- 1Department of Otorhinolaryngology—Head and Neck Surgery, Leiden University Medical Center, Leiden, Netherlands
- 2Apeldoorn Dizziness Centre, Gelre Hospital, Apeldoorn, Netherlands
- 3Department of Neuro-otology, Imperial College London, London, United Kingdom
Introduction: Menière’s disease (MD) is an inner ear disorder characterized by episodic vertigo, fluctuating sensorineural hearing loss, tinnitus, and aural fullness. As of yet, the etiology of MD remains unknown, which contributes to the lack of an evidence-based treatment. Outcomes and outcome measurement instruments (OMIs) used in trials assessing the effectiveness of potential MD treatment are randomly selected due to the absence of established guidelines on this matter. The objective of this review is to give an overview of the outcome domains, outcomes and OMIs used in randomized controlled trials (RCTs) evaluating treatment effects in MD to 2024. This will be the first step of developing a Core Outcome Set (COS) for MD trials.
Methods: A literature search of the PubMed, Embase and Cochrane library databases was conducted from inception to November 2024. All RCTs on the treatment effect of various therapies for patients suffering from MD were included. Among other details, we extracted and analyzed all outcome domains, outcomes, and OMIs used in these RCTs.
Results: A total of 76 RCTs were included, revealing a diverse range of outcomes and OMIs used across the included studies. Outcome domains encompassed dizziness, hearing, tinnitus, aural fullness, quality of life (QoL) and other. Outcomes used most frequently included: the severity of vertigo attacks, the number of vertigo attacks, vestibular function, hearing loss, severity of hearing loss, QoL related to dizziness, and Qol related to tinnitus. The latter two were most commonly measured with the Dizziness Handicap Inventory (DHI), the Functional Level Scale (FLS) and the Tinnitus Handicap Inventory (THI) respectively. For the other outcomes, there was little uniformity in the use of OMIs. Moreover, there was a notable lack of validated OMIs used in the included RCTs.
Conclusion: This scoping review highlights the need for standardizing outcome selection for RCTs focusing on the treatment of MD. In this first step of developing a Core Outcome Set for MD, we identified a potential list of outcomes to be used in the next steps of ‘the Core Outcome Set for Menière’s Disease (COSMED)’ study.
1 Introduction
Menière’s disease (MD) is an inner ear disorder defined by intermittent spontaneous episodes of vertigo, objectified fluctuating bass-perceptive sensorineural hearing loss, tinnitus, and/or aural fullness (1). The etiology of MD is poorly understood and the definite diagnosis is made based on the above-mentioned symptoms. Partly due to its unknown etiology, an evidence-based treatment has not yet been determined (2). Clinical research on the treatment of MD is being conducted globally, but outcome measures typically differ.
In research, an outcome domain refers to a key area of interest that is relevant to a particular clinical field and can be divided in multiple outcomes. An outcome refers to what is being measured to assess the effect of an exposure or health intervention. An outcome measurement instrument (OMI) is a tool to measure that outcome. OMIs can have various forms such as a single question, a quantitative test or a questionnaire. In MD, outcomes and OMIs used in clinical trials to evaluate effectiveness are generally randomly selected due to the absence of established guidelines on this matter (1). Therefore, multiple studies have emphasized the need for identifying and standardizing relevant outcomes and OMIs used in research focused on assessing treatment options for MD (3, 4). A correct selection of outcomes and its accompanying OMIs in clinical trials is crucial because of the significant impact on patient care. Poor choice of OMIs affects the quality and clinical relevance of trial results. Moreover, inconsistency in the selection of outcomes and OMIs between trials results in failure of the synthesis of evidence and makes comparison of treatment options complicated if not impossible.
A core outcome set (COS) is a consensus-derived set for the least amount of data that ought to be measured and reported in each clinical trial for a particular condition. Implementing a COS in all trials conducted for a specific condition helps to minimize the inefficient and unethical measurement of irrelevant outcomes, thereby minimizing costs, improving quality and comparability. More importantly, the COS reflects the perspectives of various stakeholders worldwide and contains the outcomes that are most important to patients. The use of a COS enhances consistency across different trials, facilitating effective comparison and pooling of results, while also minimizing selective reporting bias (5). Due to the lack of uniformity in selecting outcomes in research focused on MD, ‘the Core Outcome Set for Menière’s Disease (COSMED)’ study aims to develop a standardized, minimal set of outcomes for randomized controlled trials on the treatment of MD.
The development of a COS requires a multi-step consensus process that includes key stakeholder groups. The Core Outcome Measures in Effectiveness Trials organization (COMET) has developed a gold-standard approach to develop a COS which consists of two stages: (1) identify outcomes that should be measured and reported and (2) assess the OMIs most appropriate to measure these outcomes.
As a first step of developing the COSMED, we have conducted a scoping review to give an overview of the outcome domains, outcomes and OMIs used in randomized controlled trials evaluating treatment effects in MD to 2024. The results of this scoping review can be used in subsequent stages of the COS development.
2 Methods
This scoping review was conducted in accordance with the PRISMA guidelines for scoping reviews (6). The first step in developing a COS is defining a scope. This refers to the particular area of health care of interest. The scope includes information on the target population, health condition, and interventions the COS intends to be applied to. We developed a search strategy for this scoping review based on the scope we formulated.
2.1 Search strategy and data sources
PubMed, Embase and Cochrane library databases were searched for eligible Randomized Controlled Trials (RCTs) on the treatment effect of various therapies for patients suffering from MD from inception to November 2024. With the assistance of a clinical librarian of the Leiden University Medical Center (LUMC), the following research strategy was comprised: (“Meniere Disease”[Majr] OR “Meniere’s”[tiab] OR “Menieres”[tiab] OR “Meniere”[tiab] OR “Ménière’s”[tiab] OR “Ménières”[tiab] OR “Ménière”[tiab] OR “Méniere’s”[tiab] OR “Ménieres”[tiab] OR “Méniere”[tiab] OR “Menière’s”[tiab] OR “Menières”[tiab] OR “Menière”[tiab] OR “Endolymphatic Hydrops”[Majr] OR “Endolymphatic Hydrops”[ti] OR ((cochlea*[ti] OR labyrinth*[ti] OR aural[ti] OR auditory[ti] OR otogenic[ti]) AND (“Vertigo”[Majr] OR “Vertigo”[ti] OR hydrops[ti] OR “Syndrome”[majr] OR syndrom*[ti]))) AND (“study”[tw] OR “studies”[tw] OR clinical trial*[tw] OR “Clinical Trial”[Publication Type] OR RCT*[tw] OR “Therapeutics”[Mesh] OR therap*[tw] OR treatment*[tw] OR intervention*[tw] OR “Treatment Outcome”[Mesh] OR outcome*[tw] OR “effectiveness”[tw] OR “efficacy”[tw] OR “end point”[tw] OR “endpoint”[tw]) NOT (Case Reports[ptyp] OR Case Report*[ti])).
