
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 26 February 2025
Sec. Neurorehabilitation
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1499133
Introduction: Chronic pelvic pain syndrome is a common condition characterized by persistent symptoms that are difficult to treat. Repetitive transcranial magnetic stimulation (rTMS) is considered a safe treatment option for alleviating chronic pelvic pain, but different stimulation protocols can affect pain relief outcomes. Establishing an optimal stimulation protocol can enhance the uniformity and consistency of rTMS to provide a potentially effective therapeutic intervention. This review sought to systematically review and assess the existing literature on transcranial magnetic stimulation in patients experiencing chronic pelvic pain syndrome, evaluate the therapeutic efficacy, and determine the most effective stimulation protocol.
Methods: A comprehensive search was conducted across three databases, supplemented by manual searches. Two researchers independently reviewed and extracted relevant studies and subsequently performed a thorough analysis of all available clinical data.
Results: A total of eight studies were ultimately incorporated into the analysis. These comprised two randomized controlled trials, one self-controlled trial, two case reports, and three prospective studies. All studies demonstrated a notable reduction in pain scores post-treatment.
Conclusion: rTMS has demonstrated efficacy in alleviating pain in individuals suffering from chronic pelvic pain syndrome. It is regarded as a safe intervention with minimal adverse effects. Nonetheless, the variability observed across studies hindered our ability to conclusively determine the most effective stimulation sites and parameters. Additional research is essential to reduce bias, enhance methodological rigor, and ascertain the optimal conditions and indications for brain stimulation to optimize the therapeutic effectiveness of rTMS.
Systematic Review Registration: https://inplasy.com/projects/, identifier INPLASY2023120112.
Chronic pelvic pain syndrome (CPPS) is characterized by enduring or recurrent pain in the pelvic region lasting for at least 3 months without a definitive pathological explanation, and it affects both males and females. The main symptoms include pelvic floor issues, bowel problems, lower urinary tract problems, sexual dysfunction, and gynecological concerns. Moreover, negative emotional, behavioral, and cognitive reactions are often elicited by these symptoms (1). Globally, estimates indicate that the prevalence of CPPS is between 2 and 16% in the male population and up to 24% in the female population (2, 3). CPPS can significantly impact patients’ social engagement and overall quality of life, resulting in heightened feelings of generalized anxiety and depression. As a result, this situation causes great stress for families and society as a whole (4). It is projected that the annual cost of CPPS management will reach $880 million (5).
Individuals with CPPS frequently have impaired pain regulation and increased sensitivity in both the peripheral and central neural systems (6–8). Physical therapy, medication, and nerve block treatments are the current choices for treating CPPS (9, 10). In modern physiotherapy, neuromodulation is being used for the treatment of CPPS patients more and more frequently (11). Transcranial magnetic stimulation (TMS) is a non-invasive, painless neuromodulatory technique that uses pulsed magnetic fields to change the brain metabolism and neural electrical activity. This can result in a range of physiological and biochemical effects (12). Repetitive TMS, or rTMS, is a technique that has been used in the therapeutic management of a variety of neurological and psychiatric conditions since groundbreaking research was published in 1985 that detailed the use of magnetic fields to alter electrical signals within the brain (13–15). Recent developments in neuro-navigation and non-invasive stimulation technology have extended the use of rTMS to the treatment of various types of chronic refractory pain viable (16). Some small-scale studies have revealed that rTMS could be useful in reducing pelvic discomfort in people with CPPS (17, 18). The methodologies employed in the various studies into rTMS have exhibited significant variability, particularly in factors such as the specific rTMS device utilized, the configuration of the coil, and the designated stimulation site, as well as the frequency and intensity of the stimulation. Additionally, differences in the number and duration of stimulations, the total number of sessions conducted, and the overall number of pulses administered have further contributed to this variability. Consequently, it is clear that the outcomes may differ based on the targeted area of stimulation and the selected frequency. At present, there are no standardized protocols for the treatment of CPPS utilizing TMS. The treatment protocols that incorporate TMS for individuals with CPPS remain to be validated through extensive randomized controlled trials. This review seeks to assess the efficacy and safety of TMS in alleviating pain associated with CPPS by examining the current body of literature.
To identify studies, we conducted a comprehensive search of the PubMed, Embase, and Cochrane Library databases, utilizing the term “transcranial magnetic stimulation (TMS)” as a medical MESH term, with no time restrictions applied to any of the search fields. The last search date was February 19, 2024. This search encompassed all relevant studies available in these databases. The search was further refined by including one of the following four keywords or MESH terms in the title or abstract: Term A: “Neuralgia*, Perineal,” “Perineal Neuralgia*,” “Pelvic Pain,” “Pelvic Girdle Pain” (n = 78); Term B: “Anorectal disease,” “Rect* pain,” “an* pain” (n = 49); Term C: “Pelvic Inflammatory Disease,” “Pelvic Infection” (n = 15); Term D: “Prostatitis,” “Chronic prostate pain” (n = 23). The reference lists of the retrieved articles were manually examined, resulting in the identification of a total of 7 additional articles.
The criteria for inclusion were as follows: (1) original research articles; (2) research involving human subjects; (3) publications in the English language; (4) research activities were concentrated on rTMS; and (5) studies evaluating patients showing chronic pelvic pain without any clear underlying pathology.
The exclusion criteria were as follows: (1) book chapters, commentaries, meta-analyses, systematic reviews, letters to the editor, and comments; (2) articles in which rTMS was not employed as a therapy option; (3) articles that did not provide data on the outcomes of pain treatment; and (4) articles involving patients under the age of 18.
The complete set of articles retrieved was used in the final screening, and additional publications meeting the inclusion and exclusion criteria were selected from the reference lists of the retrieved articles.
Based on previous literature searches, we anticipated that the included studies would employ diverse therapeutic intervention parameters and exhibit heterogeneity in their dimensions of effect measurement. Due to certain obstacles that prevented us from conducting a thorough and convincing quantitative meta-analysis, we chose to provide a narrative overview of the study results instead (19). All studies were grouped and summarized based on the applied therapeutic parameter models, and the treatment effects were described.
