
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 27 February 2025
Sec. Stroke
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1512913
Background: The aim of this study was to construct and validate a new nomogram to predict the risk of poor outcome in patients with acute ischemic stroke (AIS) after intravenous thrombolytic therapy (IVT).
Methods: A total of 425 patients who received IVT within 4.5 h of stroke onset were included in a retrospective study. All the patients were divided into training (70%, n = 298) and validation cohorts (30%, n = 127). Poor outcome (defined as a 90-day modified Rankin Scale score 3–5) was the primary outcome. Logistic regression was used for analysis of independent risk factors for poor outcome in patients with AIS. Nomograms of poor outcome in AIS patients were constructed using R software. Discrimination and calibration of the models were assessed using area under the receiver operating characteristic (ROC) curve (AUC) and calibration plots.
Results: Multifactorial logistic regression analysis showed that SII (OR = 1.001, 95% CI: 1.000–1.002, p = 0.008), SIRI (OR = 1.584, 95% CI: 1.122–2.236, p = 0.009), NIHSS (OR = 1.101, 95% CI: 1.044–1.160, p < 0.001), and history of diabetes mellitus (OR = 2.582, 95% CI: 1.285–5.188, p = 0.008) were the independent risk factors for the occurrence of poor outcome in AIS patients. The poor outcome nomogram for AIS patients was constructed based on the above independent risk factors. The training and validation cohort AUCs of the nomogram were 0.854 (95% CI: 0.807–0.901) and 0.855 (95% CI: 0.783–0.927), respectively. The prediction models were well calibrated in both the training and validation cohorts. The net benefit of the nomograms was better when the threshold probability ranges were 4.28–66.4% and 4.01–67.8% for the training and validation cohorts, respectively.
Conclusion: New nomogram includes NIHSS, SII, SIRI and diabetes as variables with the potential to predict the risk of 90-day outcomes in patients with AIS following IVT.
Stroke, a sudden neurological disorder, is a leading cause of disability and death in adults (1). Among stroke cases, acute ischemic stroke (AIS) accounts for 60 to 80% (2). Intravenous thrombolysis with recombinant tissue-type plasminogen activator (rt-PA) within 4.5 h of onset of symptoms is the treatment of choice and significantly improves neurological function in patients (3). However, a certain percentage of patients continue to experience poor prognostic outcomes (4). Therefore, the early identification of patients at risk for poor outcome, along with timely and accurate therapeutic interventions, is crucial for improving patient recovery and outcomes (5, 6). A nomogram is a visual scoring model that utilizes biological and clinical variables to accurately calculate the probability of an individual patient’s risk for a specific clinical event (7). The chart is widely used for clinical decision-making in a wide range of conditions (8, 9). Nomograms surpass traditional scoring systems in their ability to more accurately identify patients with poor outcome, assist in selecting optimal treatment options, and enhance the quality of patient survival (10). The aim of this study was to construct a nomogram to predict the risk probability of poor outcome in AIS patients following intravenous thrombolytic therapy.
The data utilized in this study were sourced from the Hospital of North China University of Science and Technology, covering the period from June 2021 to October 2023. This retrospective cohort study included 425 patients diagnosed with acute ischemic stroke who underwent intravenous thrombolysis at our facility.
The inclusion criteria were as follows: (1) patients met the diagnostic criteria outlined in the Chinese AIS diagnostic and treatment guidelines; (2) age was ≥18 years; (3) the time from symptom onset to the administration of rt-PA intravenous thrombolysis was less than 4.5 h; (4) the modified Rankin Scale (mRS) score was ≤2 prior to the onset of the disease; and (5) patients provided signed informed consent. Patients with the following conditions were excluded from the study: (1) those who underwent intravenous thrombolysis followed by arterial thrombolysis or endovascular thrombolysis; (2) individuals with autoimmune diseases; and (3) patients with incomplete clinical data. This study received review and approval from the Institutional Research Review Board of North China University of Science and Technology Hospital.
Demographic characteristics of all participating patients were collected, including age, National Institutes of Health Stroke Scale (NIHSS) score, sex, and vascular risk factors such as history of alcohol consumption, hypertension, smoking, diabetes mellitus, previous stroke, hyperlipidemia, coronary heart disease, hyperhomocysteinemia, and atrial fibrillation. Clinical information gathered included systolic blood pressure, diastolic blood pressure, and blood glucose levels. Laboratory data encompassed SII, SIRI, platelet count, total cholesterol, LDL cholesterol, triglycerides, and HDL cholesterol. All blood samples used for laboratory testing were obtained from the first blood draw conducted just prior to the patient’s admission for intravenous thrombolysis. Hyperhomocysteinemia is defined as an elevated serum homocysteine concentration of more than 15 μmol/L (11). SIRI is defined as the product of neutrophil count and monocyte count divided by lymphocyte count, while SII is calculated as the product of platelet count and neutrophil count divided by lymphocyte count.
