- 1 Rotman Research Institute, Baycrest, Toronto, ON, Canada
- 2 Department of Psychology, University of Toronto, Toronto, ON, Canada
- 3 Department of Psychiatry, University of Toronto, Toronto, ON, Canada
- 4 Clinical Neuroscience, St George’s University of London, London, UK
We explored age differences in auditory perception by measuring fMRI adaptation of brain activity to repetitions of sound identity (what) and location (where), using meaningful environmental sounds. In one condition, both sound identity and location were repeated allowing us to assess non-specific adaptation. In other conditions, only one feature was repeated (identity or location) to assess domain-specific adaptation. Both young and older adults showed comparable non-specific adaptation (identity and location) in bilateral temporal lobes, medial parietal cortex, and subcortical regions. However, older adults showed reduced domain-specific adaptation to location repetitions in a distributed set of regions, including frontal and parietal areas, and to identity repetition in anterior temporal cortex. We also re-analyzed data from a previously published 1-back fMRI study, in which participants responded to infrequent repetition of the identity or location of meaningful sounds. This analysis revealed age differences in domain-specific adaptation in a set of brain regions that overlapped substantially with those identified in the adaptation experiment. This converging evidence of reductions in the degree of auditory fMRI adaptation in older adults suggests that the processing of specific auditory “what” and “where” information is altered with age, which may influence cognitive functions that depend on this processing.
Introduction
Neural responses to stimulus repetitions are smaller in magnitude than responses to the first presentation of a stimulus. In the animal literature this reduction in response has been measured in single or multi-neuron spiking (e.g., Li et al., 1993; Desimone, 1996; Anderson et al., 2008), and has been termed repetition suppression. In humans, when studied with functional MRI, it has been called fMRI adaptation. fMRI adaptation has been studied most extensively in the visual system (e.g., Buchel et al., 1999; Grill-Spector et al., 1999; Goh et al., 2004; Winston et al., 2004) and has been observed for immediate repetitions of a stimulus (Grill-Spector et al., 2006) and to repeated stimulus presentations occurring days after the initial test session (van Turennout et al., 2000), although it is not clear whether the mechanisms are the same across time scales.
In the auditory system, repetition effects have primarily been studied using electrophysiological responses (e.g., event-related potentials, or ERPs). These consist of reduced amplitude of responses to tones, especially when repetition times are short (Budd et al., 1998; Sable et al., 2004), although reductions over minutes (Polich and McIsaac, 1994) and weeks (Shelley et al., 1991) also have been observed. Although auditory fMRI adaptation has not been as widely studied as that in the visual system, reductions of activity to repeated sounds have been reported to pure tones and noise bursts (Inan et al., 2004; Petkov et al., 2004). In addition, a recent study showed that different brain areas adapted to specific categories of meaningful environmental sounds (Doehrmann et al., 2008). Domain-specific adaptation also has been reported in anterior temporal cortex for repetition of sound identity, and in posterior temporal cortex and the inferior parietal lobe (IPL) for repetition of a sound’s location (Altmann et al., 2007, 2008).
The neuronal mechanisms underlying adaptation are not well understood, although several models have been proposed, including neuronal fatigue and response time facilitation (Grill-Spector et al., 2006). Another theory emphasizes a sharpening of the stimulus representation, such that the overall response of a brain area becomes sparser with repeated presentations (Desimone, 1996; Wiggs and Martin, 1998). Other models have emphasized top-down factors rather than those operating within a single neuronal population. For instance, Friston (2005) proposed that repetition suppression indexes a top-down driven decrease in computational demand that occurs when expected and observed sensory information coincide, and evidence consistent with this idea has been reported recently (Summerfield et al., 2008). Also in line with the notion of top-down influences are reports that areas outside of sensory association cortices also show repetition effects, notably regions thought to be involved in cognitive control, such as the inferior frontal gyri (van Turennout et al., 2000; Deouell et al., 2007).
There is some evidence suggesting that adaptation in sensory systems is influenced by aging. Altered fMRI adaptation to immediate or delayed repetitions of visually presented objects has been found in older adults in some studies (Chee et al., 2006; Goh et al., 2010), but not all (Soldan et al., 2008). Smaller repetition effects in older relative to younger adults in electrophysiological responses to repeated auditory stimuli within trials (Fabiani et al., 2006) or across trials (Aine et al., 2005) have been reported, as have smaller repetition effects for visual stimuli (Joyce et al., 1998; Lawson et al., 2007). In an earlier fMRI study utilizing an auditory 1-back working memory task (Grady et al., 2008), we found adaptation in superior temporal cortex to repetition of both sound category and location (i.e., for targets). Older adults showed reduced adaptation to category repetitions and the magnitude of the adaptation was related to accuracy on the task.
However, that experiment was not designed to look at adaptation specifically, so here we report the result of an experiment that addressed this question directly. We tested for age differences in fMRI adaptation to sound identity and location to see if there were effects of age on both “what” and “where” auditory pathways (Alain et al., 2001). The design of this experiment allowed us to examine non-specific aspects of adaptation (when both identity and location were repeated), as well as domain-specific aspects (when only one feature was repeated). One might expect a larger age reduction for specific adaptation since reduced selectivity of brain responses to stimulus features has been reported in some studies (Payer et al., 2006; Carp et al., in press). We also re-analyzed the data from our previous working memory experiment (Grady et al., 2008), restricting the analysis to just the repeated location and category targets, to explore common patterns of age differences in repetition effects to sound identity and location across the two fMRI studies. For both analyses we used a multivariate approach that allowed a sensitive assessment of adaptation patterns across the whole brain (Lukic et al., 2002; McIntosh and Lobaugh, 2004), to provide a broader perspective on age differences in adaptation and whether they might be found in areas throughout the what and where pathways, and beyond.
Materials and Methods
Participants
A total of 19 young adults and 20 older adults were recruited for the fMRI adaptation experiment. Four older adults were excluded because of hearing loss. Data from three young adults had to be discarded due to excessive head motion or other technical difficulties, resulting in a final sample of 16 young (mean age = 26.7 ± 3.8 years; 11 men) and 16 older adults (mean age = 66.3 ± 4.5 years; 10 men). All were screened to exclude health problems and/or medications that might affect cognitive function and brain activity, including diabetes, stroke, and severe white matter abnormalities. Older adults scored in the normal range, between 28 and 30 (mean 29.69 ± 0.60), on a commonly used test of mental status (Folstein et al., 1975).
Full details of the sample of participants in the working memory study have been described previously (Grady et al., 2008). In brief, 16 younger (mean age = 26.1 ± 3.7 years; eight men) and 18 older (mean age = 65.8 ± 4.5 years; nine men) right handed, healthy adults completed the experiment. No individual had evidence of severe white matter abnormalities or prior stroke.
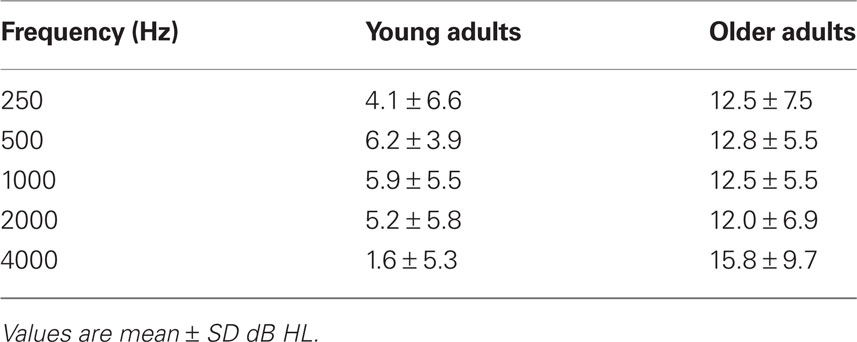
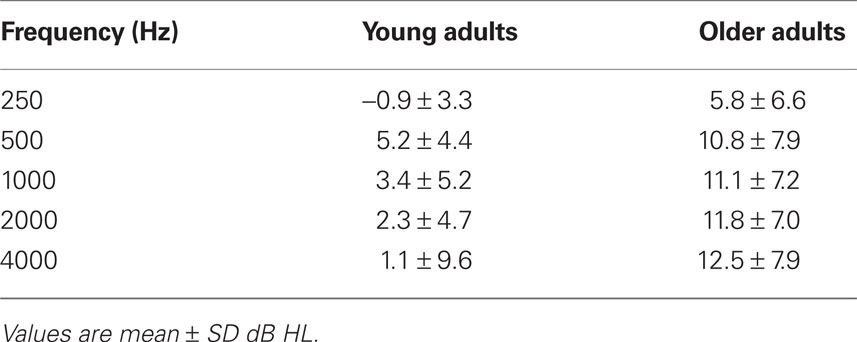
All participants were recruited from the local community and laboratory personnel, and provided informed consent in accordance with the guidelines established by the University of Toronto and Baycrest Centre. Both experiments were approved by the Baycrest Research Ethics Board. All participants had pure-tone thresholds measured between the range of 250 and 4000 Hz in both ears (see Tables 1 and 4).
