- 1Dipartimento di Medicina dei Sistemi, Clinica Psichiatrica, Università di Roma Tor Vergata, Rome, Italy
- 2Laboratorio di Neurologia Clinica e Comportamentale, Fondazione Santa Lucia IRCCS, Rome, Italy
- 3Temerty Centre for Therapeutic Brain Intervention, Centre for Addiction and Mental Health, University of Toronto, Toronto, ON, Canada
Recently, a growing body of data has revealed that beyond a dysfunction of connectivity among different brain areas in schizophrenia patients (SCZ), there is also an abnormal asymmetry of functional connectivity compared with healthy subjects. The loss of the cerebral torque and the abnormalities of gyrification, with an increased or more complex cortical folding in the right hemisphere may provide an anatomical basis for such aberrant connectivity in SCZ. Furthermore, diffusion tensor imaging studies have shown a significant reduction of leftward asymmetry in some key white-matter tracts in SCZ. In this paper, we review the studies that investigated both structural brain asymmetry and asymmetry of functional connectivity in healthy subjects and SCZ. From an analysis of the existing literature on this topic, we can hypothesize an overall generally attenuated asymmetry of functional connectivity in SCZ compared to healthy controls. Such attenuated asymmetry increases with the duration of the disease and correlates with psychotic symptoms. Finally, we hypothesize that structural deficits across the corpus callosum may contribute to the abnormal asymmetry of intra-hemispheric connectivity in schizophrenia.
Introduction
Left–right asymmetries of brain and behavior are known to be widespread among both vertebrates and invertebrates, and can arise through a number of genetic, epigenetic, or neural mechanisms (Corballis, 2014). In human beings, such brain asymmetry is widely associated with complementary functions, with the left-hemisphere regarded as being dominant for language and handedness (Corballis, 2014), and the right hemisphere as being dominant for some non-verbal functions, such as spatial attention (Cai et al., 2013) and the processing of faces (Dundas et al., 2014). The healthy brain exhibits both structural (e.g., in gray matter volume, in cytoarchitecture and dendritic arborization, or in white-matter integrity) and functional asymmetries (Rentería, 2012). On the contrary, a reduction of this phenomenon has been reported in schizophrenia, mostly as a consequence for the failure of the left-hemisphere dominance (Mitchell and Crow, 2005). Furthermore, a growing body of evidence has revealed that in schizophrenia, such a reduced brain asymmetry is not only structural (Friston, 2002; Koch et al., 2008; Ribolsi et al., 2009; Baker et al., 2014; Zhang et al., 2014) but also functional, as there is an abnormal asymmetry of functional connections compared to healthy subjects (Jalili et al., 2010). Starting with a brief overview of the structural and functional asymmetry in healthy brains, in this manuscript, we will focus our attention on the recent works that investigated the asymmetry of functional connectivity in schizophrenia, suggesting that this aspect may represent a neurophysiological feature that is unique to this disorder. Moreover, we will discuss the possibility that an impaired inter-hemispheric connectivity may influence this phenomenon. Finally, the hypothetical relationship between abnormal functional connectivity and psychotic symptomatology will be also taken into consideration.
Brain Asymmetry of Connectivity in Healthy Subjects
Structural Brain Asymmetry in Healthy Subjects
Gray matter
The brain exhibits macroscopic asymmetries (e.g., in volume, fissurization), microscopic asymmetries (e.g., in cytoarchitecture and dendritic arborization), and neurochemical asymmetries, such as dopaminergic and noradrenergic sensitivity (Toga and Thompson, 2003). It has been observed, initially from autopsy and later from imaging, that the frontal right-hemisphere protrudes more anteriorly and is often wider than the left, and the left occipital lobe extends beyond and is often wider than the right in many individuals (see figure of the cerebral torque from Mitchell and Crow, 2005). This phenomenon is refered to as the so-called “Yakovlevian torque” (LeMay, 1976; Toga and Thompson, 2003). This structural morphology reflects differences of gray matter volume (Weinberger et al., 1982; Takao et al., 2013) and cortical thickness (Luders et al., 2006) in frontal (greater in the right), middle (greater in the left planum temporale) and occipital regions (greater in the left). Furthermore, focal asymmetries, which are more easily interpretable with regards to their relationships with brain function, were also demonstrated. Interstingly, the leftward asymmetry of the planum temporale, an auditory cortex region at the back of the superior temporal surface that is involved in phonetic processing (Jäncke et al., 2002) is considered as an anatomical marker of left hemispheric functional specialization for language (Geschwind and Levitsky, 1968; Shapleske et al., 1999). Notably, in this article, we use the expression leftward/rightward asymmetry to refer to the distribution of asymmetry toward the left or the right hemisphere.
White matter
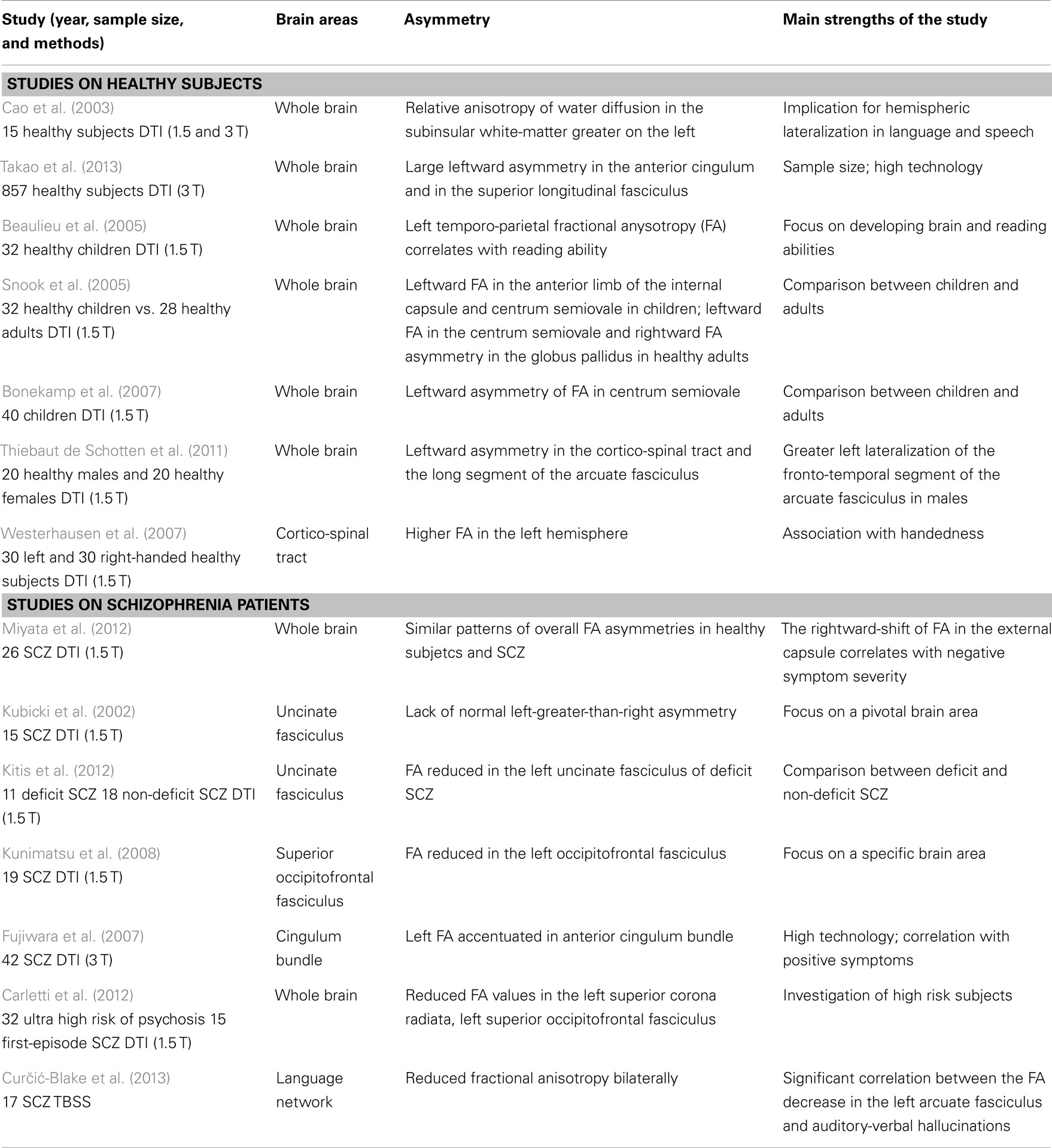
Diffusion tensor imaging (DTI) is one of the most widely used magnetic resonance (MR) imaging techniques for assessing brain tissue integrity, and white matter in particular. It is sensitive to the random thermal motions of water and is useful for probing white-matter microstructure and connectivity in vivo. Through this technique, white-matter asymmetry has been revealed in several studies in healthy adults (Cao et al., 2003), in the developing human brain (Beaulieu et al., 2005; Snook et al., 2005; Bonekamp et al., 2007), and in a variety of neuropsychological disorders (Fletcher et al., 2010; Kubicki et al., 2011). Leftward asymmetry of the anterior cingulum and of the arcuate fasciculus (Takao et al., 2013), the cortico-spinal tract (Thiebaut de Schotten et al., 2011), and the posterior limb of the internal capsule (Westerhausen et al., 2007) are among the most consistent white-matter asymmetries in healthy conditions. A selection of studies on white-matter asymmetry in healthy subjects and of their main findings is provided in Table 1.
