
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 24 January 2025
Sec. Stroke
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1537755
This article is part of the Research TopicQuality of Stroke Care: What Could Be Improved, and How? - Volume IIView all 9 articles
 Zhen Wang1†
Zhen Wang1† Jiacheng Yu2†
Jiacheng Yu2† Yu Zhang2†
Yu Zhang2† Jiaping Ruan2
Jiaping Ruan2 Xiaojie Liu2
Xiaojie Liu2 Sijia Ma2
Sijia Ma2 Jun Xie3
Jun Xie3 Mimi Wu2*
Mimi Wu2* Jinhua Bo2*
Jinhua Bo2* Yu’e Sun1,2*
Yu’e Sun1,2*Background: The new-onset cerebral infarction is frequent after revascularization of moyamoya disease (MMD) in adults, serving as a major public health issue worldwide. The present study aims to construct a nomogram to predict postoperative new-onset cerebral infarction (POCI) after revascularization of adult MMD.
Materials and methods: Clinical data of 653 cases of adult MMD treated with revascularization were retrospectively analyzed. They were randomly divided into a training set (n = 457) and a validation set (n = 196) at a ratio of 7:3. Based on the risk factors of POCI after revascularization of adult MMD identified by logistic regression analysis and the corresponding regression coefficients, a nomogram was constructed. Its performance to predict POCI after revascularization of adult MMD was validated by calculating the area under the curve (AUC) and the decision curve analysis.
Results: Univariate and multivariate logistic regression analyses showed that preoperative cerebral infarction (OR 2.548, 95% CI 1.357–4.787; p = 0.004), posterior cerebral artery anomalies (OR 2.106, 95% CI 1.157–3.834; p = 0.015), post-transit arterial development (OR 2.983, 95% CI 1.336–6.661; p = 0.008), pre-anesthesia mean arterial pressure > 102.830 mmHg (OR 3.329, 95% CI 1.938–5.721; p < 0.001), total operating time > 212.500 min (OR 2.256, 95% CI 1.239–4.140; p = 0.008), preoperative fibrinogen level > 2.750 g/L (OR 1.852, 95% CI 1.072–3.200; p = 0.027), and mean corpuscular hemoglobin concentration (OR 1.021, 95% CI 1.001–1.040; p = 0.038) were independent risk factors of POCI after revascularization of adult MMD. The AUC was 0.772 (95% CI 0.714–0.772) in the training set, and 0.718 (95% CI 0.603–0.833) in the validation set.
Conclusion: Collectively, the newly established nomogram effectively and intuitively predicts the POCI after revascularization of adult MMD.
Clinical trial registration: www.chictr.org, identifier ChiCTR2400087946.
Moyamoya disease (MMD) is a progressive cerebrovascular disease characterized by stenosis of the cerebral arteries surrounding the circle of Willis and development of abnormal moyamoya vessels, which has become a leading cause of ischemic and hemorrhagic strokes (1, 2). Presently, revascularization is preferred to adult MMD that effectively halts disease progression and prevents future ischemic strokes (3). However, postoperative new-onset cerebral infarction (POCI) after revascularization remains high (4.0–33.0%) (4–7), resulting in a poor outcome, long length of stay and high economic costs.
Prior studies have identified several risk factors of postoperative cerebral ischemic complications of MMD and most of them cannot be modified (6–11). So far, the vascular condition of MMD is the mostly concerned predictive factor of POCI (5, 12). Besides the vascular condition, POCI is also closely linked with perioperative factors like preoperative comorbidities, intraoperative blood pressure (13), coagulation state, and surgical methods (8). A prediction model comprehensively incorporating the above-mentioned factors is urgently needed to visualize the possibility of POCI after revascularization of adult MMD, thus providing survival benefits to affected people.
In the present study, we comprehensively analyzed clinical data of POCI in adult MMD patients treated with revascularization, and constructed a nomogram. Its performance was later validated, aiming to provide a useful tool to screen high-risk population of POCI.
This was a single-center, large-scale retrospective observational cohort study with the approval of the institutional review board (No. 2023-455-01) on October 9, 2023 and waiver of informed consent. It was registered at www.chictr.org (No. ChiCTR2400087946) on August 7, 2024. Elective intracranial extracranial vascular bypass between February 2018 and February 2024 at Nanjing Drum Tower Hospital were retrospectively screened. The exclusion criteria were as follows: pediatric MMD, geriatric MMD, those lacking magnetic resonance angiography, digital subtraction angiography, or other preoperative and postoperative data, and individuals with cerebrovascular lesions caused by atherosclerosis, autoimmune disease, meningitis, brain tumors, Down syndrome, von Recklinghausen’s disease, head injury, or head irradiation (14).
A standardized anesthesia protocol was established in our center to ensure a homogeneous management during the induction and maintenance of anesthesia, and regulatory phases of blood pressure, arterial blood carbon dioxide partial pressure, and fluid therapy. Detailed protocols during the anesthesia management were described in Supplementary Table S1.
POCI was defined as newly detected cerebral infarctions on imaging scans within 7 days of surgery compared to preoperative assessments. New-onset cerebral infarctions were low-density foci visualized on postoperative electron computed tomography (CT) scans or high-signal areas on magnetic resonance diffusion-weighted imaging sequences that were not associated with ischemic symptoms. New-onset cerebral hemorrhage must be excluded (5, 15). Representative imaging scans before and after surgery were shown in Figure 1.

Figure 1. Representative imaging scans of 4 cases of POCI. In Case A classified as POCI (−), there lacked obvious manifestations of cerebral infarction before (A1) or after surgery (A2), only showing intracranial softening foci. In Case B classified as POCI (−), a preoperative subacute cerebral infarction (B1) without postoperative progression (B2) was visualized on imaging scans. In Case C classified as POCI (+), no obvious infarction foci were preoperatively observed in the corpus callosum (C1) or the right frontoparietal lobe (C2). Postoperatively, new acute infarction was identified in the corpus callosum (C3), while the preoperatively identified infarct foci on the right side remained unchanged (C4). In Case D classified as POCI (+), there lacked obvious manifestations of cerebral infarction preoperatively (D1), but presented a new-onset acute cerebral infarction in the left hemi-oval center postoperatively (D2).
