- 1 Leiden University Institute for Psychological Research, Leiden, Netherlands
- 2 Leiden Institute for Brain and Cognition, Leiden, Netherlands
- 3 Centre for Human Drug Research, Leiden, Netherlands
- 4 University of Amsterdam, Amsterdam, Netherlands
Animal research and computational modeling have indicated an important role for the neuromodulatory locus coeruleus–norepinephrine (LC–NE) system in the control of behavior. According to the adaptive gain theory, the LC–NE system is critical for optimizing behavioral performance by regulating the balance between exploitative and exploratory control states. However, crucial direct empirical tests of this theory in human subjects have been lacking. We used a pharmacological manipulation of the LC–NE system to test predictions of this theory in humans. In a double-blind parallel-groups design (N = 52), participants received 4 mg reboxetine (a selective norepinephrine reuptake inhibitor), 30 mg citalopram (a selective serotonin reuptake inhibitor), or placebo. The adaptive gain theory predicted that the increased tonic NE levels induced by reboxetine would promote task disengagement and exploratory behavior. We assessed the effects of reboxetine on performance in two cognitive tasks designed to examine task (dis)engagement and exploitative versus exploratory behavior: a diminishing-utility task and a gambling task with a non-stationary pay-off structure. In contrast to predictions of the adaptive gain theory, we did not find differences in task (dis)engagement or exploratory behavior between the three experimental groups, despite demonstrable effects of the two drugs on non-specific central and autonomic nervous system parameters. Our findings suggest that the LC–NE system may not be involved in the regulation of the exploration–exploitation trade-off in humans, at least not within the context of a single task. It remains to be examined whether the LC–NE system is involved in random exploration exceeding the current task context.
Introduction
The locus coeruleus (LC) is one of the major brainstem neuromodulatory nuclei, with widely distributed, ascending projections throughout the neocortex. LC activation results in the release of norepinephrine (NE) in cortical areas, which increases the responsivity of these areas to their afferent input (Servan-Schreiber et al., 1990; Berridge and Waterhouse, 2003). Traditionally, the LC–NE system has been associated with basic functions such as arousal and the sleep–wake cycle (Jouvet, 1969; Aston-Jones et al., 1984), but recent studies have suggested that this system also plays a more specific role in the control of behavior (Aston-Jones et al., 1997; Usher et al., 1999; Clayton et al., 2004). According to an influential recent theory of LC function, the adaptive gain theory (Aston-Jones and Cohen, 2005), the LC–NE system plays an important role in regulating the balance between exploiting known sources of reward versus exploring alternative options.
Neurophysiological studies in monkeys have revealed spontaneous fluctuations of tonic (baseline) LC activity over the course of a test session (Kubiak et al., 1992; Aston-Jones et al., 1996). Interestingly, these variations in tonic LC activity were closely related to the monkeys’ control state: periods of moderate tonic LC activity were consistently associated with task engagement and accurate task performance, whereas periods of elevated tonic LC activity were associated with distractible behavior and poor task performance. Periods of very low or absent tonic LC activity were associated with drowsiness and inattention. Furthermore, periods of moderate tonic LC activity were accompanied by large phasic increases in LC activity following task-relevant stimuli, whereas such phasic LC responses were diminished during periods of elevated or low tonic LC activity. Thus, during alert task performance, the pattern of LC activity varied between moderate tonic/large phasic activity, and elevated tonic/small phasic activity, which are referred to as the phasic and the tonic LC mode, respectively.
According to the adaptive gain theory (Aston-Jones and Cohen, 2005), the phasic and tonic LC modes promote, respectively, exploitative and exploratory control states. In the phasic mode, NE is released selectively in response to task-relevant events, which promotes task engagement and the optimization of performance in the current task (exploitation). In the tonic mode the sustained release of NE indiscriminately facilitates processing of all events, including non-task-related events, which promotes task disengagement and exploration. The theory further proposes that transitions between the phasic and tonic LC modes are driven by assessments of task-related costs and rewards (task utility), carried out in ventral and medial frontal structures.
The adaptive gain theory has been supported by computational modeling and neurophysiological studies in monkeys (Usher et al., 1999; Aston-Jones and Cohen, 2005) and, indirectly, by recent pupillometry studies in humans (Gilzenrat et al., 2010; Jepma and Nieuwenhuis, in press). However, crucial direct empirical tests of the theory in human participants have been lacking.
In the present study, we used a pharmacological manipulation to test in humans one of the central tenets of the adaptive gain theory, namely the assumption that the tonic LC mode promotes an exploratory control state. Participants received a single dose of reboxetine (a selective NE reuptake inhibitor), citalopram (a selective serotonin reuptake inhibitor), or placebo. Acute administration of reboxetine has opposing effects in the forebrain (increased NE levels via the inhibition of NE reuptake) and in the LC (reduction of firing activity via the increased activation of inhibitory α2-autoreceptors; Szabo and Blier, 2001). However, microdialysis studies have shown that the net effect of these two actions is an increase in NE levels in various regions of the brain (for a wide range of reboxetine doses; Page and Lucki, 2002; Invernizzi and Garattini, 2004), which supposedly resembles the effects of elevated NE release in the tonic LC mode. To determine whether potential effects were selective for manipulations of the LC–NE system, we used citalopram as a control drug; it increases serotonin but not NE levels (Bymaster et al., 2002). To confirm that these drugs at the doses employed in this study were pharmacologically active, we determined pupil size and several of the most drug-sensitive central nervous system (CNS) effects, including adaptive-tracking performance (index of visuomotor coordination and vigilance; Van Steveninck et al., 1991, 1993) and saccadic peak velocity (index of alertness; Van Steveninck et al., 1991, 1999).
The adaptive gain theory predicted that the increased tonic NE levels that were presumably induced by reboxetine would result in more task disengagement and exploratory behavior in the reboxetine group compared to the citalopram and placebo groups. We used two cognitive tasks to test these predictions. We measured task (dis)engagement using a diminishing-utility task (Gilzenrat et al., 2010), in which task difficulty and potential reward – two determinants of task utility – increased over time. Importantly, participants had the opportunity to reset the level of task difficulty and reward, and hence disengage from the current task set. We measured exploratory behavior using a gambling task with a gradually changing pay-off structure (Daw et al., 2006; Figure 2), in which optimal performance required a delicate balance between exploitative and exploratory choices.
Materials and Methods
Participants
Fifty-two healthy university students, aged 18–25 years, took part in a single experimental session in return for €100,-. After signing an informed consent, participants were medically screened within 3 weeks before study participation. Exclusion criteria included history or presence of psychiatric disease and evidence of relevant clinical abnormalities.