Asymmetry of the Functional Connectivity in Healthy Subjects
However, beyond structural brain asymmetry, we are now aware of various studies that focus on the asymmetry of the functional connectivity between the two hemispheres (Table 2). Functional magnetic resonance imaging (fMRI) has been used to examine the hemispheric differences in the functional networks. For instance, in a resting-state fMRI study on the hemispheric asymmetry in the cognitive division of anterior cingulate cortex, the connections linking the anterior cingulate cortex to the frontal and parietal lobes are stronger in the right than in the left hemisphere (Yan et al., 2009). Similarly, a comparison between left and right fronto-parietal network by means of EEG during an auditory location oddball paradigm has revealed a rightward hemispheric asymmetry in this network that conforms to the right-hemisphere dominance model in audiospatial perception (Dietz et al., 2014). To this regard, a recent fMRI study has reported two distinct forms of lateralization for the right and the left hemisphere: while the left hemisphere shows a preference to interact more exclusively with itself, particularly for cortical regions involved in language and fine motor coordination, the right-hemisphere cortical regions involved in visuospatial and attentional processing interact in a more integrative fashion with both hemispheres (Gotts et al., 2013). Using the same methods, Saenger et al. (2012) investigated the hemispheric asymmetries of functional connectivity and gray matter volume in the default mode network. For both right-handed and left-handed subjects, a greater leftward functional connectivity was observed in the posterior cingulate gyrus. Interestingly, in this study functional asymmetries are not always reflected or determined by structural asymmetries (Saenger et al., 2012).
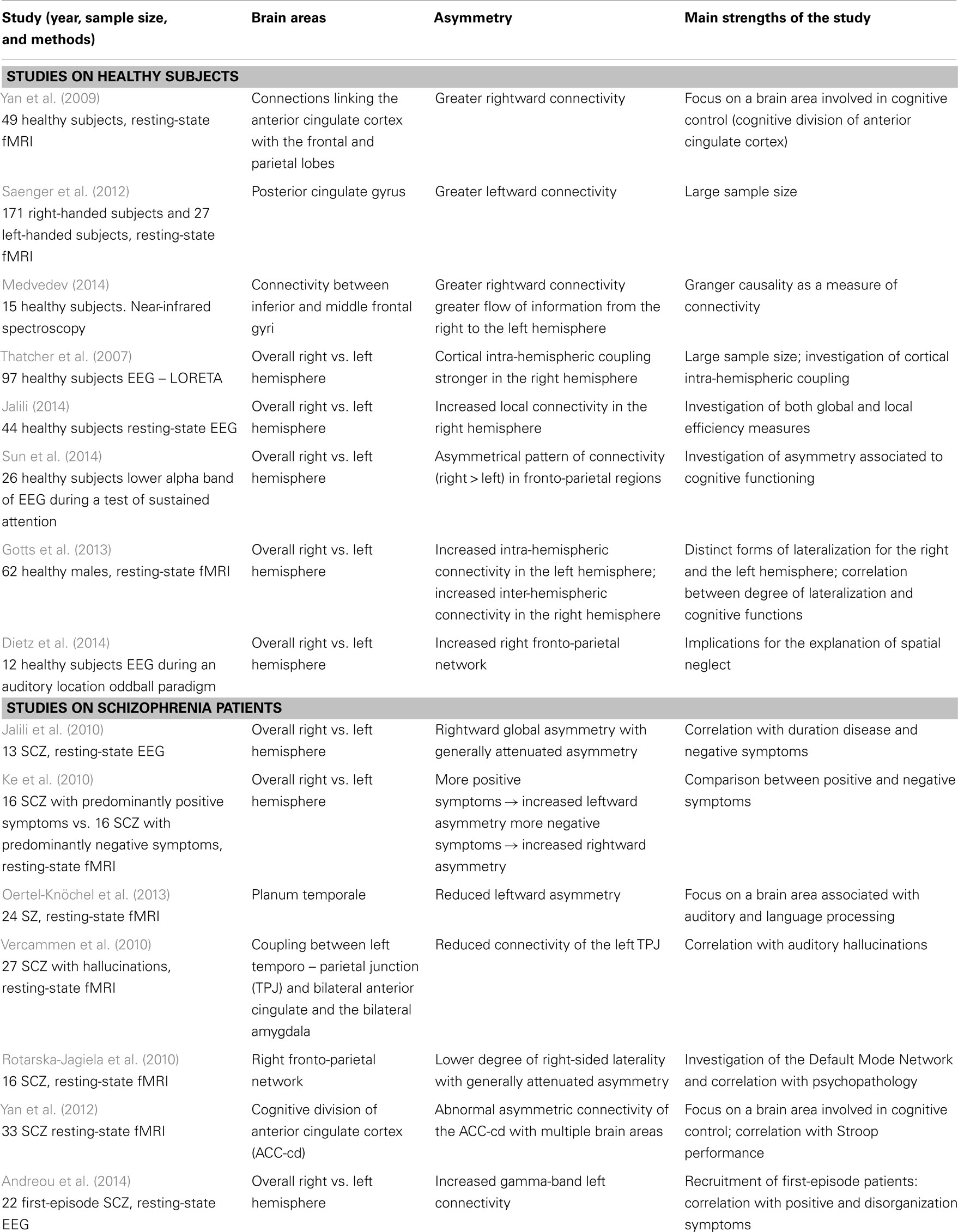
Table 2. Studies on asymmetry of functional connectivity on healthy subjects and schizophrenia patients.
A recent study used a different approach, by utilizing near-infrared spectroscopy to measure connectivity in each hemisphere between inferior and middle frontal gyri (“unilateral connectivity”), as well as connectivity between homologous structures of both hemispheres (“bilateral connectivity”). There was greater flow of information from the right to the left hemisphere in both left- and right-handed subjects (Medvedev, 2014). In addition, asymmetry of cortical connectivity has been investigated using current source correlations derived from the resting-state EEG. Higher connectivity in the right hemisphere was found in the majority of right-handed subjects (Jalili, 2014). Similarly, Thatcher et al. (2007) have shown that, in general, the right hemisphere exhibited higher intra-hemispheric source correlations than did the left hemisphere. This study confirmed earlier results based on surface EEG coherence, which also found higher right versus left hemispheric coherence (Tucker et al., 1986; Voineskos et al., 2010).
Thus, both electrophysiological and brain imaging studies seem to provide converging evidence that functional connectivity is higher in the right hemisphere (Medvedev, 2014). This pattern has been confirmed during a test of sustained attention (Sun et al., 2014). This right-shifted asymmetric pattern of functional connectivity is not fixed during the life course, but seems to be dependent on both development and aging. For instance, in the neonatal brain, recent studies have revealed a greater structural efficiency in the left hemisphere than in the right, suggesting that the brain regions in the left hemisphere interconnect in better integration and segregation as compared to the right hemisphere (Ratnarajah et al., 2013). Similarly, in a sample of middle age healthy subjects (with a mean age of 53 years), there is a more prominent leftward asymmetry in cortical connectivity, i.e., a larger number of regions demonstrated a higher degree of connectivity in the left hemisphere (Bonilha et al., 2014).
Likewise, cognitive activation studies have consistently demonstrated that prefrontal activation tends to be less lateralized in older adults as compared with younger adults (Cabeza et al., 1997, 2004, Cabeza, 2002; Morcom et al., 2003; Dennis et al., 2007). This phenomenon is known as the HAROLD (hemispheric asymmetry reduction in older adults) effect (Cabeza, 2002), which has also been documented with electroencephalography (Bellis et al., 2000) as well as near-infrared spectroscopy (Herrmann et al., 2006).
Brain Asymmetry of Connectivity in Schizophrenia
Structural Brain Asymmetry in Schizophrenia
Gray matter
Several neuroimaging studies have reported a loss of the cerebral torque (greater width of the right frontal and left occipital lobes relative to their contra-lateral counterparts) in patients with schizophrenia compared to normal controls (Crow, 2013). This phenomenon arises out of an abnormality in the genetic control for the development of normal cerebral asymmetry and underlies alterations in language processing (Crow, 1997). In healthy conditions (see figure of the cerebral torque from Mitchell and Crow, 2005), the structure of the cerebral torque produces some effects on the asymmetry of brain connectivity, as the torque drives the transmission within the system in the direction left occipito-temporo-parietal → right occipito-temporo-parietal → right dorso-lateral prefrontal → left dorso-lateral prefrontal (Mitchell and Crow, 2005). Therefore, the core symptoms of schizophrenia may reflect this failure of the left-hemisphere dominance for the faculty of language (DeLisi, 2001; Crow, 2008).
