- 1Department of Neurophysiology and Pathophysiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
- 2Department of Psychology, University of Amsterdam, Amsterdam, Netherlands
- 3Amsterdam Center for Brain and Cognition, Institute for Interdisciplinary Studies, Amsterdam, Netherlands
Brain activity fluctuates continuously, even in the absence of changes in sensory input or motor output. These intrinsic activity fluctuations are correlated across brain regions and are spatially organized in macroscale networks. Variations in the strength, topography, and topology of correlated activity occur over time, and unfold upon a backbone of long-range anatomical connections. Subcortical neuromodulatory systems send widespread ascending projections to the cortex, and are thus ideally situated to shape the temporal and spatial structure of intrinsic correlations. These systems are also the targets of the pharmacological treatment of major neurological and psychiatric disorders, such as Parkinson’s disease, depression, and schizophrenia. Here, we review recent work that has investigated how neuromodulatory systems shape correlations of intrinsic fluctuations of large-scale cortical activity. We discuss studies in the human, monkey, and rodent brain, with a focus on non-invasive recordings of human brain activity. We provide a structured but selective overview of this work and distil a number of emerging principles. Future efforts to chart the effect of specific neuromodulators and, in particular, specific receptors, on intrinsic correlations may help identify shared or antagonistic principles between different neuromodulatory systems. Such principles can inform models of healthy brain function and may provide an important reference for understanding altered cortical dynamics that are evident in neurological and psychiatric disorders, potentially paving the way for mechanistically inspired biomarkers and individualized treatments of these disorders.
Introduction
Neural population activity in the cerebral cortex fluctuates continuously, even in the absence of changes in sensory input or motor output. These so-called intrinsic cortical activity fluctuations show remarkable structure across time and space: activity fluctuations correlate across sets of distributed brain areas, on the basis of which macroscale functional networks can be delineated (Biswal et al., 1995; Fox and Raichle, 2007). Such intrinsic activity correlations are commonly studied in a setting that is often referred to as the “resting state”: the absence of motor output or structured sensory input (often with eyes-closed). However, intrinsic activity fluctuations that correlate across time and space also occur during active processing of sustained, unchanging, sensory input (Donner et al., 2013; Meindertsma et al., 2017; Pfeffer et al., 2018). We therefore use the term “intrinsic activity correlations,” as it is agnostic about the behavioral context.
We focus on intrinsic correlations between cortical population signals that pool the activity across thousands of individual neurons. Intrinsic activity fluctuations have also been investigated at the level of single-neuron spiking, in this context commonly referred to as “noise correlations” (Zohary et al., 1994; Cohen and Kohn, 2011; Nienborg et al., 2012; Kohn et al., 2016). Similar to the intrinsic correlations between cortical population signals reviewed below, noise correlations between single neurons are state-dependent (e.g., Harris and Thiele, 2011; Reimer et al., 2014; Joshi and Gold, 2019). An important open question, beyond the scope of this review, is whether or not the same mechanistic principles account for the impact of state variations on neural correlations at these different (microscopic vs. macroscopic) scales. In this article, we use the term “intrinsic (cortical activity) correlations” to exclusively refer to correlations between cortical population signals.
Although predominantly studied with functional magnetic resonance imaging (fMRI), intrinsic correlations have also been shown to occur using electro-/magnetoencephalography (E/MEG), electrocorticography, and other imaging modalities (Friston et al., 1993; Mao et al., 2001; Nir et al., 2008; de Pasquale et al., 2010; Hipp et al., 2012; Lewis et al., 2016; Siems et al., 2016; Stitt et al., 2018; Hollensteiner et al., 2019) and show spatiotemporal correspondence across modalities (Hipp and Siegel, 2015; Siems et al., 2016). The temporal structure of intrinsic activity varies across cortical areas, which may reflect inter-areal variation in computational properties such as intrinsic timescales (Murray et al., 2014) that depend on inter-areal projections (Chaudhuri et al., 2015) and, possibly, functional interactions (Baria et al., 2013). Moreover, intrinsic activity correlations are largely predictive of task-related activation patterns (Cole et al., 2016; Tavor et al., 2016), and provide useful diagnostic and prognostic markers of neurological and psychiatric disorders (Fox and Greicius, 2010; van den Brink et al., 2018b). Thus, intrinsic activity correlations are a ubiquitous phenomenon and their quantitative features (Box 1) are potentially revealing indicators of the functional architecture of the brain.
Box 1. Quantifying features of intrinsic activity correlations.
In this article, we discuss three characteristics of intrinsic activity correlations:
• The strength/magnitude of correlations in activity. Correlation strength can be used to examine the extent to which activity between any two brain regions is correlated (so-called seed-based correlation analysis), or to examine the overall “connectedness” of the brain by averaging the correlation coefficient across all brain region or deriving summary statistics such as “degree” (Rubinov and Sporns, 2010) or “functional connectivity density” (Tomasi and Volkow, 2010).
• Topography: A spatial representation of (some property of) a system. For example, the spatial distribution of a particular resting state network (RSN), defined as a set of brain regions or voxels that shows consistent spatio-temporal dynamics (usually above a particular threshold). The topography is often used to characterize the structure of individual RSNs, or experimental manipulation-related changes therein (Smith et al., 2009).
• Topology: The geometrical relationship between elements of a system. The human brain has been argued to approximate a small world topology, which forms a mixture of dense connections between neighboring brain regions and sparse long-range connections (Watts and Strogatz, 1998; Sporns and Zwi, 2004; Bassett and Bullmore, 2006; Bassett et al., 2006). Such a topology allows for both distributed and integrated processing, the balance between which relates to task-performance (Shine et al., 2016; Shine and Poldrack, 2018).
A number of observations indicate that the features of intrinsic activity correlations are shaped by the architecture of anatomical connections of the cerebral cortex. First, intrinsic correlations within the visual system reflect established principles of cortico-cortical projections, such as retinotopic organization (Heinzle et al., 2011; Donner et al., 2013; Gravel et al., 2014; Bock et al., 2015). Second, computational models that are equipped with realistic anatomical connectivity can predict the topological features and temporal dynamics of empirical intrinsic correlations reasonably well (Honey et al., 2007, 2009; Cabral et al., 2012). Third, causal manipulation of anatomical connections alters the strength of functional interactions (O’Reilly et al., 2013). This line of inquiry thus suggests that the full repertoire of functional interactions across the brain is shaped by the anatomical substrate upon which these interactions unfold (Deco et al., 2011).
However, other observations indicate that the anatomical connectome alone is not sufficient to account for the features of intrinsic activity correlations. The correspondence between the anatomical and functional connectome varies with attentional (Baria et al., 2013) and conscious (Barttfeld et al., 2015) state, and shows substantial temporal variability even within periods of rest (Chang and Glover, 2010; Sakoglu et al., 2010; Allen et al., 2014; Zalesky et al., 2014; Lurie et al., 2018). What are the sources of these variations of intrinsic activity correlations?
Here, we focus on one candidate source that has received surprisingly little attention in the resting-state literature, but, as we propose, is crucial for understanding the origin, dynamics, and diagnostic value of intrinsic activity correlation: the neuromodulatory systems of the brainstem. The term refers to a small set of brainstem nuclei with widespread projections to the forebrain, which synthesize and release specific modulatory neurotransmitters (“neuromodulators”; Figure 1 and Box 2). By virtue of their widespread projection profiles and effects on the state of cortical target networks, these systems can shape neural activity across the cortex in a coordinated fashion. Consequently, these systems are in an ideal position to shape intrinsic activity correlations. What is more, these brainstem systems are disturbed in several major psychiatric disorders, which also coincide with changes in intrinsic activity correlations (Calhoun et al., 2009; Rosazza and Minati, 2011; Wang et al., 2012; Vargas et al., 2013; Baggio et al., 2015; Dichter et al., 2015; Mulders et al., 2015; Giraldo-Chica and Woodward, 2017).
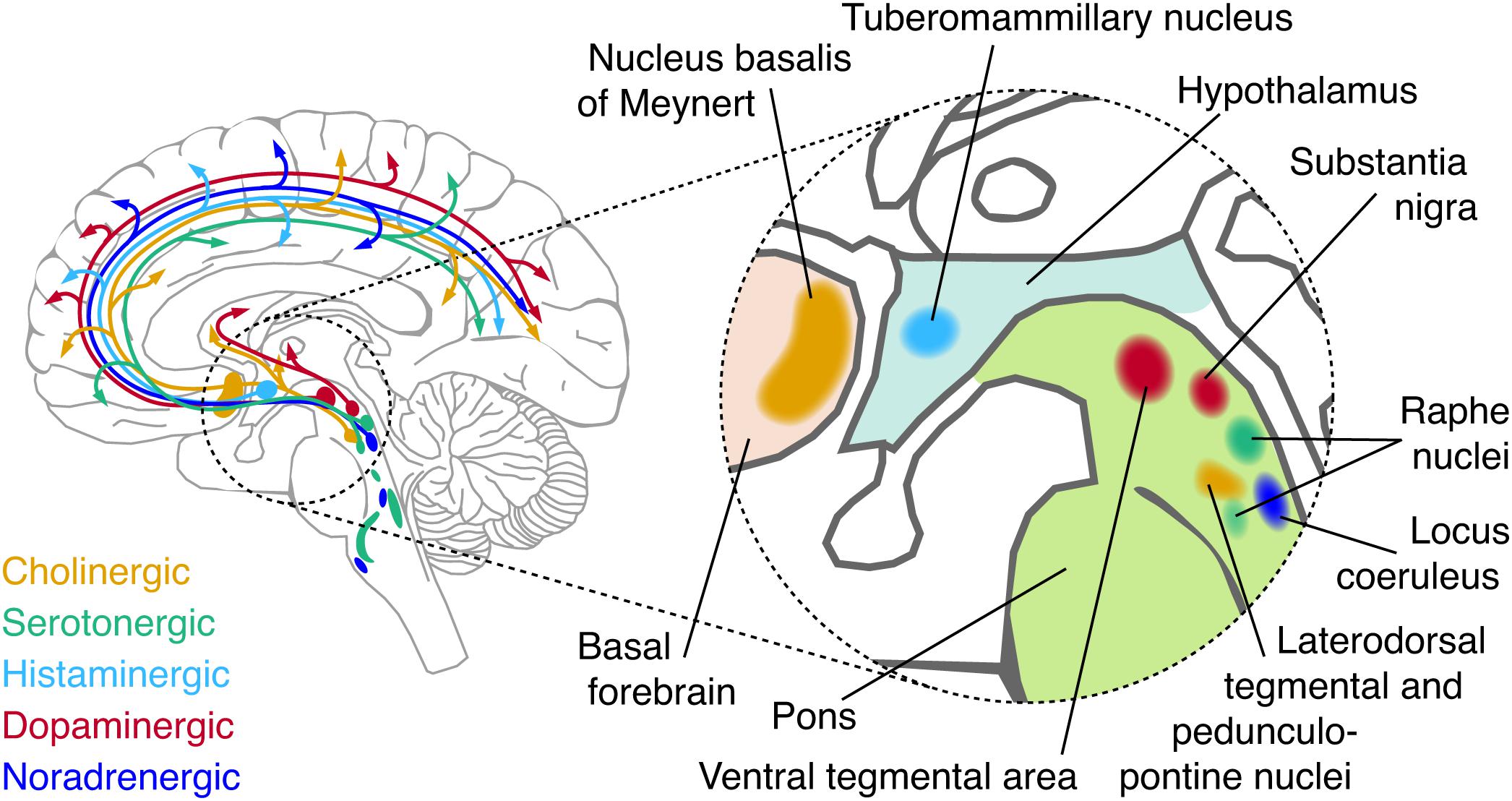
Figure 1. Schematic of major neuromodulatory systems. Cerebellar, spinal, and temporal projections are omitted for brevity. The inset shows the approximate anatomical location of each nucleus that sends major afferents to the forebrain.
Box 2. Major brainstem neuromodulatory systems.
Besides various peptides, five major neuromodulatory systems have been identified:
• Norepinephrine (NE) is released by the locus coeruleus (LC), and the A1/A2 regions of the brainstem (Sara, 2009). The LC projects to virtually all areas of the forebrain with the exception of the basal ganglia. Projection specificity of sub-populations of LC neurons has recently been shown (Chandler and Waterhouse, 2012; Chandler et al., 2014; Schwarz and Luo, 2015; Schwarz et al., 2015; Uematsu et al., 2015, 2017; Rho et al., 2018; Cerpa et al., 2019).