Stimuli
Stimuli consisted of meaningful sounds from four categories (62 sounds in each category): human non-speech sounds (e.g., laughter), animal sounds (e.g., a rooster crowing), musical sounds (e.g., a cello being played), and machine noise (e.g., road construction). The sounds were chosen from a larger databank1 and only those that could be unambiguously categorized (by a group of individuals not included in the current report) were used in the study. Stimuli were digitally generated at a sampling rate of 24,414 Hz using a System 3 Real-Time Processor from Tucker Davis Technologies (Alachua, FL, USA). Onsets and offsets of the sounds were shaped by two halves of an 8-ms Kaiser window, respectively, and all of the stimuli had the same averaged root mean square (RMS).
We used a head-related transfer function (HRTF) to present sounds at four virtual locations along the azimuth: +30°, −30°, +95°, −95°. A variety of HRTF coefficients were tested and those that resulted in the most accurate responses during an initial discrimination test of four different locations were used for each participant. During scanning, sounds were presented with Avotec headphones (rated 30 dB sound reduction, with scanner noise measured at 90 dB).
For stimulus presentation we used a modification of a procedure reported by Goh et al. (2004), in which each trial consisted of four sounds, presented for 1005 ms each with a 295-ms interval between sounds. This design takes advantage of the fact that immediate repetitions produce larger adaptation than delayed repetitions (Grill-Spector et al., 2006), so it should be maximally sensitive to age differences, but retains the flexibility provided by an event-related design to intermix trial types. The inter-trial interval randomly varied between 6, 8, 10, and 12 s. There were four types of trials: same sound, same location (SSSL); same sound, different location (SSDL); different sound, same location (DSSL); different sound, different location (DSDL). In DSSL and DSDL trials the four different sounds were from different categories. Participants were administered five runs in total (lasting 350 s each). In each scanning run there were five trials of each type, and all participants received the same pseudorandom order of trials. There were 100 trials in total and 25 per trial type. A given sound was used in only one trial, so that each trial across the five runs used unique stimuli. Participants’ eyes were open throughout the scanning runs. The nature of the stimuli and trials were described to the participants, and they were instructed to listen to the sounds and note if there were any repetitions of the sounds or location within a trial. Sounds were presented through the headphones at approximately 90 dB SPL. No response was required.
The environmental sounds in the working memory experiment were from the same set used in the adaptation experiment, although only three categories were used (human, animal, and musical sounds) and presentation of stimuli occurred at only three possible locations (−90°, 0°, +90°). Participants performed 1-back tasks in which sound category or sound location was occasionally repeated. Prior to a block of trials, a visual word prompt was presented for 10 s and remained visible for the entire block. This prompt cued the participant as to which feature (category or location) should be attended for the 1-back task. Within each run the conditions were alternated three times (three category blocks interspersed with three location blocks) for 30 s per block; up to six runs were performed (minimum of three runs). The stimulus onset asynchrony was 2 s and the inter-target intervals varied between 4 and 12 s. The same headphones and presentation level were used as in the adaptation experiment.
fMRI Data Acquisition and Pre-Processing
Images for the adaptation experiment were acquired with a Siemens Trio 3 T magnet. We first obtained a T1 weighted anatomical volume using SPGR [time echo (TE) = 2.6 ms, time repetition (TR) = 2000 ms, FOV = 256 mm, slice thickness = 1 mm] for co-registration with the functional images and to ensure that there were no significant brain abnormalities in any of the participants. T2* functional images (TE = 30 ms, TR = 2000 ms, flip angle = 70°, FOV = 200 mm) were obtained using EPI acquisition. Each functional sequence consisted of twenty-eight 5-mm thick axial slices (in-plane resolution = 3.12 mm × 3.12 mm), positioned to image the whole brain. Functional data were adjusted for pulse and respiration, slice timing and motion corrected, spatially normalized to MNI space, and smoothed (8 mm) using AFNI2. Images were saved in nifti (.nii) format and imported into Matlab for analysis. The final image resolution was 4 mm isotropic.
Images for the working memory experiment were acquired on a General Electric Signa 3 T magnet. A T1-weighted anatomical volume using spoiled gradient echo recall (TE = 3.4 ms, TR = 35 ms, flip angle = 35°) was acquired to aid co-registration and inspect for brain abnormalities. T2* functional images (TE = 40 ms, TR = 2000 ms, flip angle = 80°) were obtained using single-shot spiral in–out acquisition (Glover and Lai, 1998; Preston et al., 2004). Each functional sequence consisted of twenty-five 5-mm thick axial slices, positioned to image the whole brain (in-plane resolution = 3.12 mm × 3.12 mm). Images were pre-processed as described above. Only those target trials with correct responses were included in the analysis. For young adults there were, on average, 64 category target trials and 62 location trials included in the analysis (minimum of 32). For older adults the mean number of trials included in the analysis was 48 and 54 for category and location, respectively (minimum of 27).
fMRI Data Analysis
The analysis procedure for the two fMRI experiments was identical. The first 20 s of each run were discarded to allow for stabilization of the magnetization. We used a whole-brain multivariate approach, partial least squares (PLS;McIntosh et al., 1996), in order to identify large-scale patterns of activity across the entire brain. PLS operates on the covariance between brain voxels and the experimental design across participants to identify a new set of variables (so-called latent variables or LVs) that optimally relate brain activity to contrasts across the conditions. PLS is similar to other data-driven multivariate techniques, such as principal component analysis, in that contrasts across conditions or groups typically are not specified in advance. Instead, the algorithm extracts a set of LVs that each identify some contrast across the experimental conditions, and the brain activity associated with each contrast. The LVs are extracted in order of the amount of covariance explained (with the LV accounting for the most covariance extracted first). That is, the first LV identifies the most prominent pattern of task contrasts and brain activity present in the data, with subsequent LVs identifying additional patterns that are expressed in the data to a lesser degree. The specific comparisons examined in each fMRI experiment are described in the Section “Results” for each experiment; the general methods are detailed here.
Partial least squares was carried out after averaging all events for each trial type of interest. The analysis included six TRs for each event (i.e., 12 s starting from trial onset) and activity at each time point was normalized to activity in the first TR of the trial. In event-related PLS, there is no baseline condition per se; instead, normalization to the first time point in the event ensures that the changes in signal represent either increases or decreases of activity relative to the beginning of each trial. PLS as applied to event-related data results in a set of brain regions that are reliably related to the task contrasts for each TR on each LV, thus providing temporal as well as spatial information (McIntosh et al., 2004).
Task PLS begins with a covariance matrix between the experimental conditions and each voxel’s signal; covariances are calculated across subjects within each group. The covariance matrix is then decomposed using singular value decomposition (SVD) to produce orthogonal LVs that optimally represent relations between brain voxels and experimental conditions. Each LV has a “singular value” that indicates that amount of covariance accounted for by the LV. The significance for each LV as a whole was determined by using a permutation test using 500 permutations (McIntosh et al., 1996). These permutations used sampling without replacement to reassign the order of conditions for each participant. A PLS analysis was run for each new sample, and the number of times the permuted singular values exceeded the observed singular values was calculated. This procedure provides exact probabilities for all LVs, and an objective means for determining the number of LVs to be retained (p < 0.05 was used here). In addition, because the decomposition of the data matrix is done in a single analytic step, no correction for multiple comparisons is required for this approach.
In addition to the permutation test, a second and independent step was to determine the reliability of the weights (saliences) for the brain voxels that showed the pattern of condition contrasts identified by the LVs. To do this, all saliences were submitted to a bootstrap estimation of the standard errors (Efron and Tibshirani, 1986) using 100 bootstraps. For the bootstrap procedure, participants are randomly resampled, with replacement, 100 times and each voxel’s standard error is calculated. Voxels with a salience/standard error ratio (bootstrap ratio, BSR) greater than |3.0| were considered to be robust, as these ratios are analogous to Z scores (Sampson et al., 1989), so that a BSR of 3 would be equivalent to p < 0.005. Using this BSR threshold, we identified clusters containing at least 10 reliable voxels, and a local maximum for each cluster was defined as the voxel with a BSR higher than any other voxel in a 2-cm cube centered on that voxel. Locations of these maxima are reported as coordinates in MNI space.
To obtain summary measures of each participant’s expression of an LV’s pattern, we calculated “brain scores” by multiplying each voxel’s salience by the normalized BOLD signal in the voxel, and summing over all brain voxels for each participant. The mean brain score at each TR (called “temporal brain scores”) can be plotted for each condition (analogous to a hemodynamic response function for a given region) to characterize the time course of whole-brain activity across the conditions. Confidence intervals (95%) for the mean brain scores in each condition and group (mean centered and collapsed across all TRs) also were calculated from the bootstrap. To obtain a conservative measure of differences in activity between conditions and age groups, we determined these differences via a lack of overlap in the confidence intervals (CIs). That is, non-overlapping CIs between conditions within a group, or between groups within a condition, indicated a significant difference.