Both structural and functional evidence suggest that schizophrenia is associated with reduced lateralization of language to the left hemisphere, with some studies reporting a reversal of lateralization to the right hemisphere (Gruzelier et al., 1999; Gur and Chin, 1999; Kwon et al., 1999; Aydin et al., 2001; Sommer et al., 2001; Kircher et al., 2002; Sumich et al., 2002). Interestingly, a loss of left-hemisphere dominance and structural anomalies of cerebral asymmetry are related to the progression of the disease (Lam et al., 2012). A second morphological aspect in schizophrenia concerns the abnormal cortical folding (Cachia et al., 2008; Palaniyappan et al., 2014). In the normal population, structural cortical asymmetries, including the lateral temporal cortex, the planum temporale and the superior temporal sulcus are well-documented in adults and children (Toga and Thompson, 2003) and, interestingly, are already present in term infants (Hill et al., 2010). Some studies have revealed an increased or more complex cortical folding in the right hemisphere compared to the left one in both patients and high-risk subjects (Harris et al., 2004, 2007; Narr et al., 2004; Stanfield et al., 2008).
In contrast, regions with significant reductions in gyrification (hypogyria) are predominantly in the left hemisphere (Palaniyappan and Liddle, 2012), confirming the hypothesis of an abnormal brain lateralization. Interestingly, schizophrenia patients (SCZ) with resistant auditory hallucinations show a greater local sulcal index decrease in the superior temporal sulcus bilaterally, and in a peculiar way, also in the left middle frontal sulcus and the diagonal branch of left sylvian fissure (Cachia et al., 2008).
A strict link between gyrification and disruption in neural connectivity in schizophrenia has been hypothesized (White and Hilgetag, 2011). For example, an increased prefrontal gyrification in schizophrenia has been related to increased local short range prefrontal connectivity and reduced long range connectivity (Dauvermann et al., 2012), although this study didn’t investigate the asymmetry of these connections. Therefore, morphological evidence for both the loss of the cerebral torque and the abnormalities of gyrification may provide some anatomical basis for the aberrant connectivity in schizophrenia.
White matter
Diffusion tensor imaging investigation has revealed wide white-matter changes in schizophrenia, even at the illness’ onset, as well as before (Kubicki and Shenton, 2014). Interestingly, either patterns of reduced connectivity can be observable across the different stages of the disorder (Wheeler and Voineskos, 2014) in medication-naïve: first-episode patients (Zhang et al., 2014), high-risk subjects (Bloemen et al., 2010; Carletti et al., 2012), and schizotypal subjects (Smallman et al., 2014), suggesting that they may represent a neurobiological trait marker for the schizophrenia spectrum disorders.
With regard to the asymmetry between the two hemispheres, in schizophrenia, there are patterns of overall fractional anysotropy (FA) asymmetries, similar to healthy controls (Miyata et al., 2012). However, SCZ show a significant reduction of leftward asymmetry in some peculiar white-matter tracts, like the uncinate fasciculus (Kubicki et al., 2002; Kitis et al., 2012; Miyata et al., 2012), the inferior occipitofrontal fasciculi (Miyata et al., 2012), the superior occipitofrontal fasciculus (Kunimatsu et al., 2008), and the anterior cingulum bundle (Fujiwara et al., 2007).
Interestingly, the rightward-shift of the FA of the uncinate fasciculus correlates with negative symptom severity (Miyata et al., 2012). Abnormalities in this fasciculus have been more frequently associated with several psychiatric disorders, suggesting that this peculiar alteration of white matter may be involved in the impairment of some cognitive functions, such as episodic memory, language, and social emotional processing (Von Der Heide et al., 2013). Also interesting, different studies showing that left-hemisphere white-matter tracts are more impaired than are right-hemisphere ones are included in a meta-analysis, which investigated a total of 407 patients affected by schizophrenia or related diagnoses (Ellison-Wright and Bullmore, 2009). Two major clusters of altered white-matter integrity were isolated, both of them in the left hemisphere. These clusters are the cerebello-thalamo-cortical circuit and the temporal network interconnecting the frontal lobe, insula, hippocampus/amygdala, and occipital lobe (Ellison-Wright and Bullmore, 2009). Moreover more reduced FA values have been found in high-risk subjects who later developed schizophrenia, as compared to those who did not, in the left temporal white matter at baseline (Bloemen et al., 2010) and in the left superior corona radiata, left superior occipitofrontal fasciculus after the onset of illness (Carletti et al., 2012). A selection of studies and of their main findings on white-matter asymmetry in schizophrenia subjects is provided in Table 1.
On the whole, the DTI findings seem to confirm the hypothesis of a reduced leftward asymmetry. Although most studies revealed decreased FA, some studies found increased FA in specific white-matter tracts in patients with schizophrenia, compared to controls (Alba-Ferrara et al., 2013). These findings of increased FA in schizophrenia are thought to reflect hyperconnectivity (Zhang et al., 2012) or deficient axonal pruning (Alba-Ferrara et al., 2013). Interestingly, some studies have shown a positive correlation between FA and the degree of negative symptoms (Bijanki et al., 2014; Lee et al., 2014). Similarly, in the study conducted by Szeszko et al. (2008), even if lower FA were found in the uncinate fasciculus, inferior fronto-occipital fasciculus, and superior longitudinal fasciculus, higher FA in the left inferior fronto-occipital fasciculus correlated significantly with greater severity of hallucinations and delusions. According to the authors, a possible explanation for the association between positive symptoms and FA values in this tract is that increased FA may render patients more vulnerable to experiencing such symptoms (Szeszko et al., 2008).
However, a growing body of research on the left arcuate fasciculus, which is the key pathway of the language network, has revealed reduced FA in this tract in SCZ with auditory hallucinations (Seok et al., 2007; Catani et al., 2011; de Weijer et al., 2011; Abdul-Rahman et al., 2012; Curčić-Blake et al., 2013). To this regard, a recent meta-analysis of DTI studies has revealed for the first time a significant correlation between the FA decrease in the left arcuate fasciculus and auditory-verbal hallucinations, although a high heterogeneity within the entire dataset of the studies included should be also considered (Geoffroy et al., 2014). These data seem to support our hypothesis of a reduced leftward asymmetry in schizophrenia, and, in addition, that such attenuated left lateralization is related to an increase in positive symptoms.
Asymmetry of the Functional Connectivity in Schizophrenia
Apart from the above-described evidence for patterns of reduced asymmetry of gray and white matter, functional connectivity was recently investigated in order to account for possible functional asymmetries. A review of the literature on this theme was conducted in the PubMed database by combining the keywords “connectivity,” “schizophrenia,” and “asymmetry” or “laterality” (Table 2). In a specific study on the asymmetry of functional connectivity, a generally attenuated asymmetry between the two hemispheres was found in chronic SCZ as compared to healthy controls (Jalili et al., 2010). Furthermore, the degree of such abnormality of asymmetry increases with the duration of the disease and correlates with the negative symptoms (Jalili et al., 2010). The result of an attenuated asymmetry of functional connectivity was found also by Rotarska-Jagiela et al. (2010), as patients showed a lower degree of right-sided laterality for the right fronto-parietal network. Interestingly, such decreased hemispheric laterality correlates with increasing symptoms of disorganization.
Other fMRI studies investigated specifically the relationship between abnormal asymmetry of functional connectivity and the psychotic symptomatology (Ke et al., 2010). Notably, patients exhibiting positive symptoms showed significantly increased leftward asymmetry of functional connectivity, as measured by a resting-state fMRI. Interestingly, also first-episode SCZ show an increased left connectivity in the gamma-band compared to controls (Andreou et al., 2014). In contrast, patients with more pronounced negative symptoms exhibited increased rightward asymmetry of functional connectivity (Ke et al., 2010). These findings are consistent with the results of some previous studies, e.g., Gruzelier et al. (1999), who reported that active syndrome SCZ (characterized by positive symptoms) showed left-greater-than-right asymmetry, while patients suffering from deficit syndrome schizophrenia (characterized by more negative symptoms), on the other hand, showed right-greater-than-left asymmetry. A paradigmatic example is the analysis of functional connectivity of the auditory perception network (Rotarska-Jagiela et al., 2009; Gavrilescu et al., 2010; Vercammen et al., 2010).