The findings of the literature search and the selected articles are depicted in the PRISMA flowchart presented in Figure 1. Comprehensive articles were ultimately chosen, and further full-text publications that satisfied the inclusion criteria were identified through a review of the references in these articles. Duplicate publications were eliminated by utilizing EndNote software. After that, the titles and abstracts of the articles were scrutinized, with two researchers embarked on a thorough independent review. They carefully selected and kept the full-text versions of all articles that met the inclusion criteria. Patient characteristics, treatment details, and clinical results data were also extracted. Any inconsistencies were addressed through discussions between the two researchers.
This review was based on previous research and did not involve any studies conducted by the authors on human participants or animals.
Our literature search strategy identified a total of 172 articles, of which nine met the inclusion criteria. However, one study used pelvic floor surface electromyography values as the outcome measure and was, therefore, excluded from the analysis. The remaining eight studies (two randomized controlled trials, one pre-post self-controlled study, two case reports, and three prospective studies) were included. The patients included those with chronic prostatitis/CPPS, chronic pelvic pain (CPP), irritable bowel syndrome, perineal pain, and urological CPP syndrome. Table 1 summarizes the characteristics of the included studies and provides a detailed summary of the treatment regimens and results.
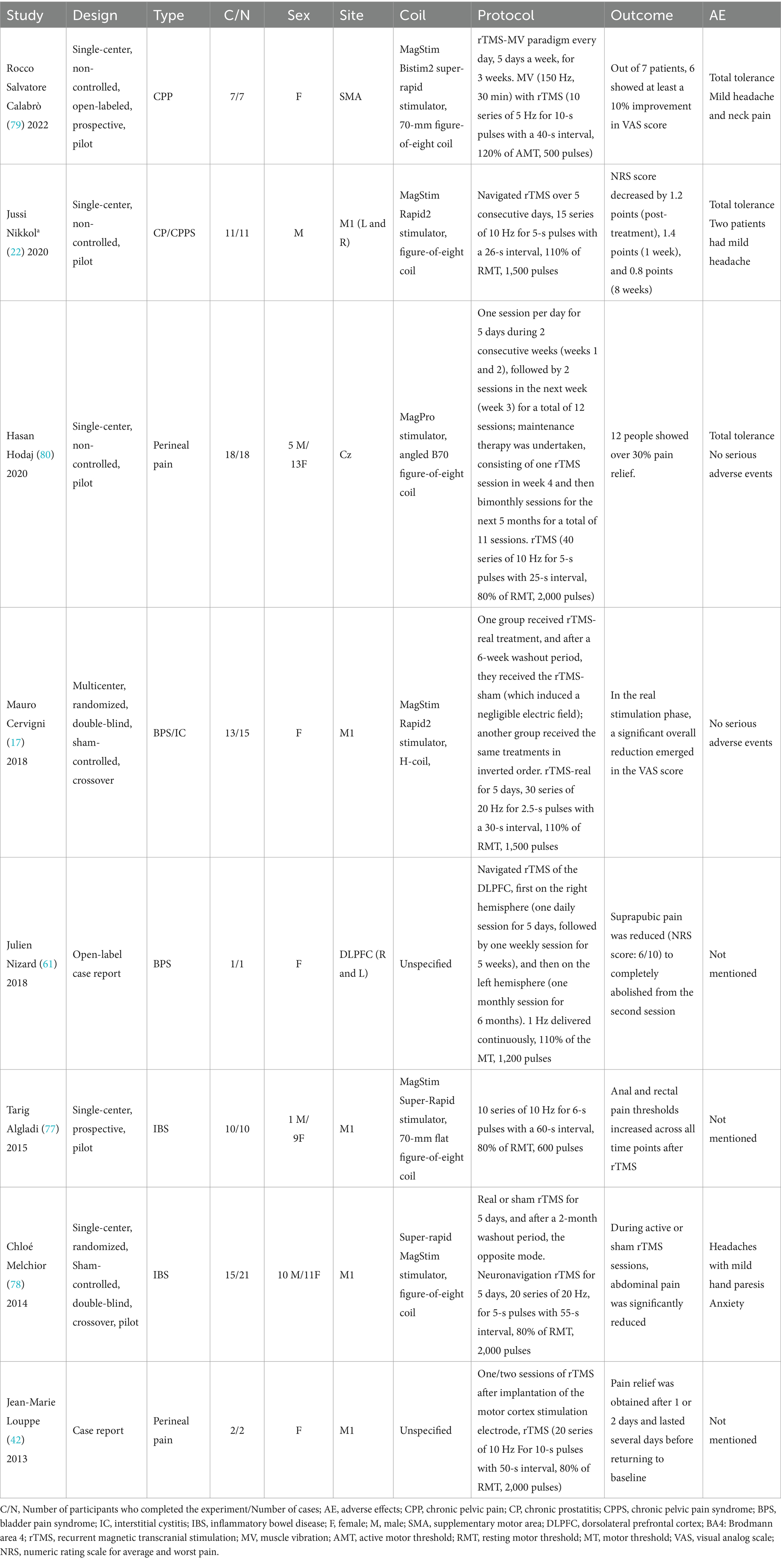
Table 1. An overview of the protocols and results documented in the studies incorporated within this review.
Analysis of data from the eight studies showed that seven reported a decrease in pain scores, whereas one reported an increase in the pain threshold. The included literature comprised single-arm clinical studies; therefore, the JBI scale was utilized for quality assessment, as shown in Table 2. A total of 85 patients received rTMS, and 77 completed the treatment, with 58 experiencing a decrease in pain scores and 10 showing an increase in the pain threshold. After excluding two case reports and one study with elevated pain thresholds, the effectiveness rates of pain treatment from the remaining five studies were combined using RevMan software and presented in a forest plot, as shown in Figure 2. The visual analog scale (VAS) is the most frequently utilized instrument for pain assessment.
