- Helen Wills Neuroscience Institute, University of California at Berkeley, Berkeley, CA, USA
We investigated the effect of bromocriptine, a dopamine agonist, on individual differences in behavior as well as frontal–striatal connectivity during a working memory task. After dopaminergic augmentation, frontal–striatal connectivity in low working memory capacity individuals increases, corresponding with behavioral improvement whereas decreases in connectivity in high working memory capacity individuals are associated with poorer behavioral performance. These findings corroborate an inverted U-shape response of dopamine function in behavioral performance and provide insight on the corresponding neural mechanisms.
Introduction
Dopamine is critical for working memory (Cools and D’Esposito, 2009), which refers to the temporary retention of information that was just experienced but no longer exists in the external environment, or was just retrieved from long-term memory. Numerous studies in animals and humans have shown that working memory depends on the function of the prefrontal cortex (PFC; Fuster, 2008). The PFC contains a high concentration of dopamine receptors receiving diffuse ascending inputs from the midbrain (Goldman-Rakic et al., 1992; Robbins, 2000). In monkeys, depletion of PFC dopamine or pharmacological blockade of dopamine receptors induces impairment on working memory tasks (Brozoski et al., 1979; Sawaguchi and Goldman-Rakic, 1991) and administration of dopamine agonists reverses these impairments (Brozoski et al., 1979; Arnsten et al., 1994). Dopamine also plays a prominent role in the striatal function, which differs from its role in PFC function (Crofts et al., 2001). Dopamine in the PFC is proposed to stabilize task-relevant working memory representations by reducing susceptibility to distraction (Durstewitz et al., 2000; Seamans and Yang, 2004). Conversely, dopamine in the striatum may rapidly update working memory representations in a task-relevant manner (Frank et al., 2001; Gruber et al., 2006). In support of these hypotheses, a human pharmacological fMRI study found that administration of the dopaminergic agonist bromocriptine modulated striatal and PFC activity during the flexible updating and stable maintenance of representations, respectively (Cools et al., 2007).
Given the strong anatomical connectivity between the PFC and striatum (Alexander et al., 1986), coordinated activity between these two brain regions is likely critical for optimum working memory function. For example, in rats, destruction of dopamine terminals within PFC leads to functional changes in the striatum in terminal regions of dopaminergic midbrain projections (Pycock et al., 1980). Similar findings in monkeys (Roberts et al., 1994) provide further support that these two dopaminergic systems exhibit functional interactions. Moreover, the importance of investigating connectivity is highlighted by a study that demonstrated that alterations in dopaminergic-related movement, either hyperkinesia or akinesia, resulted not from changes in overall levels of cortical or striatal activity but instead from dopamine-related changes in the coordinated activity of neurons between the cortex and the striatum (Costa et al., 2006). Thus, the goal of the present human pharmacological fMRI study is to assess the effects of dopaminergic augmentation with bromocriptine on functional connectivity between the PFC and striatum during working memory function.
In a previous human pharmacological fMRI study, we found that bromocriptine modulated different working memory processes during the performance of a verbal delayed recognition task (Gibbs and D’Esposito, 2005). The relationship between items retrieved from working memory and PFC activity was correlated, but only in the bromocriptine session (i.e., slower retrieval rate was associated with more PFC activity and faster retrieval rate with less PFC activity). Additionally, bromocriptine did not modulate the encoding or retention stages of the task. In the present study, we have re-analyzed this fMRI data using a multivariate method to investigate the effect of administration of a bromocriptine on functional connectivity between the PFC and striatum during working memory function.
Materials and Methods
Subjects
The same 13 subjects’ data (ages 21–30) were used from the original study (Gibbs and D’Esposito, 2005). All procedures were approved by The University of California Berkeley Committee for the Protection of Human Subjects.