Clinical data were available from the medical record system and anesthesia management system. All biochemical indices were obtained within 4 days preoperatively and independently verified by two investigators. Missing values were addressed using the list deletion method.
Briefly, three categories of preoperative data were recorded. First, baseline and demographic data, including gender, age, body mass index, the American Society of Anesthesiologists physical status classification, preoperative history and comorbid symptoms (e.g., comorbid hypokinesia, speech dysfunction, visual impairment and loss of consciousness), preoperative medications (e.g., antihypertensive agents, hypoglycemic agents, aspirin, clopidogrel), and preoperative comorbidities (e.g., hypertension, diabetes mellitus, cerebral infarction, stroke, cerebral hemorrhage, intracranial aneurysm, brain atrophy). Second, preoperative laboratory testing, including complete blood count (CBC), liver and kidney function, coagulation function, and biochemical testing. Third, the severity and vascular involvement of MMD, including the type of MMD (hemorrhagic, ischemic, and asymptomatic), and vascular involvement (involvement of the anterior cerebral arteries [ACA], middle cerebral arteries [MCA], internal carotid arteries, posterior cerebral arteries [PCA], vertebral basilar arteries, and opening of the anterior and posterior traffic arteries).
Revascularization was performed by the same chief surgeon. The following surgery-related indicators were recorded: surgical approach (direct, indirect, and combined revascularization), number of surgeries (first or second revascularization), side of the surgery (left or right), recipient vessel for revascularization (posterior branch, anterior branch, apical branch and frontal branch of the superficial temporal artery), and the total operating time (TOT).
The following anesthesia-related indicators were recorded: preoperative noninvasive blood pressure (systolic blood pressure [SBP], diastolic blood pressure [DBP], and mean arterial pressure [MAP]), total anesthesia time (TAT), fluid intake and output (total outflow, total inflow, hemorrhage, colloid replenishment, and crystalloid replenishment), total consumption of analgesic medication, and maximum and minimum values of end-expiratory carbon dioxide.
Most of clinical data in this study were continuous variables. To enhance the clinical applicability, continuous variables were converted into dichotomous variables using specific cutoff values for subsequent statistical analyses. The optimal cutoff values were selected by referencing normal physiological values provided by our laboratory center, or constructing a receiver operating characteristic (ROC) curve to calculate the Youden index. Detailed cutoff values were listed in Supplementary Table S2.
Descriptive analysis and binary logistic regression analyses were performed by SPSS 27 (IBM, Armonk, NY, United States), and other statistical analyses were performed by R software (version 4.4.0, http://www.R-project.org). The data was randomly divided into training and validation sets at a ratio of 7:3. The training set was used for feature selection and model building, while the validation set was used to evaluate the effect of the trained model. Categorized variables were described by the frequency and percentage; continuous variables obeying normal distribution were described as mean ± standard deviation; and skewed distribution data were described as median and interquartile range (P25, P75). Chi-square test for categorical data, t-test for continuous variables obeying normal distribution, and Mann–Whitney U-test for continuous variables not obeying normal distribution were performed. All tests were two-tailed and p < 0.05 was defined as statistically significant.
Initially, stepwise backward regression was performed to identify variables significantly associated with POCI in the training set (p < 0.05). Those with a significant difference were further subjected to the multivariable logistic regression. Then, a nomogram was constructed to predict POCI after revascularization of adult MMD. Its performances, including the discrimination, calibration, and clinical applicability were tested by ROC, calibration curve, decision curve analysis (DCA), respectively. The generalization of the nomogram was finally validated in the validation set. In details, an AUC > 0.70 indicated the acceptable discrimination of the nomogram. The calibration was evaluated by comparing predicted values with observed results, visualized by a calibration curve plot using a 1,000 bootstrap resampling procedure. DCA was used to quantify the net benefits at different threshold probabilities (16).
A total of 1,047 cases of MMD were initially screened. After excluding 50 cases lacking medical history, 44 cases of geriatric MMD, and 12 cases of pediatric MMD, 941 cases were saved. After review of the perioperative data, 288 cases were removed. Thus, a total of 653 cases were enrolled in the study. The included cases were randomly divided into a training set and a validation set on a 7:3 basis, involving 457 cases in the training set and 196 cases in the validation set (Figure 2).
Of the included cases, 308 (47.17%) were male and 345 (52.83%) were female patients, with a mean age of 47.90 ± 9.48 years. POCI confirmed by positive imaging manifestations was found in 102 (15.62%) cases, consisting of 48 (7.35%) cases of asymptomatic cerebral infarction and 54 (8.27%) of symptomatic cerebral infarction. Due to the randomized grouping, the incidence of POCI was comparable between the training and validation sets (p > 0.05). Demographic data between the training set and validation set were detailed in Table 1.
Of the 457 cases of adult MMD in the training set, 207 (45.30%) were male and 250 (54.70%) were female patients, with a mean age of 47.80 ± 9.77 years. Divided by the presence of POCI, there were significant differences in the SBP, DBP and mean corpuscular hemoglobin concentration (MCHC) on admission, type of MMD, and occurrence of cerebral hemorrhage (p < 0.05, Table 2).