The rTMS frequency ranged from 1 Hz to 20 Hz, with high-frequency stimulation used in seven trials, including 10 Hz in four trials, 20 Hz in two trials, and 5 Hz in one trial. Only one trial used low-frequency (1 Hz) rTMS. The results clearly indicated that high-frequency rTMS had an analgesic effect. Further research is needed to investigate the effectiveness of low-frequency 1 Hz rTMS.
In the eight trials, the primary target for stimulation was the M1 area (five times), with one describing the target area as Brodmann’s area 4, one trial targeting the Cz point, and the remaining studies targeting the dorsolateral prefrontal cortex (DLPFC) and supplementary motor area (SMA). During the baseline, treatment, and follow-up periods after stimulation, all experiments showed there was modulation of pain in response to rTMS. A comprehensive analysis indicates that transcranial magnetic stimulation of the M1 region of the brain can effectively reduce pain scores in individuals with chronic pelvic pain syndrome. However, further research is necessary to explore its application in the SMA and DLPFC.
CPPS is a pain syndrome that does not involve any distinct pathological alterations. It predominantly involves pelvic-related (pelvic pain and pelvic girdle pain), nerve-related (perineal pain and neuralgia), and anorectal diseases (rectal pain and anal pain), which occur in both sexes; gynecological diseases (pelvic inflammatory disease and pelvic infection), which occur only in females; and prostate-related diseases (chronic prostate pain and prostatitis), which occur only in males. While the etiologies of the pain associated with these conditions may differ, they all impact the pelvic floor region, are characterized by specific symptoms, and lead to chronic and challenging pain management. People have shorter lifespans and spend more of their income on healthcare as a result (20). Numerous social and psychological factors, as well as hypertense pelvic floor muscles (PFMs) and the enhanced sensitivity of peripheral and central nerves, are just some of the multi-faceted causes of CPPS (21). According to a few limited and preliminary findings from recent studies, rTMS shows promise as a treatment for CPPS (22). However, our incomplete understanding of the mechanisms underlying the effects of rTMS and the varying analgesic outcomes of rTMS under different conditions complicate the evidence regarding its effectiveness and safety in individuals with CPPS. Consequently, this discussion focuses on evaluating the efficacy and safety of rTMS by considering factors such as the stimulation device (coil), stimulation parameters, and specific brain regions targeted, all of which can influence treatment outcomes in Table 3.
The coil is a crucial factor in determining TMS’s safety and effectiveness, as the outcomes are limited by the device’s hardware (23). The manner in which the electric fields diffuse from several coils positioned on the surface of the brain can have a significant impact on the efficacy and safety of rTMS (24). As such, a careful evaluation of the accuracy and penetration depth of the various stimulation coils is essential in clinical settings. The placement and orientation of the coil are factors that significantly affect the effectiveness of TMS therapy (25). Thus, accurate spatial localization is essential for the effective application of functional magnetic stimulation. In our review of the literature, five studies utilized figure-of-eight coils, one employed an H-coil, while two case reports did not specify the shape of the coil used. In terms of pain relief, one study indicated an elevation in the pain threshold occurred, while the remaining seven all reported noteworthy reductions in pain. Consequently, when selecting a TMS coil, careful evaluation of the benefits and drawbacks of the stimulation focus and depth are important, along with a thorough assessment of the safety considerations associated with TMS.
The precise settings used during the stimulation and the targeted brain regions are intimately associated with the therapeutic effects of rTMS (26). By using various stimulation settings to elicit a variety of neurophysiological reactions, rTMS has the potential to improve recovery (27). TMS acts by coordinating specific neural network patterns of brain activity when specific frequencies are targeted (28). Determining the optimal rTMS frequency for each distinct region of the brain could greatly improve the efficacy of treatments for neurological disorders because different parts of the brain react differently to different frequencies (28, 29). This could pave the way for better brain plasticity adjustments, leading to more successful treatment outcomes. The present study revealed that, in the context of CPPS, most studies used high rTMS frequencies (5–20 Hz), with only one study evaluating the effects of low-frequency (1 Hz) rTMS. Notably, the most commonly utilized frequency was 10 Hz, which was employed in four studies. This insight is completely in line with other studies on the use of rTMS for pelvic floor diseases (18). In a parallel exploration, studies on chronic pain have unveiled comparable findings, highlighting the effectiveness of using high-frequency rTMS at frequencies of between 10 and 20 Hz. Numerous conditions, including migraines, fibromyalgia, peripheral neuropathic pain, and various other types of chronic pain, may be alleviated by this innovative technique (30). Moreover, a narrative analysis of neuropathic pain indicated that the dynamic approach of high-frequency 10 Hz rTMS, targeting the primary motor cortex (M1) and administered over multiple sessions, exhibited a notably enhanced therapeutic impact compared to the more muted low-frequency and one-time TMS techniques (26).
