- 1National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA
- 2National Institutes of Health, National Institute on Drug Abuse, Baltimore, MD, USA
Obesity is associated with physical inactivity, which exacerbates the negative health consequences of obesity. Despite a wide consensus that people with obesity should exercise more, there are few effective methods for increasing physical activity in people with obesity. This lack is reflected in our limited understanding of the cellular and molecular causes of physical inactivity in obesity. We hypothesize that impairments in dopamine signaling contribute to physical inactivity in people with obesity, as in classic movement disorders such as Parkinson's disease. Here, we review two lines of evidence supporting this hypothesis: (1) chronic exposure to obesogenic diets has been linked to impairments in dopamine synthesis, release, and receptor function, particularly in the striatum, and (2) striatal dopamine is necessary for the proper control of movement. Identifying the biological determinants of physical inactivity may lead to more effective strategies for increasing physical activity in people with obesity, as well as improve our understanding of why it is difficult for people with obesity to alter their levels of physical activity.
Introduction
Obesity is associated with reductions in motor output, often termed “physical inactivity” (Tudor-Locke et al., 2010; Bouchard et al., 2015), although whether this relationship is causal remains a point of debate (Simon et al., 2008; Haskell et al., 2009; Dwyer-Lindgren et al., 2013; Swift et al., 2014). Despite the importance of physical activity for health, there are few effective methods for increasing physical activity levels in people with obesity, leading some researchers to conclude that, “there are presently no evidence-based interventions that can reliably and sustainably increase the level of physical activity among obese adults” (Ekkekakis et al., 2016). This point is reflected in our limited understanding of the cellular and molecular determinants of physical inactivity in people with obesity. We believe that a cellular understanding of why obesity is associated with physical inactivity is needed to understand, and ultimately alter, the relationship between obesity and physical inactivity. In this review, we propose that impairments in striatal dopamine contribute to physical inactivity in obesity, akin to classic movement disorders such as Parkinson's disease.
The striatum is a forebrain structure that controls movement, as well as learning and emotional states. There are two main projection cell types in the striatum, the “direct” and the “indirect” pathway medium spiny neurons (dMSNs and iMSNs), as well as several classes of interneurons. dMSNs and iMSNs exhibit distinct protein expression patterns, projection targets, and support distinct behavioral functions (Alexander and Crutcher, 1990; DeLong, 1990; Gerfen et al., 1990; Graybiel et al., 1994; Le Moine and Bloch, 1995; Obeso et al., 2000; Figure 1A). dMSNs express the excitatory Gs-coupled dopamine D1 receptor (D1R), while iMSNs express the inhibitory Gi-coupled dopamine D2 receptor (D2R; Gerfen et al., 1990). Dopamine can facilitate movement by binding to D1Rs and enhancing the output of dMSNs, or binding to D2Rs and inhibiting the output of iMSNs (Sano et al., 2003; Buch et al., 2005; Durieux et al., 2009; Kravitz et al., 2010). In this way, dopaminergic signaling controls the downstream signaling of dMSNs and iMSNs, and resulting motor output. We have simplified this discussion for the purposes of this review, but striatal function is also influenced by several additional layers of complexity (Mink, 1996; Calabresi et al., 2014). For example, the dorsal striatum is commonly linked to motor control, while the ventral striatum is linked to motivation and effortful movement (Mogenson et al., 1980; Voorn et al., 2004; Kreitzer and Malenka, 2008).
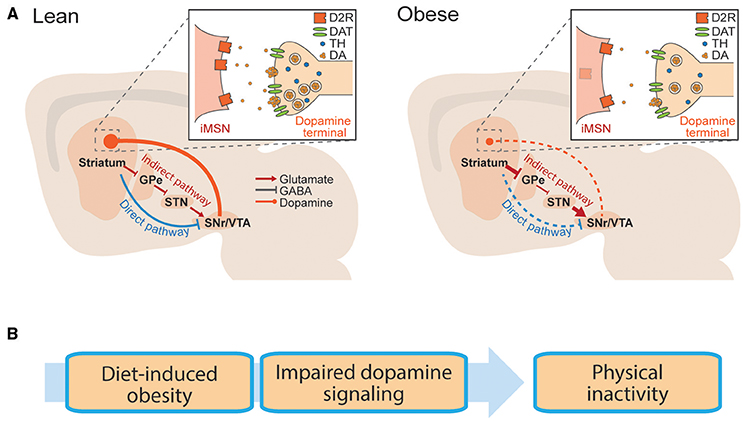
Figure 1. Basal ganglia circuitry in lean and obese conditions. (A) Striatal neurons send projections to the midbrain via the direct pathway or indirect pathway. Schematic is replicated in lean (left) and obese (right) conditions, to show reported dopaminergic alterations in obesity. Inlay: Dopaminergic synapse onto striatal iMSNs. GPe, globus pallidus; STN, subthalamic nucleus; SNr, substantia nigra; VTA, ventral tegmental area. (B) Hypothetical progression by which diet induced obesity is associated with impaired striatal dopamine transmission, leading to physical inactivity.
The importance of dopamine for proper control of movement is evident in neurological disorders. Hypokinetic states such as Parkinson's disease are the result of too little striatal dopamine (Hornykiewicz, 2010), whereas hyperactive states such as bipolar mania are associated with too much (Logan and McClung, 2016). Drugs that increase dopamine release (e.g., amphetamine) increase motor output (Schindler and Carmona, 2002) and dopamine antagonists (used clinically to reduce manic episodes) often result in motor impairments as a side effect (Janno et al., 2004; Parksepp et al., 2016). Genetic manipulations in animals further support the role of striatal dopamine transmission in motor control, as mice lacking dopamine receptors have reduced movement (Drago et al., 1994; Xu et al., 1994; Baik et al., 1995; Kelly et al., 1997; Beeler et al., 2016), whereas those that overexpress dopamine receptors are hyperactive (Ikari et al., 1995; Ingram et al., 1998; Dracheva et al., 1999; Thanos et al., 2001; Trifilieff et al., 2013). In particular, cell-type specific reductions of the D2R in iMSNs reduce open field movement, demonstrating the sufficiency of the D2R to regulate physical activity, by controlling the output of iMSNs (Anzalone et al., 2012; Lemos et al., 2016). In summary, striatal dopamine promotes movement in animals, due to actions on its striatal target neurons.