Univariate logistic regression initially identified 14 variables significantly correlated with POCI in adult MMD patients after revascularization (Supplementary Table S3), including the type of MMD (OR 3.218, 95% CI 1.523–6.798; p = 0.002), history of cerebral infarction (OR 3.152, 95% CI 1.752–5.670; p < 0.001), PCA anomalies (OR 2.484, 95% CI 1.442–2.272; p = 0.001), post-transit arterial development (PTAD, OR 2.250, 95% CI 1.120–4.524; p = 0.023), pre-anesthesia MAP>102.83 mmHg (OR 3.733, 95% CI 2.244–6.209; p < 0.001), pre-anesthesia SBP > 140 mmHg (OR 2.848, 95% CI 1.720–4.718; p < 0.001), pre-anesthesia DBP > 85.5 mmHg (OR 2.810, 95% CI 1.697–4.653; p < 0.001), TOT>212.5 min (OR 2.848, 95% CI 1.720–4.718; p = 0.026), TAT > 245 min (OR 2.156, 95% CI 1.180–3.940; p = 0.013), MCHC (OR 1.021, 95% CI 1.003–1.040; p = 0.002), alkaline phosphatase (OR 1.013, 95% CI 1.002–1.024; p = 0.022), direct bilirubin (OR 1.220, 95% CI 1.035–1.437; p = 0.017), fibrinogen (FIB) >2.75 g/L (OR 1.982, 95% CI 1.202–3.266; p = 0.007), and history of cerebral hemorrhage (OR 0.486, 95% CI 0.252–0.937; p = 0.031) (Table 3).
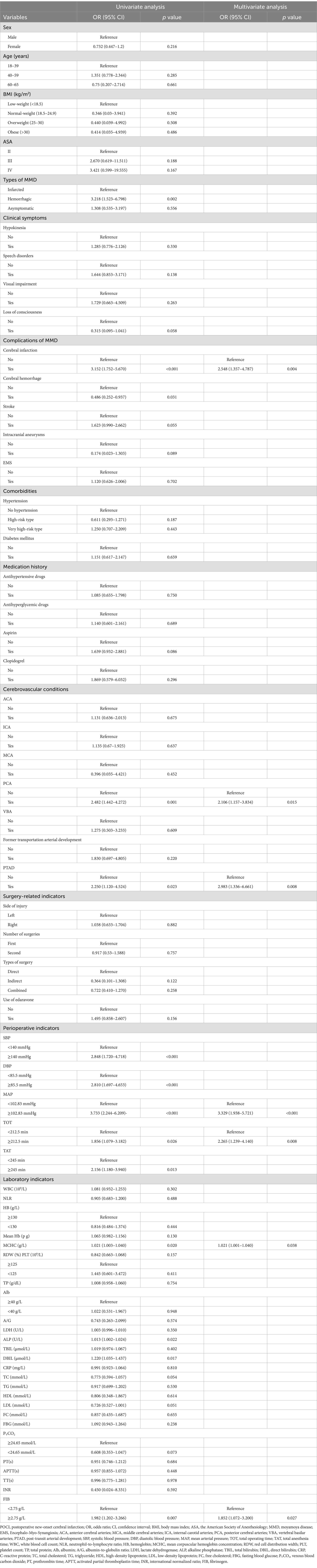
Table 3. Independent risk factors of POCI identified by univariate and stepwise regression multivariate analyses.
Finally, 7 independent risk factors of POCI were screened by stepwise backward regression analysis, including the history of cerebral infarction (OR 2.548, 95% CI 1.357–4.787; p = 0.004), PCA anomalies (OR 2.106, 95% CI 1.157–3.834; p = 0.015), PTAD (OR 2.983, 95% CI 1.336–6.661; p = 0.008), pre-anesthesia MAP>102.83 mmHg (OR 3.329, 95% CI 1.938–5.721; p < 0.001), TOT>212.5 min (OR 2.265, 95% CI 1.239–4.140; p = 0.008), MCHC (OR 1.021, 95% CI 1.001–1.040; p = 0.038), and preoperative FIB>2.75 g/L (OR 1.852, 95% CI 1.072–3.200; p = 0.027) (Table 3).
Incorporating the 7 independent risk factors of POCI, we constructed a nomogram (Figure 3) and the corresponding risk score of each variable was listed in Supplementary Table S4. By calculating the total score of the 7 parameters, a higher score predicted a greater possibility of POCI after revascularization of adult MMD.
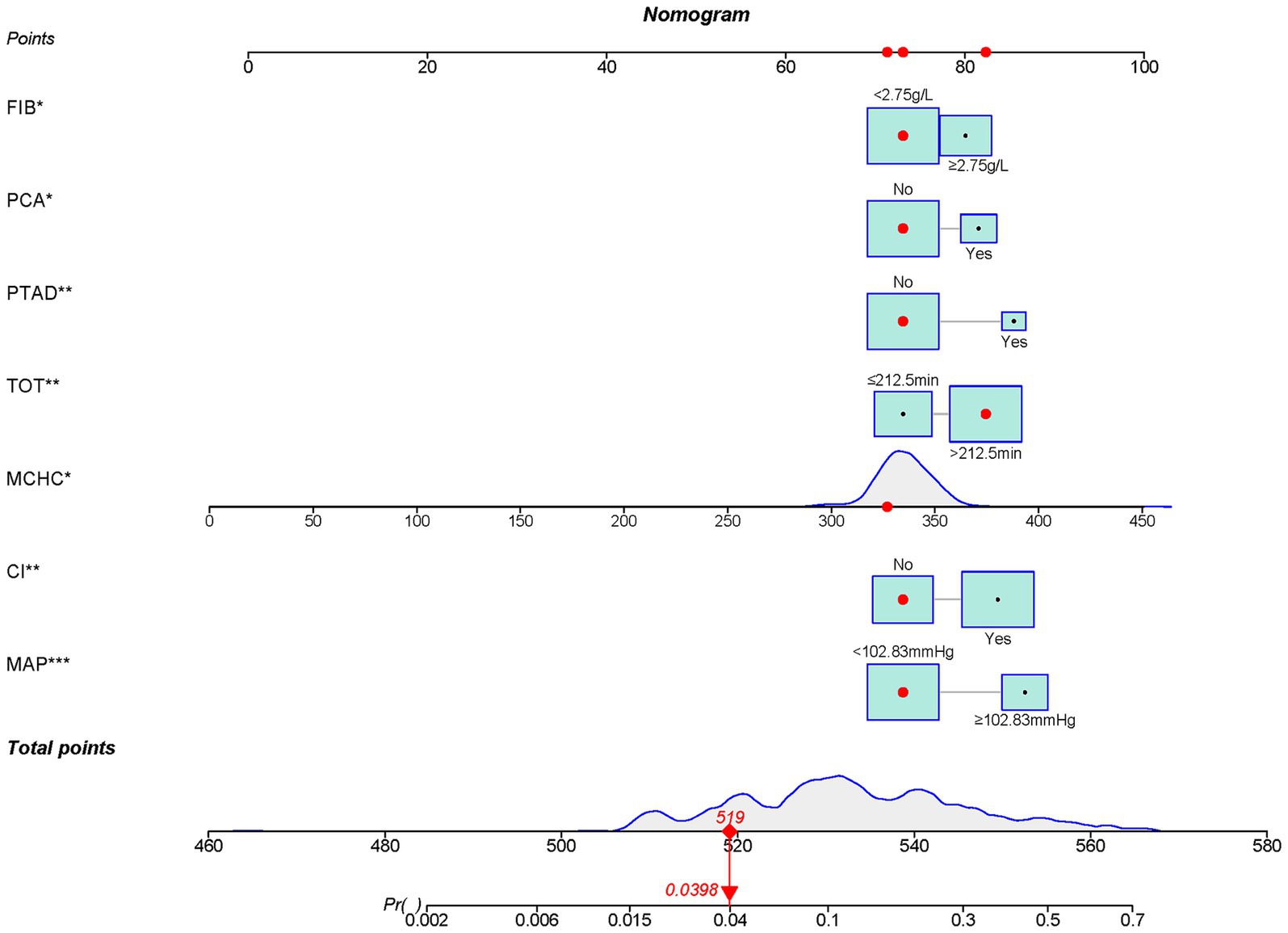