Results
Adaptation Experiment
Audiometric thresholds were averaged across right and left ears (Table 1), and then were analyzed with a 1 (age group) by 5 (repeated factor of frequency) mixed ANOVA. The older adults had higher thresholds across all frequencies, as expected, F(1,30) = 23.8, p < 0.001. The interaction of age and frequency also was significant, F(4,120) = 4.2, p < 0.01, due to a larger age difference at the highest frequency.
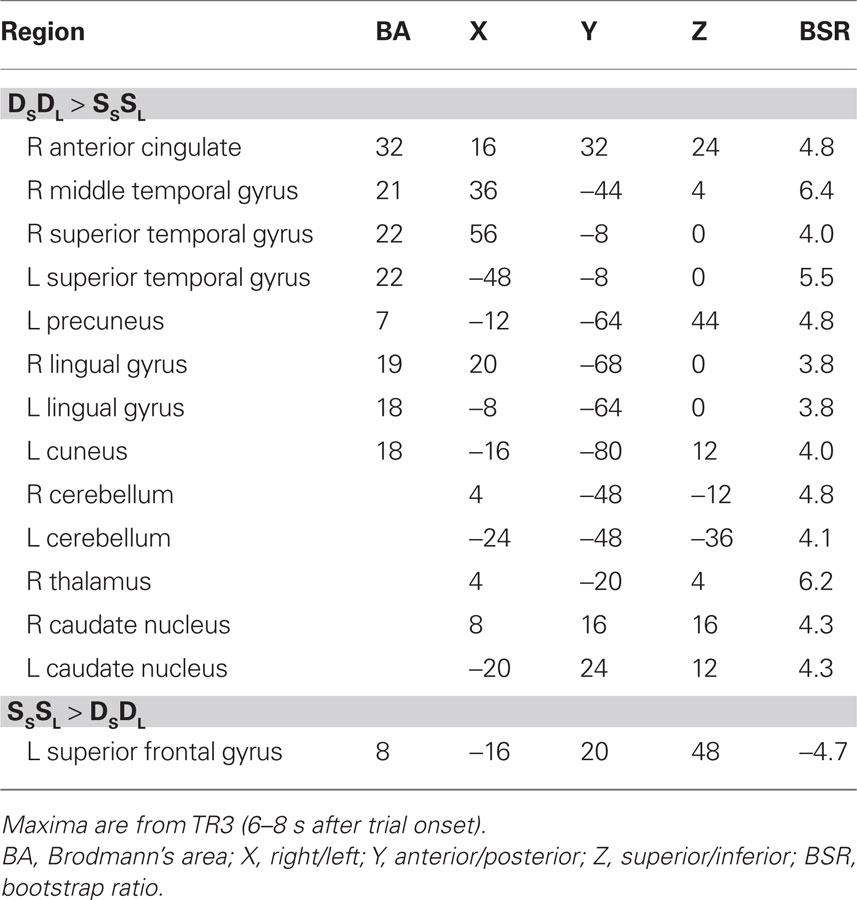
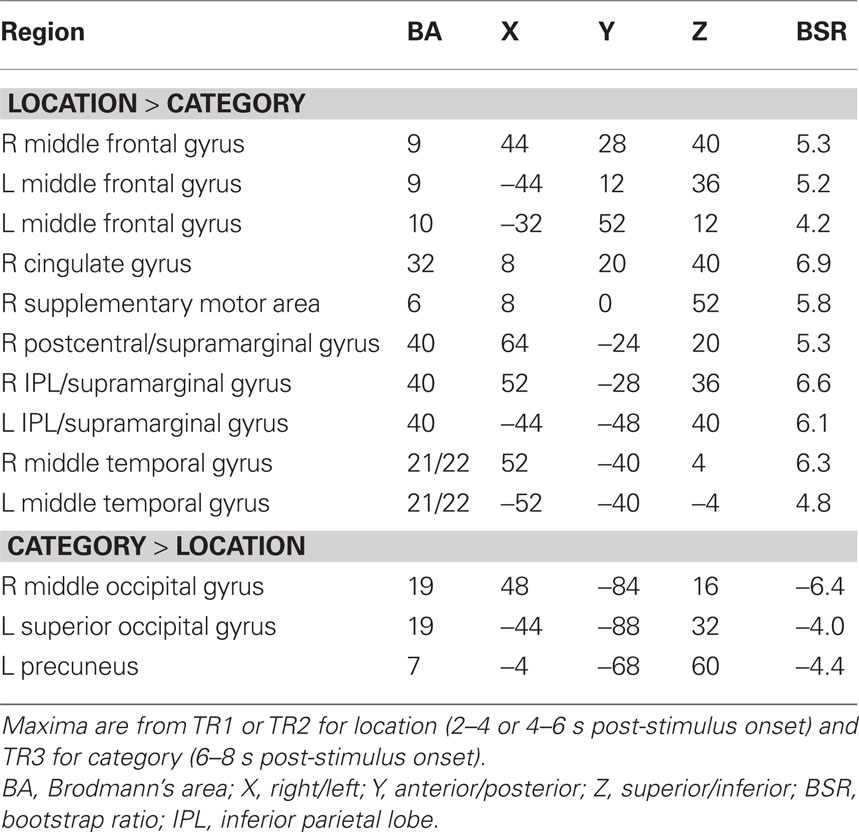
For this experiment, two analyses of the fMRI data were carried out. The first analysis examined the condition where both sound and location changed (DSDL) and the condition where both features were repeated (SSSL) to assess non-specific adaptation, i.e., adaptation that is not specific to one feature or the other. This analysis identified one significant LV (p = 0.012, accounting for 63% of the covariance) that showed a set of regions with robust adaptation effects in both young and older adults (Figure 1). Both groups showed less activity in these regions during SSSL relative to DSDL (Figure 1B), and there were no age differences in activity (Figure 1C). Adaptation to repetition of both sound identity and location, relative to changes in both features, was seen in middle and superior temporal gyri bilaterally and medial parietal cortex (Figure 1A; Table 2). Adaptation also was seen in right thalamus (extending into midbrain regions), bilateral caudate nucleus, and in some medial areas of visual cortex. A single region in left superior frontal gyrus showed more activity when both identity and location were repeated.
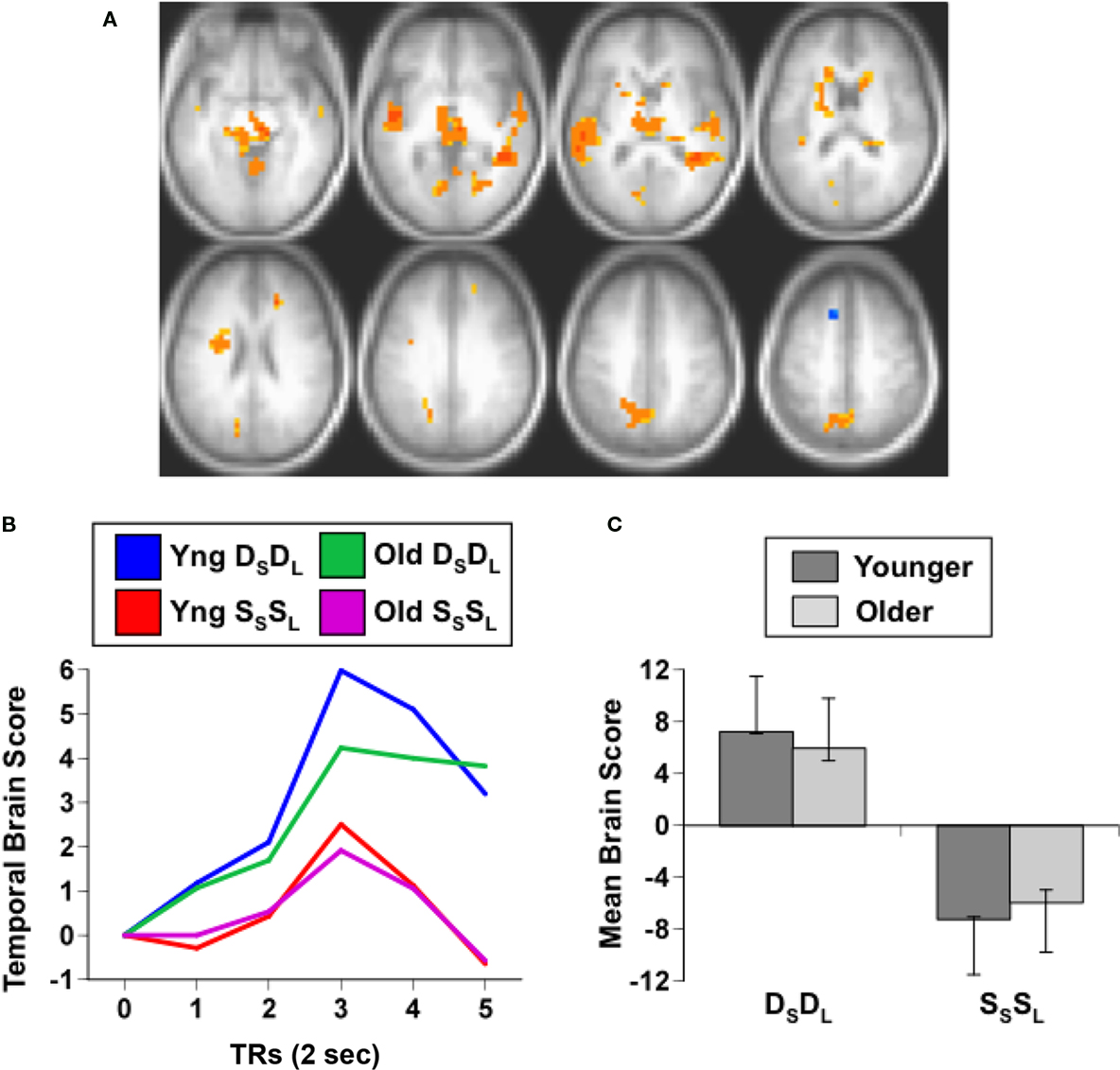
Figure 1. Results from the comparison of the DSDL and SSSL conditions. (A) Brain areas showing differential activity across conditions (images are from TR3). Orange regions showed greater activity when the sound identity and location changed relative to repetitions of both sound identity and location (non-specific adaptation). In this and subsequent figures the right side of the brain is shown on the right side of the image. (B) Temporal brain scores (i.e., brain scores plotted over 12 s after stimulus onset) showing the time course for the pattern of brain activity seen in (A) for the two conditions in each age group. (C) Mean brain scores collapsed over all TRs and mean centered (zero in the graph represents overall mean activity). Error bars are the 95% confidence intervals (CIs) calculated from the bootstrap. Non-overlapping CIs across conditions indicate adaptation in SSSL relative to DSDL. Overlap of CIs for the two groups within each condition indicates no age differences in activity for either condition.