The main motor cortex (M1) located in the precentral gyrus (Brodmann’s area 4) of the human brain is a crucial component of the motor cortex (31). By collaborating with other motor regions, it aids in the regulation of human movement. A multitude of anatomical, functional, and organizational characteristics of the motor cortex are associated with persistent pain (32), and the motor cortex may be impacted by various persistent pain conditions in different ways (33). Studies have revealed that the M1 region is important in affecting our perception of experimental pain as well as in reducing the symptoms of chronic neuropathic pain (34). Pain is influenced by M1 activity, which is linked to pain perception in a number of ways, entailing the inhibition of ascending nociceptive signals in the thalamus and the activation of the descending pain modulation route via the midbrain periaqueductal gray matter (35). One groundbreaking study that looked at the use of rTMS for bladder pain syndrome/interstitial cystitis (BPS/IC) (17) obtained promising results suggesting that rTMS could be a game-changer in easing pelvic pain and related urinary issues. By fine-tuning brain plasticity and reshaping neuronal pathways in the cortex, this innovative treatment may offer new hope for those suffering from these challenging conditions. The SMA is a region of the cerebral cortex that is essential to our capacity for movement and motor coordination (36). When we look at the structure of the brain, the SMA, which is part of Brodmann area 6, is tucked away in the posterior section of the superior frontal gyrus, and is necessary to synchronize and balance the body’s movements (37). The SMA is more important for coordinating and adjusting our motions than the main motor cortex. It is essential for regulating the flow of activity in the primary motor cortex (M1) and for coordinating the performance of motor sequences (36, 38). This regulatory powerhouse is crucial for achieving the smoothness of body movements, optimizing motor function, and ensuring the flawless execution of unilateral brain activity (39). In addition to compensating for injury to M1 and its corticospinal tract fibers, the structural integrity of the SMA and the corticospinal tract nerve fibers emanating from the proximal SMA might affect contralateral motor performance (40). The SMA plays a critical function in pain regulation and the emergence of neuropathic pain by participating in the neural network for pain processing in the cingulate cortex and hippocampal regions (41). The SMA exhibits morphological abnormalities and functional hyperactivity in PFM disorders, including CPPS and urinary incontinence (42–49). Two studies included in this review examined the role of the SMA in regulating the PFM, where excessive tension in the PFM is the primary mechanism contributing to CPP. rTMS has the potential to modulate both the increase and decrease in SMA activity, thereby facilitating either an enhancement or reduction in PFM activity. A prior investigation suggested that low-frequency rTMS aimed at the SMA may enhance PFM activity to address PFM relaxation. On the other hand, by reducing PFM activity, high-frequency rTMS may be very helpful in releasing tension in such muscles (50).
Tucked away in the frontal lobes on both sides of the brain, the DLPFC serves as a hub of neural connections to numerous brain regions (51). Due to its multiple connections to the motor and sensory cortices, this region is crucial for controlling behavior, attention, and influencing cognitive function (52). Research on healthy individuals indicates that the left DLPFC has an inhibitory effect on the ipsilateral M1, whereas the right DLPFC has a comparatively smaller impact on the ipsilateral M1 (53). Additionally, some studies suggest that chronic pain is associated with a reduction in gray matter within the DLPFC (54). Non-invasive brain stimulation techniques have the power to reshape the structure and operation of the DLPFC. Using rTMS to stimulate the DLPFC can change how we perceive pain, as it not only modifies the emotional significance we attach to pain but also counteracts the shifts in motor cortex excitability that painful experiences can trigger. In the end, this helps to relieve discomfort (55). By interacting with other regulatory systems, such as the opioid pathways and our cognitive and emotional reactions, this alteration can also alter how we feel pain (56). In addition, rTMS stimulation of the DLPFC can alter the activity of the emotional regulation network by affecting the functional connectivity of the amygdala, thereby modulating pain perception (57–60). A study using rTMS of the DLPFC in patients with bladder pain syndrome (BPS) found that low-frequency TMS of the right DLPFC improved the symptoms. In addition, treatment outcomes were enhanced and depression symptoms were reduced when the left DLPFC was activated (61). Based on these studies, we can infer that the M1 area of the brain is associated with central sensitization, alterations in neural plasticity, and the inhibitory pathways of both ascending and descending transmission. The SMA is closely linked to PFM tension, and the DLPFC area mutually influences the cognitive experience and emotional value of the patients.
Our preference for using our left or right hand is strongly connected with our brain’s reaction to TMS (62, 63). This is because unique structural and functional differences in our brains lead to varying reactions to pain. For example, a study discovered a connection between our sensitivity to pain and how we utilize our dominant and non-dominant hands (64). Moreover, a plethora of research suggests that left-handed individuals experience different degrees of pain sensitivity than their right-handed counterparts. This might be connected to their non-dominant hand’s increased sensitivity to pain, which results in a decreased degree of total lateralization (65). Studies focusing on infants have revealed that their hand dominance could play a role in how they experience pain. This connection is explained by the activation of the prefrontal lobe in reaction to negative experiences (66). Human handedness is associated with the integrity of the white matter pathways in the brain (67). Variations in white matter connectivity may provide insight into the effects of TMS on brain activity and cognitive function (63). Consequently, when evaluating TMS’s efficacy in modulating brain function, the influence of handedness is an important consideration.
In TMS, the pulsed electromagnetic field generated by the coil can produce significant noise that might disrupt and obscure the desired effects of the treatment. Tinnitus, hearing loss, and a lowered tolerance for sound are among some of the safety concerns (68). The risk associated with sound pulses should be considered while selecting coils, even if it can be mitigated by the patients donning the appropriate protection gear (69, 70). Since the issuance of safety guidelines on TMS by the International Federation of Clinical Neurophysiology (IFCN) in 1998, the incidence of TMS/rTMS-induced seizures has been notably low. Seizures caused by rTMS are also unlikely to recur after the operation, unless the patient has a history of epilepsy (23). The moderate side effects of TMS, which are rather common, may depend on the participants’ initial expectations or anxiety levels (71). Recent evaluations have demonstrated that TMS is a safe and effective therapy option for a range of patient populations, including children, teens, adults, peripartum women, and older adults (72–75). The studies assessed in this review indicated that adherence to the recommended stimulation frequency and intensity parameters generally results in well-tolerated responses among individuals, with only minor adverse events and no instances of severe reactions documented. These findings demonstrate the safety of TMS treatment for CPPS, which is consistent with earlier safety assessments of TMS treatment for chronic neuropathic pain (76).
The primary study limitations identified include the heterogeneity across various studies, the incomplete assessment of the overall extent of pain relief, discrepancies in the targeted stimulation sites, and inconsistencies in the parameters used for stimulation.
Evaluations of the safety of rTMS suggest that it is a promising non-invasive approach for managing CPPS. The reviewed studies indicated that targeting the M1 motor area with rTMS can enhance brain plasticity, regional brain connectivity, and brain–spinal-cord pathways, and improve the effectiveness of pain mitigation (17, 77, 78). However, when CPPS results from elevated PFM tension, stimulating the SMA with rTMS frequently yields better results (48, 79). Lastly, in patients with concurrent mental diseases, stimulation of the DLPFC region can help regulate mood and significantly lessen symptoms (61). Another important factor to consider is whether someone is left-handed or right-handed.
