Summary
Treatment for Post-Traumatic Stress Disorder (PTSD) is not always effective, and as the increasing demand for better management of PTSD and combat-related PTSD (CR-PTSD) infiltrates the UK media, so does a pressing need to understand individual variance in disease aetiology. Recent research in psychology, neuroscience and genetics has separately investigated how and why PTSD affects individuals differently. Here, we report on research on trauma, spatial processing and genetics to demonstrate that the hippocampus, part of the medial temporal lobe, is key to understanding how genes and environment interact to determine susceptibility to, and successful recovery from, PTSD. We argue that the integration of these research disciplines will bring new possibilities for prevention and treatment of PTSD within the Ministry of Defence (MOD), emergency services, National Health Service (NHS) and beyond.
Trauma and Treatment Success
Individual variations within (CR-)PTSD are a challenge for PTSD research and for treatment approaches. For example, overall lifetime prevalence of exposure to traumatic events varies between 40 and 90% (Hemmings et al., 2013a) with extensive differences between individuals, military and civilian populations, and between social and occupational domains (Zaidi and Foy, 1994; Alonso et al., 2004; Evans et al., 2006; Iversen et al., 2007; Druss et al., 2009), whereas lifetime prevalence of PTSD is estimated at only 9% (Breslau et al., 2013). Further complications of under (Gee, 2013) over (Palmer, 2012) and self-reporting (Richardson et al., 2010) PTSD render its UK prevalence rate at 3% (McManus, 2009) to serve as only an estimation of the impact this condition has on individuals, communities and the wider economy.
Although the causes of variation in (CR-)PTSD prevalence and treatment success remain widely unknown (Acheson et al., 2012), age at which individuals are exposed to trauma is known to influence PTSD aetiology (Carrion et al., 2001): both in terms of early life stress (Brewin et al., 2000; Vasterling and Brewin, 2005; McGowan and Szyf, 2010) and the development of skills required to both verbalize and spatially contextualize trauma (van der Kolk, 2003; Betts et al., 2012); and with regard to dementia (Duax et al., 2013).
With the spectrum of treatment (Lindauer et al., 2005) and its success (Gaskell and British Pyshcological Society, 2005) being broad and the UK military charity Combat Stress delivering PTSD treatment on the principal1 that over one third of veterans will not recover well, there is an increasing and genuine demand for efficient assessment, referrals and treatment in a very diverse PTSD population. We propose that understanding individual variance in hippocampal integrity will provide a stable and objective means of quantifying PTSD susceptibility and treatment success.
PTSD, the Hippocampus, and Spatial Processing
PTSD has been associated with hippocampal integrity and volume (Gilbertson et al., 2002; Apfel et al., 2011), with chronic PTSD in veterans being associated with a 6% reduction in hippocampal volume compared to recovered veterans (Gilbertson et al., 2002; Apfel et al., 2011). Moreover, it is understood that:
(1) Hippocampal integrity is affected by stress (Sapolsky, 2000; Vasterling and Brewin, 2005; Wang et al., 2010);
(2) Stress from trauma exposure poses a real threat to hippocampal functionality (Brewin et al., 2010; Acheson et al., 2012; Pitman et al., 2012); and
(3) PTSD symptoms (such as management of intrusions, inadequate integration of sensory memories in recall, lack of “self-referential perspective” and fear contextualization) are related to hippocampal activity (Jeansok and Fanselow, 1992; Philips and Le Doux, 1992; Ehlers and Clark, 2000; Astur et al., 2006; Bisby et al., 2010; Brewin et al., 2010; Acheson et al., 2012; Pitman et al., 2012).
While there is growing evidence for the importance of hippocampal integrity for PTSD resilience and recovery, the question how to best assess it remains. Hippocampal function can be measured in many ways, including pattern separation (Clelland et al., 2009) and context-dependent fear conditioning (Gerlai, 2001; Ji and Maren, 2008). We suggest hippocampal-dependent spatial processing (King et al., 2002; Bird and Burgess, 2008; Bisby et al., 2010) is particularly useful for trauma research. Spatial processing abilities are known to be negatively affected by trauma (Gilbertson et al., 2007; Bisby et al., 2010; Tempesta et al., 2012, Smith et al., manuscript in preparation), are highly relevant for those occupations which demand navigation competence and simultaneously elevate risk of trauma exposure (such as the Armed Forces and emergency services), and can be objectively quantified (Bird and Burgess, 2008).