Obesity is associated with impairments in striatal dopamine function. Reported impairments include deficiencies in dopamine synthesis and release, as well as alterations in striatal dopamine receptors. While alterations in striatal DA transmission are commonly discussed in relation to reward processing (Kenny et al., 2013; Volkow et al., 2013), we hypothesize that these impairments may also contribute to the link between obesity and physical inactivity (Figure 1B).
Obesity and Physical Inactivity
An inverse relationship between weight gain and physical activity has been observed in humans (Hemmingsson and Ekelund, 2007; Chaput et al., 2012; Hjorth et al., 2014), non-human primates (Wolden-Hanson et al., 1993), domesticated animals (Morrison et al., 2013), and rodents (Jürgens et al., 2006; Bjursell et al., 2008). The cross-species nature of this relationship indicates that it is a conserved phenomenon that may stem from the evolutionary benefit of storing energy in times of caloric excess, a state that is rare in nature. However, in modern environments physical inactivity exacerbates the negative health effects of obesity, increasing the risk of cardiac disease and diabetes (Al Tunaiji et al., 2014; Bao et al., 2014; Bouchard et al., 2015). It is possible that physical inactivity precedes, and thereby contributes to, weight gain (Jürgens et al., 2006; Haskell et al., 2009). Indeed animals with high levels of spontaneous physical activity are partially protected against diet-induced obesity (Teske et al., 2012; Zhang et al., 2012). While pre-existing differences in activity levels may contribute to the relationship between obesity and physical inactivity, at a cellular level it remains unclear why people with obesity are inactive.
Part of the difficulty in understanding this relationship stems from the multifaceted nature of the two variables. For instance, the weight of excess adiposity restricts joint and muscle mobility and increases joint pain, which may make it more difficult for people to move (Belczak et al., 2014; Muramoto et al., 2014). However, weight alone does not appear sufficient to explain physical inactivity in people with obesity. Several researchers have tracked physical activity levels across periods of weight loss, to see whether physical activity levels increase as people lose weight, and experience fewer mobility-restricting effects of excess adiposity. Surprisingly, weight loss is generally associated with decreases, and not increases, in physical activity (de Boer et al., 1986; de Groot et al., 1989; Martin et al., 2007; Redman et al., 2009). These results have been explained in terms of metabolic adaptations, as the body seeks to reduce energy expenditure to compensate for the caloric deficit induced by the diet. However, when subjects were tracked during maintained periods of weight loss lasting a year, physical activity levels still did not increase above pre-diet obese levels (Camps et al., 2013). Similar results have been reported following gastric bypass surgery. Despite large amounts of weight loss (>30 kg), objectively measured physical activity levels did not increase in patients that received gastric bypass surgery, even up to 12 months after the peak of the weight loss (Bond et al., 2010; Ramirez-Marrero et al., 2014; Berglind et al., 2015, 2016). Studies in animals also support these conclusions, as loss of adiposity is again associated with decreases, and not increases, in physical activity (Sullivan and Cameron, 2010; Morrison et al., 2014; Vitger et al., 2016). We conclude that the weight of excess adiposity does not sufficiently explain the association between obesity and physical inactivity. Rather, the evidence suggests that obesity-induced adaptations continue to contribute to physical inactivity, even after weight loss. While these adaptations may include chronic mobility issues in joints or muscles, we hypothesize that motor circuitry in the brain is also a large contributor. Specifically, we hypothesize that deficits in striatal dopaminergic signaling contribute to the persistent reductions in physical activity in obesity.
Further supporting the conclusion that the weight of adiposity does not adequately explain physical inactivity in obesity, not all groups of obese animals, or people with obesity, have low levels of physical activity. Even in studies that report deficits in striatal dopamine, physical activity levels can remain unaltered (Davis et al., 2008). Similar findings have been reported under controlled conditions in humans as well. In an 8-week study in which subjects were over-fed by 1000 calories per day, subjects significantly increased their spontaneous physical activity, despite gaining an average of 4.7 kg. The authors linked this increase to a mechanism for dissipating excess energy to preserve body weight (Levine et al., 1999). A similar increase in physical activity was reported in an 8-week over-eating study, despite an average weight gain of 5.3 kg (Apolzan et al., 2014). While physical inactivity is a correlate of obesity in large populations, there is considerable variability on this point among individuals. This variability may be another avenue for unraveling the cellular underpinnings of the relationship between physical activity and obesity.
Obesity and Disruptions in Dopamine Production and Release
A wealth of animal research has described alterations in the dopamine system in obesity. The majority of studies in obese rodents have focused on dopamine transmission in the nucleus accumbens (NAc), which resides in the ventral striatum and is involved in effortful movement (Salamone et al., 2007; Schmidt et al., 2012). Based on this role, the NAc may be particularly important for explaining the lack of vigorous physical activity in obesity (Ekkekakis et al., 2016). Long-term ad libitum high-fat diet decreased tonic dopamine in the NAc of mice (Carlin et al., 2013) as well as dopamine turnover in the NAc of rats (Davis et al., 2008). This specific deficit was distinct from adiposity, as rats that were fed an iso-caloric amount of high-fat diet also had decreased dopamine turnover (Davis et al., 2008). Whereas both chow and high-fat diet increased phasic dopamine in the NAc of lean rats, obese rats had a blunted response to these diets (Geiger et al., 2009). Chronic exposure may be necessary for deficits in phasic dopamine signaling, as they are seen following 6, but not 2, weeks of high-fat diet (Cone et al., 2013). Similar to differences observed in phasic dopamine release in the NAc of obese animals, rats that were bred to be prone to weight gain had reduced dopaminergic responses to both chow (Geiger et al., 2008) and high-fat diet (Rada et al., 2010).