Figure 3. A nomogram to predict POCI after revascularization of adult MMD. In the nomogram, parameters marked with an asterisk (*) indicate that they remain statistically significant in the final model, whereas those without an asterisk indicate that they are not statistically significant. For continuous variables, the distribution is represented as a frequency distribution curve; for categorical variables, the distribution is depicted using green squares, where larger squares represent higher frequencies of individuals at the corresponding level. Additionally, the first observation in the dataset is highlighted on the graph with a red dot, and its corresponding points are also indicated by red dots.
For example, a male adult patient with MMD who FIB<2.75 g/L, PCA = No, PATD = No, TOT>212.5 min, MCHC =325, CI = No, MAP <102.83 mmHg, and was graded with 73, 73, 73, 82, 72, 73 and 73 points, respectively. A total score of 519 points in this nomogram indicated a 3.98% probability of POCI. In addition, we constructed a web-based online dynamic nomogram to estimate the probability of new-onset cerebral infarction after revascularization of moyamoya disease, which can be accessed at https://predict-calculate.shinyapps.io/DynNomapp/.
Validated in the training set, the AUC was 0.772 (95% CI 0.714–0.772) (Figure 4A). The calibration curves showed bias-correction and an apparent curve similar to the ideal line (Figure 4B), demonstrating a good agreement between the predicted and observed extent of POCI. The DCA curve further demonstrated the gain of net benefit at a threshold of 3–60% (Figure 4C).
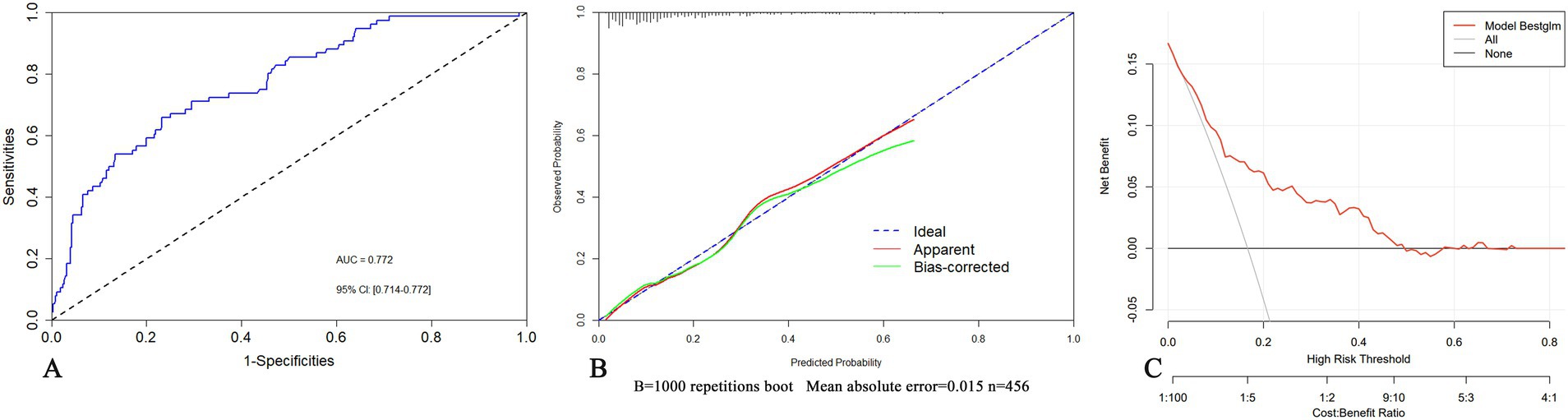
Figure 4. The validation of the nomogram in the training set by ROC curves (A), calibration curves (B) and DCA (C).
In the validation set, the AUC of the nomogram was 0.718 (95% CI: 0.603–0.833), showing no significant difference in comparison to that of the training set (Figure 5A). For the calibration curves, the apparent curves approached to the ideal curves were indicative of a good performance of prediction (Figure 5B). Overall, the nomogram exhibited good discrimination, calibration, and clinical applicability.
