
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 29 January 2025
Sec. Applied Neuroimaging
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1532883
This article is part of the Research Topic Applied Neuroimaging for the Diagnosis and Prognosis of Cerebrovascular Disease View all 6 articles
Introduction: Extracranial carotid calcification is a common marker of advanced atherosclerosis. However, its impact on stroke risk is not consistent across studies, and examining the type of calcification and the presence of systemic diseases might be helpful. We aimed to investigate extracranial carotid calcification and its association with risk factors for ischemic cerebrovascular diseases.
Materials and methods: Among 1,863 consecutive patients in the Atherosclerotic Plaque Characteristics Associated with a Progression Rate of the Plaque and a Risk of Stroke in Patients with the Carotid Bifurcation Plaque Study (ANTIQUE), 132 symptomatic or asymptomatic patients (177 carotid plaques) with >30% carotid stenosis examined through computed tomography (CT) and magnetic resonance imaging (MRI) were included. Statistical data were assessed using the χ2-test, Fisher’s exact test, t-test, and Mann–Whitney test to investigate the calcification risk factors.
Results: Compared to the absence of calcifications, spotty calcifications were associated with male sex [odds ratio (OR): 3.72, 95% confidence interval (CI): 1.06–13.05], while large calcifications were associated with older patients (OR: 1.60 per 5 years of age, 95% CI: 1.20–2.13). Large calcifications were also strongly associated with coronary heart disease (OR: 4.07, 95% CI: 1.15–14.44) and atrial fibrillation (p = 0.025). In comparison between only spotty and large calcifications, spotty calcifications were associated with male sex (OR: 3.72, 95% CI: 1.06–13.05), smoking (p = 0.020) in more significant quantities (p = 0.014), and lipid plaque (p < 0.001), while large calcifications with contralateral stenosis degree (p = 0.044). No significant relationship was found between cerebrovascular events and the type of calcification.
Conclusion: Although the presence and type of extracranial carotid calcification were not related to ipsilateral ischemic events, large calcifications were strongly associated with coronary heart disease and atrial fibrillation.
Clinical trial registration: ClinicalTrials.gov, identifier NCT02360137.
Carotid artery stenosis caused by atherosclerosis represents a substantial global epidemiological burden, with the prevalence of carotid plaque at 21% among individuals aged 30–79 years (1). Importantly, carotid atherosclerosis accounts for up to 25% of all ischemic strokes, which are among the leading causes of mortality and disability worldwide (2, 3). Extracranial carotid calcification represents a well-known clinical marker of atherosclerosis, which is characteristic of arterial aging. It is present in up to 75% of the population over the age of 75 years (1, 4).
The immediate inflammatory response that results in microcalcifications represents the pathophysiology of carotid calcification, which can be detected by non-invasive imaging methods, such as computed tomography (CT) and magnetic resonance imaging (MRI) (5). While microcalcifications and spotty calcifications indicate active vascular calcification related to inflammation, causing plaque instability, macrocalcification is strongly inversely related to macrophage infiltration, causing plaque stabilization (6). However, the results of the previous studies are not consistent. Several comprehensive studies have shown that carotid plaque calcification is a protective plaque characteristic not associated with stroke (7–9), but some studies found a positive association between calcification and stroke (10–12). Therefore, examining the relationship between extracranial carotid calcification and systemic diseases that play a role in stroke risk might be useful for risk stratification in patients (13). To the best of our knowledge, when considering the calcification type and multiple atherosclerosis-related systematic diseases, there exists a lack of evidence of these types of relationships.
This study aimed to assess the association between extracranial carotid plaque calcification and risk factors for ischemic cerebrovascular diseases in both symptomatic and asymptomatic patients.
This study presents a post hoc analysis of data from the prospective multicenter observational and cross-sectional Atherosclerotic Plaque Characteristics Associated with a Progression Rate of the Plaque and a Risk of Stroke in Patients with the Carotid Bifurcation Plaque Study (ANTIQUE; ClinicalTrials.gov Identifier: NCT02360137).
For the present study, we enrolled all consecutive patients from the ANTIQUE study who has carotid stenosis of at least 30% and underwent clinical and diagnostic (CT and MRI) examinations. The patients were recruited into the comprehensive stroke center between October 2016 and March 2019 from those indicated for neurosonology examination in stroke prevention or acute stroke diagnostics (14, 15). The inclusion criteria were as follows: Patients aged above 30 years; atherosclerotic plaque in the carotid bifurcation or the proximal part of the internal carotid artery with a thickness of ≥2 mm in the transverse plane of the ultrasound B-mode measurement; calcification detected in the mentioned area of carotid bifurcation in CT examination; sufficient image quality from CT and MRI examinations; and patient self-sufficiency (modified Rankin scale score, 0–2 points). A carotid plaque, representing the most stenotic lesion when multiple plaques were present, causing stenosis at least 30% on ultrasound B-mode (transition from laminar to turbulent blood flow) was included and further assessed (16).
The exclusion criteria were as follows: Patients whose CT or MRI of the neck was not performed; insufficient CT and MR image quality of the patients; non-cooperative patients for the examinations; patients detected with carotid artery occlusion; patients undergoing stenting in the carotid bifurcation; and patients after invasive treatment of ipsilateral carotid artery (carotid endarterectomy or stenting).