The above deficits in dopamine release may be explained by alterations in genes involved in the synthesis and metabolism of dopamine. Midbrain dopamine regions including the substantia nigra and the ventral tegmental area (VTA) provide the main dopaminergic innervation to the striatum (Figure 1). Expression of tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis, is reduced in the VTA of mice fed a high-fat diet (Vucetic et al., 2012; Carlin et al., 2013). Again, this did not depend on fat storage, as similar effects were observed in mice that were pair-fed a high fat diet (Li et al., 2009). The effect of high-fat diet on co-acetyl methyl transferase (COMT), a key enzyme responsible for the degradation of dopamine is less clear, with studies reporting either decreased (Carlin et al., 2013) or unchanged (Alsio et al., 2010; Vucetic et al., 2012) expression following diet-induced obesity. Interestingly, in humans, polymorphisms that confer low activity of monoamine-oxidases (the other main enzyme responsible for degrading dopamine) have been linked to obesity (Camarena et al., 2004; Ducci et al., 2006; Need et al., 2006). Overall, the evidence supports two conclusions: (1) exposure to high-fat diets can impair dopamine synthesis and striatal dopamine release and processing, but (2) heterogeneity exists among these reports, indicating that the impact of high-fat diets on the dopamine system is complex and may occur differently among different individuals.
Obesity and Dysfunction of Dopamine Receptors
Multiple researchers have observed alterations in dopamine receptors in people with obesity. Individuals with at least one copy of the drd2 Taq1A allele have reduced brain D2R availability of ~30–40% (Noble et al., 1991; Thompson et al., 1997) and an increased prevalence of obesity (Blum et al., 1996; Stice et al., 2008, 2010; Davis et al., 2009; Carpenter et al., 2013). An inverse relationship between obesity and D2R availability, assayed via positron emission tomography (PET), has also been reported in humans. This was first reported by Wang et al. (2001) and was initially supported by others (Volkow et al., 2008; de Weijer et al., 2011; Kessler et al., 2014; van de Giessen et al., 2014). However, several other groups have failed to replicate this finding (Dunn et al., 2012; Caravaggio et al., 2015; Cosgrove et al., 2015; Karlsson et al., 2015, 2016; Tuominen et al., 2015), or found opposing associations in different regions of the striatum (Guo et al., 2014). Interestingly, Guo and colleagues noted a negative relationship between body mass index (BMI) and D2R binding only in the ventral striatum, which may be linked to effortful movements (Salamone et al., 2007; Schmidt et al., 2012). Several possibilities may account for the discrepancy among studies of D2R binding and BMI. Different D2R radio-ligands were used among these studies, which may bind differentially to D2R or D3Rs (Gaiser et al., 2016). Changes in striatal dopamine tone could impact binding potential (Horstmann et al., 2015). Finally, experimental factors including the amount of time after meal consumption or individual variability among subjects may contribute to observed differences (Small et al., 2003).
Animal studies have more consistently linked impairments in D2Rs to obesity, via analysis of mRNA (Mathes et al., 2010; Zhang et al., 2015), protein (Johnson and Kenny, 2010; Adams et al., 2015), and receptor binding (Huang et al., 2006; Hajnal et al., 2008; Thanos et al., 2008; Michaelides et al., 2012; van de Giessen et al., 2012, 2013; Narayanaswami et al., 2013). Interestingly, rats maintained on an iso-caloric high-fat (but not high-sugar) diet also had lower levels of D2Rs in ventral (but not dorsal) striatum (Adams et al., 2015), supporting the conclusion that exposure to high-fat diet may be a better predictor of dopaminergic dysfunction than weight gain itself (van de Giessen et al., 2013). To date, no published work has examined associations between D1-type dopamine receptors (D1Rs) and obesity in humans, so an evaluation of potential changes here is limited to a small number of animal studies. D1R mRNA was decreased in obese rats relative to lean controls (Vucetic et al., 2012; Zhang et al., 2015), while another study reported a decrease in D1Rs only in female rats (Ong et al., 2013). We conclude that reduced function of D2Rs appears to be a particularly important alteration in obesity, although there is considerable variability in D2R alterations among studies and individuals. Unfortunately, studies of the D1R are too sparse to make strong conclusions about its relationship to obesity.
Do Alterations in Dopamine Function Recover with Weight Loss?
It is unclear whether changes in dopamine signaling in people with obesity persist after weight loss. The few studies that exist on this topic point to dopaminergic alterations being at least partly resistant to change, and at times even exacerbated by weight loss. High-fat diet reduced the levels of several enzymes involved in dopamine production in the VTA and NAc, and switching these obese mice to low-fat chow caused even further decreases in these enzymes (Carlin et al., 2013; Sharma et al., 2013). Two PET imaging studies reported a lack of recovery of D2R binding following Roux-en-Y gastric bypass surgery (RYGB) in humans, with one showing an even further decrease in binding (Dunn et al., 2010; de Weijer et al., 2014). A small study of five women reported a partial recovery of D2R binding 6-weeks after RYGB (Steele et al., 2010). An increase in D2R binding was also reported during food restriction and associated weight alterations in obese rats (Thanos et al., 2008). Although the data on this topic are limited, it appears that diet-induced changes in dopamine function are at least partly persistent following weight loss. Consistent with this conclusion, physical activity levels remain low in people with obesity, even months after the peak of weight loss (Bond et al., 2010; Camps et al., 2013; Ramirez-Marrero et al., 2014; Berglind et al., 2015, 2016). Again, the small number of studies of this topic precludes firm conclusions, and underscores the need for further research on the persistence of dopaminergic alterations in people with obesity.
Obesity and Physical Inactivity: Conclusions
Chronic exposure to obesogenic diets is associated with changes in both physical activity levels and dopaminergic function. Diet-induced changes in the dopamine system may be sufficient to explain the development of physical inactivity in people with obesity. Increased understanding of obesity-related changes in dopamine and related systems may support evidence-based approaches for increasing physical activity in people with obesity. In addition, such an understanding may reveal genetic or environmental contributions to dopaminergic dysfunction, and physical inactivity, in obesity.
Author Contributions
AK, TO, and DF conceived of the idea and wrote and edited this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by the NIH Intramural research program. We thank Kavya Devarakonda for comments on this manuscript.
