- 1Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
- 2Departments of Molecular and Cellular Biology and Statistics, Harvard University, Cambridge, MA, United States
- 3Department of Neurology, Oregon Health & Science University, Portland, OR, United States
- 4Population Health and Equity Research Institute, Center for Health Care Research and Policy, Case Western Reserve University at MetroHealth Medical Center, Cleveland, OH, United States
- 5Parkinson and Other Movement Disorders Center, Department of Neurosciences, UC San Diego, La Jolla, CA, United States
- 6Movement Disorder Program, Department of Neurology, University of Chicago Medicine, Chicago, IL, United States
- 7Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 8Department of Neurology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
- 9Tanz Centre for Research in Neurodegenerative Diseases, University of Toronto, Toronto, ON, Canada
- 10Krembil Brain Institute, University Health Network, Toronto, ON, Canada
- 11Edmond J. Safra Program in Parkinson's Disease, Rossy PSP Centre, Morton and Gloria Shulman Movement Disorders Clinic, Krembil Brain Institute, Toronto Western Hospital, University Health Network and Division of Neurology, University of Toronto, ON, Canada
- 12Department of Neurology, Mount Sinai Beth Israel, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 13Memory and Aging Center, Department of Neurology, University of California, San Francisco, San Francisco, CA, United States
- 14Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, United States
Background: The Montreal Cognitive assessment (MoCA) is a well-validated global cognitive screening instrument. Its validity in progressive supranuclear palsy (PSP) has not been assessed.
Objectives: To evaluate the MoCA as an outcome measure in PSP clinical trials.
Methods: Cognitive data from 162 participants in the placebo arm of the Biogen PASSPORT study (NCT03068468) were analyzed using linear mixed-effects modeling (LMM) and repeated measures correlation.
Results: There was a significant decline in the MoCA score over time of −1.4 (95% CI −0.84 to −1.97) points over a 48-week period (p < 0.0001). Small but significant changes (p < 0.01) were observed in all MoCA domains except abstraction. The MoCA correlated weakly with the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) over time (rrm = 0.1, p = 0.02) but exhibited a stronger correlation with the PSP Rating Scale (PSPRS) (rrm = −0.25, p < 0.0001).
Conclusion: The MoCA appears to have limited sensitivity in capturing cognitive decline in PSP.
1 Introduction
Progressive supranuclear palsy (PSP) is a form of atypical parkinsonism and tauopathy characterized by neuronal cell death in critical regions of the central nervous system such as the substantia nigra, basal ganglia, and subthalamic nucleus (1). Postural instability and ocular motor abnormalities are among the classic features of the disease. As part of their clinical presentation, PSP patients also frequently develop multi-domain cognitive dysfunction, including deficits in attention, memory, communication, visuospatial perception, and executive function (2). Accurate monitoring of these symptoms in clinical trial settings is crucial for determining the efficacy of pharmacological interventions.
The Repeatable Battery Assessment of Neuropsychological Status (RBANS) (3) is among the few cognitive assessments validated for use in PSP, assessing five critical domains: immediate memory, visuospatial/constructional ability, language, attention, and delayed recall. Notably, in longitudinal studies of participants with PSP, the RBANS has demonstrated clinically significant cognitive decline over 1 year as well as validity as a clinical trial outcome measure (4, 5).
The Montreal Cognitive Assessment (MoCA) (6) is a commonly used clinical scale for measuring cognitive impairment in clinical practice and clinical trials, including in PSP (7–9). Its brevity and ease of administration make it an attractive alternative to the RBANS. While RBANS administration takes approximately 20–30 min, MoCA takes roughly 10 min, with the time ranging based on the functional ability of the individual for both assessments. The MoCA comprises eight domains including visuospatial and executive functioning, naming, memory, attention, language, abstraction, delayed recall, and orientation, totaling 30 points. In North American populations, a score of 18–26 indicates mild cognitive impairment (MCI) (6), and a score of <18 is consistent with dementia in Parkinson’s disease (PD) (10), though this cutoff has not been specifically evaluated in PSP. Although initially developed as a screening tool, the MoCA has been used as a longitudinal measure of cognition across various neurological disorders such as MCI (11, 12), dementia (12), stroke (13–15) and ALS (16, 17). However, there are still conflicting reports about its sensitivity to detect progression of cognitive decline in related diseases like PD (18–22). Additionally, while an observational longitudinal study has reported on the change in MoCA over time (23), its utility as an outcome measure in clinical trials has yet to be studied. Here, we assess the longitudinal performance of the MoCA in a large well-characterized clinical trial cohort of participants with PSP.