Cognitive Tasks
Subjects were administered 1.25 mg bromocriptine, a D2 agonist, or a lactose placebo in a two session, double-blind, counter-balanced design. Scanning occurred approximately 120 min after administration of the drug or placebo and sessions occurred approximately 1 week apart. fMRI scans were acquired while the subjects performed four types of working memory trials, as described previously (Gibbs and D’Esposito, 2005). Briefly, two or six letters were presented for 4 s (cue period), followed by a 12-s delay period in which the screen was blank. Following the delay, a single-case probe letter appeared for 2 s (probe period) and subjects indicated with a manual button press whether the single letter was part of the set of letters seen during the cue period. The probe period was followed by a jittered inter-trial interval that ranged from 8 to 12 s. The probe letter was presented either superimposed upon (e.g., intact) or masked (e.g., degraded) by a background of visual noise. Behaviorally, the subjects showed no difference between the masked or superimposed probes, therefore this data was collapsed between the two stimuli types. Four trials of each of the four types (2-letters intact, 2-letters degraded, 6-letters intact, and 6-letters degraded) were presented during each 7-min run. There were six runs per session, for a total of 96 trials per scanning session.
Different memory load levels allowed us to calculate the reaction time (RT) slope (RT at the 6-letter condition minus the 2-letter condition divided by change across memory load) and RT intercept for each subject. We chose two levels based on prior reports that RT increases linearly with set size (Sternberg, 1966) and that RT slope is sensitive to memory retrieval efficiency and RT intercept estimates motor speed under conditions of no memory load (Sternberg, 1969). Control tasks to assess motor speed and vigilance on drug and placebo sessions were also performed. These included the box completion task and the numerical cancelation task (Lewis and Kupke, 1977). ANOVAs were first used to test the effects of load, perceptual degradation, and bromocriptine on RT and accuracy for all subjects, and are reported in the original paper (Gibbs and D’Esposito, 2005). Planned comparisons used t-tests to investigate the effect of drug on accuracy in low- and high-span subjects, as well as RT slope in low- and high-span subjects.
Baseline working memory capacity was measured since previous human and pharmacological studies have shown that the effect of dopaminergic agonists on behavioral performance and neural activity depends on an individual’s baseline working memory capacity (Cai and Arnsten, 1997; Kimberg et al., 1997). Subjects with higher working memory capacity perform worse on working memory tasks after dopaminergic augmentation, whereas subjects with lower working memory capacity perform better. Also, in a recent human PET study, we found that an individual’s working memory capacity positively correlated with their dopamine synthesis capacity (Cools et al., 2008). We measured working memory capacity by performance on the listening version (Salthouse and Babcock, 1991) of the Daneman and Carpenter (1980) reading span task. Subjects were divided into low- and high-span groups based on the span task score, with individuals with a score of 4.0 and above being high-span. Eight subjects were classified as high span, and five subjects as low-span.
MRI Methods
MRI data acquisition
Functional and structural images were acquired with a Varian INOVA 4.0T scanner and a TEM send-and-receive RF head coil. Functional images were acquired using a 2-shot gradient echo EPI sequence, providing 18 5.0-mm thick axial slices with a 0.5-mm inter-slice gap, TR of 2,000 ms, 22.4 cm × 22.4 cm field of view, and a 64 × 64-matrix size, resulting in an in-plane resolution of 3.5 mm × 3.5 mm. The first 10 images from each run were removed to approach steady-state tissue magnetization. High-resolution MP-Flash 3-D T1-weighted scans were also acquired.
MRI data analysis
Image volumes were corrected for slice timing skew using temporal sync-interpolation and corrected for movement using rigid-body transformation parameters. Image preprocessing and statistical analyses were performed using SPM21. Images were resampled to 2 mm × 2 mm × 2 mm and smoothed with an 8-mm FWHM Gaussian kernel. A high-pass filter removed frequencies below 0.01 Hz from the data. Structural T1-weighted images were normalized to the Montreal Neurological Institute (MNI) reference brain. Transformations calculated by normalizing each subject’s structural images were applied to the functional images collected in each run. Data were analyzed using the general linear model (GLM). For each subject, BOLD signal during the cue, delay, and probe periods in each trial type were modeled as impulses of neural activity convolved with the SPM canonical hemodynamic response function. A covariate at the onset of the cue period of the task (first TR, 0 s) modeled early encoding processes; one at the third TR (4 s) modeled late encoding processes. Since encoding processes may continue into the delay period of the task, this late cue period activity was modeled to reduce noise in the estimate of the baseline but was not included in the univariate analyses (Zarahn et al., 1999). The early and late phases of the delay period were modeled with covariates at the fifth and seventh TRs, respectively (8 and 12 s). We only investigated the late phase due to potential overlap between the late encoding and the early delay period processes. The probe period was modeled with a covariate at the onset of the probe (ninth TR, 16 s).