Brain imaging studies have specifically investigated the lateralization of the planum temporale, finding not only a reduced hemispheric asymmetry (Oertel et al., 2010), which correlates with hallucination severity (Sumich et al., 2005) but also a reduced leftward asymmetry of intrinsic functional connectivity within this area in SCZ and their relatives (Oertel-Knöchel et al., 2013). Therefore, there are complex interactions between the morphological alterations and the resulting dysfunctional connectivity of the network associated with auditory hallucinations. However, despite these preliminary results showing an overall reduction of the asymmetry of functional connectivity, there are still relatively few studies addressing this topic systematically in other cortical networks.
Inter-Hemispheric Connectivity
Healthy Subjects
The corpus callosum is the largest commissure in the brain and acts as a “bridge” of nerve fibers that connect the two cerebral hemispheres. It plays a crucial role in inter-hemispheric integration and is responsible for normal communication and cooperation between the two hemispheres (Nowicka and Tacikowski, 2011). Several studies have demonstrated a relationship between the corpus callosum size and measures of inter-hemispheric transfer time in split-brain and acallosal patients (Marzi et al., 1991; Forster and Corballis, 1998, 2000; Roser and Corballis, 2002).
A decrease in fiber size and transcallosal connectivity might be related to a reduced need for inter-hemispheric communication due, in part, to increased intrahemispheric connectivity and specialization (Doron and Gazzaniga, 2008). DTI findings on healthy individuals demonstrated an increase in molecular diffusion of the corpus callosum in strongly left-lateralized subjects as compared to moderately left-lateralized, bilateral, or right-lateralized subjects (Westerhausen et al., 2006). Furthermore, patterns of directional symmetry/asymmetry of transcallosal transfer time may be related to the degree of brain lateralization (Nowicka and Tacikowski, 2011).
However, it is still uncertain how the corpus callosum regulates transfer and communication between the two hemispheres. Some studies suggest that the corpus callosum could play an inhibitory role, whereas others say that the corpus callosum serves an excitatory function (Bloom and Hynd, 2005). Anatomical and functional lateralization can be explained by either of the two theories. Lateralization could have originated from an inhibitory function of the corpus callosum by inhibiting the opposing hemisphere, thereby hindering development and allowing for asymmetrical hemisphere development. The excitatory model could have allowed for a unilateral flow of information toward one hemisphere, therefore explaining both the origin of lateralization and the integration between the two parts of the brain (van der Knaap and van der Ham, 2011).
Schizophrenia Patients
Structural deficiencies of the corpus callosum in schizophrenia further point to disrupted inter-hemispheric information transferring (Beaumont and Dimond, 1973). At this moment, there is evidence of reduced functional inter-hemispheric connectivity (Ribolsi et al., 2011) and that it goes along with abnormal brain asymmetry. For example, it has been shown that disrupted inter-hemispheric connectivity is related to diminished lateralization of activation during language processing, and that this finding is specific to schizophrenia (Bleich-Cohen et al., 2012). Additionally, insufficient communication between hemispheres might further affect the developmental process of each individual hemisphere, resulting in the relative lack of asymmetry observed in schizophrenic brains (Innocenti et al., 2003).
Many morphological and neuroimaging schizophrenia studies have detected abnormalities in callosal shape (DeQuardo, 1999; Downhill et al., 2000; Narr et al., 2000; Frumin et al., 2002), size (Arnone et al., 2008; Rotarska-Jagiela et al., 2008), density (Hulshoff Pol et al., 2004; Seok et al., 2007; Wolf et al., 2008), structure (Flynn et al., 2003; Diwadkar et al., 2004; Kubicki et al., 2005), and function (Innocenti et al., 2003). Differences in area and volume of the corpus callosum were greatest in patients whose condition was chronic relative to patients with a first episode, as well as the controls (Collinson et al., 2014).
Recent DTI evidence suggests an inter-hemispheric hypoconnectivity in patients with schizophrenia and their relatives (Whitford et al., 2010; Knöchel et al., 2012), which predicts inter-hemispheric transfer time (Whitford et al., 2011) and psychotic symptoms (Whitford et al., 2010). Using a technique called voxel-mirrored homotopic connectivity (VMHC), a recent study found that the correlation between homologous brain regions was reduced in patients with schizophrenia and schizoaffective disorder. According to the authors, deficits in white-matter connectivity in the corpus callosum could disrupt the synchrony between homotopically connected regions because neural signals are not transmitted with fidelity. Another, not mutually exclusive, explanation is that dysfunctions in local gray matter structure could account for the deficits (Hoptman et al., 2012). Interestingly, the inter-hemispheric resting-state FC of VMHC is also disrupted in unaffected siblings of SCZ (Guo et al., 2014).
Conclusion
As reported above in this manuscript, several studies on schizophrenia have reported a loss of the developmental brain torsion (the so-called cerebral torque) with resulting failure of the left-hemisphere dominance (Mitchell and Crow, 2005). Interestingly, significant reductions in gyrification (hypogyria) are predominantly in the left hemisphere (Palaniyappan and Liddle, 2012). This morphologic evidence may play a role in the impairment and abnormal asymmetry of neural connectivity (White and Hilgetag, 2011), as well as in confirming the hypothesis of a failure of this hemisphere dominance and an abnormal brain lateralization.
A revision of the more recent functional connectivity studies reveals an overall rightward global asymmetry, both in healthy subjects (Medvedev, 2014) and in SCZ (Jalili et al., 2010). However, the patients show a more generally attenuated asymmetry, which increases with the duration of the disease and correlates with the psychotic symptoms (Jalili et al., 2010; Rotarska-Jagiela et al., 2010). Interestingly, patients exhibiting positive symptoms have significantly increased leftward asymmetry of functional connectivity, while the negative symptom group, in contrast, exhibits increased rightward asymmetry of functional connectivity (Ke et al., 2010). The DTI studies also confirm these data with the SCZ showing a significant reduction of leftward asymmetry in some peculiar white-matter tracts, like the uncinate fasciculus (Kubicki et al., 2002; Kitis et al., 2012; Miyata et al., 2012). Furthermore, different studies have also shown that left-hemisphere white-matter tracts are more impaired than are the right-hemisphere ones (Ellison-Wright and Bullmore, 2009).
An important aspect of brain lateralization in the healthy brain is that the left hemisphere has a greater preference for within-hemisphere interactions, whereas the right hemisphere has interactions that are more strongly bilateral. At a macroscopic scale, this is broadly consistent with proposals that hold that cortical representations are more focal in the left hemisphere and more diffuse in the right hemisphere (Gotts et al., 2013). Therefore, in the healthy subjects, left-hemisphere regions are biased to interact more strongly within the same hemisphere, whereas right-hemisphere regions interact more strongly with both hemispheres. These two different patterns of interaction are associated with left-lateralized functions, such as language and motor abilities, and right-lateralized functions, such as visuospatial attention (Gotts et al., 2013). Therefore, as a consequence of abnormal brain asymmetry and of the failure of the left-hemisphere dominance in schizophrenia, lateralized functions are compromised in schizophrenia. Indeed, different studies have revealed abnormal patterns of connectivity in the left hemisphere in relation to specific psychotic domains (Rotarska-Jagiela et al., 2009; Gavrilescu et al., 2010; Vercammen et al., 2010).
In this paper, we also hypothesize that the abnormal asymmetry of the functional connectivity may be partly due to the well-observed inter-hemispheric communication in schizophrenia. As reported above, there is evidence that reduced functional inter-hemispheric connectivity goes along with abnormal brain asymmetry (Innocenti et al., 2003). Furthermore, deficits in white-matter connectivity in the corpus callosum could disrupt the synchrony between homotopically connected regions because neural signals are not transmitted with fidelity (Hoptman et al., 2012).