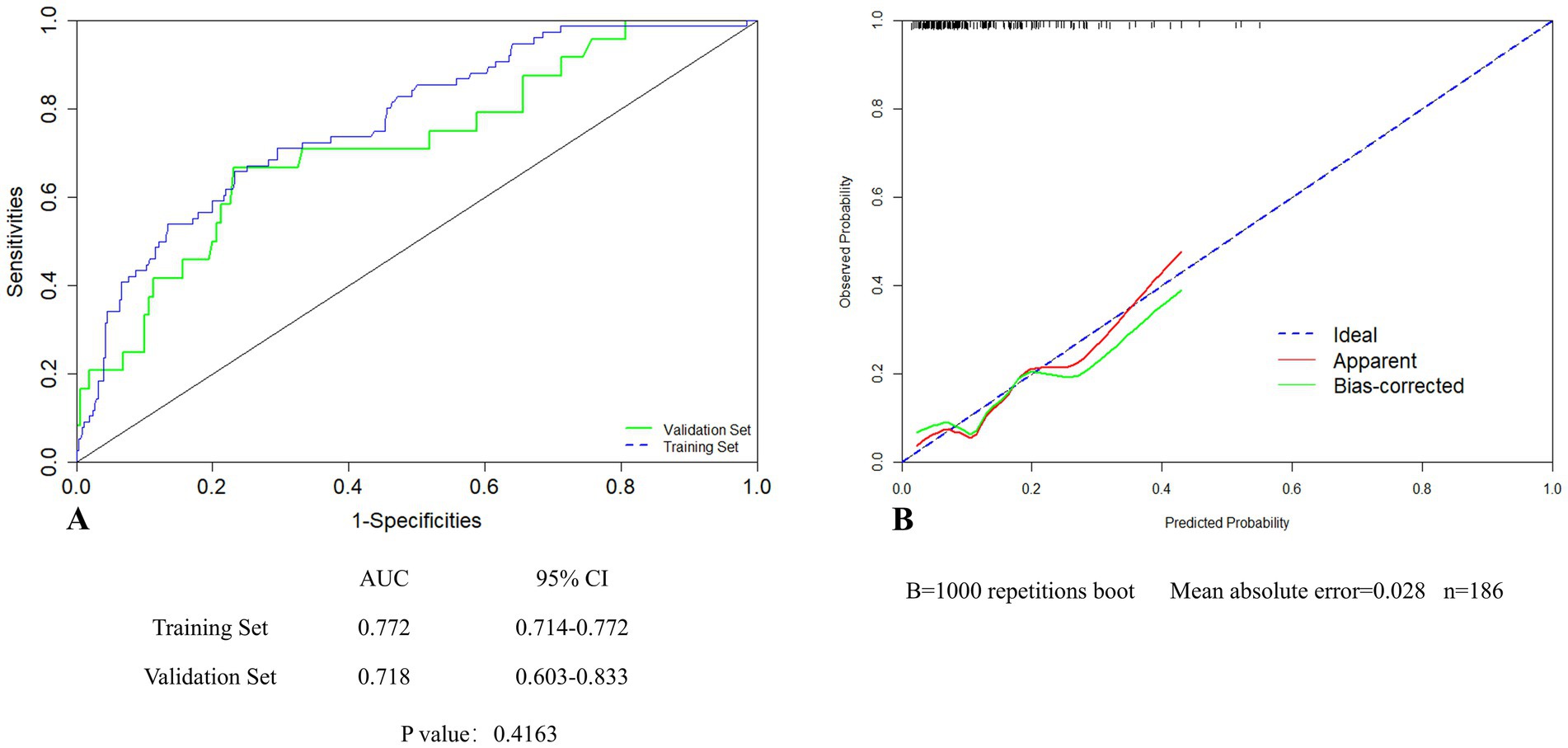
Figure 5. Internal validation of the nomogram in the validation set by ROC (A) and calibration curves (B).
Through thoroughly analyzed perioperative data of adult MMD patients treated with revascularization, including the generation information, demographic data, laboratory testing, imaging findings, surgery-related indicators and anesthesia-related indicators, we found that history of cerebral infarction, PCA anomalies, PTAD, pre-anesthesia MAP>102.83 mmHg, TOT>212.5 min, MCHC, and preoperative FIB>2.75 g/L were significantly correlated with POCI. In addition, a nomogram based on these factors was established to identify high-risk patients of POCI.
The incidence of POCI in our study was 15.62%, and that of symptomatic cerebral infarction was 8.27%. In line with our study, a multicenter retrospective study reported an ischemic stroke incidence of 15.6% within 30 days after revascularization (17). In addition, William et al. (18) reported a 30-day incidence of postoperative stroke in MMD patients at 14.4%. Given the high incidence of POCI in MMD patients, early identification of the high-risk population is of great importance to favor the individualized treatment and long-term prognosis.
Through analyzing baseline and demographic data of participants, especially the vascular conditions associated with MMD, we found that history of cerebral infarction, PCA anomalies and PTAD were significantly correlated with POCI. Accordingly, preoperative cerebral infarction was reported to be an indicator for instability of regional cerebral blood flow (rCBF) and have inadequate collateralization pathways to compensate for hemodynamic impairment (19–21), thus resulting in postoperative ischemic complications. Besides, the involvement of abnormal collateral vessels as a compensatory mechanism is important in MMD patients at a high risk of POCI. PCA anomalies (e.g., stenosis, occlusion, formation of collateral vessels), either in the main trunk or its branches, threaten the risk of cerebral infarction. Notably, smoky vessels in the PCA are associated with higher Suzuki stages and higher probability of POCI in MMD patients (5). Furthermore, meningeal collateral branches arising from the PCA provide the collateral blood flow in patients with advanced MMD. Individuals with the involvement of PCA were reported to be more vulnerable to the hemodynamic stress of general anesthesia and surgical revascularization (7). Although the rCBF and perfusion in the operated hemisphere with the involved PCA can be preserved or even enhanced by a direct revascularization, those in the contralateral hemisphere often remain inadequate and thus result in POCI (22). Posterior circulatory compensation typically refers to the supply of blood from the PCA to the original donor areas of MCA and ACA through various collateral and anastomotic branches (5). In our study, there was an increased probability of POCI in adult MMD patients with PTAD, which might be attributed to more severe Suzuki’s angiographic stage of MMD and more reduced tolerance to cerebral ischemia. The resulted severe hemodynamic failure can lead to the ischemic emergence (8, 12).