Overall, this review summarizes the analgesic effect of rTMS on CPPS. Although TMS is a safe and promising technique that can reduce long-term refractory pain, its clinical application is still hindered by the variability in stimulation parameters and inclusion criteria, diverse etiologies, varied outcome assessment methods, and limited randomization of studies, all of which lead to a high risk of bias. Therefore, additional research efforts are necessary to determine the most effective stimulation protocols and to standardize all pertinent parameters. This will contribute to improving the long-term effectiveness of rTMS as a noninvasive treatment modality for the management of CPPS.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
CL: Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing, Funding acquisition. BZ: Data curation, Investigation, Methodology, Writing – review & editing, Writing – original draft. JZ: Formal analysis, Methodology, Writing – original draft. KY: Software, Validation, Writing – original draft. DC: Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Chengdu Medical Research Project (grant no. 2024232) and the Tianfu Scientific Research Incubation Fund (grant no. 2022QN02).
The authors would like to thank Yaodong You of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine for helpful discussions on topics related to this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1499133/full#supplementary-material
1. Engeler, D, Baranowski, AP, Berghmans, B, Birch, J, Borovicka, J, and Cottrell, AM, et al. EAU guidelines on chronic pelvic pain. (2022). Available at: www.uroweb.org
2. Smith, CP. Male chronic pelvic pain: An update. Indian J Urol. (2016) 32:34–9. doi: 10.4103/0970-1591.173105
3. Latthe, P, Latthe, M, Say, L, Gülmezoglu, M, and Khan, KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. (2006) 6:177. doi: 10.1186/1471-2458-6-177
4. Kwon, J, Lee, HJ, Joo, JH, and Park, E-C. Urinary incontinence status changes and depressive symptoms among middle-aged and older women: using data from a survey of the Korean longitudinal study of aging. J Affect Disord. (2021) 279:549–53. doi: 10.1016/j.jad.2020.10.039
5. Stones, W, Cheong, YC, Howard, FM, and Singh, S. Interventions for treating chronic pelvic pain in women. Cochrane Collab. (2010) 11:1–43. doi: 10.1002/14651858.cd000387
6. Allaire, C, Williams, C, Bodmer-Roy, S, Zhu, S, Arion, K, Ambacher, K, et al. Chronic pelvic pain in an interdisciplinary setting: 1-year prospective cohort. Am J Obstet Gynecol. (2018) 218:114.e1–114.e12. doi: 10.1016/j.ajog.2017.10.002
7. Thomtén, J, and Karlsson, A. Psychological factors in genital pain: the role of fear-avoidance, pain catastrophizing and anxiety sensitivity among women living in Sweden. Scand J Pain. (2014) 5:193–9. doi: 10.1016/j.sjpain.2014.01.003
8. Grinberg, K, Weissman-Fogel, I, Lowenstein, L, Abramov, L, and Granot, M. How does myofascial physical therapy attenuate pain in chronic pelvic pain syndrome? Pain Res Manag. (2019) 2019:1–11. doi: 10.1155/2019/6091257
9. Pena, VN, Engel, N, Gabrielson, AT, Rabinowitz, MJ, and Herati, AS. Diagnostic and management strategies for patients with chronic prostatitis and chronic pelvic pain syndrome. Drugs Aging. (2021) 38:845–86. doi: 10.1007/s40266-021-00890-2
10. Tadros, NN, Shah, AB, and Shoskes, DA. Utility of trigger point injection as an adjunct to physical therapy in men with chronic prostatitis/chronic pelvic pain syndrome. Transl Androl Urol. (2017) 6:534–7. doi: 10.21037/tau.2017.05.36
11. Patel, CB, Patel, AA, and Diwan, S. The role of neuromodulation in chronic pelvic pain: a review article. Pain Phys. (2022) 25:531–E542.
12. Lanza, G, Fisicaro, F, Cantone, M, Pennisi, M, Cosentino, FII, Lanuzza, B, et al. Repetitive transcranial magnetic stimulation in primary sleep disorders. Sleep Med Rev. (2023) 67:101735. doi: 10.1016/j.smrv.2022.101735
13. Barker, AT, Jalinous, R, and Freeston, IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. (1985) 325:1106–7. doi: 10.1016/s0140-6736(85)92413-4
14. Eldaief, MC, Press, DZ, and Pascual-Leone, A. Transcranial magnetic stimulation in neurology: a review of established and prospective applications. Neur Clin Pract. (2013) 3:519–26. doi: 10.1212/01.cpj.0000436213.11132.8e
15. Aleman, A. Use of repetitive transcranial magnetic stimulation for treatment in psychiatry. Clin Psychopharmacol Neurosci. (2013) 11:53–9. doi: 10.9758/cpn.2013.11.2.53
16. Lefaucheur, J-P, André-Obadia, N, Antal, A, Ayache, SS, Baeken, C, Benninger, DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. (2014) 125:2150–206. doi: 10.1016/j.clinph.2014.05.021
17. Cervigni, M, Onesti, E, Ceccanti, M, Gori, MC, Tartaglia, G, Campagna, G, et al. Repetitive transcranial magnetic stimulation for chronic neuropathic pain in patients with bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. (2018) 37:2678–87. doi: 10.1002/nau.23718
18. Mazeaud, C, Salazar, BH, and Khavari, R. Noninvasive brain stimulation in the treatment of functional urological and pelvic floor disorders: a scoping review. Neurourol Urodyn. (2023) 42:1318–28. doi: 10.1002/nau.25205
19. Rodgers, M, Sowden, AJ, Petticrew, M, Arai, L, Roberts, HM, Britten, N, et al. Testing methodological guidance on the conduct of narrative synthesis in systematic reviews. Eval. (2009) 15:47–71. doi: 10.1177/1356389008097871