References
Adams, W. K., Sussman, J. L., Kaur, S., D'Souza, A. M., Kieffer, T. J., and Winstanley, C. A. (2015). Long-term, calorie-restricted intake of a high-fat diet in rats reduces impulse control and ventral striatal D2 receptor signalling - two markers of addiction vulnerability. Eur. J. Neurosci. 42, 3095–3104. doi: 10.1111/ejn.13117
Alexander, G. E., and Crutcher, M. D. (1990). Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271. doi: 10.1016/0166-2236(90)90107-L
Alsiö, J., Olszewski, P. K., Norbäck, A. H., Gunnarsson, Z. E., Levine, A. S., Pickering, C., et al. (2010). Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience 171, 779–787. doi: 10.1016/j.neuroscience.2010.09.046
Al Tunaiji, H., Davis, J. C., Mackey, D. C., and Khan, K. M. (2014). Population attributable fraction of type 2 diabetes due to physical inactivity in adults: a systematic review. BMC Public Health 14:469. doi: 10.1186/1471-2458-14-469
Anzalone, A., Lizardi-Ortiz, J. E., Ramos, M., De Mei, C., Hopf, F. W., Iaccarino, C., et al. (2012). Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J. Neurosci. 32, 9023–9034. doi: 10.1523/JNEUROSCI.0918-12.2012
Apolzan, J. W., Bray, G. A., Smith, S. R., de Jonge, L., Rood, J., Han, H., et al. (2014). Effects of weight gain induced by controlled overfeeding on physical activity. Am. J. Physiol. Endocrinol. Metab. 307, E1030–E1037. doi: 10.1152/ajpendo.00386.2014
Baik, J. H., Picetti, R., Saiardi, A., Thiriet, G., Dierich, A., Depaulis, A., et al. (1995). Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377, 424–428. doi: 10.1038/377424a0
Bao, W., Tobias, D. K., Bowers, K., Chavarro, J., Vaag, A., Grunnet, L. G., et al. (2014). Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: a prospective cohort study. JAMA Intern. Med. 174, 1047–1055. doi: 10.1001/jamainternmed.2014.1795
Beeler, J. A., Faust, R. P., Turkson, S., Ye, H., and Zhuang, X. (2016). Low dopamine D2 receptor increases vulnerability to obesity via reduced physical activity not increased appetitive motivation. Biol. Psychiatry 79, 887–897. doi: 10.1016/j.biopsych.2015.07.009
Belczak, C. E., de Godoy, J. M., Belzack, S. Q., Ramos, R. N., and Caffaro, R. A. (2014). Obesity and worsening of chronic venous disease and joint mobility. Phlebology 29, 500–504. doi: 10.1177/0268355513492510
Berglind, D., Willmer, M., Eriksson, U., Thorell, A., Sundbom, M., Uddén, J., et al. (2015). Longitudinal assessment of physical activity in women undergoing Roux-en-Y gastric bypass. Obes. Surg. 25, 119–125. doi: 10.1007/s11695-014-1331-x
Berglind, D., Willmer, M., Tynelius, P., Ghaderi, A., Näslund, E., and Rasmussen, F. (2016). Accelerometer-measured versus self-reported physical activity levels and sedentary behavior in women before and 9 months after Roux-en-Y gastric bypass. Obes. Surg. 26, 1463–1470. doi: 10.1007/s11695-015-1971-5
Bjursell, M., Gerdin, A. K., Lelliott, C. J., Egecioglu, E., Elmgren, A., Törnell, J., et al. (2008). Acutely reduced locomotor activity is a major contributor to Western diet-induced obesity in mice. Am. J. Physiol. Endocrinol. Metab. 294, E251–E260. doi: 10.1152/ajpendo.00401.2007
Blum, K., Braverman, E. R., Wood, R. C., Gill, J., Li, C., Chen, T. J., et al. (1996). Increased prevalence of the Taq I A1 allele of the dopamine receptor gene (DRD2) in obesity with comorbid substance use disorder: a preliminary report. Pharmacogenetics 6, 297–305. doi: 10.1097/00008571-199608000-00003
Bond, D. S., Jakicic, J. M., Unick, J. L., Vithiananthan, S., Pohl, D., Roye, G. D., et al. (2010). Pre- to postoperative physical activity changes in bariatric surgery patients: self report vs. objective measures. Obesity 18, 2395–2397. doi: 10.1038/oby.2010.88
Bouchard, C., Blair, S. N., and Katzmarzyk, P. T. (2015). Less sitting, more physical activity, or higher fitness? Mayo Clin. Proc. 90, 1533–1540. doi: 10.1016/j.mayocp.2015.08.005
Buch, T., Heppner, F. L., Tertilt, C., Heinen, T. J., Kremer, M., Wunderlich, F. T., et al. (2005). A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat. Methods 2, 419–426. doi: 10.1038/nmeth762
Calabresi, P., Picconi, B., Tozzi, A., Ghiglieri, V., and Di Filippo, M. (2014). Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat. Neurosci. 17, 1022–1030. doi: 10.1038/nn.3743
Camarena, B., Santiago, H., Aguilar, A., Ruvinskis, E., González-Barranco, J., and Nicolini, H. (2004). Family-based association study between the monoamine oxidase A gene and obesity: implications for psychopharmacogenetic studies. Neuropsychobiology 49, 126–129. doi: 10.1159/000076720
Camps, S. G., Verhoef, S. P., and Westerterp, K. R. (2013). Weight loss-induced reduction in physical activity recovers during weight maintenance. Am. J. Clin. Nutr. 98, 917–923. doi: 10.3945/ajcn.113.062935
Caravaggio, F., Raitsin, S., Gerretsen, P., Nakajima, S., Wilson, A., and Graff-Guerrero, A. (2015). Ventral striatum binding of a dopamine D2/3 receptor agonist but not antagonist predicts normal body mass index. Biol. Psychiatry 77, 196–202. doi: 10.1016/j.biopsych.2013.02.017
Carlin, J., Hill-Smith, T. E., Lucki, I., and Reyes, T. M. (2013). Reversal of dopamine system dysfunction in response to high-fat diet. Obesity 21, 2513–2521. doi: 10.1002/oby.20374
Carpenter, C. L., Wong, A. M., Li, Z., Noble, E. P., and Heber, D. (2013). Association of dopamine D2 receptor and leptin receptor genes with clinically severe obesity. Obesity 21, E467–E473. doi: 10.1002/oby.20202
Chaput, J. P., Lambert, M., Mathieu, M. E., Tremblay, M. S., O'Loughlin, J., and Tremblay, A. (2012). Physical activity vs. sedentary time: independent associations with adiposity in children. Pediatr. Obes. 7, 251–258. doi: 10.1111/j.2047-6310.2011.00028.x
Cone, J. J., Chartoff, E. H., Potter, D. N., Ebner, S. R., and Roitman, M. F. (2013). Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS ONE 8:e58251. doi: 10.1371/journal.pone.0058251
Cosgrove, K. P., Veldhuizen, M. G., Sandiego, C. M., Morris, E. D., and Small, D. M. (2015). Opposing relationships of BMI with BOLD and dopamine D2/3 receptor binding potential in the dorsal striatum. Synapse 69, 195–202. doi: 10.1002/syn.21809
Davis, C. A., Levitan, R. D., Reid, C., Carter, J. C., Kaplan, A. S., Patte, K. A., et al. (2009). Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity 17, 1220–1225. doi: 10.1038/oby.2009.52
Davis, J. F., Tracy, A. L., Schurdak, J. D., Tschöp, M. H., Lipton, J. W., Clegg, D. J., et al. (2008). Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav. Neurosci. 122, 1257–1263. doi: 10.1037/a0013111
de Boer, J. O., van Es, A. J., Roovers, L. C., van Raaij, J. M., and Hautvast, J. G. (1986). Adaptation of energy metabolism of overweight women to low-energy intake, studied with whole-body calorimeters. Am. J. Clin. Nutr. 44, 585–595.