Participants received a single oral dose of 4 mg reboxetine, 30 mg citalopram, or placebo in a double-blind, parallel-groups design. The doses of reboxetine and citalopram were based on previous studies that have found significant behavioral effects using these doses of reboxetine (e.g., Tse and Bond, 2002; Miskowiak et al., 2007; De Martino et al., 2008) and citalopram (e.g., Chamberlain et al., 2006). Unfortunately, the random-block design intended to produce equal numbers of men and women in each treatment group was thwarted by early dropouts and planning problems, causing a somewhat unbalanced sex distribution. The reboxetine group (8 men, 10 women, mean age = 20.6), the citalopram group (8 men, 8 women, mean age = 21.6), and the placebo group (10 men, 8 women, mean age = 21.5) had similar mean ages (F(2, 49) = 1.66, p = 0.20). The study was approved by the medical ethics committee of the Leiden University Medical Center and conducted according to the Declaration of Helsinki.
Procedure
All participants came to the research center at 8 AM after an overnight fast (except from water). We instructed participants to abstain from caffeine, nicotine, alcohol and other psycho-active substances from 10 PM the night prior to the study day. On arrival, participants underwent a medical screening. Approximately 1 h after arrival, participants in the citalopram group received a capsule with 2 mg granisetron, to prevent nausea as a potential side effect of citalopram. Participants in the reboxetine and placebo groups received a placebo capsule instead of granisetron. Sixty minutes later, participants received a capsule with reboxetine, citalopram or placebo.
Peak plasma concentrations of reboxetine and citalopram occur, respectively, 2 and 2–4 h after drug administration (Hyttel, 1994; Edwards et al., 1995; Dostert et al., 1997; Noble and Benfield, 1997). Accordingly, the experimental tasks designed to measure task (dis)engagement and exploratory behavior were performed between 2 and 3 h post-treatment. All participants started with the diminishing-utility task, followed by the gambling task1. We measured participants’ pupil–iris ratio (Twa et al., 2004) and subjective state at several time points during the study day. Subjective state was assessed by means of sixteen 100-mm visual analog scales measuring alertness, calmness and contentment (Bond and Lader, 1974). In addition, at several time points during the study day, we measured participants’ adaptive-tracking performance (Borland and Nicholson, 1984; see Supplementary Material for a description of the task) and saccadic eye movements (Van Steveninck et al., 1989). These measures were part of a more extensive CNS test battery, the results of which will be reported more comprehensively elsewhere. To assess drug-related effects on subjective state, pupil size, adaptive-tracking performance, and saccadic eye movements, we compared the pre-treatment values with the average values from the time points surrounding performance of the diminishing-utility task and the gambling task (i.e., 2-3 h post-treatment). The complete time courses of these measures will be reported elsewhere.
Diminishing-Utility Task
Participants performed an auditory pitch-discrimination task (Gilzenrat et al., 2010). Each trial began with a sequence of two 250-ms sinusoidal tones: a reference tone, followed 3 s later by a comparison tone. Participants were instructed to indicate whether the comparison tone was higher or lower in pitch than the reference tone, and earned points for each correct response. If participants responded correctly on a particular trial, the value of that trial was added to the participant’s total score. In addition, in the next trial, the reward that could be earned increased by five points, and the pitch discrimination was made more difficult by halving the difference in pitch between the reference and comparison tones. Following an incorrect response, the reward value of the subsequent trial decreased by 10 points (but with a floor value of zero points), and the level of task difficulty remained the same. Importantly, prior to each trial, participants had the opportunity to “escape” from the current series of discriminations without score penalty and receive a new discrimination task (i.e., comparison against a new reference tone), with the point value reset to five points and the easiest pitch discriminability. Participants were instructed to maximize their total score over the 20 min of the experiment.
The task procedure is illustrated in Figure 1. At the start of each trial participants were shown a score/value screen that displayed the total score accumulated thus far and the point value of the next trial. Participants then indicated with a key press whether they wanted to “accept” this trial or “escape”. If the participant accepted the trial, a reference/comparison tone pair followed after a delay of 1 s. Participants were instructed to indicate as quickly and accurately as possible whether the comparison tone was lower or higher in pitch than the reference tone. After a delay of 1 s, the accuracy of the participant’s response was indicated by a 250-ms feedback sound: a bell sound for correct responses and a buzzer sound for incorrect responses. Two seconds after the feedback sound, the next trial started. If participants pressed the “escape” button at the score/value screen, a 250-ms “escape sound” was played, immediately followed by a new score/value screen. We refer to a series of trials accepted by a participant as an “epoch” of play. Electing to escape begins a new epoch. We considered the average number of trials in an epoch as an index of task (dis)engagement.
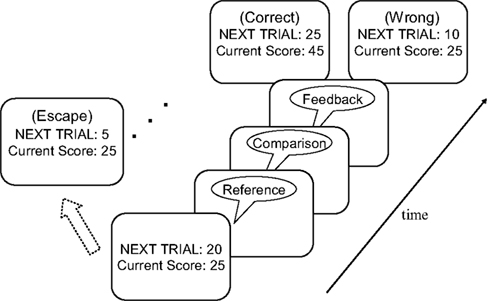
Figure 1. Illustration of a sample trial in the diminishing-utility task. See text for further details.
In the first trial of each epoch, the difference in pitch between the two tones was 64 Hz. As noted above, this difference was halved following each correct response. If participants correctly discriminated a ¼-Hz difference, the tones presented in the next trial were impossible to discriminate (i.e., 0 Hz difference), and impossible discrimination trials continued to be presented until the participant elected to escape. Accordingly, participants would exhaust any real discriminable differences between reference and comparison tone after nine correct trials; the tenth and subsequent trials within an epoch were impossible to discriminate. The feedback signal on impossible-discrimination trials was randomly picked. The same reference tone was presented on each trial within a given epoch. After an escape, a new reference tone was selected randomly without replacement from the set (400, 550, 700, and 850 Hz). The set was replenished if all reference tones were exhausted. On 50% of the trials, the comparison tone was higher in pitch and on the remaining trials it was lower in pitch than the reference tone.
Gambling Task
Participants performed a “four-armed bandit” task (Daw et al., 2006). On each trial, participants were presented with pictures of four different-colored slot machines, and selected one by pressing the “q”-, “w”-, “a”-, or “s”- key. Participants had a maximum of 1.5 s in which to make their choice; if no choice was made during that interval, a red X appeared in the center of the screen for 4.2 s to signal a missed trial (average number = 2.5). If participants responded within 1.5 s, the lever of the chosen slot machine was lowered and the number of points earned was displayed in the chosen machine for 1 s after which the next trial started. The task consisted of 300 trials. Importantly, the number of points paid off by the four slot machines gradually and independently changed from trial to trial (Figure 2; Supplementary Material).
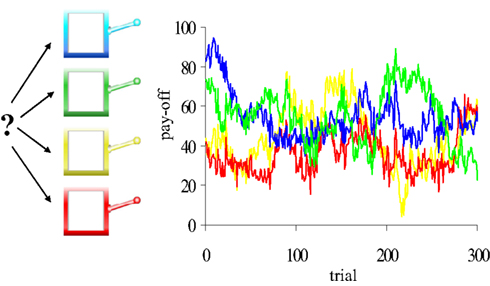
Figure 2. The four-armed bandit task. Participants made repeated choices between four slot machines. Unlike standard slots, the mean pay-offs of the four machines changed gradually and independently from trial to trial (four colored lines). Participants were encouraged to earn as many points as possible during the task. Each choice was classified as exploitative or exploratory, using a computational model of reinforcement learning.