2 Methods
All secondary analyses were performed in accordance with the ethical standards of Mass General Brigham Institutional Review Board in accordance with the Declaration of Helsinki. Written informed consent was obtained at the time of data collection and reconsent was not required for this secondary analysis.
Data were obtained from N = 162 members of the placebo arm of the Study of BIIB092 in Participants with Progressive Supranuclear Palsy (PASSPORT, clinicaltrials.gov identifier: NCT03068468), a phase 2, randomized, placebo-controlled trial evaluating the safety and efficacy of gosuranemab (a tau monoclonal antibody) in adults with PSP-Richardson syndrome (RS). Enrollment was limited to patients with probable or possible PSP based on the MDS PSP diagnosis criteria, symptom onset ≤5 years prior to baseline, aged 41–86. Patients with a score of ≤20 on the Mini-Mental State Examination or who were unable to ambulate independently were excluded. Participants were enrolled at 90 outpatient sites spanning 13 countries and underwent a 52-week double-blind phase, followed by an open-label extension period. Scores were initially recorded at baseline and then approximately every 12 weeks throughout the 124-week duration of the study (24). Owing to the high rate of dropout after week 52, we truncated the data after 52 weeks. Of note, the MoCA was performed at week 48 while the RBANS was performed at week 52. For the purposes of this analysis, we merged the data from these two time points.
An appropriate adaptation of all assessments, accounting for language and cultural relevance, was administered in each country that participated in the trial. Each domain of the RBANS yields scores ranging from 40 to 154, contributing to a total scaled score between 40 and 160 points (3), while each domain of the MoCA is scored on a scale of 0–6 points, with a maximum score of 30 points (6). Four parallel versions of the RBANS were administered sequentially every 3 months (A, B, C and D) resulting in a repetition of version A at week 52. MoCA version 7.1 was used repeatedly throughout the study.
All statistical analyses were performed using RStudio statistical software version 4.3.1. Linear mixed-effects models (LMM), fitted with the lme4 and lmerTest packages, were used to assess the temporal changes in the MoCA total and individual domain scores over the 52-week double-blind treatment period. LMM analysis is well-suited for longitudinal data as it can account for individual variability among subjects while effectively handling missing data under the missing at random (MAR) assumption (i.e., data missing dependent on data at hand), a frequent challenge encountered in longitudinal studies (25). Models included week (categorical), age at baseline, and sex as fixed effects with subject-specific random intercepts. Restricted Maximum Likelihood was used as the estimation method.