20. Brünahl, CA, Riegel, B, Höink, J, Kutup, A, Eichelberg, E, and Löwe, B. Psychosomatische Aspekte des chronischen Unterbauchschmerzsyndroms. Psychometrische Ergebnisse der Pilotphase einer interdisziplinären Sprechstunde psychosomatic aspects of chronic pelvic pain syndrome. Psychometric results from the pilot phase of an interdisciplinary outpatient clinic. Schmerz. (2014) 28:311–8. doi: 10.1007/s00482-014-1422-6
21. Bharucha, AE, and Lee, TH. Anorectal and pelvic pain. Mayo Clin Proc. (2016) 91:1471–86. doi: 10.1016/j.mayocp.2016.08.011
22. Nikkola, J, Holm, A, Seppänen, M, Joutsi, T, Rauhala, E, and Kaipia, A. Repetitive transcranial magnetic stimulation for chronic prostatitis/chronic pelvic pain syndrome: a prospective pilot study. Int Neurourol J. (2020) 24:144–9. doi: 10.5213/inj.1938258.129
23. Rossi, S, Antal, A, Bestmann, S, Bikson, M, Brewer, C, Brockmöller, J, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin Neurophysiol. (2021) 132:269–306. doi: 10.1016/j.clinph.2020.10.003
24. Iwahashi, M, Gomez-Tames, J, Laakso, I, and Hirata, A. Evaluation method for in situ electric field in standardized human brain for different transcranial magnetic stimulation coils. Phys Med Biol. (2017) 62:2224–38. doi: 10.1088/1361-6560/aa5b70
25. Lu, S, Jiang, H, Li, C, Hong, B, Zhang, P, and Liu, W. Genetic algorithm for TMS coil position optimization in stroke treatment. Front Public Health. (2022) 9:794167. doi: 10.3389/fpubh.2021.794167
26. Tsai, Y-Y, Wu, W-T, Han, D-S, Mezian, K, Ricci, V, Özçakar, L, et al. Application of repetitive transcranial magnetic stimulation in neuropathic pain: a narrative review. Lifestyles. (2023) 13:258. doi: 10.3390/life13020258
27. Klomjai, W, Katz, R, and Lackmy-Vallée, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med. (2015) 58:208–13. doi: 10.1016/j.rehab.2015.05.005
28. Salinas, FS, Franklin, C, Narayana, S, Szabó, CÁ, and Fox, PT. Repetitive transcranial magnetic stimulation educes frequency-specific causal relationships in the motor network. Brain Stimul. (2016) 9:406–14. doi: 10.1016/j.brs.2016.02.006
29. Vasant, DH, Michou, E, Mistry, S, Rothwell, JC, and Hamdy, S. High-frequency focal repetitive cerebellar stimulation induces prolonged increases in human pharyngeal motor cortex excitability. J Physiol. (2015) 593:4963–77. doi: 10.1113/jp270817
30. Fernandes, AM, Graven-Nielsen, T, and De Andrade, DC. New updates on transcranial magnetic stimulation in chronic pain. Curr Opin Support Palliat Care. (2022) 16:65–70. doi: 10.1097/spc.0000000000000591
31. Bhattacharjee, S, Kashyap, R, Abualait, T, Annabel Chen, S-H, Yoo, W-K, and Bashir, S. The role of primary motor cortex: more than movement execution. J Mot Behav. (2021) 53:258–74. doi: 10.1080/00222895.2020.1738992
32. Yang, Q, Wang, Z, Yang, L, Xu, Y, and Chen, LM. Cortical thickness and functional connectivity abnormality in chronic headache and low back pain patients. Hum Brain Mapp. (2017) 38:1815–32. doi: 10.1002/hbm.23484
33. Chang, W-J, O’Connell, NE, Beckenkamp, PR, Alhassani, G, Liston, MB, and Schabrun, SM. Altered primary motor cortex structure, organization, and function in chronic pain: a systematic review and meta-analysis. J Pain. (2018) 19:341–59. doi: 10.1016/j.jpain.2017.10.007
34. Salo, N-T, Vaalto, SMI, Koponen, LM, Nieminen, JO, and Ilmoniemi, RJ. The effect of experimental pain on short-interval intracortical inhibition with multi-locus transcranial magnetic stimulation. Exp Brain Res. (2019) 237:1503–10. doi: 10.1007/s00221-019-05502-5
35. Kisler, L-B, Weissman-Fogel, I, Sinai, A, Sprecher, E, Chistyakov, AV, Shamay-Tsoory, S, et al. Bi-phasic activation of the primary motor cortex by pain and its relation to pain-evoked potentials − an exploratory study. Behav Brain Res. (2017) 328:209–17. doi: 10.1016/j.bbr.2017.04.006
36. Albishi, AM. Why do different motor cortical areas activate the same muscles? Brain Struct Funct. (2023) 228:2017–24. doi: 10.1007/s00429-023-02703-1
37. Sheets, JR, Briggs, RG, Young, IM, Bai, MY, Lin, Y-H, Poologaindran, A, et al. Parcellation-based modeling of the supplementary motor area. J Neurol Sci. (2021) 421:117322. doi: 10.1016/j.jns.2021.117322
38. Lu, Y, Kim, J, and Kim, T. A neurophysiological approach to the distinction between motor and cognitive skills: a functional magnetic resonance imaging study. Front Neurosci. (2023) 17:1178800. doi: 10.3389/fnins.2023.1178800
39. Côté, SL, Elgbeili, G, Quessy, S, and Dancause, N. Modulatory effects of the supplementary motor area on primary motor cortex outputs. J Neurophysiol. (2020) 123:407–19. doi: 10.1152/jn.00391.2019
40. Xu, YW, Lin, P, Yao, PS, Zheng, SF, and Kang, DZ. Structure and function of corticospinal projection originating from supplementary motor area. Neuroradiology. (2021) 63:1283–92. doi: 10.1007/s00234-021-02669-z
41. Rana, M, Yani, MS, Asavasopon, S, Fisher, BE, and Kutch, JJ. Brain connectivity associated with muscle synergies in humans. J Neurosci. (2015) 35:14708–16. doi: 10.1523/JNEUROSCI.1971-15.2015
42. Louppe, J, Nguyen, J, Robert, R, Buffenoir, K, De Chauvigny, E, Riant, T, et al. Motor cortex stimulation in refractory pelvic and perineal pain: report of two successful cases. Neurourol Urodyn. (2013) 32:53–7. doi: 10.1002/nau.22269
43. Kairys, AE, Schmidt-Wilcke, T, Puiu, T, Ichesco, E, Labus, JS, Martucci, K, et al. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. J Urol. (2015) 193:131–7. doi: 10.1016/j.juro.2014.08.042
44. Kilpatrick, LA, Kutch, JJ, Tillisch, K, Naliboff, BD, Labus, JS, Jiang, Z, et al. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol. (2014) 192:947–55. doi: 10.1016/j.juro.2014.03.093