Before the start of the experimental session, participants were given 24 practice trials. We instructed the participants that, on top of the standard payment for participation in the study, they would receive a bonus sum of money that depended on the number of points they would obtain in this task, and that the average bonus earned in this task was 9 euros. However, we did not tell participants how the number of points was converted into euros, or what their cumulative point total was. After completion of the study, each participant received a bonus of 10 euros.
Analysis
We fitted three reinforcement-learning models to the data. All models estimated the pay-offs of each machine on each trial, and selected a machine based on these estimations. The models differed in how they calculated the estimated pay-offs (Supplementary Material). All models selected a machine according to the “softmax” rule. This rule assumes that choices between different options are made in a probabilistic manner, such that the probability that a particular machine is chosen depends on its relative estimated pay-off. The exploitation–exploration balance is adjusted by a parameter referred to as gain, or inverse temperature: with higher gain, action selection is determined more by the relative estimated pay-offs of the different options (exploitation), whereas with lower gain, action-selection is more evenly distributed across the different options (exploration). We classified each choice as exploitative or exploratory according to whether the chosen slot machine was the one with the maximum estimated pay-off (exploitation) or not (exploration). In addition, we calculated the degree of exploration for each exploratory choice, by subtracting the estimated pay-off of the chosen machine from the maximum estimated pay-off. We assessed the value of the gain parameter and the proportion of exploratory choices as a function of pharmacological treatment. Only the results from the best-fitting model are reported, although the other models yielded similar results.
Results
Subjective State
The participants assigned to the three treatment groups did not differ in their pre-treatment ratings of alertness, calmness or contentment (all ps > 0.7; Table 1). To asses the effects of reboxetine and citalopram on subjective state we conducted analyses of covariance (ANCOVAs) on the subjective ratings of alertness, calmness and contentment, with treatment and sex as between-subject factors, and the pre-treatment ratings as covariate. There were no main effects of treatment or sex, and no treatment by sex interactions on any of these ratings (all ps > 0.16), suggesting that reboxetine and citalopram did not affect subjective state.
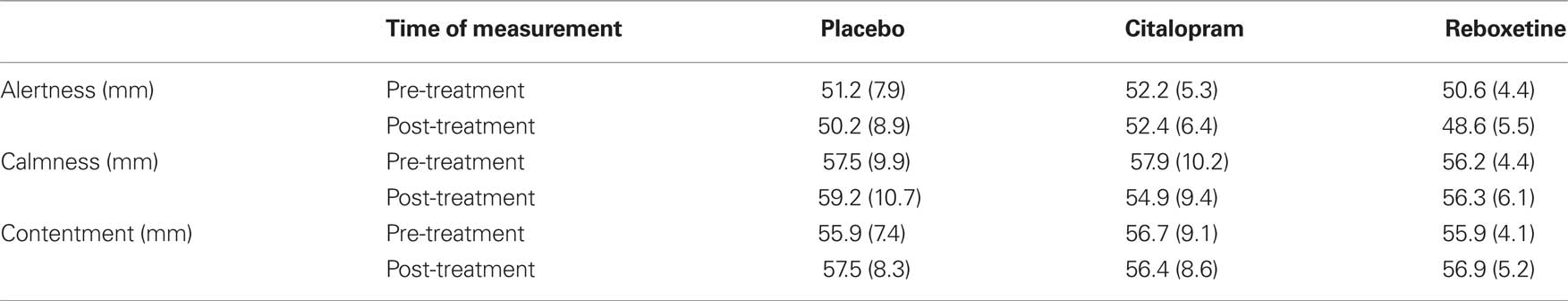
Table 1. Pre- and post-treatment ratings of alertness, calmness, and contentment in the placebo, citalopram and reboxetine group (SD in parentheses).
Non-Specific Central and Autonomic Nervous System Effects
Figure 3 (left panel) shows the adaptive-tracking performance pre-treatment (averaged across 1.5 and 0.5 h pre-treatment) and post-treatment (averaged across 2 and 3 h post-treatment) for each treatment group. We conducted an ANCOVA on the post-treatment adaptive-tracking performance with treatment and sex as between-subjects factors and pre-treatment performance as covariate. This analysis revealed a main effect of treatment [F(2, 45) = 5.2, p = 0.009]. There was no main effect of sex [F(1, 45) = 0.8, p = 0.4] and no interaction between treatment and sex [F(2, 45) = 1.1, p = 0.3]. Follow-up comparisons indicated that the reboxetine group showed worse post-treatment adaptive-tracking performance than the placebo group [F(1, 31) = 12.0, p = 0.02], whereas there was no difference between the citalopram and the placebo group [F(1, 29) = 0.5, p = 0.5]. The difference in post-treatment adaptive-tracking performance between the reboxetine and the citalopram group just failed to reach significance [F(1, 29) = 3.8, p = 0.06]. These results suggest that reboxetine led to a decrease in adaptive-tracking performance.
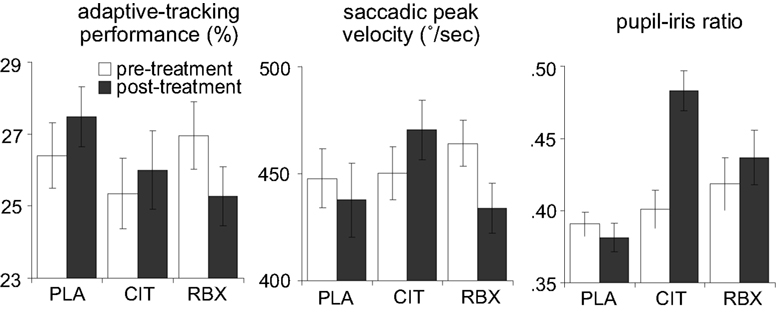
Figure 3. Adaptive-tracking performance, saccadic peak velocity and pupil–iris ratio pre-treatment and post-treatment, separately for each treatment group (error bars indicate standard errors of the mean). PLA, placebo; CIT, citalopram; RBX, reboxetine.
