
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 29 January 2025
Sec. Stroke
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1516577
This article is part of the Research Topic From bench to bedside: Inflammation in Neurovascular Disorders and Stroke View all 9 articles
Background: The systemic immune–inflammation index (SII) is a composite and easily available inflammation index, which can quantitatively reflect the degree of inflammation. This study aims to investigate the predictive value of admission SII for outcomes of large artery occlusion treated with mechanical thrombectomy (MT).
Methods: This retrospective study was conducted at Suining Central Hospital, Sichuan, China. Patients were stratified into quartiles based on their SII. The investigating outcomes included hemorrhagic transformation (HT), malignant brain edema (MBE), 90-day functional outcome, and mortality. The adverse function was defined as the modified Rankin Scale (mRS) score > 2 at the 90-day follow-up. Multivariate analysis was performed to explore the relationships between SII and outcomes. In addition, cases (distinguished from the aforementioned patients) treated with MT + mild hypothermia (MH) were also included to elucidate the relationships between SII/MH and outcomes in a new cohort.
Results: A total of 323 patients treated with MT were included. The observed HT, MBE, adverse function, and mortality rates were 31.9, 25.7, 59.4, and 27.9%, respectively. Multivariate analysis demonstrated that heightened SII was significantly related to HT (odds ratio [OR]: 1.061, 95% confidence interval [CI]: 1.035–1.086, p < 0.001), MBE (OR: 1.074, 95% CI: 1.045–1.103, p < 0.001), adverse function (OR: 1.061, 95% CI: 1.031–1.092, p < 0.001), and mortality (OR: 1.044, 95% CI: 1.018–1.070, p = 0.001), after adjusting sex, age, Glasgow Coma Scale (GCS) score at admission, initial National Institutes of Health Stroke Scale (NIHSS) score, baseline Alberta Stroke Program Early Computed Tomography Score (ASPECTS), present HMCAS, occluded vessel region, collateral score and successful revascularization. HT and MBE may partially account for patients with elevated SII’s adverse function and mortality. In addition, with the criterion of baseline ASPECTS ≤ 7, a total of 42 patients treated with MT + MH were enrolled to build up a new cohort combined with 72 patients treated with mere MT. The risk role of SII and protect effect of MH were identified for HT (SII—OR: 1.037, 95% CI: 1.001–1.074; MH—OR: 0.361, 95% CI: 0.136–0.957), MBE (SII—OR: 1.063, 95% CI: 1.019–1.109; MH—OR: 0.231, 95% CI: 0.081–0.653), and mortality (SII—OR: 1.048, 95% CI: 1.011–1.087; MH—OR: 0.343, 95% CI: 0.118–0.994).
Conclusion: Elevated SII was related to HT, MBE, 90-day adverse function, and mortality after MT. The MH may improve prognosis under high inflammation status.
Acute ischemic stroke (AIS) related with large artery occlusion (LAO), is characterized by high morbidity and mortality (1). With the development of interventional technology, mechanical thrombectomy (MT) has become the frontmost therapeutic modality for LAO patients with contraindications or ineffectiveness of thrombolysis (2). Rapid recanalization of occluded arteries and restoring blood flow to salvageable brain tissue can limit the transformation of the ischemic penumbra into infarction (3). However, despite its efficacy, substantial neurological and life-threatening complications still exist. Hemorrhagic transformation (HT) and malignant brain edema (MBE) are common complications after MT that are mainly caused by blood–brain barrier (BBB) disruption (4, 5). Severe HT and MBE prominently increase intracranial pressure, leading to cerebral hernia, and often result in poor functional outcomes that include even death (6). Therefore, identifying predictors of complications and risk factors for poor outcomes may improve patients’ prognoses.
Inflammation plays a pivotal role in the pathophysiology of AIS, involving the progression and resolution of infarction and remodeling and repair of ischemic tissue. However, inflammatory cascade also injuries vascular endothelium and disrupts the integrity of BBB, increasing the risk of HT and MBE (3, 7). Systemic immune–inflammation index (SII) is a composite inflammation index, calculated by combining neutrophil, lymphocyte, and platelet, and quantitatively reflects the inflammatory status (8). Previous studies have found relationships between high levels of SII and poor prognosis in intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (9, 10). Recently, a series of reports have separately put forward that elevated SII is related to HT, MBE, and unfavorable functional outcomes in patients treated with MT (11–13). While none has confirmed these relationships in the same cohort. Therefore, we conducted a retrospective study and set a primary purpose to investigate the predictive value of SII for early complications (HT and MBE) and late outcomes (90-day functional status and mortality). Our secondary objective was to explore the potential mechanism of SII linking to 90-day outcomes.
The institutional ethics committee of our institution approved (No. KYLLK20240133) this study; we retrospectively reviewed the database of the stroke center in Suining Central Hospital, Sichuan, China, and the data of patients undergoing MT for LAO from January 2021 to May 2024 were screened. Patient selection was based on the following inclusion criteria: (1) patients were admitted within 24 h after disease onset; (2) patients with age more than 18 years; and (3) patients suffered from LAO in anterior circulation (internal carotid artery [ICA] or middle cerebral artery [MCA]). The exclusion criteria were as follows: (1) Patients with LAO in the posterior circulation (vertebral artery or basilar artery); (2) patients with apparent infection or immune disease at admission; (3) patients who had comorbidities that required hormone or immunosuppressive agent therapy; (4) patients with progressive or decompensated comorbidities before AIS onset; (5) patients without SII data at admission or outcomes at follow-up; and (6) patients with a history of stroke had residual apparent dysfunction before AIS onset (modified Rankin Scale [mRS] score ≥ 2). In addition, patients with multiple times of MT for re-occlusion of ICA or MCA within the same hospital stay were also excluded, because this may interfere with the predictive value of SII for outcomes.
A comprehensive evaluation was deployed immediately after patients were admitted to our emergency, including blood sample collection, consciousness assessment by Glasgow Coma Scale (GCS) score, neurological deficits assessment by the National Institutes of Health Stroke Scale (NIHSS) score, and neuroimaging test. The non-enhanced computed tomography (CT) of the brain was performed to rule out ICH, and CT angiography to confirm the presence of LAO. Intravenous thrombolysis with alteplase was administered within 4.5 h after AIS onset, and MT was carried out for patients with contraindication or futile recanalization of thrombolysis. For LAO with a time window of 4.5–24 h from onset, MT was performed after confirming the mismatch of ischemic and infarction areas evaluated by CT perfusion or MRI/DWI. All MT procedures were performed under general anesthesia using a modern stent retriever and/or direct aspiration technique. Stent implantation or balloon angioplasty was performed for stenosis or dissection at the surgeon’s discretion. The vessel recanalization was measured by modified thrombolysis in cerebral infarction (mTICI) score ≥ 2b was regarded as successful recanalization. Time from onset to recanalization was defined as the time from symptom appearance to successful recanalization or abortion of procedure if successful recanalization was not achieved (14). Planned cranial CT scans were conducted immediately after surgery, 24- and 48-h postprocedure. A CT was also taken whenever there was a change in neurological symptoms.