The hippocampus has been implicated in allocentric processing, a specific type of spatial processing which involves viewpoint-independent manipulations of spatial relations between locations (Burgess et al., 2002; Burgess, 2006). Allocentric processing allows for the use of “observer” or “field” perspectives in trauma processing, and this dates back to Freudian psychoanalysis of anxiety-provoking memories (McIsaac and Eich, 2004; Eich et al., 2011, 2012). Explicit references to allocentric (or indeed egocentric) processing are unlikely to be found in trauma literature simply because this terminology is more familiar to the domain of spatial cognition. An example of therapeutic field using similar constructs is offered by Ehlers and Clark (2000) who employ the term “the self-referential,” in their theoretical model of PTSD. Neuropsychologists interested in spatial processing might refer to such self-referential processing as “egocentric” (or non-allocentric). Nonetheless using perspective (and indeed spatial perspective) in contextualizing evocative and sensory trauma is referred to in trauma literature (Steel et al., 2005; Neuner et al., 2008), and a specific example of this is offered with Bisby and colleagues' recent non-clinical samples (Bisby et al., 2010). Forthcoming findings from research into the effect of PTSD on configural memory may substantiate this relationship between allocentric processing and trauma processing further (Smith et al., manuscript in preparation). Brewin goes so far as to suggest that future clinical interventions for PTSD should involve further attempts to change information processing biases in Vasterling and Brewin (2005), and this paper adopts spatial cognition terminology (i.e., “allocentric processing”) to shed more light on role of the hippocampus in processing traumatic and spatial information.
Hippocampal Integrity
We have argued that trauma and PTSD affect hippocampal integrity (Acheson et al., 2012) and that this integrity is important for success in some treatments (Neuner et al., 2008; Bisby et al., 2010; Adenauer et al., 2011). This poses a dilemma: how can the hippocampus be appropriately employed to process the trauma, if trauma is disrupting its own function?
The answer may lie in the fact that the hippocampus is able to generate neurons throughout life (Eriksson et al., 1998; Andersen et al., 2007). It can increase in volume through spatial training (Maguire et al., 2000) and also increase in density through meta-cognition (Holzel et al., 2010). Moreover, spatial training procedures which force participants to adopt allocentric, viewpoint-independent perspectives have produced regenerative effects in the hippocampus (Whitlock et al., 2006; Lövdén et al., 2011).
These findings strongly suggest that hippocampal integrity and function can be improved using training procedures that employ spatial tasks. While this already has potential implications for PTSD recovery—particularly for interventions that make use of spatial contextualization— recent results from genetics research suggest that training success may depend on specific genotypes (Lövdén et al., 2011).
DNA
Many genes have been associated with PTSD symptomology (Koenen et al., 2009; Schmidt et al., 2011; Skelton et al., 2012)—several by means of the phenotype or “candidate approach” (Yehuda et al., 2011; Skelton et al., 2012) which selects genes already known to result in similar traits as the PTSD symptom of interest (Gottesman and Gould, 2003; Acheson et al., 2012). Whilst this has provided insight into many areas of the neurobiological system and various “symptoms” associated with PTSD, Hemmings et al. (2013b) recently stated that “no gene variant has yet been reported as unequivocally involved in the development of this disorder [PTSD].” We suggest that the Brain Derived Neurotropic Factor (BDNF) gene may be that gene: primarily because of its role in hippocampal processing and its recent associations with PTSD.
BDNF determines levels of N-acetylaspartate (NAA) in the hippocampus, which is a putative marker of neural integrity (Egan et al., 2003; Salehi et al., 2013), is crucial for maintaining a healthy hippocampal volume (Carballedo et al., 2013) and plays an important role in managing the stress response (Suliman et al., 2013). The BDNF vall66met polymorphism involves three genotypes: val/val, val/met and met/met. In the Caucasian population, 70% of the population carry the single nucleotide polymorphism val66val, 27% carry val66met and 3% carry met66met (Petryshen et al., 2010).
What Evidence Links BDNF to PTSD?
A wide literature introduces the role of BDNF and val66met in psychological wellbeing, in overall development (Casey et al., 2009), in mood disorders (Duman and Monteggia, 2006), depression (Aguilera et al., 2009; Gatt et al., 2009) and even attempted suicide (Perroud et al., 2008; Pregelj et al., 2011).
The BDNF polymorphism has been associated with childhood trauma, with carriers of the “met” variation being particularly sensitive to the impact of child abuse and recent stress (Elzinga et al., 2011). BNDF variations are also considered as modifiers of the risk of childhood trauma in obsessive-compulsive disorder (Hemmings et al., 2013a,b; Suliman et al., 2013) and as mediators of the impact of childhood adversity on lifetime depression (Carver et al., 2011). A plethora of studies demonstrate a connection between the val66met polymorphism of BDNF and PTSD in relation to: extinguishing the fear and startle response (Rattiner et al., 2004; Zhang et al., 2014); PTSD symptomology and severity (Koenen et al., 2009; Frielingsdorf et al., 2010; Hemmings et al., 2013b); psychotic PTSD (Pivac et al., 2012); and the efficacy of PTSD therapy (Felmingham et al., 2013).