The outcome was determined by the modified Rankin Score (mRs) at 90 days after thrombolysis. Poor outcome was defined as an mRs score of 3–5.
Upon admission, all patients received guideline-based therapy, specifically recombinant tissue plasminogen activator, administered within 4.5 h of stroke onset. The dosage was established at 0.9 mg/kg, with a maximum limit of 90 mg. Ten percent of the total dose was administered as an intravenous bolus, followed by a continuous intravenous infusion of the remaining 90% over a duration of 60 min.
Data analysis and nomogram construction were conducted utilizing SPSS software version 27.0 and R software version 4.4.0. Measurement data were reported as means with standard deviations (SDs) or medians with interquartile ranges (IQRs). Group comparisons were performed using t-tests or Mann–Whitney U nonparametric tests, while count data were expressed as frequencies with percentages. Comparisons between the two groups were assessed using the χ2 test.
Variables that achieved statistical significance at p < 0.05 in the univariate analysis were subsequently included in the multivariate logistic regression analysis to identify independent risk factors associated with poor prognostic outcomes at 90 days. Event outcomes were assigned values of “1” for poor outcome and “0” for good outcome. Independent risk factor variables were incorporated using R software to develop a new nomogram prediction model.
A total of 465 patients who received intravenous thrombolysis with rt-PA were enrolled in this study. A total of 42 patients were excluded from the study due to various reasons: 21 patients underwent endovascular thrombolysis, 6 patients had autoimmune diseases, and 15 patients had incomplete follow-up data. Ultimately, 425 patients were successfully enrolled in this study, among which 102 patients experienced poor outcome, resulting in an incidence rate of 24%.
All subjects who met the established criteria were randomly assigned to the training cohort (n = 298) and the validation cohort (n = 127) in a 7:3 ratio. The median age of participants was 66 (57, 72) years in the training cohort and 65 (59, 71) in the validation cohort. There were no significant differences between the two groups regarding age, gender, or medical history (all p > 0.05, Table 1).
