
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 27 January 2025
Sec. Stroke
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1519818
 Zheng Li1
Zheng Li1 Wen-qi Xu1
Wen-qi Xu1 Jiao-qi Wang1
Jiao-qi Wang1 Jia-hui Yang1
Jia-hui Yang1 Xiao-hua Shi1
Xiao-hua Shi1 Cheng-bing Wang1
Cheng-bing Wang1 Zhong-xin Xu1*†
Zhong-xin Xu1*† Jin-lan Jiang2*†
Jin-lan Jiang2*†Background: This meta-analysis aimed to investigate the effect of statins on the prognosis of patients with intracerebral hemorrhage (ICH).
Methods: We conducted a systematic search using the keywords “statin” and “intracerebral hemorrhage” across four electronic databases (PubMed, Cochrane Library, Web of Science, and Embase) from their inception to October 31, 2023, to identify studies comparing the effects of statins on the prognosis of patients with ICH. The primary outcome was total mortality after ICH. This meta-analysis was registered online (PROSPERO ID: CRD42023493063).
Results: Our initial search identified 5,543 studies. After applying inclusion criteria, 30 studies with a total of 42,298 patients were included in the final analysis. Our meta-analysis showed that statins significantly reduced overall mortality in patients with ICH (OR: 0.61; 95% CI: 0.51–0.73; I2 = 87%; p < 0.01). Subgroup analyses further demonstrated lower mortality in ICH patients treated with statins compared to those not treated, including in the propensity score matching (PSM) group (OR: 0.59; 95% CI: 0.48–0.74; I2 = 90%; p < 0.01), the prospective cohort study (PCS) group (OR: 0.56; 95% CI: 0.40–0.77; I2 = 89%, p < 0.01), and the retrospective cohort study (RCS) group (OR: 0.64; 95% CI: 0.51–0.81; I2 = 87%, p < 0.01).
Conclusion: Our meta-analysis of 30 studies suggests that statin use may be associated with improved mortality and functional outcomes in patients with intracerebral hemorrhage (ICH).
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, CRD42023493063.
Intracerebral hemorrhage (ICH) is associated with high disability (42%) and mortality rates (50%), creating a substantial burden on families and society (1, 2). In clinical practice, patients prior to ICH with ischemic cardiovascular and cerebrovascular diseases frequently use statins as primary and secondary prevention for ischemic stroke (3). However, the lipid-lowering effect of statins could be a double-edged sword (1). While statins reduce the occurrence of endothelial atherosclerosis and possess multiple antioxidative properties, they may also compromise vascular endothelial integrity, potentially increasing the risk of hemorrhage.
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial (NCT00147602), a randomized controlled trial (RCT) involving 4,731 patients and published in 2006 (4), demonstrated that atorvastatin significantly reduced the incidence of stroke and transient ischemic attack (TIA). Conversely, a secondary analysis of SPARCL in 2009 revealed that statins might increase the risk of spontaneous intracerebral hemorrhage (5). This finding prompted many clinicians to reconsider the safety of statin use in patients with a history of ICH. Subsequent research from 2010 to 2015, including 13 observational cohort studies and five meta-analyses (6–10), generally concluded that statins do not worsen the prognosis of cerebral hemorrhage. However, these meta-analyses often lacked sufficient sample sizes and robust subgroup analyses to adequately control for confounding factors. Consequently, it remains uncertain whether patients with ICH and concurrent ischemic cardiovascular disease can safely use statins for ischemic stroke prevention.
Given these uncertainties, it is imperative to incorporate the latest research into a comprehensive meta-analysis to better guide clinicians in developing more rational statin treatment strategies for patients with hemorrhagic stroke.
This meta-analysis is reported in accordance with the Meta-Analysis (PRISMA) Statement and followed the PICO principle (P: Patients with spontaneous intracranial hemorrhage, I: statin treatment; C: non-statin treatment, and O: prognosis of spontaneous intracerebral hemorrhage). In order to improve the quality of our analysis, we formulated a series of strict inclusion and exclusion criteria to ensure that only the most relevant studies were included in our analyses.
Eligible studies were observational cohort studies or randomized controlled trial (RCT) studies that conformed to the following criteria: (1) case–control or cohort studies that investigated the effect of statins on the prognosis of patients with ICH by comparing a statin-treated group and a non-statin-treated group; (2) head computed tomography (CT) or magnetic resonance imaging (MRI) that clearly met the imaging diagnostic criteria for ICH; (3) no significant differences between the statin group and non-statin group in terms of gender, mean age, and medical history; (4) a body of research data that could be extracted; (5) clear outcome indicators; (6) the full text is available and supplementary information could be obtained from the authors to obtain additional materials and data if required, and (7) published in English.