Recently, Zhang et al. (2014) reported that amongst a sample of 461 trauma exposed US soldiers deployed in Afghanistan and Iran, 10% had probable PTSD (Zhang et al., 2014). Within that group (n = 42), the frequency of met/met genotypes was nearly three-fold higher than in the controls, and the frequency of val/met genotypes was two-fold higher in individuals with probable-PTSD than in controls. The frequency of the BDNF val66met genotypes was significantly higher in those with PTSD and in those with exaggerated startle (a core symptom of PTSD) than in non-PTSD groups. Overall, the val66val genotype has been suggested to increase PTSD resilience, while the val66met allele increases vulnerability of PTSD (Koenen et al., 2009; Elzinga et al., 2011; Hemmings et al., 2013b; Zhang et al., 2014).
What is the Relation between the BDNF Polymorphism and the Hippocampus?
“Met” carriers develop smaller hippocampi (Szeszko et al., 2005), especially if they are exposed to early life stress (Gatt et al., 2009)—and as they age, are more likely to show lower hippocampal activity and resilience (Raz and Rodrigue, 2006; Fehér et al., 2013; Wiener et al., 2013), poorer performance on spatial tasks (Sanchez et al., 2011) and are less prone to explore unfamiliar environments (Chen et al., 2006). Furthermore, val66met has been shown to impair the hippocampal plasticity induced by SSRI anti-depressants (such as fluoxetine) which are often used in the treatment of PTSD (Bath et al., 2012). Kleim et al. (2006) showed that changes in neural plasticity and motor function are mediated by the val66met BDNF polymorphism, and Lövdén et al. (2011) demonstrated that increased levels of hippocampal NAA (a putative marker of neural integrity) as a result of spatial training were restricted to BDNF val homozygotes (val/val). Val/met heterozygotes and met/met homozygotes did not benefit from the spatial training which required allocentric processing, the very processing which is thought to be so useful to manage trauma.
Can We Predict Success Rates of Different PTSD Treatments?
We have reviewed research demonstrating that:
(1) PTSD is inextricably linked to the hippocampus (Astur et al., 2006; Bisby et al., 2010; Brewin et al., 2010; Acheson et al., 2012; Pitman et al., 2012);
(2) Hippocampal integrity and development has a strong genetic component (Szeszko et al., 2005; Gatt et al., 2009; Lövdén et al., 2011) and
(3) Some forms of PTSD treatments rely on hippocampal processing (McIsaac and Eich, 2004; Vasterling and Brewin, 2005; Adenauer et al., 2011).
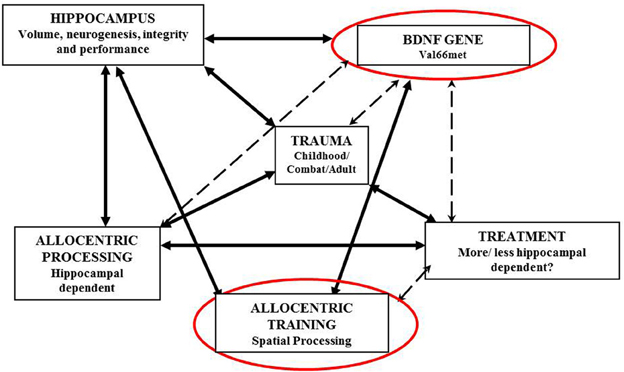
Figure 1 illustrates the interrelations between BDNF, the hippocampus, spatial processing and trauma processing.
Together, these findings allow for an intriguing conclusion: the success rate of specific PTSD treatments may well be predicted by analysing patients' BDNF genotype. Specifically, we argue that PTSD therapies involving spatial contextualization of traumatic event (such as exposure therapy) will have lower success rates in val/met heterozygotes and met/met homozygotes than in val/val homozygotes, especially if individuals have been exposed to early life stress. This is because spatial contextualization is dependent on hippocampal processing, and hippocampal integrity and plasticity is mediated by the val66met BDNF polymorphism.
In conclusion, we suggest that genetic analysis can help to predict the success of different types of PTSD treatments and methods of trauma processing, and may be used to improve referral pathways and eventually PTSD recovery rates.