Symptomatic patients were characterized as those with clinical signs of recent ipsilateral cerebrovascular ischemic events [transient ischemic attack (TIA), stroke, amaurosis fugax, and/or retinal infarction] in the last 90 days (time from symptom onset to imaging), excluding patients with other potential stroke etiologies (cardioembolic, lacunar, arterial dissection, vasculitis, other rare causes of stroke) (17). Both arteries from symptomatic patients were included: the artery ipsilateral to the cerebrovascular event (symptomatic) and the contralateral (asymptomatic). Patients without clinical signs of TIA/stroke in the relevant arterial territory within the last 90 days were classified as asymptomatic.
All patients were examined through CT (first-line modality—as soon as possible after the onset of symptoms or within 30 days of recruitment from the neurosonology laboratory for asymptomatic patients) and MRI within 7 days following the CT examination.
All patients were examined by a standard multidetector CT angiography (CTA) of carotid and brain arteries using various machines, with an intravenous iodine contrast agent Iomeron® 400 (Bracco Imaging, Milan, Italy) or Ultravist® 370 (Bayer HealthCare Pharmaceuticals LLC, Berlin, Germany) administered with 50–100-mL doses. Multiplanar axial plane reconstructions (<1-mm slices) and sagittal and coronal maximum intensity projection reconstructions (3–8 mm) were assessed with a uniform window width and center of 700 and 200 Hounsfield units (HU), respectively.
Carotid artery stenosis severity was measured based on the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria (18). Plaque morphology was analyzed using density measurement of individual characteristics in HU. Characteristics were classified as lipid (<60 HU), fibrous (60–130 HU), or calcified (>130 HU) based on voxel-level measurements within regions of interest (2–10 pixels per region, covering a minimum of three plaque slices) (19). For overall plaque evaluation (lipid, fibrous, or calcified), the predominant characteristic had to occur in >50% plaque area. Calcifications were divided according to size into spotty (<3 mm in length/width) or large (>3 mm) (20). Additionally, smooth (no irregularities), irregular (minor surface changes), or ulcerated (>1 mm deep excavation in at least two planes) plaque surface was evaluated (19).
Carotid MRI examination protocol was conducted on different 1.5-Tesla machines with head/neck coil, consisting of the following sequences: Fat-suppressed T1-weighted_TSE [turbo spin echo; echo time (TE) 19 ms, repetition time (TR) 600 ms; slice thickness 3 mm; duration 3:50 min], 3D_T1-weighted_MPRAGE (magnetization prepared rapid gradient echo; TE 4 ms; TR 670 ms; inversion time 370 ms; 1 mm; 5:49 min), T2-weighted TSE (TE 72 ms; TR 4,580 ms; 4 mm; 3:18 min), and 3D_TOF (time of flight; TE 7 ms; TR 24 ms; 1 mm; 2:43 min).
In the individual plaque characteristics evaluation, differently distributed intraplaque signal intensities were visually compared to sternocleidomastoid muscle intensity. Overall, plaque composition was evaluated by the modified American Heart Association (AHA) plaque classification for MRI (IV–V, VI: unstable soft plaques; VII, VIII: stable hard plaques) (21). Lipid-rich necrotic core (LRNC; TOF: isointense, T1-w: isointense to hyperintense, T2-w: hypointense) and LRNC covering fibrous cap status (thick, thin, or ruptured) were assessed (22). Finally, intraplaque hemorrhage (IPH) categorized into acute (<1 week old; T1-w, TOF: hyperintense, T2-w: iso to hypointense) and subacute (1–6 weeks old; T1-w, T2-w, TOF: hyperintense) was evaluated.
All mentioned CT- and MRI-derived carotid plaque characteristics were evaluated by a single experienced rater (D.P.), blinded to patient medical history and CT results, based on cited major studies and expert consensus (5).
From the patient anamnestic data, the following atherosclerosis-related risk factors were retrieved: sex, age, arterial hypertension, diabetes mellitus, hyperlipidemia, bronchial asthma, chronic obstructive pulmonary disease, nephropathy, hyperuricemia, cancer, smoking, and alcohol. Daily cigarette and alcohol consumption (1 unit/20 g of alcohol = beer 0.5 L or wine 0.2 L or spirits 0.05 L) in the last year was also recorded. Moreover, data regarding atherosclerosis-related diseases (coronary heart disease, myocardial infarction, atrial fibrillation, and peripheral arterial disease) and cerebrovascular events (ischemic stroke, hemorrhagic stroke, transient ischemic attack, amaurosis fugax, and retinal infarction) were collected.
A statistical study power calculation was carried out. For a medium effect size w = 0.3, the significance level 0.05, and the test power 0.8 in the 2 × 2 table, the total sample size equal to 88 was sufficient. To account for the low quality of data in 25%, 110 patients were considered as a minimum to be recruited for the study.
The baseline characteristics were analyzed using descriptive statistics. Continuous data were noted as means ± standard deviations (SD) or medians and ranges. The categorical data were presented as numbers and percentages. Baseline differences between asymptomatic and symptomatic arteries were analyzed using the χ2-test of independence for contingency tables for categorical variables. If the assumption that the value of the expected cell counts is 5, or more, in at least 80% of the cells, and no cell has an expected count less than one was violated, Fisher’s exact test was used. Differences in continuous variables were assessed using the independent samples’ t-test for normally distributed variables or Mann–Whitney test otherwise. The normality of data was evaluated through the Shapiro–Wilk test.
Associations between the mentioned risk factors and calcification type (spotty, large) were assessed using the χ2-test, Fisher’s exact test, t-test, or Mann–Whitney test. Relationships between calcification type (spotty and large) and other plaque characteristics (CT: plaque type, plaque surface; MRI: AHA type, LRNC, fibrous cap, IPH), side of stenosis (ipsilateral, contralateral) were assessed using χ2-test or Fisher’s exact test with post hoc comparisons using adjusted residuals, or Mann–Whitney test. Associations of calcification type (no calcification, spotty, and large) and atherosclerosis-related diseases were evaluated by a χ2-test or Fisher’s exact test with post hoc comparisons using adjusted residuals. Detailed tables of the adjusted residuals are provided in Supplementary Tables S1–S5).