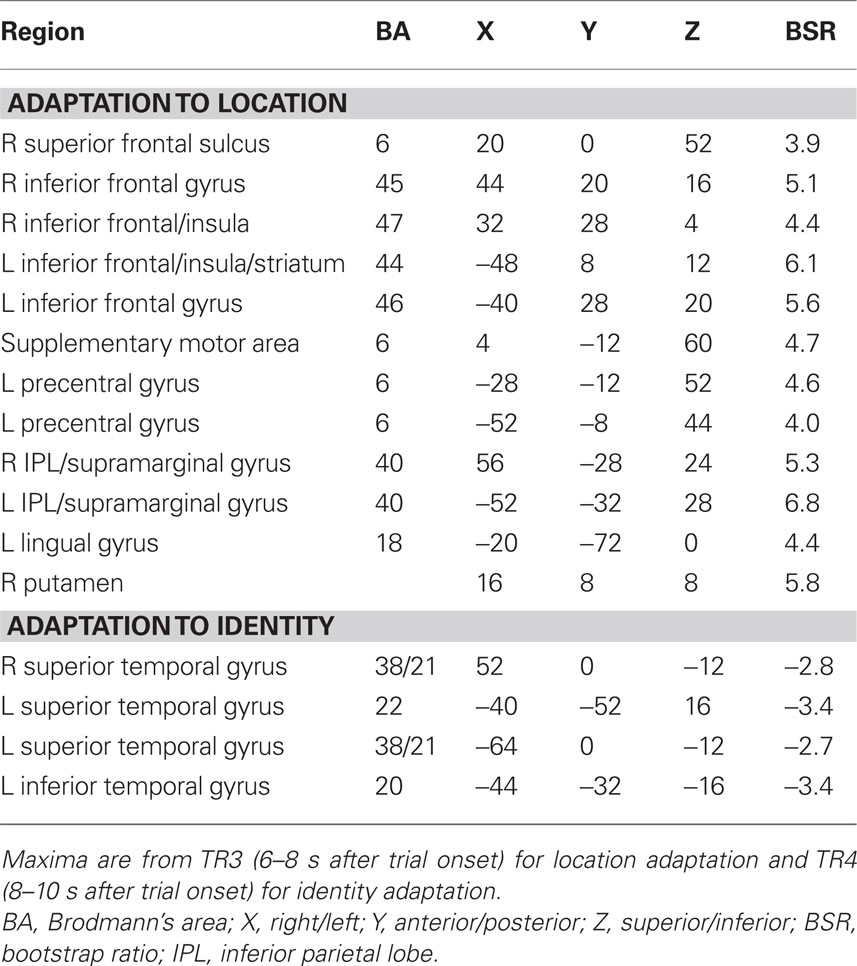
The second analysis examined adaptation specific to either repetitions of sound identity or sound location, and assessed DSSL and SSDL. This analysis should show one set of regions with adaptation when the same sound is repeated (greater activity in DSSL than in SSDL), such as anterior temporal cortex, and a different set of regions that adapt when location is repeated (more activity in SSDL than DSSL), such as parietal cortex (Alain et al., 2010). This analysis identified a single significant LV, showing a pattern of activity differentiating these conditions in younger adults, but not in older adults (p = 0.03, accounting for 66% of the covariance, Figure 2). A distributed group of regions showed adaptation in young adults when the location of the sound was repeated, i.e., more activity for SSDL than for DSSL (Figures 2C,D). This adaptation to location repetitions occurred in IPL, superior frontal cortex, premotor cortex, and inferior frontal gyri bilaterally (Figure 2A; Table 3). There were no regions showing domain-specific adaptation to changes of sound identity in the younger adults at the BSR threshold of 3.0. However when this threshold was lowered, several regions in temporal cortex were identified that showed adaptation to identity, i.e., had more activity in DSSL than in SSDL (Figure 2B; Table 3). In contrast to this pattern seen in younger adults, the older adults showed no difference in activity between these two conditions (Figures 2C,D).
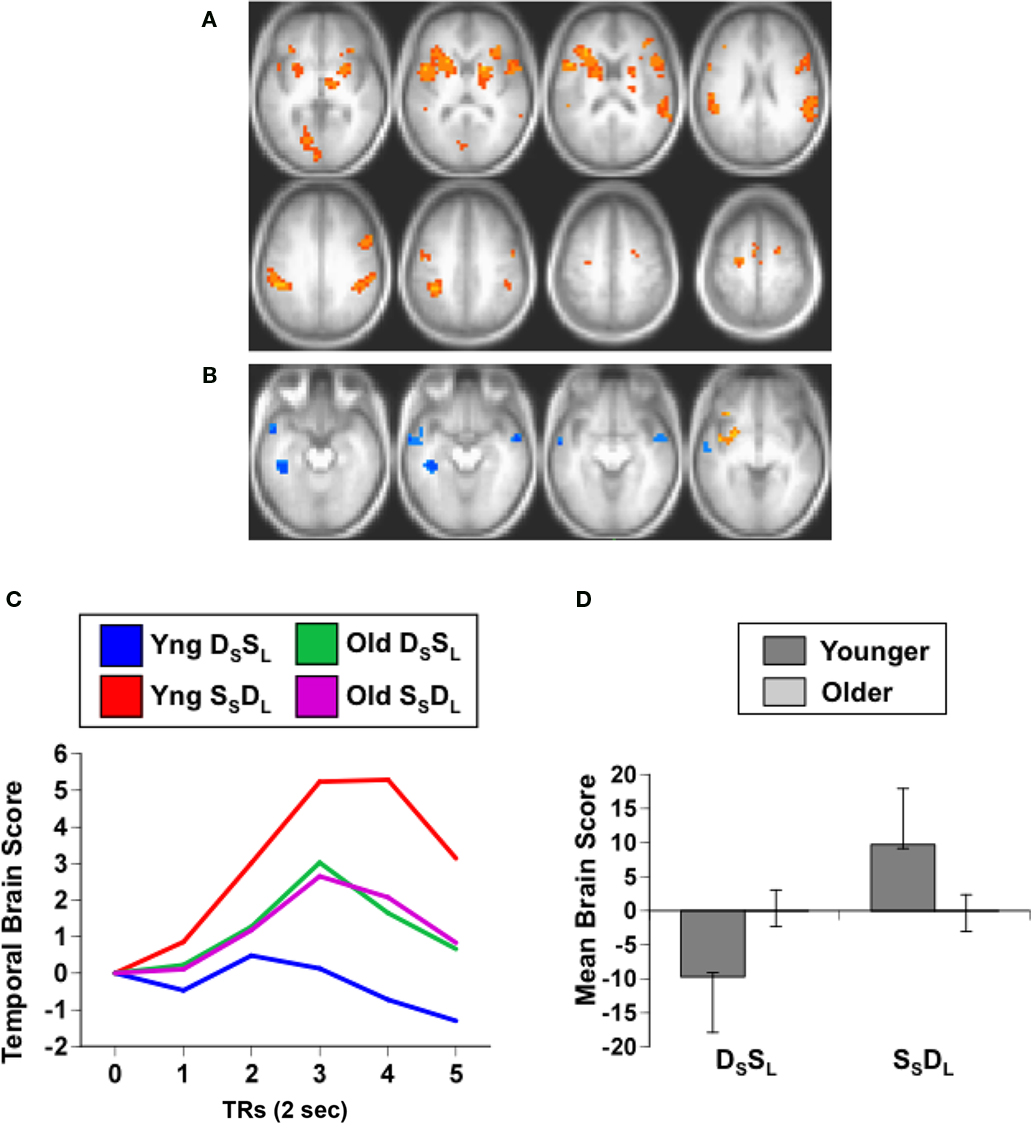
Figure 2. Results from the analysis of the DSSL and SSDL conditions. (A) Brain areas showing adaptation to location repetition (more activity in SSDL than DSSL; images are from TR3). (B) Brain areas showing the opposite pattern of adaptation to identity repetition (greater activity in DSSL than in SSDL; images are from TR4, and BSR threshold = 2). (C) Temporal brain scores (over 12 s after stimulus onset) showing the time course for the pattern of brain activity for the two conditions in each age group. (D) Mean brain scores collapsed over all TRs and mean centered (zero in the graph represents overall mean activity). Error bars are the 95% confidence intervals (CIs) calculated from the bootstrap. Non-overlapping CIs between conditions in young adults indicate different activity patterns in the two conditions. Overlapping CIs for older adults indicate no difference between conditions, and activity does not differ from the overall mean in either condition. Non-overlapping CIs between groups indicate an age difference for both conditions.