One possible hypothesis is that the abnormal asymmetry of connectivity may be related to a dysfunctional inter-hemispheric communication. Indeed, insufficient communication between hemispheres might further affect the developmental process of each individual hemisphere, resulting in the relative lack of asymmetry observed in schizophrenic brains (Innocenti et al., 2003). Future studies should investigate the degree of connectivity of a brain region in relation to the efficiency of the inter-hemispheric connectivity in schizophrenia.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdul-Rahman, M. F., Qiu, A., Woon, P. S., Kuswanto, C., Collinson, S. L., and Sim, K. (2012). Arcuate fasciculus abnormalities and their relationship with psychotic symptoms in schizophrenia. PLoS ONE 7:e29315. doi:10.1371/journal.pone.0029315
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Alba-Ferrara, L., de Erausquin, G. A., Hirnstein, M., Weis, S., and Hausmann, M. (2013). Emotional prosody modulates attention in schizophrenia patients with hallucinations. Front. Hum. Neurosci. 7:59. doi:10.3389/fnhum.2013.00059
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Andreou, C., Nolte, G., Leicht, G., Polomac, N., Hanganu-Opatz, I. L., Lambert, M., et al. (2014). Increased resting-state gamma-band connectivity in first-episode schizophrenia. Schizophr. Bull. doi:10.1093/schbul/sbu121
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Arnone, D., McIntosh, A. M., Tan, G. M., and Ebmeier, K. P. (2008). Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophr. Res. 101, 124–132. doi:10.1016/j.schres.2008.01.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Aydin, N., Dane, S., Oztürk, I., Uslu, C., Gümüstekin, K., and Kirpinar, I. (2001). Left ear (right temporal hemisphere) advantage and left temporal hemispheric dysfunction in schizophrenia. Percept. Mot. Skills 93, 230–238. doi:10.2466/pms.2001.93.1.230
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Baker, J. T., Holmes, A. J., Masters, G. A., Yeo, B. T., Krienen, F., Buckner, R. L., et al. (2014). Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry 71, 109–118. doi:10.1001/jamapsychiatry.2013.3469
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Beaulieu, C., Plewes, C., Paulson, L. A., Roy, D., Snook, L., Concha, L., et al. (2005). Imaging brain connectivity in children with diverse reading ability. Neuroimage 25, 1266–1271. doi:10.1016/j.neuroimage.2004.12.053
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Beaumont, J. G., and Dimond, S. J. (1973). Brain disconnection and schizophrenia. Br. J. Psychiatry 123, 661–662. doi:10.1192/bjp.123.6.661
Bellis, T. J., Nicol, T., and Kraus, N. (2000). Aging affects hemispheric asymmetry in the neural representation of speech sounds. J. Neurosci. 20, 791–797.
Bijanki, K. R., Hodis, B., Magnotta, V. A., Zeien, E., and Andreasen, N. C. (2014). Effects of age on white matter integrity and negative symptoms in schizophrenia. Schizophr. Res. doi:10.1016/j.schres.2014.05.031
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bleich-Cohen, M., Sharon, H., Weizman, R., Poyurovsky, M., Faragian, S., and Hendler, T. (2012). Diminished language lateralization in schizophrenia corresponds to impaired inter-hemispheric functional connectivity. Schizophr. Res. 134, 131–136. doi:10.1016/j.schres.2011.10.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bloemen, O. J., de Koning, M. B., Schmitz, N., Nieman, D. H., Becker, H. E., de Haan, L., et al. (2010). White-matter markers for psychosis in a prospective ultra-high-risk cohort. Psychol. Med. 40, 1297–1304. doi:10.1017/S0033291709991711
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bloom, J. S., and Hynd, G. W. (2005). The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol. Rev. 15, 59–71. doi:10.1007/s11065-005-6252-y
Bonekamp, D., Nagae, L. M., Degaonkar, M., Matson, M., Abdalla, W. M., Barker, P. B., et al. (2007). Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage 34, 733–742. doi:10.1016/j.neuroimage.2006.09.020
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bonilha, L., Nesland, T., Rorden, C., and Fridriksson, J. (2014). Asymmetry of the structural brain connectome in healthy older adults. Front. Psychiatry 4:186. doi:10.3389/fpsyt.2013.00186
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cabeza, R. (2002). Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol. Aging 17, 85–100. doi:10.1037/0882-7974.17.1.85
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cabeza, R., Daselaar, S. M., Dolcos, F., Prince, S. E., Budde, M., and Nyberg, L. (2004). Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb. Cortex 14, 364–375. doi:10.1093/cercor/bhg133
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cabeza, R., McIntosh, A. R., Tulving, E., Nyberg, L., and Grady, C. L. (1997). Age-related differences in effective neural connectivity during encoding and recall. Neuroreport 8, 3479–3483. doi:10.1097/00001756-199711100-00013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cachia, A., Paillère-Martinot, M. L., Galinowski, A., Januel, D., de Beaurepaire, R., Bellivier, F., et al. (2008). Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations. Neuroimage 39, 927–935. doi:10.1016/j.neuroimage.2007.08.049
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cai, Q., Van der Haegen, L., and Brysbaert, M. (2013). Complementary hemispheric specialization for language production and visuospatial attention. Proc. Natl. Acad. Sci. U.S.A. 110, E322–E330. doi:10.1073/pnas.1212956110
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cao, Y., Whalen, S., Huang, J., Berger, K. L., and DeLano, M. C. (2003). Asymmetry of subinsular anisotropy by in vivo diffusion tensor imaging. Hum. Brain Mapp. 20, 82–90. doi:10.1002/hbm.10130
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Carletti, F., Woolley, J. B., Bhattacharyya, S., Perez-Iglesias, R., Fusar Poli, P., Valmaggia, L., et al. (2012). Alterations in white matter evident before the onset of psychosis. Schizophr. Bull. 38, 1170–1179. doi:10.1093/schbul/sbs053
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Catani, M., Craig, M. C., Forkel, S. J., Kanaan, R., Picchioni, M., Toulopoulou, T., et al. (2011). Altered integrity of perisylvian language pathways in schizophrenia: relationship to auditory hallucinations. Biol. Psychiatry 70, 1143–1150. doi:10.1016/j.biopsych.2011.06.013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Collinson, S. L., Gan, S. C., Woon, P. S., Kuswanto, C., Sum, M. Y., Yang, G. L., et al. (2014). Corpus callosum morphology in first-episode and chronic schizophrenia: combined magnetic resonance and diffusion tensor imaging study of Chinese Singaporean patients. Br. J. Psychiatry 204, 55–60. doi:10.1192/bjp.bp.113.127886
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Corballis, M. C. (2014). Left brain, right brain: facts and fantasies. PLoS Biol. 12:e1001767. doi:10.1371/journal.pbio.1001767
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Crow, T. J. (1997). Is schizophrenia the price that Homo sapiens pays for language? Schizophr. Res. 28, 127–141. doi:10.1016/S0920-9964(97)00110-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Crow, T. J. (2008). The ‘big bang’ theory of the origin of psychosis and the faculty of language. Schizophr. Res. 102, 31–52. doi:10.1016/j.schres.2008.03.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Crow, T. J. (2013). The XY gene hypothesis of psychosis: origins and current status. Am. J. Med. Genet. B Neuropsychiatr. Genet. 162, 800–824. doi:10.1002/ajmg.b.32202
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Curčić-Blake, B., Nanetti, L., van der Meer, L., Cerliani, L., Renken, R., Pijnenborg, G. H., et al. (2013). Not on speaking terms: hallucinations and structural network disconnectivity in schizophrenia. Brain Struct. Funct. doi:10.1007/s00429-013-0663-y
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dauvermann, M. R., Mukherjee, P., Moorhead, W. T., Stanfield, A. C., Fusar-Poli, P., Lawrie, S. M., et al. (2012). Relationship between gyrification and functional connectivity of the prefrontal cortex in subjects at high genetic risk of schizophrenia. Curr. Pharm. Des. 18, 434–442. doi:10.2174/138161212799316235
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
de Weijer, A. D., Mandl, R. C., Diederen, K. M., Neggers, S. F., Kahn, R. S., Hulshoff Pol, H. E., et al. (2011). Microstructural alterations of the arcuate fasciculus in schizophrenia patients with frequent auditory verbal hallucinations. Schizophr Res. 130, 68–77. doi:10.1016/j.schres.2011.05.010
DeLisi, L. E. (2001). Speech disorder in schizophrenia: review of the literature and exploration of its relation to the uniquely human capacity for language. Schizophr. Bull. 27, 481–496. doi:10.1093/oxfordjournals.schbul.a006889
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dennis, N. A., Kim, H., and Cabeza, R. (2007). Effects of aging on true and false memory formation: an fMRI study. Neuropsychologia 45, 3157–3166. doi:10.1016/j.neuropsychologia.2007.07.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