As a direct outcome, relationships between the calcification type (none, spotty, and large) and mentioned cerebrovascular events were assessed via the χ2-test or Fisher’s exact test where appropriate. Statistical significance was assumed at a p-value of <0.05. All analyses were performed using IBM-SPSS Statistics version 29.0 for Windows.
Overall, 132 patients (264 carotid bifurcations) were examined by CT and MRI from 1,863 patients enrolled in the ANTIQUE study. Only a symptomatic artery was included from symptomatic patients (not the contralateral asymptomatic artery) to reach homogeneous groups of plaques. From 264 carotid arteries, 177 plaques (68.9% male individuals; median age of 69 years) were included, and 87 arteries were excluded due to asymptomatic artery of symptomatic patient (60 cases), carotid occlusion (17 cases), and carotid stenosis <30% (10 cases). The study flow chart is presented in Figure 1. Symptomatic patients were significantly more likely to consume alcohol in larger quantities, have hyperuricemia, and have less frequent coronary heart disease compared to asymptomatic patients. All the details about the study population are available in Table 1.
When assessing the risk factors regarding absent calcification, spotty calcifications were associated with male sex [crude odds ratio (OR) 3.72, 95% confidence interval (CI) 1.06–13.05; Supplementary Table S6]. For large calcification, logistic regression analysis showed that these patients were significantly older (crude OR 1.60 per 5 years of age, 95% CI 1.20–2.13; Supplementary Table S6). Detailed results are presented in Supplementary Table S6 (crude ORs) and Supplementary Table S7 (adjusted ORs).
In comparison between only spotty and large calcifications, men had more often spotty calcifications, while women had more frequent large calcifications (p = 0.015). Higher age was associated with the presence of large calcification (p = 0.027). Smoking in more significant quantities was significantly related to spotty calcification (p = 0.014). At the same time, alcohol consumption, along with other atherosclerosis risk factors and chronic diseases, did not differ between the groups. All the above-mentioned results are presented in Table 2.
The calcification type was not related to the degree of ipsilateral stenosis but to contralateral stenosis. Large calcification was associated with a high degree of stenosis contralaterally (p = 0.044). Lipid plaque on CT was associated with spotty calcification (p < 0.001), while a large calcification was associated with calcified plaque (p < 0.001). Spotty calcification showed a non-significant trend toward more frequent presence in plaques with irregular surfaces than large calcification (p = 0.16). No significant associations were found between calcification patterns and MRI-derived carotid plaque characteristics. However, non-significant trends suggested spotty calcifications were more common in AHA type IV–V plaques (LRNC surrounded by fibrous tissue with possible calcification), plaques with LRNC, and thin or ruptured fibrous cap; and large calcification were more common in AHA type VII (calcified plaque) and subacute IPH. Detailed results on CT- and MRI-derived plaque characteristics are presented in Table 3.
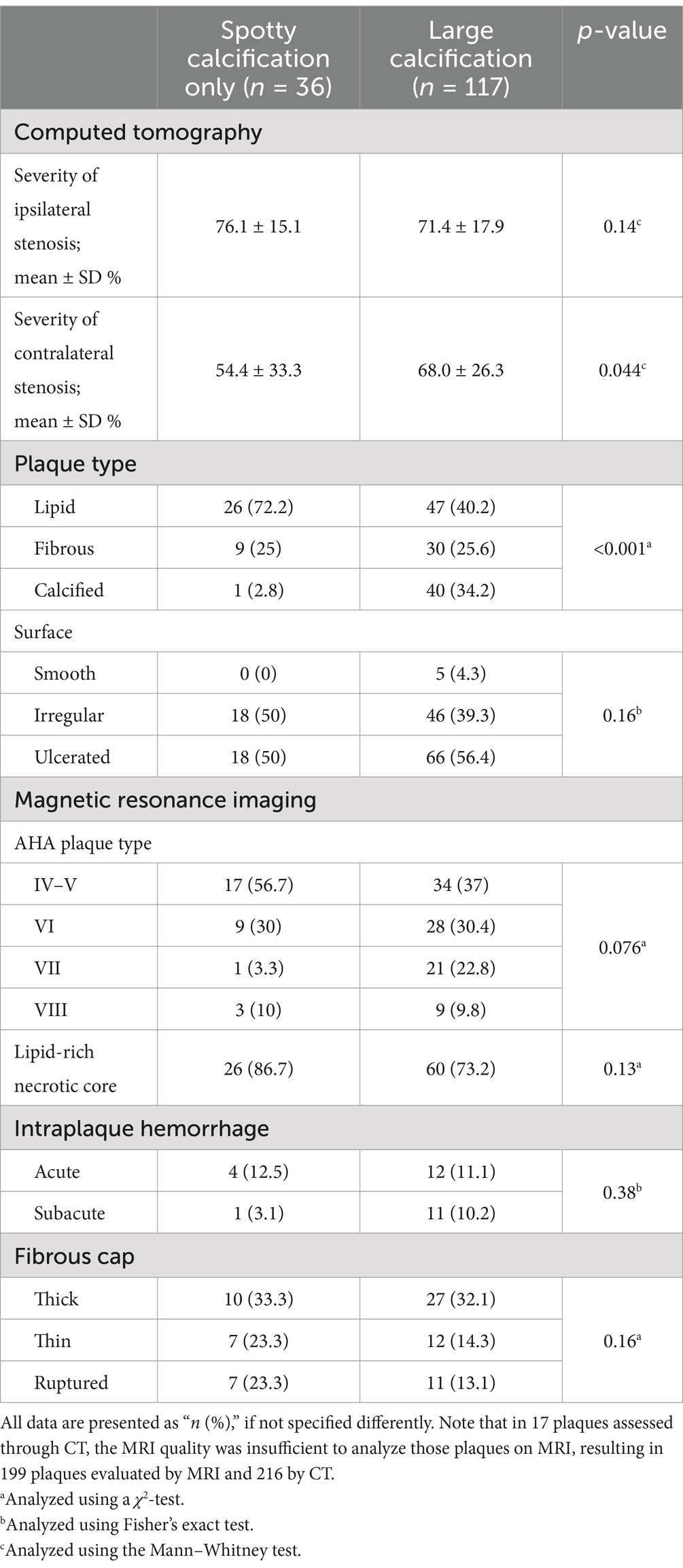
Table 3. Extracranial carotid plaque calcification and other plaque characteristics evaluated on computed tomography (CT) and magnetic resonance imaging (MRI).