Research between UCL, Bournemouth University, the NHS and Combat Stress is currently being undertaken to quantify the relationship between the BDNF gene, combat and childhood trauma processing and hippocampal-dependent navigation, with the intention of providing new insight into the experience of PTSD in the UK.
Acknowledgments
The authors would like to thank: Army of Angels (registered charity 1143612) for contributing funding to this research; Simon Wessely and Combat Stress; Sue Clarke for her clinical support; and Chris Brewin, Ian Palmer, and Kate Adie for their sound advice and guidance.
Footnotes
- ^Based on the model used by the Australian Veterans Rehabilitation programme (Combat Stress Annual Report 2011–12).
References
Acheson, D. T., Gresack, J. E., and Risbrough, V. B. (2012). Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology 62, 674–685. doi: 10.1016/j.neuropharm.2011.04.029
Adenauer, H., Catani, C., Gola, H., Keil, J., Ruf, M., Schauer, M., et al. (2011). Narrative exposure therapy for PTSD increases top-down processing of aversive stimuli—evidence from a randomized controlled treatment trial. BMC Neurosci. 12:127. doi: 10.1186/1471-2202-12-127
Aguilera, M., Arias, B., Wichers, M., Barrantes-Vidal, N., Moya, J., Villa, H., et al. (2009). Early adversity and 5-HTT/BDNF genes: new evidence of geneeenvironment interactions on depressive symptoms in a general population. Psychol. Med. 39, 1425–1432. doi: 10.1017/S0033291709005248
Alonso, J., Angermeyer, M. C., Bernert, S., Bruffaerts, R., Brugha, T. S., Bryson, H., et al. (2004). Disability and quality of life impact of mental disorders in Europe: results from the European study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr. Scand. Suppl. 109, 38–46. doi: 10.1111/j.1600-0047.2004.00329.x
Andersen, P., Morris, R., Amaral, D., Bliss, T., and O'Keefe, J. (2007). The Hippocampus Book. New York, NY: Oxford University Press.
Apfel, B. A., Ross, J., Hlavin, J., Meyerhoff, D. J., Metzler, T. J., Marmar, C. R., et al. (2011). Hippocampal volume differences in gulf war veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol. Psychiatry 69, 541–548. doi: 10.1016/j.biopsych.2010.09.044
Astur, R. S., St. Germain, S. A., Tolin, D., Ford, J., Russell, D., and Stevens, M. (2006). Hippocampus function predicts severity of post-traumatic stress disorder. Cyberpsychol. Behav. 9, 234–240. doi: 10.1089/cpb.2006.9.234
Bath, K. G., Jing, D. Q., Dincheva, I., Neeb, C. C., Pattwell, S. S., Chao, M. V., et al. (2012). BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. Neuropsychopharmacology 37, 1297–1304. doi: 10.1038/npp.2011.318
Betts, K., Williams, G. M., Najman, J. M., Bor, W., and Alati, R. (2012). Pre-trauma verbal ability at five years of age and the risk of post-traumatic stress disorder in adult males and females. J. Psychiatr. Res. 46, 933–939. doi: 10.1016/j.jpsychires.2012.04.002
Bird, C. M., and Burgess, N. (2008). The hippocampus and memory: insights from spatial processing. Nature 9, 182–194. doi: 10.1038/nrn2335
Bisby, J. A., King, J. A., Brewin, C. R., Burgess, N., and Curran, H. V. (2010). Acute effects of alcohol on intrusive memory development and viewpoint dependence in spatial memory support a dual representation model. Biol. Psychiatry 68, 280–286. doi: 10.1016/j.biopsych.2010.01.010
Breslau, N., Troost, J. P., Bohnert, K., and Luo, Z. (2013). Influence of predispositions on post-traumatic stress disorder: does it vary by trauma severity? Psychol. Med. 43, 381–390. doi: 10.1017/S0033291712001195
Brewin, C., Gregory, J. D., Lipton, M., and Burgess, N. (2010). Intrusive images in psychosocial disorders: characteristics, neural mechanisms, and treatment implications. Psychol. Rev. 117, 210–232. doi: 10.1037/a0018113