DeQuardo, J. R. (1999). Landmark analysis of corpus callosum shape in schizophrenia. Biol. Psychiatry 46, 1712–1714. doi:10.1016/S0006-3223(99)00196-1
Dietz, M. J., Friston, K. J., Mattingley, J. B., Roepstorff, A., and Garrido, M. I. (2014). Effective connectivity reveals right-hemisphere dominance in audiospatial perception: implications for models of spatial neglect. J. Neurosci. 34, 5003–5011. doi:10.1523/JNEUROSCI.3765-13.2014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Diwadkar, V. A., DeBellis, M. D., Sweeney, J. A., Pettegrew, J. W., and Keshavan, M. S. (2004). Abnormalities in MRI-measured signal intensity in the corpus callosum in schizophrenia. Schizophr. Res. 67, 277–282. doi:10.1016/S0920-9964(03)00098-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Doron, K. W., and Gazzaniga, M. S. (2008). Neuroimaging techniques offer new perspectives on callosal transfer and interhemispheric communication. Cortex 44, 1023–1029. doi:10.1016/j.cortex.2008.03.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Downhill, J. E. Jr., Buchsbaum, M. S., Wei, T., Spiegel-Cohen, J., Hazlett, E. A., Haznedar, M. M., et al. (2000). Shape and size of the corpus callosum in schizophrenia and schizotypal personality disorder. Schizophr. Res. 42, 193–208. doi:10.1016/S0920-9964(99)00123-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dundas, E. M., Plaut, D. C., and Behrmann, M. (2014). An ERP investigation of the co-development of hemispheric lateralization of face and word recognition. Neuropsychologia 61, 315–323. doi:10.1016/j.neuropsychologia.2014.05.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ellison-Wright, I., and Bullmore, E. (2009). Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr. Res. 108, 3–10. doi:10.1016/j.schres.2008.11.021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fletcher, P. T., Whitaker, R. T., Tao, R., DuBray, M. B., Froehlich, A., Ravichandran, C., et al. (2010). Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage 51, 1117–1125. doi:10.1016/j.neuroimage.2010.01.083
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Flynn, S. W., Lang, D. J., Mackay, A. L., Goghari, V., Vavasour, I. M., Whittall, K. P., et al. (2003). Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol. Psychiatry 8, 811–820. doi:10.1038/sj.mp.4001337
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Forster, B., and Corballis, M. C. (1998). Interhemispheric transmission times in the presence and absence of the forebrain commissures: effects of luminance and equiluminance. Neuropsychologia 36, 925–934. doi:10.1016/S0028-3932(98)00016-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Forster, B., and Corballis, M. C. (2000). Interhemispheric transfer of colour and shape information in the presence and absence of the corpus callosum. Neuropsychologia 38, 32–45. doi:10.1016/S0028-3932(99)00050-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Frumin, M., Golland, P., Kikinis, R., Hirayasu, Y., Salisbury, D. F., Hennen, J., et al. (2002). Shape differences in the corpus callosum in first-episode schizophrenia and first-episode psychotic affective disorder. Am. J. Psychiatry 159, 866–868. doi:10.1176/appi.ajp.159.5.866
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fujiwara, H., Namiki, C., Hirao, K., Miyata, J., Shimizu, M., Fukuyama, H., et al. (2007). Anterior and posterior cingulum abnormalities and their association with psychopathology in schizophrenia: a diffusion tensor imaging study. Schizophr. Res. 95, 215–222. doi:10.1016/j.schres.2007.05.044
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gavrilescu, M., Rossell, S., Stuart, G. W., Shea, T. L., Innes-Brown, H., Henshall, K., et al. (2010). Reduced connectivity of the auditory cortex in patients with auditory hallucinations: a resting state functional magnetic resonance imaging study. Psychol. Med. 40, 1149–1158. doi:10.1017/S0033291709991632
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Geoffroy, P. A., Houenou, J., Duhamel, A., Amad, A., De Weijer, A. D., Curčić-Blake, B., et al. (2014). The arcuate fasciculus in auditory-verbal hallucinations: a meta-analysis of diffusion-tensor-imaging studies. Schizophr. Res. 159, 234–237. doi:10.1016/j.schres.2014.07.014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Geschwind, N., and Levitsky, W. (1968). Human brain: left-right asymmetries in temporal speech region. Science 161, 186–187. doi:10.1126/science.161.3837.186
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gotts, S. J., Jo, H. J., Wallace, G. L., Saad, Z. S., Cox, R. W., and Martin, A. (2013). Two distinct forms of functional lateralization in the human brain. Proc. Natl. Acad. Sci. U.S.A. 110, E3435–E3444. doi:10.1073/pnas.1302581110
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gruzelier, J., Wilson, L., and Richardson, A. (1999). Cognitive asymmetry patterns in schizophrenia: retest reliability and modification with recovery. Int. J. Psychophysiol. 34, 323–331. doi:10.1016/S0167-8760(99)00089-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Guo, W., Jiang, J., Xiao, C., Zhang, Z., Zhang, J., Yu, L., et al. (2014). Decreased resting-state interhemispheric functional connectivity in unaffected siblings of schizophrenia patients. Schizophr. Res. 152, 170–175. doi:10.1016/j.schres.2013.11.030
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gur, R. E., and Chin, S. (1999). Laterality in functional brain imaging studies of schizophrenia. Schizophr. Bull. 25, 141–156. doi:10.1093/oxfordjournals.schbul.a033361
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Harris, J. M., Moorhead, T. W., Miller, P., McIntosh, A. M., Bonnici, H. M., Owens, D. G., et al. (2007). Increased prefrontal gyrification in a large high-risk cohort characterizes those who develop schizophrenia and reflects abnormal prefrontal development. Biol. Psychiatry 62, 722–729. doi:10.1016/j.biopsych.2006.11.027
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Harris, J. M., Whalley, H., Yates, S., Miller, P., Johnstone, E. C., and Lawrie, S. M. (2004). Abnormal cortical folding in high-risk individuals: a predictor of the development of schizophrenia? Biol. Psychiatry 56, 182–189. doi:10.1016/j.biopsych.2004.04.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Herrmann, M. J., Walter, A., Ehlis, A. C., and Fallgatter, A. J. (2006). Cerebral oxygenation changes in the prefrontal cortex: effects of age and gender. Neurobiol. Aging 27, 888–894. doi:10.1016/j.neurobiolaging.2005.04.013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hill, J., Dierker, D., Neil, J., Inder, T., Knutsen, A., Harwell, J., et al. (2010). A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J. Neurosci. 30, 2268–2276. doi:10.1523/JNEUROSCI.4682-09.2010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hoptman, M. J., Zuo, X. N., D’Angelo, D., Mauro, C. J., Butler, P. D., Milham, M. P., et al. (2012). Decreased interhemispheric coordination in schizophrenia: a resting state fMRI study. Schizophr. Res. 141, 1–7. doi:10.1016/j.schres.2012.07.027
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hulshoff Pol, H. E., Schnack, H. G., Mandl, R. C., Cahn, W., Collins, D. L., Evans, A. C., et al. (2004). Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. Neuroimage 21, 27–35. doi:10.1016/j.neuroimage.2003.09.026
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Innocenti, G. M., Ansermet, F., and Parnas, J. (2003). Schizophrenia, neurodevelopment and corpus callosum. Mol. Psychiatry 8, 261–274. doi:10.1038/sj.mp.4001205
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jalili, M. (2014). Hemispheric asymmetry of electroencephalography-based functional brain networks. Neuroreport 25, 1266–1271. doi:10.1097/WNR.0000000000000256
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jalili, M., Meuli, R., Do, K. Q., Hasler, M., Crow, T. J., and Knyazeva, M. G. (2010). Attenuated asymmetry of functional connectivity in schizophrenia: a high-resolution EEG study. Psychophysiology 47, 706–716. doi:10.1111/j.1469-8986.2009.00971.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jäncke, L., Wüstenberg, T., Schulze, K., and Heinze, H. J. (2002). Asymmetric hemodynamic responses of the human auditory cortex to monaural and binaural stimulation. Hear. Res. 170, 166–178. doi:10.1016/S0378-5955(02)00488-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ke, M., Zou, R., Shen, H., Huang, X., Zhou, Z., Liu, Z., et al. (2010). Bilateral functional asymmetry disparity in positive and negative schizophrenia revealed by resting-state fMRI. Psychiatry Res. 182, 30–39. doi:10.1016/j.pscychresns.2009.11.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kircher, T. T., Liddle, P. F., Brammer, M. J., Williams, S. C., Murray, R. M., and McGuire, P. K. (2002). Reversed lateralization of temporal activation during speech production in thought disordered patients with schizophrenia. Psychol. Med. 32, 439–449.