The following clinical and radiological data were collected: demographics, medical history, admission GCS score and NIHSS score, baseline Alberta Stroke Program Early Computed Tomography Score (ASPECTS) on non-enhanced CT before MT, intravenous thrombolysis, laboratory examinations, and procedure details. History of hypertension, diabetes mellitus, and heart disease was determined by self-report, current medication, or prior medical records. The definition of a smoker was smoking at least one cigarette per day for 1 year or more, and the definition of alcoholism was consuming 80 g of liquor per day. The etiology of stroke was assessed using the Trial of OGR 10172 in Acute Stroke Treatment (TOAST) classification (15). Collateral circulation was evaluated based on DSA at the beginning of the MT, categorized as follows: Grade 0: no significant or little filling of the occluded region or less than one-third of the occluded territory; grade 1: collateralization fills less than two-thirds of the occluded territory; and grade 2: collaterals reach more than two-thirds of the occluded territory or the proximal main stem (16). The HT was indicated as the presence of postoperative hemorrhage in the cerebral infarction area. The MBE was defined as follows: (1) More than 50% of middle cerebral artery supply territory had parenchymal hypodensity, accompanied by local brain edema, like lateral ventricle compression and sulcal effacement; and (2) midline shift of >5 mm at septum pellucidum or pineal gland with basal cistern occlusion (17). Two neuroradiologists independently evaluated the perioperative imaging characteristics blind to clinical data, including baseline ASPECTS, hyperdense middle cerebral artery sign (HMCAS), occluded vessel region, collateral score, and postoperative HT and MBE. Any discrepancies were figured out through discussion. The laboratory test data included peripheral blood cell counts, high-sensitivity C-reactive protein (hsCRP), coagulation function, serum electrolyte, liver and kidney function, blood lipid, cholesterol, and glucose. SII was calculated by the following formula: Neutrophil × Platelet/Lymphocyte. Qualified personnel or physicians evaluated the 90-day outcome measures through in-person interviews at the clinic or by telephone. The mRS score was used to assess the 90-day functional outcome, of which mRS score 0–2 was defined as favorable function, and mRS score 3–6 as adverse function (17).
From July 2023, a therapeutic method of mild hypothermia (MH) was conducted for patients with LAO treated by MT in our institution. Patients were included if (1) baseline ASPECTS ≤ 7 with or without core infarct volume > 70 mL measured by CT perfusion; and (2) their family members endorsed MH therapy. There was no different preoperative evaluation and surgical procedure strategy for these patients. After the MT procedure, patients were cooled using two cool blankets (Richeng, Changchun, Jilin, China) with a sandwiched method to reach the targeted temperature of 35°C at the posterior pharyngeal wall. All patients were endotracheally intubated with assisted ventilation. Pharmacological sedation with midazolam and analgesia with sufentanil were employed to prevent shivering during hypothermia. Arterial blood gas analysis was performed to monitor PH value, and arterial partial pressure of oxygen and carbon dioxide every 3–6 h. Hematological examinations for peripheral blood cells, hepatorenal function, electrolyte, coagulation function, and myocardial enzyme were scrutinized every 12–24 h. When the target temperature was achieved, the blanket temperature was adjusted to maintain the core temperature at 35.0°C. Slowly rewarming to 36.5°C of core temperature with a controlling rate of no faster than 0.5°C/h was initiated at the final phase of MH therapy by turning off the cooling blankets (18).
The patients (without mild hypothermia) were classified according to quartiles of SII levels (Q1–Q4). The categorical variables were presented as numbers (percentage) and were compared using chi-square test or Fisher’s exact test. The continuous variables with normal distribution were expressed as mean ± standard deviation (SD) and compared using analysis of variance (ANOVA) or Student’s t-test, otherwise, presented as median with interquartile range, and compared using Kruskal–Wallis test or Mann–Whitney U-test. Univariate analysis was performed for outcomes (HT, MBE, adverse function, and mortality). To avoid overfitting, variables that reached variate significance in all four outcomes were selected to build the “regression model”; in addition, the variable of age was also enrolled. All multivariate analyses in this study were adjusted for this model. The relationships between SII and four outcomes were quantified using multivariate logistic regression, generating odds ratio (OR) and 95% confidence interval (95% CI) after adjusting for multiple covariates. The median SII of each quartile was also treated as a continuous variable for the linear trend test. Weighted linear regression was employed to calculate multicollinearity among variables in the multivariable model, defined as variance inflation factor (VIF) >5. Correlations among variables in the “regression model” were assessed using the Spearman correlation test. Kaplan–Meier curve was depicted to reveal the cumulative incidence of mortality in quartiles of SII using a log–rank test for comparison. The receiver operator characteristic (ROC) curves were plotted to show the diagnostic effect of SII on outcomes. Meanwhile, the AUC values of SII and other composite inflammatory indices that have been verified to correlate with stroke prognosis, including systemic inflammation response index (SIRI; Neutrophil × Monocyte/Lymphocyte), neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), neutrophil percentage-to-albumin ratio (NPAR), aggregate inflammation systemic index (AISI; Neutrophil × Monocyte × Platelet/Lymphocyte), were calculated and compared using Delong test to explore whether SII possessed better diagnostic effect. Subgroup analysis was performed stratifying sex, age (either below or above 70 years), history of hypertension, TOAST classification (large-artery atherosclerosis [LAA] or others), occluded vessel region (ICA or MCA), collateral score (grades 0 + 1, or 2), and baseline ASPECTS (either below or above 7). The median value was determined as the threshold of SII in the subgroup analysis, using the L50 as a reference. In subgroup analysis, the likelihood ratio tests were employed to probe for interactions between SII and stratified variables. The Sobel test was used for mediation analysis to explore whether HT/MBE mediated elevated SII to 90-day adverse function and mortality.
Since the baseline ASPECTS was the primary indicator for MH, patients underwent mere MT with ASPECTS ≤ 7, and those with MH were selected to form a new cohort. The multivariate logistic regression was performed to explore the role of SII and MH in predicting the four outcomes after controlling for the “regression model.” All statistical analyses were carried out using Statistical Package for the Social Sciences (SPSS) version 25.0 (IBM, Armonk, NY, USA) and R-program version 4.4.1;1001 p < 0.05 was considered statistically significant.
A total of 445 patients with LAO have been surgically treated in our stroke center, and 323 patients treated with mere MT were eligible for this study. The process of patient selection is depicted in Figure 1. The mean age of patients was 70.73 ± 11.87 years, and 46.7% (151) were women. The ASPECTS, GCS, NIHSS, and NIHSS scores at admission were 8.20 ± 1.27, 12 (IQR: 10–14), and 15 (IQR: 12–19), respectively. One hundred and eighteen (36.5%) patients presented HMCAS. The occluded vessel region was categorized as ICA (124, 38.4%) and MCA (199, 61.6%). The number of patients with collateral scores of grades 0, 1, and 2 was 72 (22.3%), 133 (41.2%), and 118 (36.5%), respectively. A total of 103 (31.9%) patients experienced HT for the early complications and 82 (25.4%) patients suffered from MBE. At 90-day follow-up, 192 (59.4%) patients had adverse function (mRS score: 3–6) and 90 (27.9%) died.
The baseline characteristics of patients classified by quartiles of SII are presented in Table 1. There were 80, 81, 81, and 81 patients in Q1–Q4 Compared to patients with lower SII values, those with higher SII values were more likely to have lower baseline ASEPECTS, longer time from onset to recanalization, and higher serum glucose at admission, while less likely to experience intravenous thrombolysis, and successful revascularization. Critically, these patients possessed a higher proportion of HT, MBE, adverse function, and mortality (all p < 0.05).
The Kaplan–Meier curve demonstrated that individuals with elevated SII exhibited an increased mortality risk at 90-day follow-up (Figure 2). Univariate analysis for HT, MBE, adverse function, and mortality indicated that sex, GCS score at admission, initial NIHSS score, baseline ASPECTS, present HMCAS, occluded vessel region, collateral score, and successful revascularization were the standard significant variables that selected to build up the “regression model” (Supplementary Tables S1–S4), and age was also enrolled. The detailed relationships between SII and outcomes were summarized in Table 2. Multivariate analyses, controlled for sex, age, GCS score at admission, initial NIHSS score, baseline ASPECTS, present HMCAS, occluded vessel region, collateral score, and successful revascularization, demonstrated that patients in the highest quartile had significantly increased risk of HT (OR: 3.691; 95% CI: 1.634–8.340; p = 0.002), MBE (OR: 6.755; 95% CI: 2.288–19.940; p = 0.001), 90-day adverse function (OR: 4.969; 95% CI: 2.326–10.614; p < 0.001), and 90-day mortality (OR: 3.021; 95% CI: 1.159–7.876; p = 0.024), compared to those in the lowest quartile, after categorizing the SII as an ordinary variable. When presented as a continuous variable, the SII was also found as a significant predictor of HT (OR: 1.061; 95% CI: 1.035–1.086; p < 0.001), MBE (OR: 1.074; 95% CI: 1.045–1.103; p < 0.001), 90-day adverse function (OR: 1.061; 95% CI: 1.031–1.092; p < 0.001), and 90-day mortality (OR: 1.044; 95% CI: 1.018–1.070; p = 0.001). No obvious multicollinearity among variables was detected (VIF from 1.033 to 2.866).