2.2 Study selection
We included RCTs on pharmacological and non-pharmacological treatments for patients with MD. Studies with patients under the age of 18, with a sample size of less than 10, and studies that included patients with a variety of vertigo-related conditions, in which the proportion of results related to patients with MD remained unclear, were excluded. Reviews, animal studies, opinion papers and case reports were also excluded. There were no restrictions on the type of interventions or language. First, all titles and summaries were screened for potentially eligible RCTs. Secondly, articles were evaluated for eligibility by studying abstracts and full-text if necessary. Subsequently, the final decision on inclusion of the study was made.
2.3 Data extraction
Data was extracted using a data extraction form in Excel. For all included studies, details such as title, authors, study design, study year, country, number of participants, intervention specifics, time points and outcomes were systematically assessed and documented. The outcomes and OMIs were extracted from the method and results section of each paper. Subsequently, for each study, we categorized outcomes and OMIs into 6 different outcome domains: dizziness, hearing, tinnitus, aural fullness, quality of life and other. At the end of the data extraction process, a comprehensive list of outcomes and OMIs was conducted. This final list also specified which studies utilized which outcomes and OMIs. For each outcome domain, we provided an overview outlining the used outcomes and OMIs across the included studies.
3 Results
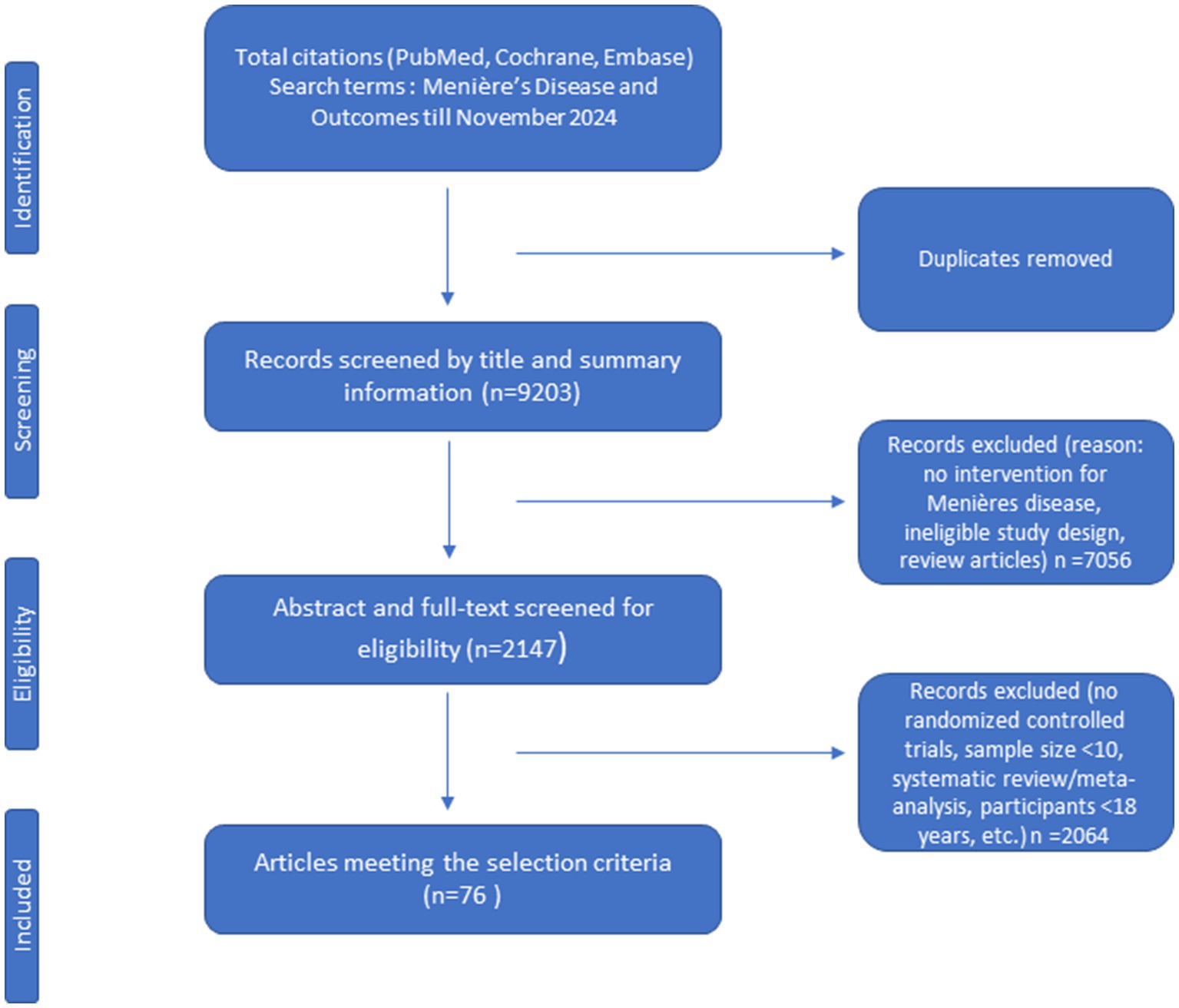
The PubMed, Embase and Cochrane library databases were searched to November 2024. In total, 9,203 studies were initially identified. Then 7,056 articles were excluded after evaluating the title and summary information. After reviewing both the abstracts and full-texts, 2,147 articles were excluded. As a result, a total of 76 RCTs were included in this study (Figure 1).
