.1016/j.arr.2016.03.003
Brambati, S. M., Termine, C., Ruffino, M., Stella, G., Fazio, F., Cappa, S. F., et al. (2004). Regional reductions of gray matter volume in familial dyslexia. Neurology 63, 742–745. doi: 10.1212/01.wnl.0000134673.95020.ee
Del Maschio, N., Sulpizio, S., Gallo, F., Fedeli, D., Weekes, B. S., and Abutalebi, J. (2018). Neuroplasticity across the lifespan and aging effects in bilinguals and monolinguals. Brain Cogn. 125, 118–126. doi: 10.1016/j.bandc.2018.06.007
Deluca, V., Rothman, J., and Pliatsikas, C. (2018). Linguistic immersion and structural effects on the bilingual brain: a longitudinal study. Biling. Lang. Cogn. 27, 1160–1175. doi: 10.1017/S1366728918000883
Desmond, J. E., and Fiez, J. A. (1998). Neuroimaging studies of the cerebellum: language, learning and memory. Trends Cogn. Sci. 2, 355–362. doi: 10.1016/s1364-6613(98)01211-x
Douaud, G., Smith, S., Jenkinson, M., Behrens, T., Johansen-Berg, H., Vickers, J., et al. (2007). Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130, 2375–2386. doi: 10.1093/brain/awm184
Eckert, M. A., Leonard, C. M., Wilke, M., Eckert, M., Richards, T., Richards, A., et al. (2005). Anatomical signatures of dyslexia in children: unique information from manual and voxel based morphometry brain measures. Cortex 41, 304–315. doi: 10.1016/s0010-9452(08)70268-5
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F., and Fox, P. T. (2012). Activation likelihood estimation meta-analysis revisited. NeuroImage 59, 2349–2361. doi: 10.1016/j.neuroimage.2011.09.017
Eickhoff, S. B., Laird, A. R., Grefkes, C., Wang, L. E., Zilles, K., and Fox, P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30, 2907–2926. doi: 10.1002/hbm.20718
Emmorey, K., Luk, G., Pyers, J. E., and Bialystok, E. (2008). The source of enhanced cognitive control in bilinguals: evidence from bimodal bilinguals. Psychol. Sci. 19, 1201–1206. doi: 10.1111/j.1467-9280.2008.02224.x
Fusar-Poli, P., Borgwardt, S., Crescini, A., Deste, G., Kempton, M. J., Lawrie, S., et al. (2011). Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci. Biobehav. Rev. 35, 1175–1185. doi: 10.1016/j.neubiorev.2010.12.005
García-Pentón, L., Fernández, Y., Duñabeitia, J. A., Pérez, A., and Carreiras, M. (2019). Neuroanatomical correlates in bilinguals: the case of children and elderly. bioRxiv [Preprint]
Garcia-Penton, L., Fernandez Garcia, Y., Costello, B., Andoni Dunabeitia, J., and Carreiras, M. (2016). The neuroanatomy of bilingualism: how to turn a hazy view into the full picture. Lang. Cogn. Neurosci. 31, 303–327. doi: 10.1080/23273798.2015.1068944
Gold, B. T., Kim, C., Johnson, N. F., Kryscio, R. J., and Smith, C. D. (2013). Lifelong bilingualism maintains neural efficiency for cognitive control in aging. J. Neurosci. 33, 387–396. doi: 10.1523/JNEUROSCI.3837-12.2013
Green, D. W., and Abutalebi, J. (2013). Language control in bilinguals: the adaptive control hypothesis. J. Cogn. Psychol. 25, 515–530. doi: 10.1080/20445911.2013.796377
Grogan, A., Green, D. W., Ali, N., Crinion, J. T., and Price, C. J. (2009). Structural correlates of semantic and phonemic fluency ability in first and second languages. Cereb. Cortex 19, 2690–2698. doi: 10.1093/cercor/bhp023
Grogan, A., Parker Jones, Ô, Ali, N., Crinion, J., Orabona, S., Mechias, M. L., et al. (2012). Structural correlates for lexical efficiency and number of languages in non-native speakers of English. Neuropsychologia 50, 1347–1352. doi: 10.1016/j.neuropsychologia.2012.02.019
Grundy, J. G., Anderson, J. A. E., and Bialystok, E. (2017). Neural correlates of cognitive processing in monolinguals and bilinguals: neural correlates of bilingualism. Ann. N. Y. Acad. Sci. 1396, 183–201. doi: 10.1111/nyas.13333