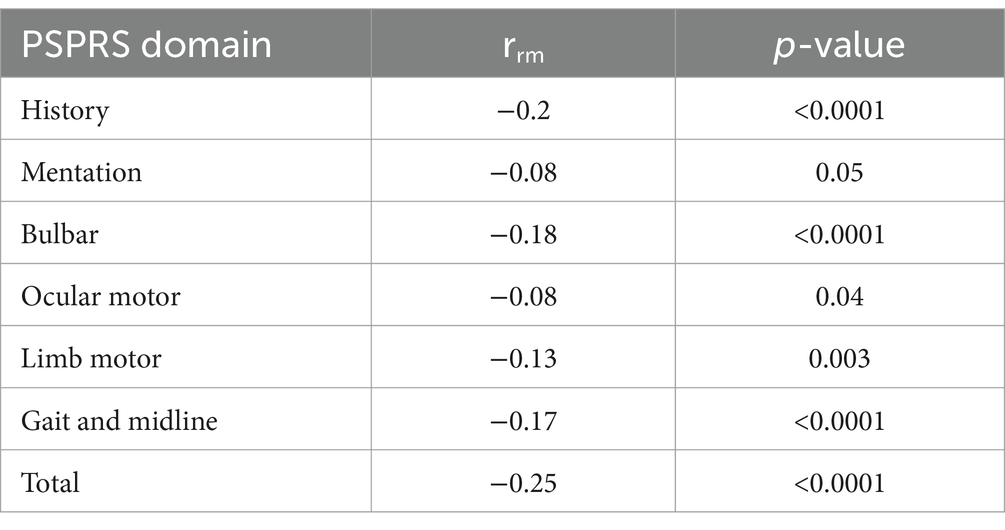
To begin to establish the criterion validity of MoCA as a repeated measures outcome, we evaluated its repeated measures correlations with those of the RBANS and PSPRS during the blinded period. We chose the RBANS as the gold standard assessment of cognitive function given its prior validation in PSP. The PSPRS was selected as the standard global PSP outcome measure for disease severity, which captures 6 domains (including ocular motor and limb motor, which can impact MoCA assessment) in addition to mentation (4 of 28 items, accounting for 16 of the 100 points) (26). We utilized an atypical application of ANCOVA (rmcorr function in R). This repeated measures correlation can account for the non-independence of repeated measures within individuals, making it especially fitting for longitudinal analyses. The rmcorr coefficient (rrm), ranging from −1 to 1, quantifies the strength of the linear association between the two variables. These effect sizes of the repeated measures correlation (r) can be interpreted as r = 0.1 small, r = 0.3 medium, r = 0.5 large (27).
3 Results
A total of 162 PSP patients were included in this analysis. Assessment data after week 52 were excluded due to the steep decline in participants after this timepoint. Demographic information and baseline scores for the MoCA, RBANS, PSPRS, and phonemic fluency are presented in Table 1. Longitudinal changes in these assessment scores are summarized in Supplementary Table 1. As shown