The correlations among variables in the multivariate model are depicted in Figure 3. The SII was significantly correlated with the initial NIHSS score (rs = 0.167, p = 0.003), baseline ASPECTS (rs = −0.170, p = 0.002), collateral score (rs = −0.197, p < 0.001), and mTICI score (rs = −0.201, p < 0.001).
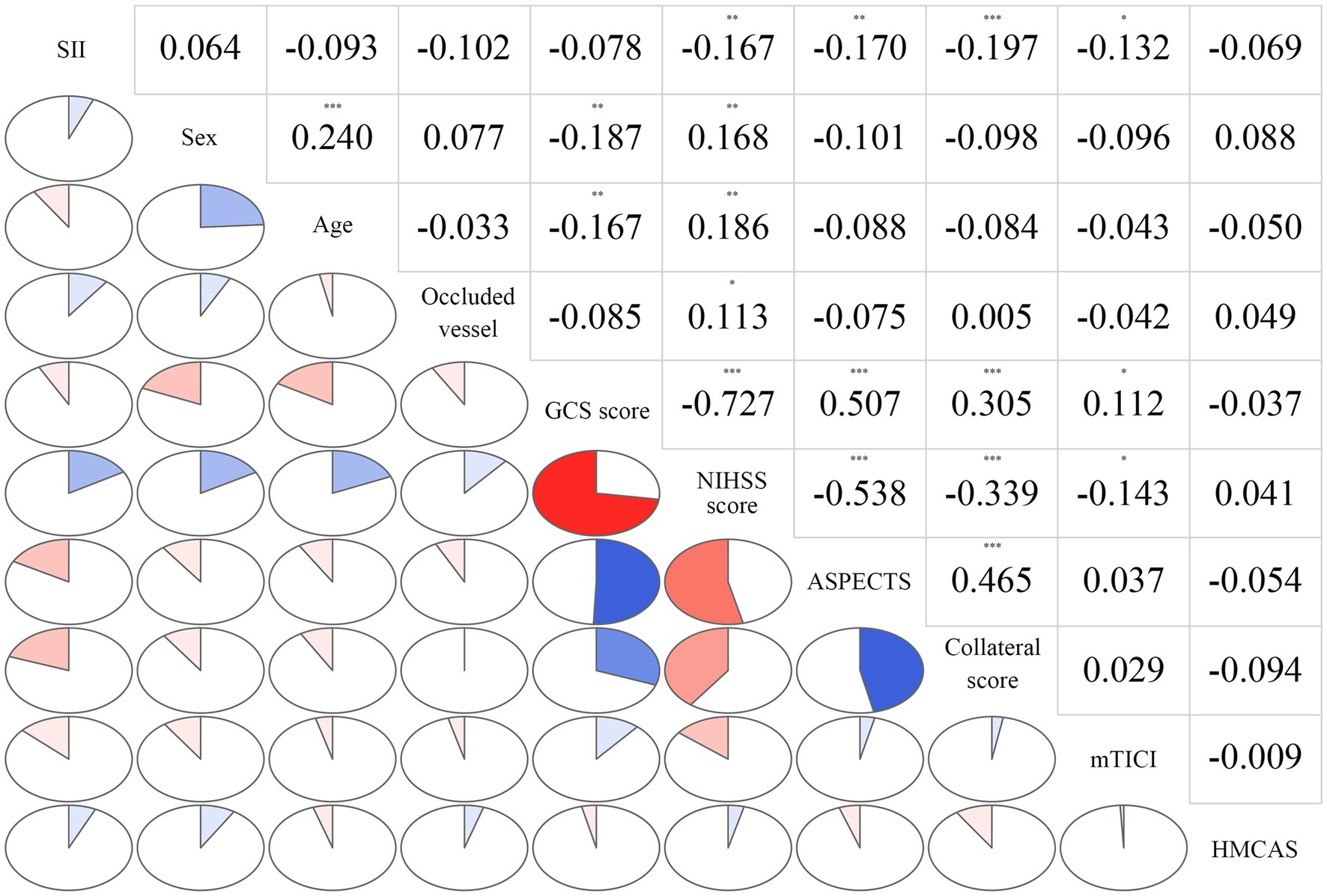
Figure 3. Correlations among variables in the multivariate model. The clockwise and counterclockwise graphs demonstrate positive and negative correlations, respectively. *p < 0.05; **p < 0.01, and ***p < 0.001, respectively.
The ROC curve suggested the moderate diagnostic performance of SII for HT, MBE, 90-day adverse function, and mortality (AUC 0.6294–0.7512, Figure 4). Importantly, SII showed a better diagnostic effect for these four outcomes than the other inflammatory indices, including SIRI, NLR, LMR, NPAR, and AISI (Table 3).
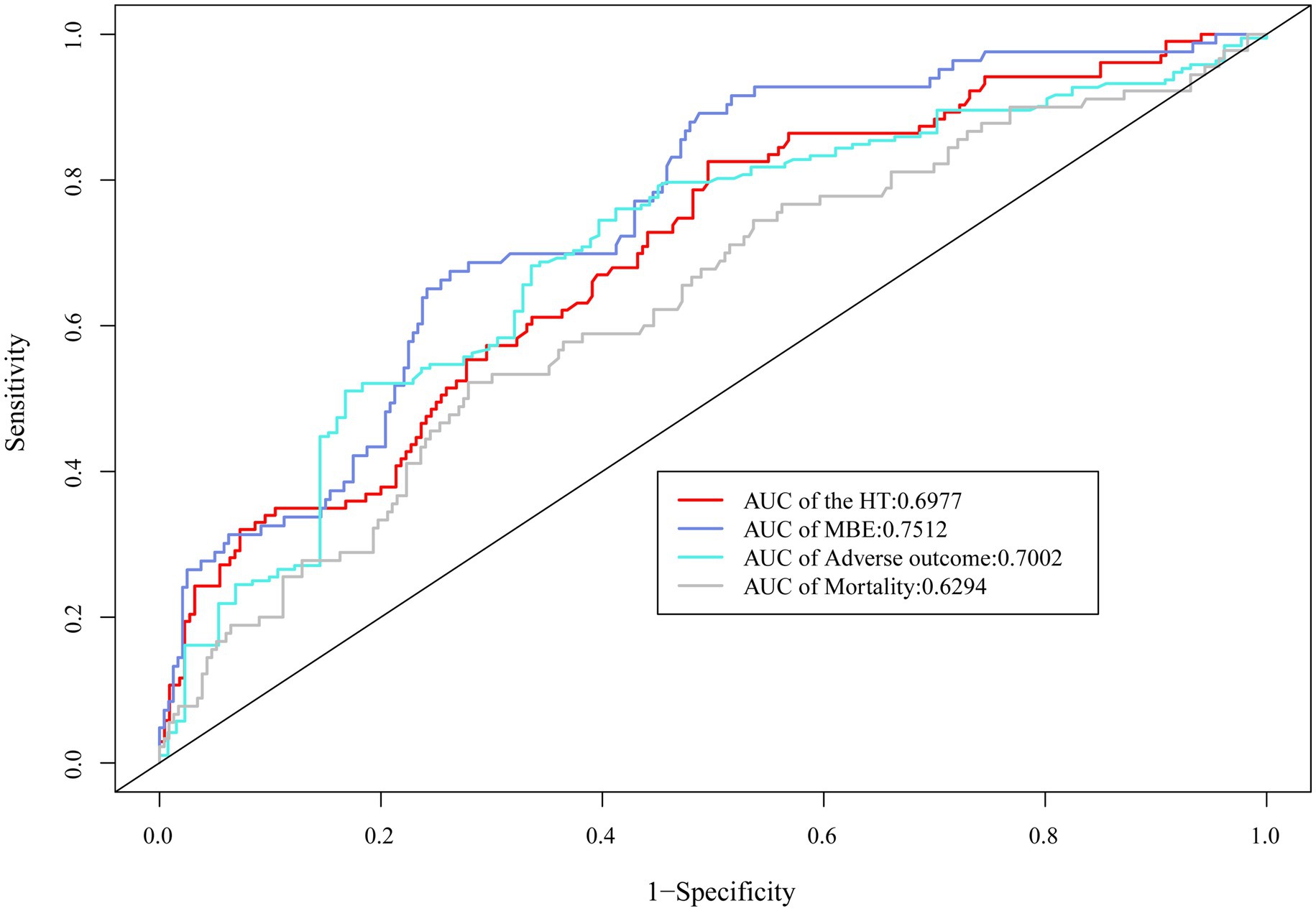
Figure 4. ROC curve demonstrated the moderate diagnostic effect of SII on HT, MBE, 90-day adverse function, and mortality.