To assess whether hearing thresholds in the older adults might have influenced the pattern of activity seen in the analysis of domain-specific adaptation to location and identity, we correlated these thresholds, averaged across ears and frequencies, with the brain scores from the DSSL and SSDL conditions in the older group. Neither of these correlations between hearing threshold and brain score was significant (DSSL r = 0.04; SSDL r = 0.20), indicating that hearing sensitivity per se did not influence brain activity in either condition in the older group.
These results indicate that both younger and older adults show non-specific adaptation, i.e., adaptation that occurs when both identity and location are repeated, in temporal cortex bilaterally, as well as in subcortical areas. However, domain-specific adaptation to either identity or location was seen only in the younger adults, and this pattern was consistent with previous work showing regional differences in brain activity for these two features of sounds. Older adults showed no evidence of any differential adaptation to sound identity and location. Next, we wished to address the question of whether we could find similar age differences in data from a previous study using similar stimuli but a different paradigm. In an earlier study we examined working memory for sound category and location in young and older adults using a 1-back task (Grady et al., 2008). We found that activity for targets was reduced relative to non-repeated stimuli in a number of regions, including auditory cortex, and that this type of adaptation effect was reduced in older adults. We reasoned that if we contrasted activity elicited by category and location targets, i.e., during repetitions, that we should find a pattern of activity similar to that seen for domain-specific adaptation in the first experiment that would be expressed to a greater degree in older adults than in young adults. That is, if older adults show less adaptation to either identity or location target events relative to younger adults, then activity for targets per se should be greater in older adults. The previous paper did not assess this difference for target activity, so we addressed this question here.
Working Memory Experiment
As in the previous experiment, audiometric thresholds were averaged across right and left ears (Table 4), and then were analyzed with a 1 (age group) by 5 (repeated factor of frequency) mixed ANOVA. The older adults had higher thresholds across all frequencies as expected, F(1,32) = 21.1, p < 0.001, but the interaction of age and frequency was not significant, F(4,128) = 2.0, p > 0.10.
The PLS analysis of the data from the working memory task assessed the activity for category and location repetitions in the two age groups, analogous to the domain-specific adaptation comparison reported above. If older adults have less adaptation to repetitions, then their brain activity for location or category targets, which are repetitions in this 1-back task, should be larger than activity seen in younger adults. This analysis revealed a single significant LV, showing a pattern of activity that differentiated category vs. location targets in both groups (p < 0.001, accounting for 64% of the covariance; Figure 3A). As would be expected if older adults have less adaptation to repeated targets, the older adults had greater expression of this pattern compared to young adults (Figures 3B,C). The predominant activity was seen during the location condition, during which substantial activity was noted in the middle temporal gyri, IPLs, and dorsolateral prefrontal regions bilaterally. More activity during the category condition, relative to location, was seen in only a few areas, including the left inferior frontal gyrus and posterior occipito-parietal regions (see Table 5).
Figure 3. Results from the working memory study. (A) Orange regions showed greater activity for the location condition. (B) Temporal brain scores plotted over 12 s after stimulus onset, showing the time course for the pattern of brain activity seen in (A) for the two conditions in each age group. (C) Mean brain scores collapsed over all TRs and mean centered (zero in the graph represents overall mean activity). Error bars are the 95% confidence intervals (CIs) calculated from the bootstrap. Non-overlapping CIs indicate a difference between category and location conditions in both groups as well as an age difference within each condition.
As with the adaptation experiment, we assessed whether there was any relation between hearing sensitivity in the older adults and their brain activity for repeated targets. We calculated the correlation between hearing threshold, averaged over ears and frequencies, and the brain scores for the category and location target conditions. These correlations were not significant (category r = 0.10; location r = 0.34), indicating that hearing sensitivity did not influence activity to target repetition in the older adults. Also, since behavioral data were available from this experiment, we correlated the brain scores from the category and location target conditions with accuracy (percent correct) in these two working memory conditions in the older adults. Neither of the correlations for accuracy was significant (category r = −0.16; location r = −0.02).
Finally, we compared the brain areas in the working memory experiment seen in Figure 3 to the set of regions where older adults had reduced adaptation relative to younger adults (Figure 2A). In order to assess the extent of the overlap in the spatial patterns identified in the two studies, for each experiment the BSR maps (from the LVs shown in Figures 2 and 3, using positive BSRs thresholded at 3.0) were summed across TRs 1–5 and the overlap of the two cumulative maps was determined, i.e., those voxels with non-zero values in both experiments (see Figure 4). Despite the fact that the two experiments were measuring different functions (passive listening vs. working memory) in different samples and using different scanners, substantial overlap is noted between the studies. Regions showing an age effect in both experiments included middle temporal, inferior/middle frontal, and inferior parietal regions bilaterally.
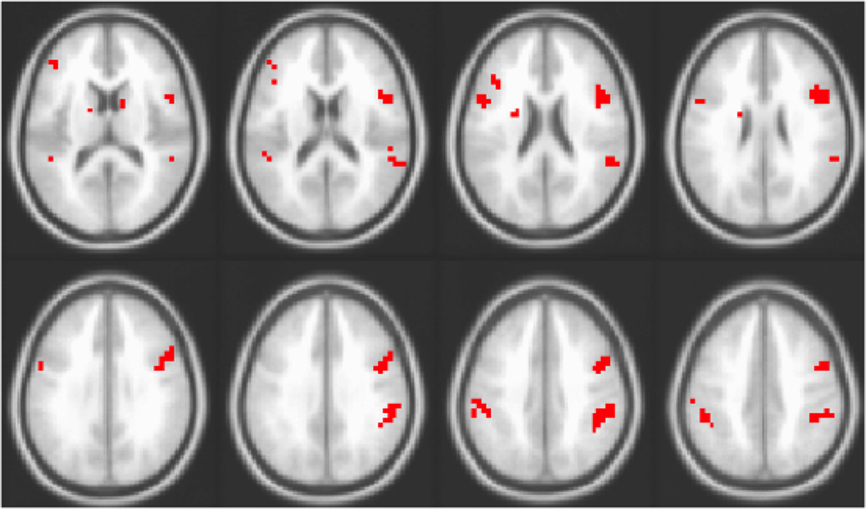
Figure 4. Overlap of areas with reliable effects in the adaptation study (contrast of DSSL and SSDL) and working memory study (i.e., overlap of areas seen in Figures 2 and 3). Areas in red are those where older adults showed no adaptation to location in the adaptation experiment and greater activity for repeated location targets in the working memory experiment.
Discussion
In this paper we have shown that both younger and older adults modulate their brain activity in response to repetitions of sound identity and location when both are repeated simultaneously. In addition, young adults show adaptation in a distributed set of brain regions to specific repetitions of the location of a sound, relative to changes in location, and weaker adaptation in temporal cortex when sound identity is repeated, whereas these repetition effects are absent in older adults. Some of the regions with age differences in location adaptation also showed age differences to repeated location targets during a working memory task in a different group of older adults. These data provide converging evidence that in older adults the ability of brain activity to track whether or not some feature of an auditory stimulus has changed may not be altered, but selective adaptation to a particular feature is reduced.