• Dopamine (DA) is predominantly released by two nuclei: the substantia nigra pars compacta (SNpc) and the ventral tegmental area (VTA) (Foote and Morrison, 1987). Four major dopaminergic branches exist of which three are ascending: the mesolimbic (VTA to ventral striatum), mesocortical (VTA to cortex) and nigrostriatal (SNpc to dorsal striatum) pathways. In addition, DA co-release by the LC (Takeuchi et al., 2016; Beas et al., 2018) and serotonergic dorsal raphe nuclei (Cho et al., 2017) have recently been shown. Other DA-producing neurons are found in the olfactory bulb (Pignatelli and Belluzzi, 2017) and pedunculopontine nucleus (French and Muthusamy, 2018). DA and NE have a similar chemical composition and are collectively known as catecholamines.
• Acetylcholine (ACh) is released by neurons in the basal forebrain (BF), which is comprised of several subdivisions, termed Ch1-Ch4, that contain cholinergic neurons. Ch4 corresponds to the nucleus basalis of Meynert, and is the major source of cortical ACh (Mesulam and van Hoesen, 1976; Mesulam et al., 1983; Mesulam and Changiz, 1988). This nucleus contains topographically organized clusters of neurons that preferentially innervate select portions of the cortex (Rho et al., 2018; Zaborszky et al., 2018; Ahmed et al., 2019). Other sources of ACh include the pedunculopontine nucleus and laterodorsal tegmental nucleus of the brainstem, which project to the thalamus, basal ganglia, hypothalums, and cortex (Statoh and Fibiger, 1986; Garcia-Rill, 1991; French and Muthusamy, 2018).
• Serotonin (5HT) originates from the raphe nuclei, which is a constellation of nuclei scattered throughout the brainstem (Törk, 1990). The raphe nuclei can be roughly subdivided in rostral and caudal portions, of which the rostral portion can be further subdivided in a dorsal (B6-B7) and median (B5/B8) portion. Both the dorsal rand median raphe project heavily to the cortex (Törk, 1990).
• Histamine is released by the tuberomammilary nucleus of the hypothalamus that projects to virtually the entire forebrain (Haas and Panula, 2003).
Most neuromodulatory brainstem nuclei do not consist of a single type of neuron releasing one neuromodulator, but contain a mixture of multiple types of neurons that can include GABAergic or glutamatergic types as well as other neuromodulators (Lin et al., 2015; Cho et al., 2017; Beas et al., 2018; Breton-Provencher and Sur, 2019). Furthermore, some of these nuclei are reciprocally connected and their activity tends to co-fluctuate in unison with changes in arousal and wakefulness (Foote and Morrison, 1987; Haas and Panula, 2003; Sara, 2009).
In what follows, we review the dependence of intrinsic activity correlations on neuromodulatory systems. An increasing number of studies over the past decade, conducted in humans, macaques, and rodents (rats and mice), have begun to provide insight into this dependence. Our goal is to provide a structured overview of this nascent literature and distil from it a number of emerging principles. This article is a selective review, which focuses on emerging principles rather than a comprehensive coverage of the literature. Moreover, we focus on the neuromodulatory systems on which most work has been conducted. In particular, the catecholaminergic systems (norepinephrine, NE; and dopamine, DA) were among the first systems to be studied in relation to intrinsic activity correlations. This article also covers more recent work into the acetylcholine (ACh) and serotonin (5HT) systems. To date, comparatively little work has been conducted on the system-level effects of histamine, and it will therefore not be discussed in the current review.
Candidate Mechanisms of Brainstem Modulation of Intrinsic Cortical Activity Correlations
The distinction between two modes of cortical state provides a useful heuristic for conceptualizing potential effects of brainstem neuromodulatory systems on cortical network dynamics: “activity state” and “dynamic state” (Curto et al., 2009; Safaai et al., 2015). This (likely oversimplified) dichotomy can be formalized by means of dynamical systems models. Curto et al. (2009) and Safaai et al. (2015) used the FitzHugh–Nagumo model [originally developed for describing action potential generation (FitzHugh, 1961; Nagumo et al., 1962)] for modeling cortical population dynamics (Curto et al., 2009; Safaai et al., 2015). In this framework, activity state refers to the set of parameters that vary on timescales from milliseconds to hundreds of milliseconds, and dynamic state refers to the set of parameters that vary more slowly (from seconds to tens of seconds) and interact multiplicatively with (i.e., modulate) the fast variations of activity states (Curto et al., 2009; Luczak et al., 2009; Harris and Thiele, 2011; Safaai et al., 2015).
In physiological terms, activity state can be conceptualized as common measures of “neuronal activity”: membrane potential or spiking activity. Changes in these measures of neuronal activity are caused by excitatory or inhibitory postsynaptic potentials, mediated by point-by-point synaptic transmission via ionotropic receptors (predominantly for glutamate and GABA). This form of synaptic transmission is the means of intracortical interactions and lies at the heart of current large-scale computational models of intrinsic activity correlations (Honey et al., 2007, 2009; Breakspear et al., 2009; Deco et al., 2013, 2014).
By contrast, variations in dynamic state can be conceptualized as the slower effects of neuromodulators, mediated by “volume transmission” (i.e., not point-by-point synapses) and by metabotropic receptors that do not alter the postsynaptic membrane potential directly. Activation of metabotropic receptors sets in motion intracellular signaling cascades that alter the way in which neurons respond to input over protracted periods of time, from changing the conductance of ionotropic receptors to altering the expression of genes. For example, catecholamines (in particular noradrenaline) change the balance between excitation and inhibition in the local microcircuit (Froemke, 2015; Martins and Froemke, 2015; Pfeffer et al., 2018). This circuit effects in turn increases the responsivity of cortical neurons to synaptic input (Moises et al., 1979; Rogawksi and Aghajanian, 1980; Seamans et al., 2001a, b; Wang and O’Donnell, 2001), an effect referred to as “neural gain”: an increase in the slope of the input-output function (Berridge and Waterhouse, 2003; Murphy and Miller, 2003; Winterer and Weinberger, 2004; Disney et al., 2007; Polack et al., 2013). This mechanism of action corresponds to the common notion of “neuromodulation.”
Importantly, while the majority of receptors for neuromodulators are metabotropic, some of them are ionotropic – in particular, the nicotinic class of ACh receptors (Itier and Bertrand, 2001), and the 5HT3 subclass of serotonin receptors (Barnes et al., 2009). Activation of both receptor types leads to rapid activation of cortical networks (Puig et al., 2004; Fu et al., 2014; McCormick and Nusbaum, 2014; Wester and McBain, 2014; McGinley et al., 2015). Moreover, changes in the activity of neuromodulatory nuclei coincide with (Eschenko et al., 2012), and cause (Pinto et al., 2013) rapid fluctuations in activity state (e.g., the transition from the “down” to “up” state of synchronized cortical population activity). Serotonergic or cholinergic activation of ionotropic receptors thus constitutes a mechanism by which neuromodulatory brainstem system can rapidly change the cortical activity state.
In sum, neuromodulators may, in principle, alter intrinsic activity correlations in two ways. First, by rapidly changing the activity state of distributed sets of cortical regions through common (excitatory or inhibitory) drive (Drew et al., 2008). Second, neuromodulators may change the dynamic state (e.g., excitation–inhibition balance) of sets of cortical regions in a coordinated fashion but on slower timescales. Such coordinated changes in dynamic state, in turn, may directly produce correlations between population activity, but they can also modulate the correlations produced by cortical interactions through altering local dynamics (Deco et al., 2014; Pfeffer et al., 2018). The two principal mechanisms (modulation of activity state vs. dynamic state) reflect the distinct effects of cortical (ionotropic vs. metabotropic) receptors. Importantly, both mechanisms can produce intrinsic activity correlations (Leopold et al., 2003), even in the absence of any effect on cortico–cortical interactions.
While the projections of neuromodulatory nuclei to the cortex are commonly known as widespread or diffuse, there is substantial heterogeneity and specificity in these projections, part of which is only now being uncovered through novel anatomical tracing techniques (Foote and Morrison, 1987; Chandler and Waterhouse, 2012; Chandler et al., 2014; Schwarz and Luo, 2015; Schwarz et al., 2015; Uematsu et al., 2015, 2017; Kebschull et al., 2016; Breton-Provencher and Sur, 2019). What is more, the cortical distributions of the various different receptors for each neuromodulator are heterogeneous (Ramos and Arnsten, 2007; Zilles and Amunts, 2009; Nahimi et al., 2015; Salgado et al., 2016), which is evident for the human cortex in recent maps of receptor gene expression (Figure 2). Consequently, input from any neuromodulatory nucleus to the cortex, changing activity state, dynamic state, or both, might translate into spatially structured correlations of neural population signals in the cortex. For this reason, it is critical to consider the potential impact of neuromodulatory brainstem systems when making inferences about physiological cortico–cortical interactions (and “cortical networks”) from the correlation of intrinsic cortical activity alone.
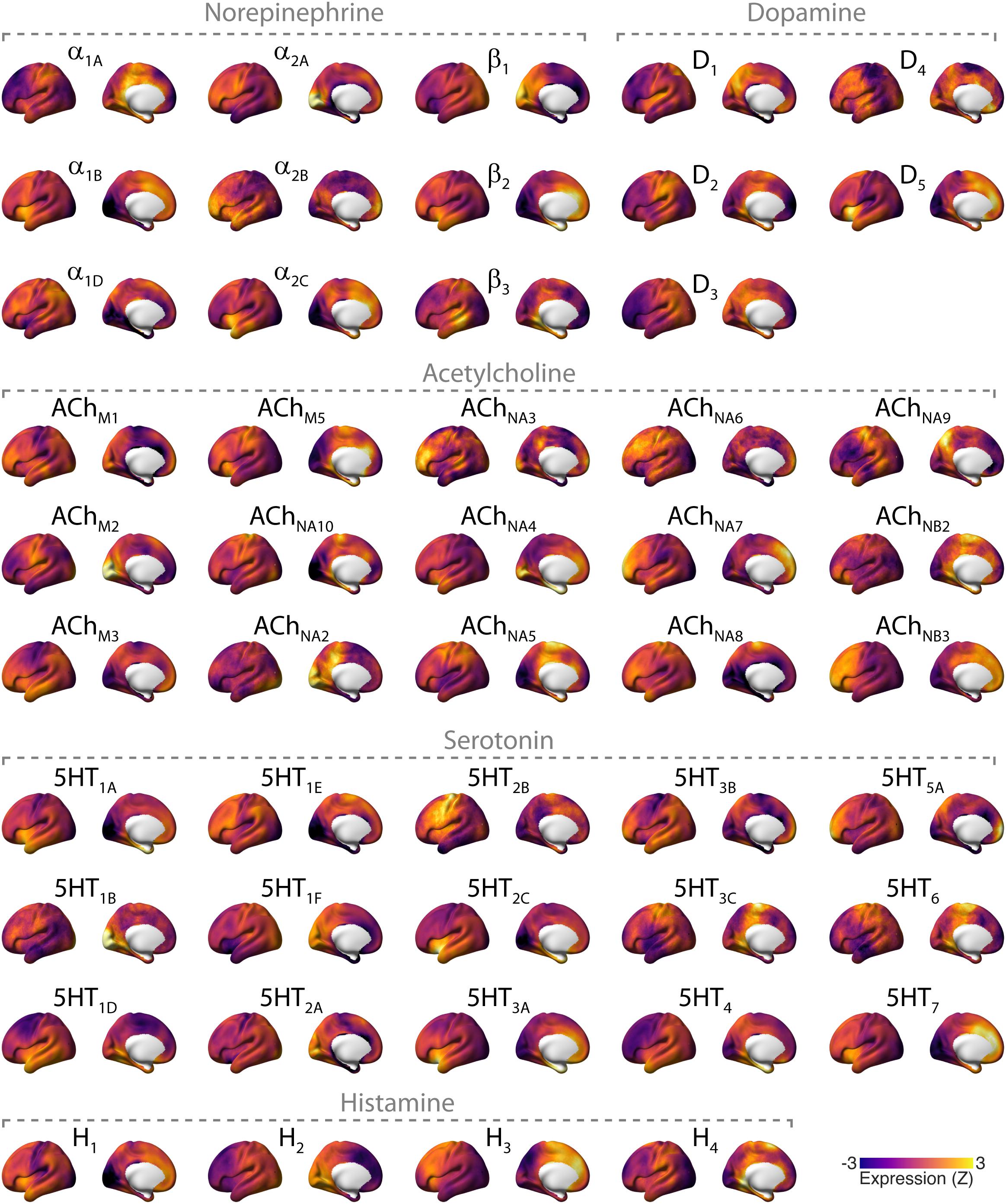
Figure 2. Overview of cortical distributions of genetic expression of neuromodulator receptors in the human brain. Receptor maps were taken from Gryglewski et al. (2018), projected onto the cortical surface, and Z-scored across space. Files and tool for plotting these maps can be found here: github.com/rudyvdbrink/receptormaps. Abbreviations: D: dopamine; ACh: acetylcholine; M: muscarinic; N: nicotinic; 5HT: serotonin; H: histamine.
