
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci. , 01 July 2014
Sec. Speech and Language
Volume 8 - 2014 | https://doi.org/10.3389/fnhum.2014.00473
This article is part of the Research Topic Neural bases of speech motor control: have we made progress since Broca’s era? View all 7 articles
 Martine Hoogman1*
Martine Hoogman1* Tulio Guadalupe1
Tulio Guadalupe1 Marcel P. Zwiers2
Marcel P. Zwiers2 Patricia Klarenbeek2
Patricia Klarenbeek2 Clyde Francks1,2
Clyde Francks1,2 Simon E. Fisher1,2*
Simon E. Fisher1,2*The FOXP2 transcription factor is one of the most well-known genes to have been implicated in developmental speech and language disorders. Rare mutations disrupting the function of this gene have been described in different families and cases. In a large three-generation family carrying a missense mutation, neuroimaging studies revealed significant effects on brain structure and function, most notably in the inferior frontal gyrus, caudate nucleus, and cerebellum. After the identification of rare disruptive FOXP2 variants impacting on brain structure, several reports proposed that common variants at this locus may also have detectable effects on the brain, extending beyond disorder into normal phenotypic variation. These neuroimaging genetics studies used groups of between 14 and 96 participants. The current study assessed effects of common FOXP2 variants on neuroanatomy using voxel-based morphometry (VBM) and volumetric techniques in a sample of >1300 people from the general population. In a first targeted stage we analyzed single nucleotide polymorphisms (SNPs) claimed to have effects in prior smaller studies (rs2253478, rs12533005, rs2396753, rs6980093, rs7784315, rs17137124, rs10230558, rs7782412, rs1456031), beginning with regions proposed in the relevant papers, then assessing impact across the entire brain. In the second gene-wide stage, we tested all common FOXP2 variation, focusing on volumetry of those regions most strongly implicated from analyses of rare disruptive mutations. Despite using a sample that is more than 10 times that used for prior studies of common FOXP2 variation, we found no evidence for effects of SNPs on variability in neuroanatomy in the general population. Thus, the impact of this gene on brain structure may be largely limited to extreme cases of rare disruptive alleles. Alternatively, effects of common variants at this gene exist but are too subtle to be detected with standard volumetric techniques.
A significant proportion of children have unexpected problems with acquiring proficient spoken language, despite adequate intelligence and opportunity. Family and twin studies indicate that genetic factors make substantial contributions to the risk of developmental speech and language impairments (Graham and Fisher, 2013). One of the most well-known genes to have been implicated in such disorders is FOXP2 (Fisher and Scharff, 2009). FOXP2 encodes a transcription factor, a protein that directly binds to regulatory regions of other target genes and thereby modulates their expression (Vernes et al., 2007).
Disruption of one copy of FOXP2 leads to problems with mastering coordinated sequences of speech movements (known as childhood apraxia of speech, CAS, or developmental verbal dyspraxia, DVD), accompanied by broad difficulties in language expression and comprehension, also affecting written modalities (Watkins et al., 2002a). A number of distinct etiological mutations affecting this gene have been discovered, in different families and cases (Fisher and Scharff, 2009). These range from missense mutations (Lai et al., 2001; Laffin et al., 2012), non-sense mutations (MacDermot et al., 2005) and indels (Turner et al., 2013), to gross chromosomal abnormalities like translocations (Shriberg et al., 2006; Kosho et al., 2008) and deletions (Zeesman et al., 2006; Palka et al., 2012; Rice et al., 2012; Zilina et al., 2012).
The most thoroughly studied FOXP2 disruption is a heterozygous missense mutation that co-segregates with speech and language disorder in 15 members of a three generation pedigree, known as the KE family (Fisher et al., 1998). The mutation, which is exclusive to this particular family, yields an arginine-to-histidine substitution in the DNA-binding domain of the encoded protein (Lai et al., 2001), which impairs its function (Vernes et al., 2006). Magnetic resonance imaging (MRI) of the KE family indicates overtly normal brain structure in the affected members, but in-depth statistical analysis using voxel-based morphometry (VBM) has uncovered a number of distributed sites showing significant differences from unaffected people. Bilateral reductions in gray-matter density were noted in the inferior frontal gyrus, caudate nucleus, precentral gyrus, temporal pole, and cerebellum, while increases were reported in the posterior superior temporal gyrus, angular gyrus, and putamen (Watkins et al., 2002b; Belton et al., 2003). Positron Emission Tomography of affected KE subjects on word repetition tasks revealed overactivation of left caudate nucleus, and left premotor cortex, with an extension into Brodmann Area (BA) 44 (Vargha-Khadem et al., 1998). Moreover, in functional magnetic resonance imaging (fMRI) studies with verb-generation tasks, affected family members showed underactivation of the left inferior gyrus and the putamen, even when no vocal output was required (Liégeois et al., 2003). Overall, the inferior frontal gyrus, striatum (in particular the caudate nucleus), and cerebellum are sites of pathology that have been most consistently associated with FOXP2 disruption in multiple studies (Vargha-Khadem et al., 1998; Watkins et al., 2002b; Belton et al., 2003; Liégeois et al., 2003). Intriguingly, analyses of human brain tissue have shown that deep layers of the cortex, medium spiny neurons of the striatum, and Purkinje cells of the cerebellum, are crucial neuronal subpopulations that most highly express FOXP2 during early development (Lai et al., 2003). These independent findings indicate remarkable overlaps with the neuroimaging findings (Lai et al., 2003).
The imaging studies in the KE family have clearly shown that a rare high-penetrant mutation which severely disrupts FOXP2 is linked with alterations in brain structure and function in the people who carry it, with major consequences for their development of speech and language skills. These intriguing findings have raised new research questions, such as whether or not the same genetic locus harbors common DNA variants with more modest effects on brain structure and function. Do such gene variants have detectable impacts on aspects of brain anatomy, neural activation and/or behavior, in other language-related disorders or in the general population? Researchers have sought to answer these questions by assessing single-nucleotide-polymorphisms (SNPs) in a range of studies with different disorders, and in typically developing people (see Table 1 and Figure 1, for summary).