No significant relation was found between ipsilateral cerebrovascular events and the presence or type of calcification. However, atrial fibrillation was significantly more often in patients with large calcification within carotid plaque (p = 0.015, overall test, Table 4). Large calcifications were associated with coronary heart disease (crude OR 4.07, 95% CI 1.15–14.44; Supplementary Table S8) and atrial fibrillation (p = 0.025; Supplementary Table S8) compared to no calcification. Further results are provided in Table 4, and crude and adjusted OR values are given in the Supplementary Tables S8, S9, respectively.
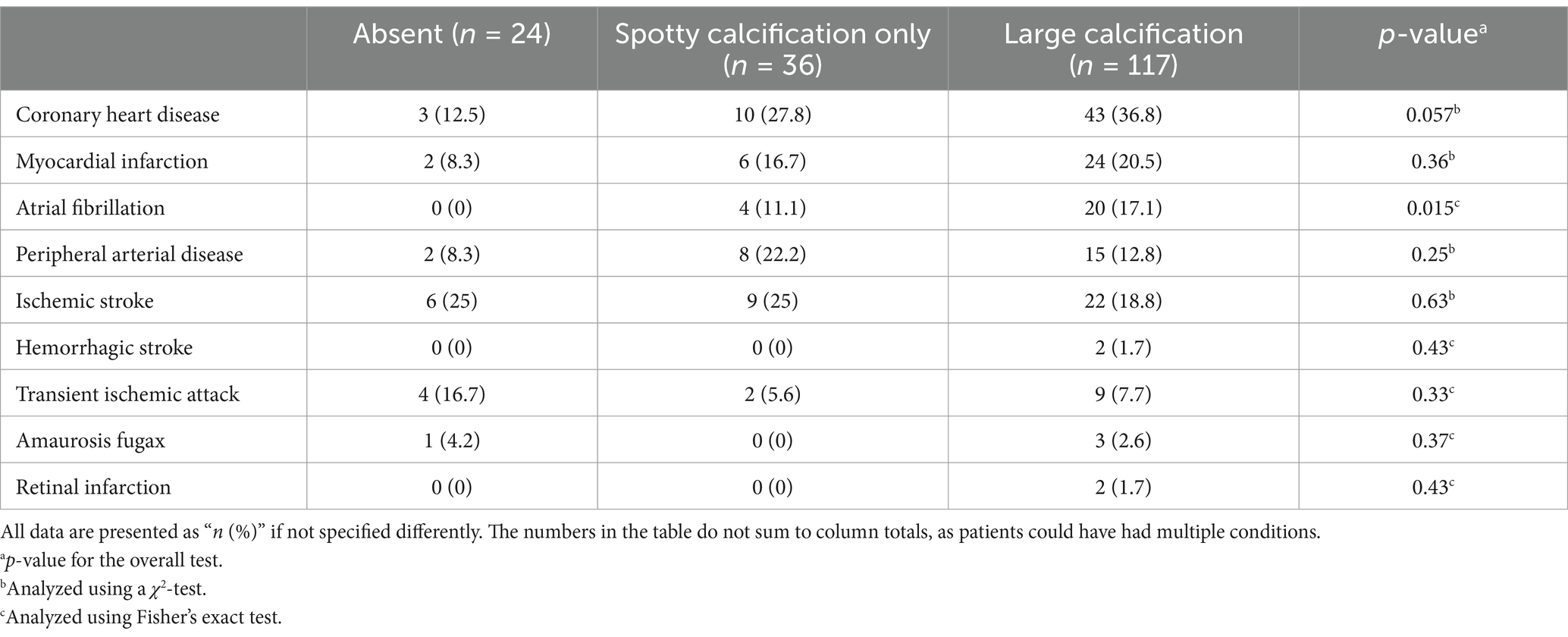
Table 4. Extracranial carotid plaque calcification in association with atherosclerosis-related diseases and cerebrovascular events.
We could not find an association between the presence and type of plaque calcifications and ipsilateral ischemic events (stroke, TIA, amaurosis fugax, or retinal infarction) in our study population. The presence of large carotid plaque calcification represented the highest association with coronary heart disease and atrial fibrillation, followed by higher patient age and female sex. On the other hand, spotty calcifications were associated with male sex, higher levels of smoking, and a greater prevalence in soft plaques.