Correlated Cortical Activity Driven by Intrinsic Fluctuations of Brainstem Activity
If neuromodulatory nuclei rapidly drive cortical activity in widespread target networks via ionotropic mechanisms then (i) removing neuromodulatory drive on the cortex should attenuate correlated activity within the cortex, and (ii) manipulating the time-varying activity of neuromodulatory nuclei should similarly affect the time-varying activity within the cortex, and (iii) activity within neuromodulatory nuclei should co-vary with intrinsic activity in large areas of the cortex. These predictions also hold if neuromodulatory nuclei potentiate drive from other sources such as the thalamus.
Causal Manipulation of Time-Varying Neuromodulatory Activity
Evidence for the first two predictions comes from studies in which the time varying activity of neuromodulatory nuclei is manipulated. Turchi et al. (2018) reversibly inactivated portions of the BF using the GABAA agonist muscimol in rhesus macaques, thus removing potential fluctuating common input to cortical areas. This manipulation reduced correlations between the “global” BOLD-fMRI signal (i.e., the average across all gray matter voxels) and voxel-wise activity topographically aligned with the afferents of the inactivated BF location. Because the BF sends GABAergic as well as cholinergic projections to the cortex, these findings do not necessarily reflect cholinergic effects. Grandjean et al. (2019) rhythmically stimulated serotonergic neurons in the DR with optogenetics in rodents, and measured the cerebral blood volume (CBV) response with fMRI (CBV was used because of putative signal-to-noise advantages over BOLD-fMRI). Cortical CBV showed widespread, correlated troughs in amplitude in response to DR stimulation. Moreover, the cortical distribution of CBV responses correlated spatially with reductions in burst rate and delta-band power as measured electrophysiologically.
Combined, these two studies (Turchi et al., 2018; Grandjean et al., 2019) provide the strongest evidence to date of a mediation of (a part of) intrinsic activity correlations through rapid, common input from neuromodulatory brainstem nuclei that are consistent with correlated changes in activity state. A critical test of this notion would, however, use as a neural marker of interest correlations between spiking activity in different cortical regions, rather than between their BOLD or CBV signals. This is because changes in the latter signals may not necessarily reflect changes in cortical activity state (i.e., spiking activity; Maier et al., 2008) but may be produced by changes in dynamic state (Logothetis, 2008).
Analogous evidence for the LC-NE system comes from older positron emission tomography (PET) work by Coull et al. (1999) who reduced LC-activity via clonidine (Florin-Lechner et al., 1996) in healthy humans. During rest, clonidine caused broad reductions in directed coupling of PET activity between several cortical and sub-cortical regions. Due to the sluggish nature of the PET signals measured, however, it is difficult to attribute these results to changes in activity or dynamic state. In addition, DA depletion has similarly been reported to cause broad reductions in fMRI activity correlation strength (Shafiei et al., 2019).
Temporal Co-variation Between the Brainstem and the Cortex
The prediction that activity in neuromodulatory nuclei should co-vary with cortical activity can be tested using seed-based correlation analyses, where (fMRI) activity in neuromodulatory nuclei is correlated with cortical activity. A limited number of such studies have been conducted. Because of the small size and spatial proximity of these nuclei to the ventricles (Figure 1) and strong effects of physiological noise, these types of measurements require non-standard approaches to fMRI measurement and data analysis (Astafiev et al., 2010; Klein-Flugge et al., 2011; Brooks et al., 2013; Beissner, 2015; de Gee et al., 2017; Forstmann et al., 2017). Therefore, unless mentioned otherwise, we only considered studies where retrospective image correction for physiological noise was applied.
Zhang et al. (2015) assessed correlations between the cortex and LC, VTA, and SN, as defined by anatomical atlases (Ahsan et al., 2007; Keren et al., 2009). They reported widespread negative correlations between the cortex and all three brainstem nuclei and predominantly positive correlations between activity in these nuclei and other subcortical areas. Liu et al. (2018) found that peaks in the global (gray matter voxel averaged) signal coincided with troughs in activity of the BF, suggesting an anti-correlation between BF activity and cortical activity. This is in line with earlier findings by Li et al. (2014), who reported widespread negative correlation between BF and cortical areas, although no correction for physiological noise was applied in this study. By contrast, Markello et al. (2018) found positive correlations between anatomically defined BF subdivisions (Zaborszky et al., 2008) and known cortical targets of BF projections, possibly due to the fact that in this study partial correlation was used to examine subdivision-specific correlations. Lastly, Beliveau et al. (2015) found relatively confined partial correlations between the cortex and anatomically and [11C]DASB PET-binding constrained delineations of the dorsal and median raphe nuclei.
Summary and Outstanding Issues
The most direct evidence for intrinsic activity correlations through common drive of distributed cortical regions by neuromodulatory systems comes from direct manipulations of the activity of the corresponding brainstem nuclei. However, such studies are sparse and have not yet been conducted for all neuromodulatory nuclei. In addition, activity in most, but not all, neuromodulatory nuclei has been reported to covary with widespread areas of the cortex. However, to the best of our knowledge, no study to date has examined the individual contribution of the full set of neuromodulatory nuclei (Figure 1 and Box 2) to intrinsic activity fluctuations within the cortex. This leaves open the possibility that any correlation between an individual nucleus and the cortex is due to shared fluctuations across neuromodulatory nuclei (de Gee et al., 2017) rather than reflecting specific drive of one neuromodulatory nucleus on cortical activity. Moreover, removal of the global signal (as done by e.g., Beliveau et al., 2015) may obscure wide-spread correlations and reveal only those correlations that are stronger than global components of the cortical signal (i.e., the mean of all cortical regions). Another important limitation of all studies assessing intrinsic activity correlations by means of the fMRI signal, is that that the latter may reflect either changes in activity state (spike rate) or changes in dynamic state produced by neuromodulatory mechanisms (Logothetis, 2008).
In sum, the available studies are consistent with the notion of common drive of distributed cortical regions by the fluctuating activity of neuromodulatory nuclei. In this light, at least part of the spatial structure of intrinsic activity correlations within the cortex may reflect the spatial distribution of the projections of these brainstem nuclei, or their receptors, rather than the topography of cortico-cortical connections. Further experiments and direct comparisons of the contribution of individual neuromodulatory nuclei on intrinsic activity correlations are warranted, bearing in mind the above-mentioned interpretational caveats.
Changes in Intrinsic Cortical Correlations Under Pharmacological Intervention
A major approach in the study of neuromodulatory systems is to manipulate neuromodulator levels via pharmacological intervention, and measure the resulting effects on cortical activity. Such pharmacological intervention will primarily exert its effects on cortical activity through sustained alterations of cortical circuit properties (i.e., shifts in cortical dynamic state), and less (or not at all) by altering the rapid drive of cortical regions, although it has been shown that the NE-reuptake inhibitor atomoxetine alters the dynamics of LC activity (e.g., via auto-receptors) (Bari and Aston-Jones, 2013). For simplicity, we here heuristically treat pharmacological intervention as manipulations of changes in cortical dynamic state, and review the effects of these manipulations on intrinsic activity correlations.
We examine three key characteristics of these correlations (Box 1) in order to delineate commonalities or inconsistencies across findings. First, given the widespread projection profile of neuromodulatory systems (Figure 1 and Box 2), pharmacological manipulation of these systems may be expected to result in changes in correlation strength that encompass large areas of the cortex. We thus discuss literature that has examined such global changes of correlation strength (i.e., whole-brain increases or decreases). Second, the spatial heterogeneity of neuromodulatory projections and receptors (Figure 2) across the cortex suggests that neuromodulatory systems may not only change the global strength of intrinsic activity correlations, but also result in spatially inhomogeneous changes of correlation strength (van den Brink et al., 2018a). Third, computational modeling work indicates that modifications of circuit properties that are subject to neuromodulatory influence such as gain or excitation–inhibition balance (Servan-Schreiber et al., 1990; Polack et al., 2013; Froemke, 2015; Pfeffer et al., 2018) can alter the geometric properties of whole-brain intrinsic activity correlations (topology), even without any heterogeneity of neuromodulatory influences (Deco et al., 2014; Shine et al., 2018a). We therefore discuss literature that has examined the effect of pharmacological manipulation of neuromodulator systems on RSN topography and whole-brain functional topology.
Changes of the Global Strength of Intrinsic Correlations
Several studies have examined the effect of the NE reuptake inhibitor atomoxetine, which increases cortical catecholamine levels (Bymaster et al., 2002; Devoto et al., 2004; Swanson et al., 2006; Koda et al., 2010), on global correlation strength. Using atomoxetine, van den Brink et al. (2016) found reductions compared to placebo in graph-theoretic metrics of the global strength of intrinsic fMRI activity correlations in humans. Similarly, Guedj et al. (2017b) found reduced atomoxetine-induced fMRI correlation strength within and between various RSNs in rhesus macaques, with an overall net change of reduced correlation. This was also reflected in a reduction in the average brain-wide weighted correlation coefficient (Guedj et al., 2017a). By contrast, Pfeffer et al. (2019) found no significant atomoxetine-induced change in graph theoretic metrics of activity correlations measured with MEG during rest. However, during viewing of a perceptually ambiguous visual stimulus task, Pfeffer et al. (2019) found that atomoxetine increased the strength of correlations. A global increase in fMRI correlations following chemogenetic LC stimulation in anesthetized mice has also been reported (Zerbi et al., 2019).
The above mentioned MEG-study by Pfeffer et al. (2019) also investigated the effect of increased cortical ACh levels on correlated activity. This study used the acetylcholinesterase inhibitor donepezil and found reduced correlation strength during rest, and no effect during the presentation of an ambiguous visual stimulus. Three studies have examined the effect of pharmacological modulation of the 5HT system on the global strength of fMRI correlations. Schaefer et al. (2014) reported widespread reductions in correlation strength (quantified as the graph theoretic metric degree) following administration of the 5HT reuptake inhibitor escitalopram. Similarly, Preller et al. (2018) found a widespread shift of cortical intrinsic activity correlations toward zero due to the 5HT2A receptor agonist LSD – an effect that did not occur when simultaneously administering the 5HT2A antagonist ketanserin. By contrast, Tagliazucchi et al. (2016) reported an LSD-induced increase in the overall strength of correlations within the cortex.
Topographically Specific Changes of Intrinsic Correlation Strength
Topographical effects of pharmacological manipulation may be informative about which sets of cortical regions (“networks”) are particularly susceptible to neuromodulatory influence through a modulation of the dynamic state. Below, we provide a summary of consistent findings in the literature (see also Tables 1, 2). We review studies that either used whole-brain “dual regression,” or correlation between a seed and the entire cortex. The term dual regression refers to sequential spatial and temporal regression of independent components with the purpose of identifying consistent spatiotemporal networks and manipulation-related changes therein (Beckmann, 2009).
Pharmacological elevations of the noradrenergic tone, while yielding a diversity of findings, have consistently produced effects that involve visual cortex. Coull et al. (1999) reported an α2 agonist clonidine-induced reduction in effective connectivity to- and from posterior extrastriate visual cortex. Reduced correlations between early visual cortex and frontoparietal cortical areas have also been reported following the α2 agonist dexmedetomidine (Akeju et al., 2016) and NET blocker atomoxetine (van den Brink et al., 2016). Similarly, Guedj et al. (2017b) reported atomoxetine-induced reductions in correlation within a frontoparietal network and peripheral visual network. One study did not report changes in correlation of visual cortical areas following chemogenetic LC stimulation in rodents (Zerbi et al., 2019), potentially due to inter-species differences or differences between the effect of chemogenetic and pharmacological manipulation (Giorgi et al., 2017).