Univariate analysis of the training set showed that NIHSS, SII, SIRI, age, and history of diabetes were significantly associated with poor outcome in patients with AIS. Further multifactorial logistic regression analysis confirmed that SII (OR = 1.001, 95% CI: 1.000–1.002, p = 0.008), SIRI (OR = 1.584, 95% CI: 1.122–2.236, p = 0.009), NIHSS (OR = 1.101, 95% CI: 1.044–1.160, p < 0.001), and history of diabetes mellitus (OR = 2.582, 95% CI: 1.285–5.188, p = 0.008) were identified as independent risk factors for the development of poor outcome in patients with AIS (Table 2).
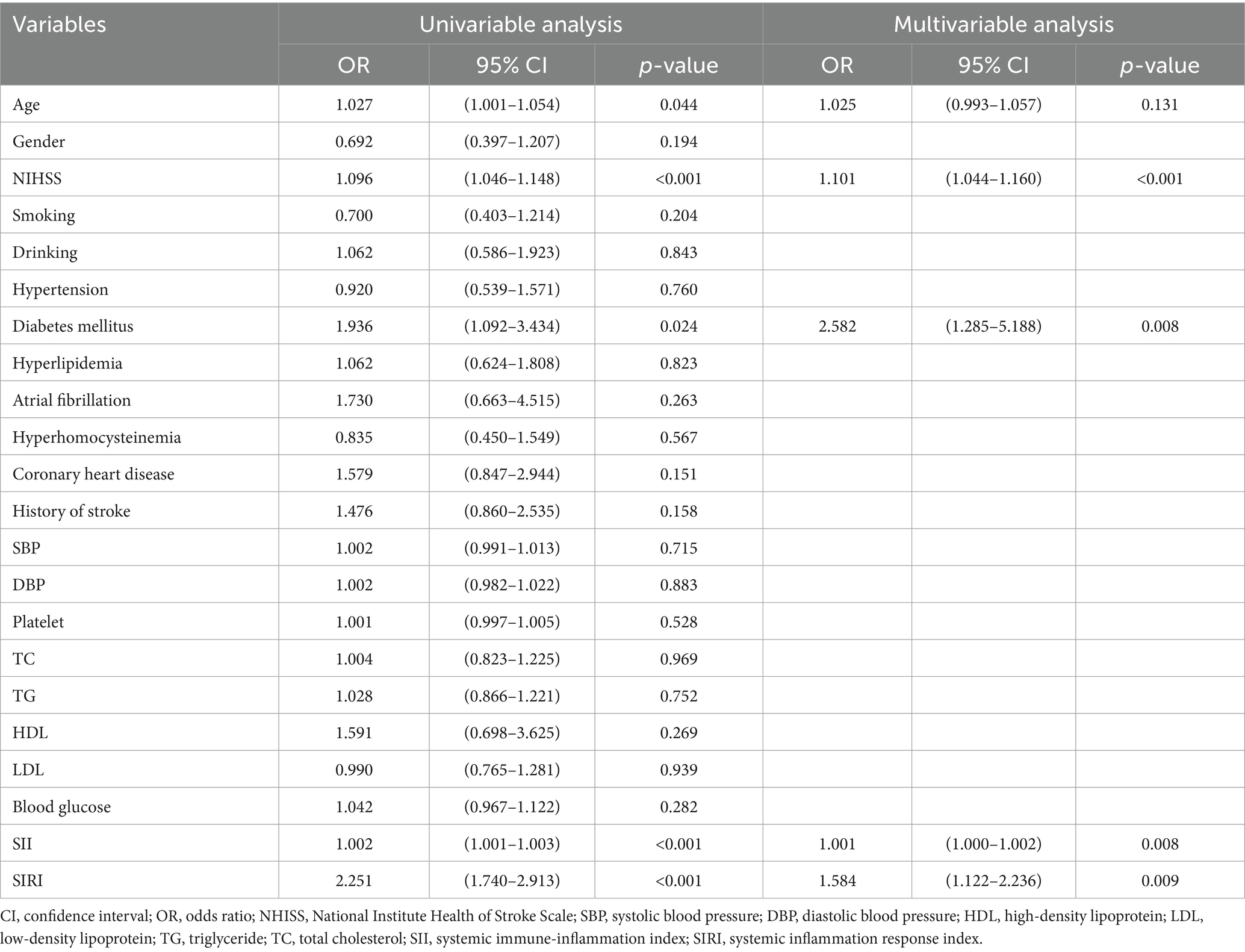
Table 2. Parameters of poor outcome risk in AIS patients after IVT therapy based on univariable and multivariable analyses of the training dataset.
A nomogram was constructed to predict poor outcome following intravenous thrombolytic therapy in patients with AIS, based on independent risk factors (Figure 1). The likelihood of poor outcome for the corresponding patients was subsequently calculated. The predictive efficacy of this line plot was evaluated using area under the receiver operating characteristic curve (ROC-AUC) analysis (Figure 2; Table 3). The AUC values for the training and validation cohorts were 0.854 (95% CI: 0.807–0.901) and 0.855 (95% CI: 0.783–0.927), respectively. Calibration plots indicated that the predicted values for both the training and validation cohorts generally aligned with the actual observed values (Figure 3). To further validate the predictive ability of this nomogram regarding the incidence of poor outcome, we employed a decision curve analysis. As illustrated in Figure 4, the net benefit of utilizing the nomogram was found to be relatively high within the threshold probability ranges of 4.28 to 66.4% for the training cohort and 4.01 to 67.8% for the validation cohort.
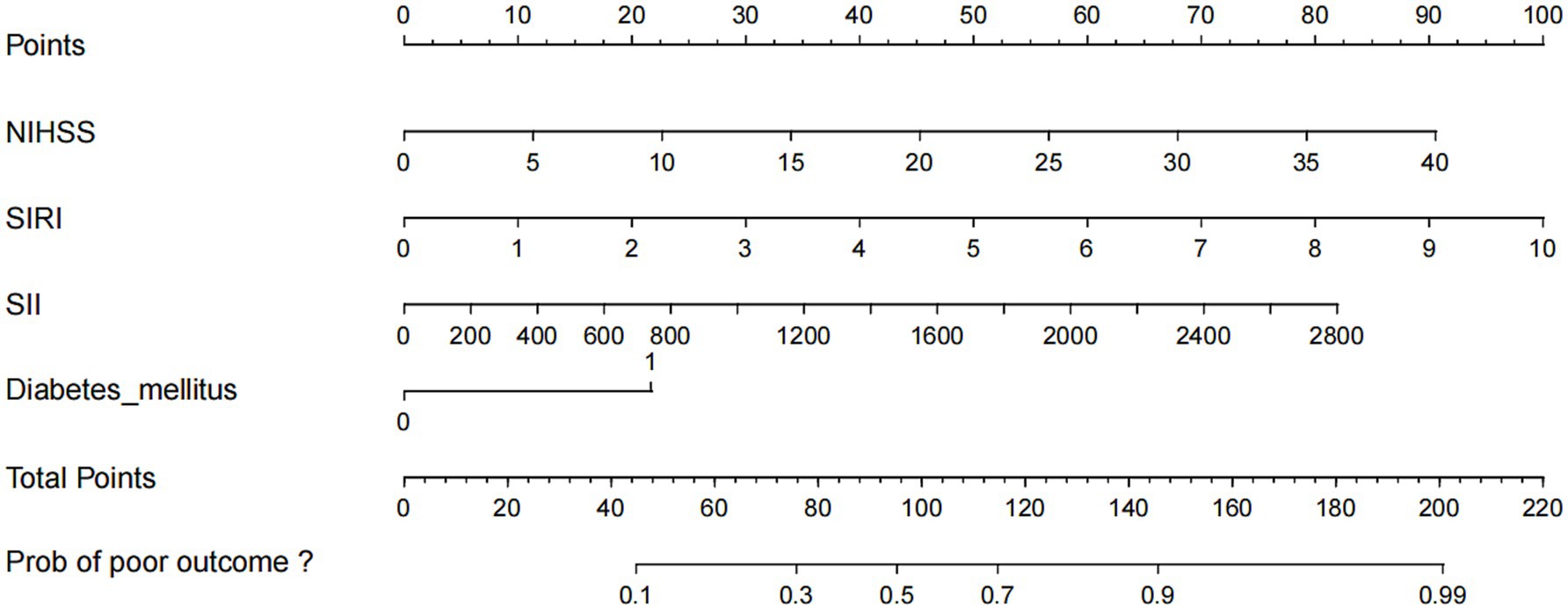
Figure 1. Nomogram for predicting the risk of poor outcome in AIS patients with IVT therapy. Each of the four indicator scores is aligned with the “Points” line. The four scores are then summed to produce a “Total Points” score, which can be utilized to predict the risk of poor outcome. NHISS, National Institute Health of Stroke Scale; SIRI, systemic inflammation response index; SII, systemic immune-inflammation index.