Figure 3 (middle panel) shows the saccadic peak velocity measured pre-treatment (averaged across 1.5 and 0.5 h pre-treatment) and post-treatment (averaged across 2 and 3 h post-treatment) for each treatment group. An ANCOVA on the post-treatment saccadic peak velocity with treatment and sex as between-subjects factors and pre-treatment saccadic peak velocity as covariate revealed a main effect of treatment [F(2, 45) = 15.3, p < 0.001]. There was no main effect of sex [F(1, 45) = 1.8, p = 0.2] and no significant interaction between treatment and sex [F(2, 45) = 0.6, p = 0.6]. Follow-up comparisons indicated that the reboxetine group showed smaller post-treatment saccadic peak velocity than the placebo group [F(1, 31) = 5.1, p = 0.03], whereas the citalopram group showed larger post-treatment saccadic peak velocity than the placebo group [F(1, 29) = 8.6, p = 0.007]. Thus, both reboxetine and citalopram affected saccadic eye movements, but the effects were in opposite directions. The time courses of saccadic peak velocity and adaptive-tracking performance showed that the effects of reboxetine and citalopram on these measures were maximal at the time points surrounding performance of the diminishing-utility task and the gambling task, suggesting that the drug-related CNS effects were maximal during performance of these tasks.
Figure 3 (right panel) shows the pupil–iris ratio measured pre-treatment (averaged across 1.5 and 0.5 h pre-treatment) and post-treatment (averaged across 2, 2.5 and 3 h post-treatment) for each treatment group. An ANCOVA on the post-treatment pupil–iris ratio with treatment and sex as between-subjects factors and pre-treatment pupil–iris ratio as covariate revealed a main effect of treatment [F(2, 45) = 22.1, p < 0.001]. There was no main effect of sex [F(1, 45) = 0.1, p = 0.7] and no significant interaction between treatment and sex [F(2, 45) = 2.8, p = 0.07]. Follow-up comparisons indicated that both the reboxetine group and the citalopram group had larger post-treatment pupil–iris ratios than the placebo group [F(1, 31) = 7.1, p = 0.01 and F(1, 29) = 44.4, p < 0.001, respectively]. In addition, post-treatment pupil–iris ratio was larger in the citalopram group than the reboxetine group [F(1, 29) = 13.7, p = 0.001]. Thus, consistent with previous studies (Phillips et al., 2000; Schmitt et al., 2002), both citalopram and reboxetine led to an increase in pupil diameter, and this effect was more pronounced in the citalopram group. There is no reliable evidence for direct projections from the LC to the autonomic nuclei that control the pupil (Aston-Jones, 2004), but there are a number of possible indirect pathways by which LC manipulation could affect the sympathetic nervous system (cf. Berntson et al., 1998). Therefore, it is possible that the increase in pupil diameter in the reboxetine group reflects drug-induced changes in LC activity. However, it is also possible that the pharmacological effects on pupil diameter were produced at the level of the autonomic nuclei controlling the pupil, and thus reflect other drug actions than changes in LC activity.
Diminishing-Utility Task
The progressive increase in both task difficulty and potential reward during each series of tone discriminations produces a non-linear development of task-related utility. Initially, the increases in reward value for correct performance outpace the increases in difficulty, such that the expected value (utility) of task performance progressively increases. However, after several trials, the increases in difficulty will lead to sufficient number of errors as to reduce the expected value of performance, even in the face of increasing reward value for correct responses.
To examine changes in performance and task-related utility leading up to and following participants’ choice to “escape” (i.e., abandon the current series and start a new one), we averaged trials as a function of their position relative to the escape events. For this analysis, we considered only escape events that were preceded and followed by a minimum of four regular (i.e., non-escape) trials. As a measure of task utility, we calculated an estimate of expected value for each trial. For a given trial, expected value was computed individually for each participant by multiplying the point value of the trial (representing the potential reward value if the trial was accepted) by the expected accuracy on that trial for that participant. Expected accuracy was defined as the probability that the participant would give a correct response, given the level of difficulty of the required pitch discrimination. To determine this, we averaged the accuracy of all other trials for that participant with the same frequency difference between reference and comparison tones.
Figure 4 (left panels) shows the average accuracy and RT on the trials flanking an escape for each treatment group. All treatment groups showed a sharp decrease in accuracy and an increase in RT over the trials leading up to an escape, which was confirmed by significant linear trends [F(1, 44) = 462.5, p < 0.001 and F(1, 44) = 14.3, p < 0.001, respectively]. As expected, performance was best on the first trial following an escape, after which accuracy gradually decreased and RT increased again [F(1, 44) = 54.5, p < 0.001 and F(1, 44) = 35.1, p < 0.001, respectively]. Figure 4 (right panels) shows how our measure of expected value and the actual point value varied across the trials surrounding an escape. In all treatment groups, participants on average selected to escape when expected value approached the start value of a new series of discriminations. Both expected value and point value gradually decreased over the trials leading up to an escape [F(1, 44) = 100.1, p < 0.001 and F(1, 44) = 30.5, p < 0.001, respectively], and gradually increased again over the trials following an escape [F(1, 44) = 422.1, p < 0.001 and F(1, 44) = 1079.0, p < 0.001, respectively]. Importantly, the effects of peri-escape trial position on performance and task utility did not interact with treatment or sex (all ps > 0.3).
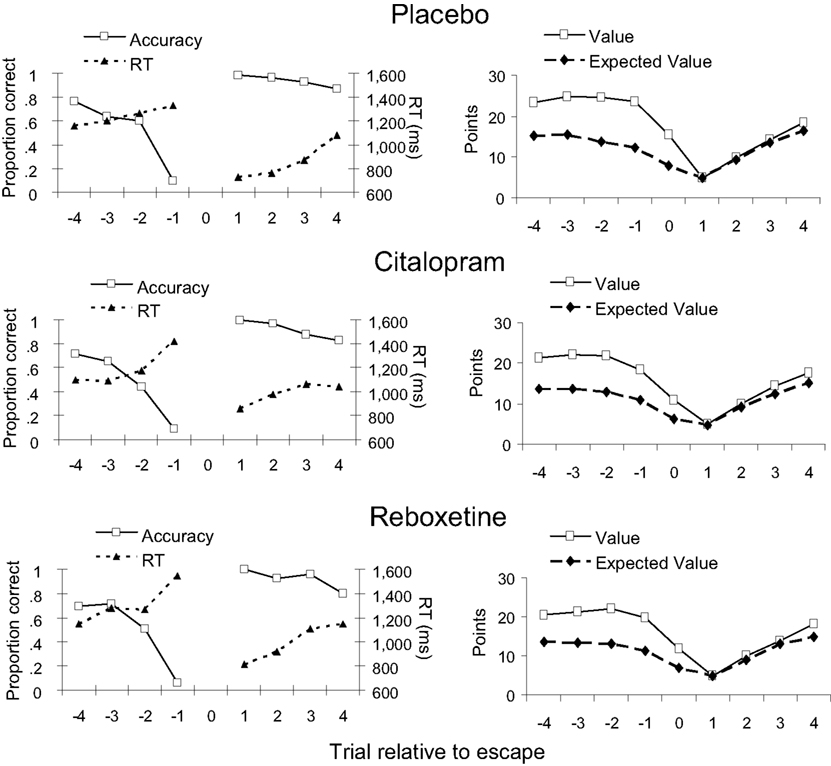
Figure 4. Dependent measures for peri-escape trials in the three treatment groups. Trial number “0” indicates the escape trial. Left panels: accuracy and response time (RT). Right panels: Trial value and its computed expected value. Note that no measures of accuracy and RT are available for escape trials, because, on these trials, no comparison tone was presented.