Kitis, O., Ozalay, O., Zengin, E. B., Haznedaroglu, D., Eker, M. C., Yalvac, D., et al. (2012). Reduced left uncinate fasciculus fractional anisotropy in deficit schizophrenia but not in non-deficit schizophrenia. Psychiatry Clin. Neurosci. 66, 34–43. doi:10.1111/j.1440-1819.2011.02293.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Knöchel, C., Oertel-Knöchel, V., Schönmeyer, R., Rotarska-Jagiela, A., van de Ven, V., Prvulovic, D., et al. (2012). Interhemispheric hypoconnectivity in schizophrenia: fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. Neuroimage 59, 926–934. doi:10.1016/j.neuroimage.2011.07.088
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Koch, G., Ribolsi, M., Mori, F., Sacchetti, L., Codecà, C., Rubino, I. A., et al. (2008). Connectivity between posterior parietal cortex and ipsilateral motor cortex is altered in schizophrenia. Biol. Psychiatry 64, 815–819. doi:10.1016/j.biopsych.2008.05.026
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kubicki, M., Alvarado, J. L., Westin, C. F., Tate, D. F., Markant, D., Terry, D. P., et al. (2011). Stochastic tractography study of inferior frontal gyrus anatomical connectivity in schizophrenia. Neuroimage 55, 1657–1664. doi:10.1016/j.neuroimage.2011.01.047
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kubicki, M., Park, H., Westin, C. F., Nestor, P. G., Mulkern, R. V., Maier, S. E., et al. (2005). DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage 26, 1109–1118. doi:10.1016/j.neuroimage.2005.03.026
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kubicki, M., and Shenton, M. E. (2014). Diffusion tensor imaging findings and their implications in schizophrenia. Curr. Opin. Psychiatry 27, 179–184. doi:10.1097/YCO.0000000000000053
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kubicki, M., Westin, C. F., Maier, S. E., Frumin, M., Nestor, P. G., Salisbury, D. F., et al. (2002). Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am. J. Psychiatry 159, 813–820. doi:10.1176/appi.ajp.159.5.813
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kunimatsu, N., Aoki, S., Kunimatsu, A., Yoshida, M., Abe, O., Yamada, H., et al. (2008). Tract-specific analysis of the superior occipitofrontal fasciculus in schizophrenia. Psychiatry Res. 164, 198–205. doi:10.1016/j.pscychresns.2008.03.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kwon, J. S., McCarley, R. W., Hirayasu, Y., Anderson, J. E., Fischer, I. A., Kikinis, R., et al. (1999). Left planum temporale volume reduction in schizophrenia. Arch. Gen. Psychiatry 56, 142–148. doi:10.1001/archpsyc.56.2.142
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lam, M., Collinson, S. L., Sim, K., Mackay, C. E., James, A. C., and Crow, T. J. (2012). Asymmetry of lexico-semantic processing in schizophrenia changes with disease progression. Schizophr. Res. 134, 125–130. doi:10.1016/j.schres.2011.10.020
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lee, J. S., Han, K., Lee, S. K., Seok, J. H., and Kim, J. J. (2014). Altered structural connectivity and trait anhedonia in patients with schizophrenia. Neurosci. Lett. 579, 7–11. doi:10.1016/j.neulet.2014.07.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
LeMay, M. (1976). Morphological cerebral asymmetries of modern man, fossil man, and nonhuman primate. Ann. N. Y. Acad. Sci. 280, 349–366. doi:10.1111/j.1749-6632.1976.tb25499.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Luders, E., Narr, K. L., Zaidel, E., Thompson, P. M., and Toga, A. W. (2006). Gender effects on callosal thickness in scaled and unscaled space. Neuroreport 17, 1103–1106. doi:10.1097/01.wnr.0000227987.77304.cc
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Marzi, C. A., Bisiacchi, P., and Nicoletti, R. (1991). Is interhemispheric transfer of visuomotor information asymmetric? Evidence from a meta-analysis. Neuropsychologia 29, 1163–1177. doi:10.1016/0028-3932(91)90031-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Medvedev, A. V. (2014). Does the resting state connectivity have hemispheric asymmetry? A near-infrared spectroscopy study. Neuroimage 85, 400–407. doi:10.1016/j.neuroimage.2013.05.092
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mitchell, R. L., and Crow, T. J. (2005). Right hemisphere language functions and schizophrenia: the forgotten hemisphere? Brain 128, 963–978. doi:10.1093/brain/awh466
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Miyata, J., Sasamoto, A., Koelkebeck, K., Hirao, K., Ueda, K., Kawada, R., et al. (2012). Abnormal asymmetry of white matter integrity in schizophrenia revealed by voxelwise diffusion tensor imaging. Hum. Brain Mapp. 33, 1741–1749. doi:10.1002/hbm.21326
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Morcom, A. M., Good, C. D., Frackowiak, R. S., and Rugg, M. D. (2003). Age effects on the neural correlates of successful memory encoding. Brain 126, 213–229. doi:10.1093/brain/awg020
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Narr, K. L., Bilder, R. M., Kim, S., Thompson, P. M., Szeszko, P., Robinson, D., et al. (2004). Abnormal gyral complexity in first-episode schizophrenia. Biol. Psychiatry 55, 859–867. doi:10.1016/j.biopsych.2003.12.027
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Narr, K. L., Thompson, P. M., Sharma, T., Moussai, J., Cannestra, A. F., and Toga, A. W. (2000). Mapping morphology of the corpus callosum in schizophrenia. Cereb. Cortex 10, 40–49. doi:10.1093/cercor/10.1.40
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nowicka, A., and Tacikowski, P. (2011). Transcallosal transfer of information and functional asymmetry of the human brain. Laterality 16, 35–74. doi:10.1080/13576500903154231
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Oertel, V., Knöchel, C., Rotarska-Jagiela, A., Schönmeyer, R., Lindner, M., van de Ven, V., et al. (2010). Reduced laterality as a trait marker of schizophrenia – evidence from structural and functional neuroimaging. J. Neurosci. 30, 2289–2299. doi:10.1523/JNEUROSCI.4575-09.2010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Oertel-Knöchel, V., Knöchel, C., Matura, S., Prvulovic, D., Linden, D. E., and van de Ven, V. (2013). Reduced functional connectivity and asymmetry of the planum temporale in patients with schizophrenia and first-degree relatives. Schizophr. Res. 147, 331–338. doi:10.1016/j.schres.2013.04.024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Palaniyappan, L., and Liddle, P. F. (2012). Aberrant cortical gyrification in schizophrenia: a surface-based morphometry study. J. Psychiatry Neurosci. 37, 399–406. doi:10.1503/jpn.110119
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Palaniyappan, L., Park, B., Balain, V., Dangi, R., and Liddle, P. (2014). Abnormalities in structural covariance of cortical gyrification in schizophrenia. Brain Struct. Funct. doi:10.1007/s00429-014-0772-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ratnarajah, N., Rifkin-Graboi, A., Fortier, M. V., Chong, Y. S., Kwek, K., Saw, S. M., et al. (2013). Structural connectivity asymmetry in the neonatal brain. Neuroimage 75, 187–194. doi:10.1016/j.neuroimage.2013.02.052
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rentería, M. E. (2012). Cerebral asymmetry: a quantitative, multifactorial, and plastic brain phenotype. Twin Res. Hum. Genet. 15, 401–413. doi:10.1017/thg.2012.13
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ribolsi, M., Koch, G., Magni, V., Di Lorenzo, G., Rubino, I. A., Siracusano, A., et al. (2009). Abnormal brain lateralization and connectivity in schizophrenia. Rev. Neurosci. 20, 61–70.