The regions of the brain that adapted to repetitions of the SSSL were primarily in anterior parts of the superior and middle temporal lobes, consistent with activation of these regions in auditory perception (e.g., Zatorre et al., 1999; Alain et al., 2001; Maeder et al., 2001; Belin and Zatorre, 2003), particularly for selectively processing the identity of sounds (Alain et al., 2001; Maeder et al., 2001). Adaptation in medial parietal cortex also was seen, in line with activation of this region during processing of sound location (Alain et al., 2001). Adaptation in both temporal identity-sensitive areas and parietal regions sensitive to sound location would be expected in this contrast that examined adaptation to both features. In addition, we found adaptation in the caudate nucleus, thalamus, and lingual gyrus. The cluster containing the thalamus extended into the midbrain, including the inferior colliculus, where some have reported activation to auditory stimuli with fMRI (Harms and Melcher, 2002; Steinmann and Gutschalk, 2010). This would indicate adaptation in this auditory subcortical region, as well as in auditory regions of the cortex. Activity in the lingual gyrus could conceivably be related to imagery during the presentation of these meaningful sounds, as imagery can activate these ventral extrastriate regions (Amedi et al., 2005; Slotnick et al., 2005). Caudate activity has been noted in auditory studies that involve some aspect of time perception or paced responding (Nenadic et al., 2003; Riecker et al., 2003). The finding of no age differences for this pattern of adaptation indicates that the general capacity of the auditory system to respond to repetitions of stimuli in the auditory environment is relatively maintained into older age.
We also examined domain-specific adaptation to repetitions of either sound identity or location, expecting that we would observe different patterns depending on which feature was repeated, as others have found for auditory adaptation (Altmann et al., 2007), and for brain activation associated with tasks based on attention to these two features (Alain et al., 2001; Maeder et al., 2001). We found that adaptation to sound identity and sound location involved different areas of the brain in the younger adults, although the adaptation pattern for location was more robust than that for identity. Indeed, the anterior temporal regions showing adaptation to identity were only revealed at a lower statistical threshold, although these areas were consistent with those identified using a univariate analysis of the data from these young adults (Alain et al., 2010) and with our earlier experiments (Alain et al., 2001; Grady et al., 2008). Adaptation to repetitions of the same sound in anterior regions of temporal cortex is in line with the idea that this region is part of the ventral auditory processing stream that represents sound identity (Alain et al., 2001; Tian et al., 2001), and with evidence that stimulus-selectivity increases toward the temporal pole (Kikuchi et al., 2010). In contrast, activity in inferior parietal and superior frontal regions was modulated by repetitions of location, consistent with the participation of these regions in the dorsal auditory stream for representing location of sound sources (e.g., Alain et al., 2008). In addition, adaptation to location was seen in premotor regions, suggesting that some motor processes might also have adapted, which would be consistent with the idea that the dorsal stream may play a role in responding to stimuli as well as perceiving their location (Goodale and Milner, 1992).
Unlike the non-specific adaptation effect, there was an age difference in domain-specific adaptation, such that older adults failed to show any differentiation between the DSSL and SSDL conditions, suggesting reduced adaptation to both identity and location when each feature was manipulated independently. In addition, a similar effect was seen in the analysis of target activity during the working memory experiment, which showed more activity in older adults for repeated location targets as well as more activity for category targets. In fact, there was considerable overlap in the regions of the brain that showed these effects, with regions in temporal, frontal, and inferior parietal cortex showing age differences in location adaptation in both experiments. Therefore, this overlap in activity patterns between the DSSL and SSDL contrast in the adaptation experiment and that seen for repeated targets in the memory experiment provides converging evidence that the distributed set of regions responding to location repetitions in younger adults is less sensitive to these repetitions in older adults. Given that the specific effects for identity in the two experiments were less robust, the conclusions regarding age differences in adaptation to these features are more tentative.
Our finding of age differences in adaptation to repetition of sound location is consistent with a number of studies showing age reductions on auditory localization tasks (e.g., Cranford et al., 1993; Abel et al., 2000; Ross et al., 2007; Wambacq et al., 2009), visual localization tasks (e.g., Cherry et al., 1993; Owsley et al., 2000), and navigation tasks (Head and Isom, 2010). It may be that smaller repetition effects for location in older adults could influence their performance on cognitive processes that would make use of this information. We did not find any correlations between working memory performance and the patterns of activity distinguishing older from younger adults in the analysis reported here that would support such a notion, although we have shown that smaller repetition effects for category targets, relative to sustained activity for non-targets, are related to working memory performance for category in older adults (Grady et al., 2008). It remains an open question as to how much age differences in adaptation can influence differences seen on tasks with higher cognitive demands.
Although our results provide evidence of age differences in auditory adaptation, they do not speak to any particular mechanism underlying this effect. That is, fMRI adaptation is simply a reduction in the BOLD signal when stimuli are repeated, and this reduction itself, as well as any age differences in it, could be due to a number of factors (as we note in the Section “Introduction”). As one model suggests (Desimone, 1996), it may be that adaptation is the result of sparser or more selective representations that arise with repeated presentations of the same stimulus or stimulus feature. If neural responses to different auditory features are less selective and more distributed in older adults, as has been shown in stimulus-selective regions in visual cortex (Payer et al., 2006; Carp et al., in press), then it is possible that this would have the effect of broadening the response in general and maintaining the response across repetitions, thus reducing the adaptation effect. Indeed, less differentiated responses to specific visual features (Carp et al., in press) or across visual tasks (Grady, 2002) have been demonstrated in older adults in areas outside of visual cortex, including the frontal lobes, similar to the frontal areas with age differences reported here. Thus, reduced selectivity of responses across the brain could be the mechanism underlying an age reduction in adaptation, but clearly this will need to be addressed in future work.
Another factor contributing to reduced adaptation to repeated stimuli in older adults could be reduced inhibitory function, such as the decline in intracortical inhibition reported in the brains of old rats (Schmidt et al., 2010) and monkeys (Schmolesky et al., 2000; Leventhal et al., 2003). A number of behavioral studies have suggested reduced inhibitory function in older adults (for reviews see Hasher et al., 1999; Healey et al., 2008) as have some recent fMRI studies (Gazzaley et al., 2005; Schmitz et al., 2010). Such a decline in inhibitory function could lead to inefficient filtering of repeated information (Fabiani et al., 2006) and smaller adaptation effects.
There is a further unresolved issue regarding adaptation per se, and that is whether adaptation over distributed areas of the brain is an indication that all of these areas adapt because of the same mechanism. For example, some have suggested that frontal repetition effects are due to conceptual processing rather than perceptual processing, and have shown that adaptation effects in anterior and posterior regions can be elicited somewhat independently (Wig et al., 2005). If this is the case, then some of the adaptation effects that we observed, particularly those in frontal cortex, may be due to our use of meaningful stimuli that might encourage semantic processing. On the other hand, a very recent paper showed essentially identical repetition effects for perceptual and semantic repetitions of auditory stimuli (De Lucia et al., 2010), suggesting that similar mechanisms are invoked regardless. Another possibility is that adaptation effects can be seen widely in the brain because reduced responses in sensory areas get passed on to higher association areas, consistent with a recent paper showing an increase in the synchrony of responses across multiple brain areas during adaptation (Gilbert et al., 2010). Clearly this is an important question for our understanding of adaptation in general, and age differences in adaptation, and will need further research.
There are several limitations of this study that should be noted. The fMRI environment is a noisy one and there is the possibility that this noise could differentially influence adaptation in the older adults. However, the age difference we observed is unlikely to be due to a differential influence of scanner noise on auditory responses in older adults because if there were such an effect it should be a general one that would have an impact across all experimental conditions. Since we found no age difference in the adaptation effect that characterized the SSSL condition relative to the DSDL condition, there does not appear to be any overall effect of scanner noise. A similar argument could be made that this lack of an age difference in non-specific adaptation makes it unlikely that there was an influence of hearing loss on adaptation in our older adults, although we cannot know this for certain. That is, it is possible that some combination of scanner noise, headphone attenuation and hearing loss made it more difficult for our older adults to discriminate differences among stimuli. An additional limitation is that we did not have our participants engage in a task during the adaptation experiment, and so cannot rule out fluctuations in attention or fatigue, particularly in the older adults. Again, the lack of an age difference in non-specific adaptation would argue against any general effects of attention on adaptation in older vs. younger adults. In addition, the substantial similarity between the age differences noted for domain-specific adaptation and those seen for target related activity in the working memory experiment, where there was an ongoing task, also argues against an attentional account of the observed age difference in adaptation.
Finally, it is of interest to relate our findings to the literature on priming, which is a behavioral facilitation to repeated stimuli that bears some resemblance to adaptation. The age reduction in adaptation that we and others have found differs from the behavioral literature on priming, which has generally shown that behavioral priming, including repetition priming and auditory priming, is generally preserved in older adults (Sommers, 1999; Wiggs et al., 2006). Repetition priming effects do appear to be less stable over time in older adults (Wiggs et al., 2006), so reduced adaptation may be involved in this instability. However, although stimulus repetition leads to both adaptation and behavioral priming, the link between them is not that well understood (Anderson et al., 2008) and may not be very strong (McMahon and Olson, 2007). Regardless of what adaptation means in terms of priming, it appears to be a very robust response of the nervous system to the repetition of stimuli in the environment, and as such may reflect a process for stimulus representation. It seems clear from our study, and from others in both the auditory and visual systems, that age influences the degree of adaptation, although the link between brain adaptation and implicit or explicit memory in older adults remains to be determined.
