- 1Neurorehabilitation Unit, IRCCS C. Mondino Foundation, Pavia, Italy
- 2Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy
- 3Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain
- 4Faculty of Law, Giustino Fortunato University, Benevento, Italy
- 5Headache Science Center, IRCCS Mondino Foundation, Pavia, Italy
- 6ICREA, Barcelona, Spain
- 7Departament de Cognició, Desenvolupament i Psicologia de l’Educació, Facultat de Psicologia, Universitat de Barcelona, Barcelona, Spain
A significant body of experimental evidence has demonstrated that it is possible to induce the illusion of ownership of a fake limb or even an entire fake body using multisensory correlations. Recently, immersive virtual reality has allowed users to experience the same sensations of ownership over a virtual body inside an immersive virtual environment, which in turn allows virtual reality users to have the feeling of being “embodied” in a virtual body. Using such virtual embodiment to manipulate body perception is starting to be extensively investigated and may have clinical implications for conditions that involve altered body image such as chronic pain. Here, we review experimental and clinical studies that have explored the manipulation of an embodied virtual body in immersive virtual reality for both experimental and clinical pain relief. We discuss the current state of the art, as well as the challenges faced by, and ideas for, future research. Finally, we explore the potentialities of using an embodied virtual body in immersive virtual reality in the field of neurorehabilitation, specifically in the field of pain.
Introduction
Embodiment is defined as the sense of having a body, and the body can be considered to be both the subject and object of medical science and practice (Gallagher, 2001). One of the main goals in the field of cognitive neuroscience is to investigate how we experience ourselves inside a body as it interacts continuously with the environment. Historically, the bodily self has been described as “obvious and unproblematic” (James, 1890) and connected to a single somatic sensory system such as visceral interception (Damasio, 2000); however, more recently, embodiment has been described as being composed of several different structurally organized subjective components (Longo et al., 2008), as opposed to a single dimension. Hence, we feel our self as being inside a body, a body that moves according to our intentions (Kilteni et al., 2012a) and that interacts with the environment. Indeed, the sense of embodiment is thought to emerge from a complex interaction between bottom–up and top–down signals (Longo et al., 2008).
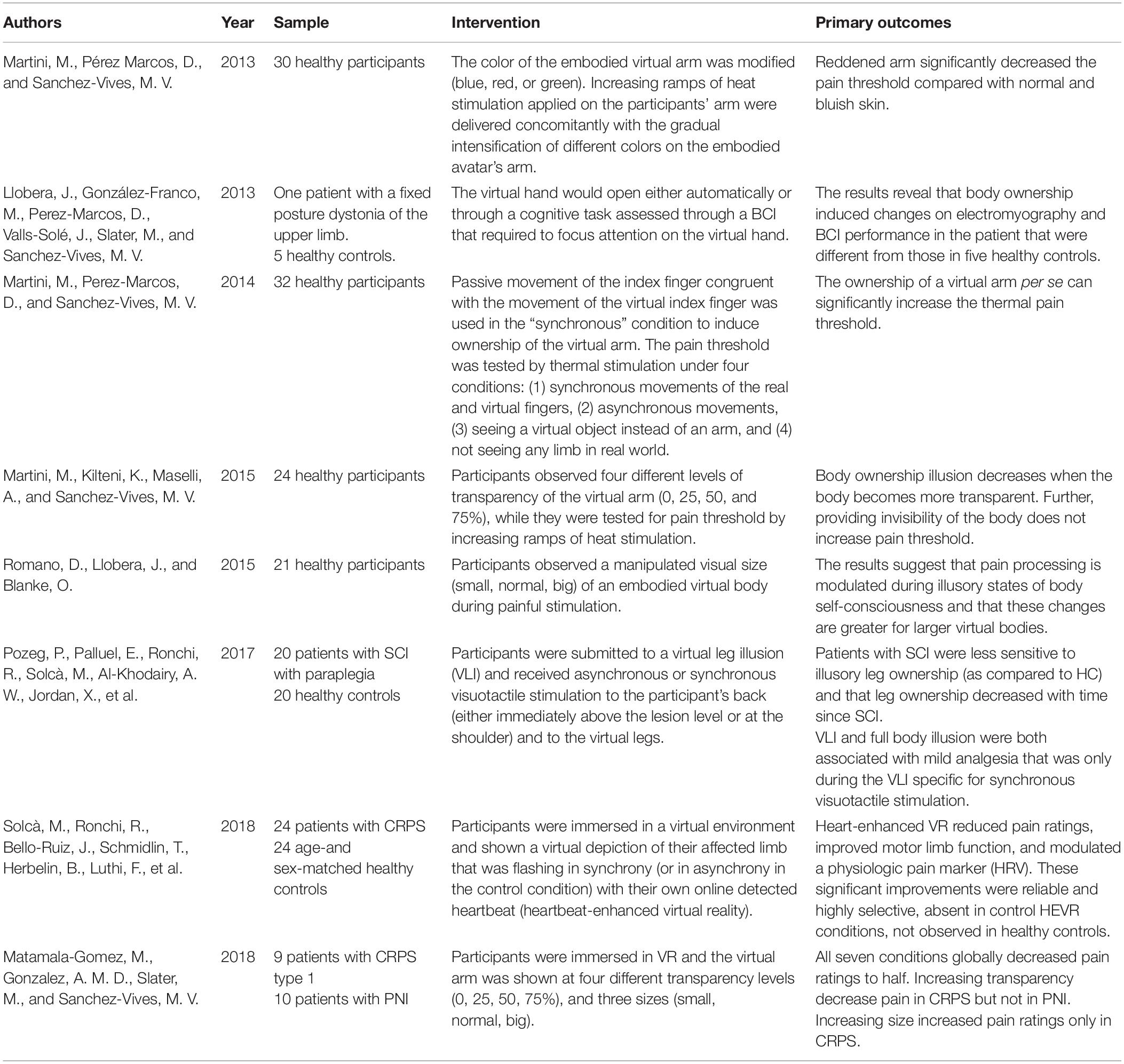
At first glance, experimental manipulation of embodiment might seem problematic; however, in the last few years, many studies have investigated bodily perception and revealed alternative ways of manipulating embodiment by using fake body parts. One example of this is the rubber hand illusion (RHI) study, in which synchronous visuotactile stimulation of both a rubber hand located within the visual field of the participant, and the participant’s real hand, located outside the visual field of the participant, confers an illusion of ownership over the rubber hand (Botvinick and Cohen, 1998). Since this study, many researchers have investigated how to manipulate body perception through the use of fake bodies such as a mannequins (Ehrsson and Petkova, 2008), mirrors (Ramachandran et al., 2009a), and virtual reality (VR) (Slater et al., 2008, 2010). Slater et al. (2008) were the first to replicate the RHI study in VR inducing ownership of a virtual hand based on visuo-tactile correlations, in an experience termed the “virtual hand illusion,” while a similar ownership was successfully induced by means of visuomotor correlations in Sanchez-Vives et al. (2010) (see Figure 1). A number of studies have focused on the use of body illusions to address pathological conditions such as chronic pain, with the focus being on the analgesic effects of cross-modal perception (e.g., pain and vision) (for reviews, see Boesch et al., 2015, 2016; Martini, 2016).
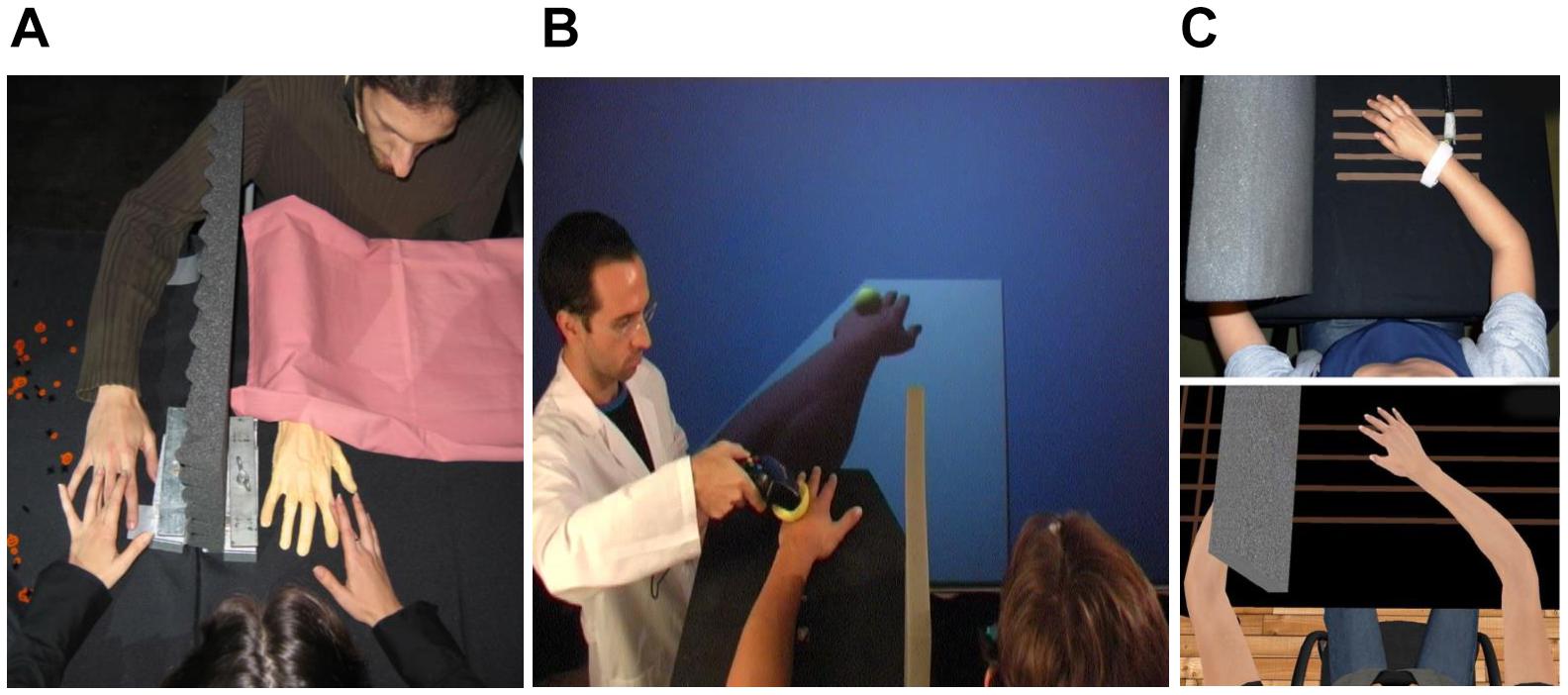
Figure 1. Experimental setups for (A) the rubber hand illusion (RHI), (B) the virtual hand illusion in non-immersive virtual reality, and (C) the virtual hand illusion in immersive virtual reality. Part (C) taken from Martini et al. (2015), reprinted with permission from Springer Nature.