45. Kutch, JJ, Yani, MS, Asavasopon, S, Kirages, DJ, Rana, M, Cosand, L, et al. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: a MAPP: research network neuroimaging study. NeuroImage Clin. (2015) 8:493–502. doi: 10.1016/j.nicl.2015.05.013
46. Woodworth, D, Mayer, E, Leu, K, Ashe-Mcnalley, C, Naliboff, BD, Labus, JS, et al. Unique microstructural changes in the brain associated with urological chronic pelvic pain syndrome (UCPPS) revealed by diffusion tensor MRI, super-resolution track density imaging, and statistical parameter mapping: a MAPP network neuroimaging study. PLoS One. (2015) 10:e0140250. doi: 10.1371/journal.pone.0140250
47. Groat, D, Griffiths, WC, and Yoshimura, DN. Neural control of the lower urinary tract. Compr Physiol. (2015) 5:327–96. doi: 10.1002/cphy.c130056
48. Griffiths, D. Neural control of micturition in humans: a working model. Nat Rev Urol. (2015) 12:695–705. doi: 10.1038/nrurol.2015.266
49. Griffiths, D, Clarkson, B, Tadic, SD, and Resnick, NM. Brain mechanisms underlying urge incontinence and its response to pelvic floor muscle training. J Urol. (2015) 194:708–15. doi: 10.1016/j.juro.2015.03.102
50. Yani, MS, Fenske, SJ, Rodriguez, LV, and Kutch, JJ. Motor cortical neuromodulation of pelvic floor muscle tone: potential implications for the treatment of urologic conditions. Neurourol Urodyn. (2019) 38:1517–23. doi: 10.1002/nau.24014
51. Panikratova, YR, Vlasova, RM, Akhutina, TV, Korneev, AA, Sinitsyn, VE, and Pechenkova, EV. Functional connectivity of the dorsolateral prefrontal cortex contributes to different components of executive functions. Int J Psychophysiol. (2020) 151:70–9. doi: 10.1016/j.ijpsycho.2020.02.013
52. Smucny, J, Dienel, SJ, Lewis, DA, and Carter, CS. Mechanisms underlying dorsolateral prefrontal cortex contributions to cognitive dysfunction in schizophrenia. Neuropsychopharmacology. (2022) 47:292–308. doi: 10.1038/s41386-021-01089-0
53. Wang, Y, Cao, N, Lin, Y, Chen, R, and Zhang, J. Hemispheric differences in functional interactions between the dorsal lateral prefrontal cortex and ipsilateral motor cortex. Front Hum Neurosci. (2020) 14:202. doi: 10.3389/fnhum.2020.00202
54. Davis, KD, and Moayedi, M. Central mechanisms of pain revealed through functional and structural MRI. J NeuroImmune Pharmacol. (2013) 8:518–34. doi: 10.1007/s11481-012-9386-8
55. Fierro, B, De Tommaso, M, Giglia, F, Giglia, G, Palermo, A, and Brighina, F. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex excitability. Exp Brain Res. (2010) 203:31–8. doi: 10.1007/s00221-010-2206-6
56. Seminowicz, DA, and Moayedi, M. The dorsolateral prefrontal cortex in acute and chronic pain. J Pain. (2017) 18:1027–35. doi: 10.1016/j.jpain.2017.03.008
57. Eshel, N, Keller, CJ, Wu, W, Jiang, J, Mills-Finnerty, C, Huemer, J, et al. Global connectivity and local excitability changes underlie antidepressant effects of repetitive transcranial magnetic stimulation. Neuropsychopharmacol: Official Pub American College of Neuropsychopharmacol. (2020) 45:1018–25. doi: 10.1038/s41386-020-0633-z
58. Ishida, T, Dierks, T, Strik, W, and Morishima, Y. Converging resting state networks unravels potential remote effects of transcranial magnetic stimulation for major depression. Front Psychol. (2020) 11:836. doi: 10.3389/fpsyt.2020.00836
59. Chen, FJ, Gu, CZ, Zhai, N, Duan, HF, Zhai, AL, and Zhang, X. Repetitive transcranial magnetic stimulation improves amygdale functional connectivity in major depressive disorder. Front Psychol. (2020) 11:732. doi: 10.3389/fpsyt.2020.00732
60. Gandhi, W, Rosenek, NR, Harrison, R, and Salomons, TV. Functional connectivity of the amygdala is linked to individual differences in emotional pain facilitation. Pain. (2020) 161:300–7. doi: 10.1097/j.pain.0000000000001714
61. Nizard, J, Esnault, J, Bouche, B, Suarez Moreno, A, Lefaucheur, J-P, and Nguyen, J-P. Long-term relief of painful bladder syndrome by high-intensity, low-frequency repetitive transcranial magnetic stimulation of the right and left dorsolateral prefrontal cortices. Front Neurosci. (2018) 12:925. doi: 10.3389/fnins.2018.00925
62. Julkunen, P, Määttä, S, Säisänen, L, Kallioniemi, E, Könönen, M, Jäkälä, P, et al. Functional and structural cortical characteristics after restricted focal motor cortical infarction evaluated at chronic stage – indications from a preliminary study. Clin Neurophysiol. (2016) 127:2775–84. doi: 10.1016/j.clinph.2016.05.013
63. Cazzoli, D, and Chechlacz, M. A matter of hand: causal links between hand dominance, structural organization of fronto-parietal attention networks, and variability in behavioural responses to transcranial magnetic stimulation. Cortex. (2017) 86:230–46. doi: 10.1016/j.cortex.2016.06.015
64. Northon, S, Deldar, Z, and Piché, M. Spinal and cerebral integration of noxious inputs in left-handed individuals. Brain Topogr. (2021) 34:568–86. doi: 10.1007/s10548-021-00864-y
65. Pud, D, Golan, Y, and Pesta, R. Hand dominancy—a feature affecting sensitivity to pain. Neurosci Lett. (2009) 467:237–40. doi: 10.1016/j.neulet.2009.10.048
66. Ozawa, M, Kanda, K, Hirata, M, Kusakawa, I, and Suzuki, C. Effect of gender and hand laterality on pain processing in human neonates. Early Hum Dev. (2011) 87:45–8. doi: 10.1016/j.earlhumdev.2010.09.371
67. Ocklenburg, S, Friedrich, P, Güntürkün, O, and Genç, E. Intrahemispheric white matter asymmetries: the missing link between brain structure and functional lateralization? Rev Neurosci. (2016) 27:465–80. doi: 10.1515/revneuro-2015-0052
68. Peterchev, AV, Murphy, DLK, and Goetz, SM. Proceedings of the 2015 IEEE engineering in medicine and biology society. (EMBS). (2015) 226–229.