While some evidence suggested a positive relationship between extracranial carotid calcification and ipsilateral ischemic events (10–12), particularly for spotty calcifications (23, 24), our study results are in agreement with the two recent comprehensive meta-analyses that identified negative association between carotid calcification and stroke (risk ratio: 0.75, OR: 0.5) (7, 9). In the interventional treatment, a large recent study found that a greater severity of carotid calcification (>50% of the plaque volume) is a significant risk factor for in-hospital stroke or death in 21,860 patients undergoing carotid artery stenting (25). Another study differentiated two calcium salts using dispersive X-ray microanalysis (hydroxyapatite, presented more in unstable plaque, and calcium oxalate, associated with plaque stability), suggesting different implications on plaque biology and subsequent stability (26). The distinction between these two types of calcium salts could have important clinical implications and can be investigated using dual-energy CT scanners to identify differences in tissue chemical composition (27, 28). Large calcifications relate with a gene transcriptional profile typical for stable plaques, repressed inflammation, and extracellular matrix organization (29). However, the association between spotty calcification and inflammatory markers, plaque instability, and accelerated disease progression should be noted (30). Finally, macrophages crucially control the mineralization process from microcalcification to bone-like tissue but are having accelerative and decelerative association with calcification. The bilateral interaction remains rather unexplored and should be studied (31).
Our study results proved the association between large calcification and generalized atherosclerosis manifested in a strong relationship with coronary heart disease, atrial fibrillation, and the severity of contralateral carotid stenosis. Two large population-based studies found the same results regarding the presence and extent of calcification and the risk of coronary heart disease (32, 33). However, a large meta-analysis revealed less prevalent carotid calcification in non-significant compared with significant coronary artery disease and moderate relation between carotid and coronary stenosis (34). Atherosclerosis affects both carotid and coronary systems, although not always in an identical phenotypic manner, so examination of carotid arteries is beneficial whenever coronary artery disease is suspected, mainly when large carotid calcification is detected. Despite the findings that patients with carotid atherosclerosis are at high risk of developing atrial fibrillation or both diseases coexist (35–37), no evidence of an association between carotid calcification and atrial fibrillation was found, which has been investigated in our study. Our positive risk association between large carotid calcification and atrial fibrillation was found only in a recent study but significantly after adjustments only in coronary plaques (38). The higher degree of contralateral carotid stenosis associated with carotid calcification demonstrated the presence of generalized atherosclerosis. However, possible overestimation of stenosis severity on CTA due to blooming artifacts from large carotid calcification should be considered (39).
Active smoking or exposure to cigarette smoke is responsible as a catalyst for the formation and development of unstable plaques (40). In particular, carotid calcification is promoted by nicotine (41), but no study was found with evidence of the influence of smoking on spotty calcification. In our study, only spotty calcifications were more often in smokers in greater quantities. The coexistence of spotty carotid calcifications and soft plaque characteristics (LRNC and IPH) (42, 43), typically associated with ipsilateral cerebrovascular events, is suggested in studies even in non-stenosing plaques (24). We found only an association between spotty calcification and lipid plaque but not with IPH or ischemic events. Although spotty calcifications might be at risk of stroke, meta-analyses confirmed that other carotid plaque characteristics are more associated with stroke (44, 45). Male sex was associated with carotid calcification compared to women (46), particularly when looking only at spotty calcification, similar to our study results (47). Calcification growth is mainly associated with increasing age, calcification load, hypertension, or smoking over time (48).
Additionally, extracranial calcification was associated with diabetes mellitus, hypertension (49), or hyperlipidemia (50) in previous studies, but we did not find any difference between them and spotty and large calcification in our study. Regarding the treatment of carotid calcification, high-density lipoprotein appears to benefit vascular calcification (51). Beneficial changes in serum calcification markers were found after ipsilateral carotid artery stenting with intensive lipid-lowering therapy to enhance contralateral carotid plaque stability in patients with bilateral carotid stenosis (52). However, no preferred treatment for extracranial carotid calcification is recommended by current guidelines. To our knowledge, this is the first study that complexly investigated the type of CT-derived extracranial carotid calcification associated with multiple atherosclerotic-related systematic diseases. Large or spotty plaque calcifications were not associated with cerebrovascular events, suggesting an association with plaque stability with no need for acute treatment. However, larger prospective studies and future efforts are warranted to study the effect of, particularly, carotid spotty calcifications on stroke risk.
This study has the following limitations. (1) Approximately 90% of all patients enrolled in the ANTIQUE study were excluded from our analysis due to stenosis degree >30% or mostly because of missing CT and MRI examination together because ultrasound was the first-line imaging modality accompanied by CT if needed or before invasive intervention (MRI underwent only a minority of patients). (2) Laboratory markers were not measured, as our primary focus was on the imaging-based presence of calcification and its relation with various atherosclerosis and stroke risk factors and other diseases. (3) Various CT and MRI devices were utilized due to the multicenter study design, which could introduce minor discrepancies in evaluating calcification and other plaque characteristics. Diagnostic modalities were calibrated using five plaques in vitro to minimize this variation.
Although the presence and type of extracranial carotid plaque calcification were not related to ipsilateral ischemic events, large calcification was strongly associated with coronary heart disease and atrial fibrillation. Higher levels of smoking was responsible for the presence of spotty calcification associated with male sex and the occurrence of soft plaques.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study involving humans was reviewed and approved by the Ethics Committee of the University Hospital Ostrava (July 31, 2014, approval no. 605/2014) and performed according to the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. All patients gave written informed consent to participate in the study.