Hämäläinen, S., Joutsa, J., Sihvonen, A. J., Leminen, A., and Lehtonen, M. (2018). Beyond volume: a surface-based approach to bilingualism-induced grey matter changes. Neuropsychologia 117, 1–7. doi: 10.1016/j.neuropsychologia.2018.04.038
Kaiser, A., Eppenberger, L. S., Smieskova, R., Borgwardt, S., Kuenzli, E., Radue, E.-W., et al. (2015). Age of second language acquisition in multilinguals has an impact on gray matter volume in language-associated brain areas. Front. Psychol. 6:368. doi: 10.3389/fpsyg.2015.00638
Klein, D., Mok, K., Chen, J.-K., and Watkins, K. E. (2014). Age of language learning shapes brain structure: a cortical thickness study of bilingual and monolingual individuals. Brain Lang. 131, 20–24. doi: 10.1016/j.bandl.2013.05.014
Krafnick, A. J., Flowers, D. L., Napoliello, E. M., and Eden, G. F. (2011). Gray matter volume changes following reading intervention in dyslexic children. NeuroImage 57, 733–741. doi: 10.1016/j.neuroimage.2010.10.062
Li, P., Legault, J., and Litcofsky, K. A. (2014). Neuroplasticity as a function of second language learning: anatomical changes in the human brain. Cortex 58, 301–324. doi: 10.1016/j.cortex.2014.05.001
Linkersdörfer, J., Lonnemann, J., Lindberg, S., Hasselhorn, M., and Fiebach, C. J. (2012). Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: an ALE meta-analysis. PLoS ONE 7:e43122. doi: 10.1371/journal.pone.0043122
Marien, P., Engelborghs, S., Pickut, B. A., and De Deyn, P. P. (2000). Aphasia following cerebellar damage: fact or fallacy? J. Neurolinguistics 13, 145–171. doi: 10.1016/s0911-6044(00)00009-9
Martínez-Horta, S., Moreu, A., Perez-Perez, J., Sampedro, F., Horta-Barba, A., Pagonabarraga, J., et al. (2019). The impact of bilingualism on brain structure and function in Huntington’s disease. Park. Relat. Disord. 60, 92–97. doi: 10.1016/j.parkreldis.2018.09.017
Mechelli, A., Crinion, J. T., Noppeney, U., O’Doherty, J., Ashburner, J., Frackowiak, R. S., et al. (2004). Structural plasticity in the bilingual brain. Nature 431, 757–757. doi: 10.1038/431757a
Mechelli, A., Price, C., Friston, K., and Ashburner, J. (2005). Voxel-based morphometry of the human brain: methods and applications. Curr. Med. Imaging Rev. 1, 105–113. doi: 10.2174/1573405054038726
Müller, V. I., Cieslik, E. C., Laird, A. R., Fox, P. T., Radua, J., Mataix-Cols, D., et al. (2018). Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 84, 151–161. doi: 10.1016/j.neubiorev.2017.11.012
Olulade, O. A., Jamal, N. I., Koo, D. S., Perfetti, C. A., LaSasso, C., and Eden, G. F. (2016). Neuroanatomical evidence in support of the bilingual advantage theory. Cereb. Cortex 26, 3196–3204. doi: 10.1093/cercor/bhv152
Osterhout, L., Poliakov, A., Inoue, K., McLaughlin, J., Valentine, G., Pitkanen, I., et al. (2008). Second-language learning and changes in the brain. J. Neurolinguistics 21, 509–521. doi: 10.1016/j.jneuroling.2008.01.001
Panizzon, M. S., Fennema-Notestine, C., Eyler, L. T., Jernigan, T. L., Prom-Wormley, E., Neale, M., et al. (2009). Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex 19, 2728–2735. doi: 10.1093/cercor/bhp026
Pliatsikas, C., Johnstone, T., and Marinis, T. (2014). Grey matter volume in the cerebellum is related to the processing of grammatical rules in a second language: a structural voxel-based morphometry study. Cerebellum 13, 55–63. doi: 10.1007/s12311-013-0515-6
Rajagopalan, V., and Pioro, E. P. (2015). Disparate voxel based morphometry (VBM) results between SPM and FSL softwares in ALS patients with frontotemporal dementia: which VBM results to consider? BMC Neurol. 15:32. doi: 10.1186/s12883-015-0274-8