Our results showed that MCHC and FIB>2.75 g/L were independent risk factors of POCI after revascularization of adult MMD. These results are similar to those of a previous clinical trial conducted by Bao et al. (23) who found that populations residing in plateau regions experience increased blood viscosity due to heightened metabolic activity in the brain and a greater reliance on oxygen, leading to elevated risks of vasospasm, thrombosis and infarction. We consistently identified a significant correlation of high-level MCHC with increased risk of POCI. However, MCHC could be influenced by confounding factors like sampling, lipid levels, hemolysis, erythrocyte agglutination, and spherocytes, which should be adjusted in future prospective studies (24). In addition, elevated FIB was associated with increased risks of cardiovascular diseases like coronary heart disease and stroke (25). Serving as a critical influencing factor of coagulation cascade, an increase in FIB indicates a hypercoagulable state that impairs endothelial function, reduces blood flow and forms thrombosis. Previous findings have illustrated the correlation of high FIB with Alzheimer’s disease and vascular dementia, rather than CRP (26), which are consistent with our findings and further highlight the vascular property of FIB involved in POCI.
Surgery also linked with POCI in adult patients with MMD. A meta-analysis suggested that combined bypass and direct bypass offer significant benefits to the outcomes of advanced stroke and cerebral hemorrhage (27). Conversely, a retrospective multicenter study did not identify significant differences in the recurrence of stroke, perioperative stroke, and mortality between MMD patients treated with indirect and direct bypass (or combined bypass) (17). In our study, surgical procedures did not influence the incidence of POCI. In addition, we found that the incidence of POCI was significantly higher in MMD patients with prolonged TOT, which may be attributed to the more severe ischemia in the affected vessel that greatly challenged the surgical procedures of intraoperative bypass, such as the management of massive hemostasis, selection of the recipient vessel, and prevention of recurrent local bleeding (28).
Anesthesia is a crucial event involved in the surgical treatment of MMD. Our findings indicated that MAP on admission higher than 102.83 mmHg was a significant predictor of POCI in adult MMD. Effective perioperative anesthesia management of blood pressure and carbon dioxide partial pressure is the cornerstone of few postoperative complications and mortality. Our data showed that elevated SBP, DBP and MAP on admission were significantly correlated with POCI, which were consistent with previous findings (13). Due to preoperative ischemia, an elevated peripheral blood pressure is essential to maintain cerebral perfusion. MMD patients at advanced stage experienced greater ischemia, indirectly requiring the more pronounced increase in the preoperative MAP. The use of antihypertensive agents during the perioperative period to reduce intraoperative hemorrhage and expand the surgical field may further decrease cerebral perfusion, exacerbate ischemia and cause cerebral infarction.
In the present study, we did not focus on the duration of hypotension. With the strict perioperative anesthesia management, the incidence of hypotension during the surgery of MMD remained low. Besides, our anesthesia management emphasized the stability of perioperative hemodynamics. Intraoperative hypotension is less detrimental than blood pressure instability to MMD patients (13). Chronic mild cerebral ischemia allows a tolerance to stable hypotension, whereas unstable blood pressure fluctuations, particularly rapid and significant drops, increase the risk of ischemic stroke.
Given that the steno-occlusive pathology mainly involves the terminal portions of bilateral hemispheres, hence, ischemic complications following revascularization surgery for MMD are not isolated to the ipsilateral hemisphere (29). However, the precise mechanism underlying acute contralateral cerebral infarctions has not been clearly defined and was regarded as multifactorial. A relevant study, conducted by Kim et al. (30) found that the potential mechanism for contralateral stroke was the redistribution of intracranial blood away from the contralateral side after revascularization. Specifically, the cerebral blood flow of the ipsilateral side was restored by the revascularization, and this might result in a reduction of the contralateral collateral flow. In addition, Parray et al. (31) indicated that the hemodynamic interrelationship between the two hemispheres and potential connections of bi-hemispheric vascular networks might be another potential mechanism. Though we have investigated the risk factors for POCI in adults MMD, our study did not differentiate between ipsilateral and contralateral cerebral infarctions. Besides, as outlined in prior work, contralateral cerebral infarctions associated with MMD may occur less frequently than ipsilateral cerebral infarctions (22, 29, 32). Considering different incidence and mechanism among ipsilateral and contralateral cerebral infarctions, further studies are warranted to identify their respective risk factors.
Our study presented several advantages. First, we thoroughly analyzed clinical data in multiple aspects, including the baseline characteristics, demographic data, imaging findings, laboratory testing, surgery-related variables, and anesthesia-related variables. Second, univariable and multivariable logistic regression with stepwise backward greatly prevented overfitting of the nomogram. Third, a large sample size elevated the reliability. However, limitations should also be concerned. First, this is a retrospective analysis based on case-system data, leading to potential recall and reporting biases. Second, the application of our nomogram to pediatric MMD patients requires a further exploration. Furthermore, our study did not differentiate between ipsilateral and contralateral cerebral infarctions. Identifying new cerebral infarctions at different anatomical sites and on different operative sides may enable more precise management of patients with MMD. Lastly, with the advancements in machine learning technology, emerging feature selection methods could be integrated into our study to enhance the performance of the prediction model.