In conclusion, we found no age difference in auditory adaptation when both identity and location were repeated, but did find reduced adaptation in older adults when there was a repetition of only one feature. This suggests that non-specific repetition effects are maintained into older age, whereas responses to changes in individual features may be more vulnerable. The lack of any differentiation of brain activity to the DSSL and SSDL conditions in older adults is similar to previous reports of less differentiated activity in visual areas sensitive to different object categories, and a similar reduction of stimulus-selective activity in the auditory system may be related to reduced adaptation. Our finding of age differences in adaptation to meaningful sounds extends the work done on adaptation in the visual system, and invites further investigation into how adaptation is related to dedifferentiation of brain activity in older adults and how it influences other cognitive processes.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a Canadian Institutes of Health Research grant to Cheryl L. Grady and Claude Alain (MOP 67131). Additional support was provided by the Canada Research Chairs Program, the Ontario Research Fund, the Canadian Foundation for Innovation, and the Heart and Stroke Foundation Centre for Stroke Recovery. Rebecca Charlton was supported by a short-term Fellowship to the Rotman Research Institute, Baycrest Centre, awarded by The International Human Frontier Science Program.
Footnotes
- ^http://www.findsounds.com/types.html
- ^afni.nimh.nih.gov/afni
References
Abel, S. M., Giguere, C., Consoli, A., and Papsin, B. C. (2000). The effect of aging on horizontal plane sound localization. J. Acoust. Soc. Am. 108, 743–752.
Aine, C. J., Adair, J. C., Knoefel, J. E., Hudson, D., Qualls, C., Kovacevic, S., Woodruff, C. C., Cobb, W., Padilla, D., Lee, R. R., and Stephen, J. M. (2005). Temporal dynamics of age-related differences in auditory incidental verbal learning. Brain Res. Cogn. Brain Res. 24, 1–18.
Alain, C., Arnott, S. R., Hevenor, S., Graham, S., and Grady, C. L. (2001). “What” and “where” in the human auditory system. Proc. Natl. Acad. Sci. U.S.A. 98, 12301–12306.
Alain, C., Shen, D., Yu, H., and Grady, C. L. (2010). Dissociable memory- and response-related activity in parietal cortex during auditory spatial working memory. Front. Psychol. 1:202. doi: 10.3389/fpsyg.2010.00202
Alain, C., Yu, H., and Grady, C. (2008). The inferior parietal lobe contributes to auditory spatial working memory and sensorimotor integration. J. Cogn. Neurosci. 20, 285–295.
Altmann, C. F., Bledowski, C., Wibral, M., and Kaiser, J. (2007). Processing of location and pattern changes of natural sounds in the human auditory cortex. Neuroimage 35, 1192–1200.
Altmann, C. F., Henning, M., Doring, M. K., and Kaiser, J. (2008). Effects of feature-selective attention on auditory pattern and location processing. Neuroimage 41, 69–79.
Amedi, A., Malach, R., and Pascual-Leone, A. (2005). Negative BOLD differentiates visual imagery and perception. Neuron 48, 859–872.
Anderson, B., Mruczek, R. E., Kawasaki, K., and Sheinberg, D. (2008). Effects of familiarity on neural activity in monkey inferior temporal lobe. Cereb. Cortex 18, 2540–2552.
Belin, P., and Zatorre, R. J. (2003). Adaptation to speaker’s voice in right anterior temporal lobe. Neuroreport 14, 2105–2109.
Buchel, C., Coull, J. T., and Friston, K. J. (1999). The predictive value of changes in effective connectivity for human learning. Science 283, 1538–1541.
Budd, T. W., Barry, R. J., Gordon, E., Rennie, C., and Michie, P. T. (1998). Decrement of the N1 auditory event-related potential with stimulus repetition: habituation vs. refractoriness. Int. J. Psychophysiol. 31, 51–68.
Carp, J., Park, J., Polk, T. A., and Park, D. C. (in press). Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. Neuroimage. doi: 10.1016/j.neuroimage.2010.04.267. [Epub ahead of print].
Chee, M. W., Goh, J. O., Venkatraman, V., Tan, J. C., Gutchess, A., Sutton, B., Hebrank, A., Leshikar, E., and Park, D. (2006). Age-related changes in object processing and contextual binding revealed using fMR adaptation. J. Cogn. Neurosci. 18, 495–507.
Cherry, K. E., Park, D. C., and Donaldson, H. (1993). Adult age differences in spatial memory: effects of structural context and practice. Exp. Aging Res. 19, 333–350.
Cranford, J. L., Andres, M. A., Piatz, K. K., and Reissig, K. L. (1993). Influences of age and hearing loss on the precedence effect in sound localization. J. Speech Hear. Res. 36, 437–441.
De Lucia, M., Cocchi, L., Martuzzi, R., Meuli, R. A., Clarke, S., and Murray, M. M. (2010). Perceptual and semantic contributions to repetition priming of environmental sounds. Cereb. Cortex 20, 1676–1684.
Deouell, L. Y., Heller, A. S., Malach, R., D’Esposito, M., and Knight, R. T. (2007). Cerebral responses to change in spatial location of unattended sounds. Neuron 55, 985–996.
Desimone, R. (1996). Neural mechanisms for visual memory and their role in attention. Proc. Natl. Acad. Sci. U.S.A. 93, 13494–13499.
Doehrmann, O., Naumer, M. J., Volz, S., Kaiser, J., and Altmann, C. F. (2008). Probing category selectivity for environmental sounds in the human auditory brain. Neuropsychologia 46, 2776–2786.
Efron, B., and Tibshirani, R. (1986). Bootstrap methods for standard errors, confidence intervals and other measures of statistical accuracy. Stat. Sci. 1, 54–77.
Fabiani, M., Low, K. A., Wee, E., Sable, J. J., and Gratton, G. (2006). Reduced suppression or labile memory? Mechanisms of inefficient filtering of irrelevant information in older adults. J. Cogn. Neurosci. 18, 637–650.
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini Mental State” – a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198.
Friston, K. (2005). A theory of cortical responses. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 360, 815–836.
Gazzaley, A., Cooney, J. W., Rissman, J., and D’Esposito, M. (2005). Top-down suppression deficit underlies working memory impairment in normal aging. Nat. Neurosci. 8, 1298–1300.
Gilbert, J. R., Gotts, S. J., Carver, F. W., and Martin, A. (2010). Object repetition leads to local increases in the temporal coordination of neural responses. Front. Hum. Neurosci. 4:30. doi: 10.3389/fnhum.2010.00030
Glover, G. H., and Lai, S. (1998). Self-navigated spiral fMRI: interleaved versus single-shot. Magn. Reson. Med. 39, 361–368.
Goh, J. O., Siong, S. C., Park, D., Gutchess, A., Hebrank, A., and Chee, M. W. (2004). Cortical areas involved in object, background, and object-background processing revealed with functional magnetic resonance adaptation. J. Neurosci. 24, 10223–10228.
Goh, J. O., Suzuki, A., and Park, D. C. (2010). Reduced neural selectivity increases fMRI adaptation with age during face discrimination. Neuroimage 51, 336–344.
Goodale, M. A., and Milner, A. D. (1992). Separate visual pathways for perception and action. Trends Neurosci. 15, 20–25.
Grady, C. L. (2002). Age-related differences in face processing: a meta-analysis of three functional neuroimaging experiments. Can. J. Exp. Psychol. 56, 208–220.
Grady, C. L., Yu, H., and Alain, C. (2008). Age-related differences in brain activity underlying working memory for spatial and nonspatial auditory information. Cereb. Cortex 18, 189–199.
Grill-Spector, K., Henson, R., and Martin, A. (2006). Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 10, 14–23.
Grill-Spector, K., Kushnir, T., Edelman, S., Avidan, G., Itzchak, Y., and Malach, R. (1999). Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron 24, 187–203.
Harms, M. P., and Melcher, J. R. (2002). Sound repetition rate in the human auditory pathway: representations in the waveshape and amplitude of fMRI activation. J. Neurophysiol. 88, 1433–1450.
Hasher, L., Zacks, R. T., and May, C. P. (1999). “Inhibitory control, circadian arousal, and age,” in Attention and Performance XVII: Cognitive Regulation of Performance. Interaction of Theory and Application, eds D. Gopher and A. Koriat (Cambridge, MA: MIT Press), 653–675.
Head, D., and Isom, M. (2010). Age effects on wayfinding and route learning skills. Behav. Brain Res. 209, 49–58.