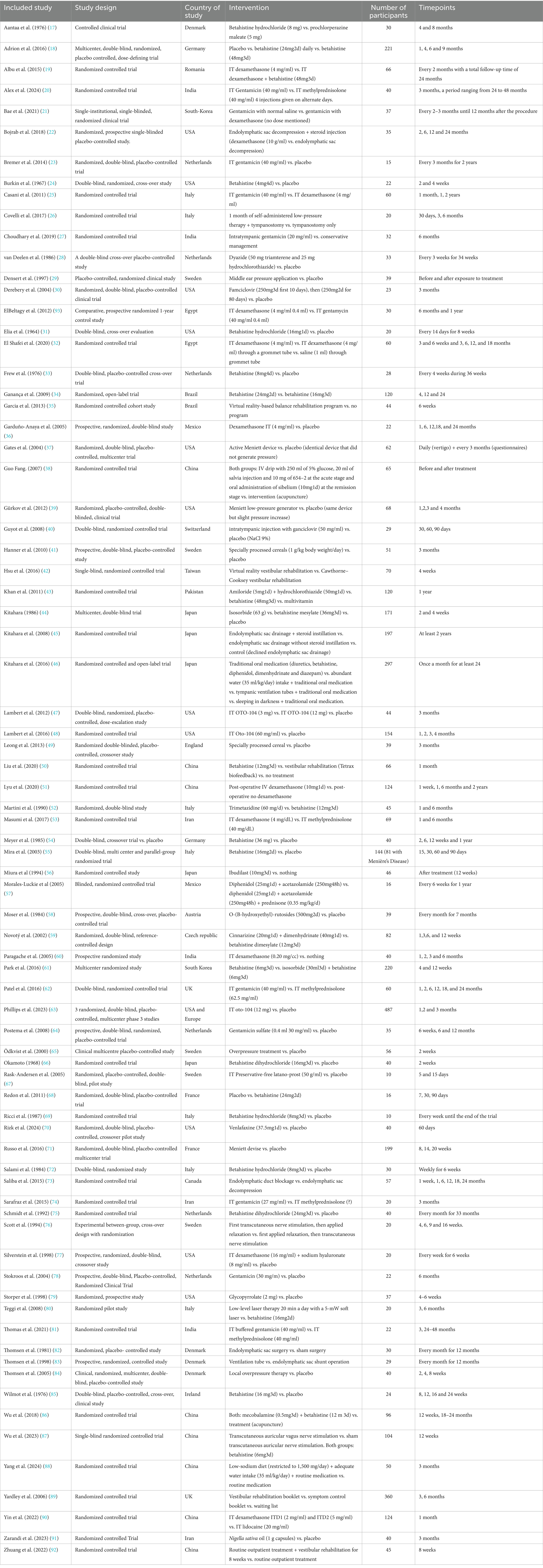
From these 76 included studies a total of 38different outcomes and 208 OMIs were identified. An overview of the included studies is presented in Table 1. An overview of all outcome domains, outcomes and OMIs used in the included studies, is presented in Supplementary Table 1.
3.1 Dizziness
The first and most frequently reported outcome domain in all studies was dizziness. Within this outcome domain, we extracted several outcomes including the number of vertigo attacks, vertigo severity, duration of a vertigo attack, type of episode and vertigo control/improvement. A wide variety of OMIs were used to evaluate these outcomes. Most of these OMIs were non-validated, self-created, symptom assessments or rating scales. The OMI that was used most frequently was the ‘class of vertigo control’, defined according to the AAO-HNS criteria (1). Additionally, within this outcome domain, a wide range of rating scales have been used to measure vertigo severity and vertigo control. Various questionnaires including the Dizziness Handicap Inventory (DHI) (7) and the Functional Level Score (1) were commonly used, however, these instruments assess vertigo-related quality of life (QoL) and thus are categorized under the outcome domain Qol.
3.2 Hearing
Given that hearing loss, similar to dizziness, serves as a diagnostic prerequisite for MD, numerous studies included hearing loss as an outcome. However, little uniformity was found in the frequencies of pure tone average (PTA) to be measured, with some studies not even specifying the frequencies assessed. In line with the AAO-HNS criteria (1), frequencies most measured were 0.5, 1, 2 and 3 kHz. Seventeen studies measured word recognition scores in addition to PTA (WRS). To determine the statistical significance of hearing loss, most studies assessing hearing, used the AAO-HNS scale where a change of more than 10 decibels in PTA, or a change of 15% in WRS was defined as a clinically significant difference (1).
3.3 Tinnitus
Articles including tinnitus as an outcome frequently faced challenges in specifying their measurement methods. Frequency of tinnitus, tinnitus prevalence, tinnitus presence or absence and the degree of tinnitus improvement were terms that were used in the articles but were not explicitly defined. Eleven different likert scales were used to measure tinnitus severity by 19 different studies. The Tinnitus Handicap Inventory (THI) (8) was most commonly used, but, similar to the DHI, we categorized this under the core domain QoL.
3.4 Aural fullness
Only 22 out of the 71 RCTs included, measured aural fullness as an outcome measure. All of these studies used OMIs that were not validated such as a variety of different likert scales, recorded in a diary or presence or absence. Furthermore, several studies did not provide details regarding the method used to measure aural fullness.
3.5 Quality of life
The assessment of QoL was categorized into several outcome domains, including QoL related to dizziness and tinnitus. Among the included RCTs, for the evaluation of QoL the Dizziness Handicap Inventory (DHI) (7), Tinnitus Handicap Inventory (THI) (8), and the Functional Level Scale (FLS) defined by the AAO-HNS criteria (1), were predominantly used. Additionally, a variety of questionnaires were used to measure quality of life in relation to dizziness, including the European Evaluation of Vertigo (EEV) scale (9), Vertigo Symptom Scale short form (VSS-SF) (10), Menière’s Disease Patient-Oriented Symptom Severity Index (MD-POSI) (11), Dizziness Beliefs Questionnaire (DBQ) (12), Vestibular Disorders Activities of Daily Living (VDADL) score (13), the Menière’s Disease Outcomes Questionnaire (MDOQ) (14) and the Neuropsychological Vertigo Inventory (NVI) (15). Furthermore, some studies addressed general health-related QoL or aspects related to psychological health, although these aspects were explored in a limited number of studies.
3.6 Other
The most frequently used outcomes which were categorized under ‘other’ were vestibular function, auditory function and balance. The most commonly utilized clinical tests were electronystagmography and electrocochleography. Balance was assessed using a wide range of clinical tests and rating scales.
Other outcome measurements that were evaluated included compliance, tolerability and overall judgment of treatment, quality of appointments, laboratory tests, activity level and other general symptoms, possibly related to MD. These outcome measurements were primarily related to interventions and were highly specific for the respective studies.
3.7 Adverse events
Among all studies included, a wide variety of adverse events was reported. Mostly, the adverse events reported were depending on the specific intervention that was performed and reflecting the risks associated with each intervention type. Due to this wide variation, which was often linked to the type of intervention, we decided not to include adverse events in the list of outcomes for MD.
4 Discussion
The key finding of this scoping review was the significant diversity observed in outcome domains, outcomes and OMIs reported across all RCTs included. The outcome domain dizziness was most commonly used and used in 64 out of the 76 studies. Eight additional studies exclusively used the DHI or the VSS-sf to evaluate dizziness, which were categorized under QoL related to dizziness. In total, four articles did not assess dizziness in any capacity. Hearing and tinnitus, which were the next most used domains, and were reported in 61 and 33 studies respectively, whereas 16 studies reported the quality of life related to tinnitus using the THI. Quality of life (QoL) was assessed in 35 studies, with a trend indicating that QoL was evaluated less frequently in the older studies. Aural fullness was the least reported outcome domain, featuring in only 22 studies. A notable issue within the outcome domains aural fullness and tinnitus was the lack of clarity regarding the assessment method used to measure outcomes. For instance, some studies evaluated tinnitus loudness or frequency without specifying the measurement method. We concede that tinnitus and aural fullness are challenging symptoms to classify, however, the lack of clarity on how outcome domains were assessed raises concerns about the reliability of these findings. The vestibular function, auditory function and balance categorized under the outcome domain ‘other’ were measured in 34 studies where in the vast majority of studies electronystagmography or electrocochleography was performed.