Region-of-interest definition
Prefrontal cortex region-of-interests (ROIs) from the left and right middle frontal gyrus (MFG) were generated from the original study (Gibbs and D’Esposito, 2005), which were the only regions that showed a relationship between activity and working memory function. ROIs were created using the MarsBar software package2 in SPM2 (Brett et al., 2002) with 7 mm radius spheres centered on the coordinates derived from the original study (MNI coordinates: 28, 36, 26, right; −28, 36, 26, left). Whole caudate ROIs were also created from the MNI brain atlas. All ROIs were reversed normalized for each subject, and multivariate analyses were conducted in native space.
Multivariate data analysis
We implemented a functional connectivity analysis method that examines beta-series correlations which imply how strongly two regions are interacting (Gazzaley et al., 2004; Rissman et al., 2004). This method allows for the examination of the functional connectivity between brain regions during distinct stages in a task. To compute functional connectivity, a unique parameter estimate (beta value) for the events in each trial are computed for each subject and then sorted by task period (i.e., cue, delay, probe), yielding a beta time series. The extent to which two regions interact is quantified by the extent to which their respective beta time series are correlated. If the beta time series of one region is correlated with another region’s beta time series, this will result in a relatively larger r value. The correlation of beta-series averaged across the voxels were calculated between the MFG and caudate ROIs (left MFG-right MFG, left MFG-left caudate, right MFG-right caudate, left caudate-right caudate) for both the placebo and drug sessions, during each stage of the task. An arc-hyperbolic tangent transform was then applied to the correlation coefficients, and Fisher’s r to z transformation was implemented to yield z-scores (see Rissman et al., 2004 for detailed methods). Subjects were then divided into low and high-span groups and t-tests were conducted between drug and placebo sessions. Only data from correct trials was utilized. Mean z-values are reported, and asterisks indicate significant differences between placebo and drug sessions, p < 0.05, corrected for multiple comparisons. Pearson correlation coefficient R-values between z-scores and behavioral measures are shown at p < 0.05 and a trend of p < 0.07.
Results
Behavioral Results
As previously reported (Gibbs and D’Esposito, 2005), on placebo, high-span subjects exhibited a faster memory retrieval rate (i.e., lower RT slope scores) as compared to low-span individuals (t = −1.83, p < 0.05, Figure 1). The speed of retrieval in low-span subjects was 46 ms/item as compared to 25 ms/item in high-span subjects. After bromocriptine administration, RT slope scores and accuracy significantly improved in low-span individuals (RT slope: t = −2.86, p < 0.05; accuracy: t = 6.67, p < 0.003; Figure 1). In contrast, 6/8 high-span individuals exhibited worsened RT slope scores on bromocriptine compared to placebo, although the group effect was not statistically significant. There was also a trend toward reduced accuracy (t = −1.81, p < 0.06; Figure 1). No drug effect was found on the control tasks that assessed motor speed and vigilance.
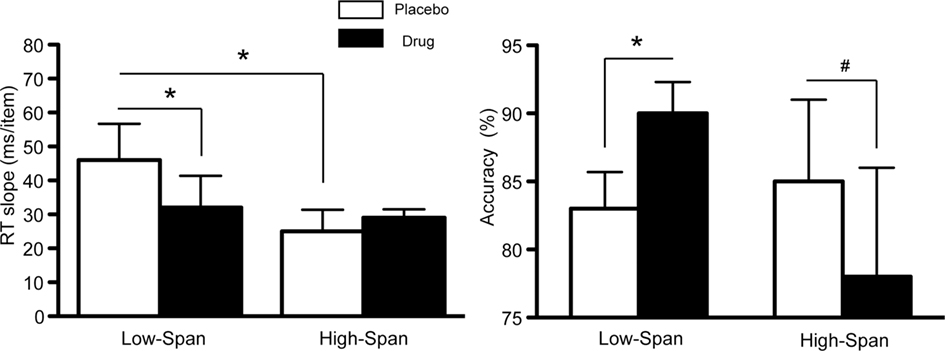
Figure 1. The effect of bromocriptine on RT slope and accuracy on high-load trials on a delayed recognition task is dependent on working memory span. Data shown represents mean ± SE of the mean. Asterisks indicate p < 0.05; # indicate p < 0.06.