We next examined the average number of accepted trials in an epoch. The average number of trials in an epoch did not differ between the three treatment groups [F(2, 44) = 0.26, p = 0.77]. There was no main effect of sex either [F(1, 44) = 1.08, p = 0.30], and no interaction between treatment and sex [F(2, 44) = 0.33, p = 0.72]. Furthermore, there was no significant across-subject correlation between the mean epoch length and the reboxetine-related change in adaptive-tracking performance [r = 0.43, p = 0.08]. Note that, if anything, this correlation showed a trend in the opposite direction than predicted by the adaptive gain theory. Mean epoch length was not significantly correlated with the drug-related increase in pupil diameter either [r = −0.13, p = 0.62 in the reboxetine group; r = 0.24, p = 0.38 in the citalopram group].
There were no effects of treatment or sex on the total number of trials completed or total number of points obtained (all ps > 0.3), except for a significant interaction between treatment and sex on the total number of point obtained [F(2, 44) = 3.68, p = 0.03]. Follow-up contrasts indicated that the male participants obtained significantly more points than the female participants in the reboxetine group [t(16) = 3.08, p = 0.007], whereas there were no significant sex effects in the placebo and citalopram groups (ps > 0.48). An overview of the dependent variables in this task as a function of treatment and sex is shown in Table 2. An analysis of the improvement in tone-discrimination performance over the course of the task (i.e., learning curve) is reported in the Supplementary Material.
Table 2. Overview of the dependent variables in the diminishing-utility task, as a function of treatment and sex (SD in parentheses).
Gambling Task
Each participant’s tendency to make exploratory choices is reflected in the estimated gain parameter of the reinforcement-learning model: a lower value of the gain parameter indicates a more exploratory choice strategy (Materials and methods; Supplementary Material). The value of the gain parameter did not differ between the three treatment groups [F(2, 45) = 0.70, p = 0.51; Table S1] or between the male and female participants [F(2, 45) = 2.50, p = 0.12]. In addition, we classified each choice as exploitative or exploratory according to whether the chosen slot machine was the one with the maximum estimated pay-off (exploitation) or not (exploration). The proportion of exploratory choices did not differ between the three treatment groups [28%, 32%, and 27% in the placebo, citalopram and reboxetine group, respectively; F(2, 45) = 0.92, p = 0.41] or between male and female participants [26% versus 31%; F(2, 45) = 2.43, p = 0.13]. The three treatment groups did not differ in the degree of exploration of the exploratory choices either (section Analysis); the degrees of exploration in the placebo, citalopram and reboxetine groups were 0.39, 0.37, and 0.37, respectively (F(2, 45) = 0.43, p = 0.65).
Neither the value of the gain parameter nor the proportion of exploratory decisions was significantly correlated with the reboxetine-related change in adaptive-tracking performance [gain parameter: r = 0.41, p = 0.09; proportion exploration: r = −0.25, p = 0.32]. Our measures of exploration were not significantly correlated with the drug-related increase in pupil diameter either (ps > 0.15 in the reboxetine group; ps > 0.35 in the citalopram group).
There were no across-subject correlations between our measure of task disengagement in the diminishing-utility task (mean epoch length) and our measures of exploration in the gambling task (value gain parameter and proportion of exploratory choices; ps > 0.8). This suggests that the disengagement and exploration measures in these tasks reflect separate aspects of the exploratory control state hypothesized to be mediated by the tonic LC mode.
Discussion
The present study provided the first direct test in humans of one of the central tenets of the adaptive gain theory of LC function (Aston-Jones and Cohen, 2005), namely the assumption that an elevated level of tonic LC–NE activity (tonic LC mode) promotes a more exploratory control state. Contrary to predictions of the adaptive gain theory, we found no evidence that the increased NE levels induced by reboxetine were associated with task disengagement or exploratory behavior in our experimental tasks.
Our null effects cannot be explained by a general ineffectiveness of our pharmacological manipulations, since there were significant drug effects on several central and autonomic nervous system parameters. Reboxetine caused reductions in adaptive-tracking performance and in saccadic peak velocity, which corroborates previous findings suggesting the involvement of the noradrenergic system in visuomotor control of movements (Wang et al., 2009). Citalopram increased saccadic peak velocity, which is in line with the mild stimulating properties of the SSRI on the electroencephalogram (Itil et al., 1984; Saletu et al., 2002). The time course of the effects suggests that reboxetine was maximally effective during performance of the diminishing-utility task and gambling task. In addition, both citalopram and reboxetine resulted in an increase in pupil diameter, but it is unknown whether these pupil modulations were produced by changes in LC activity or by other drug influences peripheral to the LC (e.g., on lower medullary NE cell groups or autonomic nervous system). Furthermore, previous studies using the same dose of reboxetine, between-subject designs, and similar group sizes have found significant group differences in behavioral measures (Tse and Bond, 2002; Miskowiak et al., 2007; De Martino et al., 2008). The absence of significant across-subject correlations between our measures of disengagement/exploration and the reboxetine-related effects on adaptive-tracking performance suggests that the effectiveness of the reboxetine manipulation in individual participants did not predict their tendency to disengage or explore.
The two experimental tasks we used to measure exploratory behavior and task (dis)engagement seem well suited for detecting individual differences in control state. The n-armed bandit task with non-stationary pay-off structure is the most commonly used paradigm for studying the exploration–exploitation trade-off in reinforcement-learning research (Sutton and Barto, 1998). Combined with computational modeling, it allows a formal description of participants’ choice behavior and provides an index of their tendency to explore. The diminishing-utility task is a more novel paradigm in which task engagement is modulated by means of dynamic changes in task-related utility. Importantly, the opportunity to “escape” from the current task set provides an overt behavioral index of disengagement. In line with a previous study using this task (Gilzenrat et al., 2010), we found that participants behaved optimally on average, and chose to disengage from the current task set when estimated task utility approached the baseline utility of a new task set. In addition, in a recent study using the same gambling task as used here (Jepma and Nieuwenhuis, in press) we have found that changes in utility measures and pupil diameter leading up to the switch from an exploitative to an exploratory choice strategy were similar to those leading up to an “escape” in the diminishing-utility task (Gilzenrat et al., 2010). This suggests that disengagement in the diminishing-utility task and exploration in the gambling task are both driven by decreases in task utility. That said, optimal exploration strategies in our experimental tasks may differ from those needed in the real world; the changes in pay-offs and task-related utility in our tasks developed gradually and relatively slowly over time, which may not correspond to the dynamics of utility changes in real-world environments (Cohen et al., 2007).