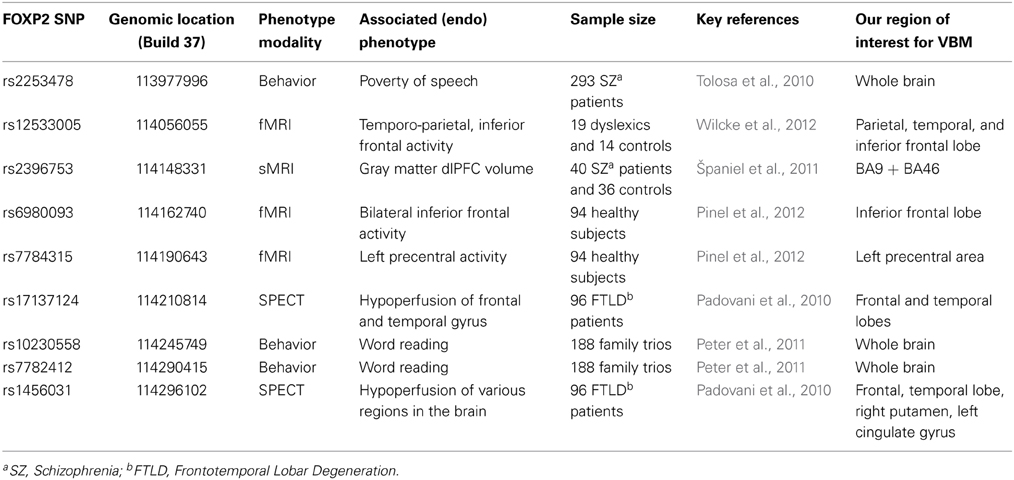
Table 1. Overview of previous studies of common variation in FOXP2 and our corresponding regions of interest for VBM analysis.
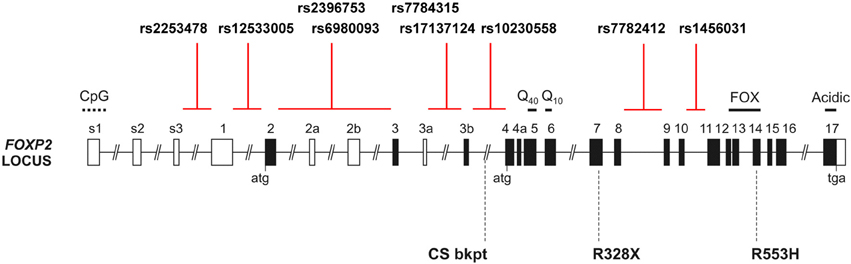
Figure 1. The human FOXP2 locus. Schematic of the human FOXP2 locus, which spans >600 kb in chromosomal band 7q31, showing the intronic locations of candidate SNPs from prior studies of common variation. Black shading indicates translated exons; “atg” and “tga” denote positions of initiation and termination codons. Known domains encoded by exons include polyglutamine tracts (Q40 and Q10), the forkhead domain (FOX), and an acidic C-terminus. Exons 3b and 4a are alternatively spliced coding exons yielding amino acid insertions, whereas alternatively spliced exons 2a, 2b, and 3a are predicted to be non-coding. Exons s1–s3 and 1 represent alternative 5′ UTR regions. CpG marks the site of a CpG island. Three rare disruptive mutations reported in children with severe speech and language impairment are indicated below the locus schematic: the R553H mutation initially discovered in the KE family, an R328X mutation identified in another family and a translocation breakpoint found in an unrelated case (CS) (Lai et al., 2001; MacDermot et al., 2005). Multiple additional point mutations and chromosomal rearrangements have been reported (Graham and Fisher, 2013).
One study assessed four common FOXP2 SNPs in patients suffering from frontotemporal lobar degeneration (FTLD), a neurodegenerative disorder which can involve breakdown of speech fluency, and reported that alleles of rs1456031 and rs17137124 were additively associated with scores on verbal fluency tasks (Padovani et al., 2010). Based on single-photon emission computed tomography (SPECT) imaging of 96 of the FTLD patients, the authors suggested that alleles of rs1456031 were associated with differential hypoperfusion (local decreased blood flow possibly leading to cell death) in frontal and temporal gyri, right putamen, and left cingulate gyrus. They also reported that rs17137124 variants were associated with differential hypoperfusion in frontal and temporal regions (Padovani et al., 2010). In a subsequent investigation of 34 patients with primary progressive aphasia, the same team proposed that the putative risk alleles of these SNPs were associated with greater hypoperfusion in frontal areas, particularly the left inferior frontal gyrus and the right cingulate gyrus (Premi et al., 2012).
In addition, FOXP2 polymorphisms have been investigated in relation to schizophrenia, which some researchers propose to be a language-related disorder (Tolosa et al., 2010). A VBM study of neuroanatomy in 40 schizophrenia patients targeted rs2396753, a SNP that had been previously associated with auditory hallucinations (Sanjuán et al., 2006), and reported that the C allele was correlated with reductions in gray matter volume in the dorsolateral prefrontal cortex (dlPFC) (Španiel et al., 2011). Another study in 293 schizophrenia patients suggested that a different SNP, rs2253478, was associated with poverty of speech in schizophrenia, but the relevance of this polymorphism for neuroanatomy was not investigated (Tolosa et al., 2010).
The effects of common variants of FOXP2 have also been investigated using functional neuroimaging. Wilcke and colleagues assessed rs12533005 in relation to fMRI data from a rhyming task in a cohort of 19 dyslexics and 14 controls, and reported a main effect of the SNP in two temporo-parietal brain areas (the angular and the supramarginal gyrus), as well as an interaction between dyslexia status and SNP alleles, reported to affect activation of inferior frontal regions (Wilcke et al., 2012). Another study, in 94 healthy adults, reported associations of rs6980093 with variations in bilateral inferior frontal activity and rs7784315 with variations in left precentral activity, as assessed by fMRI during a reading task (Pinel et al., 2012). In a behavioral study of 188 family trios with dyslexia, rs10230558, rs12533005, and rs7782412 were associated with articulation and word reading phenotypes (Peter et al., 2011). However, common variation in FOXP2 remains relatively under-studied with regard to natural variability in language performance in the general population. A recent behavioral study of 456 healthy subjects reported that rs2396753 and rs12533005 were associated with performance on a dichotic listening task; the authors thus proposed that these SNPs modulate hemispheric asymmetries for speech perception, although again there was no neuroimaging data included (Ocklenburg et al., 2013).