Manipulations of the cholinergic system have, likewise, yielded topographical effects that involve visual cortex. Tanabe et al. (2011) reported a nicotine-induced increase in correlation strength within an extrastriate network, and Klaassens et al. (2017) reported a galantamine (cholinesterase inhibitor) induced increase in correlation strength between a polar occipital network and widespread areas of the cortex. In addition, some, but not all (Guedj et al., 2017b), studies using noradrenergic or cholinergic agents have reported effects that involve the default mode network (DMN) (Tanabe et al., 2011; Akeju et al., 2016; van den Brink et al., 2016, 2018a; Klaassens et al., 2017; Zerbi et al., 2019). The observation that noradrenergic or cholinergic agents consistently produced effects on intrinsic correlations in visual cortical areas could be due to the fact that NE receptors α2A and β1, and ACh receptors NA10 and M2 are prominently expressed these regions (Figure 2).
In contrast, primarily dopaminergic agents produced no effects on visual cortex, but instead on somatosensory and (pre-)motor cortex. For example, a positive relationship between DA levels and correlation strength between the basal ganglia and sensorimotor cortex (Cole et al., 2013), reduced correlation between the caudate and nodes of the sensorimotor network including pre- and postcentral gyri following D2 receptor agonism (Ye et al., 2017), and reduced correlation within the sensorimotor network following DA depletion (Shafiei et al., 2019). Dopaminergic effects in motor cortical regions may reflect direct modulations of dynamic state within the cortex, or down-stream effects of modulations of the efficacy of dopaminergic projections from the brainstem to the basal ganglia (Figure 1).
Catecholaminergic manipulations have also been studied in the context of stress-related (re)activation patterns (Hermans et al., 2011; Gerlicher et al., 2018). These studies show prominent noradrenergic and dopaminergic effects on stress-induced changes in activation patterns or later reemergence thereof.
Studies using serotonergic agents have reported effects on intrinsic correlations resembling a combination of noradrenergic/cholinergic and dopaminergic effects: in other words, effects in both visual cortical and sensorimotor areas (Klaassens et al., 2015, 2017; Carhart-Harris et al., 2016; Tagliazucchi et al., 2016; Preller et al., 2018). In particular, studies that used the 5HT2A receptor agonist LSD consistently reported effects on visual cortex. Indeed, the 5HT2A receptor is prominently expressed in visual cortex (Figure 2).
Topologically Specific Changes of Intrinsic Correlation Strength
Various analytical tools exist to characterize the topology of functional brain organization (Rubinov and Sporns, 2010; Shine and Poldrack, 2018). For instance, the balance between topological segregation and integration of cortical ensembles is determined by the ratio of activity correlation strength within versus between separate modules (Mattar et al., 2015; Shine et al., 2016), and has been related to behavioral performance (Shine and Poldrack, 2018). Topological variations in the segregation-integration balance of fMRI activity during rest covary with pupil diameter (Shine et al., 2016), a non-invasive proxy for activity in neuromodulatory nuclei (Murphy et al., 2014; Varazzani et al., 2015; Joshi et al., 2016; Breton-Provencher and Sur, 2019). Thus, topological features of intrinsic activity correlations may be under neuromodulatory control.
Indeed, pharmacological upregulation of cortical NE levels using atomoxetine has been shown to result in a shift toward segregated processing during rest, and a converse shift toward integrated processing during the performance of a cognitively demanding (N-back) task (Shine et al., 2018b). Similarly, van den Brink et al. (2016) found that atomoxetine reduced between-module correlation strength, and reduced metrics of integration (clustering coefficient and transitivity) during rest. Guedj et al. (2017a) reported reduced global efficiency, a metric of integration (Rubinov and Sporns, 2010), but reduced clustering, due to atomoxetine in rhesus macaques.
Studies using DA manipulations seem to indicate that DA facilitates integration. Achard and Bullmore (2007) reported reduced metrics of global and local efficiency due to the D2 antagonist sulpride. Shafiei et al. (2019) reported that DA depletion reduced the participation coefficient (between-module correlation) of the sensorimotor and salience networks. Thus, DA and NE may have dichotomous effects on network topology. However, null effects of DA agonism on various topological metrics, including metrics of integration, have also been reported (Ye et al., 2017). To the best of our knowledge, no studies to date have examined the effect of ACh manipulation on network topology, and one study has examined the effect of 5HT2A agonism (via LSD) on fMRI intrinsic correlation topology (Tagliazucchi et al., 2016). This study reported reduced modularity (increased integration), increased participation coefficient of frontal and midline regions (increased between-network correlation at the expense of within-network correlation), and reduced rich-club coefficient (less correlation with hub regions).
Summary and Outstanding Issues
Pharmacological manipulation of tonic neuromodulatory action, and the resulting, putative change in cortical dynamic state, consistently alters the global strength of intrinsic correlations. Less consistent, however, is the direction of these effects, even within classes of neuromodulators. Further studies are needed to corroborate or exclude the following possible reasons for these discrepancies: cross-study differences in preprocessing such as global signal regression (Preller et al., 2018); dose-dependence of effects (Zahrt et al., 1997); or dependence of effects on cognitive/behavioral context (Coull et al., 1999; Shine et al., 2018b; Pfeffer et al., 2019) or baseline arousal (Warren et al., 2016).
Topographical effects following noradrenergic or cholinergic manipulation consistently involve visual cortex. Studies that used predominantly dopaminergic agents consistently report effects involving motor-related brain areas, but not visual brain areas. Studies that used a serotonergic agent report both visual and motor areas. This literature suggests that the regions that are most likely to be affected by pharmacological manipulation of neuromodulators are potentially distributed in accordance with the distribution of receptors across areas.
The predominant finding from studies on topological effects is that neuromodulators alter functional network topology, in particularly the catecholamines. These studies also suggest a possible distinction between the effects of DA and NE on network-level integration. Whereas NE reuptake during rest reduces topological metrics of integration, DA antagonism and depletion have the same effect, suggesting that DA facilitates integration. Similar to DA, 5HT seems to increase metrics of integration, but only one study has examined these effects. The influence of ACh on functional network topology remains to be studied.
A caveat with the findings on network topology is that some metrics of integration (in particular efficiency and clustering) are susceptible to changes in degree, even when artificially fixing degree of adjacency matrices by applying a fixed threshold (van Wijk et al., 2010). Since neuromodulators have been reported to alter degree as well (see section changes of the global strength of intrinsic correlations), future studies should carefully consider alterations in global degree when examining topological metrics. In addition, studies that have examined changes in the time-varying topology should take into account the influence of temporal fluctuations of the community structure on topological metrics (Thompson et al., 2019).
Conclusion and Future Directions
Both (de)activation studies and seed-based correlation studies provide supporting evidence for ongoing fluctuations in the activity of neuromodulatory brainstem nuclei as a possible driving source of intrinsic activity correlations within the cortex. Temporary BF inactivation, NE release inhibition, DA synthesis inhibition, and rhythmic optogenetic serotonergic DR neuron stimulation all reduce intrinsic activity correlations. Furthermore, activity fluctuations in most neuromodulatory nuclei (BF; VTA; SN; LC) but not all (raphe nuclei) predict correlated activity fluctuations in broad areas of the cortex.
Pharmacological manipulation of cortical neuromodulator levels, which putatively alters cortical dynamic state, results in diverse changes of intrinsic activity correlations. First, pharmacological upregulation consistently changes the global strength of cortical correlations. Yet, in what direction the individual modulators exert their effects needs further study, since these effects are likely to be dependent on several factors, such as drug dose or behavioral context.
Second, several pharmacological studies have quantified neuromodulator-induced changes in the topography of cortical activity. Noradrenergic and cholinergic manipulation consistently alter activity correlations in visual cortical areas, dopaminergic manipulation affects motor cortical networks, and serotonergic manipulation affects both.
Finally, a number of studies have demonstrated that neuromodulators alter the topological properties of intrinsic activity correlations. These studies suggest a possible distinction between the effects of DA and NE on network-level functional integration. Similar to DA, 5HT seems to increase metrics of functional integration.
The studies discussed in this review may well have only scratched the surface the full spectrum of effects that neuromodulatory systems exert on intrinsic activity correlations. Even so, they open up exciting avenues for future work. An effort to map the contribution of each neuromodulatory system to the spatial and temporal features of intrinsic correlations may aid the identification of shared, independent, or antagonistic principles between the actions of different neuromodulatory systems and their diverse receptor classes. Such principles will inform models of healthy brain function and provide an important reference for the mechanistic understanding of neurological and psychiatric disorders. Ultimately, such principles may guide the way toward identification of specific molecular targets for mechanistically inspired and individualized pharmacological interventions in disorders of higher brain function. In what follows, we outline a number of important avenues for future research. Each of these could help advance our understanding of the principles that govern intrinsic brain dynamics and its dysfunctions in critical ways.
Dissecting the Mechanisms of Brainstem Modulation of Cortical Correlations
We have highlighted that neuromodulatory brainstem systems may alter intrinsic correlations of cortical population activity through diverse mechanistic pathways. The fluctuating activity of brainstem nuclei may provide common drive to large swathes of cortical regions, or drive other subcortical regions (such as the pulvinar nucleus of the thalamus) that can in turn cause widespread changes in cortical activity (Nakajima and Halassa, 2017; Arcaro et al., 2018). Second, neuromodulatory brainstem systems may also modulate the cortical dynamic state, thereby altering correlations in cortical population activity indirectly. These two mechanisms are non-mutually exclusive: the physiological effect of any neuromodulatory action likely results from a complex mixture of both. Nevertheless, careful experimental manipulations should tease these mechanisms apart and provide insight into the consequences of each mechanism for intrinsic activity fluctuations within the cortex.
For example, in order to test whether neuromodulatory systems induce temporal correlations in the cortex through common drive, one can manipulate the time varying activity of brainstem nuclei, and examine the time varying signature of this manipulation in cortical activity. Using optogenetics, specific neuron types can be targeted such that non-neuromodulatory (e.g., GABAergic) long-range projections that emanate from these nuclei are not directly affected by the experimental manipulation. Moreover, electrophysiological recordings within the cortex would circumvent interpretational caveats that are inherent to the transformation of neural activity into the fMRI signal (Logothetis, 2008). Experiments of this kind have shown promise (Grandjean et al., 2019). Combining such manipulations with the administration of pharmacological blockade of ionotropic receptors could ultimately provide decisive evidence in favor of or against the notion of drive of intrinsic activity correlations by neuromodulators.
Additionally, seed-based correlation studies can provide evidence of co-fluctuating activity in brainstem neuromodulatory nuclei and the cortex in humans. In order to elucidate the relationship between each neuromodulatory system and correlated activity within the cortex, studies are needed in which activity in all nuclei is measured simultaneously, and the covariation between the individual nuclei is taken into account. Such analyses have not yet been conducted, but can be readily implemented with existing techniques.
Another possible means to distinguishing multiple mechanisms of action is to examine the spatial correspondence between the effect of a manipulation of neuromodulators on cortical intrinsic activity correlations, and the distribution of specific receptors. These comparisons are now possible using open-access databases of genetic expression of receptor types (Hawrylycz et al., 2012), validated for use in neuroimaging (Gryglewski et al., 2018). Such studies may benefit from analytical tools that are tailored to distil manipulation-related effects on cortical correlations in a specific direction, without relying on a priori selection of correlated networks (e.g., van den Brink et al., 2018a). Examining the spatial relationship between the effect of a manipulation and the receptors may also be indicative of whether the cortical effect of a neuromodulator is determined primarily by the anatomical projection profile of the nucleus that releases it, or if the receptor distributions weigh more heavily (Grandjean et al., 2019). Moreover, such analyses should be used to contrast the impact of ionotropic and metabotropic receptors.
Developing Mechanistic Models and Biomarkers for Neuropsychiatric Disorders
A detailed understanding of how neuromodulators shape intrinsic activity correlations may aid the development of novel biomarkers for neurological and psychiatric disorders, as well as mechanistic models of these disorders. Several disorders are associated with dysfunctions in one or multiple neuromodulatory brainstem systems. For example, Parkinson’s disease is caused by degeneration of the dopaminergic midbrain nuclei, along with the noradrenergic LC. Cognitive decline in aging, in particular Alzheimer’s disease, coincides with degeneration of the cholinergic BF and possibly the LC. Major depression and schizophrenia are associated with disturbances in catecholaminergic and serotonergic systems.