There was no single outcome that was reported in all studies. However, in the outcome domain QoL, there was a considerable overlap in the OMIs used. QoL related to dizziness was assessed using the DHI in 21 studies and using the FLS in 13 studies. Similarly, QoL related to tinnitus was measured using the THI in 16 studies. The OMI electronystagmography (caloric test) was the OMI most frequently used and was measured in 19 studies. In contrast, there was not much similarity in the OMIs used to measure the outcomes in the other domains. The ‘severity of vertigo attacks’ was the most commonly utilized outcome, though it was assessed using 24 distinct outcome measurement instruments (OMIs). Additionally, the outcome measure number of vertigo attacks was measured in as many as 35 studies using 17 different OMIs. Similarly for the outcome hearing loss, where various combinations of frequencies were used to calculate the PTA. The variation in OMIs used to measure a specific outcome, complicates the comparison of findings between studies. For example, when measuring vertigo severity, studies may use patient self-reported scales, frequency counts of vertigo attacks, or standardized questionnaires like the DHI. A study focusing on attack frequency alone may show different treatment effects than one measuring intensity or overall handicap. This variability makes it challenging to interpret which treatments are genuinely effective across different vertigo dimensions. Such inconsistencies also complicate the development of clinical recommendations, as different aspects of vertigo may influence treatment guidance differently.
Alongside the variation in the use of OMIs, there was an evident absence of validated measurement instruments. For example, to measure vertigo severity, many different rating scales were used. To our knowledge, these scales are not validated.Without proper validation, it is uncertain whether the instrument captures the relevant aspects of the outcome and if it is sensitive enough to detect meaningful changes and which cut-off point are defined as ‘clinically relevant’. This could lead to a misinterpretation of the true effect of the intervention. Furthermore validation establishes that an OMI can be reliably applied across different populations and settings. Non-validated instruments may perform inconsistently across diverse demographic or clinical groups, limiting the comparability of findings. In addition, we could not find any validation studies on the OMIs proposed by the AAO-HNS such as the Functional level score, the class of vertigo control, or the hearing severity scale. However, even though validated questionnaires, like the DHI, are commonly used, their quality may not always be optimal. In a study by Koppelaar-van Eijsden et al. (16), the quality of DHI was assessed using the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) methodology. They concluded that the current evidence for several measurement properties is suboptimal. Furthermore, the DHI might not be the best tool measure vertigo in MD specifically. Furthermore, the DHI may not be the most suitable instrument for assessing vertigo in MD specifically. Firstly, it is not well-suited for episodic syndromes, particularly those characterized by fluctuating symptom severity or clustered attacks. Additionally, the term ‘dizziness’ may not accurately capture the experiences of many MD patients, who often suffer from severe vertigo rather than generalized dizziness.
One of the key strengths of this scoping review is that we did not exclude any studies based on language or availability. In case studies were not available in full text through our institution, we purchased them, if possible, online. Four studies were not available in English and were translated with the aid of artificial intelligence tools. This comprehensive approach ensures that we have captured all outcomes used in recent RCTs in this study.
A limitation of this scoping review was the variability in outcome measures and OMIs across studies, which made data categorization challenging. Several OMIs did not fit into any specific domain, resulting in their classification under the domain “other,” which may reduce the clarity of the overview. Another limitation of this study is that we only assessed studies with two reviewers when there was uncertainty about their inclusion. This may affected the reliability of the selection process.
As previously mentioned, this review is part of the COSMED-study, which will be conducted in two distinct stages. The first stage will focus on identifying outcome domains for inclusion in the COS while the second stage will determine the specific outcomes to assess these domains. To reach consensus on the outcome domains and outcomes to be incorporated into the COS, a Delphi procedure will be conducted at each stage, engaging experts on MD to give their valuable opinion.
This review has collected an extensive list of all outcome domains, outcomes, and OMIs reported in recent RCTs on the treatment of MD and will serve as a basis for the upcoming Delphi procedures. However, it is important to acknowledge that this list primarily reflects the perspectives of clinicians and researchers, potentially overlooking the valuable input of patients. Patients often provide unique perspectives on disease symptoms and how these symptoms affect their daily lives, which may differ significantly from the view of clinicians and researchers. Thus, patient involvement is critical in selecting the outcomes and OMIs to be included in the COS.
To integrate the patient perspective, a focus group meeting with patients will be conducted. In this initial meeting, we will discuss outcome domains that are particularly meaningful for patients with MD. In a subsequent patient focus group during the second stage of the study, we will focus specifically on identifying outcomes that are highly relevant to patients. These patient-derived domains and outcomes, together with those identified through the review, will form the basis of the Delphi procedures for the COSMED-study.
In conclusion, there is a lack of standardized outcomes and OMIs used in RCTs on the treatment of MD. To address this problem, the COSMED study aims to develop a COS for use in RCTs evaluating MD treatments. As a first step, in this study we identified outcome domains, outcomes, and OMIs used in RCTs on the treatment of MD to 2024. The identified outcome domains encompass dizziness, hearing, tinnitus, aural fullness, Qol and other, with a notable variability in outcomes and OMIs observed within these outcome domains. Additionally, we found a lack in the use of validated OMIs. Together with the inconsistency in outcomes and OMIs reported, we emphasize the need for a standardized COS for MD. In this first step of developing a COS for MD, we identified a potential list of outcomes to be used in the next steps of our COSMED study. The development of a COS holds promise for enhancing consistency, comparability, and relevance in evaluating treatment outcomes for MD, ultimately improving patient care and advancing clinical research in this field.