Chronic pain, where the symptoms last beyond normal tissue healing times, is the most burdensome health issue worldwide in terms of years lived with disability (Vos et al., 2012) and economic cost (Gaskin and Richard, 2012). In some cases, the negative emotional experience of pain can even lead to suicidal intention (Campbell et al., 2016). Current management strategies including physical activity/exercise and psychological interventions such as cognitive behavioral therapy show short-term effects only, with small effect sizes (Williams et al., 2012; Geneen et al., 2017), while pharmacological agents, such as opioids, have limited efficacy and carry significant risks and side effects (Hofmann et al., 2012; Carter et al., 2014). Indeed, the economic burden of prescription opioid misuse alone in the United States is estimated at $78.5 billion a year, including healthcare costs, lost productivity, addiction treatment, and criminal justice system involvement (Florence et al., 2016). Many investigators have therefore attempted to look for new ways to manage pain states via non-pharmacological means (Carter et al., 2014). This paper presents a review of experimental and clinical studies that have explored the manipulation of an embodied virtual body in immersive VR for both experimental and clinical pain relief.
What is Embodiment?
The capability of our brain of having a representation of our body results in a mental construction composed of perceptions and ideas about the dynamic organization of our own body, involving vision, touch, proprioception, interoception, motor control, and vestibular sensations (Maselli and Slater, 2013). In this regard, embodiment is defined as the sense of having a body. But to what are we referring when we talk about having a body? Longo et al. (2008) described it as follows:
The sense of [having] one’s own body, variously termed “embodiment” (Arzy et al., 2006), “coenaesthesia” (Critchley, 1953), “bodily self-consciousness” (Bermúdez, 1998; Legrand, 2006), or “corporeal awareness” (Critchley, 1979; Berlucchi and Aglioti, 1997), has often been described as a non-conceptual, somatic form of knowledge, different in kind from other types of knowledge (e.g., Kant, 1965; Bermúdez, 1998).
Longo et al. (2008, p. 978)
These different descriptions of embodiment refer to the fact that we are able to feel the sense of having a body by integrating the different sensory signals arriving to our body, which our brain interprets to create a coherent representation of our self. In this regard, Longo et al. (2008) discuss the fact that others have described embodiment as the “storm-center of experience” arriving to our body, resulting in an essential factor for the construction of our internal life (James, 1905), and that other authors support the idea that embodiment is key for the construction of our inner self representation by demonstrating that the sense of embodiment is also closely related to the sense of self, and is strongly related to our individual psychological identity (Edelman, 2005; Cassam, 2012).
However, some investigations have shown that embodiment is divided into different subcomponents that form our body representation, such as body image and body schema (Gallagher and Cole, 1995). In this regard, it is known that body image and body schema play a fundamental, but clearly differentiated, role in understanding the sense of self and in individual psychological identity.
Conceptual Clarifications of Body Image and Body Schema
Gallagher (2001) has described body image as “an intentional content of consciousness that consists of a system of perceptions, attitudes, and beliefs pertaining from one’s own body.” In contrast, body schema has been described as an “automatic system of processes that constantly regulates posture and movement” and is mostly controlled by the sensorimotor system (Gallagher, 2001). One clear example of the difference between body image and body schema is the difference between perception of movement (conscious awareness of movement), related to body image, and the final execution of that movement (motor performance), related to body schema.
Studies aimed at analyzing body image have distinguished three different intentional elements: (1) the subject’s perceptual experience of his/her own body, (2) the subject’s conceptual understanding of the body, and (3) the subject’s emotional attitude toward his/her own body (Cash and Brown, 1987; Powers et al., 1987; Gardner and Moncrieff, 1988). The body image relies in the congruent inputs for all sensory and motor systems, and it has been described that experimental asynchronous multisensory stimulation results in distortion of body image (Perez-Marcos et al., 2018). In contrast, body schema is not the result of mental perception, beliefs, or attitudes, involving instead a system of motor functions or programs that operate “below” the level of self-referential intentionality, playing a dynamic role in governing posture and movement in a close automatic/subconscious way (Gallagher, 2001). While subconscious and automatic, body schema is not just a matter of mere reflex. Actions controlled by the body schema can be precisely shaped by the intentional experience or goal-directed behavior of one’s own body (Gallagher, 2001). Therefore, once one becomes aware of perceptual limb position, movement, posture, pleasure, pain, and kinesthetic experience, such awareness contributes to the perceptual aspect of one’s body image and such awareness may interact with one’s body schema (Gallagher, 2001).
The Body in the Brain
According to Melzack (1990), the body schema is controlled by a distributed neural network, or neuromatrix, mostly prewired by genetics, but flexible and open to the continuous shaping influence of experience. This network includes the somatosensory system, reticular afferents to the limbic system, and cortical regions that are important for self-recognition and recognition of external objects and entities. Somatosensory inputs to the brain from different modalities are essential for bodily awareness, especially those from proprioceptors, as demonstrated by Lackner (1988), in which he showed changes in body awareness using muscle vibration and other somatic manipulations. The sense of vision is also very important, as demonstrated by the evident anatomical distortions when congenitally blind subjects attempt to draw their own and other people’s bodies (Critchley, 1979). Further, visual information regarding the hand’s position is normally in accordance with the proprioceptive information regarding its position (van Beers et al., 1999). Tactile events regarding the body are strongly coupled with visual information (if available) of the same event (Pavani et al., 1999). Similarly, execution of movements is normally corroborated by congruent visual and tactile feedback (Janczyk et al., 2009).
Brain Lesions and Body Representation
In addition to body perception disturbances in congenitally blind subjects, it has also been shown that brain lesions can induce profound changes in body perception and body representation (Aglioti et al., 2016). For example, some patients with right-hemisphere lesions report the delusional perception that their contralateral limb or side of their body does not belong to them—a syndrome called “somatoparaphrenia” (Vallar and Ronchi, 2009; Jenkinson et al., 2013). These types of lesions allow us to explore the relationship between patients’ subjective delusory perceptions and their structural brain deficits (de Vignemont, 2011), especially if those deficits concern areas that are traditionally considered to be multisensory. Further, some brain lesions, such as stroke and/or the resultant neuroplastic changes in the brain, might result in a specific alteration of the body schema or parts of it, as for example in stroke patients who have anosognosia (lack of self-awareness) for their motor and sensory defects and refuse to believe they are affected at all (McGlynn and Schacter, 1989; Levine et al., 1991), or stroke patients with personal neglect (Guariglia and Antonucci, 1992). Disownership of affected body parts can occur after right-sided brain damage (Loetscher et al., 2006), and has also been observed in chronic pain patients suffering from complex regional pain syndrome (CRPS) (Birklein and Schlereth, 2015). In addition, brain-damaged patients without amputations have reported the presence of multiple supernumerary body parts, mostly hands or feet (Halligan et al., 1993; Ramachandran and Blakeslee, 1999). Regarding neuropathic pain patients, limb amputee patients often present with body perception disturbances, such as the affected limb changing in size and form over time (Halligan et al., 1999). Body perception disturbances have also been demonstrated in patients with CRPS (Pleger et al., 2006; Lewis et al., 2007), chronic low back pain (Moseley, 2008), and other chronic pain conditions (Lotze and Moseley, 2007). Finally, body perception disturbances, specifically affecting body image, have been demonstrated in patients with spinal cord injury without brain damage (Fuentes et al., 2013). Part of these body perception disturbances are caused by alterations in the afferent inputs. When a body part is deafferented (deprived of sensory input), the feeling of an increased size of that body part often occurs. Such an effect is observed under local anesthesia, as well as in patients with spinal cord injury that perceived their torso and limbs elongated (Fuentes et al., 2013). Similarly, anomalous multisensory information provided experimentally on the body have been found to elicit a recalibration of the body image with an elongation of the stimulated body part (Perez-Marcos et al., 2018).
In order to study the mechanisms of body perception disturbances, early investigations were conducted in healthy people using devices such as fake limbs, prisms, mirrors, and cameras, which permitted the manipulation of body-related visual cues relative to other body-related sensory information, for example, tactile and proprioceptive cues. On the basis of these techniques, experimental studies on body perception used scenarios in which an external non-self-object was experienced as part of one’s own body through multisensory and/or sensorimotor correlations between the real and the fake body or body part. For many psychologists and neuroscientists, these so-called body ownership illusions (BOIs) have constituted the main experimental method for disentangling body perception in healthy adults over the last 15 years (Blanke et al., 2015).
Body Ownership Illusions
How the brain represents our body is a fundamental question in cognitive neuroscience (Slater and Sanchez-Vives, 2016). How can we tell that our hand is part of our body and a physical object like a book is not? We generally believe that our own internal body representation is stable; however, some investigations have elicited the illusion of body ownership over objects that are not part of the body at all, which suggests that our body representation is actually highly malleable. In addition, out-of-body illusion research was reignited by Botvinick and Cohen (1998) with their RHI study. In the RHI study, perceived ownership of the rubber hand occurs because the brain’s perceptual system resolves the sensory conflict between the congruent visuotactile information (the visual position of the rubber hand together with the tactile stimulus from the stroking) and the proprioceptive input (which indicates the position of the real hand) by prioritizing the importance of the visuotactile input over the proprioceptive input, integrating the two separate but synchronous inputs (visual and tactile) into a single prediction, as a result of which participants have the perceptual illusion that the rubber hand is their real hand. The visuotactile input is sufficient to override any contradicting proprioceptive input and produce the (incorrect) prediction that the real hand is located closer to where the rubber hand is, a phenomenon known as “proprioceptive drift.” Interestingly, if the visual and tactile stimulation are asynchronous, the illusion does not occur, suggesting that congruous multisensory input is required to produce the illusion. Later, Armel and Ramachandran (2003) demonstrated than when the rubber hand is threatened, there is a strong skin conductance response (SCR), indicating a physiological response to the threat. In this study, they argue that our body representation is continuously updated based on the stimuli being received. With synchronous multisensory perception, we can feel that a rubber hand is our real hand because the brain quickly generates the corresponding illusion as a way of resolving the contradiction between the visuotactile and the proprioceptive inputs (Slater and Sanchez-Vives, 2016).