Functional MRI Results
We investigated the effects of bromocriptine on frontal–striatal functional connectivity in high- and low-span individuals while performing a delayed recognition task during each task period. Since behavioral differences between high- and low-span groups were found only on high-load trials, we focus solely on this condition.
Cue and delay periods
No differences in MFG-caudate functional connectivity between drug and placebo sessions were found in either the cue or delay periods.
Probe period
After bromocriptine administration, low-span individuals exhibited significant increases in functional connectivity between left MFG and left caudate compared to placebo (t = 2.73, p < 0.05, Figure 2). Drug-induced increases in connectivity between right MFG and right caudate were also found but were not statistically significant. No other differences in connectivity between the ROIs were found. Differences in functional connectivity between left MFG and caudate on drug versus placebo negatively correlated significantly with the drug effect on RT slope, in that individuals who exhibited the greatest improvement in RT slope after bromocriptine administration also exhibited the greatest increase in MFG-caudate connectivity (r2 = 0.72, p < 0.05; Figure 3).
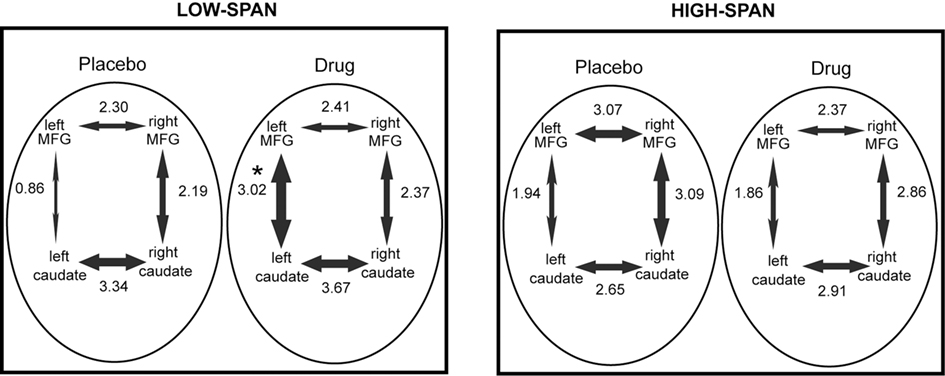
Figure 2. The effect of bromocriptine on fronto-striatal connectivity during performance of a delayed recognition memory task is dependent on working memory span. The mean correlation coefficient (expressed as a z-score) between each pair of regions is indicated alongside each arrow and the thickness of the arrows is scaled proportionally to this value. Asterisk indicates p < 0.05, for significant difference between placebo and drug session, corrected for multiple comparisons.
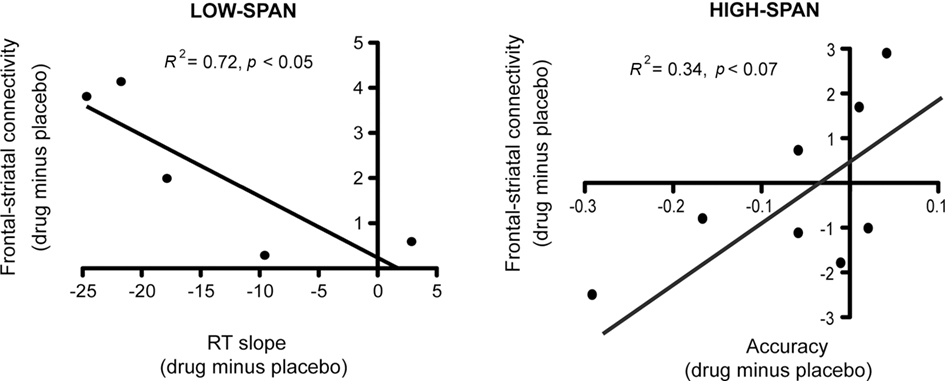
Figure 3. After bromocriptine administration, changes in frontal–striatal connectivity during the probe period of the delayed recognition task correlated with changes in behavioral performance (RT slope and accuracy on high-load trials).