de Groot, L. C., van Es, A. J., van Raaij, J. M., Vogt, J. E., and Hautvast, J. G. (1989). Adaptation of energy metabolism of overweight women to alternating and continuous low energy intake. Am. J. Clin. Nutr. 50, 1314–1323.
DeLong, M. R. (1990). Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285. doi: 10.1016/0166-2236(90)90110-V
de Weijer, B. A., van de Giessen, E., Janssen, I., Berends, F. J., van de Laar, A., Ackermans, M. T., et al. (2014). Striatal dopamine receptor binding in morbidly obese women before and after gastric bypass surgery and its relationship with insulin sensitivity. Diabetologia 57, 1078–1080. doi: 10.1007/s00125-014-3178-z
de Weijer, B. A., van de Giessen, E., van Amelsvoort, T. A., Boot, E., Braak, B., Janssen, I. M., et al. (2011). Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 1:37. doi: 10.1186/2191-219x-1-37
Dracheva, S., Xu, M., Kelley, K. A., Haroutunian, V., Holstein, G. R., Haun, S., et al. (1999). Paradoxical locomotor behavior of dopamine D1 receptor transgenic mice. Exp. Neurol. 157, 169–179. doi: 10.1006/exnr.1999.7037
Drago, J., Gerfen, C. R., Lachowicz, J. E., Steiner, H., Hollon, T. R., Love, P. E., et al. (1994). Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc. Natl. Acad. Sci. U.S.A. 91, 12564–12568. doi: 10.1073/pnas.91.26.12564
Ducci, F., Newman, T. K., Funt, S., Brown, G. L., Virkkunen, M., and Goldman, D. (2006). A functional polymorphism in the MAOA gene promoter (MAOA-LPR) predicts central dopamine function and body mass index. Mol. Psychiatry 11, 858–866. doi: 10.1038/sj.mp.4001856
Dunn, J. P., Cowan, R. L., Volkow, N. D., Feurer, I. D., Li, R., Williams, D. B., et al. (2010). Decreased dopamine type 2 receptor availability after bariatric surgery: preliminary findings. Brain Res. 1350, 123–130. doi: 10.1016/j.brainres.2010.03.064
Dunn, J. P., Kessler, R. M., Feurer, I. D., Volkow, N. D., Patterson, B. W., Ansari, M. S., et al. (2012). Relationship of dopamine type 2 receptor binding potential with fasting neuroendocrine hormones and insulin sensitivity in human obesity. Diabetes Care 35, 1105–1111. doi: 10.2337/dc11-2250
Durieux, P. F., Bearzatto, B., Guiducci, S., Buch, T., Waisman, A., Zoli, M., et al. (2009). D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat. Neurosci. 12, 393–395. doi: 10.1038/nn.2286
Dwyer-Lindgren, L., Freedman, G., Engell, R. E., Fleming, T. D., Lim, S. S., Murray, C. J., et al. (2013). Prevalence of physical activity and obesity in US counties, 2001–2011: a road map for action. Popul. Health Metr. 11:7. doi: 10.1186/1478-7954-11-7
Ekkekakis, P., Vazou, S., Bixby, W. R., and Georgiadis, E. (2016). The mysterious case of the public health guideline that is (almost) entirely ignored: call for a research agenda on the causes of the extreme avoidance of physical activity in obesity. Obes. Rev. 17, 313–329. doi: 10.1111/obr.12369
Gaiser, E. C., Gallezot, J. D., Worhunsky, P. D., Jastreboff, A. M., Pittman, B., Kantrovitz, L., et al. (2016). Elevated Dopamine D2/3 Receptor Availability in Obese Individuals: a PET Imaging Study with [11C](+)PHNO. Neuropsychopharmacology. doi: 10.1038/npp.2016.115. [Epub ahead of print].