Further, it has been shown that BOI may also be induced over the entire body in healthy subjects by using a mannequin (Petkova et al., 2011). In this study, healthy subjects observed an artificial body (a mannequin) through a head-mounted display connected to a two-synchronized-color video cameras oriented down at the mannequin body. As in the RHI study and in order to induce a BOI, participants received synchronous visuotactile stimulation at the same place in both the artificial and the real body. This whole body illusion is commonly known as the full BOI (Slater et al., 2010; Maselli and Slater, 2013). The full body ownership illusion from a first-person perspective is described as the feeling of owning an artificial body, which substitutes the real body as the origin of perceptual sensations. In this regard, some investigations have demonstrated that in order to induce a BOI, first-person visual perspective of the artificial body part or full body is key (Ehrsson and Petkova, 2008; Slater et al., 2010; Petkova et al., 2011). In addition to visuotactile stimulation and visual perspective, it has been shown that subjects may also experience the illusion when visuotactile stimulation is substituted by other modalities of multisensory and/or sensorimotor stimulation, such as sensorimotor contingencies in active or passive movements (Tsakiris et al., 2006; Sanchez-Vives et al., 2010; Kalckert and Ehrsson, 2012).
Hence, in the context of full-body illusions, self-location can be advantageously regarded as the combination of two parallel spatial representations: (1) an abstract allocentric representation of the body, mainly associated with visual perspective (first-or third-person visual perspective), and (2) an egocentric mapping of somatosensory sensations (visuotactile or visuomotor sensations) into the external space, mainly associated with peripersonal space. As reported by specific experimental paradigms adopted to induce out-of-body illusions, if these spatial representations are selectively or simultaneously altered, this could have implications for the sense of ownership of an artificial body (Maselli, 2015).
Embodiment in VR
Nowadays, the integration of technology in the field of applied neuroscience such as VR systems allows the replacement of a person’s real body with a virtual body representation, allowing the subject to feel embodied in a virtual body. In this regard, several investigations demonstrate that one may experience the sense of ownership over a virtual limb (Slater et al., 2008) and even an entire virtual body (Slater et al., 2010) by using immersive VR. In the latter study, Slater and colleagues demonstrated a full-body transfer illusion in which male subjects were able to embody a virtual female body. This finding was demonstrated subjectively (by questionnaire) and physiologically (through heart-rate changes) in response to an attack on the virtual body.
In addition, VR has been defined as a way to simulate reality and real-life situations (Slater and Sanchez-Vives, 2016). For example, it has been demonstrated that when a virtual knife stabs an embodied virtual body in an immersive VR environment, participants demonstrate an autonomic response and motor cortex activation in preparation to move the hand out of the way, just as they would in real life (González-Franco et al., 2014). Hence, anything that can happen in reality can be programed to happen in VR and be experienced as a real situation (Slater and Sanchez-Vives, 2016).
VR allows the experimenter to manipulate not only the virtual environment but also the embodied virtual body in ways that would be impossible in physical reality (Bohil et al., 2011). For example, immersive VR allows the manipulation of body representation in terms of structure, shape, size, and color, in ways that can contrast sharply with our own body image (Kilteni et al., 2012a, b; Banakou et al., 2013; Peck et al., 2013). Further, it has been shown that manipulating the characteristics of the virtual body may influence the physiological responses of the real body (Martini et al., 2013; Bergström et al., 2016), and may also modulate behavioral responses of the subjects (Osimo et al., 2015; Seinfeld et al., 2018). For this reason, immersive VR has been shown to have many potential applications in the fields of psychotherapy, rehabilitation, and behavioral neuroscience (for reviews, see Tarr and Warren, 2002; Martini, 2016; Riva et al., 2018), and even consciousness studies (for a review, see Sanchez-Vives and Slater, 2005).
VR and Pain Management
At the beginning of the 21st century, VR was introduced to the field of pain management (Hoffman et al., 2000a). The first application of VR in clinical pain was a video game in which adolescent and adult burnt patients experienced less pain while they were playing (Hoffman et al., 2000b). Later, Hoffman and colleagues conducted an fMRI brain scan study in which they found that VR greatly and significantly reduced pain in five brain regions of interest related to pain (the anterior cingulate cortex, primary and secondary somatosensory cortex, insula, and thalamus) in healthy subjects exposed to thermal stimulation (Hoffman et al., 2004). Some years later, a second fMRI study demonstrated that the pain reduction experienced by using VR was comparable to the analgesic effect of a moderate dose of hydromorphone pain medication (Hoffman et al., 2007). Up to this point, the analgesic properties of VR had been mostly attributed to its powerful distractive capacity. However, its effectiveness has been demonstrated in the management of mild and severe pain states (Doctor et al., 2002; Hoffman et al., 2011, 2014). In addition, the positive pain-relieving effects of VR may also be mediated through a reduction in anxiety and through the user experiencing positive emotions such as a sense of fun (Triberti et al., 2014).
One reason in favor of the distractive effect on pain associated to VR in the studies from Hoffman and colleagues is because of the lack of embodiment in a virtual body in the VR scenarios of their studies, in which patients were observing fun and distractive situations in a display instead of being embodied in a virtual environment through an immersive VR system. In addition, Malloy and Milling, in a review on the effectiveness of VR intervention for pain relief, reported that immersive VR is more effective in promoting analgesia than non-immersive VR systems (Malloy and Milling, 2010). The difference between these two systems is the lack of embodiment in the non-immersive VR systems, whereas using immersive VR systems, one may be embodied in a virtual body and immersed in the virtual world, feeling present in the generated VR scenario (Sanchez-Vives and Slater, 2005). It has been reported that this “transportation of consciousness to another place” involved in the sense of presence in a virtual environment might be strong enough to diminish sensations of pain (Sanchez-Vives and Slater, 2005). Hence, although Hoffman and colleagues used an immersive VR system in their pain studies, these early pain studies using VR did not include embodiment in a virtual body.
Immersive VR and Pain
The sense of being present in an immersive VR scenario while being embodied in a virtual body offers the possibility of modulating pain perception by observing the embodied virtual body from a first-person perspective (for a review, see Martini, 2016). The representation of the body is modulated by the integration of different sensory signals, and this has been extensively investigated (Macaluso and Maravita, 2010; Medina and Coslett, 2010; Serino and Haggard, 2010; Wesslein et al., 2014). In this regard, in IVR, we can therefore act on the virtual body seen from a first-person perspective and experimentally manipulate the multisensory integration in a highly controlled way.
The Vision of the Body in Pain
It has been shown that watching clips of another person’s hand receiving painful stimuli, while concomitantly receiving painful laser stimulations on one’s one hand, modulates the pain system in the second somatosensory area that reflects the sensory qualities of pain (Valeriani et al., 2008). Later, Longo and colleagues demonstrated, again using laser-evoked potentials, that the vision of one’s painful part of the body is analgesic (Longo et al., 2009). In this study, they conducted three different experiments in which they showed that when participants observed their own painfully stimulated hand (without observing the painful stimulation), they felt less pain compared to when they were looking at a box or at someone else’s hand. The authors postulated that reduction of pain perception while observing one’s own hand was due to a visually induced activation of inhibitory GABAergic interneurons in somatosensory areas. Similarly, Cardini and coworkers showed that vision of the hand, compared to vision of a box, caused a suppression of the early somatosensory potential when electrical stimulation was applied to two fingers at the same time, thus revealing an augmented inhibitory interneuronal activity within the somatosensory cortex (Cardini et al., 2011). This finding was supported by an EEG study by Mancini et al. (2013), in which they demonstrated that vision of the body, compared to vision of a neutral object, increased noxious-related beta oscillatory activity bilaterally in sensorimotor areas, which probably reflects cortical inhibitory activity of nociceptive stimuli processing.
Other neuroimaging studies have found that vision of the painful body part (subjected to painful mechanical stimulations) increases the functional connectivity between brain areas of the so-called “pain matrix” and the posterior parietal and occipito-temporal brain areas related to vision of the body (Longo et al., 2012). Further, in this study, the authors observed that the vision of one’s own hand led to a reduction in the activation of the primary somatosensory cortex and the operculo-insular cortex following painful stimulation (Longo et al., 2012). Specifically, the analgesic effects of the vision of the body part seem to be site-specific, which means that less pain is perceived when looking at the body region where the painful stimuli is applied (Diers et al., 2013). Another factor that modulates pain perception while observing the painful part of the body is visual size modification. One example of this is the study by Mancini et al. (2011), in which the authors found a direct correlation between thermal pain threshold and hand size. Specifically, they found that enlargement of the stimulus-receiving hand enhanced analgesia (i.e., increased the pain threshold), whereas visual reduction of the hand decreased analgesia (reduced the pain threshold). However, there are contradictory results about how visual size modification affects pain perception. For instance, while enlargement of the affected hand had an analgesic effect in healthy subjects (Mancini et al., 2011; Romano et al., 2015), the opposite occurred in patients with chronic arm pain (Moseley et al., 2008), while enlarging the hand had no effect in patients with hand osteoarthritis (Preston and Newport, 2011). In addition, when visual enlargement is shown in a single direction (i.e., a “stretch” illusion) and is accompanied by tactile feedback (emphasizing the stretch by simultaneously pulling on the limb), there is a marked analgesic effect in both hand (Preston and Newport, 2011) and knee osteoarthritis (Stanton et al., 2018). It is worth noting that in both these aforementioned studies, a minority of subjects experienced a greater analgesic effect when the opposite (i.e., a shrink/compression) illusion was shown. The authors suggest that the effect may be specific to the individual (Stanton et al., 2018), which raises the intriguing possibility that greater analgesic effects may be achieved with tailored VR experiences that address cognitive aspects of the patient’s unique pain experience. For example, in osteoarthritis, if patients believe that their pain is caused by compression of the bony surfaces, a stretch illusion may be effective; in other patients who believe that swelling is the primary driver of their pain, a shrink illusion may be more effective.