Our exclusion criteria were as follows: (1) the research content was not related to the effect of statins on ICH; (2) a lack of head CT scans or MRI data as diagnostic criteria for ICH; (3) incomplete records of ICH prognosis; (4) patients with traumatic subarachnoid hemorrhage, intracranial aneurysm rupture bleeding, venous thrombolysis related bleeding, bleeding after mechanical thrombectomy, cerebral infarction bleeding transformation; (5) article was a summary, review, case report, lecture, reply to the editor, or Trail registry record; (6) animal experiments, and (7) articles for which the full text was not available, data are not available, no clear outcome measures, and articles for which the authors could not be contacted.
By applying Boolean logic operations, we searched four key online databases (PubMed, Embase, the Cochrane Library and the Web of Science) for ‘intracerebral hemorrhage’ and ‘statin’. This systematic literature search was conducted on the 31st of October 2023 (For detailed information on subject words, free words, and search terms, please refer to the Supplementary material). We restricted our search to articles published in English. Our meta-analysis protocol was registered online (PROSPERO ID: CRD42023493063) and followed a prespecified plan of analysis.
Two investigators (JQ-W and JH-Y) independently screened the titles, abstracts and full texts of the articles identified by our literature search. Irrelevant and duplicate publications were rejected to ensure that the remaining articles met our inclusion criteria. Disagreements were resolved by consensus or by consulting a senior reviewer (XH-S). Next, we extracted specific data from the original research articles, as required. Data extraction was performed independently and in duplicate by two reviewers (CB-W and JL-J) using a predetermined data-extraction table which included the following information: including basic features (author, publication date, the country in which the subjects resided, the number of patients involved in the statin and non-statin groups, trial duration, mean population age, and male proportion), intervention characteristics (statin type, dose, time), study design (study center, propensity score matching (PSM), follow-up times, bias) and outcomes (outcome indicators, primary outcome).
Two of the authors (ZL and WQ-X) independently reviewed the risk of bias in the included observational cohort studies by using the Newcastle-Ottawa Scale (NOS) and in the RCTs by using the updated version of the Cochrane Risk of Bias Tool. Articles with a NOS score > 7 were considered to represent high-quality research studies and were included in our meta-analysis. Disagreements between the reviewers were resolved by discussion and a third reviewer (XZ-X) was consulted to reach a consensus.
Binary classification variables were compared by odds ratios (ORs) and 95% confidence intervals (CIs). The standard I2 test was used to evaluate heterogeneity between the studies; an I2 > 50% indicated that a random effect model (RM) should be applied, while an I2 < 50% indicated that the fixed effect model (FM) should be applied. Following the generation of forest and funnel maps, research studies with higher levels of heterogeneity were eliminated and the data were re-analyzed. All calculations were performed using statistical software provided by the Cochrane Collaboration (RevMan 5.3) and R 4.3.2.
The primary outcome of our meta-analysis was total mortality after ICH, and the secondary outcome was functional prognosis. In order to improve the consistency of data between groups, we grouped patients with different study conditions for analysis. Our subgroup analysis included mortality at different times after the occurrence of ICH [period of hospitalization: 30 days, 90 days, and long term (0.5–1 year)], and a better functional prognosis (a modified Rankin Scale [mRS] score of 0–3) at different times after the occurrence of ICH (at discharge, 90 days, and long term). In addition, observational cohort studies were divided into several subgroups according to their characteristics: design (prospective cohort study, retrospective cohort study) and propensity score matching.
Initially, our literature search identified 5,543 reports; however, 1,049 of these articles were removed due to duplication. After screening the titles and abstracts, we removed a further 4,112 reports that were irrelevant to our research focus, and 301 reviews. After screening the full text of the remaining 81 articles, 50 articles were excluded as they were unavailable. Supplementary Table S1 lists the specific reasons for exclusion. It was not possible to extract key data from Wubshet et al. (11) due to the lack of detailed information relating to the number of deaths from ICH after taking statins. Finally, 30 eligible studies (5, 10, 12–39) were included in our final meta-analysis. Figure 1 depicts the process used to retrieve data. Our analysis included 42,298 people, including 13 articles (10, 14, 15, 22, 26–28, 31, 32, 36, 38, 39) from the Americas, five articles (12, 17, 23, 29, 34) from Europe, eight articles (13, 16, 19–21, 24, 25, 30) from Asia, two articles (35, 37) from the Middle East, and two international articles (5, 18). In addition, we included eight studies (13, 15, 16, 19–22, 24) that contained propensity score matching and detailed information relating to other key experimental characteristics, such as population. Design and outcome indicators are shown in Supplementary Table S2. With regards to the quality of the 30 research articles included in this meta-analysis, 28 observational cohort studies were analyzed by the NOS rating scale (7–9 points), and two RCT studies (5, 14) were analyzed by the Cochrane risk assessment tool. All of the studies included in our analysis were high-quality studies with a low risk of bias.