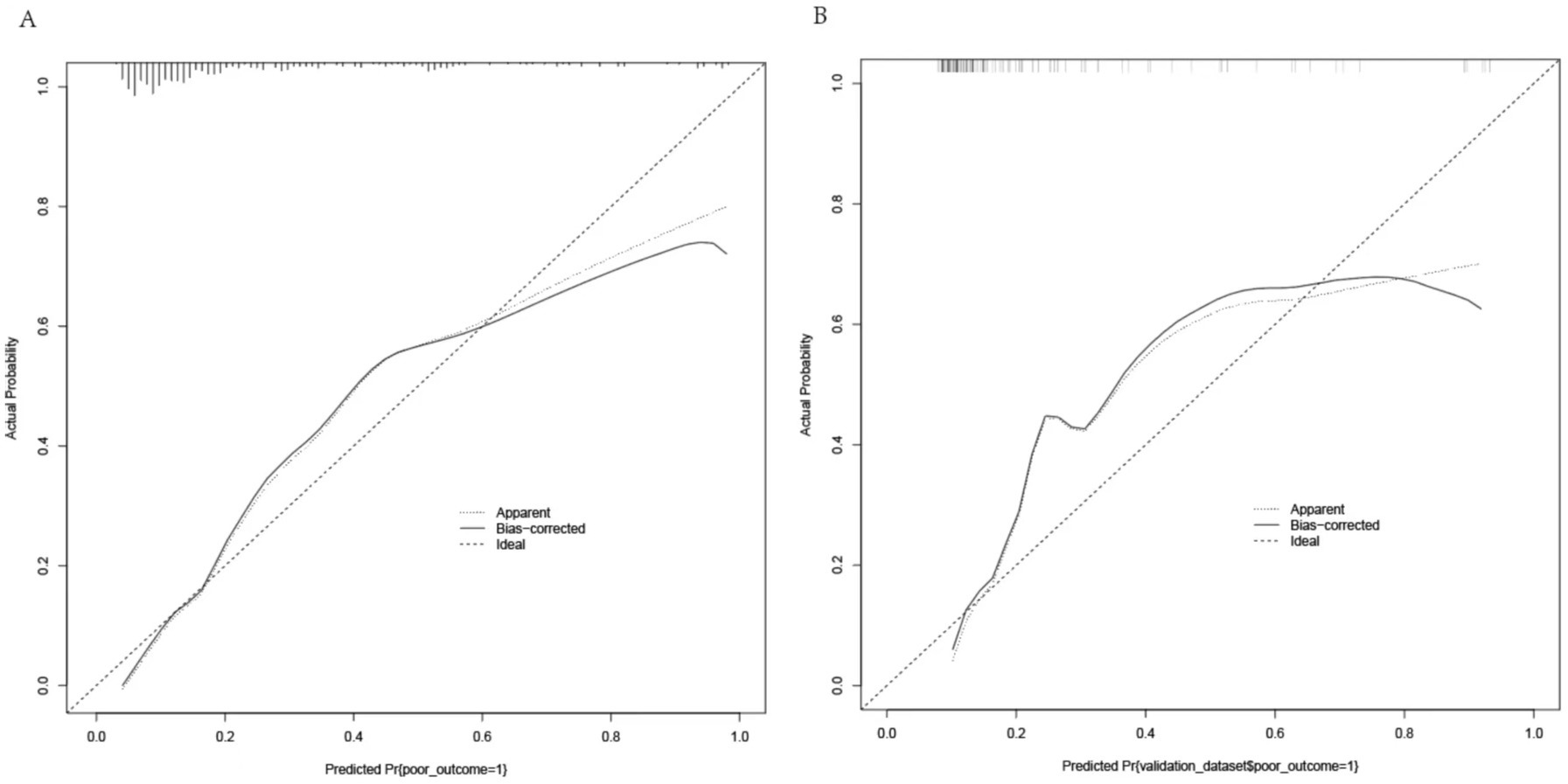
Figure 3. Calibration curves for the nomogram. (A) Training cohort: MAD = 0.042, n = 298. (B) Validation cohort: MAD = 0.067, n = 127. Each cohort was repeated 1,000 times.