It has been also shown that the observation of a downscaled back in chronic back pain patients reduced their pain perception, while no effect was reported for the enlarged back visual condition (Diers et al., 2016). The latter study supports the results found in a case study of phantom limb pain conducted by Ramachandran et al. (2009b), in which by using mirrors, they found that minimizing the size of the lost left forearm reduced the patients’ pain perception, while magnifying it had no effect. One explanation for the contradictory results between pain-free participants and chronic pain patients is the complex relationship between pain and the neural representation of the body (Lotze and Moseley, 2007; Gilpin et al., 2015). Related to this, while the temporary painful stimulation in pain-free participants for experimental purposes does not modulate the representation of the body, it is known that patients suffering from chronic pain have associated changes in the central neural system, including a modified cortical representation of the painful part of the body (Moseley and Flor, 2012).
Taken together, these studies demonstrate an important modulatory effect of the vision of one’s own painful part of the body, both in healthy subjects and in subjects with chronic pain. However, it has been recently suggested that, in order to be effective at decreasing pain perception, the visual feedback has to be “realistic” by using real-time video or realistic representations of the painful part of the body, instead of a static or neutral image, at least with chronic lower back pain patients (Diers et al., 2016). For this reason, pain management using immersive VR, which allows subjects to be embodied in a virtual body capable of movement, seems to be a potential alternative for studying pain perception in both healthy and clinical populations.
Embodiment in VR for Pain Relief
In the context of these studies, Martini and colleagues investigated the effect of virtual body ownership on pain perception and found that looking at one’s own virtual hand also had analgesic properties, as described for the real hand (Longo et al., 2009) (Figure 2). An increased experimental pain threshold was found when compared with the observation of either a real or a virtual object (Martini et al., 2014). Further, they found that the feeling of ownership over the virtual arm was crucial to accomplish the analgesic effect. Regardless, the analgesic effect experienced while observing one’s own body seems to be effective even when observing an embodied virtual body if participants experienced high levels of ownership of the body. The fact that looking at one’s own “rubber hand” (after inducing the RHI) is not analgesic (Mohan et al., 2012) opened up a debate regarding the extent to which looking at a surrogate body was actually analgesic. This issue was sorted out by Nierula et al. (2017), who demonstrated the relevance of the position of the surrogate with respect to the real hand. While the rubber hand cannot be co-located with the real hand (since they both occupy physical space), the virtual hand can be co-located (or not) with the real hand. Nierula et al. (2017) demonstrated that as the distance between the real and the virtual hand increases, the analgesic effect decreases (Figure 3A). In agreement with this, previous findings by Romano and colleagues also reported reduced physiological responses to painful stimuli measured via SCRs, when participants observed a virtual body from a first-person perspective co-located with their real body compared with observing the virtual body turned 90° from the real body (Romano et al., 2015). Moreover, in the same study, the authors observed that physiological responses were negatively correlated with the size of the virtual body: the bigger the virtual body, the lower the SCRs (Romano et al., 2015). These results are in line with the observation of a magnified body part increased experimental heat pain thresholds (Mancini et al., 2011; Romano and Maravita, 2014).
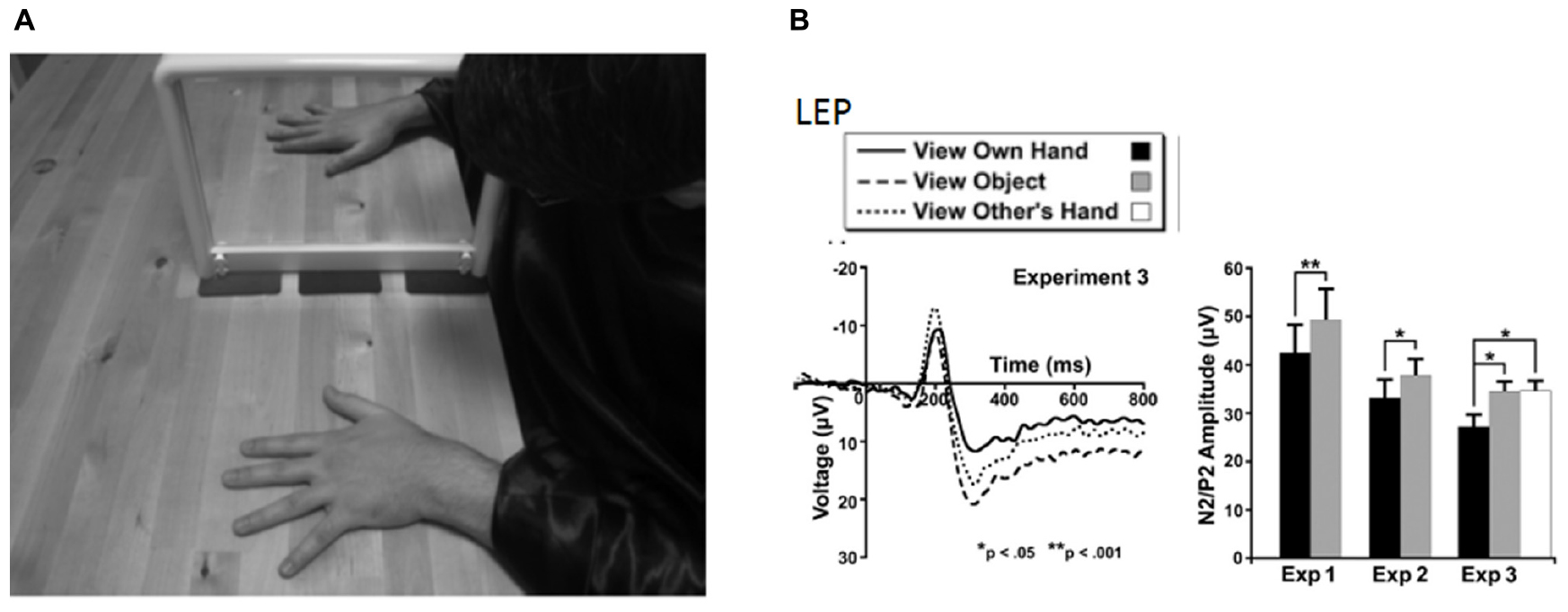
Figure 2. Experimental setup and results from Longo et al. (2009) in which vision of the body was shown to be analgesic, subjectively (using self-report pain ratings) and objectively using laser-evoked potentials. (A) The mirror box technique in which the subject has the experience of viewing their right hand, while in fact seeing their left hand reflected in a mirror. (B) Laser-evoked potentials (left) and peak-to-peak amplitudes (right) for the three experimental conditions. Error bars are one SEM. Reprinted from Copyright [2009] Society for Neuroscience. ∗p < 0.05, ∗∗p < 0.01.
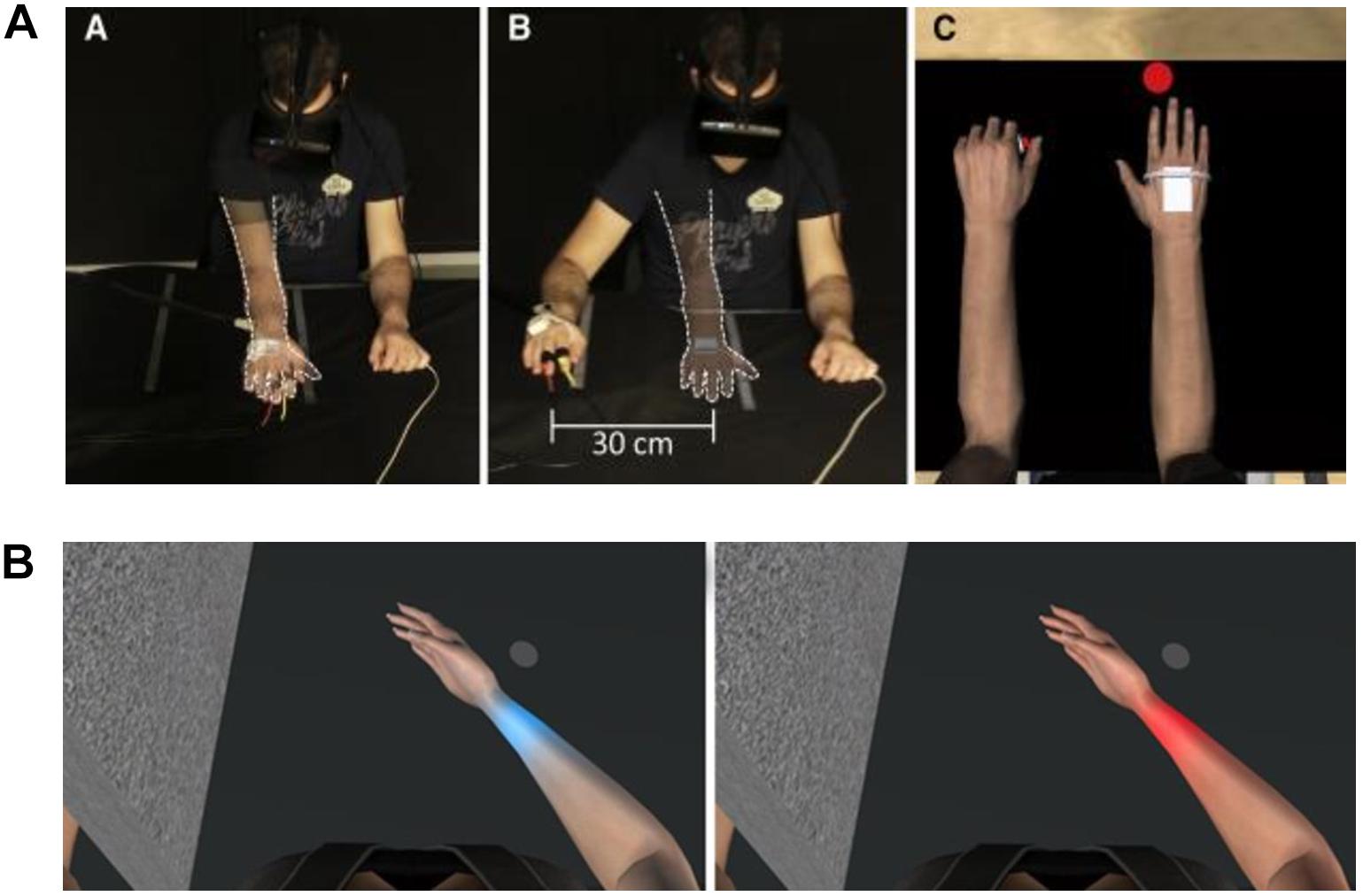
Figure 3. (A) Experimental setup of co-location experiment by Nierula et al. (2017). The participant wore a head-mounted display that provided an immersive virtual environment including a virtual own body that was perceived from a first-person perspective. The transparent arm outlined with a white dashed line indicated the positions of the virtual arm. Position of participant during (left panel) co-location, where the virtual and real arm were co-located, and (middle panel) when there was a distance of 30 cm between the real and virtual arm (right panel). The virtual body from the participant’s point of view. Reprinted with permission from Elsevier. (B) Participant’s view of virtual arm in the experiment by Martini et al. (2013). The right arm is co-located with the virtual arm, with congruent finger movements, in order to induce embodiment of the virtual limb. Heat stimulation is provided to the wrist while the skin color changed. Pain threshold was increased in the blue arm condition (left) versus the red arm condition (right).