All 30 of the studies included in our analysis included total mortality as the primary outcome measure. There was a significant reduction in the total mortality of patients with ICH who received statins compared to those who did not (OR: 0.61; 95% CI: 0.51–0.73; I2 = 87%; p < 0.01; Figure 2). Subgroup analysis at other independent time points revealed that compared with patients with ICH who did not receive statins, those who did receive statins during hospitalization showed a reduction in mortality (OR: 0.63, 95%CI: 0.46–0.88; I2 = 74%; p < 0.01; Supplementary Figure S3). When compared with patients with ICH who did not receive statins, those who did received statins 30 days after ICH showed a reduction in mortality (OR: 0.52; 95% CI: 0.29–0.92; I2 = 93%; p = 0.03; Supplementary Figure S4). When evaluated 90 days after ICH, patients who received statins showed a reduction in mortality when compared to those who did not (OR: 0.62; 95% CI: 0.47–0.82; I2 = 70%; p < 0.01; Supplementary Figure S5). The long-term administration of statins after ICH reduced the mortality of ICH patients when compared to non-administration (OR: 0.54; 95% CI: 0.33–0.91; I2 = 91%; p = 0.02; Supplementary Figure S6).

Figure 2. Forest plot of total mortality between statin and non-statin group. OR, odds ratio; CI, confidence interval.
Our subgroup analysis also included experimental quality and design. Compared with patients with ICH who did not receive statins, the propensity score matching group showed a significant reduction in mortality (OR: 0.59; 95% CI: 0.48–0.74; I2 = 90%; p < 0.01; Supplementary Figure S7). Patients with ICH in the prospective cohort study group who received statins showed a significant reduction in mortality when compared with those who did not receive statins (OR: 0.56; 95% CI: 0.40–0.77; I2 = 89%; p < 0.01; Supplementary Figure S8). Furthermore, patients with ICH in the retrospective cohort study group who received statins showed a reduction in mortality when compared to those who did not receive statins (OR: 0.64; 95% CI: 0.51–0.81; I2 = 87%; p < 0.01; Supplementary Figure S9).
Ten of the studies (5, 14, 18, 22, 25, 29, 31, 34, 35, 37) selected for analysis included secondary outcome indicators, including a good functional prognosis. Our analysis revealed that the administration of statins at different timepoints improved the prognosis of patients with ICH. There was no significant difference between the statin and no-statin groups in terms of achieving a better functional prognosis at discharge (OR: 1.78; 95% CI: 1.39–2.29; I2 = 23%; p < 0.01; Figure 3). Ninety days of statin therapy after ICH was associated with better functional outcomes than no statin therapy (OR: 1.71; 95% CI: 1.16–2.51; I2 = 84%; p = 0.01; Table 1). Long-term analysis (6 months to 1 year) showed that those who received statin therapy achieved better functional outcomes than those who did not receive statin therapy (OR: 1.26; 95% CI: 0.43–3.74; I2 = 96%; p = 0.71; Table 1).
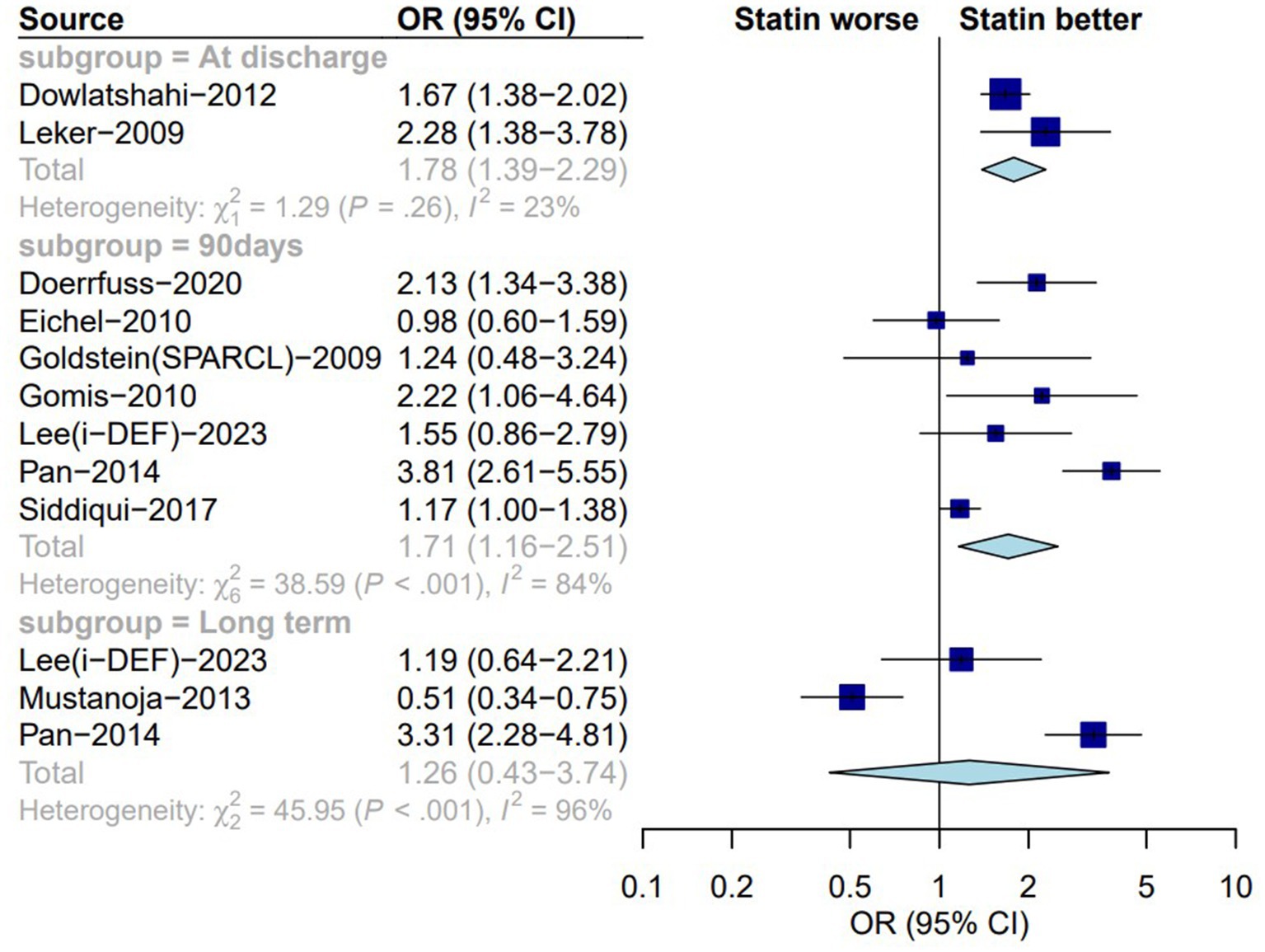
Figure 3. Forest plot of functional prognosis between statin group and non-statin group. OR, odds ratio; CI, confidence interval.
In order to verify the stability of the meta-analysis results, we reanalyzed the results using the “leave one out” method of R software. We conducted sensitivity analyses for the total mortality associated with the use of statins by excluding one study from our analysis at a time followed by re-evaluation. One study (13) excluded from our analysis that had the greatest impact on the OR for statin on total mortality. Analysis of the remaining studies showed that the exclusion of this particular study did not affect the outcome of statin use on total mortality (OR: 0.63; 95% CI: 0.52–0.75; I2 = 84%; p < 0·01; Supplementary Figure S19). In addition, the study of other groups did not have a significant impact on the overall results (Supplementary Figures S10–S27).