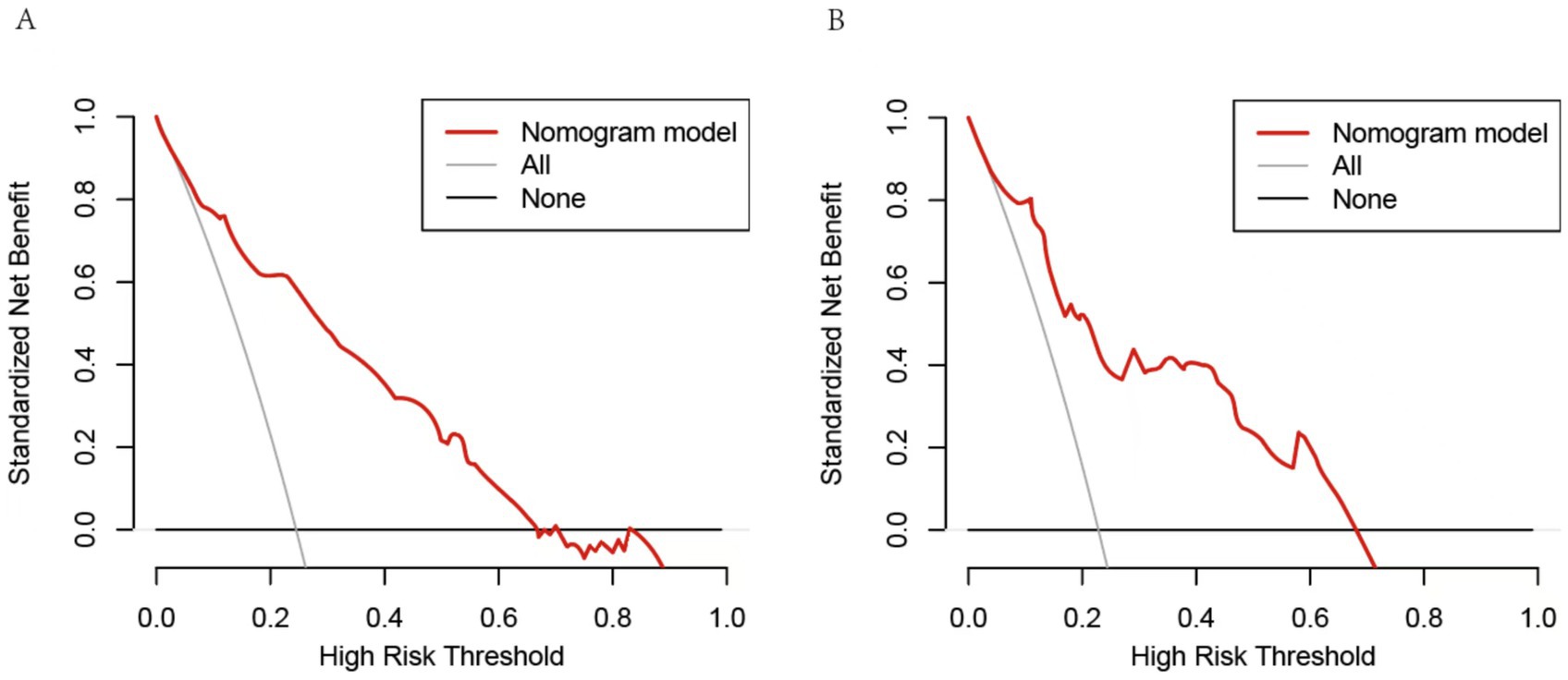
Figure 4. Decision curve analysis of the nomogram. (A) The threshold probability range for the training set was 4.28 to 66.4%. (B) The threshold probability range for the testing cohort was 4.01 to 67.8%. The horizontal line represents a scenario in which all factors are associated with a net benefit of zero. The dotted line means all patients who accept intravenous thrombolysis will develop poor outcome. The curves above were compared based on net benefit, represented by a backslash with a negative slope.
This study included a total of 425 patients with acute ischemic stroke (AIS) who were treated with intravenous thrombolysis. Among these patients, 102 (24.0%) exhibited a poor outcome, indicating a high probability of adverse outcomes in AIS patients. Furthermore, the existing prediction models appear inadequate for accurately assessing patient outcome. Consequently, there is an urgent need for the development of new prediction models to assist clinicians in providing precise risk assessments and improving patient outcomes.