Ribolsi, M., Mori, F., Magni, V., Codecà, C., Kusayanagi, H., Monteleone, F., et al. (2011). Impaired inter-hemispheric facilitatory connectivity in schizophrenia. Clin. Neurophysiol. 122, 512–517. doi:10.1016/j.clinph.2010.08.013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Roser, M., and Corballis, M. C. (2002). Interhemispheric neural summation in the split brain with symmetrical and asymmetrical displays. Neuropsychologia 40, 1300–1312. doi:10.1016/S0028-3932(01)00219-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rotarska-Jagiela, A., Oertel-Knoechel, V., DeMartino, F., van de Ven, V., Formisano, E., Roebroeck, A., et al. (2009). Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry Res. 174, 9–16. doi:10.1016/j.pscychresns.2009.03.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rotarska-Jagiela, A., Schönmeyer, R., Oertel, V., Haenschel, C., Vogeley, K., and Linden, D. E. (2008). The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. Neuroimage 39, 1522–1532. doi:10.1016/j.neuroimage.2007.10.063
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rotarska-Jagiela, A., van de Ven, V., Oertel-Knöchel, V., Uhlhaas, P. J., Vogeley, K., and Linden, D. E. (2010). Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 117, 21–30. doi:10.1016/j.schres.2010.01.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Saenger, V. M., Barrios, F. A., Martinez-Gudino, M. L., and Alcauter, S. (2012). Hemispheric asymmetries of functional connectivity and grey matter volume in the default mode network. Neuropsychologia 50, 1308–1315. doi:10.1016/j.neuropsychologia.2012.02.014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Seok, J. H., Park, H. J., Chun, J. W., Lee, S. K., Cho, H. S., Kwon, J. S., et al. (2007). White matter abnormalities associated with auditory hallucinations in schizophrenia: a combined study of voxel-based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry Res. 156, 93–104. doi:10.1016/j.pscychresns.2007.02.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shapleske, J., Rossell, S. L., Woodruff, P. W., and David, A. S. (1999). The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res. Brain Res. Rev. 29, 26–49. doi:10.1016/S0165-0173(98)00047-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Smallman, R. P., Barkus, E., Azadbakht, H., Embleton, K. V., Haroon, H. A., Lewis, S. W., et al. (2014). MRI diffusion tractography study in individuals with schizotypal features: a pilot study. Psychiatry Res. 221, 49–57. doi:10.1016/j.pscychresns.2013.10.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Snook, L., Paulson, L. A., Roy, D., Phillips, L., and Beaulieu, C. (2005). Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage 26, 1164–1173. doi:10.1016/j.neuroimage.2005.03.016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sommer, I. E., Ramsey, N. F., and Kahn, R. S. (2001). Language lateralization in schizophrenia, an fMRI study. Schizophr. Res. 52, 57–67. doi:10.1016/S0920-9964(00)00180-8
Stanfield, A. C., Moorhead, T. W., Harris, J. M., Owens, D. G., Lawrie, S. M., and Johnstone, E. C. (2008). Increased right prefrontal cortical folding in adolescents at risk of schizophrenia for cognitive reasons. Biol. Psychiatry 63, 80–85. doi:10.1016/j.biopsych.2007.04.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sumich, A., Chitnis, X. A., Fannon, D. G., O’Ceallaigh, S., Doku, V. C., Faldrowicz, A., et al. (2005). Unreality symptoms and volumetric measures of Heschl’s gyrus and planum temporal in first-episode psychosis. Biol. Psychiatry 57, 947–950. doi:10.1016/j.biopsych.2004.12.041
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sumich, A., Chitnis, X. A., Fannon, D. G., O’Ceallaigh, S., Doku, V. C., Falrowicz, A., et al. (2002). Temporal lobe abnormalities in first-episode psychosis. Am. J. Psychiatry 159, 1232–1235. doi:10.1176/appi.ajp.159.7.1232
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sun, Y., Lim, J., Kwok, K., and Bezerianos, A. (2014). Functional cortical connectivity analysis of mental fatigue unmasks hemispheric asymmetry and changes in small-world networks. Brain Cogn. 85, 220–230. doi:10.1016/j.bandc.2013.12.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Szeszko, P. R., Robinson, D. G., Ashtari, M., Vogel, J., Betensky, J., Sevy, S., et al. (2008). Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology 33, 976–984. doi:10.1038/sj.npp.1301480
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Takao, H., Hayashi, N., and Ohtomo, K. (2013). White matter microstructure asymmetry: effects of volume asymmetry on fractional anisotropy asymmetry. Neuroscience 231, 1–12. doi:10.1016/j.neuroscience.2012.11.038
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Thatcher, R. W., Biver, C. J., and North, D. (2007). Spatial-temporal current source correlations and cortical connectivity. Clin. EEG Neurosci. 38, 35–48. doi:10.1177/155005940703800109
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Thiebaut de Schotten, M., Ffytche, D. H., Bizzi, A., Dell’Acqua, F., Allin, M., Walshe, M., et al. (2011). Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage 54, 49–59. doi:10.1016/j.neuroimage.2010.07.055
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Toga, A. W., and Thompson, P. M. (2003). Temporal dynamics of brain anatomy. Annu. Rev. Biomed. Eng. 5, 119–145. doi:10.1146/annurev.bioeng.5.040202.121611
Tucker, D. M., Roth, D. L., and Bair, T. B. (1986). Functional connections among cortical regions: topography of EEG coherence. Electroencephalogr. Clin. Neurophysiol. 63, 242–250. doi:10.1016/0013-4694(86)90092-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
van der Knaap, L. J., and van der Ham, I. J. (2011). How does the corpus callosum mediate interhemispheric transfer? A review. Behav. Brain Res. 223, 211–221. doi:10.1016/j.bbr.2011.04.018
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vercammen, A., Knegtering, H., Liemburg, E. J., den Boer, J. A., and Aleman, A. (2010). Functional connectivity of the temporo-parietal region in schizophrenia: effects of rTMS treatment of auditory hallucinations. J. Psychiatr. Res. 44, 725–731. doi:10.1016/j.jpsychires.2009.12.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Voineskos, A. N., Farzan, F., Barr, M. S., Lobaugh, N. J., Mulsant, B. H., Chen, R., et al. (2010). The role of the corpus callosum in transcranial magnetic stimulation induced interhemispheric signal propagation. Biol. Psychiatry 68, 825–831. doi:10.1016/j.biopsych.2010.06.021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Von Der Heide, R. J., Skipper, L. M., Klobusicky, E., and Olson, I. R. (2013). Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain 136, 1692–1707. doi:10.1093/brain/awt094
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Weinberger, D. R., Luchins, D. J., Morihisa, J., and Wyatt, R. J. (1982). Asymmetrical volumes of the right and left frontal and occipital regions of the human brain. Ann. Neurol. 11, 97–100. doi:10.1002/ana.410110118
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Westerhausen, R., Huster, R. J., Kreuder, F., Wittling, W., and Schweiger, E. (2007). Corticospinal tract asymmetries at the level of the internal capsule: is there an association with handedness? Neuroimage 37, 379–386. doi:10.1016/j.neuroimage.2007.05.047
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Westerhausen, R., Kreuder, F., Woerner, W., Huster, R. J., Smit, C. M., Schweiger, E., et al. (2006). Interhemispheric transfer time and structural properties of the corpus callosum. Neurosci. Lett. 409, 140–145. doi:10.1016/j.neulet.2006.09.028
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wheeler, A. L., and Voineskos, A. N. (2014). A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front. Hum. Neurosci. 8:653. doi:10.3389/fnhum.2014.00653
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
White, T., and Hilgetag, C. C. (2011). Gyrification and neural connectivity in schizophrenia. Dev. Psychopathol. 23, 339–352. doi:10.1017/S0954579410000842
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Whitford, T. J., Kubicki, M., Ghorashi, S., Schneiderman, J. S., Hawley, K. J., McCarley, R. W., et al. (2011). Predicting inter-hemispheric transfer time from the diffusion properties of the corpus callosum in healthy individuals and schizophrenia patients: a combined ERP and DTI study. Neuroimage 54, 2318–2329. doi:10.1016/j.neuroimage.2010.10.048
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Whitford, T. J., Kubicki, M., Schneiderman, J. S., O’Donnell, L. J., King, R., Alvarado, J. L., et al. (2010). Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biol. Psychiatry 68, 70–77. doi:10.1016/j.biopsych.2010.03.025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wolf, R. C., Höse, A., Frasch, K., Walter, H., and Vasic, N. (2008). Volumetric abnormalities associated with cognitive deficits in patients with schizophrenia. Eur. Psychiatry 23, 541–548. doi:10.1016/j.eurpsy.2008.02.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yan, H., Tian, L., Yan, J., Sun, W., Liu, Q., Zhang, Y. B., et al. (2012). Functional and anatomical connectivity abnormalities in cognitive division of anterior cingulate cortex in schizophrenia. PLoS ONE 7:e45659. doi:10.1371/journal.pone.0045659
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yan, H., Zuo, X. N., Wang, D., Wang, J., Zhu, C., Milham, M. P., et al. (2009). Hemispheric asymmetry in cognitive division of anterior cingulate cortex: a resting-state functional connectivity study. Neuroimage 47, 1579–1589. doi:10.1016/j.neuroimage.2009.05.080
Zhang, D., Guo, L., Hu, X., Li, K., Zhao, Q., and Liu, T. (2012). Increased cortico-subcortical functional connectivity in schizophrenia. Brain Imaging Behav. 6, 27–35. doi:10.1007/s11682-011-9138-z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhang, R., Wei, Q., Kang, Z., Zalesky, A., Li, M., Xu, Y., et al. (2014). Disrupted brain anatomical connectivity in medication-naïve patients with first-episode schizophrenia. Brain Struct. Funct. doi:10.1007/s00429-014-0706-z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: schizophrenia, connectivity, asymmetry, interhemispheric, diffusion tensor imaging, fMRI
Citation: Ribolsi M, Daskalakis ZJ, Siracusano A and Koch G (2014) Abnormal asymmetry of brain connectivity in Schizophrenia. Front. Hum. Neurosci. 8:1010. doi: 10.3389/fnhum.2014.01010
Received: 23 June 2014; Accepted: 26 November 2014;
Published online: 22 December 2014.
Edited by:
Silvio Ionta, Centre Hospitalier Universitaire Vaudois and University of Lausanne SwitzerlandReviewed by:
Wi Hoon Jung, University of Pennsylvania, USAThilo Kellermann, RWTH Aachen University, Germany
Branislava Curcic-Blake, University Medical Center Groningen, Netherlands
Copyright: © 2014 Ribolsi, Daskalakis, Siracusano and Koch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michele Ribolsi, Dipartimento di Medicina dei Sistemi, Clinica Psichiatrica, Università Tor Vergata, Via Nomentana 1362, Rome 00137, Italy e-mail:bWljaGVsZS44MUBsaXZlLml0
 Michele Ribolsi
Michele Ribolsi Zafiris J. Daskalakis
Zafiris J. Daskalakis Alberto Siracusano1
Alberto Siracusano1 Giacomo Koch
Giacomo Koch