Although disengagement and exploration are both considered behaviors indicative of an exploratory control state associated with the tonic LC mode, it is important to note that disengagement in the diminishing-utility task (i.e., choosing to “escape” from the current series of tone discriminations) is not equivalent to exploration in the gambling task, which may explain the absence of a correlation between our measures of disengagement and exploratory behavior. The development of a computational model for the diminishing-utility task is an important objective for future studies, as this will allow a more formal description of participants’ behavior in this task and a better comparison with exploratory behavior in other tasks.
One possible explanation for the absence of reboxetine effects on our measures of task disengagement and exploratory behavior is that the LC–NE system is not involved in regulating the balance between exploitative and exploratory control states in humans. The adaptive gain theory is based on findings from neurophysiological studies in monkeys using relatively simple target-detection tasks, and it is possible that the results from these studies cannot be generalized to the regulation of control state in humans. Moreover, although it is intuitively appealing to interpret the observations of increased distractibility, labile attention, and impaired focused performance during elevated tonic LC–NE activity in animals as reflections of an exploratory control state (Aston-Jones and Cohen, 2005), it is important to note that the neurophysiological studies did not explicitly investigate the exploration–exploitation trade-off; the proposed link between the tonic LC mode and an exploratory control state is an assumption. Because we did not find evidence for this assumption, it seems appropriate to consider alternative explanations for the distractible behavior associated with the tonic LC mode. When taking a reinforcement-learning model perspective, it may be possible to explain the behaviors observed in the tonic LC mode by changes in reinforcement-learning parameters other than the exploration parameter. One possibility is that high LC–NE activity increases the rate at which action values are updated based on new information (i.e., the learning rate parameter). This hypothesis would be compatible with a recent proposal that increased NE levels boost the learning of new task contingencies (Yu and Dayan, 2005). In line with this hypothesis, the estimated learning rate of the reinforcement-learning model that we fit to the choice data of the gambling task was somewhat larger in the reboxetine group than in the other treatment groups (Table S2 and Figure S1 in Supplementary Material). However, because of the very high learning rates associated with this task, this result must be interpreted with caution. Alternatively, high LC–NE activity may increase the importance attached to immediate versus delayed rewards (i.e., the future-reward discount factor). Support for this hypothesis comes from findings from a recent study in mice that suggest that drug-induced increases in NE levels impair the ability to take future rewards into account, which would lead to the impulsive selection of options with short-term rewards (Luksys et al., 2009). Luksys et al. suggested that the distractible behavior observed in animals with elevated LC–NE activity can be produced by an increased devaluation of future, relative to immediate, rewards combined with high exploitation (as opposed to exploration; see Doya, 2002, for a similar proposal). Thus, the behaviors associated with the tonic LC mode that have been interpreted as indices of an exploratory control state by the adaptive gain theory may also be explained by modulations of other reinforcement-learning parameters. To further address this issue, future studies need to dissociate the role of the LC–NE system and other neuromodulatory systems in the regulation of different components of reinforcement learning and decision making.
Another possibility is that the tonic LC mode promotes a type of exploratory behavior and disengagement that was not measured in the present study. It is likely that exploration is not a single process but comprises several distinct functions involving different neural mechanisms. An important aspect may be whether exploration is driven by top–down motives or by bottom–up stimulation. Exploratory behavior in the four-armed bandit task may be referred to as “controlled” or “systematic” exploration, since it is aimed at obtaining information in order to optimize performance in the current task. Similarly, disengaging from the current task set in the diminishing-utility task serves the higher-level goal of maximizing the total score obtained in the task. Such controlled, top–down driven exploration and disengagement within the current task context might be mediated by different neural mechanisms and/or neuromodulatory systems than random, bottom–up driven exploration exceeding the current task context. Controlled exploration presumably requires cognitive control functions that rely on the prefrontal cortex (PFC), which is supported by the finding of PFC activation during exploratory decisions in the four-armed bandit task (Daw et al., 2006). There is also some evidence that the dopamine system plays a role in the regulation of a particular type of controlled exploration (Frank et al., 2009). Our findings suggest that the LC–NE system may not be involved in controlled exploration. However, our study leaves open the possibility that the LC–NE system is involved in random exploration exceeding the current task context. Random exploration is likely to be associated with an increased sensitivity to bottom–up activation, resulting from a global increase in neuronal responsivity. The widespread projection system of the LC and the neuromodulatory effects of NE on cortical neurons suggest that the LC–NE system is well suited to produce such global changes in responsivity.
The idea that the tonic LC mode promotes a more random type of exploration outside the current task context is supported by findings that drug-related increases in tonic NE levels improve attentional-set shifting and reversal learning in rats and monkeys (Devauges and Sara, 1990; Lapiz and Morilak, 2006; Lapiz et al., 2007; Seu et al., 2008), whereas noradrenergic lesions impair attentional-set shifting (Tait et al., 2007; McGaughy et al., 2008; Newman et al., 2008). These functions require the adaptation of behavior according to unexpected changes in the task environment, which depends on a shift of attention to previously irrelevant stimulus dimensions. These types of attention shifts are likely to be facilitated by random exploration (although an increased learning rate may provide an alternative explanation). Investigating the noradrenergic modulation of random exploration outside the current task context in humans is an important objective for future studies.
The distinction between controlled and random exploration might be related to the proposed distinction between expected and unexpected uncertainty (Yu and Dayan, 2005). Yu and Dayan have proposed that acetylcholine signals expected uncertainty (i.e., anticipated variation in task outcome), whereas NE signals unexpected uncertainty (i.e., unanticipated changes in the task context resulting in strong violations of top–down expectations; see Bouret and Sara, 2005, for a similar account). Yu and Dayan have also proposed that the NE-related signaling of unexpected uncertainty facilitates the learning of predictive relationships within a behavioral context, and therefore accelerates the detection of a change in task contingencies, which could explain the improvements in attentional-set shifting associated with increased tonic NE levels. Yu and Dayan’s account thus suggests that the tonic LC mode boosts learning about new predictive relationships in noisy and changing environments. This account is closely related to the adaptive gain theory’s assumption that the tonic LC mode promotes exploration, at least when applied to random exploration exceeding the current task context, since this type of exploration is likely to facilitate the learning of contextual changes. The detection of unexpected uncertainty might be an important factor in driving the LC towards a more tonic LC mode. However, how assessments of unexpected uncertainty interact with assessments of task-related utility on different timescales to regulate LC mode and control state remains to be investigated. An interesting speculation is that the degree of unexpected uncertainty determines how much weight is given to assessments of long versus short-term utility, such that long-term utility has relatively less influence in situations of high unexpected uncertainty. In terms of reinforcement-learning models, this would be similar to the suggested modulation of the learning rate parameter by the volatility of the environment (Behrens et al., 2007).