Our study identified that the systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), National Institutes of Health Stroke Scale (NIHSS) score, and a history of diabetes were independent risk factors for poor short-term outcome following intravenous thrombolytic therapy in patients with acute ischemic stroke (AIS). Utilizing these independent risk factors, we developed and validated a nomogram prediction model aimed at forecasting poor outcome after intravenous thrombolytic therapy in AIS patients. Among these, SII and SIRI serve as stable markers of the inflammatory response and can be easily obtained through blood cell counts, making them both practical and reproducible. Originally proposed to be associated with poor outcomes in cancer patients (12, 13), SII and SIRI incorporate a composite index of three distinct leukocyte and platelet subpopulations, offering new insights by integrating the interplay of platelet increase, inflammation, and immunity (14, 15). As novel indicators of inflammation, SII and SIRI are linked to the inflammatory response and secondary brain damage in patients with AIS (16). Following the onset of cerebral ischemia, inflammatory cells respond rapidly, with neutrophils being the first to appear in the penumbra and infarct core (17). Once activated, neutrophils exacerbate brain edema and damage the blood-brain barrier by releasing pro-inflammatory mediators and metalloproteinases. Concurrently, additional monocytes migrate to the ischemic brain tissue, further worsening cerebral ischemia-reperfusion injury (18). An animal experiment showed that necrotic platelets interact with neutrophils during reperfusion, and rapid platelet exposure is extremely critical for inducing CypD-mediated platelet necrosis and necrosis-dependent brain injury (19). In contrast, lymphocytes primarily serve a protective function, capable of modulating the inflammatory response following a stroke, mitigating the disruption of the blood–brain barrier, and facilitating the recovery of neurological function (20). Furthermore, previous studies have indicated that elevated SII and SIRI can serve as predictors for post-stroke cognitive impairment in patients (21, 22). Additionally, research has demonstrated that SIRI and SII values correlate with a poor 90-day outcome in patients with AIS who have undergone intravenous thrombolysis, aligning with the findings of this study (23, 24).
This study demonstrates that the NIHSS score is an independent risk factor for poor 90-day outcome in patients with AIS, aligning with findings from previous research (25, 26). The NIHSS score serves as an indicator of disease severity in AIS patients; an elevated score suggests greater vascular occlusion or compromised collateral circulation capacity (27). Furthermore, the NIHSS score can predict the location and extent of infarction in stroke patients (28). Research indicates that the baseline NIHSS score also predicts post-thrombolytic hemorrhagic transformation in patients undergoing rt-PA intravenous thrombolysis (29, 30). In this study, a history of diabetes emerged as an independent risk factor for poor outcome following intravenous thrombolysis in AIS patients, with those having diabetes being 2.582 times more likely to experience poor outcomes compared to their non-diabetic counterparts. Tang et al. demonstrated that diabetes is an independent predictor of early neurologic improvement after IVT and a risk factor for incomplete recanalization at 24 h post-treatment (31). Additionally, previous studies have indicated that stroke patients with diabetes mellitus exhibit higher rates of disability and mortality following hospital discharge (32).
In the present study, novel inflammatory markers SII and SIRI were incorporated into the Short-term Adverse Outcome Scale for patients with AIS. Independent risk factors, including the NIHSS score and a history of diabetes mellitus, were also integrated into the nomogram to explore the risk of poor outcomes in AIS patients following intravenous thrombolysis in a comprehensive and cohesive manner. Almost all healthcare organizations, even those in economically disadvantaged areas, have these predictors readily available. Furthermore, validation of the nomogram indicated that the predictive model exhibited good differentiation and calibration, suggesting it may serve as a reliable tool for predicting the risk of poor outcomes in AIS patients treated with rt-PA. For patients identified by our nomogram as being at high risk for poor outcomes following thrombolysis, clinicians may consider alternative treatments to enhance clinical benefits. However, this study has certain limitations: first, it is a retrospective analysis; second, it is a single-center study, and its results have not been fully validated in an external cohort. Future prospective multicenter studies are necessary. More importantly, external validation in other cohorts is still needed before formal routine clinical practice can be established. This will provide a more reliable scientific basis for the therapeutic and prognostic assessment of patients with AIS.
Our study proposes a novel and practical nomogram that incorporates SII, SIRI, the NIHSS score, and a history of diabetes to effectively predict the probability of an unfavorable outcome at 90-day following intravenous thrombolysis in patients with ischemic stroke.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by North China University of Science and Technology Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YZ: Writing – original draft, Writing – review & editing, Data curation, Resources. RZ: Writing – review & editing. PL: Writing – review & editing. ZZ: Writing – review & editing. HY: Writing – review & editing. ZS: Writing – review & editing. YX: Writing – review & editing. AM: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Hebei Province Medical Applicable Technology Tracking Project (GZ2020041) and Clinical Medicine Training Programme of Hebei Provincial Health and Wellness Commission (Ji Wei Ban Science and Education [2021] No. 9).