As the above literature review shows, the potential impact of common variation in FOXP2 remains open to debate. In particular, while common FOXP2 SNPs have been the subject of multiple neuroimaging genetics studies, all such investigations have involved notably small sample sizes with low power and high susceptibility to false positive findings (Button et al., 2013), and there are no reports of independent replications. In the current investigation, we assessed the effects of common variants of FOXP2 on brain structure using a substantial dataset of 1301 typically developing adult subjects from the general population, a sample which is more than 10 times larger than those used for previous neuroimaging genetic studies of this gene. To provide a statistically robust study design, we carried out our investigation in stages.
First, we checked the common variants of FOXP2 that have been proposed to have effects on neuroanatomy, function or behavior/cognition in the prior smaller studies (Table 1 and outlined above). Where possible, we tested specific hypotheses regarding particular brain regions, based on the claims made in these previous reports (Table 1). For SNPs that have been argued to affect neuroanatomy, we could focus our analyses on regions highlighted in the relevant earlier study. If a SNP was previously proposed to alter functional activation, we again targeted the site(s) implicated from the prior report, looking in our sample for effects on structure of that candidate region. This strategy is based on well-established findings of convergent functional and structural effects due to rare severe FOXP2 disruptions; people carrying such mutations show altered activation on language tasks as well as structural changes detectable by volumetric approaches, affecting the same regions (Vargha-Khadem et al., 1998; Watkins et al., 2002b; Liégeois et al., 2003). Moreover, the downstream pathways regulated by FOXP2 include targets that affect both structural and functional properties of neural circuits (Vernes et al., 2011; French et al., 2012). Since we could not make a clear prediction about the expected direction of effect, we carried out statistical tests that were two-tailed. Some of the candidate SNPs had only been assessed in relation to behavior/cognition in prior studies, so in those cases we did not have a predefined brain region of interest (Table 1). Thus, for all candidate SNPs we went on to carry out a broader evaluation of potential effects anywhere in the brain. In the final stage of our investigation, we performed a gene-wide analysis that captured the majority of common variation in FOXP2, to systematically assess associations with relevant neuroanatomical phenotypes in our large sample.
The study sample consisted of healthy adult subjects taking part in the Brain Imaging Genetics (BIG) study in Nijmegen, The Netherlands (Franke et al., 2010). This study was initiated in 2007 and comprises self-reportedly healthy volunteers who participate in studies at the Donders Centre for Cognitive Neuroimaging, Nijmegen, The Netherlands. All subjects have structural MRI data available as part of their involvement in diverse smaller-scale studies and gave their consent to be part of the BIG study. In addition, for 1301 subjects genome-wide genotyping was also available (Guadalupe et al., 2014) and these subjects were selected for the current study. Subjects were of Caucasian descent with no self-reported neurological or psychiatric history, and mainly had a high level of education (80% with a bachelor student level or higher). The median age was 22.0 years (range 18–55 years) and 41% of the sample was male. All participants gave written informed consent and the study was approved by the local ethics committee (CMO Region Arnhem-Nijmegen, The Netherlands).
To obtain DNA, saliva was collected using Oragene containers (DNA Genotek, Ottawa, ON, Canada). Isolation of DNA was done by the Human Genetics Department of the Radboud University Medical Centre, Nijmegen, The Netherlands. Whole genome genotyping was done using Affymetrix GeneChip SNP, 6.0 (Affymetrix Inc., Santa Clara, CA). For the first stage of analyses, candidate SNPs from prior studies rs2253478, rs12533005, rs2396753, rs6980093, rs7784315, rs17137124, rs10230558, rs7782412, and rs1456031 were extracted from this dataset using PLINK v1.07 (http://pngu.mgh.harvard.edu/purcell/plink) (Purcell et al., 2007). For the second stage of analyses, all SNPs within the gene boundaries of FOXP2 (USCS Genome Bioinformatics Site, http://genome.ucsc.edu/) including 25 kb flanking regions to capture regulatory sequences, were extracted. SNPs were excluded when they showed a minor allele frequency of less than 1%, failed the Hardy–Weinberg Equilibrium test (p < 0.000005) or had a genotyping rate below 95%. This resulted in 1180 SNPs.
Anatomical T1-weighted whole brain MPRAGE scans were acquired at the Donders Centre for Cognitive Neuroimaging using a 1.5T scanner (Sonata and Avanto, Siemens, Erlangen, Germany) or a 3T scanner (Trio and TrioTim, Siemens, Erlangen, Germany). The imaging protocols of the T1 scans included small variations, due to the fact that the images were acquired during several studies. The most common variations included the following parameters and values: TR/TI/TE/sagittal-slices: 2300/1100/3.03/192; 2730/1000/2.95/176; 2250/850/2.95/176; 2250/850/3.93/176; 2250/850/3.68/176; 2300/1100/3.03/192; 2300/1100/2.92/192; 2300/1100/2.96/192; 2300/1100/2.99/192; 1940/1100/3.93/176; and 1960/1100/4.58/176. Slight variations in these imaging parameters have been shown not to affect the reliability of morphometric results (Jovicich et al., 2009).
To study local differences in gray and white matter related to genetic variation we used a VBM protocol. For this analysis, T1-images were processed using the default procedures of the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/), implemented in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Using a unified model, T1-images were bias-field corrected, segmented into gray, white matter, and cerebro-spinal fluid and normalized to standard space (as defined by the Montreal Neurological Institute; MNI) by high-dimensional DARTEL warping (Ashburner, 2007). The resulting images were modulated by the non-linear part of their DARTEL warp field and smoothed with an 10 mm FWHM Gaussian smoothing kernel, providing for an analysis of relative differences in regional gray and white matter volume, corrected for individual brain size.