DP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. DŠa: Conceptualization, Data curation, Methodology, Writing – review & editing, Formal analysis, Visualization, Writing – original draft. DŠk: Conceptualization, Data curation, Methodology, Writing – review & editing, Funding acquisition, Project administration, Supervision.
David Netuka, Jiří Vrána, František Charvát (Military University Hospital Prague, Prague), Petra Kešnerová (University Hospital Motol, Prague); Tomáš Hrbáč, Tomáš Jonszta (University of Ostrava and University Hospital Ostrava, Ostrava); Roman Herzig (Charles University and University Hospital Hradec Králové, Hradec Králové).
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Ministry of Health of the Czech Republic (Grant Nos. NV-19-04-00270, NV-19-08-00362, and NU22-09-00389).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1532883/full#supplementary-material
1. Song, P, Fang, Z, Wang, H, Cai, Y, Rahimi, K, Zhu, Y, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. (2020) 8:e721–9. doi: 10.1016/S2214-109X(20)30117-0
2. Hart, RG, Diener, HC, Coutts, SB, Easton, JD, Granger, CB, O’Donnell, MJ, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. (2014) 13:429–38. doi: 10.1016/S1474-4422(13)70310-7
3. Martin, SS, Aday, AW, Almarzooq, ZI, Anderson, CAM, Arora, P, Avery, CL, et al. 2024 heart disease and stroke statistics: a report of US and global data from the American Heart Association. Circulation. (2024) 149:e347–913. doi: 10.1161/CIR.0000000000001209
4. Tesauro, M, Mauriello, A, Rovella, V, Annicchiarico-Petruzzelli, M, Cardillo, C, Melino, G, et al. Arterial ageing: from endothelial dysfunction to vascular calcification. J Intern Med. (2017) 281:471–82. doi: 10.1111/joim.12605
5. Saba, L, Loewe, C, Weikert, T, Williams, MC, Galea, N, Budde, RPJ, et al. State-of-the-art CT and MR imaging and assessment of atherosclerotic carotid artery disease: standardization of scanning protocols and measurements-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur Radiol. (2023) 33:1063–87. doi: 10.1007/s00330-022-09024-7
6. Ahmed, M, McPherson, R, Abruzzo, A, Thomas, SE, and Gorantla, VR. Carotid artery calcification: what we know so far. Cureus. (2021) 13:e18938. doi: 10.7759/cureus.18938
7. Baradaran, H, Al-Dasuqi, K, Knight-Greenfield, A, Giambrone, A, Delgado, D, Ebani, EJ, et al. Association between carotid plaque features on CTA and cerebrovascular ischemia: a systematic review and meta-analysis. Am J Neuroradiol. (2017) 38:2321–6. doi: 10.3174/ajnr.A5436
8. Nandalur, KR, Hardie, AD, Raghavan, P, Schipper, MJ, Baskurt, E, and Kramer, CM. Composition of the stable carotid plaque: insights from a multidetector computed tomography study of plaque volume. Stroke. (2007) 38:935–40. doi: 10.1161/01.STR.0000257995.74834.92
9. Homssi, M, Saha, A, Delgado, D, RoyChoudhury, A, Thomas, C, Lin, M, et al. Extracranial carotid plaque calcification and cerebrovascular ischemia: a systematic review and meta-analysis. Stroke. (2023) 54:2621–8. doi: 10.1161/STROKEAHA.123.042807
10. Nandalur, KR, Baskurt, E, Hagspiel, KD, Finch, M, Phillips, CD, Bollampally, SR, et al. Carotid artery calcification on CT may independently predict stroke risk. Am J Roentgenol. (2006) 186:547–52. doi: 10.2214/AJR.04.1216
11. Elias-Smale, SE, Odink, AE, Wieberdink, RG, Finch, M, Phillips, CD, Bollampally, SR, et al. Carotid, aortic arch and coronary calcification are related to history of stroke: the Rotterdam study. Atherosclerosis. (2010) 212:656–60. doi: 10.1016/j.atherosclerosis.2010.06.037
12. Kan, Y, He, W, Ning, B, Li, H, Wei, S, and Yu, T. The correlation between calcification in carotid plaque and stroke: calcification may be a risk factor for stroke. Int J Clin Exp Pathol. (2019) 12:750–8.
13. Agacayak, KS, Guler, R, and Sezgin Karatas, P. Relation between the incidence of carotid artery calcification and systemic diseases. Clin Interv Aging. (2020) 15:821–6. doi: 10.2147/CIA.S256588
14. Školoudík, D, Kešnerová, P, Hrbáč, T, Netuka, D, Vomáčka, J, Langová, K, et al. Visual and digital analysis of the ultrasound image in a stable and progressive carotid atherosclerotic plaque. Cesk Slov Neurol N. (2021) 84/117:38–44. doi: 10.48095/cccsnn202138
15. Školoudík, D, Kešnerová, P, Hrbáč, T, Netuka, D, Vomáčka, J, Langová, K, et al. Risk factors for carotid plaque progression after optimising the risk factor treatment: substudy results of the Atherosclerotic Plaque Characteristics Associated with a Progression Rate of the Plaque and a Risk of Stroke in Patients with the carotid Bifurcation Plaque Study (ANTIQUE). Stroke Vasc Neurol. (2022) 7:132–9. doi: 10.1136/svn-2021-001068
16. von Reutern, GM, and von Büdingen, HJ. Ultrasound diagnosis of cerebrovascular disease: Doppler sonography of the extra- and intracranial arteries duplex scanning. 2nd ed. Stuttgart: Thieme (1993).