The authors gratefully thank physicians and nurses in neurological critical care unit of Affiliated Hospital of North China University of Science and Technology for their hard work in contributing data to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1512913/full#supplementary-material
1. Mishra, A, Malik, R, Hachiya, T, Jürgenson, T, Namba, S, Posner, DC, et al. Stroke genetics informs drug discovery and risk prediction across ancestries. Nature. (2022) 611:115–23. doi: 10.1038/s41586-022-05165-3
2. Chang, RW, Tucker, LY, Rothenberg, KA, Lancaster, E, Faruqi, RM, Kuang, HC, et al. Incidence of ischemic stroke in patients with asymptomatic severe carotid stenosis without surgical intervention. JAMA. (2022) 327:1974–82. doi: 10.1001/jama.2022.4835
3. Hacke, W, Kaste, M, Bluhmki, E, Brozman, M, Dávalos, A, Guidetti, D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. (2008) 359:1317–29. doi: 10.1056/NEJMoa0804656
4. Jiang, B, Zhu, G, Xie, Y, Heit, JJ, Chen, H, Li, Y, et al. Prediction of clinical outcome in patients with large-vessel acute ischemic stroke: performance of machine learning versus SPAN-100. AJNR Am J Neuroradiol. (2021) 42:240–6. doi: 10.3174/ajnr.A6918
5. Zeinhom, MG, Khalil, MFE, Kamel, IFM, Kohail, AM, Ahmed, SR, Elbassiouny, A, et al. Predictors of the unfavorable outcomes in acute ischemic stroke patients treated with alteplase, a multi-center randomized trial. Sci Rep. (2024) 14:5960. doi: 10.1038/s41598-024-56067-5
6. Berge, E, Whiteley, W, Audebert, H, De Marchis, GM, Fonseca, AC, Padiglioni, C, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. (2021) 6:I–LXII. doi: 10.1177/2396987321989865
7. Iasonos, A, Schrag, D, Raj, GV, and Panageas, KS. How to build and interpret a nomogram for cancer outcome. J Clin Oncol. (2008) 26:1364–70. doi: 10.1200/JCO.2007.12.9791
8. Miao, X, Guo, Y, Ding, L, Xu, X, Zhao, K, Zhu, H, et al. A dynamic online nomogram for predicting the heterogeneity trajectories of frailty among elderly gastric cancer survivors. Int J Nurs Stud. (2024) 153:104716. doi: 10.1016/j.ijnurstu.2024.104716
9. Gao, Y, and Gan, X. A novel nomogram for the prediction of subsyndromal delirium in patients in intensive care units: a prospective, nested case-controlled study. Int J Nurs Stud. (2024) 155:104767. doi: 10.1016/j.ijnurstu.2024.104767
10. Zhang, X, Peng, M, Feng, C, Wang, H, Gong, P, Jiang, T, et al. Nomogram predicting early neurological improvement in ischaemic stroke patients treated with endovascular thrombectomy. Eur J Neurol. (2021) 28:152–60. doi: 10.1111/ene.14510
11. Zaric, BL, Obradovic, M, Bajic, V, Haidara, MA, Jovanovic, M, and Isenovic, ER. Homocysteine and hyperhomocysteinaemia. Curr Med Chem. (2019) 26:2948–61. doi: 10.2174/0929867325666180313105949
12. Hu, B, Yang, XR, Xu, Y, Sun, YF, Sun, C, Guo, W, et al. Systemic immune-inflammation index predicts outcome of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
13. Geng, Y, Zhu, D, Wu, C, Wu, J, Wang, Q, Li, R, et al. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. Int Immunopharmacol. (2018) 65:503–10. doi: 10.1016/j.intimp.2018.10.002
14. Dong, W, Gong, Y, Zhao, J, Wang, Y, Li, B, and Yang, Y. A combined analysis of TyG index, SII index, and SIRI index: positive association with CHD risk and coronary atherosclerosis severity in patients with NAFLD. Front Endocrinol. (2024) 14:1281839. doi: 10.3389/fendo.2023.1281839
15. Cui, S, Cao, S, Chen, Q, He, Q, and Lang, R. Preoperative systemic inflammatory response index predicts the outcome of patients with hepatocellular carcinoma after liver transplantation. Front Immunol. (2023) 14:1118053. doi: 10.3389/fimmu.2023.1118053
16. Wu, F, Liu, Z, Zhou, L, Ye, D, Zhu, Y, Huang, K, et al. Systemic immune responses after ischemic stroke: from the center to the periphery. Front Immunol. (2022) 13:911661. doi: 10.3389/fimmu.2022.911661
17. Jayaraj, RL, Azimullah, S, Beiram, R, Jalal, FY, and Rosenberg, GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. (2019) 16:142. doi: 10.1186/s12974-019-1516-2
18. Xie, L, Zhang, S, Huang, L, Peng, Z, Lu, H, He, Q, et al. Single-cell RNA sequencing of peripheral blood reveals that monocytes with high cathepsin S expression aggravate cerebral ischemia-reperfusion injury. Brain Behav Immun. (2023) 107:330–44. doi: 10.1016/j.bbi.2022.11.001