Brewin, C. R., Andrews, B., and Valentine, J. D. (2000). Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J. Consult. Clin. Psychol. 68, 748–766. doi: 10.1037/0022-006X.68.5.748
Burgess, N. (2006). Spatial memory: how egocentric and allocentric combine. Trends Cogn. Sci. 10, 551–557. doi: 10.1016/j.tics.2006.10.005
Burgess, N., O'Keefe, J., and Maguire, E. A. (2002). The human hippocampus and review spatial and episodic memory. Neuron 35, 625–641. doi: 10.1016/S0896-6273(02)00830-9
Carballedo, A., Morris, D., Zill, P., Fahey, C., Reinhold, E., Meisenzahl, E., et al. (2013). Brain-derived neurotrophic factor Val66Met polymorphism and early life adversity affect hippocampal volume. Am. J. Med. Genet. B Neuropsychiatr. Genet. 162, 183–190. doi: 10.1002/ajmg.b.32130
Carrion, V. G., Weems, C. F., Ray, R. D., Glaser, B., and Reiss, A. L. (2001). Toward and emprical definition of pedeatric PTSD: the phenomenology of PTSD symptoms in youth. J. Am. Acad. Child Adolesc. Psychiatry 41, 166–173. doi: 10.1097/00004583-200202000-00010
Carver, C. S., Johnson, S. L., Joormann, J., Lemoult, J., and Cuccaro, M. L. (2011). Childhood adversity interacts separately with 5-HTTLPR and BDNF to predict lifetime depression diagnosis. J. Affect. Disord. 132, 89–93. doi: 10.1016/j.jad.2011.02.001
Casey, B. J., Glatt, C. E., Tottenham, N., Soliman, F., Bath, K., and Amso, D., et al. (2009). Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience 164, 108–120. doi: 10.1016/j.neuroscience.2009.03.081
Chen, Z. Y., Jing, D., Bath, K. G., Ieraci, A., Khan, T., Siao, C. J., et al. (2006). Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 314, 140–143. doi: 10.1126/science.1129663
Clelland, C. D., Choi, M., Romberg, C., Clemenson, G. D. Jr., Fragniere, A., Tyers, P., et al. (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213. doi: 10.1126/science.1173215
Combat Stress (2012). Annual Report 2011–12. Registered charity 206002. Available online at: http://www.combatstress.org.uk/media/56674/combat_stress_annual_review_2011-12.pdf
Druss, B. G., Hwang, I., Petukhova, M., Sampson, N. A., Wang, P. S., and Kessler, R. C. (2009). Impairment in role functioning in mental and chronic medical disorders in the United States: results from the National Comorbidity Survey Replication. Mol. Psychiatry 14, 728–737. doi: 10.1038/mp.2008.13
Duax, J. M., Waldron-Perrine, B., Rauch, S. A. M., and Adams, K. M. (2013). Prolonged exposure therapy for a Vietnam veteran with PTSD and early-stage dementia. Cogn. Behav. Pract. 20, 64–73. doi: 10.1016/j.cbpra.2012.02.001
Duman, R. S., and Monteggia, L. M. (2006). A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 59, 1116–1127. doi: 10.1016/j.biopsych.2006.02.013
Egan, M. F., Kojima, M., Callicott, J. H., Goldberg, T. E., Kolachana, B. S., Bertolino, A., et al. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269. doi: 10.1016/S0092-8674(03)00035-7
Ehlers, A., and Clark, D. M. (2000). A cognitive model of posttraumatic stress disorder. Behav. Res. Ther. 38, 313–345. doi: 10.1016/S0005-7967(99)00123-0
Eich, E., Handy, T. C., Holmes, E. A., Lerner, J., and McIsaac, H. (2011). “Field and observer perspectives in autobiographical memory,” in 14th Sydney Symposium on Social Psychology, (New York, NY: University of South Wales).
Eich, E., Handy, T. C., Holmes, E. A., Lerner, J., and McIsaac, H. K. (2012). “Field and observer perspectives in autobiographical memory,” in Social Thinking and Interpersonal Behaviour, eds J. P. Forgas, K. Fiedler, and C. Sedikides (New York, NY: Psychology Press).