To study the effects of all common variation in FOXP2 on candidate brain regions from prior studies of rare variation, volumes of the caudate nucleus, cerebellum and inferior frontal cortex were segmented using FreeSurfer version 5.1 using labels “caudate right” + “caudate left” as caudate nucleus volume; “cerebellum cortex right” + “cerebellum cortex left” as cerebellum volume; and “left and right pars orbitalis” + “left and right pars triangularis” + “left and right pars opercularis” as inferior frontal cortex volume. These volumes were produced with the standard “-recon-all” processing pipeline and default parameters. Estimates of total brain volume (TBV), for inclusion as a covariate, were calculated as the voxel-wise sum of the native gray matter and white matter probability maps from our VBM processing pipeline.
For the VBM analysis the smoothed images were used in multiple regression analysis implemented in SPM8, to test for volumetric differences in relation to SNP genotypes. The effect of each candidate SNP was tested in a separate multiple regression. Genotypes of each SNP were coded to represent a linear allelic additive effect, and age and sex were used as linear covariates. Scanner field strength was also included as a covariate. Gray and white matter analyses were done separately. After grouping according to genotype, outlier analysis implemented in the VBM toolbox identified images with poor quality or artifacts. Images that showed a deviation of more than 1.5 times the interquartile range from the median were excluded from further analysis. To first assess specific claims about SNP associations from the prior literature we applied small volume corrections (pFWE < 0.05) to the regions proposed by the original report. We used regions defined by the WFU pickatlas and included BA 9+46 as dlPFC, the parietal, temporal and (inferior) frontal lobe, left precentral area, putamen, and cingulate (see Table 1). If in the original VBM study, effects on white matter were reported, then association was only tested in white matter, whereas if the original study posited effects on gray matter, then association was only tested in gray matter. If the original study was an fMRI or SPECT study, we tested both gray and white matter. If a FOXP2 SNP had been reported to be associated only with a cognitive/behavioral trait (i.e., without investigating a neuroimaging phenotype) in the prior study, then we performed brain-wide analyses using cluster-extent statistics (pFWE < 0.05) instead of testing for peaks within predefined regions of interest. Clusters were formed using puncorrected < 0.001 and corrected for non-stationarity in the data (Hayasaka et al., 2004). For the candidate FOXP2 SNPs from the prior neuroimaging genetics reports, we also went on to perform a final exploratory search, testing not only in the brain regions of the previous association, but also across the entire brain using a puncorrected < 0.001. In such analyses, we split our sample into a 1.5 Tesla discovery cohort (n = 648) and a 3 Tesla replication cohort (n = 653), a strategy that has been adopted in earlier published investigations of the Nijmegen BIG sample (e.g., Cousijn et al., 2012).
In the second stage of our analyses, we systematically assessed all common variants of FOXP2 in the BIG dataset. We tested these for effects on volumetric measures of three brain regions, based on prior neuroimaging studies of rare FOXP2 mutations: the inferior frontal gyrus, caudate nucleus, and cerebellum (Vargha-Khadem et al., 1998; Watkins et al., 2002b; Belton et al., 2003; Liégeois et al., 2003). A whole gene linear regression analysis was performed for each of 1180 SNPs and 3 regional volumes separately using PLINK, and with covariates age, gender, TBV, and field strength of the scanner. A multiple-testing correction was performed by running 10,000 max (T) permutation test using the “mperm” command and saving all the observed and permuted data using the “mperm-save-all” command. These data were combined to create a summed statistic per run for all SNPs at the same time (10,001 in total, one for the observed data and 10,000 for the permuted data). The empirical p-value was then estimated by the number of times the sum of the observed summed statistic was smaller than the sum of the permuted statistic, divided by the total number of permutations (10,000) (Bralten et al., 2011). To find out where in the gene the effect was most prominent, the single SNP p-values were evaluated. Adding the mperm command in PLINK gives empirical p-values for each SNP, corrected for the number of SNPs in the analysis.
The genotype distributions in our sample are displayed in Table 2. The genotype distribution of rs7784315 resulted in a relatively small group of minor allele homozygotes and therefore we combined this group with the heterozygotes for our association analysis. The imputation quality of rs7782412 was below standard; 39% of the genotype calls had a probability of lower than 0.9, necessitating the use of another SNP as a proxy. The best SNP that could act as a proxy was rs12705966, located 41 kb upstream of rs7782412, and the two markers have an R2 of 0.66 and a d′ of 1.0.
An effect of rs2396753 on gray matter volume in the dlPFC that was suggested in a previous VBM study (Španiel et al., 2011) could not be replicated in the current study. There were no significant voxels at puncorrected = 0.001, nor were there any other effects elsewhere in the brain.
Variation in rs17137124, previously associated with frontal degeneration (Padovani et al., 2010), showed a weak association with white matter in the frontal lobe, F = 18.82, pFWE = 0.04, peak voxel at 16, 66, −3, cluster size = 1263, Figure 2. White matter density was highest in the group of C-allele homozygotes.
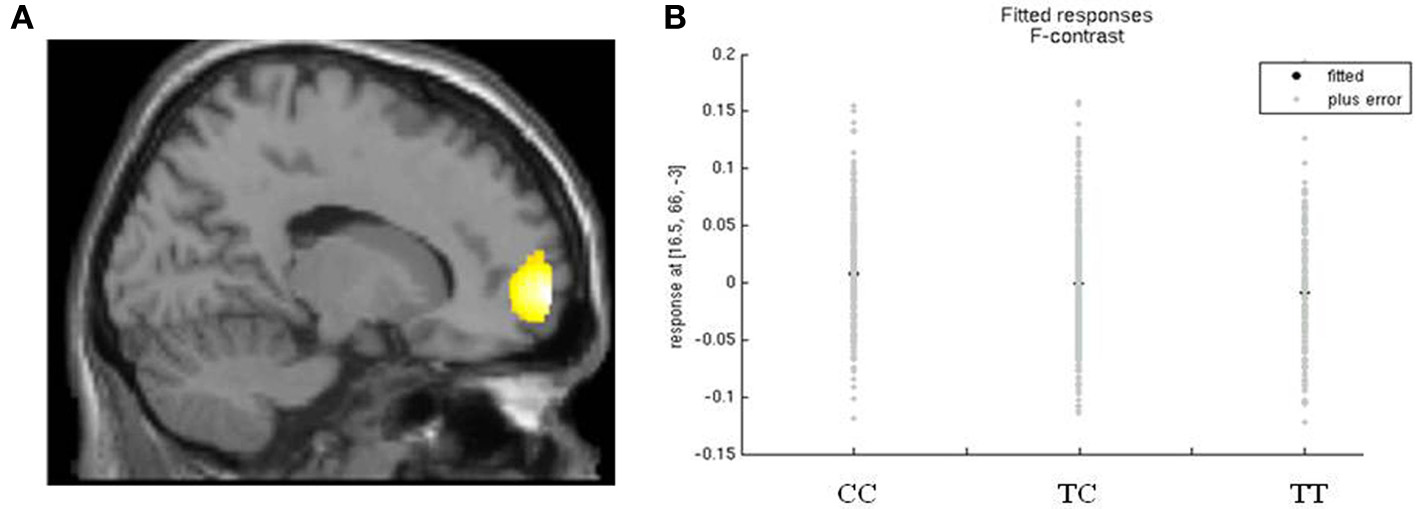
Figure 2. rs17137124 and white matter density in the frontal lobe. (A) Relationship between white matter volume in the frontal lobe and variation in rs17137124, at x = 16. (B) Direction of the effect of rs17137124 at peak voxel x = 16, y = 66, z = −3, with the CC group having the highest white matter values. Voxel co-ordinates are given in MNI (Montreal Neurological Institute) space.