19. Denorme, F, Manne, BK, Portier, I, Eustes, AS, Kosaka, Y, Kile, BT, et al. Platelet necrosis mediates ischemic stroke outcome in mice. Blood. (2020) 135:429–40. doi: 10.1182/blood.2019002124
20. Macrez, R, Ali, C, Toutirais, O, Le Mauff, B, Defer, G, Dirnagl, U, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. (2011) 10:471–80. doi: 10.1016/S1474-4422(11)70066-7
21. Chu, M, Luo, Y, Wang, D, Liu, Y, Wang, D, Wang, Y, et al. Systemic inflammation response index predicts 3-month outcome in patients with mild acute ischemic stroke receiving intravenous thrombolysis. Front Neurol. (2023) 14:1095668. doi: 10.3389/fneur.2023.1095668
22. Cheng, Y, Zhu, H, Liu, C, Li, L, Lin, F, Guo, Y, et al. Systemic immune-inflammation index upon admission correlates to post-stroke cognitive impairment in patients with acute ischemic stroke. Aging. (2024) 16:8810–21. doi: 10.18632/aging.205839
23. Ma, F, Li, L, Xu, L, Wu, J, Zhang, A, Liao, J, et al. The relationship between systemic inflammation index, systemic immune-inflammatory index, and inflammatory prognostic index and 90-day outcomes in acute ischemic stroke patients treated with intravenous thrombolysis. J Neuroinflammation. (2023) 20:220. doi: 10.1186/s12974-023-02890-y
24. Weng, Y, Zeng, T, Huang, H, Ren, J, Wang, J, Yang, C, et al. Systemic immune-inflammation index predicts 3-month functional outcome in acute ischemic stroke patients treated with intravenous thrombolysis. Clin Interv Aging. (2021) 16:877–86. doi: 10.2147/CIA.S311047
25. Ping, Z, Min, L, Qiuyun, L, Xu, C, and Qingke, B. Prognostic nomogram for the outcomes in acute stroke patients with intravenous thrombolysis. Front Neurosci. (2022) 16:1017883. doi: 10.3389/fnins.2022.1017883
26. Mehta, A, Mahale, R, Buddaraju, K, Majeed, A, Sharma, S, Javali, M, et al. Intravenous thrombolysis for acute ischemic stroke: review of 97 patients. J Neurosci Rural Pract. (2017) 8:038–43. doi: 10.4103/0976-3147.193558
27. Heldner, MR, Zubler, C, Mattle, HP, Schroth, G, Weck, A, Mono, ML, et al. National Institutes of Health Stroke Scale score and vessel occlusion in 2,152 patients with acute ischemic stroke. Stroke. (2013) 44:1153–7. doi: 10.1161/STROKEAHA.111.000604
28. Agis, D, Goggins, MB, Oishi, K, Oishi, K, Davis, C, Wright, A, et al. Picturing the size and site of stroke with an expanded National Institutes of Health Stroke Scale. Stroke. (2016) 47:1459–65. doi: 10.1161/STROKEAHA.115.012324
29. Jiang, Z, Xu, D, Li, H, and Wu, X. A novel nomogram to predict symptomatic intracranial hemorrhage in ischemic stroke patients after intravenous thrombolysis. Ther Clin Risk Manag. (2023) 19:993–1003. doi: 10.2147/TCRM.S436145
30. Guo, H, Xu, W, Zhang, X, Zhang, S, Dai, Z, Li, S, et al. A nomogram to predict symptomatic intracranial hemorrhage after intravenous thrombolysis in Chinese patients. Neuropsychiatr Dis Treat. (2021) 17:2183–90. doi: 10.2147/NDT.S320574
31. Tang, H, Zhang, S, Yan, S, Liebeskind, DS, Sun, J, Ding, X, et al. Unfavorable neurological outcome in diabetic patients with acute ischemic stroke is associated with incomplete recanalization after intravenous thrombolysis. J Neurointerv Surg. (2016) 8:342–6. doi: 10.1136/neurintsurg-2014-011643
32. Saposnik, G, Fang, J, Kapral, MK, Tu, JV, Mamdani, M, Austin, P, et al. The iScore predicts effectiveness of thrombolytic therapy for acute ischemic stroke. Stroke. (2012) 43:1315–22. doi: 10.1161/STROKEAHA.111.646265
Keywords: acute ischemic stroke, SII, SIRI, intravenous thrombolysis, nomogram
Citation: Zhao Y, Zhang R, Li P, Zhang Z, Yu H, Su Z, Xia Y and Meng A (2025) A new nomogram for predicting 90-day outcomes of intravenous thrombolysis in patients with acute ischaemic stroke. Front. Neurol. 16:1512913. doi: 10.3389/fneur.2025.1512913
Received: 17 October 2024; Accepted: 07 February 2025;
Published: 27 February 2025.
Edited by:
Majaz Moonis, UMass Memorial Medical Center, United StatesReviewed by:
Ibraheem Alkhawaldeh, Mutah University, JordanCopyright © 2025 Zhao, Zhang, Li, Zhang, Yu, Su, Xia and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aiguo Meng, bWFnbWFzdGVyQHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.