Elzinga, B. M., Molendijk, M. L., Oude Voshaar, R. C., Bus, B. A., Prickaerts, J., Spinhoven, P., et al. (2011). The impact of childhood abuse and recent stress on serum brain-derived neurotrophic factor and the moderating role of BDNF Val66Met. Psychopharmacology 214, 319–328. doi: 10.1007/s00213-010-1961-1
Eriksson, P. S., Perfilieva, E., Bjork-Eriksson, T., Alborn, A.-M., Nordborg, C., Peterson, D. A., et al. (1998). Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317. doi: 10.1038/3305
Evans, S., Giodan, C., Spielman, L., and Difede, J. (2006). Anger and its association to distress and social/occupational functioning in symptomatic disaster relief workers responding to the September 11 2001 World Trade Cenre Disaster. J. Trauma. Stress 19, 147–152. doi: 10.1002/jts.20107
Fehér, A., Juhász, A., Rimanóczy, A., Kálmán, J., and Janka, Z. (2013). Association between BDNF Val66Met polymorphism and Alzheimer disease, dementia with Lewy bodies, and Pick disease. Alzheimer Dis. Assoc. Disord. 23, 224–228. doi: 10.1097/WAD.0b013e318199dd7d
Felmingham, K. L., Dobson-Stone, C., Schofield, P. R., Quirk, G. J., and Bryant, R. A. (2013). The brain-derived neurotrophic factor val66met polymorphism predicts response to exposure therapy in posttraumatic stress disorder. Biol. Psychiatry 73, 1059–1063. doi: 10.1016/j.biopsych.2012.10.033
Frielingsdorf, H., Bath, K. G., Soliman, F., Difede, J., Casey, B. J., and Lee, F. S. (2010). Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications for posttraumatic stress disorder. Ann. N.Y. Acad. Sci. 1208, 150–157. doi: 10.1111/j.1749-6632.2010.05722.x
Gaskell and British Pyshcological Society. (2005). Post-traumatic Stress Disorder: The Management of PTSD in Adults and Children in Primary AND Secondary Care. Trowbridge: National Institute for Clinical Excellence, National Clinical Guidelines Number 26.
Gatt, J. M., Nemeroff, C. B., Dobson-Stone, C., Paul, R. H., Bryant, R. A., Schofield, P. R., et al. (2009). Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol. Psychiatry 14, 681–695. doi: 10.1038/mp.2008.143
Gee, D. (2013). The Last Ambush? Aspects of Mental Health in the British Armed Forces. London: ForcesWatch.
Gerlai, R. (2001). Behavioral tests of hippocampal function: simple paradigms complex problems. Behav. Brain Res. 125, 269–277. doi: 10.1016/S0166-4328(01)00296-0
Gilbertson, M. W., Shenton, M. E., Ciszewski, A., Kasai, K., Lasko, N. B., Orr, S. P., et al. (2002). Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 5, 1242–1247. doi: 10.1038/nn958
Gilbertson, M. W., Williston, S. K., Paulus, L. A., Lasko, N. B., Gurvits, T. V., Shenton, M. E., et al. (2007). Configural cue performance in identical twins discordant for posttraumatic stress disorder: theoretical implications for the role of hippocampal function. Biol. Psychiatry 62, 513–520. doi: 10.1016/j.biopsych.2006.12.023
Gottesman, I. I., and Gould, T. D. (2003). The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160, 636–645. doi: 10.1176/appi.ajp.160.4.636
Hemmings, S. L., Lochner, C., van der Merwe, L., Cath, D. C., Seedat, S., and Stein, D. J. (2013b). BDNF Val66Met modifies the risk of childhood trauma on obsessive-compulsive disorder. J. Psychiatr. Res. 47, 1857–1863. doi: 10.1016/j.jpsychires.2013.08.012
Hemmings, S. M. J., Martin, L. I., Klopper, M., van der Merwe, L., Aitken, L., De Wit, E., et al. (2013a). Bdnf val66met and drd2 taq1a polymorphisms interact to influence ptsd symptom severity: a preliminary investigation in a south african population. Progr. Neuropsychopharmacol. Biol. Psychiatry 40, 273–280. doi: 10.1016/j.pnpbp.2012.10.011
Holzel, B. K., Carmody, J., Congleton, C., Yerramsetti, S. M., Gard, T., and Lazar, S. W. (2010). Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 191, 36–43. doi: 10.1016/j.pscychresns.2010.08.006
Iversen, A., Fear, N., Simonoff, E., Hull, L., Horn, O., Greenberg, N., et al. (2007). Influence of childhood adversity on health among male UK military personnel. Br. J. Psychiatry 191, 506–511. doi: 10.1192/bjp.bp.107.039818
Jeansok, K. J., and Fanselow, M. S. (1992). Modality specific retrograde amnesia of fear. Science 256, 675–677. doi: 10.1126/science.1585183