The other proposed candidate SNPs listed in Table 1 (Padovani et al., 2010; Tolosa et al., 2010; Peter et al., 2011; Pinel et al., 2012; Wilcke et al., 2012) did not show any association with the structural brain phenotypes measured with VBM either using a region of interest or in a brain wide analysis.
The results of the gene-wide analysis revealed no significant whole gene FOXP2 effects on caudate nucleus (pempirical = 0.81), cerebellar gray matter (pempirical = 0.71) or inferior frontal volume (pempirical = 0.84). Some SNPs were suggestive (uncorrected p-value < 0.05) of an association with the caudate nucleus volume. However, these results did not survive correction for multiple testing (Table 3). The SNP rs144807019 had the lowest p-value (puncorrected = 0.002, pcorrected = 0.16). This SNP is in close proximity (~4 kb) to rs1456031, a SNP previously associated with poverty of speech (Tolosa et al., 2010).
The present study went beyond the established impact of rare highly penetrant mutations of FOXP2 on brain structure, to investigate whether frequent polymorphisms at this locus have effects on normal variation in neuroanatomy in the general population. In the first stage, we targeted common SNPs that have been claimed to have phenotypic effects in prior studies. Despite using a large sample of 1301 healthy participants, more than 10 times larger than any prior neuroimaging genetics study of FOXP2, we did not detect significant associations. The sole exception was rs17137124, showing a borderline significant association with white matter density in the frontal lobe, an effect that would not be robust to adjustment for multiple testing.
The lack of effects may be explained by several different factors, which are not mutually exclusive. First, we note that the previous positive findings in neuroimaging genetics of common variations in FOXP2 have all come from studies with small sample sizes, in scanned groups ranging from a maximum of 96 (Padovani et al., 2010) to as few as 14 participants (Wilcke et al., 2012). Small sample sizes in imaging genetics studies not only lead to reduced power, they make the analyses susceptible to an elevated rate of type I errors (Button et al., 2013). Therefore, at least some of the original reports of SNP associations may represent false-positive findings, especially since the p-values in many of these studies were only marginally significant. Second, some of the prior studies of common FOXP2 variants involved analyses of task-related activations via functional neuroimaging or associations with behavioral traits, whereas the current study focused on effects on brain structure. Thus, it is conceivable that the candidate SNPs are associated with alterations in aspects of brain function or behavioral output without detectable impacts on neuroanatomy. On the other hand, as discussed further below, prior studies of FOXP2 disruptions have demonstrated effects on both function and structure of the relevant brain circuits (Vargha-Khadem et al., 1998; Watkins et al., 2002b; Liégeois et al., 2003), and the gene is known to regulate targets with roles in neurite outgrowth, axon guidance, and synaptogenesis (Vernes et al., 2011). Future genetic association studies involving functional neuroimaging during language-related tasks in large samples (hundreds, rather than tens of individuals) are needed to properly address this issue. Third, a number of the previous investigations targeted disease cohorts (FTLD, schizophrenia or dyslexia) while this study involved an unselected general population sample. Thus, it might be argued that effects of some of these variants are only relevant for modulating phenotypes within people who have a disorder. However, these are all common disorders, and given the high frequency of the relevant SNPs in healthy individuals, one might expect to uncover some evidence of association with a relevant endophenotype in a large sample such as that used here. Moreover, while FOXP2 is itself poorly investigated in relation to language skills in the normal range, it has been shown that targets downstream of this transcription factor have effects that are not only relevant to disorder but also to language performance in the general population (Vernes et al., 2008; Whitehouse et al., 2011).
As far as we are aware, for the candidate SNPs of FOXP2 that have been claimed to have effects on brain structure or function in prior studies, no empirical studies have been carried out to determine their likely impact at the molecular or cellular level. None of the known candidate SNPs change the amino-acid sequence of the encoded protein, so they do not affect its shape or its functional properties (Figure 1). DNA variants that do not alter protein sequences can still have effects on function, for example, by altering how much of the relevant protein is made in any particular cell, how the protein levels are able to change in respond to signals, and/or another aspect of its regulation (Fisher, 2006). The effects of such common regulatory SNPs are typically subtle and can be difficult to demonstrate. Moreover, when multiple common SNPs lie close to each other and tend to be coinherited (i.e., they are in linkage disequilibrium) it is hard to determine which of the neighboring variants provides the functional explanation for an observed association with a phenotypic trait. So far, studies of common FOXP2 variation have simply assumed that the associated SNPs must be regulatory variants (or are in linkage disequilibrium with regulatory variants) that modulate the expression of the gene, in some undetermined way, without testing this assumption in a cellular assay or other model system. The lack of experimental studies on common variants, in cellular and animal models, is in stark contrast to the in-depth work that has been performed for rare mutations of this gene (Vernes et al., 2006, 2011; Groszer et al., 2008; French et al., 2012; Kurt et al., 2012). It will be important in future to use functional genomics in model systems to increase our understanding of how non-coding regulatory sequences at the FOXP2 locus affect its expression and function. Findings from such efforts should be closely integrated with ongoing work on phenotypic associations in human datasets, whether from disease cohorts or healthy populations, to increase chance of uncovering biologically valid results (Deriziotis and Fisher, 2013).