Geiger, B. M., Behr, G. G., Frank, L. E., Caldera-Siu, A. D., Beinfeld, M. C., Kokkotou, E. G., et al. (2008). Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 22, 2740–2746. doi: 10.1096/fj.08-110759
Geiger, B. M., Haburcak, M., Avena, N. M., Moyer, M. C., Hoebel, B. G., and Pothos, E. N. (2009). Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 159, 1193–1199. doi: 10.1016/j.neuroscience.2009.02.007
Gerfen, C. R., Engber, T. M., Mahan, L. C., Susel, Z., Chase, T. N., Monsma, F. J. Jr., et al. (1990). D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432. doi: 10.1126/science.2147780
Graybiel, A. M., Aosaki, T., Flaherty, A. W., and Kimura, M. (1994). The basal ganglia and adaptive motor control. Science 265, 1826–1831. doi: 10.1126/science.8091209
Guo, J., Simmons, W. K., Herscovitch, P., Martin, A., and Hall, K. D. (2014). Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol. Psychiatry 19, 1078–1084. doi: 10.1038/mp.2014.102
Hajnal, A., Margas, W. M., and Covasa, M. (2008). Altered dopamine D2 receptor function and binding in obese OLETF rat. Brain Res. Bull. 75, 70–76. doi: 10.1016/j.brainresbull.2007.07.019
Haskell, W. L., Blair, S. N., and Hill, J. O. (2009). Physical activity: health outcomes and importance for public health policy. Prev. Med. 49, 280–282. doi: 10.1016/j.ypmed.2009.05.002
Hemmingsson, E., and Ekelund, U. (2007). Is the association between physical activity and body mass index obesity dependent? Int. J. Obes. 31, 663–668. doi: 10.1038/sj.ijo.0803458
Hjorth, M. F., Chaput, J. P., Ritz, C., Dalskov, S. M., Andersen, R., Astrup, A., et al. (2014). Fatness predicts decreased physical activity and increased sedentary time, but not vice versa: support from a longitudinal study in 8- to 11-year-old children. Int. J. Obes. 38, 959–965. doi: 10.1038/ijo.2013.229
Hornykiewicz, O. (2010). A Brief history of levodopa. J. Neurol. 257, S249–S252. doi: 10.1007/s00415-010-5741-y
Horstmann, A., Fenske, W. K., and Hankir, M. K. (2015). Argument for a non-linear relationship between severity of human obesity and dopaminergic tone. Obes. Rev. 16, 821–830. doi: 10.1111/obr.12303
Huang, X. F., Zavitsanou, K., Huang, X., Yu, Y., Wang, H., Chen, F., et al. (2006). Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav. Brain Res. 175, 415–419. doi: 10.1016/j.bbr.2006.08.034
Ikari, H., Zhang, L., Chernak, J. M., Mastrangeli, A., Kato, S., Kuo, H., et al. (1995). Adenovirus-mediated gene transfer of dopamine D2 receptor cDNA into rat striatum. Brain Res. Mol. Brain Res. 34, 315–320. doi: 10.1016/0169-328X(95)00185-U
Ingram, D. K., Ikari, H., Umegaki, H., Chernak, J. M., and Roth, G. S. (1998). Application of gene therapy to treat age-related loss of dopamine D2 receptor. Exp. Gerontol. 33, 793–804. doi: 10.1016/S0531-5565(98)00043-6
Janno, S., Holi, M., Tuisku, K., and Wahlbeck, K. (2004). Prevalence of neuroleptic-induced movement disorders in chronic schizophrenia inpatients. Am. J. Psychiatry 161, 160–163. doi: 10.1176/appi.ajp.161.1.160
Johnson, P. M., and Kenny, P. J. (2010). Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 13, 635–641. doi: 10.1038/nn.2519
Jürgens, H. S., Schürmann, A., Kluge, R., Ortmann, S., Klaus, S., Joost, H. G., et al. (2006). Hyperphagia, lower body temperature, and reduced running wheel activity precede development of morbid obesity in New Zealand obese mice. Physiol. Genomics 25, 234–241. doi: 10.1152/physiolgenomics.00252.2005
Karlsson, H. K., Tuominen, L., Tuulari, J. J., Hirvonen, J., Parkkola, R., Helin, S., et al. (2015). Obesity is associated with decreased mu-opioid but unaltered dopamine D2 receptor availability in the brain. J. Neurosci. 35, 3959–3965. doi: 10.1523/JNEUROSCI.4744-14.2015
Karlsson, H. K., Tuulari, J. J., Tuominen, L., Hirvonen, J., Honka, H., Parkkola, R., et al. (2016). Weight loss after bariatric surgery normalizes brain opioid receptors in morbid obesity. Mol. Psychiatry. 21, 1057–1062. doi: 10.1038/mp.2015.153
Kelly, M. A., Rubinstein, M., Asa, S. L., Zhang, G., Saez, C., Bunzow, J. R., et al. (1997). Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19, 103–113. doi: 10.1016/S0896-6273(00)80351-7
Kenny, P. J., Voren, G., and Johnson, P. M. (2013). Dopamine D2 receptors and striatopallidal transmission in addiction and obesity. Curr. Opin. Neurobiol. 23, 535–538. doi: 10.1016/j.conb.2013.04.012
Kessler, R. M., Zald, D. H., Ansari, M. S., Li, R., and Cowan, R. L. (2014). Changes in dopamine release and dopamine D2/3 receptor levels with the development of mild obesity. Synapse 68, 317–320. doi: 10.1002/syn.21738
Kravitz, A. V., Freeze, B. S., Parker, P. R., Kay, K., Thwin, M. T., Deisseroth, K., et al. (2010). Regulation of Parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626. doi: 10.1038/nature09159
Kreitzer, A. C., and Malenka, R. C. (2008). Striatal plasticity and basal ganglia circuit function. Neuron 60, 543–554. doi: 10.1016/j.neuron.2008.11.005
Le Moine, C., and Bloch, B. (1995). D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J. Comp. Neurol. 355, 418–426. doi: 10.1002/cne.903550308
Lemos, J. C., Friend, D. M., Kaplan, A. R., Shin, J. H., Rubinstein, M., Kravitz, A. V., et al. (2016). Enhanced gaba transmission drives bradykinesia following loss of dopamine D2 receptor signaling. Neuron 90, 824–838. doi: 10.1016/j.neuron.2016.04.040
Levine, J. A., Eberhardt, N. L., and Jensen, M. D. (1999). Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 283, 212–214. doi: 10.1126/science.283.5399.212
Li, Y., South, T., Han, M., Chen, J., Wang, R., and Huang, X. F. (2009). High-fat diet decreases tyrosine hydroxylase mRNA expression irrespective of obesity susceptibility in mice. Brain Res. 1268, 181–189. doi: 10.1016/j.brainres.2009.02.075