Ji, J., and Maren, S. (2008). Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn. Mem. 15, 244–251. doi: 10.1101/lm.794808
King, J. A., Burgess, N., Hartley, T., Vargha-Khadem, F., and O'Keefe, J. (2002). Human hippocampus and viewpoint dependence in spatial memory. Hippocampus 12, 811–820. doi: 10.1002/hipo.10070
Kleim, J. A., Chan, S., Pringle, E., Schallert, K., Procaccio, V., Jimenez, R., et al. (2006). BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat. Neurosci. 9, 735–737. doi: 10.1038/nn1699
Koenen, K. C., Amstadter, A. B., and Nugent, N. R. (2009). Gene-environment interaction in posttraumatic stress disorder: an update. J. Trauma. Stress 22, 416–426. doi: 10.1002/jts.20435
Lindauer, R. J., Vlieger, E. J., Jalink, M., Olff, M., Carlier, I. V., Majoie, C. B., et al. (2005). Effects of psychotherapy on hippocampal volume in out-patients with post-traumatic stress disorder: a MRI investigation. Psychol. Med. 35, 1421–1431. doi: 10.1017/S0033291705005246
Lövdén, M., Schaefer, S., Noack, H., Kanowski, M., Kaufmann, J., Tempelmann, C., et al. (2011). Performance-related increases in hippocampal N-acetylaspartate (NAA) induced by spatial navigation training are restricted to BDNF Val homozygotes. Cereb. Cortex 21, 1435–1442. doi: 10.1093/cercor/bhq230
Maguire, E. A., Gadian, D. G., Johnsrude, I. S., Good, C. D., Ashburner, J., Frackowiak, R. S., et al. (2000). Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. U.S.A. 97, 4398–4403. doi: 10.1073/pnas.070039597
McGowan, P., and Szyf, M. (2010). The epigenetics of social adversity in early life: implications for mental health outcomes. Neurobiol. Dis. 39, 66–72. doi: 10.1016/j.nbd.2009.12.026
McIsaac, H. K., and Eich, E. (2004). Vantage point in traumatic memory. Psychol. Sci. 15, 248–253. doi: 10.1111/j.0956-7976.2004.00660.x
McManus, S., Meltzer, H., Brugha, T., Bebbington, P., and Jenkins, R. (2009). Adult Psychiatric Morbidity in England, 2007: Results of a Household Survey. The Health and Social Care Information Centre.
Neuner, F., Catani, C., Ruf, M., Schauer, E., Schauer, M., and Elbert, T. (2008). Narrative exposure therapy for the treatment of traumatized children and adolescents (KidNET): from neurocognitive theory to field intervention. Child Adolesc. Psychiatr. Clin. N. Am. 17, 641–664. doi: 10.1016/j.chc.2008.03.001
Palmer, I. (2012). UK extended medical assessment programme for ex-service personnel: the first 150 individuals seen. Psychiatr. Online 36, 263–227. doi: 10.1192/pb.bp.110.033266
Perroud, N., Courtet, P., Vincze, I., Jaussent, I., Jollant, F., Bellivier, F., et al. (2008). Interaction between BDNF Val66Met and childhood trauma on adult's violent suicide attempt. Genes Brain Behav. 7, 314–322. doi: 10.1111/j.1601-183X.2007.00354.x
Petryshen, T. L., Sabeti, P. C., Aldinger, K. A., Fry, B., Fan, J. B., Schaffner, S. F., et al. (2010). Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol. Psychiatry 15, 810–815. doi: 10.1038/mp.2009.24
Philips, R. G., and Le Doux, L. E. (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106, 274–285. doi: 10.1037/0735-7044.106.2.274
Pitman, R. K., Rasmusson, A. M., Koenen, K. C., Shine, L. M., Orr, S. P., Gilbertson, M. W., et al. (2012). Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 13, 769–787. doi: 10.1038/nrn3339
Pivac, N., Kozaric-Kovacic, D., Grubisic-Ilic, M., Nedic, G., Rakos, I., Nikolac, M., et al. (2012). The association between brain-derived neurotrophic factor val66met variants and psychotic symptoms in posttraumatic stress disorder. World J. Biol. Psychiatry 13, 306–311. doi: 10.3109/15622975.2011.582883
Pregelj, P., Nedic, G., Paska, A. V., Zupanc, T., Nikolac, M., Balazic, J., et al. (2011). The association between brain-derived neurotrophic factor polymorphism (BDNF Val66Met) and suicide. J. Affect. Disord. 128, 287–290. doi: 10.1016/j.jad.2010.07.001
Rattiner, L. M., Davis, M., French, C. T., and Ressler, K. J. (2004). Brain-derived neurotrophic factor and tyrosine kinase receptor b involvement in amygdala-dependent fear conditioning. J. Neurosci. 24, 4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004