Rajagopalan, V., Yue, G. H., and Pioro, E. P. (2014). Do preprocessing algorithms and statistical models influence voxel-based morphometry (VBM) results in amyotrophic lateral sclerosis patients? A systematic comparison of popular VBM analytical methods: VBM Results in ALS. J. Magn. Reson. Imaging 40, 662–667. doi: 10.1002/jmri.24415
Reiterer, S. M., Hu, X., Erb, M., Rota, G., Nardo, D., Grodd, W., et al. (2011). Individual differences in audio-vocal speech imitation aptitude in late bilinguals: functional neuro-imaging and brain morphology. Front. Psychol. 2:271. doi: 10.3389/fpsyg.2011.00271
Ressel, V., Pallier, C., Ventura-Campos, N., Diaz, B., Roessler, A., Avila, C., et al. (2012). An effect of bilingualism on the auditory cortex. J. Neurosci. 32, 16597–16601. doi: 10.1523/JNEUROSCI.1996-12.2012
Richardson, F. M., and Price, C. J. (2009). Structural MRI studies of language function in the undamaged brain. Brain Struct. Funct. 213, 511–523. doi: 10.1007/s00429-009-0211-y
Richlan, F., Kronbichler, M., and Wimmer, H. (2013). Structural abnormalities in the dyslexic brain: a meta-analysis of voxel-based morphometry studies: meta-analysis developmental dyslexia. Hum. Brain Mapp. 34, 3055–3065. doi: 10.1002/hbm.22127
Rotge, J.-Y., Langbour, N., Guehl, D., Bioulac, B., Jaafari, N., Allard, M., et al. (2010). Gray matter alterations in obsessive–compulsive disorder: an anatomic likelihood estimation meta-analysis. Neuropsychopharmacology 35, 686–691. doi: 10.1038/npp.2009.175
Stein, M., Federspiel, A., Koenig, T., Wirth, M., Strik, W., Wiest, R., et al. (2012). Structural plasticity in the language system related to increased second language proficiency. Cortex 48, 458–465. doi: 10.1016/j.cortex.2010.10.007
Stein, M., Winkler, C., Kaiser, A., and Dierks, T. (2014). Structural brain changes related to bilingualism: does immersion make a difference? Front. Psychol. 5:1116. doi: 10.3389/fpsyg.2014.01116
Titova, O. E., Hjorth, O. C., Schiöth, H. B., and Brooks, S. J. (2013). Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: a meta-analysis of VBM studies. BMC Psychiatry 13:110. doi: 10.1186/1471-244X-13-110
Turkeltaub, P. E., Eickhoff, S. B., Laird, A. R., Fox, M., Wiener, M., and Fox, P. (2012). Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 33, 1–13. doi: 10.1002/hbm.21186
Wei, M., Joshi, A. A., Zhang, M., Mei, L., Manis, F. R., He, Q., et al. (2015). How age of acquisition influences brain architecture in bilinguals. J. Neurolinguistics 36, 35–55. doi: 10.1016/j.jneuroling.2015.05.001
Winkler, A. M., Kochunov, P., Blangero, J., Almasy, L., Zilles, K., Fox, P. T., et al. (2010). Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage 53, 1135–1146. doi: 10.1016/j.neuroimage.2009.12.028
Keywords: bilingualism, activation likelihood estimate analysis (ALE), voxel-based morphometry (VBM), gray matter, meta-analysis
Citation: Danylkiv A and Krafnick AJ (2020) A Meta-Analysis of Gray Matter Differences Between Bilinguals and Monolinguals. Front. Hum. Neurosci. 14:146. doi: 10.3389/fnhum.2020.00146
Received: 28 November 2019; Accepted: 31 March 2020;
Published: 23 April 2020.
Edited by:
Hidenao Fukuyama, Kyoto University, JapanReviewed by:
Mark Antoniou, Western Sydney University, AustraliaGuosheng Ding, Beijing Normal University, China
Copyright © 2020 Danylkiv and Krafnick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anthony J. Krafnick, YWtyYWZuaWNrQGRvbS5lZHU=
 Anastasiya Danylkiv
Anastasiya Danylkiv Anthony J. Krafnick
Anthony J. Krafnick