These clear associations with neuromodulatory brainstem systems are currently not exploited for the early detection and classification of such disorders. In particular, the current classification and diagnosis of psychiatric disorders is solely based on subjective assessments of behavioral symptoms, irrespective of the underlying pathophysiological mechanisms (Insel et al., 2010; Krystal and State, 2014). One consequence of this coarse and phenomenological classification scheme is that patient populations in one diagnostic category are often heterogeneous in terms of the neural circuit deficits that give rise to the behavioral symptoms (Seaton et al., 2001; Insel et al., 2010). This hampers the development of individualized treatment plans that target the key circuit disorder that underlies the cognitive or behavioral deficits of a given patient.
The insight that neuromodulators profoundly shape intrinsic activity correlations that are evident with non-invasive neuroimaging techniques opens the door for overcoming these limitations in current clinical practice. The insight sets the stage for the development of neural markers of psychiatric disorders that are directly grounded in the underlying pathomechanisms and cortical signatures of neuromodulatory action. Specifically, the changes in correlation patterns associated with (manipulations of) a specific neuromodulatory system can provide a “reference template” to which alterations of correlation patterns associated with specific disorders can be compared. Such markers may prove to reflect an individual patient’s precise deficit more reliably and help identify molecular targets for pharmacological intervention.
In this light, it is important to evaluate the influence of behavioral context on the effect that neuromodulators exert on intrinsic activity correlations. Manipulation of neuromodulator levels has been shown to result in opposing effects on intrinsic activity correlations under different behavioral contexts (Coull et al., 1999; Shine et al., 2018b; Pfeffer et al., 2019). Direct comparisons of various neuromodulators within the same- and between different cognitive contexts can thus provide interpretational constraints on alterations of correlation patterns that are associated with psychiatric disorders. Moreover, such an approach may help resolve standing discrepancies in the literature regarding the direction of pharmacological effects on intrinsic activity correlations.
Lastly, neuromodulators interact, through reciprocal connections between the brainstem nuclei, shared cortical afferents, and cortical receptor co-expression. Because of this, dysfunction in a single neuromodulatory system is unlikely to occur without affecting others. Moreover, any neuromodulatory dysfunction that is associated with a psychiatric disorder may not be observable in intrinsic activity correlations as a linear summation of the above described reference templates of the individual neuromodulatory systems that are dysfunctional. Thus, further study on how the joint actions of neuromodulators shape cortical interactions is needed. A starting point is to incorporate receptor co-expression into large-scale computational models of cortical function, combined with coupling terms that link activity of one neuromodulatory system to that of another. This may capture interactions between these systems more accurately, and yield mechanistic insight about how dysfunction in these systems manifests itself at the level of intra-cortical processes and behavior.
Author Contributions
RB and TD conceived the idea for this article. RB wrote the manuscript. RB, TP, and TD commented on and edited the manuscript.
Funding
This work was supported by a fellowship for postdoctoral researchers funded by the Alexander von Humboldt Foundation (to RB), and the following grants from the German Research Foundation (to TD): DO 1240/4-1, SFB 936/A7, and SFB 936/Z3.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Achard, S., and Bullmore, E. (2007). Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 3:e17. doi: 10.1371/journal.pcbi.0030017
Ahmed, N. Y., Knowles, R., and Dehorter, N. (2019). New insights into cholinergic neuron diversity. Front. Mol. Neurosci. 12:204. doi: 10.3389/fnmol.2019.00204
Ahsan, R. L., Allom, R., Gousias, I. S., Habib, H., Turkheimer, F. E., Free, S., et al. (2007). Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. NeuroImage 38, 261–270. doi: 10.1016/j.neuroimage.2007.06.004
Akeju, O., Loggia, M. L., Catana, C., Pavone, K. J., Vazquez, R., Rhee, J., et al. (2016). Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. eLife 3:e04499.
Allen, E. A., Damaraju, E., Plis, S. M., Erhardt, E. B., Eichele, T., and Calhoun, V. D. (2014). Tracking whole-brain connectivity dynamics in the resting state. Cereb. Cortex 24, 663–676. doi: 10.1093/cercor/bhs352
Arcaro, M. J., Pinsk, M. A., Chen, J., and Kastner, S. (2018). Organizing principles of pulvino-cortical functional coupling in humans. Nat. Commun. 9:5382. doi: 10.1038/s41467-018-07725-6
Astafiev, S. V., Snyder, A. Z., Shulman, G. L., and Corbetta, M. (2010). Comment on “modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI” and “homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area”. Science 328:309. doi: 10.1126/science.1177200
Baggio, H. C., Segura, B., and Junque, C. (2015). Resting-state functional brain networks in Parkinson’s disease. CNS Neurosci. Ther. 21, 793–801. doi: 10.1111/cns.12417
Bari, A., and Aston-Jones, G. (2013). Atomoxetine modulates spontaneous and sensory-evoked discharge of locus coeruleus noradrenergic neurons. Neuropharmacology 64, 53–64. doi: 10.1016/j.neuropharm.2012.07.020
Baria, A. T., Mansour, A., Huang, L., Baliki, M. N., Cecchi, G. A., Mesulam, M. M., et al. (2013). Linking human brain local activity fluctuations to structural and functional network architectures. NeuroImage 73, 144–155. doi: 10.1016/j.neuroimage.2013.01.072
Barnes, N. M., Hales, T. G., Lummis, S. C., and Peters, J. A. (2009). The 5-HT3 receptor - the relationship between structure and function. Neuropharmacology 56, 273–284. doi: 10.1016/j.neuropharm.2008.08.003
Barttfeld, P., Uhrig, L., Sitt, J. D., Sigman, M., Jarraya, B., and Dehaene, S. (2015). Signature of consciousness in the dynamics of resting-state brain activity. Proc. Natl. Acad. Sci. U.S.A. 112, 887–892. doi: 10.1073/pnas.1418031112
Bassett, D. S., Meyer-Lindenberg, A., Achard, S., Duke, T., and Bullmore, E. (2006). Adaptive reconfiguration of fractal small-world human brain functional networks. Proc. Natl. Acad. Sci. U.S.A. 103, 19518–19523. doi: 10.1073/pnas.0606005103
Beas, B. S., Wright, B. J., Skirzewski, M., Leng, Y., Hyun, J. H., Koita, O., et al. (2018). The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat. Neurosci. 21, 963–973. doi: 10.1038/s41593-018-0167-4
Beckmann, C. F. (2009). Group Comparison of Resting-State FMRI Data Using Multi-Subject ICA and Dual Regression. Minnesota: OHBM.
Beissner, F. (2015). Functional MRI of the brainstem: common problems and their solutions. Clin. Neuroradiol. 25, (Suppl. 2), 251–257. doi: 10.1007/s00062-015-0404-0
Beliveau, V., Svarer, C., Frokjaer, V. G., Knudsen, G. M., Greve, D. N., and Fisher, P. M. (2015). Functional connectivity of the dorsal and median raphe nuclei at rest. NeuroImage 116, 187–195. doi: 10.1016/j.neuroimage.2015.04.065
Berridge, C. W., and Waterhouse, B. D. (2003). The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 42, 33–84. doi: 10.1016/s0165-0173(03)00143-7
Biswal, B., Yetkin, F. Z., Haughton, V. M., and Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541. doi: 10.1002/mrm.1910340409
Bock, A. S., Binda, P., Benson, N. C., Bridge, H., Watkins, K. E., and Fine, I. (2015). Resting-state retinotopic organization in the absence of retinal input and visual experience. J. Neurosci. 35, 12366–12382. doi: 10.1523/JNEUROSCI.4715-14.2015
Breakspear, M., Terry, J. R., and Friston, K. J. (2009). Modulation of excitatory synaptic coupling facilitates synchronization and complex dynamics in a biophysical model of neuronal dynamics. Network 14, 703–732. doi: 10.1088/0954-898x_14_4_305
Breton-Provencher, V., and Sur, M. (2019). Active control of arousal by a locus coeruleus GABAergic circuit. Nat. Neurosci. 22, 218–228. doi: 10.1038/s41593-018-0305-z
Brooks, J. C., Faull, O. K., Pattinson, K. T., and Jenkinson, M. (2013). Physiological noise in brainstem FMRI. Front. Hum. Neurosci. 7:623. doi: 10.3389/fnhum.2013.00623
Bymaster, F. P., Katner, J. S., Nelson, D. L., Hemrick-luecke, S. K., Threlkeld, P. G., Heiligenstein, J. H., et al. (2002). Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27, 699–711. doi: 10.1016/s0893-133x(02)00346-9
Cabral, J., Hugues, E., Kringelbach, M. L., and Deco, G. (2012). Modeling the outcome of structural disconnection on resting-state functional connectivity. NeuroImage 62, 1342–1353. doi: 10.1016/j.neuroimage.2012.06.007
Calhoun, V. D., Eichele, T., and Pearlson, G. (2009). Functional brain networks in schizophrenia: a review. Front. Hum. Neurosci. 3:17. doi: 10.3389/neuro.09.017.2009
Carhart-Harris, R. L., Muthukumaraswamy, S., Roseman, L., Kaelen, M., Droog, W., Murphy, K., et al. (2016). Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc. Natl. Acad. Sci. U.S.A. 113, 4853–4858. doi: 10.1073/pnas.1518377113
Cerpa, J. C., Marchand, A. R., and Coutureau, E. (2019). Distinct regional patterns in noradrenergic innervation of the rat prefrontal cortex. J. Chem. Neuroanat. 96, 102–109. doi: 10.1016/j.jchemneu.2019.01.002
Chandler, D., and Waterhouse, B. D. (2012). Evidence for broad versus segregated projections from cholinergic and noradrenergic nuclei to functionally and anatomically discrete subregions of prefrontal cortex. Front. Behav. Neurosci. 6:20. doi: 10.3389/fnbeh.2012.00020
Chandler, D. J., Gao, W.-J., and Waterhouse, B. D. (2014). Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc. Natl. Acad. Sci. U.S.A. 111, 6816–6821. doi: 10.1073/pnas.1320827111
Chang, C., and Glover, G. H. (2010). Time-frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage 50, 81–98. doi: 10.1016/j.neuroimage.2009.12.011
Chaudhuri, R., Knoblauch, K., Gariel, M. A., Kennedy, H., and Wang, X. J. (2015). A large-scale circuit mechanism for hierarchical dynamical processing in the primate cortex. Neuron 88, 419–431. doi: 10.1016/j.neuron.2015.09.008
Cho, J. R., Treweek, J. B., Robinson, J. E., Xiao, C., Bremner, L. R., Greenbaum, A., et al. (2017). Dorsal raphe dopamine neurons modulate arousal and promote wakefulness by salient stimuli. Neuron 94, 1205.e8–1219.e8.
Cohen, M. R., and Kohn, A. (2011). Measuring and interpreting neuronal correlations. Nat. Neurosci. 14, 811–819. doi: 10.1038/nn.2842
Cole, D. M., Beckmann, C. F., Oei, N. Y., Both, S., van Gerven, J. M., and Rombouts, S. A. (2013). Differential and distributed effects of dopamine neuromodulations on resting-state network connectivity. NeuroImage 78, 59–67. doi: 10.1016/j.neuroimage.2013.04.034
Cole, M. W., Ito, T., Bassett, D. S., and Schultz, D. H. (2016). Activity flow over resting-state networks shapes cognitive task activations. Nat. Neurosci. 19, 1718–1726. doi: 10.1038/nn.4406
Coull, J. T., Büchel, C., Friston, K. J., and Firth, C. D. (1999). Noradrenergically mediated plasticity in a human attentional neuronal network. NeuroImage 10, 705–715. doi: 10.1006/nimg.1999.0513
Curto, C., Sakata, S., Marguet, S., Itskov, V., and Harris, K. D. (2009). A simple model of cortical dynamics explains variability and state dependence of sensory responses in urethane-anesthetized auditory cortex. J. Neurosci. 29, 10600–10612. doi: 10.1523/JNEUROSCI.2053-09.2009
de Gee, J. W., Colizoli, O., Kloosterman, N. A., Knapen, T., Nieuwenhuis, S., and Donner, T. (2017). Dynamic modulation of decision biases by brainstem arousal systems. eLife 6:e23232.
de Pasquale, F., Della Penna, S., Snyder, A. Z., Lewis, C., Mantini, D., Marzetti, L., et al. (2010). Temporal dynamics of spontaneous MEG activity in brain networks. Proc. Natl. Acad. Sci. U.S.A. 107, 6040–6045. doi: 10.1073/pnas.0913863107
Deco, G., Jirsa, V. K., and McIntosh, A. R. (2011). Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 12, 43–56. doi: 10.1038/nrn2961
Deco, G., Ponce-Alvarez, A., Mantini, D., Romani, G. L., Hagmann, P., and Corbetta, M. (2013). Resting-state functional connectivity emerges from structurally and dynamically shaped slow linear fluctuations. J. Neurosci. 33, 11239–11252. doi: 10.1523/JNEUROSCI.1091-13.2013
Deco, G., Ponce-Alvarez, A., Hagmann, P., Romani, G. L., Mantini, D., and Corbetta, M. (2014). How local excitation-inhibition ratio impacts the whole brain dynamics. J. Neurosci. 34, 7886–7898. doi: 10.1523/JNEUROSCI.5068-13.2014
Deco, G., Cruzat, J., Cabral, J., Knudsen, G. M., Carhart-Harris, R. L., Whybrow, P. C., et al. (2018). Whole-brain multimodal neuroimaging model using serotonin receptor maps explains non-linear functional effects of LSD. Curr. Biol. 28, 3065.e6–3074.e6.