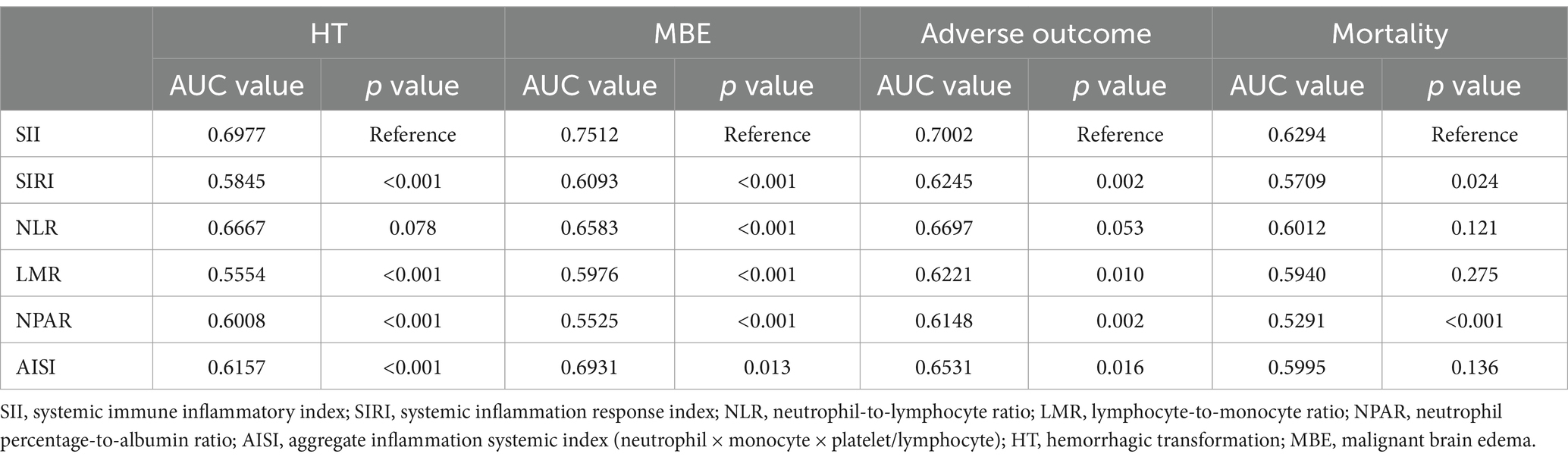
Table 3. Comparisons of diagnostic value for outcomes between SII and other combined inflammatory markers.
The subgroup analysis was performed to meticulously evaluate the prognostic utility of SII for predicting the above-mentioned four outcomes, stratifying age, sex, history of hypertension, TOAST classification, occluded vessel region, collateral score, and baseline ASPECTS. No interaction was detected between SII and these variables (all p > 0.05). The SII was identified as a substantial predictor of HT, MBE, and 90-day adverse functional outcomes across majority of the patient subgroups. However, no significant correlation was observed between SII and 90-day mortality in all subgroups (all p > 0.05, Figure 5).
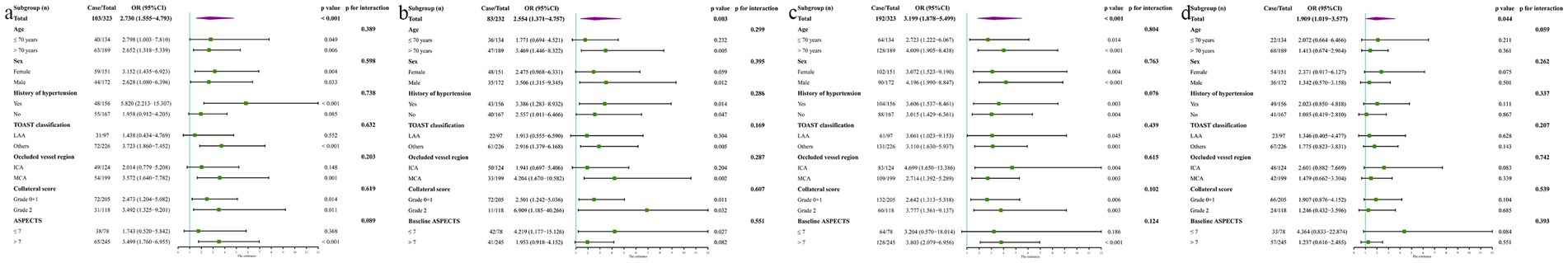
Figure 5. Subgroup analysis evaluated the relationship between SII with HT (A), MEB (B), 90-day adverse function (C), and mortality (D) by stratifying age, sex, history of hypertension, TOAST classification, occluded vessel region, collateral score, and baseline ASPECTS, using median value as the threshold of SII.
The mediation analysis was conducted to explore whether the relationships between elevated SII and increased risk of 90-day adverse function/mortality were mediated by HT or MBE. Using HT or MBE as a mediator respectively, we revealed the mediating effect of HT and MBE in the effect of the SII on 90-day adverse function and mortality (Figure 6).
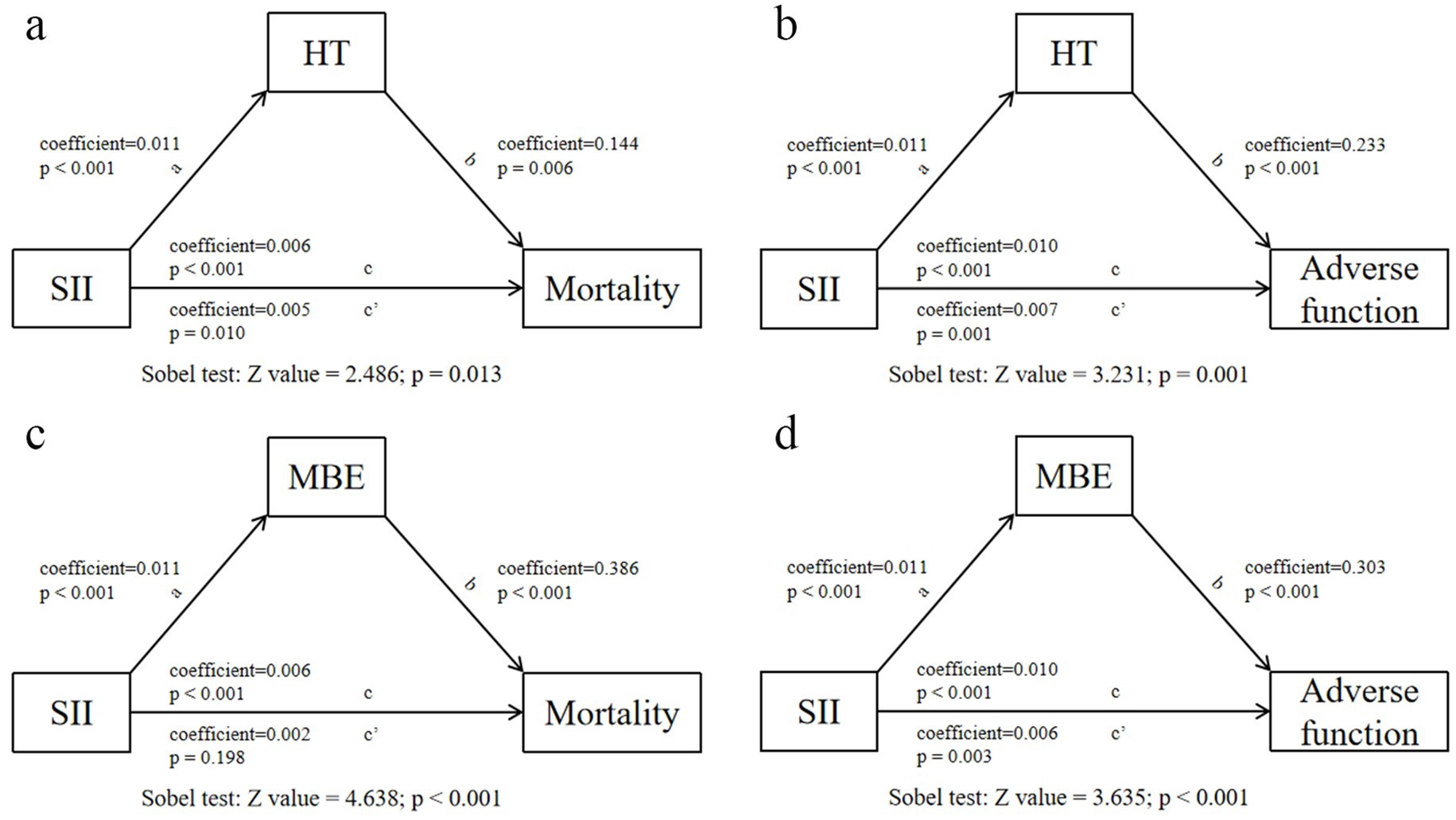
Figure 6. Analysis of the mediating effects of postoperative HT/MBE on the relationship between SII and 90-day adverse function/mortality.