Visual manipulations of the body modulate pain perception. One example is the study conducted by Martini et al. (2013) in which the color of a virtual arm was modified and the pain threshold was measured in healthy subjects (see Figure 3B). Specifically, observation of a bluish “cold” virtual arm increased heat pain thresholds, whereas observation of a reddened “hot” virtual arm decreased heat pain thresholds. Co-location of the virtual body with the real one seems to be another key factor for increasing pain thresholds in healthy subjects (Nierula et al., 2017).
Although evidence suggests that observing one’s own body while experiencing a painful stimulus reduces pain perception, what would happen if the painful part of the body were to fade away? To answer this question, Martini and co-workers conducted an experimental study in which the virtual body was rendered with different levels of transparency while participants were exposed to a painful heat stimulus. They found that the higher levels of transparency were inversely correlated with levels of ownership, but where the body was semi-transparent, higher levels of ownership over a see-through body resulted in an increased pain sensitivity (Martini et al., 2015). Nevertheless, in clinical populations, the effect of transparency is less clear. In this regard, in a study by Matamala-Gomez et al. (2018), two different groups of chronic arm pain patients [CRPS and peripheral nerve injury (PNI)] were immersed in VR and the virtual arm was observed by the patients at four different transparency levels (transparency test) and three different sizes (size test). In contrast to the study conducted on healthy subjects by Martini et al. (2015), Matamala-Gomez et al. (2018) found that increasing transparency levels of the observed virtual arm decreased pain ratings in CRPS, but this did not occur in PNI. Size increase slightly increased pain ratings only in CRPS patients. Further, the authors found that patients with chronic pain can achieve levels of ownership and agency over a virtual arm similar to healthy participants. Moreover, the VR exposure to all of the conditions globally decreased the mean pain ratings by half by the end of the experiment compared to pain ratings at baseline (see Figure 4). This study highlights the possibility that embodiment in VR decreases, at least temporarily, pain ratings in patients with chronic pain. The specific underlying mechanisms of each type of pain probably have a role in the type of strategy that is more effective for reducing pain perception in clinical populations. Further research is required to ascertain optimal dosage and duration of the effects.
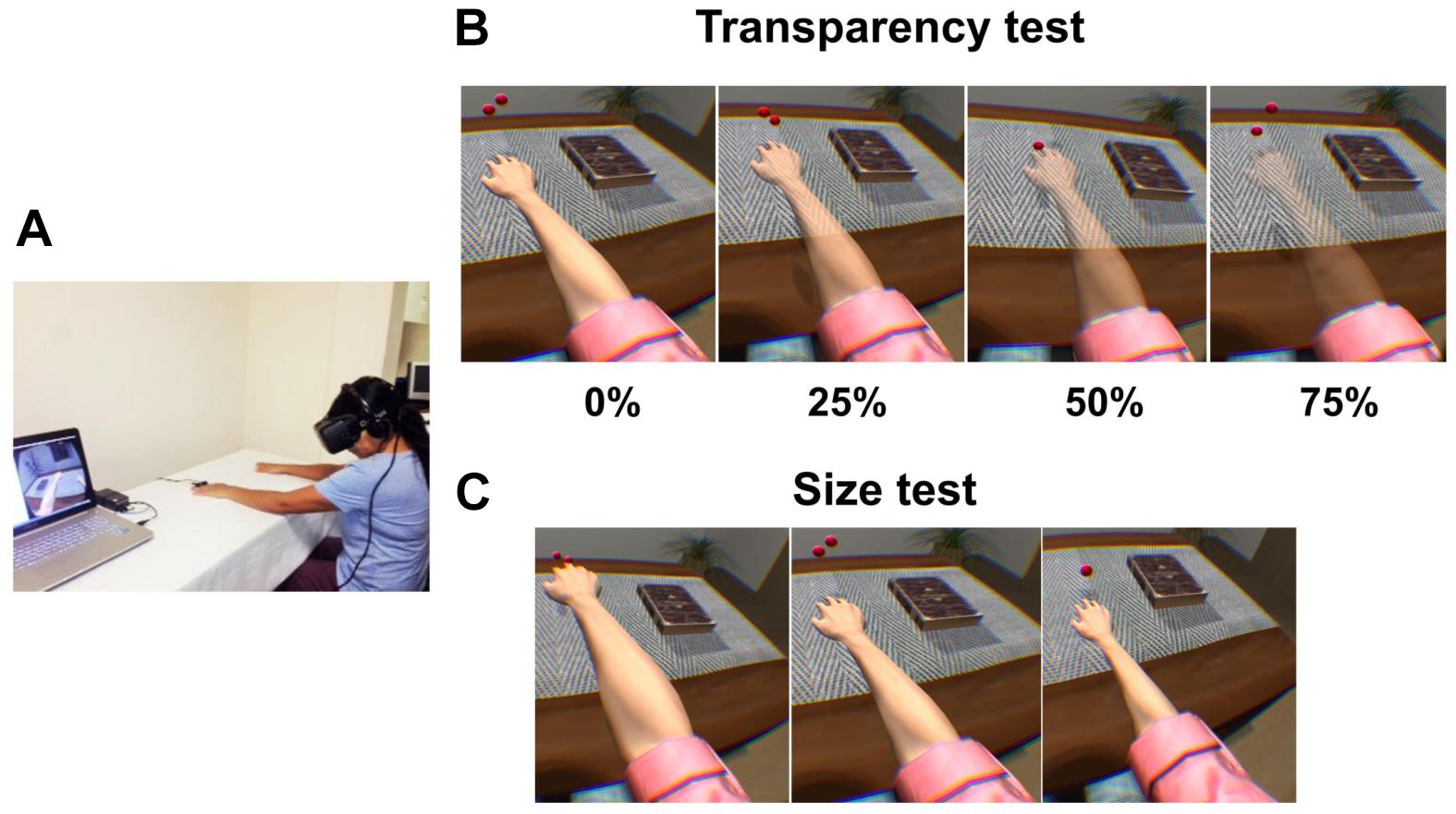
Figure 4. Experimental setup, and transparency and size tests for Matamala-Gomez et al. (2018). (A) Patients wore a head-mounted display (HMD) that immersed them in a virtual environment, which allowed participants to feel embodied in a virtual body viewed from a first-person perspective that was co-located with their real body. Virtual balls tapped the fingers during each stimulus presentation, which was accompanied by visuo-tactile stimulation to induce ownership over the virtual arm. (B) Transparency test including all four conditions: virtual arm transparency set at 0% (maximum opacity), 25, 50, and 75% (low opacity). (C) Size test including all three conditions: virtual arm presented in a big size, in its normal size, and in a small size. Reprinted with permission from Elsevier.
Other investigations have also used embodiment in a virtual body to modulate pain perception in clinical populations. In a recent study by Solcà et al. (2018), 24 CRPS patients were immersed in VR, embodied in a virtual body, and observed their affected virtual limb flashing in synchrony with their own detected heartbeat, or asynchronously in the control condition. Here, the authors observed reduced pain ratings and improved motor limb function while observing the synchronous heartbeat condition compared with the asynchronous control condition. Moreover, in another recent study that attempted to modulate neuropathic pain in spinal cord injury patients, the authors showed that VR exposure using multisensory stimulation is associated with mild analgesia, to suggest potential implications for spinal cord injury neurorehabilitation protocols (Pozeg et al., 2017). Finally, Llobera et al. (2013) used body ownership illusions induced using immersive VR combined with a brain–computer interface (BCI) system in a single patient with dystonia of the upper limb suffering from chronic pain. The patient was embodied in a virtual body while observing a virtual hand opening either automatically or through a cognitive task assessed using a BCI that required patient effort. The evaluation was conducted also on a group of five healthy controls. The authors found that embodiment in the virtual body induced changes in electromyography and BCI tasks in the patient that were different from those observed in the controls (see Table 1 for a review).
Discussion
This review has discussed the potentialities of using an embodied virtual body in immersive VR for pain modulation. Specifically, we have discussed the use of multisensory integration applications, by means of body ownership illusions, to decrease pain perception in healthy and clinical populations.
In a systematic review conducted by Boesch et al. (2016) of non-virtual body illusions (illusory changes of size, mirror therapy, etc.) on clinical pain, they found that there is limited evidence to suggest that bodily illusions can alter pain, but some illusions, namely, mirror therapy, bodily resizing, and use of functional prostheses, show therapeutic promise. Concerning the effects of embodiment on clinical pain, the authors discuss two studies of patients with chronic pain that showed no effect of embodiment on pain levels and suggest that a potential explanation is that embodiment and pain modulation may be separate processes. However, the review did not examine any studies that used immersive VR studies to induce embodiment. Here, we show that through an embodied virtual body, we may modulate body representation and change pain perception in healthy and clinical populations.