High-span individuals exhibited significant functional connectivity between left MFG and left caudate, and right MFG and right caudate, on both placebo and drug sessions, with no significant drug effect (Figure 2). However, five out of eight high-span individuals exhibited drug-induced decreases in connectivity between right MFG and right caudate, and four out of eight individuals between left MFG and left caudate. There was a trend in the correlation between the drug effects on right MFG and right caudate connectivity and the drug effect on task accuracy (r2 = 0.34, p < 0.07), in that individuals who worsened the most after bromocriptine administration exhibited the greatest decrease in MFG-caudate connectivity (Figure 3).
Discussion
In our initial report of this data on the effects of bromocriptine on working memory function (Gibbs and D’Esposito, 2005), we found that the relationship between working memory retrieval processes and PFC activity was influenced by dopaminergic augmentation. Specifically, PFC activity was correlated with memory retrieval rate after bromocriptine administration (i.e., slower retrieval rate was associated with more PFC activity and faster retrieval rate with less PFC activity), whereas after placebo administration these measures were uncorrelated. These results add to the accumulating evidence in humans for a role of dopamine in working memory function, mediated, at least in part, through modulation of PFC activity (Cools and D’Esposito, 2009).
In the current study, our aim was to investigate the effect of dopaminergic augmentation on functional interactions between the PFC and striatum. Re-analyzing our original dataset, we found that during the engagement of working memory retrieval processes, bromocriptine increased fronto-striatal connectivity in low-span individuals, corresponding with improvement in their performance. In contrast, high-span individuals exhibited a decrease in fronto-striatal connectivity after bromocriptine administration, corresponding with worsening in behavioral performance. Given that the results in high span subjects did not reach statistical significance likely due to our small sample size, this particular finding will need replication. Nevertheless, the new findings that we report are consistent with previous studies in both animals and humans that suggest too much or too little dopamine can be detrimental to behavioral performance, which is reflected by changes in neural activity (e.g., Williams and Goldman-Rakic, 1995; Cools et al., 2007; Cools and D’Esposito, in press). In humans, we have shown that individuals with lower working memory span have lower dopamine synthesis capacity (Cools et al., 2008). Thus, we have proposed that low-span individuals with suboptimal baseline dopamine levels are boosted into optimal range with dopaminergic augmentation whereas high-span individuals with optimal baseline dopamine levels are “overdosed.” Our findings suggest that fronto-striatal connectivity is strengthened with optimal dopamine levels and weakened with suboptimal levels.
Only a few human studies have examined the effects of dopaminergic modulation on functional connectivity between PFC and the striatum. For example in one study of healthy individuals, increased frontal–striatal functional connectivity was associated with faster set shifting on the Wisconsin card sorting task (Nagano-Saito et al., 2008). After dopamine depletion via a tyrosine and phenylalanine deficient drink, the association between connectivity and behavioral performance was no longer present. Another study investigated fronto-striatal functional connectivity in individuals who were homozygous for the Val/Val and Met/Met polymorphism of the catechol-O-methyl transferase (COMT) gene, an enzyme which breaks down dopamine in the PFC and leads Val/Val carriers to have less PFC dopaminergic activity but presumably increased striatal dopaminergic activity, as compared to Met/Met carriers (Krugel et al., 2009). Val/Val individuals performed better on a reversal task and exhibited greater fronto-striatal functional connectivity. Additionally, a genetic study investigated differences in the dopamine and cAMP-regulated phosphoprotein-32 (DARPP-32) gene, which is known to be stimulated by dopamine signaling via phosphorylation of DARPP-32, leading to inhibition of protein phosphatase-1 (PP-1), thereby affecting other downstream target genes (Greengard, 2001). The study showed healthy individuals with the frequent haplotype of the DARPP-32 gene exhibited increased baseline cognitive performance as well as increased structural and fronto-striatal functional connectivity (Meyer-Lindenberg et al., 2007). Finally, in a PET study of Parkinson’s disease patients (Jahanshahi et al., 2010), increased frontal–striatal connectivity was demonstrated during performance of a motor timing task only on dopaminergic medication. Together, these studies provide convergent evidence that behavioral performance is correlated with fronto-striatal connectivity, which is modulated by dopamine. Our current study adds to these findings by investigating the direct effect of dopaminergic stimulation in individuals with different levels of underlying baseline working memory capacity.