We constructed a nomogram, incorporating preoperative cerebral infarction, PCA anomalies, PTAD, pre-anesthesia MAP>102.83 mmHg, TOT>212.50 min, preoperative FIB>2.75 g/L, and MCHC, to effectively predict POCI after revascularization of adult MMD. The nomogram is validated to present acceptable performances of discrimination, calibration, and clinical applicability.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Medical Ethics Committee of Drum Tower Hospital, Nanjing University School of Medicine (Approval number: 2023-455-01). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
ZW: Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Project administration, Software, Validation, Visualization. JY: Data curation, Investigation, Writing – review & editing. YZ: Writing – review & editing, Data curation, Formal analysis, Investigation. JR: Data curation, Investigation, Resources, Writing – review & editing. XL: Data curation, Investigation, Project administration, Writing – review & editing. SM: Data curation, Resources, Writing – review & editing. JX: Data curation, Resources, Validation, Writing – review & editing. MW: Resources, Supervision, Validation, Writing – review & editing. JB: Conceptualization, Funding acquisition, Methodology, Resources, Software, Supervision, Visualization, Writing – review & editing. YS: Conceptualization, Funding acquisition, Methodology, Resources, Software, Supervision, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82071229); Nanjing Health Science and Technology Development Special Fund (YKK22089); Nanjing Drum Tower Hospital 2023 Clinical Research Special Fund (2023-LCYJ-PY-07); Nanjing Drum Tower Hospital New Technology Development Fund (XJSFZJJ202016); and Nanjing Medical Science and Technique Development Foundation (YKK21084).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1537755/full#supplementary-material
1. Singh, R, McLelland, MD, De La Peña, NM, Pollock, JR, Catapano, JS, Srinivasan, VM, et al. Research advances in the diagnosis and treatment of moyamoya disease: a bibliometric analysis. Neurosurg Rev. (2022) 45:1977–85. doi: 10.1007/s10143-022-01748-w
2. Kuroda, S, and Houkin, K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. (2008) 7:1056–66. doi: 10.1016/S1474-4422(08)70240-0
3. Kim, JE, and Jeon, JS. An update on the diagnosis and treatment of adult Moyamoya disease taking into consideration controversial issues. Neurol Res. (2014) 36:407–16. doi: 10.1179/1743132814Y.0000000351
4. Hayashi, T, Kimiwada, T, Karibe, H, Shirane, R, Sasaki, T, Metoki, H, et al. Preoperative risks of cerebral infarction in pediatric Moyamoya disease. Stroke. (2021) 52:2302–10. doi: 10.1161/STROKEAHA.120.032699
5. Yu, T, Wang, R, Ye, X, Zeng, C, Chen, X, and Zhao, Y. Angioarchitectural factors associated with postoperative cerebral infarction in ischemic Moyamoya disease. Brain Sci. (2022) 12:1270. doi: 10.3390/brainsci12101270
6. Qian, Y, Huang, B, Hu, Z, Wang, J, Zhao, P, and Li, X. Analysis of factors related to cerebral infarction after direct bypass surgery in adults with Moyamoya disease. Cerebrovasc Dis. (2020) 49:55–61. doi: 10.1159/000504743
7. Park, W, Ahn, JS, Lee, HS, Park, JC, and Kwun, BD. Risk factors for newly developed cerebral infarction after surgical revascularization for adults with Moyamoya disease. World Neurosurg. (2016) 92:65–73. doi: 10.1016/j.wneu.2016.03.053
8. Wang, P, Cai, H, Luo, R, Liu, X, Zhang, D, and Zhang, Y. Assessment of surgical outcomes in children and adult ischemic moyamoya disease and its relationship with the pre-infarction cerebral perfusion status. Turk Neurosurg. (2021) 32:43–51. doi: 10.5137/1019-5149.JTN.33210-20.2
9. Kim, M, Park, W, Chung, Y, Lee, SU, Park, JC, Kwon, DH, et al. Development and validation of a risk scoring model for postoperative adult moyamoya disease. J Neurosurg. (2021) 134:1505–14. doi: 10.3171/2020.2.JNS193221
10. Chen, P, Wang, Y, Li, S, Tang, D, Yang, S, Zeng, F, et al. Development and external validation of nomogram for cerebral infarction in Moyamoya diseases. Transl Stroke Res. (2023) 14:890–8. doi: 10.1007/s12975-023-01127-7
11. Guo, Q, Fan, YN, Wang, QN, Li, J, Han, C, Zou, Z, et al. Nomogram for predicting long-term outcomes of Encephaloduroarteriosynangiosis in toddlers with Moyamoya disease: a longitudinal and cross-sectional study. Transl Stroke Res. (2023) 9:1–13. doi: 10.1007/s12975-023-01213-w
12. Sun, H, Li, W, Xia, C, Ren, Y, Ma, L, Xiao, A, et al. Angiographic and hemodynamic features in asymptomatic hemispheres of patients with Moyamoya disease. Stroke. (2022) 53:210–7. doi: 10.1161/STROKEAHA.121.035296
13. Li, J, Zhao, Y, Zhao, M, Cao, P, Liu, X, Ren, H, et al. High variance of intraoperative blood pressure predicts early cerebral infarction after revascularization surgery in patients with Moyamoya disease. Neurosurg Rev. (2020) 43:759–69. doi: 10.1007/s10143-019-01118-z
14. Kuroda, S, Fujimura, M, Takahashi, J, Kataoka, H, Ogasawara, K, Iwama, T, et al. Diagnostic criteria for Moyamoya disease – 2021 revised version. Neurol Med Chir (Tokyo). (2022) 62:307–12. doi: 10.2176/jns-nmc.2022-0072
15. Wang, J, Jiang, H, Tang, J, Lin, C, Ni, W, and Gu, Y. Postoperative cerebral infarction after revascularization in patients with moyamoya disease: incidence and risk factors. Front Neurol. (2022) 13:1053193. doi: 10.3389/fneur.2022.1053193
16. Vickers, AJ, and Elkin, EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Mak. (2006) 26:565–74. doi: 10.1177/0272989X06295361
17. Jang, DK, Lee, KS, Rha, HK, Huh, PW, Yang, JH, Park, IS, et al. Bypass surgery versus medical treatment for symptomatic moyamoya disease in adults. J Neurosurg. (2017) 127:492–502. doi: 10.3171/2016.8.JNS152875