Regarding the importance of body representation in pain perception, it is known that many chronic pain patients have a distorted representation of the affected part of the body (Lewis et al., 2007; Moseley, 2008; Senkowski and Heinz, 2016). Further, misrepresentations of the body have been associated with pain (Lotze and Moseley, 2007), and several reports support structural and functional differences between people with and without pain, both at a cortical or at a subcortical level, in brain areas involved in body awareness and body perception (Flor et al., 1997; Pleger et al., 2006; Gwilym et al., 2010). Distortions of body perception involving a painful part of the body (i.e., the body part feeling larger than it really is) have also been demonstrated (Moyer, 2005; Lewis et al., 2007). There is some evidence that treatment directed at changing these functional brain alterations, such as graded motor imagery and sensorimotor retraining (Moseley, 2004, 2006; Pleger et al., 2006), reduces pain, which suggests that there is a bidirectional link between pain and body perception. In addition to this, it has been shown that pain perception is reduced with a corresponding restoration of functional cortical representation of the painful part of the body in CRPS patients (Pleger et al., 2006).
Future Research
These studies support a link between body perception and clinical disorders such as pain, highlighting the advantages of using embodiment through VR systems in neurorehabilitation and pain management. Nonetheless, robust and suitably powered randomized control trials are needed to further explore the full potential of body illusions and embodied technologies to modulate pain perception, especially with the use of immersive VR. Furthermore, further investigations aimed at modulating pain perception through an embodied virtual body with larger sample sizes will allow a better understanding of the contribution that the subjective feeling of ownership over an embodied virtual body has on pain perception. Moreover, future studies on this topic may make use of brain imaging techniques, which will allow better identification of the neural structures underlying the complex link between modification of body perception and pain.
Interestingly, virtual body embodiment may also allow the study empathy in pain. It is known that the mere observation of other people in pain tends to elicit empathic responses regarding pain perception in one’s body (Lamm et al., 2011; Benuzzi et al., 2018). Hence, what will happen if we use embodiment to create a pain-free representation of the body? Although some authors have started to investigate how to use empathy for pain relief by using embodiment (Fusaro et al., 2016), further investigations are needed to create new behavioral and cognitive training methods for modulating pain perception in clinical populations.
Conclusion
The studies commented throughout this narrative review, especially those conducted with chronic pain patients, pave the way for the design of new rehabilitation protocols with prolonged and repeated doses of embodied virtual body in immersive VR to tackle chronic pain disorders, and enable the integration of such “digital therapy” with existing conventional pain treatments.
Author Contributions
MM-G contributed to the bibliographic review and writing of the manuscript. TD contributed to the writing and review of the manuscript. SB and GS contributed to the bibliographic suggestions and review of the manuscript. MS-V and CT contributed to the supervision of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aglioti, S. M., Berlucchi, G., Aglioti, S., and Berlucchi, G. (2016). The body in the brain: neural bases of corporeal awareness. Trends Neurosci. 2236, 560–564. doi: 10.1016/S0166-2236(97)01136-3
Armel, K. C., and Ramachandran, V. S. (2003). Projecting sensations to external objects: evidence from skin conductance response. Proc. R. Soc. B Biol. Sci. 270, 1499–1506. doi: 10.1098/rspb.2003.2364
Arzy, S., Overney, L. S., Landis, T., and Blanke, O. (2006). Neural mechanisms of embodiment: asomatognosia due to premotor cortex damage. Arch. Neurol. 63, 1022–1025. doi: 10.1001/archneur.63.7.1022
Banakou, D., Groten, R., and Slater, M. (2013). Illusory ownership of a virtual child body causes overestimation of object sizes and implicit attitude changes. Proc. Natl. Acad. Sci. U.S.A. 110, 12846–12851. doi: 10.1073/pnas.1306779110
Benuzzi, F., Lui, F., Ardizzi, M., Ambrosecchia, M., Ballotta, D., Righi, S., et al. (2018). Pain mirrors: neural correlates of observing self or others’ facial expressions of pain. Front. Psychol. 9:1825. doi: 10.3389/fpsyg.2018.01825
Bergström, I., Kilteni, K., and Slater, M. (2016). First-person perspective virtual body posture influences stress: a virtual reality body ownership study. PLoS One 11:e0148060. doi: 10.1371/journal.pone.0148060
Berlucchi, G., and Aglioti, S. (1997). The body in the brain: neural bases of corporeal awareness. Trends Neurosci. 20, 560–564. doi: 10.1016/s0166-2236(97)01136-3
Birklein, F., and Schlereth, T. (2015). Complex regional pain syndrome-significant progress in understanding. Pain 156, S94–S103. doi: 10.1097/01.j.pain.0000460344.54470.20
Blanke, O., Slater, M., and Serino, A. (2015). Behavioral, neural, and computational principles of bodily self-consciousness. Neuron 88, 145–166. doi: 10.1016/j.neuron.2015.09.029
Boesch, E., Bellan, V., Moseley, G., and Stanton, T. (2016). The effect of bodily illusions on clinical pain: a systematic review and meta-analysis. Pain 157, 516–529. doi: 10.1097/j.pain.0000000000000423
Boesch, E., Bellan, V., Moseley, G. L., and Stanton, T. R. (2015). The effect of bodily illusions on clinical pain. Pain 157, 516–529. doi: 10.1097/j.pain.0000000000000423
Bohil, C. J., Alicea, B., and Biocca, F. A. (2011). Virtual reality in neuroscience research and therapy. Nat. Rev. Neurosci. 12, 752–762. doi: 10.1038/nrn3122
Botvinick, M., and Cohen, J. (1998). Rubber hands “feel” touch that eyes see. Nature 391:756. doi: 10.1038/35784
Campbell, G., Bruno, R., Darke, S., Shand, F., Hall, W., Farrell, M., et al. (2016). Prevalence and correlates of suicidal thoughts and suicide attempts in people prescribed pharmaceutical opioids for chronic pain. Clin. J. Pain 32, 292–301. doi: 10.1097/AJP.0000000000000283
Cardini, F., Longo, M. R., and Haggard, P. (2011). Vision of the body modulates somatosensory intracortical inhibition. Cereb. Cortex 21, 2014–2022. doi: 10.1093/cercor/bhq267
Carter, G. T., Duong, V., Ho, S., Ngo, K. C., Greer, C. L., and Weeks, D. L. (2014). Side effects of commonly prescribed analgesic medications. Phys. Med. Rehabil. Clin. N. Am. 25, 457–470. doi: 10.1016/j.pmr.2014.01.007
Cash, T. F., and Brown, T. A. (1987). Body image in anorexia nervosa and bulimia nervosa: a review of the literature. Behav. Modif. 11, 487–521. doi: 10.1177/01454455870114005
Critchley, M. (1953). Parietal lobes. G. Psichiatr. Neuropatol. 81, 872–873. doi: 10.1136/bmj.2.4851.1416
Damasio, A. (2000). The Feeling of What Happens: Body and Emotion in the Making of Consciousness. Boston: Mariner Books.
de Vignemont, F. (2011). Embodiment, ownership and disownership. Conscious. Cogn. 20, 82–93. doi: 10.1016/j.concog.2010.09.004
Diers, M., Löffler, A., Zieglgänsberger, W., and Trojan, J. (2016). Watching your pain site reduces pain intensity in chronic back pain patients. Eur. J. Pain 20, 581–585. doi: 10.1002/ejp.765
Diers, M., Zieglgänsberger, W., Trojan, J., Drevensek, A. M., Erhardt-Raum, G., and Flor, H. (2013). Site-specific visual feedback reduces pain perception. Pain 154, 890–896. doi: 10.1016/j.pain.2013.02.022
Doctor, J. N., Carrougher, G. J., Furness, T. A., Patterson, D. R., and Hoffman, H. G. (2002). Virtual reality as an adjunctive pain control during burn wound care in adolescent patients. Pain 85, 305–309. doi: 10.1016/s0304-3959(99)00275-4
Ehrsson, H., and Petkova, V. (2008). If I were you: perceptual illusion of body swapping. PLoS One 3:e3832. doi: 10.1371/journal.pone.0003832
Flor, H., Braun, C., Elbert, T., and Birbaumer, N. (1997). Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci. Lett. 224, 5–8. doi: 10.1016/S0304-3940(97)13441-3
Florence, C. S., Zhou, C., Luo, F., and Xu, L. (2016). The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med. Care 54, 901–906. doi: 10.1097/MLR.0000000000000625
Fuentes, C. T., Pazzaglia, M., Longo, M. R., Scivoletto, G., and Haggard, P. (2013). Body image distortions following spinal cord injury. J. Neurol. Neurosurg. Psychiatry 84, 201–207. doi: 10.1136/jnnp-2012-304001
Fusaro, M., Tieri, G., and Aglioti, S. M. (2016). Seeing pain and pleasure on self and others: behavioral and psychophysiological reactivity in immersive virtual reality. J. Neurophysiol. 116, 2656–2662. doi: 10.1152/jn.00489.2016
Gallagher, S. (2001). Dimensions of embodiment: body image and body schema in medical contexts. Br. J. Clin. Pharmacol. 68, 147–175. doi: 10.1007/978-94-010-0536-4
Gallagher, S., and Cole, J. (1995). Body schema and body image in a deafferented subject. J. Mind Behav. 16, 369–390. doi: 10.1016/j.neuropsychologia.2009.09.022
Gardner, R. M., and Moncrieff, C. (1988). Body image distortion in anorexics as a non-sensory phenomenon: a signal detection approach. J. Clin. Psychol. 44, 101–107. doi: 10.1002/1097-4679(198803)44:2<101::aid-jclp2270440203>3.0.co;2-u
Gaskin, D. J., and Richard, P. (2012). The economic costs of pain in the United States. J. Pain 13, 715–724. doi: 10.1016/j.jpain.2012.03.009
Geneen, L. J., Moore, R. A., Clarke, C., Martin, D., Colvin, L. A., and Smith, B. H. (2017). “Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews,” in Cochrane Database of Systematic Reviews, ed. L. J. Geneen (Chichester: John Wiley & Sons, Ltd.).