To further explore the potential sources of heterogeneity, we conducted subgroup analyses on the effect of statin use on overall mortality in patients with intracerebral hemorrhage. The subgroups included: age (>65 years vs. ≤65 years), study center (single-center vs. multi-center), study design (prospective cohort study [PCS] vs. retrospective cohort study [RCS]), propensity score matching (PSM: Yes vs. No), and publication year (before 2015 vs. after 2015). However, the results demonstrated that the heterogeneity remained substantial across all subgroup analyses, indicating that these factors did not significantly contribute to the observed heterogeneity (Supplementary Figures S28–S32).
Compared to previous publications, this meta-analysis has a number of advantages, including a sufficient sample size, the analysis of subgroups by propensity score matching (PSM), and a more efficient means of controlling for confounding factors. Over the past decade, a large amount of data have been collated by multiple centers; this has allowed prospective cohort studies to include more comprehensive designs and allowed for the publication of many randomized controlled trials (RCTs). Since 2019, nine high-quality studies have been published, accounting for one-third of the total number of articles included in the present analysis. Furthermore, some of the existing meta-analyses relating to the impact of statins on the prognosis of ICH included subgroup analyzes at different timepoints after the onset of ICH. But these existing studies relating to the prognosis of patients with ICH, including total mortality and functional prognosis, did not consider design and PSM as indicators for subgroup analysis. Therefore, in the current meta-analysis, we aimed to investigate whether statins can exert adverse effects on the prognosis of patients with ICH by performing objective and systematic analyses.
In the present study, we comprehensively searched several online databases and identified relevant literature by formulating strict inclusion and exclusion criteria. Finally, we screened 30 high-quality publications involving 42,298 patients, including 16,482 cases in the statin group and 25,816 cases in the non-statin group. We extracted key data from the literature and performed statistical analysis with Revman version 5.3 software and R version 4.3.2.
The results of our meta-analysis showed that statin treatment significantly reduced the total mortality of patients after ICH (OR: 0.61; 95% confidence interval: 0.51–0.73; p < 0.01); these findings are consistent those reported by previous meta-analyses (8, 10). In addition, we performed subgroup analysis to reduce the influence of confounding factors. Our outcomes showed that statins reduced the mortality of patients with ICH after different time periods (after 30 days, 90 days and long-term hospitalization), thus indicating that statins play a protective role from the onset of ICH to a long-term prognosis. In order to exclude the influence of confounding factors, our subgroup analysis included a PSM group (OR: 0.59; 95% CI: 0.48–0.74; p < 0.01). Our analysis showed that statins still played a protective role after the exclusion of confounding factors, such as age, hypertension, and diabetes. In order to avoid the impact of different research designs on our results, we performed subgroup analysis according to different trial designs. Analysis showed that PCS and RCS both reduced mortality after ICH.