17. Sacco, RL, Kasner, SE, Broderick, JP, Caplan, LR, Connors, JJ, Culebras, A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
18. HJM, B, Taylor, DW, Haynes, RB, Sackett, DL, Peerless, SJ, Ferguson, GG, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. (1991) 325:445–53. doi: 10.1056/NEJM199108153250701
19. de Weert, TT, Ouhlous, M, Meijering, E, Zondervan, PE, Hendriks, JM, van Sambeek, MR, et al. In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arterioscler Thromb Vasc Biol. (2006) 26:2366–72. doi: 10.1161/01.ATV.0000240518.90124.57
20. Motoyama, S, Sarai, M, Harigaya, H, Anno, H, Inoue, K, Hara, T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. (2009) 54:49–57. doi: 10.1016/j.jacc.2009.02.068
21. Cai, JM, Hatsukami, TS, Ferguson, MS, Small, R, Polissar, NL, and Yuan, C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. (2002) 106:1368–73. doi: 10.1161/01.cir.0000028591.44554.f9
22. Saam, T, Ferguson, MS, Yarnykh, VL, Takaya, N, Xu, D, Polissar, NL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. (2005) 25:234–9. doi: 10.1161/01.ATV.0000149867.61851.31
23. Zhang, F, Yang, L, Gan, L, Fan, Z, Zhou, B, Deng, Z, et al. Spotty calcium on cervicocerebral computed tomography angiography associates with increased risk of ischemic stroke. Stroke. (2019) 50:859–66. doi: 10.1161/STROKEAHA.118.023273
24. Homssi, M, Vora, A, Zhang, C, Baradaran, H, Kamel, H, and Gupta, A. Association between spotty calcification in nonstenosing extracranial carotid artery plaque and ipsilateral ischemic stroke. J Am Heart Assoc. (2023) 12:e028525. doi: 10.1161/JAHA.122.028525
25. Mota, L, Wang, SX, Cronenwett, JL, Nolan, BW, Malas, MB, Schermerhorn, ML, et al. Association of stroke or death with severity of carotid lesion calcification in patients undergoing carotid artery stenting. J Vasc Surg. (2024) 79:305–315.e3. doi: 10.1016/j.jvs.2023.10.046
26. Bischetti, S, Scimeca, M, Bonanno, E, Federici, M, Anemona, L, Menghini, R, et al. Carotid plaque instability is not related to quantity but to elemental composition of calcification. Nutr Metab Cardiovasc Dis. (2017) 27:768–74. doi: 10.1016/j.numecd.2017.05.006
27. Manglaviti, G, Tresoldi, S, Guerrer, CS, Di Leo, G, Montanari, E, Sardanelli, F, et al. In vivo evaluation of the chemical composition of urinary stones using dual-energy CT. AJR Am J Roentgenol. (2011) 197:W76–83. doi: 10.2214/AJR.10.5217
28. Mannelli, L, MacDonald, L, Mancini, M, Ferguson, M, Shuman, WP, Ragucci, M, et al. Dual energy computed tomography quantification of carotid plaques calcification: comparison between monochromatic and polychromatic energies with pathology correlation. Eur Radiol. (2015) 25:1238–46. doi: 10.1007/s00330-014-3523-0
29. Karlöf, E, Seime, T, Dias, N, Lengquist, M, Witasp, A, Almqvvist, H, et al. Correlation of computed tomography with carotid plaque transcriptomes associates calcification with lesion-stabilization. Atherosclerosis. (2019) 288:175–85. doi: 10.1016/j.atherosclerosis.2019.05.005
30. Joshi, NV, Vesey, AT, Williams, MC, Shah, AS, Calvert, PA, Craighead, FH, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. (2014) 383:705–13. doi: 10.1016/S0140-6736(13)61754-7
31. Waring, OJ, Skenteris, NT, Biessen, EAL, and Donners, MMPC. Two-faced Janus: the dual role of macrophages in atherosclerotic calcification. Cardiovasc Res. (2022) 118:2768–77. doi: 10.1093/cvr/cvab301
32. Mehta, A, Rigdon, J, Tattersall, MC, German, CA, Barringer, TA 3rd, Joshi, PH, et al. Association of carotid artery plaque with cardiovascular events and incident coronary artery calcium in individuals with absent coronary calcification: the MESA. Circ Cardiovasc Imaging. (2021) 14:e011701. doi: 10.1161/CIRCIMAGING.120.011701
33. Gepner, AD, Young, R, Delaney, JA, Budoff, MJ, Polak, JF, Blaha, MJ, et al. Comparison of carotid plaque score and coronary artery calcium score for predicting cardiovascular disease events: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. (2017) 6:e005179. doi: 10.1161/JAHA.116.005179
34. Bytyçi, I, Shenouda, R, Wester, P, and Henein, MY. Carotid atherosclerosis in predicting coronary artery disease: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. (2021) 41:e224–37. doi: 10.1161/ATVBAHA.120.315747
35. Willeit, K, Pechlaner, R, Egger, G, Weger, S, Oberhollenzer, M, Willeit, J, et al. Carotid atherosclerosis and incident atrial fibrillation. Arterioscler Thromb Vasc Biol. (2013) 33:2660–5. doi: 10.1161/ATVBAHA.113.302272
36. Heeringa, J, van der Kuip, DA, Hofman, A, Kors, JA, van Rooij, FJ, Lip, GY, et al. Subclinical atherosclerosis and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. (2007) 167:382–7. doi: 10.1001/archinte.167.4.382