A fully gene-wide view did not uncover any common FOXP2 SNPs as new candidates for having effects on brain structure, at least for the neuroanatomical phenotypes that we were able to study here. Our focus here was on brain structures that have been robustly connected with FOXP2 functions in prior work on rare mutations and animal models. It is well-established that disruption of the FOXP2 gene yields detectable alterations of distributed corticostriatal and corticocerebellar brain circuits, affecting both their structural architecture and functional properties. This conclusion is supported not only by neuroimaging studies of humans carrying heterozygous mutations that disturb FOXP2 protein function (as described in the Introduction), but also by diverse investigations of genetically manipulated animal models (Fisher and Scharff, 2009). For example, for mice that carry Foxp2 mutations, matching those implicated in speech and language disorder, there have been reports of effects on neurite outgrowth (Vernes et al., 2011), task-related neural firing (French et al., 2012), and synaptic plasticity (Groszer et al., 2008) in the relevant brain regions, associated with deficits in acquisition of motor-skills and impaired learning of auditory-motor associations (Kurt et al., 2012). Knockdown of the avian ortholog in a key striatal nucleus of the zebrafinch brain reduces spine density (Schulz et al., 2010), disturbs dopaminergic modulation of corticostriatal signaling (Murugan et al., 2013), leading to reduced vocal plasticity and impaired learning of song (Haesler et al., 2007). Thus, the choice of brain structures for the current gene-wide study of common variants was strongly grounded in existing knowledge about the roles of the gene, but no evidence of effects on the structures of relevant regions could be detected.
Given our sample size and design, we estimated our candidate region VBM analyses had sufficient power (80%) to detect allelic effects small enough to explain 1.7% of the phenotypic variance voxel-wise. Our exploratory VBM analyses had enough power to detect effects as small as 3.4% of the voxel-wise variance, while our gene-wide analysis of FOXP2 variants had enough power to detect an effect of 2% on the phenotypic variance in regional volumes (calculated in G*Power; Faul et al., 2007). It is thus possible that common variations at the FOXP2 locus do not contribute to variability in relevant aspects of neuroanatomy in the general population, and that its effects on brain structure are mainly evident in extreme cases of rare disruptive alleles. Alternatively, there might be common variants at this gene with effects of a subtle nature, or which impact on aspects of neuroanatomy that are more difficult to detect with standard volumetric techniques. In particular, given the prior evidence of a link between FOXP2 and neurite outgrowth and axon guidance, investigations of structural and functional connectivity and common SNPs in sufficiently large samples may prove informative.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research was supported by the Max Planck Society. We would like to thank all the people who kindly participated in BIG. This work makes use of the BIG (Brain Imaging Genetics) database, first established in Nijmegen, The Netherlands, in 2007. This resource is now part of Cognomics (www.cognomics.nl), a joint initiative by researchers of the Donders Centre for Cognitive Neuroimaging, the Human Genetics and Cognitive Neuroscience departments of the Radboud University Medical Centre and the Max Planck Institute for Psycholinguistics in Nijmegen. The Cognomics Initiative is supported by the participating departments and centers and by external grants, i.e., the Biobanking and Biomolecular Resources Research Infrastructure (Netherlands) (BBMRI-NL), the Hersenstichting Nederland, and the Netherlands Organisation for Scientific Research (NWO).
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113. doi: 10.1016/j.neuroimage.2007.07.007
Belton, E., Salmond, C. H., Watkins, K. E., Vargha-Khadem, F., and Gadian, D. G. (2003). Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Hum. Brain Mapp. 18, 194–200. doi: 10.1002/hbm.10093
Bralten, J., Arias-Vásquez, A., Makkinje, R., Veltman, J. A., Brunner, H. G., Fernández, G., et al. (2011). Association of the Alzheimer's Gene SORL1 with hippocampal volume in young, healthy adults. Am. J. Psychiatry 168, 1083–1089. doi: 10.1176/appi.ajp.2011.10101509
Button, K. S., Ioannidis, J. P., Mokrysz, C., Nosek, B. A., Flint, J., Robinson, E. S., et al. (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376. doi: 10.1038/nrn3475
Cousijn, H., Rijpkema, M., Harteveld, A., Harrison, P. J., Fernández, G., Franke, B., et al. (2012). Schizophrenia risk gene ZNF804A does not influence macroscopic brain structure: an MRI study in 892 volunteers. Mol. Psychiatry 17, 1155–1157. doi: 10.1038/mp.2011.181
Deriziotis, P., and Fisher, S. E. (2013). Neurogenomics of speech and language disorders: the road ahead. Genome Biol. 14:204. doi: 10.1186/gb-2013-14-4-204
Faul, F., Erdfelder, E., Lang, A. G., and Buchner, A. (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. doi: 10.3758/BF03193146
Fisher, S. E. (2006). Tangled webs: tracing the connections between genes and cognition. Cognition 101, 270–297. doi: 10.1016/j.cognition.2006.04.004