Author contributions
MB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. BvE: Conceptualization, Investigation, Methodology, Validation, Visualization, Writing – review & editing. MvB: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – review & editing. DK: Writing – review & editing. TB: Conceptualization, Methodology, Supervision, Visualization, Writing – review & editing. PvB: Conceptualization, Methodology, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to express their gratitude to Jan W. Schoones, librarian at the Walaeus Library, Leiden University Medical Center, for his valuable assistance in developing the search strategy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1516350/full#supplementary-material
References
1. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière's disease. American Academy of otolaryngology-head and neck foundation, Inc. Otolaryngol Head Neck Surg. (1995) 113:181–5. doi: 10.1016/S0194-5998(95)70102-8
2. Basura, GJ, Adams, ME, Monfared, A, Schwartz, SR, Antonelli, PJ, Burkard, R, et al. Clinical Practice Guideline: Ménière's Disease. Otolaryngol Head Neck Surg. (2020) 162:S1–s55. doi: 10.1177/0194599820909438
3. Webster, KE, Lee, A, Galbraith, K, Harrington-Benton, NA, Judd, O, Kaski, D, et al. Intratympanic corticosteroids for Ménière's disease. Cochrane Database Syst Rev. (2023) 2023:Cd015245. doi: 10.1002/14651858.CD015245.pub2
4. Lee, A, Webster, KE, George, B, Harrington-Benton, NA, Judd, O, Kaski, D, et al. Surgical interventions for Ménière's disease. Cochrane Database Syst Rev. (2023) 2:Cd015249. doi: 10.1002/14651858.CD015249.pub2
5. (BD4BO) IMIIBdfbo (2018). A practical toolkit for the identification, selection and measurement of outcomes including in realworld settings.
6. Tricco, AC, Lillie, E, Zarin, W, O'Brien, KK, Colquhoun, H, and Levac, D. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
7. Jacobson, GP, and Newman, CW. The development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg. (1990) 116:424–7. doi: 10.1001/archotol.1990.01870040046011
8. Newman, CW, Jacobson, GP, and Spitzer, JB. Development of the tinnitus handicap inventory. Arch Otolaryngol Head Neck Surg. (1996) 122:143–8. doi: 10.1001/archotol.1996.01890140029007
9. Mègnigbêto, CA, Sauvage, JP, and Launois, R. The European evaluation of Vertigo (EEV) scale: a clinical validation study. Rev Laryngol Otol Rhinol (Bord). (2001) 122:95–102.
10. Yardley, L, Gresty, M, Bronstein, A, and Beyts, J. Changes in heart rate and respiration rate in patients with vestibular dysfunction following head movements which provoke dizziness. Biol Psychol. (1998) 49:95–108. doi: 10.1016/S0301-0511(98)00029-5
11. Murphy, MP, and Gates, GA. Measuring the effects of Meniere's disease: results of the patient-oriented severity index (MD POSI) version 1. Ann Otol Rhinol Laryngol. (1999) 108:331–7. doi: 10.1177/000348949910800403
12. Yardley, L, Beech, S, and Weinman, J. Influence of beliefs about the consequences of dizziness on handicap in people with dizziness, and the effect of therapy on beliefs. J Psychosom Res. (2001) 50:1–6. doi: 10.1016/S0022-3999(00)00202-6
13. Cohen, HS, and Kimball, KT. Development of the vestibular disorders activities of daily living scale. Arch Otolaryngol Head Neck Surg. (2000) 126:881–7. doi: 10.1001/archotol.126.7.881
14. Kato, BM, LaRouere, MJ, Bojrab, DI, and Michaelides, EM. Evaluating quality of life after endolymphatic sac surgery: the Ménière’s disease outcomes questionnaire. Otol Neurotol. (2004) 25:339–44. doi: 10.1097/00129492-200405000-00023
15. Lacroix, E, Deggouj, N, Salvaggio, S, Wiener, V, Debue, M, and Edwards, MG. The development of a new questionnaire for cognitive complaints in vertigo: the neuropsychological Vertigo inventory (NVI). Eur Arch Otorrinolaringol. (2016) 273:4241–9. doi: 10.1007/s00405-016-4135-x
16. Koppelaar-van Eijsden, HM, Schermer, TR, and Bruintjes, TD. Measurement properties of the dizziness handicap inventory: a systematic review. Otol Neurotol. (2022) 43:e282–97. doi: 10.1097/MAO.0000000000003448
17. Aantaa, E, and Skinhoj, A. Controlled clinical trial comparing the effect of betahistine hydrochloride and prochlorperazine maleate on patients with Meniére's disease. Ann Clin Res. (1976) 8:284–7.
18. Adrion, C, Fischer, CS, Wagner, J, Gürkov, R, Mansmann, U, and Strupp, M. Efficacy and safety of betahistine treatment in patients with Meniere's disease: primary results of a long term, multicentre, double blind, randomised, placebo controlled, dose defining trial (BEMED trial). BMJ. (2016) 352:h6816. doi: 10.1136/bmj.h6816
19. Albu, S, Chirtes, F, Trombitas, V, Nagy, A, Marceanu, L, Babighian, G, et al. Intratympanic dexamethasone versus high dosage of betahistine in the treatment of intractable unilateral Meniere disease. Am J Otolaryngol. (2015) 36:205–9. doi: 10.1016/j.amjoto.2014.10.032
20. Alex, A, Mammen, MD, Lepcha, A, Reka, K, Augustine, AM, and Philip, A. A randomised controlled trial comparing Intratympanic gentamicin with methylprednisolone in Meniere's disease with good hearing. Indian J Otolaryngol Head Neck Surg. (2024) 76:3793–9. doi: 10.1007/s12070-024-04749-x
21. Bae, SH, Lee, JM, Lee, HJ, Na, G, and Kim, SH. Effect of dexamethasone combination with gentamicin in Chemical Labyrinthectomy on hearing preservation and Vertigo control in patients with unilateral Meniere's disease: a randomized controlled clinical trial. J Clin Med. (2021) 10:5581. doi: 10.3390/jcm10235581
22. Bojrab, DI II, LaRouere, MJ, Babu, S, Sargent, E, Chan, E, Hong, R, et al. Endolymphatic sac decompression with intra-sac dexamethasone injection in Menière's disease. Otol Neurotol. (2018) 39:616–21. doi: 10.1097/MAO.0000000000001810
23. Bremer, HG, van Rooy, I, Pullens, B, Colijn, C, Stegeman, I, van der Zaag-Loonen, HJ, et al. Intratympanic gentamicin treatment for Ménière's disease: a randomized, double-blind, placebo-controlled trial on dose efficacy - results of a prematurely ended study. Trials. (2014) 15:328. doi: 10.1186/1745-6215-15-328
25. Casani, AP, Piaggi, P, Cerchiai, N, Seccia, V, Sellari, S, and Dallan, I. Intratympanic treatment of intractable unilateral Meniere disease: gentamicin or dexamethasone? A randomized controlled trial. Otolaryngol Head Neck Surg. (2012) 146:430–7. doi: 10.1177/0194599811429432
26. Covelli, E, Volpini, L, Atturo, F, Benincasa, AT, Filippi, C, Tarentini, S, et al. Delayed effect of active pressure treatment on endolymphatic Hydrops. Audiol Neurotol. (2017) 22:24–9. doi: 10.1159/000472245
27. Choudhary, A, Biswas, K, Basak, B, Ghosh, S, and Bhattacharya, D. Intratympanic low dose gentamicin in intractable Meniere’s disease- chemical labyrinthectomy revisited. J Evol Med Dent Sci. (2019) 8:1843–7. doi: 10.14260/jemds/2019/405
28. van Deelen, GW, and Huizing, EH. Use of a diuretic (Dyazide) in the treatment of Menière's disease. A double-blind cross-over placebo-controlled study. ORL J Otorhinolaryngol Relat Spec. (1986) 48:287–92. doi: 10.1159/000275884
29. Densert, B, Densert, O, Arlinger, S, Sass, K, and Odkvist, L. Immediate effects of middle ear pressure changes on the electrocochleographic recordings in patients with Menière's disease: a clinical placebo-controlled study. Am J Otolaryngol. (1997) 18:726–33.