A total of 42 patients were treated with MT + MH, and no severe cardiac arrhythmia, coagulopathy, and hypotension were encountered in these patients. The mean time of inducing hypothermia was 3.4 ± 1.8 h, the duration of maintenance was 53.7 ± 18.1 h, and the period of rewarming was 7.2 ± 2.6 h. Seventy-eight patients who underwent mere MT with ASPECTS ≤ 7 were selected with these 42 patients to form a new cohort. Compared to remaining patients with baseline ASPECTS 8–10, those in the new cohort possessed a significantly high value of SII (759 [IQR: 497–1,467] vs. 1,137 [IQR: 714–2,060], p < 0.001). The baseline characteristics of patients treated with or without MH were presented in Supplementary Table S5. A similar value of SII was found in these two groups (MT vs. MT + MH = 1,283 [IQR 706–2,223] vs. 1,066 [IQR 694–1,802], p = 0.314). Compared to patients with mere MT, those treated with MT + MH experienced a significantly lower incidence of postoperative HT (48.7% vs. 28.6%, p = 0.033), MBE (53.8% vs. 23.8%, 0.002), and 90-day mortality (42.3% vs. 19.0%, p = 0.01). In comparison, the rate of 90-day adverse function was similar (82.1% vs. 85.7%, p = 0.608). As shown in Table 4, after adjusting for sex, age, GCS score at admission, initial NIHSS score, baseline ASPECTS, present HMCAS, occluded vessel region, collateral score, and successful revascularization, the risk effect of SII and protective effect of MH were observed in HT (SII—OR: 1.037, 95% CI: 1.001–1.074, p = 0.047; MH—OR: 0.361, 95% CI: 0.136–0.957, p = 0.04), MBE (SII—OR: 1.063, 95% CI: 1.019–1.109, p = 0.005; MH—OR: 0.231, 95% CI: 0.081–0.653, p = 0.006), and 90-day mortality (SII—OR: 1.048, 95% CI: 1.011–1.087, p = 0.011; MH—OR: 0.343, 95% CI: 0.118–0.994, p = 0.049). However, the effects were not found in 90-day adverse function (SII—OR: 1.048, 95% CI: 0.983–1.118, p = 0.153; MH—OR” 1.214, 95% CI: 0.319–4.622, p = 0.776). In addition, other common complications are listed in Table 5. Patients treated with MH + MT developed a significantly higher rate of pneumonia than those treated with only MT (MH + MT vs. MT = 54.8% vs. 30.8%, p = 0.010).
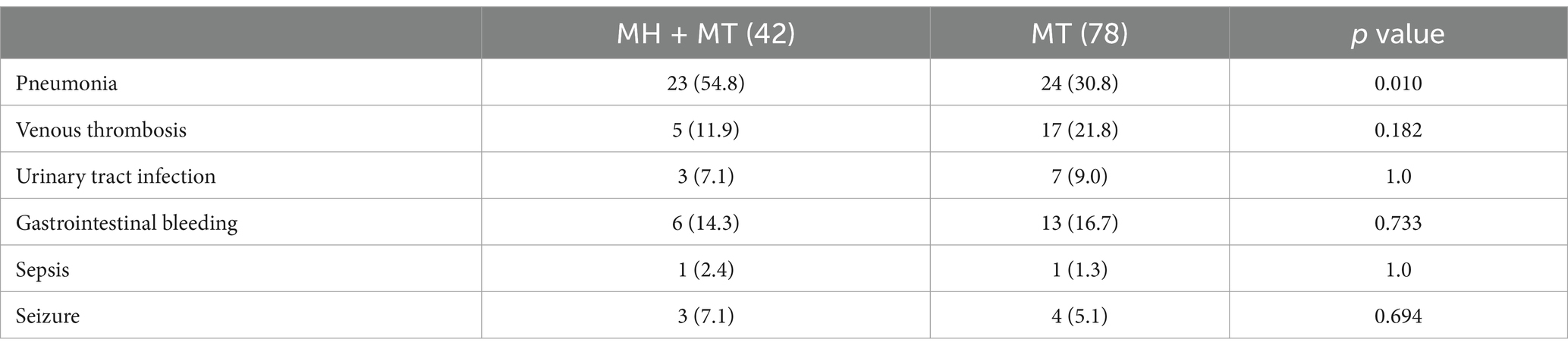
Table 5. Complications of patients treated with mild hypothermia + mechanical thrombectomy and only mechanical thrombectomy.
In this study, we investigated the relationship between SII and clinical outcomes in patients with large artery occlusion involving the anterior circulation treated by MT, revealing that elevated SII was linked to both early complications and late prognosis in these patients—even after adjusting for confounding risk factors. Furthermore, SII showed higher diagnostic value than other composite inflammation indices and may lead to 90-day adverse function and mortality by mediating HT and MBE. Therefore, SII may be an independent risk factor for poor prognosis in patients who underwent MT with LAO in the anterior circulation. In addition, first, we put forward that MH could reduce the risk of HT and MEB and be a life-saving measure for patients with high inflammation status.
Increased evidence demonstrates that inflammation plays a crucial role in the occurrence and progression of a series of diseases. As a composite measure derived from neutrophils, lymphocytes, and platelets, SII fully reflects the inflammatory status and has been revealed to be related with the prognosis of a variety of diseases, such as coronary heart disease, non-small cell lung cancer, and liver cirrhosis (19–21). As for stroke, Cao and Song et al. found the relationship between elevated SII and HT in AIS treated with MT, while the relationship between SII and MBE was not explored (11). Ji et al. identified the predictive value of SII for MBE but they did not further investigate the effect of SII on HT (13). Herein, first, we explored the association of SII with HT, MBE, and 90-day outcomes after MT in the same cohort.