Fisher, S. E., and Scharff, C. (2009). FOXP2 as a molecular window into speech and language. Trends Genet. 25, 166–177. doi: 10.1016/j.tig.2009.03.002
Fisher, S. E., Vargha-Khadem, F., Watkins, K. E., Monaco, A. P., and Pembrey, M. E. (1998). Localisation of a gene implicated in a severe speech and language disorder. Nat. Genet. 18, 168–170. doi: 10.1038/ng0298-168
Franke, B., Vasquez, A. A., Veltman, J. A., Brunner, H. G., Rijpkema, M., and Fernández, G. (2010). Genetic variation in CACNA1C, a gene associated with bipolar disorder, influences brainstem rather than gray matter volume in healthy individuals. Biol. Psychiatry 68, 586–588. doi: 10.1016/j.biopsych.2010.05.037
French, C. A., Jin, X., Campbell, T. G., Gerfen, E., Groszer, M., Fisher, S. E., et al. (2012). An aetiological Foxp2 mutation causes aberrant striatal activity and alters plasticity during skill learning. Mol. psychiatry 17, 1077–1085. doi: 10.1038/mp.2011.105
Graham, S. A., and Fisher, S. E. (2013). Decoding the genetics of speech and language. Curr. Opin. Neurobiol. 23, 43–51. doi: 10.1016/j.conb.2012.11.006
Groszer, M., Keays, D. A., Deacon, R. M., De Bono, J. P., Prasad-Mulcare, S., Gaub, S., et al. (2008). Impaired synaptic plasticity and motor learning in mice with a point mutation implicated in human speech deficits. Curr. Biol. 18, 354–362. doi: 10.1016/j.cub.2008.01.060
Guadalupe, T., Zwiers, M. P., Teumer, A., Wittfeld, K., Vasquez, A. A., Hoogman, M., et al. (2014). Measurement and genetics of human subcortical and hippocampal asymmetries in large datasets. Hum. Brain Mapp. 35, 3277–3289. doi: 10.1002/hbm.22401
Haesler, S., Rochefort, C., Georgi, B., Licznerski, P., Osten, P., and Scharff, C. (2007). Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus Area X. PLoS Biol. 5:e321. doi: 10.1371/journal.pbio.0050321
Hayasaka, S., Phan, K. L., Liberzon, I., Worsley, K. J., and Nichols, T. E. (2004). Nonstationary cluster-size inference with random field and permutation methods. Neuroimage 22, 676–687. doi: 10.1016/j.neuroimage.2004.01.041
Jovicich, J., Czanner, S., Han, X., Salat, D., Van Der Kouwe, A., Quinn, B., et al. (2009). MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage 46, 177–192. doi: 10.1016/j.neuroimage.2009.02.010
Kosho, T., Sakazume, S., Kawame, H., Wakui, K., Wada, T., Okoshi, Y., et al. (2008). De-novo balanced translocation between 7q31 and 10p14 in a girl with central precocious puberty, moderate mental retardation, and severe speech impairment. Clin. Dysmorphol. 17, 31–34. doi: 10.1097/MCD.0b013e3282f17688
Kurt, S., Fisher, S. E., and Ehret, G. (2012). Foxp2 mutations impair auditory-motor association learning. PloS ONE 7:e33130. doi: 10.1371/journal.pone.0033130
Laffin, J. J., Raca, G., Jackson, C. A., Strand, E. A., Jakielski, K. J., and Shriberg, L. D. (2012). Novel candidate genes and regions for childhood apraxia of speech identified by array comparative genomic hybridization. Genet. Med. 14, 928–936. doi: 10.1038/gim.2012.72
Lai, C. S., Fisher, S. E., Hurst, J. A., Vargha-Khadem, F., and Monaco, A. P. (2001). A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413, 519–523. doi: 10.1038/35097076
Lai, C. S., Gerrelli, D., Monaco, A. P., Fisher, S. E., and Copp, A. J. (2003). FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain 126, 2455–2462. doi: 10.1093/brain/awg247
Liégeois, F., Baldeweg, T., Connelly, A., Gadian, D. G., Mishkin, M., and Vargha-Khadem, F. (2003). Language fMRI abnormalities associated with FOXP2 gene mutation. Nat. Neurosci. 6, 1230–1237. doi: 10.1038/nn1138
MacDermot, K. D., Bonora, E., Sykes, N., Coupe, A. M., Lai, C. S., Vernes, S. C., et al. (2005). Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am. J. Hum. Genet. 76, 1074–1080. doi: 10.1086/430841
Murugan, M., Harward, S., Scharff, C., and Mooney, R. (2013). Diminished FoxP2 levels affect dopaminergic modulation of corticostriatal signaling important to song variability. Neuron 80, 1464–1476. doi: 10.1016/j.neuron.2013.09.021
Ocklenburg, S., Arning, L., Gerding, W. M., Epplen, J. T., Gunturkun, O., and Beste, C. (2013). FOXP2 variation modulates functional hemispheric asymmetries for speech perception. Brain Lang. 126, 279–284. doi: 10.1016/j.bandl.2013.07.001
Padovani, A., Cosseddu, M., Premi, E., Archetti, S., Papetti, A., Agosti, C., et al. (2010). The speech and language FOXP2 gene modulates the phenotype of frontotemporal lobar degeneration. J. Alzheimers Dis. 22, 923–931. doi: 10.3233/JAD-2010-101206
Palka, C., Alfonsi, M., Mohn, A., Cerbo, R., Guanciali Franchi, P., Fantasia, D., et al. (2012). Mosaic 7q31 deletion involving FOXP2 gene associated with language impairment. Pediatrics 129, e183–e188. doi: 10.1542/peds.2010-2094
Peter, B., Raskind, W. H., Matsushita, M., Lisowski, M., Vu, T., Berninger, V. W., et al. (2011). Replication of CNTNAP2 association with nonword repetition and support for FOXP2 association with timed reading and motor activities in a dyslexia family sample. J. Neurodev. Disord. 3, 39–49. doi: 10.1007/s11689-010-9065-0
Pinel, P., Fauchereau, F., Moreno, A., Barbot, A., Lathrop, M., Zelenika, D., et al. (2012). Genetic variants of FOXP2 and KIAA0319/TTRAP/THEM2 locus are associated with altered brain activation in distinct language-related regions. J. Neurosci. 32, 817–825. doi: 10.1523/JNEUROSCI.5996-10.2012
Premi, E., Pilotto, A., Alberici, A., Papetti, A., Archetti, S., Seripa, D., et al. (2012). FOXP2, APOE, and PRNP: new modulators in primary progressive aphasia. J. Alzheimers Dis. 28, 941–950. doi: 10.3233/JAD-2011-111541