Logan, R. W., and McClung, C. A. (2016). Animal models of bipolar mania: the past, present and future. Neuroscience 321, 163–188. doi: 10.1016/j.neuroscience.2015.08.041
Martin, C. K., Heilbronn, L. K., de Jonge, L., DeLany, J. P., Volaufova, J., Anton, S. D., et al. (2007). Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity 15, 2964–2973. doi: 10.1038/oby.2007.354
Mathes, W. F., Nehrenberg, D. L., Gordon, R., Hua, K., Garland, T. Jr., and Pomp, D. (2010). Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behav. Brain Res. 210, 155–163. doi: 10.1016/j.bbr.2010.02.016
Michaelides, M., Thanos, P. K., Kim, R., Cho, J., Ananth, M., Wang, G. J., et al. (2012). PET imaging predicts future body weight and cocaine preference. Neuroimage 59, 1508–1513. doi: 10.1016/j.neuroimage.2011.08.028
Mink, J. W. (1996). The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol. 50, 381–425. doi: 10.1016/S0301-0082(96)00042-1
Mogenson, G. J., Jones, D. L., and Yim, C. Y. (1980). From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol. 14, 69–97. doi: 10.1016/0301-0082(80)90018-0
Morrison, R., Penpraze, V., Beber, A., Reilly, J. J., and Yam, P. S. (2013). Associations between obesity and physical activity in dogs: a preliminary investigation. J. Small Anim. Pract. 54, 570–574. doi: 10.1111/jsap.12142
Morrison, R., Reilly, J. J., Penpraze, V., Pendlebury, E., and Yam, P. S. (2014). A 6-month observational study of changes in objectively measured physical activity during weight loss in dogs. J. Small Anim. Pract. 55, 566–570. doi: 10.1111/jsap.12273
Muramoto, A., Imagama, S., Ito, Z., Hirano, K., Tauchi, R., Ishiguro, N., et al. (2014). Waist circumference is associated with locomotive syndrome in elderly females. J. Orthop. Sci. 19, 612–619. doi: 10.1007/s00776-014-0559-6
Narayanaswami, V., Thompson, A. C., Cassis, L. A., Bardo, M. T., and Dwoskin, L. P. (2013). Diet-induced obesity: dopamine transporter function, impulsivity and motivation. Int. J. Obes. 37, 1095–1103. doi: 10.1038/ijo.2012.178
Need, A. C., Ahmadi, K. R., Spector, T. D., and Goldstein, D. B. (2006). Obesity is associated with genetic variants that alter dopamine availability. Ann. Hum. Genet. 70, 293–303. doi: 10.1111/j.1529-8817.2005.00228.x
Noble, E. P., Blum, K., Ritchie, T., Montgomery, A., and Sheridan, P. J. (1991). Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch. Gen. Psychiatry 48, 648–654. doi: 10.1001/archpsyc.1991.01810310066012
Obeso, J. A., Rodríguez-Oroz, M. C., Rodríguez, M., Lanciego, J. L., Artieda, J., Gonzalo, N., et al. (2000). Pathophysiology of the basal ganglia in Parkinson's disease. Trends Neurosci. 23, S8–S19. doi: 10.1016/s1471-1931(00)00028-8
Ong, Z. Y., Wanasuria, A. F., Lin, M. Z., Hiscock, J., and Muhlhausler, B. S. (2013). Chronic intake of a cafeteria diet and subsequent abstinence. Sex-specific effects on gene expression in the mesolimbic reward system. Appetite 65, 189–199. doi: 10.1016/j.appet.2013.01.014
Parksepp, M., Ljubajev, Ü., Täht, K., and Janno, S. (2016). Prevalence of neuroleptic-induced movement disorders: an 8-year follow-up study in chronic schizophrenia inpatients. Nord. J. Psychiatry 70, 498–502. doi: 10.3109/08039488.2016.1164245
Rada, P., Bocarsly, M. E., Barson, J. R., Hoebel, B. G., and Leibowitz, S. F. (2010). Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiol. Behav. 101, 394–400. doi: 10.1016/j.physbeh.2010.07.005
Ramirez-Marrero, F. A., Miles, J., Joyner, M. J., and Curry, T. B. (2014). Self-reported and objective physical activity in postgastric bypass surgery, obese and lean adults: association with body composition and cardiorespiratory fitness. J. Phys. Act. Health 11, 145–151. doi: 10.1123/jpah.2012-0048
Redman, L. M., Heilbronn, L. K., Martin, C. K., de Jonge, L., Williamson, D. A., Delany, J. P., et al. (2009). Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS ONE 4:e4377. doi: 10.1371/journal.pone.0004377
Salamone, J. D., Correa, M., Farrar, A., and Mingote, S. M. (2007). Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 191, 461–482. doi: 10.1007/s00213-006-0668-9
Sano, H., Yasoshima, Y., Matsushita, N., Kaneko, T., Kohno, K., Pastan, I., et al. (2003). Conditional ablation of striatal neuronal types containing dopamine D2 receptor disturbs coordination of basal ganglia function. J. Neurosci. 23, 9078–9088. Available online at: http://www.jneurosci.org/content/23/27/9078.long
Schindler, C. W., and Carmona, G. N. (2002). Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol. Biochem. Behav. 72, 857–863. doi: 10.1016/S0091-3057(02)00770-0
Schmidt, L., Lebreton, M., Cléry-Melin, M. L., Daunizeau, J., and Pessiglione, M. (2012). Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biol. 10:e1001266. doi: 10.1371/journal.pbio.1001266
Sharma, S., Fernandes, M. F., and Fulton, S. (2013). Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int. J. Obes. 37, 1183–1191. doi: 10.1038/ijo.2012.197
Simon, C., Schweitzer, B., Oujaa, M., Wagner, A., Arveiler, D., Triby, E., et al. (2008). Successful overweight prevention in adolescents by increasing physical activity: a 4-year randomized controlled intervention. Int. J. Obes. 32, 1489–1498. doi: 10.1038/ijo.2008.99
Small, D. M., Jones-Gotman, M., and Dagher, A. (2003). Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 19, 1709–1715. doi: 10.1016/S1053-8119(03)00253-2
Steele, K. E., Prokopowicz, G. P., Schweitzer, M. A., Magunsuon, T. H., Lidor, A. O., Kuwabawa, H., et al. (2010). Alterations of central dopamine receptors before and after gastric bypass surgery. Obes. Surg. 20, 369–374. doi: 10.1007/s11695-009-0015-4
Stice, E., Spoor, S., Bohon, C., and Small, D. M. (2008). Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322, 449–452. doi: 10.1126/science.1161550