Finally, it is important to note that although microdialysis studies have shown that a single dose of reboxetine increases NE concentrations, these studies, due to their limited temporal resolution, do not provide unequivocal evidence that this reflects purely an increase in tonic NE levels. Since the effects of selective NE reuptake inhibitors on the phasic LC response in awake animals are not known, we cannot exclude the possibility that our reboxetine manipulation also affected phasic LC activity and NE release, for example via modulations of the electrotonic coupling strength between LC neurons (Alvarez et al., 2002). Thus, determining the exact effects of selective NE reuptake inhibitors on the phasic and tonic components of LC–NE activity will be important for a better understanding of their effects on cognition. In addition, the effects of pharmacologically increasing NE levels on control state might depend on individual differences in baseline (pre-treatment) NE level. Accordingly, individual differences in baseline NE level could have been partly responsible for the absence of group differences on our measures of disengagement and exploration. Consistent with this possibility, a recent study in mice has shown that pharmacological manipulations of the LC–NE system interact with several other factors, such as individual differences in genotype and trait anxiety, stress and motivation, in modulating the exploration–exploitation trade-off (Luksys et al., 2009). Thus, it seems that multiple factors need to be taken into account to enable predictions of exploratory behavior and its modulation by NE.
To conclude, our findings suggest that the acute induction of an elevated tonic NE level does not affect people’s tendency to explore or disengage, at least not within the current task context. These findings challenge the adaptive gain theory’s claim that the LC–NE system regulates the balance between exploitative and exploratory control states (Aston-Jones and Cohen, 2005). It remains to be examined whether the LC–NE system is involved in random exploration outside the current task context, possibly driven by the detection of unexpected uncertainty. The present study contributes to our understanding of the noradrenergic modulation of human control state, and hopefully encourages further investigation of this topic.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the Netherlands Organization for Scientific Research. We thank Rafal Bogacz for providing the scripts for fitting the reinforcement-learning models to the behavioral data, Thijs Schrama for his help with the bootstrap analyses, and Marieke de Kam for her statistical advice.
Footnote
- ^Due to technical problems, three participants did not complete one of the tasks and were excluded from the corresponding analyses. For the diminishing-utility task this was the case for one female participant in the citalopram group and one male participant in the placebo group, and for the four-armed bandit task this was the case for one male participant in the placebo group.
References
Alvarez, V. A., Chow, C. C., Van Bockstaele, E. J., and Williams, J. T. (2002). Frequency-dependent synchrony in locus ceruleus: role of electrotonic coupling. Proc. Natl. Acad. Sci. U.S.A. 99, 4032–4036.
Aston-Jones, G. (2004). “Locus coeruleus, A5 and A7 noradrenergic cell groups,” In The Rat Nervous System, 3rd Edn. ed. G. Paxinos. (San Diego: Elsevier Academic Press), 259–294.
Aston-Jones, G., and Cohen, J. D. (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450.
Aston-Jones, G., Foote, S. L., and Bloom, F. E. (1984). “Anatomy and physiology of locus coeruleus neurons: functional implications,” in Frontiers of Clinical Neuroscience: Vol. 2, Norepinephrine, eds M. Ziegler and C. R. Lake (Baltimore: Williams and Wilkins), 92–116.
Aston-Jones, G., Rajkowski, J., and Kubiak, P. (1997). Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience 80, 697–715.
Aston-Jones, G., Rajkowski, J., Kubiak, P., Valentino, R. J., and Shipley, M. T. (1996). Role of the locus coeruleus in emotional activation. Prog. Brain Res. 107, 379–402.
Behrens, T. E., Woolrich, M W., Walton, M. E., and Rushworth, M. F. (2007). Learning the value of information in an uncertain world. Nat. Neurosci. 10, 1214–1221.
Berntson, G. G., Sarter, M., and Cacioppo, J. T. (1998). Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behav. Brain Res. 94, 225–248.
Berridge, C. W., and Waterhouse, B. D. (2003). The locus coeruleus- noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 42, 33–84.
Bond, A., and Lader, M. (1974). The use of analogue scales in rating subjective feelings. Br. J. Psychol. 47, 211–218.
Borland, R. G., and Nicholson, A. N. (1984). Visual motor co-ordination and dynamic visual acuity. Br. J. Clin. Pharmacol. 18(Suppl. 1), 69S–72S.
Bouret, S., and Sara, S. J. (2005). Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 28, 574–582.
Bymaster, F. P., Zhang, W., Carter, P. A., Shaw, J., Chernet, E., Phebus, L., Wong, D. T., and Perry, K. W. (2002). Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology (Berl.) 160, 353–361.
Chamberlain, S. R., Müller, U., Blackwell, A. D., Clark, L., Robbins, T. W., and Sahakian, B. J. (2006). Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311, 861–863.
Clayton, E. C., Rajkowski, J., Cohen, J. D., and Aston-Jones, G. (2004). Phasic activation of monkey locus coeruleus neurons by simple decisions in a forced choice task. J. Neurosci. 24, 9914–9920.
Cohen, J. D., McClure, S. M., and Yu, A. J. (2007). Should I stay or should I go? How the human brain manages the trade-off between exploitation and exploration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 933–942.
Daw, N. D., O’Doherty, J. P., Dayan, P., Seymour, B., and Dolan, R. J. (2006). Cortical substrates for exploratory decisions in humans. Nature 441, 876–479.
De Martino, B., Strange, B. A., and Dolan, R. J. (2008). Noradrenergic neuromodulation of human attention for emotional and neutral stimuli. Psychopharmacology (Berl.) 197, 127–136.
Devauges, V., and Sara, S. J. (1990). Activation of the noradrenergic system facilitates an attentional shift in the rat. Behav. Brain Res. 39, 19–28.
Dostert, P., Benedetti, M. S., and Poggesi, I. (1997). Review of the pharmacokinetics and metabolism of reboxetine, a selective noradrenaline reuptake inhibitor. Eur. Neuropsychopharmacol. 7(Suppl. 1), S23–S35.
Edwards, D., M., Pellizzoni, C., Breuel, H. P., Berardi, A., Castelli, M. G., Frigerio, E., Poggesi, I., Rocchetti, M., Dubini, A., and Strolin Benedetti, M. (1995). Pharmacokinetics of reboxetine in healthy volunteers. Single oral doses, linearity and plasma protein binding. Biopharm. Drug Dispos. 16, 443–460.
Frank, M. J., Doll, B. B., Oas-Terpstra, J., and Moreno, F. (2009). Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nat. Neurosci. 12, 1062–1068.
Gilzenrat, M. S., Nieuwenhuis, S., Jepma, M., and Cohen, J. D. (2010). Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn. Affect Behav. Neurosci. 10, 252–269.
Hyttel, J. (1994). Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs). Int. Clin. Psychopharmacol. 9(Suppl. 1), 19–26.
Invernizzi, R. W., and Garattini, S. (2004). Role of presynaptic alpha2- adrenoceptors in antidepressant action: recent findings from microdialysis studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 28, 819–827.
Itil, T. M., Menon, G. N., Bozak, M. M., and Itil, K. Z. (1984). CNS effects of citalopram, a new serotonin inhibitor antidepressant (a quantitative pharmaco-electroencephalography study). Prog. Neuropsychopharmacol. Biol. Psychiatry 8, 397–409.