Inflammation after AIS occurs early through the activation of both innate and adaptive immune systems. Local hypoxic and proinflammatory milieu stimulates the infiltration of peripheral leukocytes (22). Neutrophils are the earliest leukocyte subtype to react in the blood after AIS. They can rapidly migrate to ischemic brain tissue, aggravating cellular injury and extracellular matrix damage by releasing reactive oxygen species, proteases, and proinflammatory factors (8, 23). Moreover, neutrophils are a vital source of MMP9, contributing to the disruption of blood–brain barrier (BBB) that has been identified as the fundamental mechanism of HT and MBE in patients with AIS (4). In addition, overactivated neutrophils can produce neutrophil extracellular traps (NETs) that trap other blood cells, causing pathological thrombosis and exacerbating neuroinflammatory response (23). Furthermore, the NETs also contribute to the no-reflow phenomenon—defined as inadequate blood flow in certain areas within the brain even after successful recanalization—attributed to local microvascular obstructions, endothelial swelling, or other cellular debris (24, 25). hemostasis, platelets are rich sources of growth factors and play a crucial role in angiogenesis, tissue remodeling, and apoptosis (26). However, platelets are also involved in the inflammation following tissue injury, and platelet activation can directly drive local and systemic inflammatory responses (13, 26). After ischemia/reperfusion, platelets and leukocytes’ interaction aggravates tissue injury through stimulating leukocyte extravasation, oxidative rupture, and microcirculation occlusion (13). In the mice models, despite successful recanalization of the proximal MCA, microvascular occlusion caused by the adhesion of activated platelets and neutrophils was observed in the core infarction and ischemic penumbra area (25, 27). Critically, thrombocytosis and platelet activation are related to elevated disruption of BBB following AIS, which further results in an increased risk of HT and MBE after MT (28). In contrast, lymphocyte is a primary neuroprotective immunoregulator that specific subpopulations can preserve immune homeostasis and relieve cerebral damage by limiting the generation of proinflammatory mediators, regulating the activation of autoreactive cells, and promoting the process of neural repair (3). However, increased levels of catecholamine and cortisol generated by the overactivated sympathetic nervous system and hypothalamic–pituitary–adrenal axis after AIS induce apoptosis and functional inhibition of peripheral lymphocytes (3).
Previous studies on AIS treatment supported that higher inflammatory factors are related with poor prognosis, and are correlated with higher futile recanalization risk when focusing on reperfusion therapy (3, 11–13). However, few of them investigated the mechanism linking early inflammatory status to later outcomes (13). The SII weights the effects of neutrophils, platelets, and lymphocytes for AIS after MT, fully demonstrating the status of systemic inflammation and immune response. Consistent with previous studies, we revealed SII as an independent predictor of HT and MBE after MT, both of which are common complications affecting prognosis (11, 13). Since the value of SII is easily disturbed, it may not be persuasive to explain the effect of SII on later prognosis directly. Therefore, we explored the potential mechanism of SII affecting 90-day functional outcomes and mortality. The mediation analysis confirmed that the effect of an elevated SII on 90-day adverse function and mortality was partially mediated by HT and MBE, which may firstly explain the mechanism that inflammation could affect late prognosis through early complications. Future researches are required to investigate whether early suppression of inflammation reduces the risk of HT and MBE to improve prognosis in patients undergoing MT. However, the results need to be interpreted cautiously because our study only revealed the statistical correlation between SII and outcomes, rather a causal relationship, and further studies elucidating the direct effect are required.
Several immune–inflammation indicators related with stroke prognosis have been proposed in previous reports, such as LMR, NPAR, and AISI (11, 29, 30). The data extracted from the Endovascular Treatment for Acute Basilar Artery Occlusion study demonstrated that a higher neutrophil-to-lymphocyte ratio was related with futile recanalization (31). The systemic inflammatory response index was calculated with the following formula: Neutrophil × Monocyte/lymphocyte, which was related to poor prognosis after MT (3). Compared with complex inflammatory factors calculated by any two indexes, such as NLR, LMR, and NPAR, SII could more accurately evaluate the inflammatory status by assessing neutrophil, lymphocyte, and platelet (32). Considering that inflammatory indicators are susceptible to various factors, such as infection, SII may reduce the bias caused by excessive inclusion of inflammatory indicators, compared with AISI. However, Cao and Song et al. found that NLR had a higher predictive value than SII for symptomatic ICH and 90-day adverse function in patients treated with MT (symptomatic ICH AUC: NLR vs. SII = 0.758 vs. 0.707; 90-day adverse function AUC: NLR vs. SII = 0.662 vs. 0.633) (11). The discrepancy may be generated because only patients who achieved successful recanalization (mTICI of 2b-3) were enrolled in their study. We speculated that patients with futile recanalization had higher thrombus burden, and SII is superior to NLR in reflecting outcomes in these patients (33). In the current study, comparisons of SII with other composite inflammatory markers indicated the higher diagnostic value of SII for outcomes after MT for LAO, further demonstrating the better applicability of SII for clinical guidance.
Mild hypothermia protects brain injury after cerebral ischemia by inhibiting adhesion, aggregation, and infiltration of leukocytes, and blocking the following inflammatory cascade reaction (24). Wang et al. observed that MH significantly reduced neutrophil infiltration and monocyte accumulation after stroke in a model of transient focal cerebral ischemia (34). In mice models, Zhao et al. suggested that MH could reduce the invasion of neutrophils and macrophages to the brain after cerebral ischemia (35). Furthermore, MH could inhibit the aggregation and adhesion of neutrophils formed by no-reflow, which is particularly crucial for highlighting the importance of microvascular patency alongside the opening of large vessels (24). In a rat model of ischemic stroke, Zhao et al. showed that the expression of MMP-9 was significantly decreased in the MH group compared to the control group (36). All the above reports have experimentally confirmed that MH can play a role in brain protection after cerebral ischemia. Multiple prospective studies also identified the relationship between MH clinically and promoted prognosis in patients treated by MT (18, 37, 38). However, the specific indications for MH therapy are still ambiguous, because of the different inclusion criteria in each study. An early pilot study called Cooling for Acute Ischemic Brain Damage (COOL AID) identified the safety and effectiveness of MH therapy for patients treated with intravenous thrombolysis or MT (18). However, Georgiadis et al. reported that MH therapy resulted in higher mortality and higher complication rates compared with hemicraniectomy (39). Different conditions of patients treated with these two methods may be the reason for this discrepancy. In their study, hemicraniectomy was applied for patients with the non-dominant hemisphere affected, while MH therapy was used for patients with the dominant hemisphere affected. In 2016, Schneider et al. also put forward that no benefits of hypothermia in patients treated with hemicraniectomy for large ischemic stroke (40). According to the findings of their study, it might be suggested that later MH (after hemicraniectomy) for patients with large ischemic stroke was even harmful. Among the patients recruited from the Reperfusion and Cooling in Cerebral Acute Ischemia (ReCCLAIM) study, MH was protective against intracerebral hemorrhages after intra-arterial reperfusion therapy (41). Consistent with this study under similar inclusive ASPECTS (ReCCLAIM: 5–7; current study: ≤7), we also observed the protective effect of MH for postoperative HT. As a pilot study in our institution, the MH was only employed for patients with baseline ASPECTS ≤ 7, which has been proven to be an independent predictor of poor outcomes in patients treated with MT (38). In our study, elevated SII was still significantly related to HT, MBE, and 90-day mortality. Furthermore, with a similar value of SII, the MH therapy may have served as a protective factor that significantly decreased the risk of HT, MBE, and 90-day mortality while failing to improve functional outcomes. We speculated that MH suppresses cerebral ischemia’s inflammatory response after MT, causing mitigation of the BBB damage and resulting in reduced risk of HT, MBE, and followed 90-day mortality. However, for patients with large core infarction before MT, extended occlusion has led to irreversible cerebral tissue damage, making ineffectiveness of any subsequent reperfusion efforts, and even if inflammation was inhibited, the functional impairment could not be reversed (24). Nevertheless, MH substantially reduced the risk of HT and MBE, promoted the survival rate of patients, and could also be used as a life-saving measure for patients with high inflammation status.