30. Derebery, MJ, Fisher, LM, and Iqbal, Z. Randomized double-blinded, placebo-controlled clinical trial of famciclovir for reduction of Ménière's disease symptoms. Otolaryngol Head Neck Surg. (2004) 131:877–84. doi: 10.1016/j.otohns.2004.08.012
31. Elia, JC. Double-blind evaluation of a new treatment for Ménière's syndrome. JAMA. (1966) 196:187–9. doi: 10.1001/jama.1966.03100150133043
32. El Shafei, RR, and Qotb, M. Comparison of the effect of three treatment interventions for the control of Meniere’s disease: a randomized control trial. Egypt J Otolaryngol. (2020) 36:22. doi: 10.1186/s43163-020-00018-0
33. Frew, IJ, and Menon, GN. Betahistine hydrochloride in Méniére's disease. Postgrad Med J. (1976) 52:501–3. doi: 10.1136/pgmj.52.610.501
34. Ganança, MM, Caovilla, HH, and Ganança, FF. Comparable efficacy and tolerability between twice daily and three times daily betahistine for Ménière's disease. Acta Otolaryngol. (2009) 129:487–92. doi: 10.1080/00016480802273082
35. Garcia, AP, Ganança, MM, Cusin, FS, Tomaz, A, Ganança, FF, and Caovilla, HH. Vestibular rehabilitation with virtual reality in Ménière's disease. Braz J Otorhinolaryngol. (2013) 79:366–74. doi: 10.5935/1808-8694.20130064
36. Garduño-Anaya, MA, Couthino De Toledo, H, Hinojosa-González, R, Pane-Pianese, C, and Ríos-Castañeda, LC. Dexamethasone inner ear perfusion by intratympanic injection in unilateral Ménière's disease: a two-year prospective, placebo-controlled, double-blind, randomized trial. Otolaryngol Head Neck Surg. (2005) 133:285–94. doi: 10.1016/j.otohns.2005.05.010
37. Gates, GA, Green, JD Jr, Tucci, DL, and Telian, SA. The effects of transtympanic micropressure treatment in people with unilateral Meniere's disease. Arch Otolaryngol Head Neck Surg. (2004) 130:718–25. doi: 10.1001/archotol.130.6.718
38. Guo, F. Observation on treatment of dizziness mainly by acupuncture. J Tradit Chin Med. (2007) 27:16–8.
39. Gürkov, R, Filipe Mingas, LB, Rader, T, Louza, J, Olzowy, B, and Krause, E. Effect of transtympanic low-pressure therapy in patients with unilateral Menière's disease unresponsive to betahistine: a randomised, placebo-controlled, double-blinded, clinical trial. J Laryngol Otol. (2012) 126:356–62. doi: 10.1017/S0022215112000102
40. Guyot, JP, Maire, R, and Delaspre, O. Intratympanic application of an antiviral agent for the treatment of Ménière's disease. ORL J Otorhinolaryngol Relat Spec. (2008) 70:21–7. doi: 10.1159/000111044
41. Hanner, P, Rask-Andersen, H, Lange, S, and Jennische, E. Antisecretory factor-inducing therapy improves the clinical outcome in patients with Ménière's disease. Acta Otolaryngol. (2010) 130:223–7. doi: 10.3109/00016480903022842
42. Hsu, SY, Fang, TY, Yeh, SC, Su, MC, Wang, PC, and Wang, VY. Three-dimensional, virtual reality vestibular rehabilitation for chronic imbalance problem caused by Ménière's disease: a pilot study. Disabil Rehabil. (2017) 39:1601–6. doi: 10.1080/09638288.2016.1203027
43. Khan, BH, Ahmed, ZA, and Khan, RA, editors. (2011). Effects of diuretic and vasodilator therapy in Meniere's disease.
44. Kitahara, M, Watanabe, I, Hinoki, M, Mizukoshi, K, and Matsunaga, T. Clinical study of isosorbide on Meniere's disease inter-group comparative study with betahistine mesylate by multicentered double-blind trial. Otologia fukuoka. (1986) 32:44–92.
45. Kitahara, T, Kubo, T, Okumura, S, and Kitahara, M. Effects of endolymphatic sac drainage with steroids for intractable Meniere's disease: a long-term follow-up and randomized controlled study. Laryngoscope. (2008) 118:854–61. doi: 10.1097/MLG.0b013e3181651c4a
46. Kitahara, T, Okamoto, H, Fukushima, M, Sakagami, M, Ito, T, Yamashita, A, et al. A two-year randomized trial of interventions to decrease stress hormone vasopressin production in patients with Meniere's disease-a pilot study. PLoS One. (2016) 11:e0158309. doi: 10.1371/journal.pone.0158309
47. Lambert, PR, Nguyen, S, Maxwell, KS, Tucci, DL, Lustig, LR, Fletcher, M, et al. A randomized, double-blind, placebo-controlled clinical study to assess safety and clinical activity of OTO-104 given as a single intratympanic injection in patients with unilateral Ménière's disease. Otol Neurotol. (2012) 33:1257–65. doi: 10.1097/MAO.0b013e318263d35d
48. Lambert, PR, Carey, J, Mikulec, AA, LeBel, C, and S,. Intratympanic sustained-exposure dexamethasone thermosensitive gel for symptoms of Ménière's disease: randomized phase 2b safety and efficacy trial. Otol Neurotol. (2016) 37:1669–76. doi: 10.1097/MAO.0000000000001227
49. Leong, SC, Narayan, S, and Lesser, TH. Antisecretory factor–inducing therapy improves patient-reported functional levels in Meniere's disease. Ann Otol Rhinol Laryngol. (2013) 122:619–24. doi: 10.1177/000348941312201004
50. Liu, JL, Liu, JG, Chen, XB, and Liu, YH. The benefits of betahistine or vestibular rehabilitation (Tetrax biofeedback) on the quality of life and fall risk in patients with Ménière's disease. J Laryngol Otol. (2020) 134:1073–6. doi: 10.1017/S0022215120002509
51. Lyu, Y, Zhang, D, Li, X, Han, Y, Li, Y, Wang, J, et al. Dexamethasone protects the hearing of Meniere's disease patients after triple semicircular canal plugging. Acta Otolaryngol. (2020) 140:803–7. doi: 10.1080/00016489.2020.1775292
52. Martini, A, and De Domenico, F. Trimetazidine versus betahistine in Ménière's disease. A double blind method. Ann Otolaryngol Chir Cervicofac. (1990) 107:20–7.