In this study, we selected a good functional prognosis at each stage after ICH as the evaluation index, and good functional recovery was defined as the patient being able to walk independently (an mRS of 0–3 points). Our meta-analysis results showed that statins can reduce the degree of disability at different periods (at discharge and 90 days). However, there was low certainty evidence indicating that the use of statins could achieve better a prognosis over the long-term.
Historically, researchers have considered that the adverse effects of statins on patients with ICH may arise from two main aspects. Firstly, statins reduce the plasma levels of cholesterol and increase the permeability of the blood–brain barrier. Epidemiological studies have reported that hypocholesterolemia can increase the incidence and mortality of hemorrhagic stroke and that adequate cholesterol levels are necessary to maintain the integrity of the cerebral vessels (40). A previous cohort study by Xie et al. (41) confirmed that hypolipidemia (low-density lipoprotein cholesterol (LDL-C) < 70 mg/dL) represents an independent high-risk factor for severe hemorrhagic stroke (42). Secondly, statins inhibit the coagulation cascade by exerting anti-platelet and fibrinolytic effects and by influencing coagulation factors, thus causing expansion of a cerebral hematoma. However, the pleiotropic effects of statins (43), such as anti-inflammation, increased cerebral blood flow, resistance against oxidative stress, reduced cerebral edema, and other protective effects on the nervous system, cannot be ignored. Our meta-analysis showed that statins improve the prognosis of patients with ICH at different time points. Several factors may explain why statins can exert protective effects on the nervous system at different times after ICH. For example, during the acute phase of cerebral hemorrhage (in-hospital), statins can reduce perihematomal edema (PHE), resist oxidative stress and inflammation, and reduce extensive neuronal damage during the recovery period (30–90 days after onset). During this recovery period, statins improve cerebral blood flow, increase cerebral perfusion, and correct the central nervous system. In the long-term (6 months to 1 year) after the onset of ICH, statins promote vascular endothelial growth, nerve regeneration, stabilize the blood–brain barrier, and prevent the recurrence of stroke.
The Heart Protection Study (HPS) (44) and SPARCL studies (4) confirmed that statins can significantly reduce the incidence of ischemic cardiovascular and cerebrovascular diseases. The main findings of the SPACL study showed that statins significantly reduced the incidence of ischemic strokes and transient ischemic attacks and conducted secondary analyzes based on first outcome hemorrhagic events. The initial trial reported that statins increased the risk of cerebral hemorrhage, although the SPARCL secondary analysis (5) (n = 43; including 29 patients taking atorvastatin and 14 patients on placebo) was obviously limited by a small sample size and low statistical power. Furthermore, the SPARCL trial used a high dose of atorvastatin dose (80 mg/d); high doses of statins can significant reductions in blood lipids. However, an excessive reduction of blood lipid levels can increase the risk of cerebral hemorrhage and may also cause muscle and liver damage. For secondary prevention, the most commonly used dose of atorvastatin to control blood lipid levels in stroke patients is small to medium (20–40 mg/d). With the exception of the SPACRCL study, no previous trial has produced convincing evidence to confirm that statins worsen the prognosis of patients with ICH. Although statins have a double-edged sword effect of anti-atherosclerosis and increased bleeding risk, more attention should be paid to the overall condition of each patient. For example, if an ICH patient also suffers from hyperlipidemia, coronary heart disease, ischemic stroke, etc., it is recommended to actively give statins for treatment.
Our study has several strengths: it includes a large sample size with 30 studies, one-third of which were published in the past 5 years. Additionally, it features more refined subgroup analyses and a comprehensive sensitivity analysis. Although our present analysis included 14 new studies and conducted a more comprehensive subgroup analysis when compared with the previous meta-analysis reported by Jung et al. (8), we were still unable to acquire sufficient data relating to the types and dosages of statins. Furthermore, we were unable to analyze additional factors such as the dissolution characteristics and dosage of statins with regards to their impact on the prognosis of patients with ICH. Not all subjects adhered to their randomized treatment and we were unable to consider socioeconomic status, immediate poststroke complications, detailed cognitive function, family support, insurance status, and length of stay in hospital. Rehabilitation data cannot control the influence of different factors on experimental design. In general, there is currently a lack of large-scale RCTs relating to the clinical application of statins for patients with cerebral hemorrhage to fully identify the impact of statins on the prognosis of cerebral hemorrhage. The SATURN trial (NCT03936361) is a 6-year RCT study involving 1,456 patients with intracerebral hemorrhage. Data derived from this trial will further reveal the impact of continuous or discontinuous statin administration on the prognosis of ICH.