In mice models, specifically targeting neutrophils with anti-Ly6G could improve penumbral blood flow, and anti-inflammatory treatment could restore microcirculation and decrease no-reflow (42, 43). However, similar therapy has never been evaluated in clinical practice. Although the significant relationship between SII and poor prognosis is observed in the current study, the utility of SII in real-time clinical decision-making remains limited at this stage because only statistical correlation has been identified. Nevertheless, our study may partially reveal the direction of future research, namely, the relationship between anti-inflammation and outcome improvement in patients with MT for LAO. Indeed, this study also had some limitations. First, the single central retrospective design with a limited number of cases in this study relies on the accuracy of data collection and hampers the generalization of these results. Second, the severity and treatment status of comorbidities were failed to be obtained from a significant proportion of patients or their family members. The results may be influenced without considering these variables. Moreover, although there was no obvious collinearity among variables in the multivariate model (all VIF < 5), correlation analysis demonstrated a significant correlation between some variables, which may partially affect the performance of the model’s performance. Third, a single baseline measurement without additional time-point sample collection may not adjust for the variation trend of SII value for predicting outcomes. Potential bias may be generated if the change in SII is also a relevant outcome indicator. Fourth, selection bias in selecting patients for MH may be generated because preoperative ASPECTS based on no-enhanced CT may not be accurate enough to assess the actual ischemia of cerebral tissue. More advanced imaging methods, such as CTP and MRI, and more rigorous inclusion criteria will be implemented for MH therapy. In addition, only patients with ASPECTS ≤ 7 were selected for MH therapy. This hinders us from exploring whether MH therapy could improve prognosis in patients with ASPECTS > 7, and whether these patients in high inflammatory status (high SII) were recommended for MH therapy. Fifth, other potential variables that may be related with outcomes may be ignored to enroll, which may influence the accuracy of ORs of SII predicting outcomes.
SII is an easily available inflammatory index and can be used as an independent risk factor for HT, MBE, 90-day adverse function, and mortality for patients with LAO in anterior circulation treated by MT, providing a reference for clinical practice. A meticulous protocol is required for patients with high SII value, including close postoperative scrutiny, active therapeutic measures, such as high-dose dehydration or decompressive craniectomy if necessary for MBE, and cautious postoperative anti-platelet/anticoagulation regimens. Postoperative HT and MBE may be the mediators linking SII to 90-day outcomes. With a similar value of SII, MH may improve the prognosis of patients after MT by inhibiting the inflammatory response. However, the current study only provides a preliminary assessment, and more research studies, particularly in randomized controlled trials, are needed to confirm the efficacy and safety of MH in this patient population, and fundamental studies are still required to verify the mechanism.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the ethics committee of Suining Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AQ: Writing – original draft, Writing – review & editing. LZ: Formal analysis, Methodology, Software, Writing – original draft. HH: Data curation, Resources, Writing – review & editing. JD: Data curation, Software, Validation, Writing – review & editing. ST: Funding acquisition, Software, Supervision, Writing – review & editing. WX: Conceptualization, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Research Project on Medical Youth Innovation in Sichuan Province (No. Q22014) and the Medical Research Project of Sichuan Province (No. S23006).
We thank all the medical staff in our stroke center at Suining Central Hospital, Sichuan, China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1516577/full#supplementary-material
1001. ^The R-program is an open source software, we have added the a website link of this software: https://www.r-project.org/
1. Zhang, L, Li, J, Yang, B, Li, W, Wang, X, Zou, M, et al. The risk and outcome of malignant brain edema in post-mechanical thrombectomy: acute ischemic stroke by anterior circulation occlusion. Eur J Med Res. (2023) 28:435. doi: 10.1186/s40001-023-01414-x
2. Shaban, A, al, S, Chalhoub, RM, Bass, E, Maier, I, Psychogios, MN, et al. Mechanical thrombectomy for large vessel occlusion strokes beyond 24 hours. J Neurointerv Surg. (2023) 15:e331–6. doi: 10.1136/jnis-2022-019372
3. Lattanzi, S, Norata, D, Divani, AA, di Napoli, M, Broggi, S, Rocchi, C, et al. Systemic inflammatory response index and futile recanalization in patients with ischemic stroke undergoing endovascular treatment. Brain Sci. (2021) 11:1164. doi: 10.3390/brainsci11091164
4. Krishnan, R, Mays, W, and Elijovich, L. Complications of mechanical thrombectomy in acute ischemic stroke. Neurology. (2021) 97:S115–s125. doi: 10.1212/wnl.0000000000012803:doi
5. Ni, H, Lu, GD, Hang, Y, Jia, ZY, Cao, YZ, Shi, HB, et al. Association between infarct location and Hemorrhagic transformation of acute ischemic stroke following successful recanalization after mechanical thrombectomy. AJNR Am J Neuroradiol. (2023) 44:54–9. doi: 10.3174/ajnr.A7742
6. Huang, X, Yang, Q, Shi, X, Xu, X, Ge, L, Ding, X, et al. Predictors of malignant brain edema after mechanical thrombectomy for acute ischemic stroke. J Neurointerv Surg. (2019) 11:994–8. doi: 10.1136/neurintsurg-2018-014650
7. Qiu, H, Shen, R, Chen, L, Pandey, S, Sun, J, and Deng, H. Low serum magnesium levels are associated with Hemorrhagic transformation after mechanical thrombectomy in patients with acute ischemic stroke. Front Neurol. (2022) 13:831232. doi: 10.3389/fneur.2022.831232
8. Li, WC, Zhou, YX, Zhu, G, Zeng, KL, Zeng, HY, Chen, JS, et al. Systemic immune inflammatory index is an independent predictor for the requirement of decompressive craniectomy in large artery occlusion acute ischemic stroke patients after mechanical thrombectomy. Front Neurol. (2022) 13:945437. doi: 10.3389/fneur.2022.945437
9. Wang, J, du, Y, Wang, A, Zhang, X, Bian, L, Lu, J, et al. Systemic inflammation and immune index predicting outcomes in patients with intracerebral hemorrhage. Neurol Sci. (2023) 44:2443–53. doi: 10.1007/s10072-023-06632-z
10. Yun, S, Yi, HJ, Lee, DH, and Sung, JH. Systemic inflammation response index and systemic immune-inflammation index for predicting the prognosis of patients with aneurysmal subarachnoid Hemorrhage. J Stroke Cerebrovasc Dis. (2021) 30:105861. doi: 10.1016/j.jstrokecerebrovasdis.2021.105861
11. Cao, W, Song, Y, Bai, X, Yang, B, Li, L, Wang, X, et al. Systemic-inflammatory indices and clinical outcomes in patients with anterior circulation acute ischemic stroke undergoing successful endovascular thrombectomy. Heliyon. (2024) 10:e31122. doi: 10.1016/j.heliyon.2024.e31122
12. Chen, G, Wang, A, Zhang, X, Li, Y, Xia, X, Tian, X, et al. Systemic immune-inflammation response is associated with futile recanalization after endovascular treatment. Neurocrit Care. (2024) 41:165–73. doi: 10.1007/s12028-023-01930-y
13. Ji, Y, Xu, X, Wu, K, Sun, Y, Wang, H, Guo, Y, et al. Prognosis of ischemic stroke patients undergoing endovascular thrombectomy is influenced by systemic inflammatory index through malignant brain Edema. Clin Interv Aging. (2022) 17:1001–12. doi: 10.2147/CIA.S365553
14. Huang, X, Cai, Q, Xiao, L, Gu, M, Liu, Y, Zhou, Z, et al. Influence of procedure time on outcome and hemorrhagic transformation in stroke patients undergoing thrombectomy. J Neurol. (2019) 266:2560–70. doi: 10.1007/s00415-019-09451-5
15. Adams, HP Jr. B.H. Bendixen, L.J. Kappelle, et al., classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
16. Christoforidis, GA, Mohammad, Y, Kehagias, D, Avutu, B, and Slivka, AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. (2005) 26:1789–97.