It is important to note, however, that one study using a reversal task found individuals with the A1+ genotype status for the DRD2 Taq1A gene (having presumably decreased striatal D2 receptor concentration) performed better on placebo. This was associated with increased fronto-striatal functional connectivity as compared to A1− individuals (having presumably increased dopamine receptor concentration; Cohen et al., 2007). After dopaminergic augmentation, A1+ individuals’ behavior worsened, which was associated with decreased fronto-striatal functional connectivity, whereas A1− individuals’ behavior improved and fronto-striatal connectivity increased. While this study investigated genetic differences in D2 receptor concentration, our study presumably investigated differences in striatal dopamine synthesis capacity. It is not clear whether A1+ individuals in the study with decreased striatal dopamine receptor concentration have decreased dopamine synthesis capacity, or whether individuals in our study with low dopamine synthesis capacity exhibit a compensatory mechanism that leads to greater striatal dopamine receptor concentrations. Also, the Cohen study used cabergoline, which has a greater affinity for D2 receptors, and a lower affinity for D1 receptors as compared to bromocriptine (Gerlach et al., 2003). Thus, it is possible that the behavioral improvement we observed in the low-span individuals may be due in part to bromocriptine’s actions on PFC D1 receptors.
Animal studies have also observed dopaminergic modulation of coherent activity between frontal cortex and striatum. For example, in measurements of local field potential (LFP) oscillations in rats, it was observed that piriform cortex and striatum exhibit coherent rhythmic activity at ∼50 Hz, whereas the frontal cortex is coherent with striatum at higher frequencies (∼80–100 Hz; Berke, 2009). Dopamine stimulation induced a switch between cortico-striatal networks, decreasing the lower frequency striatal LFP oscillations (most coherent with piriform cortex), but increasing the higher frequency oscillations (most coherent with frontal cortex). Another study found that chemical lesions of midbrain dopaminergic neurons in rats caused increased coherence of β-frequency (15–30 Hz) oscillatory activity between frontal cortex and subthalamic nucleus (STN), as compared to non-lesioned rats. Administration of a dopaminergic agonist to the lesioned rats suppressed these oscillations and increased coherent activity at higher frequencies (Sharott et al., 2005). In a human EEG study with Parkinson’s patients, coherence between STN and frontal cortex was found at the 70 to 85-Hz band, but only when patients were on dopaminergic medication (Williams et al., 2002). Despite these empirical observations, the exact neuronal mechanisms underlying dopaminergic modulation of fronto-striatal connectivity remains unclear. In a study combining optical monitoring and electrophysiology recordings in mice (Bamford et al., 2004), dopaminergic agonists led to inhibition of cortico-striatal synaptic transmission in a subset of terminals by inhibiting synaptic vesicle exocytosis. It was concluded that dopamine strengthens the most active cortico-striatal inputs by filtering out activity of less active inputs. Whether this type of mechanism underlies dopamine’s effect on fronto-striatal connectivity observed in humans warrants further investigation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge NIH (R01-DA20600-MTD) and NIDA (F32DA027684-DLW) for funding support, and members of the D’Esposito lab for overall comments and suggestions.
Footnotes
References
Alexander, G. E., DeLong, M. R., and Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381.