Gilpin, H. R., Moseley, G. L., Stanton, T. R., and Newport, R. (2015). Evidence for distorted mental representation of the hand in osteoarthritis. Rheumatology 54, 678–682. doi: 10.1093/rheumatology/keu367
González-Franco, M., Peck, T. C., Rodríguez-Fornells, A., and Slater, M. (2014). A threat to a virtual hand elicits motor cortex activation. Exp. Brain Res. 232, 875–887. doi: 10.1007/s00221-013-3800-1
Guariglia, C., and Antonucci, G. (1992). Personal and extrapersonal space: a case of neglect dissociation. Neuropsychologia 30, 1001–1009. doi: 10.1016/0028-3932(92)90051-M
Gwilym, S. E., Filippini, N., Douaud, G., Carr, A. J., and Tracey, I. (2010). Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum. 62, 2930–2940. doi: 10.1002/art.27585
Halligan, P. W., Marshall, J. C., and Wade, D. T. (1993). Diminution and enhancement of visuo-spatial neglect with sequential trials. J. Neurol. 240, 117–120. doi: 10.1007/BF00858728
Halligan, P. W., Zeman, A., and Berger, A. (1999). Phantoms in the brain. BMJ 319, 587–588. doi: 10.1136/bmj.319.7210.587
Hoffman, H. G., Chambers, G. T., Meyer, W. J., Arceneaux, L. L., Russell, W. J., Seibel, E. J., et al. (2011). Virtual reality as an adjunctive non-pharmacologic analgesic for acute burn pain during medical procedures. Ann. Behav. Med. 41, 183–191. doi: 10.1007/s12160-010-9248-7
Hoffman, H. G., Doctor, J. N., Patterson, D. R., Carrougher, G. J., and Furness, T. A. III (2000a). Use of virtual reality for adjunctive treatment of adolescent burn pain during wound care. Pain 85, 305–309. doi: 10.1016/s0304-3959(99)00275-4
Hoffman, H. G., Patterson, D. R., and Carrougher, G. J. (2000b). Use of virtual reality for adjunctive treatment of adult burn pain during physical therapy: a controlled study. Clin. J. Pain 16, 244–250. doi: 10.1097/00002508-200009000-00010
Hoffman, H. G., Meyer, W. J., Ramirez, M., Roberts, L., Seibel, E. J., Atzori, B., et al. (2014). Feasibility of articulated arm mounted oculus rift virtual reality goggles for adjunctive pain control during occupational therapy in pediatric burn patients. Cyberpsychol. Behav. Soc. Netw. 17, 397–401. doi: 10.1089/cyber.2014.0058
Hoffman, H. G., Richards, T. L., Coda, B., Bills, A. R., Blough, D., Richards, A. L., et al. (2004). Modulation of thermal pain-related brain activity with virtual reality: evidence from fMRI. Neuroreport 15, 1245–1248. doi: 10.1097/01.wnr.0000127826.73576.91
Hoffman, H. G., Richards, T. L., Van Oostrom, T., Coda, B. A., Jensen, M. P., Blough, D. K., et al. (2007). The analgesic effects of opioids and immersive virtual reality distraction: evidence from subjective and functional brain imaging assessments. Anesth. Analg. 105, 1776–1783. doi: 10.1213/01.ane.0000270205.45146.db
Hofmann, S. G., Asnaani, A., Vonk, I. J. J., Sawyer, A. T., and Fang, A. (2012). The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit. Ther. Res. 36, 427–440. doi: 10.1007/s10608-012-9476-1
Janczyk, M., Skirde, S., Weigelt, M., and Kunde, W. (2009). Visual and tactile action effects determine bimanual coordination performance. Hum. Mov. Sci. 28, 437–449. doi: 10.1016/j.humov.2009.02.006
Jenkinson, P. M., Haggard, P., Ferreira, N. C., and Fotopoulou, A. (2013). Body ownership and attention in the mirror: insights from somatoparaphrenia and the rubber hand illusion. Neuropsychologia 51, 1453–1462. doi: 10.1016/j.neuropsychologia.2013.03.029
Kalckert, A., and Ehrsson, H. (2012). Moving a rubber hand that feels like your own: a dissociation of ownership and agency. Front. Hum. Neurosci. 6:40. doi: 10.3389/fnhum.2012.00040
Kilteni, K., Groten, R., and Slater, M. (2012a). The sense of embodiment in virtual reality. Teleoperators Virtual Environ. 21, 373–387. doi: 10.1162/pres_a_00124
Kilteni, K., Normand, J.-M., Sanchez-Vives, M. V., and Slater, M. (2012b). Extending body space in immersive virtual reality: a very long arm illusion. PLoS One 7:e40867. doi: 10.1371/journal.pone.0040867
Lackner, J. R. (1988). Some proprioceptive influences on the perceptual representation of body shape and orientation. Brain 111(Pt 2), 281–297. doi: 10.1093/brain/111.2.281
Lamm, C., Decety, J., and Singer, T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54, 2492–2502. doi: 10.1016/j.neuroimage.2010.10.014
Legrand, D. (2006). The bodily self: the sensori-motor roots of pre-reflective self-consciousness. Phenom. Cogn. Sci. 5, 89–118. doi: 10.1007/s11097-005-9015-6
Levine, D. N., Calvanio, R., and Rinn, W. E. (1991). The pathogenesis of anosognosia for hemiplegia. Neurology 41, 1770–1770. doi: 10.1212/WNL.41.11.1770
Lewis, J. S., Kersten, P., McCabe, C. S., McPherson, K. M., and Blake, D. R. (2007). Body perception disturbance: a contribution to pain in complex regional pain syndrome (CRPS). Pain 133, 111–119. doi: 10.1016/j.pain.2007.03.013
Llobera, J., González-Franco, M., Perez-Marcos, D., Valls-Solé, J., Slater, M., and Sanchez-Vives, M. V. (2013). Virtual reality for assessment of patients suffering chronic pain: a case study. Exp. Brain Res. 225, 105–117. doi: 10.1007/s00221-012-3352-9
Loetscher, T., Regard, M., and Brugger, P. (2006). Misoplegia: a review of the literature and a case without hemiplegia. J. Neurol. Neurosurg. Psychiatry 77, 1099–1100. doi: 10.1136/jnnp.2005.087163
Longo, M. R., Betti, V., Aglioti, S. M., and Haggard, P. (2009). Visually induced analgesia: seeing the body reduces pain. J. Neurosci. 29, 12125–12130. doi: 10.1523/JNEUROSCI.3072-09.2009
Longo, M. R., Iannetti, G. D., Mancini, F., Driver, J., and Haggard, P. (2012). Linking pain and the body: neural correlates of visually induced analgesia. J. Neurosci. 32, 2601–2607. doi: 10.1523/JNEUROSCI.4031-11.2012
Longo, M. R., Schüür, F., Kammers, M. P. M., Tsakiris, M., and Haggard, P. (2008). What is embodiment? a psychometric approach. Cognition 107, 978–998. doi: 10.1016/j.cognition.2007.12.004
Lotze, M., and Moseley, G. L. (2007). Role of distorted body image in pain. Curr. Rheumatol. Rep. 9, 488–496. doi: 10.1007/s11926-007-0079-x
Macaluso, E., and Maravita, A. (2010). The representation of space near the body through touch and vision. Neuropsychologia 48, 782–795. doi: 10.1016/j.neuropsychologia.2009.10.010
Malloy, K. M., and Milling, L. S. (2010). The effectiveness of virtual reality distraction for pain reduction: a systematic review. Clin. Psychol. Rev. 30, 1011–1018. doi: 10.1016/j.cpr.2010.07.001
Mancini, F., Longo, M. R., Canzoneri, E., Vallar, G., and Haggard, P. (2013). Changes in cortical oscillations linked to multisensory modulation of nociception. Eur. J. Neurosci. 37, 768–776. doi: 10.1111/ejn.12080
Mancini, F., Longo, M. R., Kammers, M. P. M., and Haggard, P. (2011). Visual distortion of body size modulates pain perception. Psychol. Sci. 22, 325–330. doi: 10.1177/0956797611398496
Martini, M. (2016). Real, rubber or virtual: the vision of “one’s own” body as a means for pain modulation. a narrative review. Conscious. Cogn. 43, 143–151. doi: 10.1016/j.concog.2016.06.005
Martini, M., Kilteni, K., Maselli, A., and Sanchez-Vives, M. V. (2015). The body fades away: investigating the effects of transparency of an embodied virtual body on pain threshold and body ownership. Sci. Rep. 5:13948. doi: 10.1038/srep13948
Martini, M., Perez-Marcos, D., and Sanchez-Vives, M. V. (2013). What color is my arm? Changes in skin color of an embodied virtual arm modulates pain threshold. Front. Hum. Neurosci. 7:438. doi: 10.3389/fnhum.2013.00438
Martini, M., Perez-Marcos, D., and Sanchez-Vives, M. V. (2014). Modulation of pain threshold by virtual body ownership. Eur. J. Pain 18, 1040–1048. doi: 10.1002/j.1532-2149.2014.00451.x
Maselli, A. (2015). Allocentric and egocentric manipulations of the sense of self-location in full-body illusions and their relation with the sense of body ownership. Cogn. Process. 16, 309–312. doi: 10.1007/s10339-015-0667-z
Maselli, A., and Slater, M. (2013). The building blocks of the full body ownership illusion. Front. Hum. Neurosci. 7:1–15. doi: 10.3389/fnhum.2013.00083
Matamala-Gomez, M., Gonzalez, A. M. D., Slater, M., and Sanchez-Vives, M. V. (2018). Decreasing pain ratings in chronic arm pain through changing a virtual body: different strategies for different pain types. J. Pain. 20, 685–697. doi: 10.1016/J.JPAIN.2018.12.001
McGlynn, S. M., and Schacter, D. L. (1989). Unawareness of deficits in neuropsychological syndromes. J. Clin. Exp. Neuropsychol. Off. J. Int. Neuropsychol. Soc. 11, 143–205. doi: 10.1080/01688638908400882
Medina, J., and Coslett, H. B. (2010). Neuropsychologia from maps to form to space: touch and the body schema. 48, 645–654. doi: 10.1016/j.neuropsychologia.2009.08.017
Melzack, R. (1990). Phantom limbs and the concept of a neuromatrix. Trends Neurosci. 13, 88–92. doi: 10.1016/0166-2236(90)90179-e
Mohan, R., Jensen, K. B., Petkova, V. I., Dey, A., Barnsley, N., Ingvar, M., et al. (2012). No pain relief with the rubber hand illusion. PLoS One 7:e52400. doi: 10.1371/journal.pone.0052400
Moseley, G. (2006). Graded motor imagery for pathologic pain. a randomized controlled trial. Neurology 67, 2129–2134. doi: 10.1212/01.wnl.0000249112.56935.32
Moseley, G., and Flor, H. (2012). Targeting cortical representations in the treatment of chronic pain a review. Neurorehabil. Neural Repair. 26, 646–652. doi: 10.1177/1545968311433209
Moseley, G., Parsons, T., and Spence, C. (2008). Visual distortion of a limb modulates the pain and swelling evoked by movement. Curr. Biol. 18, R1047–R1048.