Healey, M. K., Campbell, K. L., and Hasher, L. (2008). “Cognitive aging and increased distractibility: costs and potential benefits,” in Progress in Brain Research, Vol. 169, The Essence of Memory, eds W. S. Sossin, J.-C. Lacaille, V. F. Castellucci, and S. Belleville (Amsterdam: Elsevier), 353–363.
Inan, S., Mitchell, T., Song, A., Bizzell, J., and Belger, A. (2004). Hemodynamic correlates of stimulus repetition in the visual and auditory cortices: an fMRI study. Neuroimage 21, 886–893.
Joyce, C. A., Paller, K. A., McIsaac, H. K., and Kutas, M. (1998). Memory changes with normal aging: behavioral and electrophysiological measures. Psychophysiology 35, 669–678.
Kikuchi, Y., Horwitz, B., and Mishkin, M. (2010). Hierarchical auditory processing directed rostrally along the monkey’s supratemporal plane. J. Neurosci. 30, 13021–13030.
Lawson, A. L., Guo, C., and Jiang, Y. (2007). Age effects on brain activity during repetition priming of targets and distracters. Neuropsychologia 45, 1223–1231.
Leventhal, A. G., Wang, Y., Pu, M., Zhou, Y., and Ma, Y. (2003). GABA and its agonists improved visual cortical function in senescent monkeys. Science 300, 812–815.
Li, L., Miller, E. K., and Desimone, R. (1993). The representation of stimulus familiarity in anterior inferior temporal cortex. J. Neurophysiol. 69, 1918–1929.
Lukic, A. S., Wernick, M. N., and Strother, S. C. (2002). An evaluation of methods for detecting brain activations from functional neuroimages. Artif. Intell. Med. 25, 69–88.
Maeder, P. P., Meuli, R. A., Adriani, M., Bellmann, A., Fornari, E., Thiran, J.-P., Pittet, A., and Clarke, S. (2001). Distinct pathways involved in sound recognition and localization: a human fMRI study. Neuroimage 14, 802–816.
McIntosh, A. R., Bookstein, F. L., Haxby, J. V., and Grady, C. L. (1996). Spatial pattern analysis of functional brain images using partial least squares. Neuroimage 3, 143–157.
McIntosh, A. R., Chau, W. K., and Protzner, A. B. (2004). Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage 23, 764–775.
McIntosh, A. R., and Lobaugh, N. L. (2004). Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage 23(Suppl. 1), S250–S263.
McMahon, D. B., and Olson, C. R. (2007). Repetition suppression in monkey inferotemporal cortex: relation to behavioral priming. J. Neurophysiol. 97, 3532–3543.
Nenadic, I., Gaser, C., Volz, H. P., Rammsayer, T., Hager, F., and Sauer, H. (2003). Processing of temporal information and the basal ganglia: new evidence from fMRI. Exp. Brain Res. 148, 238–246.
Owsley, C., Burton-Danner, K., and Jackson, G. R. (2000). Aging and spatial localization during feature search. Gerontology 46, 300–305.
Payer, D., Marshuetz, C., Sutton, B., Hebrank, A., Welsh, R. C., and Park, D. C. (2006). Decreased neural specialization in old adults on a working memory task. Neuroreport 17, 487–491.
Petkov, C. I., Kang, X., Alho, K., Bertrand, O., Yund, E. W., and Woods, D. L. (2004). Attentional modulation of human auditory cortex. Nat. Neurosci. 7, 658–663.
Polich, J., and McIsaac, H. K. (1994). Comparison of auditory P300 habituation from active and passive conditions. Int. J. Psychophysiol. 17, 25–34.
Preston, A. R., Thomason, M. E., Ochsner, K. N., Cooper, J. C., and Glover, G. H. (2004). Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5 and 3 T. Neuroimage 21, 291–301.
Riecker, A., Wildgruber, D., Mathiak, K., Grodd, W., and Ackermann, H. (2003). Parametric analysis of rate-dependent hemodynamic response functions of cortical and subcortical brain structures during auditorily cued finger tapping: a fMRI study. Neuroimage 18, 731–739.
Ross, B., Fujioka, T., Tremblay, K. L., and Picton, T. W. (2007). Aging in binaural hearing begins in mid-life: evidence from cortical auditory-evoked responses to changes in interaural phase. J. Neurosci. 27, 11172–11178.
Sable, J. J., Low, K. A., Maclin, E. L., Fabiani, M., and Gratton, G. (2004). Latent inhibition mediates N1 attenuation to repeating sounds. Psychophysiology 41, 636–642.
Sampson, P. D., Streissguth, A. P., Barr, H. M., and Bookstein, F. L. (1989). Neurobehavioral effects of prenatal alcohol: part II. Partial least squares analysis. Neurotoxicol. Teratol. 11, 477–491.
Schmidt, S., Redecker, C., Bruehl, C., and Witte, O. W. (2010). Age-related decline of functional inhibition in rat cortex. Neurobiol. Aging 31, 504–511.
Schmitz, T. W., Cheng, F. H., and De Rosa, E. (2010). Failing to ignore: paradoxical neural effects of perceptual load on early attentional selection in normal aging. J. Neurosci. 30, 14750–14758.
Schmolesky, M. T., Wang, Y., Pu, M., and Leventhal, A. G. (2000). Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat. Neurosci. 3, 384–390.
Shelley, A. M., Ward, P. B., Michie, P. T., Andrews, S., Mitchell, P. F., Catts, S. V., and McConaghy, N. (1991). The effect of repeated testing on ERP components during auditory selective attention. Psychophysiology 28, 496–510.
Slotnick, S. D., Thompson, W. L., and Kosslyn, S. M. (2005). Visual mental imagery induces retinotopically organized activation of early visual areas. Cereb. Cortex 15, 1570–1583.
Soldan, A., Gazes, Y., Hilton, H. J., and Stern, Y. (2008). Aging does not affect brain patterns of repetition effects associated with perceptual priming of novel objects. J. Cogn. Neurosci. 20, 1762–1776.
Sommers, M. S. (1999). Perceptual specificity and implicit auditory priming in older and younger adults. J. Exp. Psychol. Learn. Mem. Cogn. 25, 1236–1255.
Steinmann, I., and Gutschalk, A. (2010). Potential fMRI correlates of 40-Hz phase locking in primary auditory cortex, thalamus and midbrain. Neuroimage 54, 495–504.
Summerfield, C., Trittschuh, E. H., Monti, J. M., Mesulam, M. M., and Egner, T. (2008). Neural repetition suppression reflects fulfilled perceptual expectations. Nat. Neurosci. 11, 1004–1006.
Tian, B., Reser, D., Durham, A., Kustov, A., and Rauschecker, J. P. (2001). Functional specialization in rhesus monkey auditory cortex. Science 292, 290–293.
van Turennout, M., Ellmore, T., and Martin, A. (2000). Long-lasting cortical plasticity in the object naming system. Nat. Neurosci. 3, 1329–1334.
Wambacq, I. J., Koehnke, J., Besing, J., Romei, L. L., Depierro, A., and Cooper, D. (2009). Processing interaural cues in sound segregation by young and middle-aged brains. J. Am. Acad. Audiol. 20, 453–458.
Wiggs, C. L., and Martin, A. (1998). Properties and mechanisms of perceptual priming. Curr. Opin. Neurobiol. 8, 227–233.
Wiggs, C. L., Weisberg, J., and Martin, A. (2006). Repetition priming across the adult lifespan – the long and short of it. Neuropsychol. Dev. Cogn. B. Aging Neuropsychol. Cogn. 13, 308–325.
Wig, G. S., Grafton, S. T., Demos, K. E., and Kelley, W. M. (2005). Reductions in neural activity underlie behavioral components of repetition priming. Nat. Neurosci. 8, 1228–1233.
Winston, J. S., Henson, R. N., Fine-Goulden, M. R., and Dolan, R. J. (2004). fMRI-adaptation reveals dissociable neural representations of identity and expression in face perception. J. Neurophysiol. 92, 1830–1839.
Keywords: adaptation, auditory system, aging, fMRI, spatial localization
Citation: Grady CL, Charlton R, He Y and Alain C (2011) Age differences in fMRI adaptation for sound identity and location. Front. Hum. Neurosci. 5:24. doi: 10.3389/fnhum.2011.00024
Received: 22 June 2010;
Accepted: 01 March 2011;
Published online: 11 March 2011.
Edited by:
William J. Jagust, University of California Berkeley, USAReviewed by:
Ken A. Paller, Northwestern University, USADavid L. Woods, University of California at Davis, USA
Copyright: © 2011 Grady, Charlton, He and Alain. This is an open-access article subject to an exclusive license agreement between the authors and Frontiers Media SA, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Cheryl L. Grady, Rotman Research Institute, Baycrest, 3560 Bathurst Street, Toronto, ON, Canada M6A 2E1. e-mail:Y2dyYWR5QHJvdG1hbi1iYXljcmVzdC5vbi5jYQ==