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A., Bender, D., et al. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. doi: 10.1086/519795
Rice, G. M., Raca, G., Jakielski, K. J., Laffin, J. J., Iyama-Kurtycz, C. M., Hartley, S. L., et al. (2012). Phenotype of FOXP2 haploinsufficiency in a mother and son. Am. J. Med. Genet. A 158A, 174–181. doi: 10.1002/ajmg.a.34354
Sanjuán, J., Tolosa, A., González, J. C., Aguilar, E. J., Pérez-Tur, J., Nájera, C., et al. (2006). Association between FOXP2 polymorphisms and schizophrenia with auditory hallucinations. Psychiatr. Genet. 16, 67–72. doi: 10.1097/01.ypg.0000185029.35558.bb
Schulz, S. B., Haesler, S., Scharff, C., and Rochefort, C. (2010). Knockdown of FoxP2 alters spine density in Area X of the zebra finch. Genes Brain Behav. 9, 732–740. doi: 10.1111/j.1601-183X.2010.00607.x
Shriberg, L. D., Ballard, K. J., Tomblin, J. B., Duffy, J. R., Odell, K. H., and Williams, C. A. (2006). Speech, prosody, and voice characteristics of a mother and daughter with a 7;13 translocation affecting FOXP2. J. Speech Lang. Hear. Res. 49, 500–525. doi: 10.1044/1092-4388(2006/038)
Španiel, F., Horáček, J., Tintěra, J., Ibrahim, I., Novák, T., Čermák, J., et al. (2011). Genetic variation in FOXP2 alters grey matter concentrations in schizophrenia patients. Neurosci. Lett. 493, 131–135. doi: 10.1016/j.neulet.2011.02.024
Tolosa, A., Sanjuán, J., Dagnall, A. M., Moltó, M. D., Herrero, N., and De Frutos, R. (2010). FOXP2 gene and language impairment in schizophrenia: association and epigenetic studies. BMC Med. Genet. 11:114. doi: 10.1186/1471-2350-11-114
Turner, S. J., Hildebrand, M. S., Block, S., Damiano, J., Fahey, M., Reilly, S., et al. (2013). Small intragenic deletion in FOXP2 associated with childhood apraxia of speech and dysarthria. Am. J. Med. Genet. A 161, 2321–2326. doi: 10.1002/ajmg.a.36055
Vargha-Khadem, F., Watkins, K. E., Price, C. J., Ashburner, J., Alcock, K. J., Connelly, A., et al. (1998). Neural basis of an inherited speech and language disorder. Proc. Natl. Acad. Sci. U.S.A. 95, 12695–12700. doi: 10.1073/pnas.95.21.12695
Vernes, S. C., Newbury, D. F., Abrahams, B. S., Winchester, L., Nicod, J., Groszer, M., et al. (2008). A functional genetic link between distinct developmental language disorders. New Engl. J. Med. 359, 2337–2345. doi: 10.1056/NEJMoa0802828
Vernes, S. C., Nicod, J., Elahi, F. M., Coventry, J. A., Kenny, N., Coupe, A. M., et al. (2006). Functional genetic analysis of mutations implicated in a human speech and language disorder. Hum. Mol. Genet. 15, 3154–3167. doi: 10.1093/hmg/ddl392
Vernes, S. C., Oliver, P. L., Spiteri, E., Lockstone, H. E., Puliyadi, R., Taylor, J. M., et al. (2011). Foxp2 regulates gene networks implicated in neurite outgrowth in the developing brain. PLoS Genet. 7:e1002145. doi: 10.1371/journal.pgen.1002145
Vernes, S. C., Spiteri, E., Nicod, J., Groszer, M., Taylor, J. M., Davies, K. E., et al. (2007). High-throughput analysis of promoter occupancy reveals direct neural targets of FOXP2, a gene mutated in speech and language disorders. Am. J. Hum. Genet. 81, 1232–1250. doi: 10.1086/522238
Watkins, K. E., Dronkers, N. F., and Vargha-Khadem, F. (2002a). Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain 125, 452–464. doi: 10.1093/brain/awf058
Watkins, K. E., Vargha-Khadem, F., Ashburner, J., Passingham, R. E., Connelly, A., Friston, K. J., et al. (2002b). MRI analysis of an inherited speech and language disorder: structural brain abnormalities. Brain 125, 465–478. doi: 10.1093/brain/awf057
Whitehouse, A. J., Bishop, D. V., Ang, Q. W., Pennell, C. E., and Fisher, S. E. (2011). CNTNAP2 variants affect early language development in the general population. Genes Brain Behav. 10, 451–456. doi: 10.1111/j.1601-183X.2011.00684.x
Wilcke, A., Ligges, C., Burkhardt, J., Alexander, M., Wolf, C., Quente, E., et al. (2012). Imaging genetics of FOXP2 in dyslexia. Eur. J. Hum. Genet. 20, 224–229. doi: 10.1038/ejhg.2011.160
Zeesman, S., Nowaczyk, M. J., Teshima, I., Roberts, W., Cardy, J. O., Brian, J., et al. (2006). Speech and language impairment and oromotor dyspraxia due to deletion of 7q31 that involves FOXP2. Am. J. Med. Genet. A 140, 509–514. doi: 10.1002/ajmg.a.31110
Keywords: FOXP2, imaging genetics, language, transcription factor, MRI, brain anatomy, VBM
Citation: Hoogman M, Guadalupe T, Zwiers MP, Klarenbeek P, Francks C and Fisher SE (2014) Assessing the effects of common variation in the FOXP2 gene on human brain structure. Front. Hum. Neurosci. 8:473. doi: 10.3389/fnhum.2014.00473
Received: 07 March 2014; Accepted: 09 June 2014;
Published online: 01 July 2014.
Edited by:
Angela T. Morgan, Royal Childrens Hospital and University of Melbourne, AustraliaReviewed by:
Dorothy Bishop, University of Oxford, UKCopyright © 2014 Hoogman, Guadalupe, Zwiers, Klarenbeek, Francks and Fisher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martine Hoogman, Donders Centre for Cognitive NeuroImaging, (HP 204), PO box 9101, Kapittelweg 29, 6525 EN Nijmegen, Netherlands e-mail:bWFydGluZS5ob29nbWFuQHJhZGJvdWR1bWMubmw=;
Simon E. Fisher, Language and Genetics Department, Max Planck Institute for Psycholinguistics, Wundtlaan 1, Nijmegen 6525 XD, Netherlands e-mail:c2ltb24uZmlzaGVyQG1waS5ubA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.