53. Masoumi, E, Dabiri, S, Khorsandi, MT, Erfanian, R, Sohrabpour, S, Yazdani, N, et al. Methylprednisolone versus dexamethasone for control of Vertigo in patients with definite Meniere's disease. Iran J Otorhinolaryngol. (2017) 29:341–6.
54. Meyer, ED. Treatment of Meniere disease with betahistine dimesilate (Aequamen)--double-blind study versus placebo (crossover). Laryngol Rhinol Otol (Stuttg). (1985) 64:269–72. doi: 10.1055/s-2007-1008135
55. Mira, E, Guidetti, G, Ghilardi, L, Fattori, B, Malannino, N, Maiolino, L, et al. Betahistine dihydrochloride in the treatment of peripheral vestibular vertigo. Eur Arch Otorrinolaringol. (2003) 260:73–7. doi: 10.1007/s00405-002-0524-4
56. Miura, M, Naito, E, Tsuji, J, and Naito, Y. Ketas treatment for Meniere's disease. Pract Otol. (1994) 87:1733–8. doi: 10.5631/jibirin.87.1733
57. Morales-Luckie, E, Cornejo-Suarez, A, Zaragoza-Contreras, MA, and Gonzalez-Perez, O. Oral administration of prednisone to control refractory vertigo in Ménière's disease: a pilot study. Otol Neurotol. (2005) 26:1022–6. doi: 10.1097/01.mao.0000185057.81962.51
58. Moser, M, Ranacher, G, Wilmot, TJ, and Golden, GJ. A double-blind clinical trial of hydroxyethylrutosides in Menière's disease. J Laryngol Otol. (1984) 98:265–72. doi: 10.1017/S0022215100146547
59. Novotný, M, and Kostrica, R. Fixed combination of cinnarizine and dimenhydrinate versus betahistine dimesylate in the treatment of Ménière's disease: a randomized, double-blind, parallel group clinical study. Int Tinnitus J. (2002) 8:115–23.
60. Paragache, G, Panda, NK, Ragunathan, M, and Sridhara,. Intratympanic dexamethasone application in Meniere's disease-is it superior to conventional therapy? Indian. J Otolaryngol Head Neck Surg. (2005) 57:21–3. doi: 10.1007/BF02907620
61. Park, HW, Chung, WH, Kim, SH, Kim, KS, Chung, JW, Chae, SW, et al. Multicenter randomized study on the efficacy of isosorbide in patients with Ménière’s disease. Res Vestib Sci. (2016) 15:44–50.
62. Patel, M, Agarwal, K, Arshad, Q, Hariri, M, Rea, P, Seemungal, BM, et al. Intratympanic methylprednisolone versus gentamicin in patients with unilateral Ménière's disease: a randomised, double-blind, comparative effectiveness trial. Lancet. (2016) 388:2753–62. doi: 10.1016/S0140-6736(16)31461-1
63. Phillips, J, Mikulec, AA, Robinson, JM, Skarinsky, D, and Anderson, JJ. Efficacy of intratympanic OTO-104 for the treatment of Ménière's disease: the outcome of three randomized, double-blind, placebo-controlled studies. Am J Otolaryngol. (2023) 44:584–92. doi: 10.1097/MAO.0000000000003886
64. Postema, RJ, Kingma, CM, Wit, HP, Albers, FW, and Van Der Laan, BF. Intratympanic gentamicin therapy for control of vertigo in unilateral Menire's disease: a prospective, double-blind, randomized, placebo-controlled trial. Acta Otolaryngol. (2008) 128:876–80. doi: 10.1080/00016480701762458
65. Odkvist, LM, Arlinger, S, Billermark, E, Densert, B, Lindholm, S, and Wallqvist, J. Effects of middle ear pressure changes on clinical symptoms in patients with Ménière's disease--a clinical multicentre placebo-controlled study. Acta Otolaryngol Suppl. (2000) 543:99–101. doi: 10.1080/000164800454107
66. Okamoto, K, Hazeyama, F, Taira, T, Yoshida, A, and Onoda, T. Therapeutic results of betahistine on Meniere's disease. Multi-variable analysis of the results of the double blind test and Fisher's evaluation method. Iryo. (1968) 22:650–66.
67. Rask-Andersen, H, Friberg, U, Johansson, M, and Stjernschantz, J. Effects of intratympanic injection of latanoprost in Meniere's disease: a randomized, placebo-controlled, double-blind, pilot study. Otolaryngol Head Neck Surg. (2005) 133:441–3. doi: 10.1016/j.otohns.2005.05.043
68. Redon, C, Lopez, C, Bernard-Demanze, L, Dumitrescu, M, Magnan, J, Lacour, M, et al. Betahistine treatment improves the recovery of static symptoms in patients with unilateral vestibular loss. J Clin Pharmacol. (2011) 51:538–48. doi: 10.1177/0091270010369241
69. Ricci, V, Sittoni, V, and Nicora, M. Efficaci and safety of betahistino hydrochloride versus placebo in Meniere's disease. Rivista Italiana di Otorinolaringologia Audiologia e Foniatria. (1987) 7:347–50.
70. Rizk, H, Monaghan, NP, Shah, S, Liu, Y, Keith, BA, Jeong, S, et al. Efficacy of a serotonin-norepinephrine reuptake inhibitor as a treatment for Meniere disease: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. (2024) 150:935–42. doi: 10.1001/jamaoto.2024.2241
71. Russo, FY, Nguyen, Y, de, D, Bouccara, D, Sterkers, O, Ferrary, E, et al. Meniett device in meniere disease: randomized, double-blind, placebo-controlled multicenter trial. Laryngoscope. (2017) 127:470–5. doi: 10.1002/lary.26197
72. Salami, A, Delle, PM, Tinelli, E, Jankowska, B, and Dellepiane, M. Double blind study between betahistine hydrochloride and placebo in the treatment of Meniere's syndromes. Valsalva. (1984) 60:302–12.