Our meta-analysis suggests that statin use may be associated with improved mortality and functional outcomes in patients with intracerebral hemorrhage (ICH). However, due to the observational nature of the included studies and the lack of randomized controlled trial (RCT) data, the evidence remains insufficient to establish a definitive causal relationship. Further studies, including RCTs, are needed to better understand the effects of different statin types, dosages, and durations on ICH outcomes. Additionally, more comprehensive data, including age and sex stratification, are required to refine clinical recommendations and fully assess the potential benefits of statins in this patient population.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
ZL: Writing – original draft, Writing – review & editing. W-qX: Writing – original draft. J-qW: Writing – original draft. J-hY: Writing – original draft. X-hS: Writing – original draft. C-bW: Writing – original draft. Z-xX: Writing – review & editing. J-lJ: Writing – review & editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by The National Natural Science Foundation of China (Grant No. 82172230), Life Spring AKY Pharmaceuticals (Grant No. 3R218FM83430), The Jilin Scientific and Technological Development Program (Grant No. 20240205001YY). The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1519818/full#supplementary-material
1. Lee, TC, Leung, WC, Ho, C, Chiu, MW, Leung, IY, Wong, YK, et al. Association of LDL-cholesterol <1.8 mmol/l and statin use with the recurrence of intracerebral hemorrhage. Int J Stroke. (2024) 19:695–704. doi: 10.1177/17474930241239523
2. Morris, NA, Simard, JM, and Chaturvedi, S. Surgical management for primary intracerebral hemorrhage. Neurology. (2024) 103:e209714. doi: 10.1212/WNL.0000000000209714
3. Mugawar, B, McErlean, S, O'Connor, P, and Kennedy, C. Statin intolerance and the drucebo effect. QJM. Int. J. Med. (2024) 1–3. doi: 10.1093/qjmed/hcae144
4. Amarenco, P, Bogousslavsky, J, Callahan, A 3rd, Goldstein, LB, Hennerici, M, Rudolph, AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. (2006) 355:549–59. doi: 10.1056/NEJMoa061894
5. Goldstein, LB, Amarenco, P, Zivin, J, Messig, M, Altafullah, I, Callahan, A, et al. Statin treatment and stroke outcome in the stroke prevention by aggressive reduction in cholesterol levels (sparcl) trial. Stroke. (2009) 40:3526–31. doi: 10.1161/STROKEAHA.109.557330
6. Pandit, AK, Kumar, P, Kumar, A, Chakravarty, K, Misra, S, and Prasad, K. High-dose statin therapy and risk of intracerebral hemorrhage: a meta-analysis. Acta Neurol Scand. (2016) 134:22–8. doi: 10.1111/ane.12540
7. Tapia Pérez, JH, Yildiz, OC, Schneider, T, and Nimsky, C. Meta-analysis of statin use for the acute therapy of spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis. (2015) 24:2521–6. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.036
8. Jung, JM, Choi, JY, Kim, HJ, and Seo, WK. Statin use in spontaneous intracerebral hemorrhage: a systematic review and meta-analysis. Int J Stroke. (2015) 10 Suppl A100:10–7. doi: 10.1111/ijs.12624
9. Lei, C, Wu, B, Liu, M, and Chen, Y. Association between statin use and intracerebral hemorrhage: a systematic review and meta-analysis. Eur J Neurol. (2014) 21:192–8. doi: 10.1111/ene.12273
10. Biffi, A, Devan, WJ, Anderson, CD, Ayres, AM, Schwab, K, Cortellini, L, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Stroke. (2011) 42:1314–9. doi: 10.1161/STROKEAHA.110.605923
11. Wubshet, A, Fanta, K, Gemachu, TD, Birhanu, A, and Gudina, EK. Clinical characteristics and short-term outcomes of adult stroke patients admitted to Jimma medical center, Ethiopia: a prospective cohort study. Pan Afr Med J. (2023) 44:49. doi: 10.11604/pamj.2023.44.49.37588
12. Zaryczańska, K, Pawlukowska, W, Nowacki, P, Zwarzany, Ł, Bagińska, E, Kot, M, et al. Statins and 90-day functional performance and survival in patients with spontaneous intracerebral hemorrhage. J Clin Med. (2023) 12:12. doi: 10.3390/jcm12206608
