
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Neurol. , 03 January 2025
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1540452
This article is a correction to:
Cipaglucosidase alfa plus miglustat: linking mechanism of action to clinical outcomes in late-onset Pompe disease
 Barry J. Byrne1*
Barry J. Byrne1* Giancarlo Parenti2,3
Giancarlo Parenti2,3 Benedikt Schoser4
Benedikt Schoser4 Ans T. van der Ploeg5
Ans T. van der Ploeg5 Hung Do6
Hung Do6 Brian Fox7
Brian Fox7 Mitchell Goldman7
Mitchell Goldman7 Franklin K. Johnson7
Franklin K. Johnson7 Jia Kang8
Jia Kang8 Nickita Mehta7
Nickita Mehta7 John Mondick9
John Mondick9 M. Osman Sheikh7
M. Osman Sheikh7 Sheela Sitaraman Das7
Sheela Sitaraman Das7 Steven Tuske7
Steven Tuske7 Jon Brudvig7
Jon Brudvig7 Jill M. Weimer7
Jill M. Weimer7 Tahseen Mozaffar10
Tahseen Mozaffar10A corrigendum on
Cipaglucosidase alfa plus miglustat: linking mechanism of action to clinical outcomes in late-onset Pompe disease
by Byrne, B. J., Parenti, G., Schoser, B., van der Ploeg, A. T., Do, H., Fox, B., Goldman, M., Johnson, F. K., Kang, J., Mehta, N., Mondick, J., Sheikh, M. O., Sitaraman Das, S., Tuske, S., Brudvig, J., Weimer, J. M., and Mozaffar. T. (2024). Front. Neurol. 15:1451512. doi: 10.3389/fneur.2024.1451512
In the published article, there was an error in Figure 13 as published. The error relates to the positive/negative value of some of the numbers on the axes for the lower MMT score and the GSGC total score in Figure 13A. For both lower MMT score and GSGC total score, the axis scale incorrectly read −3, −2, 1, 0, −1, −2, −3 from left to right, whereas the axis scale for lower MMT score should have been −3, −2, −1, 0, 1, 2, 3 and the axis scale for GSGC total score should have been 3, 2, 1, 0, −1, −2, −3. The corrected Figure 13 and its caption appear below.
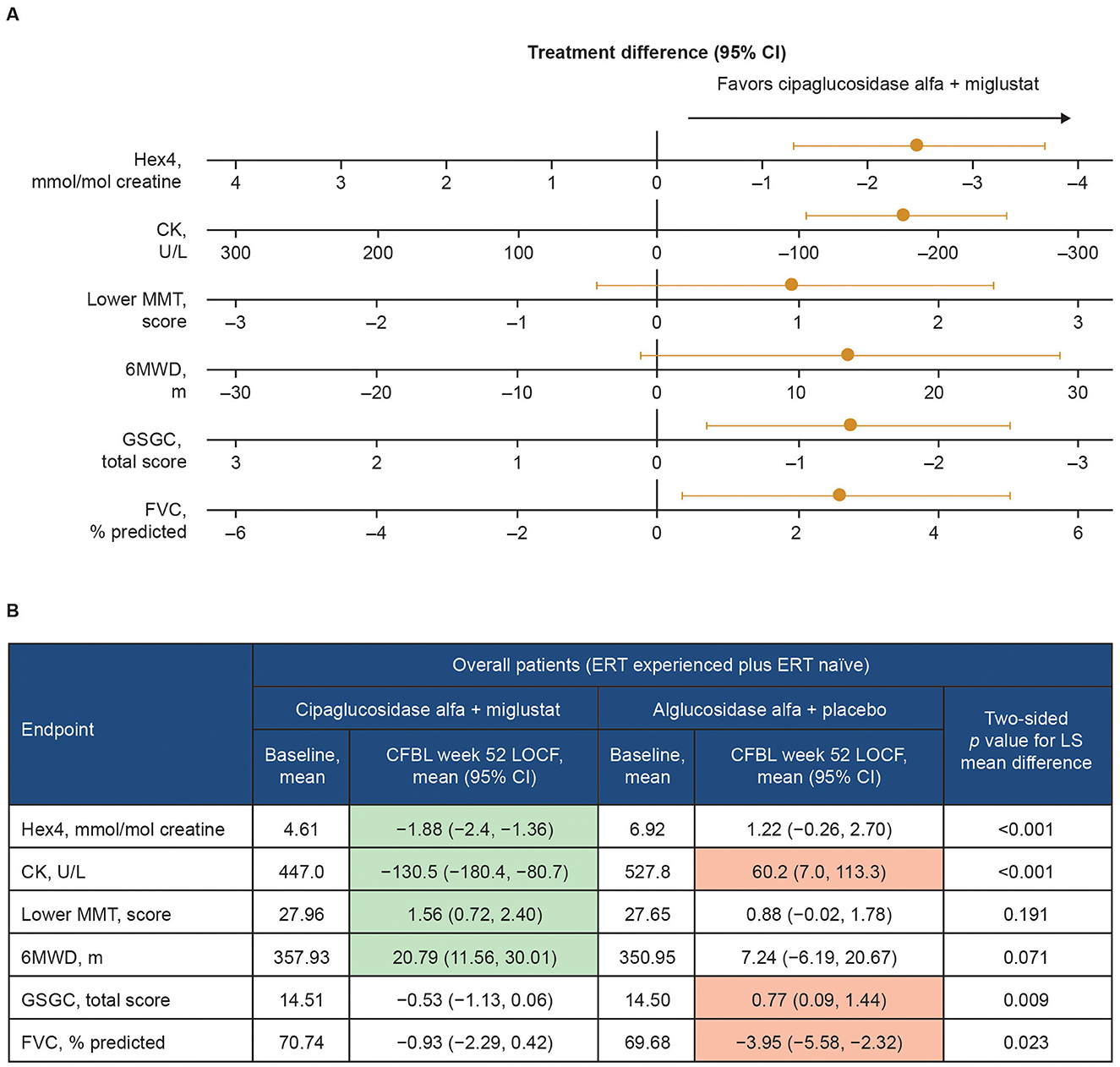