17. Zeng, W, Li, W, Huang, K, Lin, Z, Dai, H, He, Z, et al. Predicting futile recanalization, malignant cerebral edema, and cerebral herniation using intelligible ensemble machine learning following mechanical thrombectomy for acute ischemic stroke. Front Neurol. (2022) 13:982783. doi: 10.3389/fneur.2022.982783
18. Krieger, DW, de, M, Abou-Chebl, A, Andrefsky, J, Sila, C, Katzan, I, et al. Cooling for acute ischemic brain damage (cool aid): an open pilot study of induced hypothermia in acute ischemic stroke. Stroke. (2001) 32:1847–54. doi: 10.1161/01.STR.32.8.1847
19. Lei, S, Zhang, Q, Zhang, Q, Long, L, Xiong, Y, Sun, S, et al. The systemic immune inflammation index (SII) combined with the creatinine-to-cystatin C ratio (Cre/CysC) predicts sarcopenia in patients with liver cirrhosis complicated with primary hepatocellular carcinoma. Nutr Cancer. (2023) 75:1116–22. doi: 10.1080/01635581.2023.2176199
20. Dong, W, Gong, Y, Zhao, J, Wang, Y, Li, B, and Yang, Y. A combined analysis of TyG index, SII index, and SIRI index: positive association with CHD risk and coronary atherosclerosis severity in patients with NAFLD. Front Endocrinol (Lausanne). (2023) 14:1281839. doi: 10.3389/fendo.2023.1281839:doi
21. Huang, W, Luo, J, Wen, J, and Jiang, M. The relationship between systemic immune inflammatory index and prognosis of patients with non-small cell lung cancer: a meta-analysis and systematic review. Front Surg. (2022) 9:898304. doi: 10.3389/fsurg.2022.898304
22. Thomas, SE, Plumber, N, Venkatapathappa, P, and Gorantla, V. A review of risk factors and predictors for Hemorrhagic transformation in patients with acute ischemic stroke. Int J Vasc Med. (2021) 2021:4244267. doi: 10.1155/2021/4244267:doi
23. Zhang, R, Jin, F, Zheng, L, Liao, T, Guan, G, Wang, J, et al. Neutrophil to high-density lipoprotein ratio is associated with Hemorrhagic transformation in patients with acute ischemic stroke. J Inflamm Res. (2022) 15:6073–85. doi: 10.2147/JIR.S381036
24. Xu, W, Geng, X, Fayyaz, AI, and Ding, Y. The modulatory role of hypothermia in post-stroke brain inflammation: mechanisms and clinical implications. Cerebrovasc Dis. (2024) 53:1–13. doi: 10.1159/000536384
25. el, M, Glück, C, Binder, N, Middleham, W, Wyss, M, Weiss, T, et al. Neutrophils obstructing brain capillaries are a major cause of no-reflow in ischemic stroke. Cell Rep. (2020) 33:108260. doi: 10.1016/j.celrep.2020.108260
26. Lin, C, Pan, H, Qiao, Y, Huang, P, Su, J, and Liu, J. Fibrinogen level combined with platelet count for predicting Hemorrhagic transformation in acute ischemic stroke patients treated with mechanical thrombectomy. Front Neurol. (2021) 12:716020. doi: 10.3389/fneur.2021.716020
27. Yemisci, M, Gursoy-Ozdemir, Y, Vural, A, Can, A, Topalkara, K, and Dalkara, T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. (2009) 15:1031–7. doi: 10.1038/nm.2022
28. Shaik, NF, Regan, RF, and Naik, UP. Platelets as drivers of ischemia/reperfusion injury after stroke. Blood Adv. (2021) 5:1576–84. doi: 10.1182/bloodadvances.2020002888
29. Gong, P, Liu, Y, Gong, Y, Chen, G, Zhang, X, Wang, S, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. (2021) 18:51. doi: 10.1186/s12974-021-02090-6
30. Chen, Z, Xie, D, Li, Y, Dai, Z, Xiang, S, Chen, Z, et al. Neutrophil albumin ratio is associated with all-cause mortality in stroke patients: a retrospective database study. Int J Gen Med. (2022) 15:1–9. doi: 10.2147/IJGM.S323114
31. Yang, J, Jin, Z, Song, J, Guo, C, Xie, D, Yue, C, et al. Futile recanalization after endovascular treatment in patients with acute basilar artery occlusion. Neurosurgery. (2023) 92:1006–12. doi: 10.1227/neu.0000000000002313
32. Yi, HJ, Sung, JH, and Lee, DH. Systemic inflammation response index and systemic immune-inflammation index are associated with clinical outcomes in patients treated with mechanical thrombectomy for large artery occlusion. World Neurosurg. (2021) 153:e282–9. doi: 10.1016/j.wneu.2021.06.113
33. Dolu, AK, Karayiğit, O, Ozkan, C, Çelik, MC, and Kalçık, M. Relationship between intracoronary thrombus burden and systemic immune-inflammation index in patients with ST-segment elevation myocardial infarction. Acta Cardiol. (2023) 78:72–9. doi: 10.1080/00015385.2022.2035082
34. Wang, GJ, Deng, HY, Maier, CM, Sun, GH, and Yenari, MA. Mild hypothermia reduces ICAM-1 expression, neutrophil infiltration and microglia/monocyte accumulation following experimental stroke. Neuroscience. (2002) 114:1081–90. doi: 10.1016/S0306-4522(02)00350-0
35. Zhao, J, Mu, H, Liu, L, Jiang, X, Wu, D, Shi, Y, et al. Transient selective brain cooling confers neurovascular and functional protection from acute to chronic stages of ischemia/reperfusion brain injury. J Cereb Blood Flow Metab. (2019) 39:1215–31. doi: 10.1177/0271678X18808174
36. Zhao, Y, Wei, ZZ, Lee, JH, Gu, X, Sun, J, Dix, TA, et al. Pharmacological hypothermia induced neurovascular protection after severe stroke of transient middle cerebral artery occlusion in mice. Exp Neurol. (2020) 325:113133. doi: 10.1016/j.expneurol.2019.113133
37. Tian, H, Wan, Y, Zhang, H, and Zuo, J. Interrupted intraarterial selective cooling infusion combined with mechanical thrombectomy in patients with acute ischemic stroke: a prospective, nonrandomized observational cohort study. J Neurosurg. (2023) 139:1083–91. doi: 10.3171/2023.2.JNS222542
38. Wu, C, Zhao, W, An, H, Wu, L, Chen, J, Hussain, M, et al. Safety, feasibility, and potential efficacy of intraarterial selective cooling infusion for stroke patients treated with mechanical thrombectomy. J Cereb Blood Flow Metab. (2018) 38:2251–60. doi: 10.1177/0271678X18790139
39. Georgiadis, D, Schwarz, S, Aschoff, A, and Schwab, S. Hemicraniectomy and moderate hypothermia in patients with severe ischemic stroke. Stroke. (2002) 33:1584–8. doi: 10.1161/01.STR.0000016970.51004.D9
40. Schneider, H, Krüger, P, Algra, A, Hofmeijer, J, van der Worp, HB, Jüttler, E, et al. No benefits of hypothermia in patients treated with hemicraniectomy for large ischemic stroke. Int J Stroke. (2017) 12:732–40. doi: 10.1177/1747493017694388
41. Horn, CM, Sun, CH, Nogueira, RG, Patel, VN, Krishnan, A, Glenn, BA, et al. Endovascular reperfusion and cooling in cerebral acute ischemia (ReCCLAIM I). J Neurointerv Surg. (2014) 6:91–5. doi: 10.1136/neurintsurg-2013-010656
42. Huang, J, Choudhri, TF, Winfree, CJ, McTaggart, RA, Kiss, S, Mocco, J, et al. Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke. (2000) 31:3047–53. doi: 10.1161/01.STR.31.12.3047
Keywords: acute ischemic stroke, large artery occlusion, mechanical thrombectomy, systemic immune-inflammation index, mild hypothermia
Citation: Qian A, Zheng L, He H, Duan J, Tang S and Xing W (2025) Predictive value of the systemic immune–inflammation index for outcomes in large artery occlusion treated with mechanical thrombectomy—a single-center study. Front. Neurol. 15:1516577. doi: 10.3389/fneur.2024.1516577
Received: 25 October 2024; Accepted: 31 December 2024;
Published: 29 January 2025.
Edited by:
Mohd Kaisan Mahadi, National University of Malaysia, MalaysiaReviewed by:
Xin-Fang Leong, National University of Malaysia, MalaysiaCopyright © 2025 Qian, Zheng, He, Duan, Tang and Xing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuang Tang, am9obm55dGFuZ0BzbnN6eHl5Ny53ZWNvbS53b3Jr; Wenli Xing, MzkyODA4NDdAcXEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.