Devoto, P., Flore, G., Pira, L., Longu, G., and Gessa, G. L. (2004). Alpha2-adrenoceptor mediated co-release of dopamine and noradrenaline from noradrenergic neurons in the cerebral cortex. J. Neurochem. 88, 1003–1009. doi: 10.1046/j.1471-4159.2003.02239.x
Dichter, G. S., Gibbs, D., and Smoski, M. J. (2015). A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J. Affect. Disord. 172, 8–17. doi: 10.1016/j.jad.2014.09.028
Disney, A. A., Aoki, C., and Hawken, M. J. (2007). Gain modulation by nicotine in macaque v1. Neuron 56, 701–713. doi: 10.1016/j.neuron.2007.09.034
Donner, T. H., Sagi, D., Bonneh, Y. S., and Heeger, D. J. (2013). Retinotopic patterns of correlated fluctuations in visual cortex reflect the dynamics of spontaneous perceptual suppression. J. Neurosci. 33, 2188–2198. doi: 10.1523/JNEUROSCI.3388-12.2013
Drew, P. J., Duyn, J. H., Golanov, E., and Kleinfeld, D. (2008). Finding coherence in spontaneous oscillations. Nat. Neurosci. 11, 991–993. doi: 10.1038/nn0908-991
Eschenko, O., Magri, C., Panzeri, S., and Sara, S. J. (2012). Noradrenergic neurons of the locus coeruleus are phase locked to cortical up-down states during sleep. Cereb. Cortex 22, 426–435. doi: 10.1093/cercor/bhr121
FitzHugh, R. (1961). Impuses and physiological states in theoretical models of nerve membrane. Biophys. J. 1, 445–466. doi: 10.1016/s0006-3495(61)86902-6
Florin-Lechner, S. M., Druhan, J. P., Aston-Jones, G., and Valentino, R. J. (1996). Enhanced norepinephrine release in the prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 742, 89–97. doi: 10.1016/s0006-8993(96)00967-5
Foote, S. L., and Morrison, J. H. (1987). Extrathalamic modulation of cortical function. Ann. Rev. Neurosci. 10, 67–95. doi: 10.1146/annurev.neuro.10.1.67
Forstmann, B. U., Hollander, G. D., Maanen, L. V., Alkemade, A., and Keuken, M. C. (2017). Towards a mechanistic understanding of the human subcortex. Nat. Rev. Neurosci. 18, 57–65. doi: 10.1038/nrn.2016.163
Fox, M. D., and Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711. doi: 10.1038/nrn2201
Fox, M. D., and Greicius, M. (2010). Clinical applications of resting state functional connectivity. Front. Syst. Neurosci 4:19. doi: 10.3389/fnsys.2010.00019
French, I. T., and Muthusamy, K. A. (2018). A review of the pedunculopontine nucleus in Parkinson’s disease. Front. Aging Neurosci. 10:99. doi: 10.3389/fnagi.2018.00099
Friston, K. J., Firth, C. D., Liddle, P. F., and Frackowiak, R. S. J. (1993). Functional connectivity: the principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 13, 5–14. doi: 10.1038/jcbfm.1993.4
Froemke, R. C. (2015). Plasticity of cortical excitatory-inhibitory balance. Annu. Rev. Neurosci. 38, 195–219. doi: 10.1146/annurev-neuro-071714-034002
Fu, Y., Tucciarone, J. M., Espinosa, J. S., Sheng, N., Darcy, D. P., Nicoll, R. A., et al. (2014). A cortical circuit for gain control by behavioral state. Cell 156, 1139–1152. doi: 10.1016/j.cell.2014.01.050
Garcia-Rill, E. (1991). The pedunculopontine nucleus. Prog. Neurobiol. 36, 363–389. doi: 10.1016/0301-0082(91)90016-t
Gerlicher, A. M. V., Tuscher, O., and Kalisch, R. (2018). Dopamine-dependent prefrontal reactivations explain long-term benefit of fear extinction. Nat. Commun. 9:4294. doi: 10.1038/s41467-018-06785-y
Giorgi, A., Migliarini, S., Galbusera, A., Maddaloni, G., Mereu, M., Margiani, G., et al. (2017). Brain-wide mapping of endogenous serotonergic transmission via chemogenetic fMRI. Cell Rep. 21, 910–918. doi: 10.1016/j.celrep.2017.09.087
Giraldo-Chica, M., and Woodward, N. D. (2017). Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophr. Res. 180, 58–63. doi: 10.1016/j.schres.2016.08.005
Grandjean, J., Corcoba, A., Kahn, M. C., Upton, A. L., Deneris, E. S., Seifritz, E., et al. (2019). A brain-wide functional map of the serotonergic responses to acute stress and fluoxetine. Nat. Commun. 10:350. doi: 10.1038/s41467-018-08256-w
Gravel, N., Harvey, B., Nordhjem, B., Haak, K. V., Dumoulin, S. O., Renken, R., et al. (2014). Cortical connective field estimates from resting state fMRI activity. Front. Neurosci. 8:339. doi: 10.3389/fnins.2014.00339
Gryglewski, G., Seiger, R., James, G. M., Godbersen, G. M., Komorowski, A., Unterholzner, J., et al. (2018). Spatial analysis and high resolution mapping of the human whole-brain transcriptome for integrative analysis in neuroimaging. NeuroImage 176, 259–267. doi: 10.1016/j.neuroimage.2018.04.068
Guedj, C., Meunier, D., Meunier, M., and Hadj-Bouziane, F. (2017a). Could LC-NE-dependent adjustment of neural gain drive functional brain network reorganization? Neural Plast. 2017:4328015. doi: 10.1155/2017/4328015
Guedj, C., Monfardini, E., Reynaud, A. J., Farne, A., Meunier, M., and Hadj-Bouziane, F. (2017b). Boosting norepinephrine transmission triggers flexible reconfiguration of brain networks at rest. Cereb. Cortex 27, 4691–4700. doi: 10.1093/cercor/bhw262
Haas, H., and Panula, P. (2003). The role of histamine and the tuberomamillary nucleus in the nervous system. Nat. Rev. Neurosci. 4, 121–130. doi: 10.1038/nrn1034
Harris, K. D., and Thiele, A. (2011). Cortical state and attention. Nat. Rev. Neurosci. 12, 509–523. doi: 10.1038/nrn3084
Hawrylycz, M. J., Lein, S., Guillozet-Bongaarts, A. L., Shen, E. H., Ng, L., Miller, J. A., et al. (2012). An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489, 391–399. doi: 10.1038/nature11405
Heinzle, J., Kahnt, T., and Haynes, J. D. (2011). Topographically specific functional connectivity between visual field maps in the human brain. NeuroImage 56, 1426–1436. doi: 10.1016/j.neuroimage.2011.02.077
Hermans, E., Marle, H. V., Ossewaarde, L., Menckens, M., Qin, S., Kesteren, M. V., et al. (2011). Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334, 1151–1153. doi: 10.1126/science.1209603
Hipp, J. F., and Siegel, M. (2015). BOLD fMRI correlation reflects frequency-specific neuronal correlation. Curr. Biol. 25, 1368–1374. doi: 10.1016/j.cub.2015.03.049
Hipp, J. F., Hawellek, D. J., Corbetta, M., Siegel, M., and Engel, A. K. (2012). Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat. Neurosci. 15, 884–890. doi: 10.1038/nn.3101
Hollensteiner, K. J., Galindo-Leon, E., Pieper, F., Engler, G., Nolte, G., and Engel, A. K. (2019). Large-scale functional connectivity in multisensory cortex predicts performance. BioRxiv
Honey, C. J., Kotter, R., Breakspear, M., and Sporns, O. (2007). Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc. Natl. Acad. Sci. U.S.A. 104, 10240–10245. doi: 10.1073/pnas.0701519104
Honey, C. J., Sporns, O., Cammoun, L., Gigandet, X., Thiran, J. P., Meuli, R., et al. (2009). Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. U.S.A. 106, 2035–2040. doi: 10.1073/pnas.0811168106
Insel, T., Cuthbert, B., Garvey, M., Heinssen, R., Pine, D. S., Quinn, K., et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751. doi: 10.1176/appi.ajp.2010.09091379
Itier, V., and Bertrand, D. (2001). Neuronal nicotinic receptors: from protein structure to function. FEBS Lett. 504, 118–125. doi: 10.1016/s0014-5793(01)02702-8
Joshi, S., and Gold, J. I. (2019). Context-Dependent Relationships between Locus Coeruleus Activation and Coordinated Neural Activity Patterns in the Anterior Cingulate Cortex SSRN. Available at: https://ssrn.com/abstract=3413098 (accessed July 1, 2019).