Arnsten, A. F., Cai, J. X., Murphy, F. C., and Goldman-Rakic, P. S. (1994). Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology 116, 143–151.
Bamford, N. S., Zhang, H., Schmitz, Y., Wu, N. P., Cepeda, C., Levine, M. S., Schmauss, C., Zakharenko, S. S., Zablow, L., and Sulzer, D. (2004). Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 42, 653–663.
Berke, J. D. (2009). Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs. Eur. J. Neurosci. 30, 848–859.
Brett, M., Anton, J.-L., Valabregue, R., and Poline, J.-B. (2002). “Region of interest analysis using an SPM toolbox,” in 8th International Conference on Functional Mapping of the Human Brain, Sendai.
Brozoski, T. J., Brown, R., Rosvold, H. E., and Goldman, P. S. (1979). Cognitive deficit caused by regional depletion of dopamine in the prefrontal cortex of rhesus monkeys. Science 205, 929–931.
Cai, J. X., and Arnsten, A. F. (1997). Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J. Pharmacol. Exp. Ther. 282, 1–7.
Cohen, M. X., Krohn-Grimberghe, A., Elger, C. E., and Weber, B. (2007). Dopamine gene predicts the brain’s response to dopaminergic drug. Eur. J. Neurosci. 26, 3652–3660.
Cools, R., and D’Esposito, M. (2009). “Dopaminergic modulation of flexible control in humans,” in Dopamine Handbook, eds A. Bjorklund, S. B. Dunnett, L. L. Iversen, and S. D. Iversen (Oxford: Oxford University Press), 249–261.
Cools, R., and D’Esposito, M. (in press). Inverted-U shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry.
Cools, R., Gibbs, S. E., Miyakawa, A., Jagust, W., and D’Esposito, M. (2008). Working memory capacity predicts dopamine synthesis capacity in the human striatum. J. Neurosci. 28, 1208–1212.
Cools, R., Sheridan, M., Jacobs, E., and D’Esposito, M. (2007). Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J. Neurosci. 27, 5506–5514.
Costa, R. M., Lin, S. C., Sotnikova, T. D., Cyr, M., Gainetdinov, R. R., Caron, M. G., and Nicolelis, M. A. (2006). Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron 52, 359–369.
Crofts, H. S., Dalley, J. W., Van Denderen, J. C. M., Everitt, B. J., Robbins, T. W., and Roberts, A. C. (2001). Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb. Cortex 11, 1015–1026.
Daneman, M., and Carpenter, P. A. (1980). Individual differences in working memory and reading. J. Verbal Learn. Verbal Behav. 19, 450–466.
Durstewitz, D., Seamans, J., and Sejnowski, T. (2000). Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J. Neurophysiol. 83, 1733–1750.
Frank, M., Loughry, B., and O’Reilly, R. (2001). Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn. Affect. Behav. Neurosci. 1, 137–160.
Gazzaley, A., Rissman, J., and Desposito, M. (2004). Functional connectivity during working memory maintenance. Cogn. Affect. Behav. Neurosci. 4, 580–599.
Gerlach, M., Double, K., Arzberger, T., Leblhuber, F., Tatschner, T., and Riederer, P. (2003). Dopamine receptor agonists in current clinical use: comparative dopamine receptor binding profiles defined in the human striatum. J. Neural Transm. 110, 1119–1127.
Gibbs, S. E., and D’Esposito, M. (2005). Individual capacity differences predict working memory performance and prefrontal activity following dopamine receptor stimulation. Cogn. Affect. Behav. Neurosci. 5, 212–221.
Goldman-Rakic, P., Lidwo, M., Smiley, J., and Williams, M. (1992). The anatomy of dopamine in monkey and human prefrontal cortex. J. Neural Transm. 36, 163–177.
Gruber, A. J., Dayan, P., Gutkin, B. S., and Solla, S. A. (2006). Dopamine modulation in the basal ganglia locks the gate to working memory. J. Comput. Neurosci. 20, 153–166.
Jahanshahi, M., Jones, C. R., Zijlmans, J., Katzenschlager, R., Lee, L., Quinn, N., Frith, C. D., and Lees, A. J. (2010). Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson’s disease. Brain 133, 727–745.