37. Noubiap, JJ, Agbaedeng, TA, Tochie, JN, Nkeck, JR, Ndoadoumgue, AL, Fitzgerald, JL, et al. Meta-analysis comparing the frequency of carotid artery stenosis in patients with atrial fibrillation and vice versa. Am J Cardiol. (2021) 138:72–9. doi: 10.1016/j.amjcard.2020.10.017
38. Geurts, S, Bos, MM, van der Toorn, JE, Stricker, BHC, Ghanbari, M, Kors, JA, et al. Arteriosclerotic calcification and atrial fibrillation in the general population: the Rotterdam study. Am J Cardiol. (2024) 231:62–9. doi: 10.1016/j.amjcard.2024.09.002
39. Pakizer, D, Vybíralová, A, Jonszta, T, Roubec, M, Král, M, Chovanec, V, et al. Peak systolic velocity ratio for evaluation of internal carotid artery stenosis correlated with plaque morphology: substudy results of the ANTIQUE study. Front Neurol. (2023) 14:1206483. doi: 10.3389/fneur.2023.1206483
40. Csordas, A, and Bernhard, D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. (2013) 10:219–30. doi: 10.1038/nrcardio.2013.8
41. Petsophonsakul, P, Burgmaier, M, Willems, B, Heeneman, S, Stadler, N, Gremse, F, et al. Nicotine promotes vascular calcification via intracellular Ca2+-mediated, Nox5-induced oxidative stress, and extracellular vesicle release in vascular smooth muscle cells. Cardiovasc Res. (2022) 118:2196–210. doi: 10.1093/cvr/cvab244
42. van den Bouwhuijsen, QJ, Bos, D, Ikram, MA, Hofman, A, Krestin, GP, Franco, OH, et al. Coexistence of calcification, intraplaque hemorrhage and lipid Core within the asymptomatic atherosclerotic carotid plaque: the Rotterdam study. Cerebrovasc Dis. (2015) 39:319–24. doi: 10.1159/000381138
43. Kataoka, Y, Puri, R, Hammadah, M, Duggal, B, Uno, K, Kapadia, SR, et al. Spotty calcification and plaque vulnerability in vivo: frequency-domain optical coherence tomography analysis. Cardiovasc Diagn Ther. (2014) 4:460–9. doi: 10.3978/j.issn.2223-3652.2014.11.06
44. Kamtchum-Tatuene, J, Noubiap, JJ, Wilman, AH, Saqqur, M, Shuaib, A, and Jickling, GC. Prevalence of high-risk plaques and risk of stroke in patients with asymptomatic carotid stenosis: a meta-analysis. JAMA Neurol. (2020) 77:1524–35. doi: 10.1001/jamaneurol.2020.2658
45. Zhang, Y, Bai, Y, Xie, J, Wang, J, He, L, Huang, M, et al. Carotid plaque components and other carotid artery features associated with risk of stroke: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2022) 31:106857. doi: 10.1016/j.jstrokecerebrovasdis.2022.106857
46. Plank, F, Beyer, C, Friedrich, G, Wildauer, M, and Feuchtner, G. Sex differences in coronary artery plaque composition detected by coronary computed tomography: quantitative and qualitative analysis. Neth Heart J. (2019) 27:272–80. doi: 10.1007/s12471-019-1234-5
47. Kataoka, Y, Wolski, K, Uno, K, Puri, R, Tuzcu, EM, Nissen, SE, et al. Spotty calcification as a marker of accelerated progression of coronary atherosclerosis: insights from serial intravascular ultrasound. J Am Coll Cardiol. (2012) 59:1592–7. doi: 10.1016/j.jacc.2012.03.012
48. van Gils, MJ, Bodde, MC, Cremers, LG, Dippel, DW, and van der Lugt, A. Determinants of calcification growth in atherosclerotic carotid arteries; a serial multi-detector CT angiography study. Atherosclerosis. (2013) 227:95–9. doi: 10.1016/j.atherosclerosis.2012.12.017
49. Gao, X, Song, J, Watase, H, Hippe, DS, Zhao, X, Canton, G, et al. Differences in carotid plaques between symptomatic patients with and without diabetes mellitus. Arterioscler Thromb Vasc Biol. (2019) 39:1234–9. doi: 10.1161/ATVBAHA.118.312092
50. Odink, AE, van der Lugt, A, Hofman, A, Hunink, MG, Breteler, MM, Krestin, GP, et al. Risk factors for coronary, aortic arch and carotid calcification; the Rotterdam study. J Hum Hypertens. (2010) 24:86–92. doi: 10.1038/jhh.2009.42
51. Pletsch-Borba, L, Selwaness, M, van der Lugt, A, Hofman, A, Franco, OH, and Vernooij, MW. Change in carotid plaque components: a 4-year follow-up study with serial MR imaging. JACC Cardiovasc Imaging. (2018) 11:184–92. doi: 10.1016/j.jcmg.2016.12.026
Keywords: atherosclerosis, carotid artery disease, calcification, cerebrovascular disease, magnetic resonance imaging, computed tomography
Citation: Pakizer D, Šalounová D and Školoudík D (2025) Extracranial carotid plaque calcification and its association with risk factors for cerebrovascular events: insights from the ANTIQUE study. Front. Neurol. 16:1532883. doi: 10.3389/fneur.2025.1532883
Received: 22 November 2024; Accepted: 03 January 2025;
Published: 29 January 2025.
Edited by:
Jieqiong Wang, Chinese Academy of Sciences, ChinaReviewed by:
Pui Yeung Lee, Yale University, United StatesCopyright © 2025 Pakizer, Šalounová and Školoudík. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Školoudík, c2tvbG91ZGlrQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.