73. Saliba, I, Gabra, N, Alzahrani, M, and Berbiche, D. Endolymphatic duct blockage: a randomized controlled trial of a novel surgical technique for Ménière's disease treatment. Otolaryngol Head Neck Surg. (2015) 152:122–9. doi: 10.1177/0194599814555840
74. Sarafraz, M, Saki, N, Nikakhlagh, S, Mashali, L, and Arad, A. Comparison the efficacy of intratympanic injections of methylprednisolone and gentamicin to control vertigo in unilateral Ménière’s disease. Biomed Pharmacol J. (2015) 8:705–9. doi: 10.13005/bpj/772
75. Schmidt, JT, and Huizing, EH. The clinical drug trial in Menière's disease with emphasis on the effect of betahistine SR. Acta Otolaryngol Suppl. (1992) 497:1–189.
76. Scott, B, Larsen, HC, Lyttkens, L, and Melin, L. An experimental evaluation of the effects of transcutaneous nerve stimulation (TNS) and applied relaxation (AR) on hearing ability, tinnitus and dizziness in patients with Menière's disease. Br J Audiol. (1994) 28:131–40. doi: 10.3109/03005369409086560
77. Silverstein, H, Isaacson, JE, Olds, MJ, Rowan, PT, and Rosenberg, S. Dexamethasone inner ear perfusion for the treatment of Meniere's disease: a prospective, randomized, double-blind, crossover trial. Am J Otolaryngol. (1998) 19:196–201.
78. Stokroos, R, and Kingma, H. Selective vestibular ablation by intratympanic gentamicin in patients with unilateral active Ménière's disease: a prospective, double-blind, placebo-controlled, randomized clinical trial. Acta Otolaryngol. (2004) 124:172–5. doi: 10.1080/00016480410016621
79. Storper, IS, Spitzer, JB, and Scanlan, M. Use of glycopyrrolate in the treatment of Meniere's disease. Laryngoscope. (1998) 108:1442–5. doi: 10.1097/00005537-199810000-00004
80. Teggi, R, Bellini, C, Fabiano, B, and Bussi, M. Efficacy of low-level laser therapy in Ménière's disease: a pilot study of 10 patients. Photomed Laser Surg. (2008) 26:349–53. doi: 10.1089/pho.2007.2186
81. Thomas, L, Lepcha, A, Reka, K, Augustine, AM, Alex, A, Philip, A, et al. Comparing Intratympanic gentamicin with methylprednisolone in Meniere's disease with non-serviceable hearing. Indian J Otolaryngol Head Neck Surg. (2022) 74:3738–45. doi: 10.1007/s12070-021-02528-6
82. Thomsen, J, Bretlau, P, Tos, M, and Johnsen, NJ. Placebo effect in surgery for Ménière's disease. A double-blind, placebo-controlled study on endolymphatic sac shunt surgery. Arch Otolaryngol. (1981) 107:271–7. doi: 10.1001/archotol.1981.00790410009002
83. Thomsen, J, Bonding, P, Becker, B, Stage, J, and Tos, M. The non-specific effect of endolymphatic sac surgery in treatment of Meniere's disease: a prospective, randomized controlled study comparing "classic" endolymphatic sac surgery with the insertion of a ventilating tube in the tympanic membrane. Acta Otolaryngol. (1998) 118:769–73. doi: 10.1080/00016489850182413
84. Thomsen, J, Sass, K, Odkvist, L, and Arlinger, S. Local overpressure treatment reduces vestibular symptoms in patients with Meniere's disease: a clinical, randomized, multicenter, double-blind, placebo-controlled study. Otol Neurotol. (2005) 26:68–73. doi: 10.1097/00129492-200501000-00012
85. Wilmot, TJ, and Menon, GN. Betahistine in Ménière's disease. J Laryngol Otol. (1976) 90:833–40. doi: 10.1017/S0022215100082785
86. Wu, D, Liu, B, Wang, H, Rong, P, Chen, L, Duan, J, et al. Acupuncture combined with oral western medication for Meniere's disease: a randomized controlled trial. Zhongguo Zhen Jiu. (2018) 38:1047–52. doi: 10.13703/j.0255-2930.2018.10.005
87. Wu, D, Liu, B, Wu, Y, Wang, Y, Sun, J, Yang, J, et al. Meniere disease treated with transcutaneous auricular vagus nerve stimulation combined with betahistine Mesylate: a randomized controlled trial. Brain Stimul. (2023) 16:1576–84. doi: 10.1016/j.brs.2023.10.003
88. Yang, X, Lin, C, Wu, Q, Li, L, and Mei, X. Low-sodium diet with adequate water intake improved the clinical efficacy in Ménière's disease. Acta Otolaryngol. (2024) 144:14–8. doi: 10.1080/00016489.2024.2315302
89. Yardley, L, and Kirby, S. Evaluation of booklet-based self-management of symptoms in Ménière disease: a randomized controlled trial. Psychosom Med. (2006) 68:762–9. doi: 10.1097/01.psy.0000232269.17906.92
90. Yin, G, Zhicheng, L, Zhang, S, Wang, X, Yang, J, Cai, H, et al. Analysis of the efficacy and safety of Intratymapanic dexamethasone for treating Meniere’s disease: a randomized controlled trial. Ear Nose Throat J. (2022):014556132211207. doi: 10.1177/01455613221120729
91. Motesadi Zarandi, M, Rabbani, Z, Rabbani Anari, M, Kouhi, A, and Zeinaloo, M. A study of efficacy of Nigella sativa in treatment of Meniere's disease: a randomized, placebo controlled clinical trial. J Otolaryngol. (2023) 18:97–100. doi: 10.1016/j.joto.2023.02.002
92. Zhuang, Y, Wu, P, Li, W, and Xi, S. The effectiveness of vestibular rehabilitation in Ménière's disease patients with chronic imbalance. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2022) 36:675–8;84. doi: 10.13201/j.issn.2096-7993.2022.09.005
Keywords: Menière’s disease, randomized controlled trial, outcome domain, outcome measurement instrument, core outcome set
Citation: Boreel MME, van Esch BF, van Beers MA, Kaski D, Bruintjes TD and van Benthem PPG (2025) Developing a core outcome set for Menière’s disease trials, the COSMED study: a scoping review on outcomes used in existing trials. Front. Neurol. 16:1516350. doi: 10.3389/fneur.2025.1516350
Edited by:
Athanasia Korda, University of Bern, SwitzerlandReviewed by:
Marcos Rossi-Izquierdo, Lucus Augusti University Hospital, SpainEdoardo Porto, Emory University, United States
Copyright © 2025 Boreel, van Esch, van Beers, Kaski, Bruintjes and van Benthem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maud M. E. Boreel, TS5NLkUuYm9yZWVsQGx1bWMubmw=
 Maud M. E. Boreel
Maud M. E. Boreel Babette F. van Esch
Babette F. van Esch Maartje A. van Beers
Maartje A. van Beers Diego Kaski
Diego Kaski Tjasse D. Bruintjes
Tjasse D. Bruintjes Peter Paul G. van Benthem
Peter Paul G. van Benthem