Kimberg, D. Y., D’Esposito, M., and Farah, M. J. (1997). Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport 8, 3581–3585.
Krugel, L. K., Biele, G., Mohr, P. N. C., Li, S. C., and Heekeren, H. R. (2009). Genetic variation in dopaminergic neuromodulation influences the ability to rapidly and flexibly adapt decisions. Proc. Natl. Acad. Sci. U.S.A. 106, 17951–17956.
Lewis, R., and Kupke, T. (1977). “The Lafayette Clinic repeatable neuropsychological test battery: its development and research applications,” in Annual Meeting of the Southeastern Psychological Association, Hollywood, FL.
Meyer-Lindenberg, A., Straub, R. E., Lipska, B. K., Verchinski, B. A., Goldberg, T., Callicott, J. H., Egan, M. F., Huffaker, S. S., Mattay, V. S., Kolachana, B., Kleinman, J. E., and Weinberger, D. R. (2007). Genetic evidence implicating DARPP-32 in human frontostriatal structure, function, and cognition. J. Clin. Invest. 117, 672–682.
Nagano-Saito, A., Leyton, M., Monchi, O., Goldberg, Y. K., He, Y., and Dagher, A. (2008). Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J. Neurosci. 28, 3697–3706.
Pycock, C. J., Kerwin, R. W., and Carter, C. J. (1980). Effect of lesion of cortical dopamine terminals on sub-cortical dopamine-receptors in rats. Nature 286, 74–77.
Rissman, J., Gazzaley, A., and D’Esposito, M. (2004). Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage 23, 752–763.
Robbins, T. W. (2000). Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp. Brain Res. 133, 130–138.
Roberts, A. C., Desalvia, M. A., Wilkinson, L. S., Collins, P., Muir, J. L., Everitt, B. J., and Robbins, T. W. (1994). 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin card sort test – possible interactions with subcortical dopamine. J. Neurosci. 14, 2531–2544.
Salthouse, T. A., and Babcock, R. L. (1991). Decomposing adult age-differences in working memory. Dev. Psychol. 27, 763–776.
Sawaguchi, T., and Goldman-Rakic, P. S. (1991). D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 251, 947–950.
Seamans, J. K., and Yang, C. R. (2004). The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 74, 1–58.
Sharott, A., Magill, P. J., Harnack, D., Kupsch, A., Meissner, W., and Brown, P. (2005). Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur. J. Neurosci. 21, 1413–1422.
Sternberg, S. (1969). Memory-scanning: mental processes revealed by reaction-time experiments. Am. Sci. 57, 421–457.
Williams, D., Tijssen, M., Van Bruggen, G., Bosch, A., Insola, A., Di Lazzaro, V., Mazzone, P., Oliviero, A., Quartarone, A., Speelman, H., and Brown, P. (2002). Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain 125, 1558–1569.
Williams, G. V., and Goldman-Rakic, P. S. (1995). Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376, 572–575.
Keywords: fMRI, functional connectivity, working memory, dopamine
Citation: Wallace DL, Vytlacil JJ, Nomura EM, Gibbs SEB and D’Esposito M (2011) The dopamine agonist bromocriptine differentially affects fronto-striatal functional connectivity during working memory. Front. Hum. Neurosci. 5:32. doi: 10.3389/fnhum.2011.00032
Received: 21 December 2010;
Accepted: 14 March 2011;
Published online: 31 March 2011.
Edited by:
Hauke R. Heekeren, Max Planck Institute for Human Development, GermanyReviewed by:
Michael X. Cohen, University of Amsterdam, NetherlandsAtsuko Nagano-Saito, Montreal Neurological Institute, Canada
Copyright: © 2011 Wallace, Vytlacil, Nomura, Gibbs and D’Esposito. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Deanna L. Wallace, Helen Wills Neuroscience Institute, University of California at Berkeley, 132 Barker Hall, Berkeley, CA 94720-3190, USA. e-mail:ZGVhbm5hX3dhbGxhY2VAYmVya2VsZXkuZWR1


 Emi M. Nomura
Emi M. Nomura