Joshi, S., Li, Y., Kalwani Rishi, M., and Gold Joshua, I. (2016). Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89, 221–234. doi: 10.1016/j.neuron.2015.11.028
Kebschull, J. M., Garcia da Silva, P., Reid, A. P., Peikon, I. D., Albeanu, D. F., and Zador, A. M. (2016). High-throughput mapping of single-neuron projections by sequencing of barcoded RNA. Neuron 91, 975–987. doi: 10.1016/j.neuron.2016.07.036
Kelly, C., de Zubicaray, G., Di Martino, A., Copland, D. A., Reiss, P. T., Klein, D. F., et al. (2009). L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J. Neurosci. 29, 7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009
Keren, N. I., Lozar, C. T., Harris, K. C., Morgan, P. S., and Eckert, M. A. (2009). In vivo mapping of the human locus coeruleus. NeuroImage 47, 1261–1267. doi: 10.1016/j.neuroimage.2009.06.012
Klaassens, B. L., Rombouts, S. A., Winkler, A. M., van Gorsel, H. C., van der Grond, J., and van Gerven, J. M. (2017). Time related effects on functional brain connectivity after serotonergic and cholinergic neuromodulation. Hum. Brain Mapp. 38, 308–325. doi: 10.1002/hbm.23362
Klaassens, B. L., van Gorsel, H. C., Khalili-Mahani, N., van der Grond, J., Wyman, B. T., Whitcher, B., et al. (2015). Single-dose serotonergic stimulation shows widespread effects on functional brain connectivity. NeuroImage 122, 440–450. doi: 10.1016/j.neuroimage.2015.08.012
Klein-Flugge, M. C., Hunt, L. T., Bach, D. R., Dolan, R. J., and Behrens, T. E. (2011). Dissociable reward and timing signals in human midbrain and ventral striatum. Neuron 72, 654–664. doi: 10.1016/j.neuron.2011.08.024
Koda, K., Ago, Y., Cong, Y., Kita, Y., Takuma, K., and Matsuda, T. (2010). Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J. Neurochem. 114, 259–270. doi: 10.1111/j.1471-4159.2010.06750.x
Kohn, A., Coen-Cagli, R., Kanitscheider, I., and Pouget, A. (2016). Correlations and neuronal population information. Annu. Rev. Neurosci. 39, 237–256. doi: 10.1146/annurev-neuro-070815-013851
Krystal, J. H., and State, M. W. (2014). Psychiatric disorders: diagnosis to therapy. Cell 157, 201–214. doi: 10.1016/j.cell.2014.02.042
Leopold, D. A., Murayama, Y., and Logothetis, N. K. (2003). Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb. Cortex 13, 422–433. doi: 10.1093/cercor/13.4.422
Lewis, C. M., Bosman, C. A., Womelsdorf, T., and Fries, P. (2016). Stimulus-induced visual cortical networks are recapitulated by spontaneous local and interareal synchronization. Proc. Natl. Acad. Sci. U.S.A. 113, E606–E615. doi: 10.1073/pnas.1513773113
Li, C. S., Ide, J. S., Zhang, S., Hu, S., Chao, H. H., and Zaborszky, L. (2014). Resting state functional connectivity of the basal nucleus of meynert in humans: in comparison to the ventral striatum and the effects of age. NeuroImage 97, 321–332. doi: 10.1016/j.neuroimage.2014.04.019
Lin, S. C., Brown, R. E., Hussain Shuler, M. G., Petersen, C. C., and Kepecs, A. (2015). Optogenetic dissection of the basal forebrain neuromodulatory control of cortical activation, plasticity, and cognition. J. Neurosci. 35, 13896–13903. doi: 10.1523/JNEUROSCI.2590-15.2015
Liu, X., de Zwart, J. A., Scholvinck, M. L., Chang, C., Ye, F. Q., Leopold, D. A., et al. (2018). Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nat. Commun. 9:395. doi: 10.1038/s41467-017-02815-3
Logothetis, N. K. (2008). What we can do and what we cannot do with fMRI. Nature 453, 869–878. doi: 10.1038/nature06976
Lord, L.-D., Expert, P., Atasoy, S., Roseman, L., Rapuano, K., Lambiotte, R., et al. (2018). Altered trajectories in the dynamical repertoire of functional network states under psilocybin. BioRxiv
Luczak, A., Bartho, P., and Harris, K. D. (2009). Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron 62, 413–425. doi: 10.1016/j.neuron.2009.03.014
Lurie, D. J., Kessler, D., Bassett, D., Betzel, R., Breakspear, M., Keilholz, S., et al. (2018). On the nature of resting fMRI and time-varying functional connectivity. PsyRxiv
Maier, A., Wilke, M., Aura, C., Zhu, C., Ye, F. Q., and Leopold, D. A. (2008). Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nat. Neurosci. 11, 1193–1200. doi: 10.1038/nn.2173
Mao, B.-W., Hamzei-Sichani, F., Arnov, D., Froemke, R. C., and Yuste, R. (2001). Dynamics of spontaneous activity in neocortical slices. Neuron 32, 883–898. doi: 10.1016/s0896-6273(01)00518-9
Markello, R. D., Spreng, R. N., Luh, W. M., Anderson, A. K., and De Rosa, E. (2018). Segregation of the human basal forebrain using resting state functional MRI. NeuroImage 173, 287–297. doi: 10.1016/j.neuroimage.2018.02.042
Martins, A. R., and Froemke, R. C. (2015). Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat. Neurosci. 18, 1483–1492. doi: 10.1038/nn.4090
Mattar, M. G., Cole, M. W., Thompson-Schill, S. L., and Bassett, D. S. (2015). A Functional Cartography of Cognitive Systems. PLoS Comput. Biol. 11:e1004533. doi: 10.1371/journal.pcbi.1004533
McCabe, C., and Mishor, Z. (2011). Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. NeuroImage 57, 1317–1323. doi: 10.1016/j.neuroimage.2011.05.051
McCabe, C., Mishor, Z., Filippini, N., Cowen, P. J., Taylor, M. J., and Harmer, C. J. (2011). SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol. Psychiatry 16, 592–594. doi: 10.1038/mp.2010.138
McCormick, D. A., and Nusbaum, M. P. (2014). Editorial overview: neuromodulation: tuning the properties of neurons, networks and behavior. Curr. Opin. Neurobiol. 29, 4–7.
McGinley, M. J., Vinck, M., Reimer, J., Batista-Brito, R., Zagha, E., Cadwell, C. R., et al. (2015). Waking state: rapid variations modulate neural and behavioral responses. Neuron 87, 1143–1161. doi: 10.1016/j.neuron.2015.09.012
Meindertsma, T., Kloosterman, N. A., Nolte, G., Engel, A. K., and Donner, T. H. (2017). Multiple transient signals in human visual cortex associated with an elementary decision. J. Neurosci. 37, 5744–5757. doi: 10.1523/JNEUROSCI.3835-16.2017
Mesulam, M. M., and van Hoesen, G. W. (1976). Acetylcholinesterase-rich projections from the basal forebrain of the rhesus monkey to neocortex. Brain Res. 109, 152–157. doi: 10.1016/0006-8993(76)90385-1
Mesulam, M. M., and Changiz, G. (1988). Nucleus basalis (CH4) and cortial cholinergic innnervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J. Comp. Neurol. 257, 216–240. doi: 10.1002/cne.902750205
Mesulam, M. M., Mufson, E. J., Levey, A. I., and Wainer, B. H. (1983). Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia Innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol. 214, 170–197. doi: 10.1002/cne.902140206
Metzger, C. D., Wiegers, M., Walter, M., Abler, B., and Graf, H. (2015). Local and global resting state activity in the noradrenergic and dopaminergic pathway modulated by reboxetine and amisulpride in healthy subjects. Int. J. Neuropsychopharmacol. 9:pyv080. doi: 10.1093/ijnp/pyv080
Moises, H. C., Woodward, B. J., Hoffer, B. J., and Freedman, R. (1979). Interactions of norepinephrine with Purkinje cell responses to putative amino acid neurotransmitters applied by microiontophoresis. Exp. Neurol. 64, 493–515. doi: 10.1016/0014-4886(79)90227-9
Mulders, P. C., van Eijndhoven, P. F., Schene, A. H., Beckmann, C. F., and Tendolkar, I. (2015). Resting-state functional connectivity in major depressive disorder: a review. Neurosc. Biobehav. Rev. 56, 330–344. doi: 10.1016/j.neubiorev.2015.07.014
Murphy, B. K., and Miller, K. D. (2003). Multiplicative gain changes are induced by excitation or inhibition alone. J. Neurosci. 23, 10040–10051. doi: 10.1523/jneurosci.23-31-10040.2003
Murphy, P. R., O’Connell, R. G., O’Sullivan, M., Robertson, I. H., and Balsters, J. H. (2014). Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum. Brain Mapp. 35, 4140–4154. doi: 10.1002/hbm.22466
Murray, J. D., Bernacchia, A., Freedman, D. J., Romo, R., Wallis, J. D., Cai, X., et al. (2014). A hierarchy of intrinsic timescales across primate cortex. Nat. Neurosci. 17, 1661–1663. doi: 10.1038/nn.3862
Nagumo, J., Arimoto, S., and Yoshizawa, S. (1962). An active pulse transmission line simulating nerve axon. Proc. Inst. Radio Eng. 50, 2061–2070. doi: 10.1109/jrproc.1962.288235
Nahimi, A., Jakobsen, S., Munk, O. L., Vang, K., Phan, J. A., Rodell, A., et al. (2015). Mapping alpha2 adrenoceptors of the human brain with 11C-yohimbine. J. Nucl. Med. 56, 392–398. doi: 10.2967/jnumed.114.145565
Nakajima, M., and Halassa, M. M. (2017). Thalamic control of functional cortical connectivity. Curr. Opin. Neurobiol. 44, 127–131. doi: 10.1016/j.conb.2017.04.001
Nienborg, H., Cohen, M. R., and Cumming, B. G. (2012). Decision-related activity in sensory neurons: correlations among neurons and with behavior. Annu. Rev. Neurosci. 35, 463–483. doi: 10.1146/annurev-neuro-062111-150403
Nir, Y., Mukamel, R., Dinstein, I., Privman, E., Harel, M., Fisch, L., et al. (2008). Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat. Neurosci. 11, 1100–1108. doi: 10.1038/nn.2177
O’Reilly, J. X., Croxson, P. L., Jbabdi, S., Sallet, J., Noonan, M. P., Mars, R. B., et al. (2013). Causal effect of disconnection lesions on interhemispheric functional connectivity in rhesus monkeys. Proc. Natl. Acad. Sci. U.S.A. 10, 13982–13987. doi: 10.1073/pnas.1305062110
Pfeffer, T., Avramiea, A. E., Nolte, G., Engel, A. K., Linkenkaer-Hansen, K., and Donner, T. H. (2018). Catecholamines alter the intrinsic variability of cortical population activity and perception. PLoS Biol. 16:e2003453. doi: 10.1371/journal.pbio.2003453
Pfeffer, T., Ponce-Alvarez, A., van den Brink, R. L., Engel, A. K., Nolte, G., Deco, G., et al. (2019). Double-Dissociation Between Catecholaminergic and Cholinergic Effects on Cortex-Wide Intrinsic Correlations of Neural Activity. Lisabon: Cosyne.
Pignatelli, A., and Belluzzi, O. (2017). Dopaminergic neurones in the main olfactory bulb: an overview from an electrophysiological perspective. Front. Neuroanat. 11:7. doi: 10.3389/fnana.2017.00007
Pinto, L., Goard, M. J., Estandian, D., Xu, M., Kwan, A. C., Lee, S. H., et al. (2013). Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat. Neurosci. 16, 1857–1863. doi: 10.1038/nn.3552
Polack, P. O., Friedman, J., and Golshani, P. (2013). Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat. Neurosci. 16, 1331–1339. doi: 10.1038/nn.3464
Preller, K. H., Bur, J. B., Ji, J. L., Charles, H., Schleifer, Adkinson, B. D., et al. (2018). Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. eLife 7, e35082. doi: 10.7554/eLife.35082
Puig, M. V., Santana, N., Celada, P., Mengod, G., and Artigas, F. (2004). In vivo excitation of GABA interneurons in the medial prefrontal cortex through 5-HT3 receptors. Cereb. Cortex 14, 1365–1375. doi: 10.1093/cercor/bhh097
Ramos, B. P., and Arnsten, A. F. (2007). Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol. Ther. 113, 523–536. doi: 10.1016/j.pharmthera.2006.11.006
Reimer, J., Froudarakis, E., Cadwell, C. R., Yatsenko, D., Denfield, G. H., and Tolias, A. S. (2014). Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84, 355–362. doi: 10.1016/j.neuron.2014.09.033
Rho, H. J., Kim, J. H., and Lee, S. H. (2018). Function of selective neuromodulatory projections in the mammalian cerebral cortex: comparison between cholinergic and noradrenergic systems. Front. Neural Circuits 12:47. doi: 10.3389/fncir.2018.00047
Rogawksi, M. A., and Aghajanian, G. K. (1980). Modulation of lateral geniculate neurone excitability by noradrenaline microiontophoresis or locus ceuruleus stimulation. Nature 287, 731–734. doi: 10.1038/287731a0
Rosazza, C., and Minati, L. (2011). Resting-state brain networks: literature review and clinical applications. Neurol. Sci. 32, 773–785. doi: 10.1007/s10072-011-0636-y
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. NeuroImage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Safaai, H., Neves, R., Eschenko, O., Logothetis, N. K., and Panzeri, S. (2015). Modeling the effect of locus coeruleus firing on cortical state dynamics and single-trial sensory processing. Proc. Natl. Acad. Sci. U.S.A. 112, 12834–12839. doi: 10.1073/pnas.1516539112
Sakoglu, U., Pearlson, G. D., Kiehl, K. A., Wang, Y. M., Michael, A. M., and Calhoun, V. D. (2010). A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. MAGMA 23, 351–366. doi: 10.1007/s10334-010-0197-8
Salgado, H., Trevino, M., and Atzori, M. (2016). Layer- and area-specific actions of norepinephrine on cortical synaptic transmission. Brain Res. 1641, 163–176. doi: 10.1016/j.brainres.2016.01.033
Sara, S. J. (2009). The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 10, 211–223. doi: 10.1038/nrn2573
Schaefer, A., Burmann, I., Regenthal, R., Arelin, K., Barth, C., Pampel, A., et al. (2014). Serotonergic modulation of intrinsic functional connectivity. Curr. Biol. 24, 2314–2318. doi: 10.1016/j.cub.2014.08.024
Schwarz, L. A., and Luo, L. (2015). Organization of the locus coeruleus-norepinephrine system. Curr. Biol. 25, R1051–R1056.