Stice, E., Yokum, S., Bohon, C., Marti, N., and Smolen, A. (2010). Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage 50, 1618–1625. doi: 10.1016/j.neuroimage.2010.01.081
Sullivan, E. L., and Cameron, J. L. (2010). A rapidly occurring compensatory decrease in physical activity counteracts diet-induced weight loss in female monkeys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1068–R1074. doi: 10.1152/ajpregu.00617.2009
Swift, D. L., Johannsen, N. M., Lavie, C. J., Earnest, C. P., and Church, T. S. (2014). The role of exercise and physical activity in weight loss and maintenance. Prog. Cardiovasc. Dis. 56, 441–447. doi: 10.1016/j.pcad.2013.09.012
Teske, J. A., Billington, C. J., Kuskowski, M. A., and Kotz, C. M. (2012). Spontaneous physical activity protects against fat mass gain. Int. J. Obes. 36, 603–613. doi: 10.1038/ijo.2011.108
Thanos, P. K., Michaelides, M., Piyis, Y. K., Wang, G. J., and Volkow, N. D. (2008). Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse 62, 50–61. doi: 10.1002/syn.20468
Thanos, P. K., Volkow, N. D., Freimuth, P., Umegaki, H., Ikari, H., Roth, G., et al. (2001). Overexpression of dopamine D2 receptors reduces alcohol self-administration. J. Neurochem. 78, 1094–1103. doi: 10.1046/j.1471-4159.2001.00492.x
Thompson, J., Thomas, N., Singleton, A., Piggott, M., Lloyd, S., Perry, E. K., et al. (1997). D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics 7, 479–484. doi: 10.1097/00008571-199712000-00006
Trifilieff, P., Feng, B., Urizar, E., Winiger, V., Ward, R. D., Taylor, K. M., et al. (2013). Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol. Psychiatry 18, 1025–1033. doi: 10.1038/mp.2013.57
Tudor-Locke, C., Brashear, M. M., Johnson, W. D., and Katzmarzyk, P. T. (2010). Accelerometer profiles of physical activity and inactivity in normal weight, overweight, and obese U.S. men and women. Int. J. Behav. Nutr. Phys. Act. 7:60. doi: 10.1186/1479-5868-7-60
Tuominen, L., Tuulari, J., Karlsson, H., Hirvonen, J., Helin, S., Salminen, P., et al. (2015). Aberrant mesolimbic dopamine-opiate interaction in obesity. Neuroimage 122, 80–86. doi: 10.1016/j.neuroimage.2015.08.001
van de Giessen, E., Celik, F., Schweitzer, D. H., van den Brink, W., and Booij, J. (2014). Dopamine D2/3 receptor availability and amphetamine-induced dopamine release in obesity. J. Psychopharmacol. 28, 866–873. doi: 10.1177/0269881114531664
van de Giessen, E., la Fleur, S. E., de Bruin, K., van den Brink, W., and Booij, J. (2012). Free-choice and no-choice high-fat diets affect striatal dopamine D2/3 receptor availability, caloric intake, and adiposity. Obesity 20, 1738–1740. doi: 10.1038/oby.2012.17
van de Giessen, E., la Fleur, S. E., Eggels, L., de Bruin, K., van den Brink, W., and Booij, J. (2013). High fat/carbohydrate ratio but not total energy intake induces lower striatal dopamine D2/3 receptor availability in diet-induced obesity. Int. J. Obes. 37, 754–757. doi: 10.1038/ijo.2012.128
Vitger, A. D., Stallknecht, B. M., Nielsen, D. H., and Bjornvad, C. R. (2016). Integration of a physical training program in a weight loss plan for overweight pet dogs. J. Am. Vet. Med. Assoc. 248, 174–182. doi: 10.2460/javma.248.2.174
Volkow, N. D., Wang, G. J., Telang, F., Fowler, J. S., Thanos, P. K., Logan, J., et al. (2008). Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42, 1537–1543. doi: 10.1016/j.neuroimage.2008.06.002
Volkow, N. D., Wang, G. J., Tomasi, D., and Baler, R. D. (2013). The addictive dimensionality of obesity. Biol. Psychiatry 73, 811–818. doi: 10.1016/j.biopsych.2012.12.020
Voorn, P., Vanderschuren, L. J., Groenewegen, H. J., Robbins, T. W., and Pennartz, C. M. (2004). Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 27, 468–474. doi: 10.1016/j.tins.2004.06.006
Vucetic, Z., Carlin, J. L., Totoki, K., and Reyes, T. M. (2012). Epigenetic dysregulation of the dopamine system in diet-induced obesity. J. Neurochem. 120, 891–898. doi: 10.1111/j.1471-4159.2012.07649.x
Wang, G. J., Volkow, N. D., Logan, J., Pappas, N. R., Wong, C. T., Zhu, W., et al. (2001). Brain dopamine and obesity. Lancet 357, 354–357. doi: 10.1016/S0140-6736(00)03643-6
Wolden-Hanson, T., Davis, G. A., Baum, S. T., and Kemnitz, J. W. (1993). Insulin levels, physical activity, and urinary catecholamine excretion of obese and non-obese rhesus monkeys. Obes. Res. 1, 5–17. doi: 10.1002/j.1550-8528.1993.tb00003.x
Xu, M., Moratalla, R., Gold, L. H., Hiroi, N., Koob, G. F., Graybiel, A. M., et al. (1994). Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell 79, 729–742. doi: 10.1016/0092-8674(94)90557-6
Zhang, C., Wei, N. L., Wang, Y., Wang, X., Zhang, J. G., and Zhang, K. (2015). Deep brain stimulation of the nucleus accumbens shell induces anti-obesity effects in obese rats with alteration of dopamine neurotransmission. Neurosci. Lett. 589, 1–6. doi: 10.1016/j.neulet.2015.01.019
Keywords: obesity, dopamine, exercise, physical activity, physical activity promotion, Parkinson's disease, movement disorders
Citation: Kravitz AV, O'Neal TJ and Friend DM (2016) Do Dopaminergic Impairments Underlie Physical Inactivity in People with Obesity? Front. Hum. Neurosci. 10:514. doi: 10.3389/fnhum.2016.00514
Received: 26 July 2016; Accepted: 28 September 2016;
Published: 14 October 2016.
Edited by:
Daniela S. Andres, ETH Zurich, SwitzerlandReviewed by:
Ramalingam Vetrivelan, Harvard Medical School, USAAaron G. Roseberry, Georgia State University, USA
Yinghua Yu, University of Wollongong, Australia
Copyright © 2016 Kravitz, O'Neal and Friend. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexxai V. Kravitz, bGV4LmtyYXZpdHpAbmloLmdvdg==
 Alexxai V. Kravitz
Alexxai V. Kravitz Timothy J. O'Neal
Timothy J. O'Neal Danielle M. Friend1
Danielle M. Friend1