18. Powers, WJ, Clarke, WR, Grubb, RL, Videen, TO, Adams, HP, Derdeyn, CP, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the carotid occlusion surgery study randomized trial. JAMA. (2011) 306:1983–92. doi: 10.1001/jama.2011.1610
19. Chen, J, Duan, L, Xu, W-H, Han, Y-Q, Cui, L-Y, and Gao, S. Microembolic signals predict cerebral ischaemic events in patients with moyamoya disease. Eur J Neurol. (2014) 21:785–90. doi: 10.1111/ene.12392
20. Gordon, AL, Goode, S, D’Souza, O, Auer, DP, and Munshi, SK. Cerebral misery perfusion diagnosed using hypercapnic blood-oxygenation-level-dependent contrast functional magnetic resonance imaging: a case report. J Med Case Rep. (2010) 4:54. doi: 10.1186/1752-1947-4-54
21. Maddula, M, Sprigg, N, Bath, PM, and Munshi, S. Cerebral misery perfusion due to carotid occlusive disease. Stroke Vasc Neurol. (2017) 2:88–93. doi: 10.1136/svn-2017-000067
22. Jung, YJ, Ahn, JS, Kwon, DH, and Kwun, BD. Ischemic complications occurring in the contralateral hemisphere after surgical treatment of adults with Moyamoya disease. J Korean Neurosurg Soc. (2011) 50:492–6. doi: 10.3340/jkns.2011.50.6.492
23. Bao, H, Wang, D, Zhao, X, Wu, Y, Yin, G, Meng, L, et al. Cerebral edema in Chronic Mountain sickness: a new finding. Sci Rep. (2017) 7:43224. doi: 10.1038/srep43224
24. Barnes, PW, Mcfadden, SL, Machin, SJ, and Simson, E. The international consensus Group for Hematology Review: suggested criteria for action following automated CBC and WBC differential analysis. Lab Hematol. (2005) 11:83–90. doi: 10.1532/LH96.05019
25. Fibrinogen Studies Collaboration*Danesh, J, Lewington, S, Thompson, SG, Lowe, GD, Collins, R, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular MortalityAn individual participant meta-analysis. JAMA. (2005) 294:1799–809. doi: 10.1001/jama.294.14.1799
26. Van Oijen, M, Witteman, JC, Hofman, A, Koudstaal, PJ, and Breteler, MMB. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke. (2005) 36:2637–41. doi: 10.1161/01.STR.0000189721.31432.26
27. Nguyen, VN, Motiwala, M, Elarjani, T, Moore, KA, Miller, LE, Barats, M, et al. Direct, indirect, and combined extracranial-to-intracranial bypass for adult Moyamoya disease: an updated systematic review and meta-analysis. Stroke. (2022) 53:3572–82. doi: 10.1161/STROKEAHA.122.039584
28. Kuroda, S, and Houkin, K. Bypass surgery for Moyamoya disease: concept and essence of surgical techniques. Neurol Med Chir (Tokyo). (2012) 52:287–94. doi: 10.2176/nmc.52.287
29. Sussman, ES, Madhugiri, V, Teo, M, Nielsen, TH, Furtado, SV, Pendharkar, AV, et al. Contralateral acute vascular occlusion following revascularization surgery for moyamoya disease. J Neurosurg. (2019) 131:1702–8. doi: 10.3171/2018.8.JNS18951
30. Kim, TJ, Lee, JS, Hong, JM, and Lim, YC. Intracerebral steal phenomenon: A potential mechanism for contralateral stroke after carotid artery stenting. Neurologist. (2012) 18:128–9. doi: 10.1097/NRL.0b013e318253f8b5
31. Parray, T, Martin, TW, and Siddiqui, S. Moyamoya disease: A review of the disease and anesthetic management. J Neurosurg Anesthesiol. (2011) 23:100–9. doi: 10.1097/ANA.0b013e3181f84fac
32. Sato, D, Miyawaki, S, Imai, H, Hongo, H, Kiyofuji, S, Koizumi, S, et al. Clinical characteristics of immediate contralateral ischemia subsequent to revascularization for Moyamoya disease. World Neurosurg. (2024) 183:e355–65. doi: 10.1016/j.wneu.2023.12.100
95% CI - 95% confidence interval
A/G - albumin-to-globulin ratio
ACA - anterior cerebral arteries
Alb - albumin
ALP - alkaline phosphatase
APTT - activated partial thromboplastin time
AUC - area under the curve
CBC - complete blood count
CRP - C-reactive protein
CT - computerized Tomography
DBIL - direct bilirubin
DBP - diastolic blood pressure
DCA - decision curve analysis
EMS - Encephalo-Myo-Synangiosis
FBG - fasting blood glucose
FC - free cholesterol
FIB - fibrinogen
HB - hemoglobin
HDL - high-density lipoprotein
LDH - lactate dehydrogenase
LDL - low-density lipoprotein
MAP - mean arterial pressure
MCA - middle cerebral artery
MCHC - mean corpuscular hemoglobin concentration
MMD - moyamoya disease
MRI - Magnetic Resonance Imaging
NLR - neutrophil-to-lymphocyte ratio
OR - odds ratio
PCA - posterior cerebral artery
PLT - platelet count
POCI - postoperative new-onset cerebral infarction
PT - prothrombin time
PTAD - post-transit arterial development
PVCO2 - venous blood carbon dioxide
RDW - red cell distribution width
ROC - operating characteristic curve
SBP - systolic blood pressure
SD - standard deviation
STA - superficial temporal artery
TAT - total anesthesia time
TBIL - total bilirubin
TC - total cholesterol
TG - triglyceride
TOT - total operative time
TP - total protein
Keywords: adult moyamoya disease, revascularization, postoperative new-onset cerebral infarction, nomogram, retrospective study
Citation: Wang Z, Yu J, Zhang Y, Ruan J, Liu X, Ma S, Xie J, Wu M, Bo J and Sun Y (2025) A nomogram to predict postoperative new-onset cerebral infarction after revascularization of moyamoya disease in adults and its validation: a retrospective study. Front. Neurol. 16:1537755. doi: 10.3389/fneur.2025.1537755
Received: 01 December 2024; Accepted: 06 January 2025;
Published: 24 January 2025.
Edited by:
Aleksandras Vilionskis, Vilnius University, LithuaniaReviewed by:
Michael Gliem, Heinrich Heine University, GermanyCopyright © 2025 Wang, Yu, Zhang, Ruan, Liu, Ma, Xie, Wu, Bo and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu’e Sun, c3VueXVlQG5qdS5lZHUuY24=; Mimi Wu, MTM3NzAzMDY5MzVAMTYzLmNvbQ==; Jinhua Bo, Ym9qaW5odWFAbmpnbHl5LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.