69. Tringali, S, Perrot, X, Collet, L, and Moulin, A. Repetitive transcranial magnetic stimulation: hearing safety considerations. Brain Stimul. (2012) 5:354–63. doi: 10.1016/j.brs.2011.06.005
70. Kukke, SN, Brewer, CC, Zalewski, C, King, KA, Damiano, D, Alter, KE, et al. Hearing safety from single- and double-pulse transcranial magnetic stimulation in children and young adults. J Clin Neurophysiol. (2017) 34:340–7. doi: 10.1097/wnp.0000000000000372
71. Maizey, L, Allen, CPG, Dervinis, M, Verbruggen, F, Varnava, A, Kozlov, M, et al. Comparative incidence rates of mild adverse effects to transcranial magnetic stimulation. Clin Neurophysiol. (2013) 124:536–44. doi: 10.1016/j.clinph.2012.07.024
72. Iriarte, IG, and George, MS. Transcranial magnetic stimulation (TMS) in the elderly. Curr Psychiatry Rep. (2018) 20:6. doi: 10.1007/s11920-018-0866-2
73. Majumder, P, Balan, S, Gupta, V, Wadhwa, R, and Perera, TD. The safety and efficacy of repetitive transcranial magnetic stimulation in the treatment of major depression among children and adolescents: a systematic review. Cureus. (2021) 13:e14564. doi: 10.7759/cureus.14564
74. Lee, HJ, Kim, SM, and Kwon, JY. Repetitive transcranial magnetic stimulation treatment for peripartum depression: systematic review & meta-analysis. BMC Pregnancy Childbirth. (2021) 21:118. doi: 10.1186/s12884-021-03600-3
75. Liu, C, Li, L, Li, B, Liu, Z, Xing, W, Zhu, K, et al. Efficacy and safety of theta burst versus repetitive transcranial magnetic stimulation for the treatment of depression: a meta-analysis of randomized controlled trials. Neuromodulation Technol Neural Interface. (2024) 27:701–10. doi: 10.1016/j.neurom.2023.08.009
76. Gatzinsky, K, Bergh, C, Liljegren, A, Silander, H, Samuelsson, J, Svanberg, T, et al. Repetitive transcranial magnetic stimulation of the primary motor cortex in management of chronic neuropathic pain: a systematic review. Scand J Pain. (2021) 21:8–21. doi: 10.1515/sjpain-2020-0054
77. Algladi, T, Harris, M, Whorwell, PJ, Paine, P, and Hamdy, S. Modulation of human visceral sensitivity by noninvasive magnetoelectrical neural stimulation in health and irritable bowel syndrome. Pain. (2015) 156:1348–56. doi: 10.1097/j.pain.0000000000000187
78. Melchior, C, Gourcerol, G, Chastan, N, Verin, E, Menard, JF, Ducrotte, P, et al. Effect of transcranial magnetic stimulation on rectal sensitivity in irritable bowel syndrome: a randomized, placebo-controlled pilot study. Color Dis. (2014) 16:O104–11. doi: 10.1111/codi.12450
79. Calabrò, RS, Billeri, L, Porcari, B, Pignolo, L, and Naro, A. When two is better than one: a pilot study on transcranial magnetic stimulation plus muscle vibration in treating chronic pelvic pain in women. Brain Sci. (2022) 12:396. doi: 10.3390/brainsci12030396
80. Hodaj, H, Payen, J-F, Hodaj, E, Dumolard, A, Maindet, C, Cracowski, J-L, et al. Long-term treatment of chronic orofacial, pudendal, and central neuropathic limb pain with repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol. (2020) 131:1423–32. doi: 10.1016/j.clinph.2020.03.022
Keywords: transcranial magnetic stimulation, chronic pelvic pain syndrome, pelvic pain, chronic prostatitis pain, perineal pain, anal and rectal pain, prostatitis
Citation: Luo C, Zhang B, Zhou J, Yu K and Chang D (2025) Clinical application of repetitive transcranial magnetic stimulation in the treatment of chronic pelvic pain syndrome: a scoping review. Front. Neurol. 16:1499133. doi: 10.3389/fneur.2025.1499133
Received: 20 September 2024; Accepted: 04 February 2025;
Published: 26 February 2025.
Edited by:
Giorgio Scivoletto, Santa Lucia Foundation (IRCCS), ItalyReviewed by:
Hanchao Liu, Affiliated with the Zhejiang University School of Medicine, ChinaCopyright © 2025 Luo, Zhang, Zhou, Yu and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Degui Chang, NjI0NDQwMzEwQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.