Schwarz, L. A., Miyamichi, K., Gao, X. J., Beier, K. T., Weissbourd, B., DeLoach, K. E., et al. (2015). Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524, 88–92. doi: 10.1038/nature14600
Seamans, J. K., Gorelova, N., Durstewitz, D., and Yang, C. R. (2001b). Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J. Neurosci. 21, 3628–3638. doi: 10.1523/jneurosci.21-10-03628.2001
Seamans, J. K., Durstewitz, D., Christie, B. R., Stevens, C. F., and Sejnowski, T. J. (2001a). Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc. Natl. Acad. Sci. U.S.A. 98, 301–306. doi: 10.1073/pnas.011518798
Seaton, B. E., Goldstein, G., and Allen, D. N. (2001). Sources of heterogeneity in schizophrenia: the role of neropsychological functioning. Neuropsychol. Rev. 11, 45–67.
Servan-Schreiber, D., Printz, H., and Cohen, J. D. (1990). A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science 249, 892–895. doi: 10.1126/science.2392679
Shafiei, G., Zeighami, Y., Clark, C. A., Coull, J. T., Nagano-Saito, A., Leyton, M., et al. (2019). Dopamine signaling modulates the stability and integration of intrinsic brain networks. Cereb. Cortex 29, 397–409. doi: 10.1093/cercor/bhy264
Shine, J. M., and Poldrack, R. A. (2018). Principles of dynamic network reconfiguration across diverse brain states. NeuroImage 180, 396–405. doi: 10.1016/j.neuroimage.2017.08.010
Shine, J. M., Aburn, M. J., Breakspear, M., and Poldrack, R. A. (2018a). The modulation of neural gain facilitates a transition between functional segregation and integration in the brain. eLife 7, e31130. doi: 10.7554/eLife.31130
Shine, J. M., van den Brink, R. L., Hernaus, D., Nieuwenhuis, S., and Poldrack, R. A. (2018b). Catecholaminergic manipulation alters dynamic network topology across cognitive states. Netw. Neurosci. 2, 381–396. doi: 10.1162/netn_a_00042
Shine, J. M., Bissett, P. G., Bell, P. T., Koyejo, O., Balsters, J. H., Gorgolewski, K. J., et al. (2016). The dynamics of functional brain networks: integrated network states during cognitive task performance. Neuron 92, 544–554. doi: 10.1016/j.neuron.2016.09.018
Siems, M., Pape, A. A., Hipp, J. F., and Siegel, M. (2016). Measuring the cortical correlation structure of spontaneous oscillatory activity with EEG and MEG. NeuroImage 129, 345–355. doi: 10.1016/j.neuroimage.2016.01.055
Smith, S. M., Fox, P. T., Miller, K. L., Glahn, D. C., Fox, P. M., Mackay, C. E., et al. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. U.S.A. 106, 13040–13045. doi: 10.1073/pnas.0905267106
Sporns, O., and Zwi, J. D. (2004). The small world of the cerebral cortex. Neuroinformatics 2, 145–162. doi: 10.1385/ni:2:2:145
Statoh, K., and Fibiger, H. C. (1986). Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J. Comp. Neurol. 353, 277–302. doi: 10.1002/cne.902530302
Stitt, I., Zhou, Z. C., Radtke-Schuller, S., and Frohlich, F. (2018). Arousal dependent modulation of thalamo-cortical functional interaction. Nat. Commun. 9:2455. doi: 10.1038/s41467-018-04785-6
Swanson, C. J., Perry, K. W., Koch-Krueger, S., Katner, J., Svensson, K. A., and Bymaster, F. P. (2006). Effect of the attention deficit/hyperactivity disorder drug atomoxetine on extracellular concentrations of norepinephrine and dopamine in several brain regions of the rat. Neuropharmacology 50, 755–760. doi: 10.1016/j.neuropharm.2005.11.022
Tagliazucchi, E., Roseman, L., Kaelen, M., Orban, C., Muthukumaraswamy, S. D., Murphy, K., et al. (2016). Increased global functional connectivity correlates with lsd-induced ego dissolution. Curr. Biol. 26, 1043–1050. doi: 10.1016/j.cub.2016.02.010
Takeuchi, T., Duszkiewicz, A. J., Sonneborn, A., Spooner, P. A., Yamasaki, M., Watanabe, M., et al. (2016). Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537, 357–362. doi: 10.1038/nature19325
Tanabe, J., Nyberg, E., Martin, L. F., Martin, J., Cordes, D., Kronberg, E., et al. (2011). Nicotine effects on default mode network during resting state. Psychopharmacology 216, 287–295. doi: 10.1007/s00213-011-2221-8
Tavor, I., Parker, Jones O, Mars, R. B., Smith, S. M., Behrens, T. E., and Jbabdi, S. (2016). Task-free MRI predicts individual differences in brain activity during task performance. Science 352, 216–220. doi: 10.1126/science.aad8127
Thompson, W. H., Kastrati, G., Finc, K., Wright, J., Shine, J. M., and Poldrack, R. A. (2019). Time-varying nodal measures with temporal community structure: a cautionary note to avoid misquantification. BioRxiv
Tomasi, D., and Volkow, N. D. (2010). Functional connectivity density mapping. Proc. Natl. Acad. Sci. U.S.A. 107, 9885–9890. doi: 10.1073/pnas.1001414107
Törk, I. (1990). Anatomy of the serotonergic system. Ann. N. Y. Acad. Sci. 600, 9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x
Turchi, J., Chang, C., Ye, F. Q., Russ, B. E., Yu, D. K., Cortes, C. R., et al. (2018). The basal forebrain regulates global resting-state fMRI fluctuations. Neuron 97, 940.e4–952.e4. doi: 10.1016/j.neuron.2018.01.032
Uematsu, A., Tan, B. Z., and Johansen, J. P. (2015). Projection specificity in heterogeneous locus coeruleus cell populations: implications for learning and memory. Learn. Mem. 22, 444–451. doi: 10.1101/lm.037283.114
Uematsu, A., Tan, B. Z., Ycu, E. A., Cuevas, J. S., Koivumaa, J., Junyent, F., et al. (2017). Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 20, 1602–1611. doi: 10.1038/nn.4642
van de Ven, V., Wingen, M., Kuypers, K. P., Ramaekers, J. G., and Formisano, E. (2013). Escitalopram decreases cross-regional functional connectivity within the default-mode network. PLoS One 8:e68355. doi: 10.1371/journal.pone.0068355
van den Brink, R. L., Nieuwenhuis, S., and Donner, T. H. (2018a). Amplification and suppression of distinct brainwide activity patterns by catecholamines. J. Neurosci. 38, 7476–7491. doi: 10.1523/JNEUROSCI.0514-18.2018
van den Brink, R. L., Nieuwenhuis, S., van Boxtel, G. J. M., van Luijtelaar, G., Eilander, H. J., and Wijnen, V. J. M. (2018b). Task-free spectral EEG dynamics track and predict patient recovery from severe acquired brain injury. Neuroimage Clin. 17, 43–52. doi: 10.1016/j.nicl.2017.10.003
van den Brink, R. L., Pfeffer, T., Warren, C. M., Murphy, P. R., Tona, K. D., van der Wee, N. J., et al. (2016). Catecholaminergic neuromodulation shapes intrinsic MRI functional connectivity in the human brain. J. Neurosci. 36, 7865–7876. doi: 10.1523/JNEUROSCI.0744-16.2016
van Wijk, B. C. M., Stam, C. J., and Daffershofer, A. (2010). Comparing brain networks of different size and connectivity density using graph theory. PLoS One 5:e13701. doi: 10.1371/journal.pone.0013701
Varazzani, C., San-Galli, A., Gilardeau, S., and Bouret, S. (2015). Noradrenaline and dopamine neurons in the reward/effort trade-off: a direct electrophysiological comparison in behaving monkeys. J. Neurosci. 35, 7866–7877. doi: 10.1523/JNEUROSCI.0454-15.2015
Vargas, C., López-Jaramillo, C., and Vieta, E. (2013). A systematic literature review of resting state network—functional MRI in bipolar disorder. J. Affect. Disord. 150, 727–735. doi: 10.1016/j.jad.2013.05.083
Wang, J., and O’Donnell, P. (2001). D1 dopamine receptors potentiate NMDA-mediated excitability increase in layer V prefrontal cortical pyramidal neurons. Cereb. Cortex 11, 452–462. doi: 10.1093/cercor/11.5.452
Wang, L., Hermens, D. F., Hickie, I. B., and Lagopoulos, J. (2012). A systematic review of resting-state functional-MRI studies in major depression. J. Affect. Disord. 142, 6–12. doi: 10.1016/j.jad.2012.04.013
Warren, C. M., Eldar, E., van den, Brink RL, Tona, K. D., van der Wee, N. J., Giltay, E. J., et al. (2016). Catecholamine-mediated increases in gain enhance the precision of cortical representations. J. Neurosci. 36, 5699–5708. doi: 10.1523/JNEUROSCI.3475-15.2016
Watts, D. J., and Strogatz, S. H. (1998). Collective dynamics of ‘small-workd’ networks. Nature 393, 440–442. doi: 10.1038/30918
Wester, J. C., and McBain, C. J. (2014). Behavioral state-dependent modulation of distinct interneuron subtypes and consequences for circuit function. Curr. Opin. Neurobiol. 29, 118–125. doi: 10.1016/j.conb.2014.07.007
Winterer, G., and Weinberger, D. R. (2004). Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 27, 683–690. doi: 10.1016/j.tins.2004.08.002
Ye, Z., Hammer, A., and Munte, T. F. (2017). Pramipexole modulates interregional connectivity within the sensorimotor network. Brain Connect 7, 258–263. doi: 10.1089/brain.2017.0484
Zaborszky, L., Hoemke, L., Mohlberg, H., Schleicher, A., Amunts, K., and Zilles, K. (2008). Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. NeuroImage 42, 1127–1141. doi: 10.1016/j.neuroimage.2008.05.055
Zaborszky, L., Gombkoto, P., Varsanyi, P., Gielow, M. R., Poe, G., Role, L. W., et al. (2018). Specific basal forebrain-cortical cholinergic circuits coordinate cognitive operations. J. Neurosci. 38, 9446–9458. doi: 10.1523/JNEUROSCI.1676-18.2018
Zahrt, J., Taylor, J., Mathew, R., and Arnsten, A. (1997). Supranormal stimulation of d1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J. Neurosci. 17, 8528–8535. doi: 10.1523/jneurosci.17-21-08528.1997
Zalesky, A., Fornito, A., Cocchi, L., Gollo, L. L., and Breakspear, M. (2014). Time-resolved resting-state brain networks. Proc. Natl. Acad. Sci. U.S.A. 111, 10341–10346. doi: 10.1073/pnas.1400181111
Zerbi, V., Floriou-Servou, A., Markicevic, M., Vermeiren, Y., Sturman, O., Privitera, M., et al. (2019). Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103, 1–17. doi: 10.1016/j.neuron.2019.05.034
Zhang, S., Hu, S., Chao, H. H., and Li, C. R. (2015). Resting-state functional connectivity of the locus coeruleus in humans: in comparison with the ventral tegmental area/substantia nigra pars compacta and the effects of age. Cereb. Cortex 26, 3413–3427. doi: 10.1093/cercor/bhv172
Zilles, K., and Amunts, K. (2009). Receptor mapping: architecture of the human cerebral cortex. Curr. Opin. Neurol. 22, 331–339. doi: 10.1097/WCO.0b013e32832d95db
Keywords: functional connectivity, norepinepherine, dopamine, acetycholine, serotonin, brainstem, neuromodulation, resting-state
Citation: van den Brink RL, Pfeffer T and Donner TH (2019) Brainstem Modulation of Large-Scale Intrinsic Cortical Activity Correlations. Front. Hum. Neurosci. 13:340. doi: 10.3389/fnhum.2019.00340
Received: 22 July 2019; Accepted: 17 September 2019;
Published: 09 October 2019.
Edited by:
Filippo Cieri, Cleveland Clinic Lou Ruvo Center for Brain Health, United StatesReviewed by:
Wen Qin, Tianjin Medical University General Hospital, ChinaDario J. Englot, Vanderbilt University, United States
Anbupalam Thalamuthu, University of New South Wales, Australia
Copyright © 2019 van den Brink, Pfeffer and Donner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. L. van den Brink, r.van-den-brink@uke.de
 R. L. van den Brink
R. L. van den Brink T. Pfeffer1
T. Pfeffer1 T. H. Donner
T. H. Donner