13. Yang, R, Wu, J, Yu, H, Wang, S, Chen, H, Wang, M, et al. Effect of statin therapy patterns on readmission and mortality in patients with intracerebral hemorrhage. J Thromb Thrombolysis. (2023) 57:132–42. doi: 10.1007/s11239-023-02870-2
14. Lee, KH, Carvalho, F, Lioutas, V-A, Heistand, E, Das, AS, Marchina, S, et al. Relationship between prior statin therapy and radiological features and clinical outcomes of intracerebral hemorrhage. J Stroke Cerebrovasc Dis. (2023) 32:107378–8. doi: 10.1016/j.jstrokecerebrovasdis.2023.107378
15. Yuan, M, Zhou, X, Lu, X, Xiao, Z, Zhou, H, and Wang, X. Association between statin use during hospitalisation and mortality in patients with intracerebral haemorrhage: a propensity score-matched cohort study. BMJ Open. (2022) 12:e065849. doi: 10.1136/bmjopen-2022-065849
16. Li, G, Wang, S, Xiong, Y, Gu, H, Yang, K, Yang, X, et al. Prior statin and short-term outcomes of primary intracerebral hemorrhage: from a large-scale nationwide longitudinal registry. CNS Neurosci Ther. (2022) 28:1240–8. doi: 10.1111/cns.13868
17. Silva Marques, J, Ennis, G, Venade, G, João Soares, R, Monteiro, N, and Gomes, A. Association of statins with functional outcome and 30-day mortality in patients with intracerebral hemorrhage. Cureus. (2021) 13:e14421. doi: 10.7759/cureus.14421
18. Doerrfuss, JI, Abdul-Rahim, AH, Siegerink, B, Nolte, CH, Lees, KR, Endres, M, et al. Early in-hospital exposure to statins and outcome after intracerebral haemorrhage - results from the virtual international stroke trials archive. Eur Stroke J. (2020) 5:85–93. doi: 10.1177/2396987319889258
19. Lin, MS, Lin, YS, Chang, ST, Wang, PC, Chien-Chia, WV, Lin, WY, et al. Effect of initiating statin therapy on long-term outcomes of patients with dyslipidemia after intracerebral hemorrhage. Atherosclerosis. (2019) 288:137–45. doi: 10.1016/j.atherosclerosis.2019.07.009
20. Jung, M, and Lee, S. Effects of statin therapy on the risk of intracerebral hemorrhage in korean patients with hyperlipidemia. Pharmacotherapy. (2019) 39:129–39. doi: 10.1002/phar.2211
21. Chung, CM, Lin, MS, Liu, CH, Lee, TH, Chang, ST, Yang, TY, et al. Discontinuing or continuing statin following intracerebral hemorrhage from the view of a national cohort study. Atherosclerosis. (2018) 278:15–22. doi: 10.1016/j.atherosclerosis.2018.08.049
22. Siddiqui, FM, Langefeld, CD, Moomaw, CJ, Comeau, ME, Sekar, P, Rosand, J, et al. Use of statins and outcomes in intracerebral hemorrhage patients. Stroke. (2017) 48:2098–104. doi: 10.1161/STROKEAHA.117.017358
23. Tapia-Perez, JH, Zilke, R, and Schneider, T. Match-study of statin therapy in spontaneous intracerebral hemorrhage: is the discontinuation reasonable? J Neurosurg Sci. (2016) 60:301–12.
24. Chen, PS, Cheng, CL, Chang, YC, Kao Yang, YH, Yeh, PS, and Li, YH. Early statin therapy in patients with acute intracerebral hemorrhage without prior statin use. Eur J Neurol. (2015) 22:773–80. doi: 10.1111/ene.12649
25. Pan, YS, Jing, J, Wang, YL, Zhao, XQ, Song, B, Wang, WJ, et al. Use of statin during hospitalization improves the outcome after intracerebral hemorrhage. CNS Neurosci Ther. (2014) 20:548–55. doi: 10.1111/cns.12274
26. Flint, AC, Conell, C, Rao, VA, Klingman, JG, Sidney, S, Johnston, SC, et al. Effect of statin use during hospitalization for intracerebral hemorrhage on mortality and discharge disposition. JAMA Neurol. (2014) 71:1364–71. doi: 10.1001/jamaneurol.2014.2124
27. Winkler, J, Shoup, JP, Czap, A, Staff, I, Fortunato, G, McCullough, LD, et al. Long-term improvement in outcome after intracerebral hemorrhage in patients treated with statins. J Stroke Cerebrovasc Dis. (2013) 22:e541–5. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.015
28. Tapia-Pérez, JH, Rupa, R, Zilke, R, Gehring, S, Voellger, B, and Schneider, T. Continued statin therapy could improve the outcome after spontaneous intracerebral hemorrhage. Neurosurg Rev. (2013) 36:279–87; discussion 287. doi: 10.1007/s10143-012-0431-0
29. Mustanoja, S, Strbian, D, Putaala, J, Meretoja, A, Curtze, S, Haapaniemi, E, et al. Association of prestroke statin use and lipid levels with outcome of intracerebral hemorrhage. Stroke. (2013) 44:2330–2. doi: 10.1161/STROKEAHA.113.001829