Jepma, M., and Nieuwenhuis, S. (in press). Pupil diameter predicts changes in the exploration-exploitation trade-off: Evidence for the adaptive gain theory. J. Cogn. Neurosci.
Kubiak, P., Rajkowski, J., and Aston-Jones, G. (1992). Behavioral performance and sensory responsiveness of LC neurons in a vigilance task varies with tonic LC discharge rate. Soc. Neurosci. Abstr. 18, 538.
Lapiz, M. D., Bondi, C. O., and Morilak, D. A. (2007). Chronic treatment with desipramine improves cognitive performance of rats in an attentional set-shifting test. Neuropsychopharmacology (Berl. Ger.) 32, 1000–1010.
Lapiz, M. D., and Morilak, D. A. (2006). Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 137, 1039–1049.
Luksys, G., Gerstner, W., and Sandi, C. (2009). Stress, genotype and norepinephrine in the prediction of mouse behavior using reinforcement learning. Nat. Neurosci. 12, 1180–1186.
McGaughy, J., Ross, R. S., and Eichenbaum, H. (2008). Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience 153, 63–71.
Miskowiak, K., Papadatou-Pastou, M., Cowen, P. J., Goodwin, G. M., Norbury, R., and Harmer, C. J. (2007). Single dose antidepressant administration modulates the neural processing of self-referent personality trait words. Neuroimage 37, 904–911.
Newman, L. A., Darling, J., and McGaughy, J. (2008). Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology (Berl.) 200, 39–50.
Noble, S., and Benfield, P. (1997). Citalopram: a review of its pharmacology, clinical efficacy and tolerability in the treatment of depression. CNS Drugs 8, 410–431.
Page, M. E., and Lucki, I. (2002). Effects of acute and chronic reboxetine treatment on stress-induced monoamine efflux in the rat frontal cortex. Neuropsychopharmacology 27, 237–247.
Phillips, M. A., Bitsios, P., Szabadi, E., and Bradshaw, C. M. (2000). Comparison of the antidepressants reboxetine, fluvoxamine and amitriptyline upon spontaneous pupillary fluctuations in healthy human volunteers. Psychopharmacology (Berl.) 149, 72–76.
Saletu, B., Anderer, P., Saletu-Zyhlarz, G. M., Arnold, O., and Pascual-Marqui, R. D. (2002). Classification and evaluation of the pharmacodynamics of psychotropic drugs by single-lead pharmaco-EEG, EEG mapping and tomography (LORETA). Methods Find Exp. Clin. Pharmacol. 24(Suppl. C), 97S–120S.
Schmitt, J. A., Riedel, W. J., Vuurman, E. F., Kruizinga, M., and Ramaekers, J. G. (2002). Modulation of the critical flicker fusion effects of serotonin reuptake inhibitors by concomitant pupillary changes. Psychopharmacology (Berl.) 160, 381–386.
Servan-Schreiber, D., Printz, H., and Cohen, J. D. (1990). A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science 249, 892–895.
Seu, E., Lang, A., Rivera, R. J., and Jentsch, J. D. (2008). Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology (Berl.) 202, 505–519.
Sutton, R. S., and Barto, A. G. (1998). Reinforcement Learning: An Introduction. Cambridge, MA: MIT Press.
Szabo, S. T., and Blier, P. (2001). Effect of the selective noradrenergic reuptake inhibitor reboxetine on the firing activity of noradrenaline and serotonin neurons. Eur. J. Neurosci. 13, 2077–2087.
Tait, D. S., Brown, V. J., Farovik, A., Theobald, D. E., Dalley, J. W., and Robbins, T. W. (2007). Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur. J. Neurosci. 25, 3719–3724.
Tse, W. S., and Bond, A. J. (2002). Difference in serotonergic and noradrenergic regulation of human social behaviours. Psychopharmacology (Berl.) 159, 216–221.
Twa, M. D., Bailey, M. D., Hayes, J., and Bullimore, M. (2004). Estimation of pupil size by digital photography. J. Cataract Refract. Surg. 30, 381–389.
Usher, M., Cohen, J. D., Servan-Schreiber, D., Rajkowski, J., and Aston-Jones, G. (1999). The role of locus coeruleus in the regulation of cognitive performance. Science 283, 549–554.
Van Steveninck, A. L., Cohen, A. F., and Ward, T. (1989). A microcomputer based system for recording and analysis of smooth pursuit and saccadic eye movements. Br. J. Clin. Pharmacol. 27, 712–713.
Van Steveninck, A. L., Gieschke, R., Schoemaker, H. C., Pieters, M. S., Kroon, J. M., Breimer, D. D., and Cohen , A. F. (1993). Pharmacodynamic interactions of diazepam and intravenous alcohol at pseudo steady state. Psychopharmacology (Berl.) 110, 471–478.
Van Steveninck, A. L., Schoemaker, H. C., Pieters, M. S., Kroon, R., Breimer, D. D., and Cohen, A. F. (1991). A comparison of the sensitivities of adaptive tracking, eye movement analysis and visual analog lines to the effects of incremental doses of temazepam in healthy volunteers. Clin. Pharmacol. Ther. 50, 172–180.
Van Steveninck, A. L., Van Berckel, B. N., Schoemaker, R. C., Breimer, D. D., Van Gerven, J. M., and Cohen, A. F. (1999). The sensitivity of pharmacodynamic tests for the central nervous system effects of drugs on the effects of sleep deprivation. J. Psychopharmacol. 13, 10–17.
Wang, L. E., Fink, G. R., Dafotakis, M., and Grefkes, C. (2009). Noradrenergic stimulation and motor performance: differential effects of reboxetine on movement kinematics and visuomotor abilities in healthy human subjects. Neuropsychologia 47, 1302–1312.
Keywords: norepinephrine, locus coeruleus, cognitive control, exploration, decision making, reboxetine
Citation: Jepma M, te Beek ET, Wagenmakers E-J, van Gerven JMA and Nieuwenhuis S (2010) The role of the noradrenergic system in the exploration–exploitation trade-off: a psychopharmacological study. Front. Hum. Neurosci. 4:170. doi: 10.3389/fnhum.2010.00170
Received: 15 May 2010;
Paper pending published: 02 June 2010;
Accepted: 08 August 2010;
Published online: 26 August 2010
Edited by:
Francisco Barcelo, University of Illes Balears, SpainReviewed by:
Gediminas Luksys, Basel University, SwitzerlandMichael Minzenberg, University of California Davis, USA
Copyright: © 2010 Jepma, te Beek, Wagenmakers, van Gerven and Nieuwenhuis. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Marieke Jepma, Department of Psychology, Cognitive Psychology Unit, Leiden University, Wassenaarseweg 52, 2333 AK Leiden, Netherlands. e-mail:bWplcG1hQGZzdy5sZWlkZW51bml2Lm5s