Figure 13. Change from baseline at week 52 of PROPEL—effect of cipaglucosidase alfa plus miglustat compared with alglucosidase alfa plus placebo in key efficacy outcomes. (A) Forest plot illustrating mean estimated treatment differences between cipaglucosidase alfa plus miglustat versus alglucosidase alfa plus placebo and corresponding 95% CIs are shown for the combined PROPEL study population for each outcome, with units as indicated on the x-axes. For all outcomes, right-sided directionality of treatment differences indicates favorable outcomes for cipaglucosidase alfa plus miglustat compared with alglucosidase alfa plus placebo. (B) The table shows baseline mean values and Week 52 CFBL values for cipaglucosidase alfa plus miglustat and alglucosidase alfa plus placebo. Shaded CFBL indicates nominally significant improvement (green) or nominally significant worsening (red) from baseline (i.e., the 95% CI does not include zero) within each treatment group. The p-values (two-tailed LS mean difference) shown in the far-right column are for the between-group treatment differences illustrated in the forest plot.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: Pompe disease, glycogen storage disease type II, lysosomal storage disorders, enzyme replacement therapy, n-butyldeoxynojirimycin
Citation: Byrne BJ, Parenti G, Schoser B, van der Ploeg AT, Do H, Fox B, Goldman M, Johnson FK, Kang J, Mehta N, Mondick J, Sheikh MO, Sitaraman Das S, Tuske S, Brudvig J, Weimer JM and Mozaffar T (2025) Corrigendum: Cipaglucosidase alfa plus miglustat: linking mechanism of action to clinical outcomes in late-onset Pompe disease. Front. Neurol. 15:1540452. doi: 10.3389/fneur.2024.1540452
Received: 05 December 2024; Accepted: 12 December 2024;
Published: 03 January 2025.
Edited and reviewed by: Edoardo Malfatti, Hôpitaux Universitaires Henri Mondor, France
Copyright © 2025 Byrne, Parenti, Schoser, van der Ploeg, Do, Fox, Goldman, Johnson, Kang, Mehta, Mondick, Sheikh, Sitaraman Das, Tuske, Brudvig, Weimer and Mozaffar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barry J. Byrne, YmFycnkuYnlybmVAdWZsLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.