30. King, NKK, Tay, VKS, Allen, JC, and Ang, BT. Prior statin use has no effect on survival after intracerebral hemorrhage in a multiethnic Asian patient cohort. Acta Neurochir Suppl. (2012) 114:343–6. doi: 10.1007/978-3-7091-0956-4_66
31. Dowlatshahi, D, Demchuk, AM, Fang, J, Kapral, MK, Sharma, M, and Smith, EE. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. (2012) 43:1518–23. doi: 10.1161/STROKEAHA.111.645978
32. Romero, FR, Bertolini, EF, Veloso, VN, Ventunni, L, and Figueiredo, EG. Outcomes from intracerebral hemorrhage among patients pre-treated with statins. Arq Neuropsiquiatr. (2011) 69:452–4. doi: 10.1590/S0004-282X2011000400008
33. Ricard, G, Garant, MP, Carrier, N, Leblanc, N, and Boulanger, JM. Statins may increase intracerebral hemorrhage volume. Can J Neurol Sci. (2010) 37:791–6. doi: 10.1017/S0317167100051453
34. Gomis, M, Ois, A, Rodríguez-Campello, A, Cuadrado-Godia, E, Jiménez-Conde, J, Subirana, I, et al. Outcome of intracerebral haemorrhage patients pre-treated with statins. Eur J Neurol. (2010) 17:443–8. doi: 10.1111/j.1468-1331.2009.02838.x
35. Eichel, R, Khoury, ST, Ben-Hur, T, Keidar, M, Paniri, R, and Leker, RR. Prior use of statins and outcome in patients with intracerebral haemorrhage. Eur J Neurol. (2010) 17:78–83. doi: 10.1111/j.1468-1331.2009.02747.x
36. Tapia-Perez, H, Sanchez-Aguilar, M, Torres-Corzo, JG, Rodriguez-Leyva, I, Gonzalez-Aguirre, D, Gordillo-Moscoso, A, et al. Use of statins for the treatment of spontaneous intracerebral hemorrhage: results of a pilot study. Cen Eur Neurosurg. (2009) 70:15–20. doi: 10.1055/s-0028-1082064
37. Leker, RR, Khoury, ST, Rafaeli, G, Shwartz, R, Eichel, R, and Tanne, D. Prior use of statins improves outcome in patients with intracerebral hemorrhage: prospective data from the national acute stroke israeli surveys (nasis). Stroke. (2009) 40:2581–4. doi: 10.1161/STROKEAHA.108.546259
38. Naval, NS, Abdelhak, TA, Zeballos, P, Urrunaga, N, Mirski, MA, and Carhuapoma, JR. Prior statin use reduces mortality in intracerebral hemorrhage. Neurocrit Care. (2008) 8:6–12. doi: 10.1007/s12028-007-0080-2
39. FitzMaurice, E, Wendell, L, Snider, R, Schwab, K, Chanderraj, R, Kinnecom, C, et al. Effect of statins on intracerebral hemorrhage outcome and recurrence. Stroke. (2008) 39:2151–4. doi: 10.1161/STROKEAHA.107.508861
40. Boe, NJ, Hald, SM, Jensen, MM, Bojsen, JA, Elhakim, MT, Florisson, S, et al. Association between statin use and intracerebral hemorrhage location: a nested case-control registry study. Neurology. (2023) 100:e1048–61. doi: 10.1212/WNL.0000000000201664
41. Xie, YY, Liu, SM, Zhang, Q, Jia, Y, and Ding, JP. Associations between low-density lipoprotein cholesterol and haemorrhagic stroke. J Geriatr Cardiol. (2021) 18:204–9. doi: 10.11909/j.issn.1671-5411.2021.03.011
42. Yao Lim, C, Sura, H, Patel, P, Bhaskaran, A, Singh, I, Menon, V, et al. Statin use and outcomes in stroke patients with ldl<70mg/dl. J Hosp Med. (2023) 18:S125–6. doi: 10.1002/jhm.13090
43. Acton, EK, Khazaal, O, Willis, AW, Gelfand, MA, Hennessy, S, Selim, MH, et al. Statins for the prevention of post-stroke seizure and epilepsy development: a systematic review and meta-analysis: statins for post-stroke seizure and epilepsy. J Stroke Cerebrovasc Dis. (2021) 30:106024. doi: 10.1016/j.jstrokecerebrovasdis.2021.106024
Keywords: statin, intracerebral hemorrhage, mortality, meta-analysis, functional prognosis
Citation: Li Z, Xu W-q, Wang J-q, Yang J-h, Shi X-h, Wang C-b, Xu Z-x and Jiang J-l (2025) The double-edged sword of statins in intracerebral hemorrhage patients: a systematic review and meta-analysis. Front. Neurol. 16:1519818. doi: 10.3389/fneur.2025.1519818
Received: 10 November 2024; Accepted: 08 January 2025;
Published: 27 January 2025.
Edited by:
Majaz Moonis, UMass Memorial Medical Center, United StatesReviewed by:
Christian Urbanek, Klinikum Ludwigshafen, GermanyCopyright © 2025 Li, Xu, Wang, Yang, Shi, Wang, Xu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong-xin Xu, eHV6aG9uZ3hpbkBqbHUuZWR1LmNu; Jin-lan Jiang, amlhbmdqaW5sYW5Aamx1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.