Moseley, G. L. (2004). Why do people with complex regional pain syndrome take longer to recognize their affected hand? Neurology 62, 2182–2186. doi: 10.1212/01.WNL.0000130156.05828.43
Moseley, G. L. (2008). I can’t find it! distorted body image and tactile dysfunction in patients with chronic back pain. Pain 140, 167–171. doi: 10.1016/j.pain.2008.08.001
Moyer, P. (2005). Distorted body image for patients with complex regional pain syndrome. Neurol. Today 5:52. doi: 10.1097/00132985-200510000-00015
Nierula, B., Martini, M., Matamala-Gomez, M., Slater, M., and Sanchez-Vives, M. V. (2017). Seeing an embodied virtual hand is analgesic contingent on colocation. J. Pain 18, 645–655. doi: 10.1016/j.jpain.2017.01.003
Osimo, S. A., Pizarro, R., Spanlang, B., and Slater, M. (2015). Conversations between self and self as sigmund freud—a virtual body ownership paradigm for self counselling. Sci. Rep. 5:13899. doi: 10.1038/srep13899
Pavani, F., Spence, C., and Driver, J. (1999). Visual capture of touch (tactile ventriloquism); out-of-the-body experiences with rubber gloves. J. Cogn. Neurosci. 11:14.
Peck, T. C., Seinfeld, S., Aglioti, S. M., and Slater, M. (2013). Putting yourself in the skin of a black avatar reduces implicit racial bias. Conscious. Cogn. 22, 779–787. doi: 10.1016/j.concog.2013.04.016
Perez-Marcos, D., Martini, M., Fuentes, C. T., Bellido Rivas, A. I., Haggard, P., and Sanchez-Vives, M. V. (2018). Selective distortion of body image by asynchronous visuotactile stimulation. Body Image 24, 55–61. doi: 10.1016/j.bodyim.2017.11.002
Petkova, V. I., Khoshnevis, M., and Ehrsson, H. H. (2011). The perspective matters! multisensory integration in ego-centric reference frames determines full-body ownership. Front. Psychol. 2:35. doi: 10.3389/fpsyg.2011.00035
Pleger, B., Ragert, P., Schwenkreis, P., Förster, A.-F., Wilimzig, C., Dinse, H., et al. (2006). Patterns of cortical reorganization parallel impaired tactile discrimination and pain intensity in complex regional pain syndrome. Neuroimage 32, 503–510. doi: 10.1016/j.neuroimage.2006.03.045
Powers, P. S., Schulman, R. G., Gleghorn, A. A., and Prange, M. E. (1987). Perceptual and cognitive abnormalities in bulimia. Am. J. Psychiatry 144, 1456–1460. doi: 10.1176/ajp.144.11.1456
Pozeg, P., Palluel, E., Ronchi, R., Solcà, M., Al-Khodairy, A. W., Jordan, X., et al. (2017). Virtual reality improves embodiment and neuropathic pain caused by spinal cord injury. Neurology 89, 1894–1903. doi: 10.1212/WNL.0000000000004585
Preston, C., and Newport, R. (2011). Analgesic effects of multisensory illusions in osteoarthritis. Rheumatology 50, 2314–2315. doi: 10.1093/rheumatology/ker104
Ramachandran, V. S., Altschuler, E. L., Aglioti, S., Bonazzi, A., Cortese, F., Aglioti, S., et al. (2009a). The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain 132, 1693–1710. doi: 10.1093/brain/awp135
Ramachandran, V. S., Brang, D., and McGeoch, P. D. (2009b). Size reduction using mirror visual feedback (MVF) reduces phantom pain. Neurocase 15, 357–360. doi: 10.1080/13554790903081767
Ramachandran, V. S., and Blakeslee, S. (1999). Phantoms in the brain: probing the mysteries of the human mind. Am. J. Psychiatry 157, 841–842. doi: 10.1176/appi.ajp.157.5.841
Riva, G., Wiederhold, B. K., and Mantovani, F. (2018). Neuroscience of virtual reality: from virtual exposure to embodied medicine. Cyberpsychol. Behav. Soc. Netw. 22, 82–96. doi: 10.1089/cyber.2017.29099.gri
Romano, D., Llobera, J., and Blanke, O. (2015). Size and viewpoint of an embodied virtual body impact the processing of painful stimuli. J. Pain. 17, 350–358. doi: 10.1016/j.jpain.2015.11.005
Romano, D., and Maravita, A. (2014). The visual size of one’s own hand modulates pain anticipation and perception. Neuropsychologia 57, 93–100. doi: 10.1016/j.neuropsychologia.2014.03.002
Sanchez-Vives, M. V., and Slater, M. (2005). From presence to consciousness through virtual reality. Nat. Rev. Neurosci. 6, 332–339. doi: 10.1038/nrn1651
Sanchez-Vives, M. V., Spanlang, B., Frisoli, A., Bergamasco, M., and Slater, M. (2010). Virtual hand illusion induced by visuomotor correlations. PLoS One 5:e10381. doi: 10.1371/journal.pone.0010381
Seinfeld, S., Arroyo-Palacios, J., Iruretagoyena, G., Hortensius, R., Zapata, L. E., Borland, D., et al. (2018). Offenders become the victim in virtual reality: impact of changing perspective in domestic violence. Sci. Rep. 8:2692. doi: 10.1038/s41598-018-19987-7
Senkowski, D., and Heinz, A. (2016). Chronic pain and distorted body image: implications for multisensory feedback interventions. Neurosci. Biobehav. Rev. 69, 252–259. doi: 10.1016/j.neubiorev.2016.08.009
Serino, A., and Haggard, P. (2010). Touch and the body. Neurosci. Biobehav. Rev. 34, 224–236. doi: 10.1016/j.neubiorev.2009.04.004
Slater, M., Perez-Marcos, D., Ehrsson, H. H., and Sanchez-Vives, M. V. (2008). Towards a digital body: the virtual arm illusion. Front. Hum. Neurosci. 2:6. doi: 10.3389/neuro.09.006.2008
Slater, M., and Sanchez-Vives, M. V. (2016). Enhancing our lives with immersive virtual reality. Front. Robot. AI 3:74. doi: 10.3389/FROBT.2016.00074
Slater, M., Spanlang, B., Sanchez-Vives, M. V., Blanke, O., Botvinick, M., Cohen, J., et al. (2010). First person experience of body transfer in virtual reality. PLoS One 5:e10564. doi: 10.1371/journal.pone.0010564
Solcà, M., Ronchi, R., Bello-Ruiz, J., Schmidlin, T., Herbelin, B., Luthi, F., et al. (2018). Heartbeat-enhanced immersive virtual reality to treat complex regional pain syndrome. Neurology 91, e1–e11. doi: 10.1212/WNL.0000000000005905
Stanton, T. R., Gilpin, H. R., Edwards, L., Moseley, G. L., and Newport, R. (2018). Illusory resizing of the painful knee is analgesic in symptomatic knee osteoarthritis. PeerJ 6:e5206. doi: 10.7717/peerj.5206
Tarr, M. J., and Warren, W. H. (2002). Virtual reality in behavioral neuroscience and beyond. Nat. Neurosci. 5, 1089–1092. doi: 10.1038/nn948
Triberti, S., Repetto, C., and Riva, G. (2014). Psychological factors influencing the effectiveness of virtual reality–based analgesia: a systematic review. Cyberpsychol. Behav. Soc. Netw. 17, 335–345. doi: 10.1089/cyber.2014.0054
Tsakiris, M., Prabhu, G., and Haggard, P. (2006). Having a body versus moving your body: how agency structures body-ownership. Conscious. Cogn. 15, 423–432. doi: 10.1016/j.concog.2005.09.004
Valeriani, M., Betti, V., Le Pera, D., De Armas, L., Miliucci, R., Restuccia, D., et al. (2008). Seeing the pain of others while being in pain: a laser-evoked potentials study. Neuroimage 40, 1419–1428. doi: 10.1016/j.neuroimage.2007.12.056
Vallar, G., and Ronchi, R. (2009). Somatoparaphrenia: a body delusion. A review of the neuropsychological literature. Exp. Brain Res. 192, 533–551. doi: 10.1007/s00221-008-1562-y
van Beers, R. J., Sittig, A. C., and van der Gon, J. J. D. (1999). Integration of proprioceptive and visual position-information: an experimentally supported model. J. Neurophysiol. 81, 1355–1364. doi: 10.1152/jn.1999.81.3.1355
Vos, T., Flaxman, A. D., Naghavi, M., Lozano, R., Michaud, C., Ezzati, M., et al. (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 380, 2163–2196. doi: 10.1016/S0140-6736(12)61729-2
Wesslein, A. K., Spence, C., and Frings, C. (2014). Vision affects tactile target and distractor processing even when space is task-irrelevant. Front. Psychol. 5:84. doi: 10.3389/fpsyg.2014.00084
Keywords: embodiment, virtual reality, pain, ownership illusion, body illusion
Citation: Matamala-Gomez M, Donegan T, Bottiroli S, Sandrini G, Sanchez-Vives MV and Tassorelli C (2019) Immersive Virtual Reality and Virtual Embodiment for Pain Relief. Front. Hum. Neurosci. 13:279. doi: 10.3389/fnhum.2019.00279
Received: 30 May 2019; Accepted: 29 July 2019;
Published: 21 August 2019.
Edited by:
Mariella Pazzaglia, Sapienza University of Rome, ItalyReviewed by:
Eleonora Borelli, University of Modena and Reggio Emilia, ItalyBigna Lenggenhager, University of Zurich, Switzerland
Copyright © 2019 Matamala-Gomez, Donegan, Bottiroli, Sandrini, Sanchez-Vives and Tassorelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta Matamala-Gomez, bWFydGEubWF0YW1hbGExMEBnbWFpbC5jb20=
†These authors share senior authorship
 Marta Matamala-Gomez
Marta Matamala-Gomez Tony Donegan3
Tony Donegan3 Giorgio Sandrini
Giorgio Sandrini Maria V. Sanchez-Vives
Maria V. Sanchez-Vives Cristina Tassorelli
Cristina Tassorelli