Raz, N., and Rodrigue, K. M. (2006). Differential aging of the brain: patterns, cognitive correlates, modifiers. Neurosci. Biobehav. Rev. 30, 730–748. doi: 10.1016/j.neubiorev.2006.07.001
Richardson, L. K., Frueh, C. B., and Acierno, R. (2010). Prevalence estimates of combat-related PTSD: a critical review. Aust. N.Z. J. Psychiatry 44, 4–19. doi: 10.3109/00048670903393597
Salehi, B., Preuss, N., van der Veen, J. W., Shen, J., Neumeister, A., Drevets, W. C., et al. (2013). Age modulated association between prefrontal NAA and the BDNF gene. Int. J Neuropsychopharmacol. 16, 1185–1193. doi: 10.1017/S1461145712001204
Sanchez, M. M., Das, D., Taylor, J. L., Noda, A., Yesavage, J. A., and Salehi, A. (2011). BDNF polymorphism predicts the rate of decline in skilled task performance and hippocampal volume in healthy individuals. Transl. Psychiatry 1, e51. doi: 10.1038/tp.2011.47
Sapolsky, R. M. (2000). Glucocorticoids and hipocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry 57, 925–935. doi: 10.1001/archpsyc.57.10.925
Schmidt, U., Holsboer, F., and Rein, T. (2011). Epigenetic aspects of posttraumatic stress disorder. Dis. Markers 30, 77–87. doi: 10.1155/2011/343616
Skelton, K., Ressler, K. J., Norrholm, S. D., Jovanovic, T., and Bradley-Davino, B. (2012). PTSD and gene variants: new pathways and new thinking. Neuropharmacology 62, 628–637. doi: 10.1016/j.neuropharm.2011.02.013
Steel, C., Fowler, D., and Holmes, E. A. (2005). Trauma-related intrusions and psychosis: an information processing account. Behav. Cogn. Psychother. 33, 139–152. doi: 10.1017/S1352465804001924
Suliman, S., Seedat, S., and Hemmings, S. M. J. (2013). Brain-derived neurotrophic factor(BDNF) protein levels in anxiety disorders: systematic review and meta-regression analysis. Front. Integr. Neurosci. 7:55. doi: 10.3389/fnint.2013.00055
Szeszko, P. R., Lipsky, R., Mentschel, C., Robinson, D., Gunduz-Bruce, H., Sevy, S., et al. (2005). Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol. Psychiatry 10, 631–636. doi: 10.1038/sj.mp.4001656
Tempesta, D., Mazza, M., Iaria, G., De Gennaro, L., and Ferrara, M. (2012). A specific deficit in spatial memory acquisition in post-traumatic stress disorder and the role of sleep in its consolidation. Hippocampus 22, 1154–1163. doi: 10.1002/hipo.20961
van der Kolk, B. A. (2003). The neurobiology of childhood trauma and abuse. Child Adolesc. Psychiatr. Clin. N. Am. 12, 293–317. doi: 10.1016/S1056-4993(03)00003-8
Vasterling, J. J., and Brewin, C. R. (2005). Neuropsychology of PTSD: Biological, Cognitive and Clinical Perspectives. London: Guildford Press.
Wang, Z., Neylan, T. C., Mueller, S. G., Lenoci, M., Truran, D., Marmar, C. R., et al. (2010). Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch. Gen. Psychiatry 67, 296. doi: 10.1001/archgenpsychiatry.2009.205
Whitlock, J., Heynen, A., Shuler, M. G., and Bear, M. F. (2006). Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097. doi: 10.1126/science.1128134
Wiener, J. M., de Condappa, O., Harris, M. A., and Wolbers, T. (2013). Maladaptive bias for extrahippocampal navigation strategies in aging humans. J. Neurosci. 33, 6012–6017. doi: 10.1523/JNEUROSCI.0717-12.2013
Yehuda, R., Koenen, K. C., Galea, S., and Flory, J. D. (2011). The role of genes in defining a molecular biology of PTSD. Dis. Markers 30, 67–76. doi: 10.3233/DMA20110794
Zaidi, L. Y., and Foy, D. W. (1994). Childhood abuse experiences and combat-related PTSD. J. Trauma. Stress 7, 33–42. doi: 10.1002/jts.2490070105
Keywords: trauma, childhood adversity, psychology, spatial processing, navigation, hippocampus, BDNF, val66met
Citation: Miller JK and Wiener JM (2014) PTSD recovery, spatial processing, and the val66met polymorphism. Front. Hum. Neurosci. 8:100. doi: 10.3389/fnhum.2014.00100
Received: 10 December 2013; Accepted: 10 February 2014;
Published online: 26 February 2014.
Edited by:
Hugo Spiers, University College London, UKReviewed by:
Kirsten V. Smith, South London and the Maudsley NHS Foundation Trust, UKNicola T. Fear, King's College London, UK
Copyright © 2014 Miller and Wiener. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence:bWlsbGVyakBib3VybmVtb3V0aC5hYy51aw==
 Jessica K. Miller
Jessica K. Miller Jan M. Wiener
Jan M. Wiener