in Supplementary Table 1, there was a decline in the number of participants who completed the MoCA from 155 at baseline to 133 at week 48, while participants who completed the RBANS declined from 152 at baseline to 113 at week 52 (compared to the 139 participants with PSPRS data at week 52).
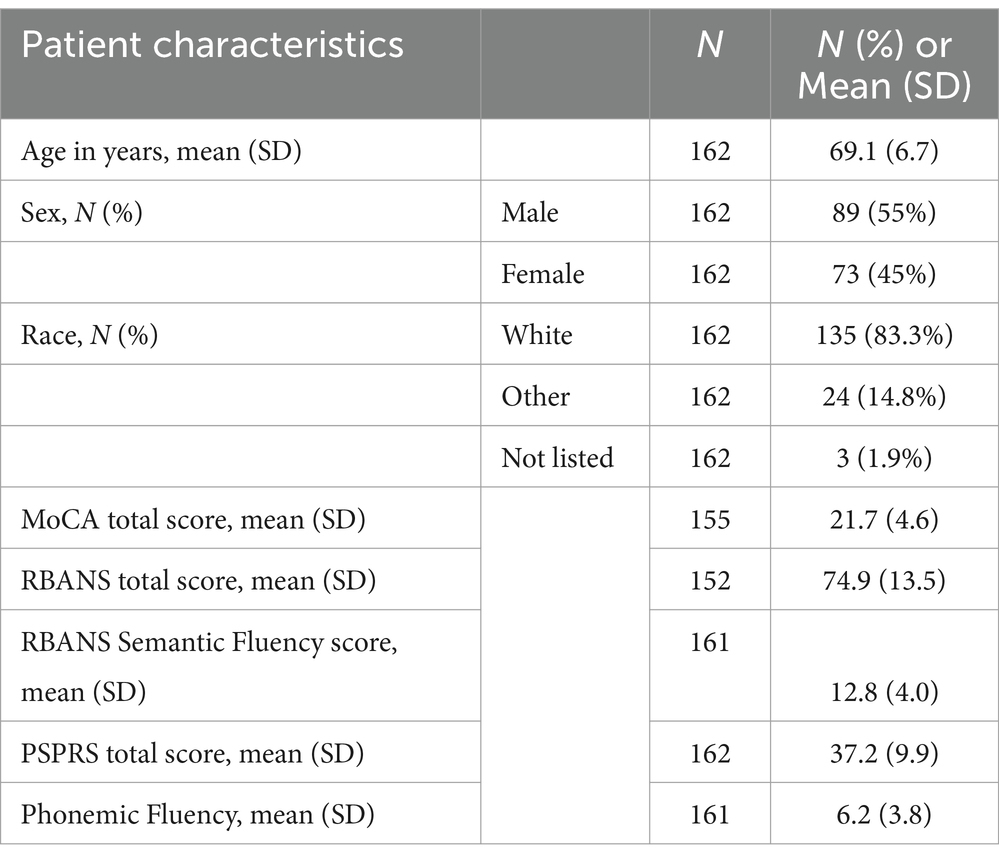
Table 1. Baseline demographics and characteristics of participants in the placebo arm of the PASSPORT study.
LMM revealed a small but statistically significant time effect on the MoCA score (p < 0.0001). The adjusted mean MoCA score estimated at baseline was 21.7 with a 95% confidence interval (CI) of 20.8 to 22.5. Over the course of 48 weeks, a mean change of −1.4 (95% CI −0.84 to −1.97) points in MoCA scores was observed (Figure 1A).
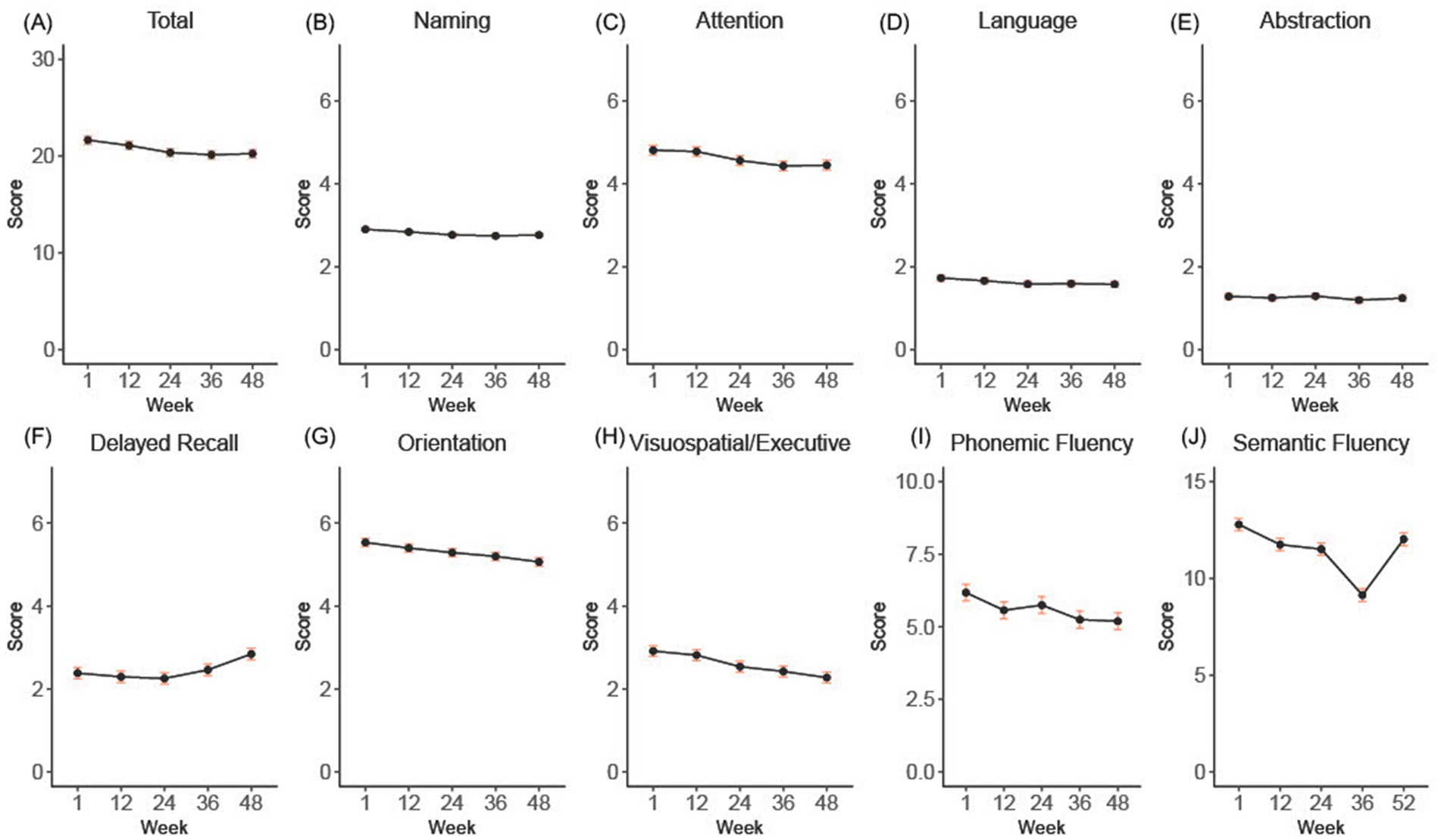
Figure 1. (A-H) shows the overall and subdomain progression of the total and subdomains of the Montreal Cognitive Assessment (MoCA) over the 52-week double-blind period. Panel (I) shows the raw phonemic fluency scores, and panel (J) shows the semantic fluency results extracted from the RBANS. Error bars indicate standard error.
To assess the contributions of each MoCA domain to the overall score decline over the 48-week period, we employed LMM for each individual domain (Figure 1B). Significant changes included: a decrease of −0.36 in attention (95% CI −0.16 to −0.56), an improvement of +0.46 in delayed recall (95% CI 0.20 to 0.72), a decrease of −0.15 in language (95% CI −0.03 to −0.28), a decrease of −0.14 in naming (95% CI −0.04 to − 0.23), a decrease of −0.47 in orientation (95% CI −0.29 to −0.65), a decrease of −0.64 in visuospatial/executive (95% CI −0.43 to −0.85), all p < 0.01. Abstraction decreased non-significantly by −0.04 (95% CI −0.16 to 0.07, p = 0.47).
To account for the binary manner in which the language data are captured with the MoCA, we also separately examined phonemic fluency, which was captured in a separately administered test, and semantic fluency, which was measured as part of the RBANS. From baseline to week 52, there was a statistically significant, although small decrease in mean phonemic fluency of −0.98 points (with a 95% confidence interval of −0.61 to −1.35, p < 0.0001) (Figure 1). In comparison, the semantic fluency sub score of the RBANS demonstrated a mean decline of −3.64 points (95% CI −3.07 to −4.22, p < 0.0001) by week 36, but increased 2.89 points by week 52 (95% CI 2.28 to 3.49), such that overall decline from baseline to week 52 was −0.76 (95% CI −0.17 to −1.35) (Figure 1).
Our repeated measures correlation analysis revealed a weak correlation of the MoCA with the RBANS (rrm = 0.1, p = 0.02, 95% CI 0.02 to 0.19) (Figure 2) and a modest correlation with the PSPRS (rrm = −0.22, p < 0.0001, 95% CI −0.14 to −0.29) (Table 2). When evaluating the PSPRS subdomains individually, we found significant correlations between total MoCA scores and all domains except mentation and ocular motor. These results are summarized in Table 2.
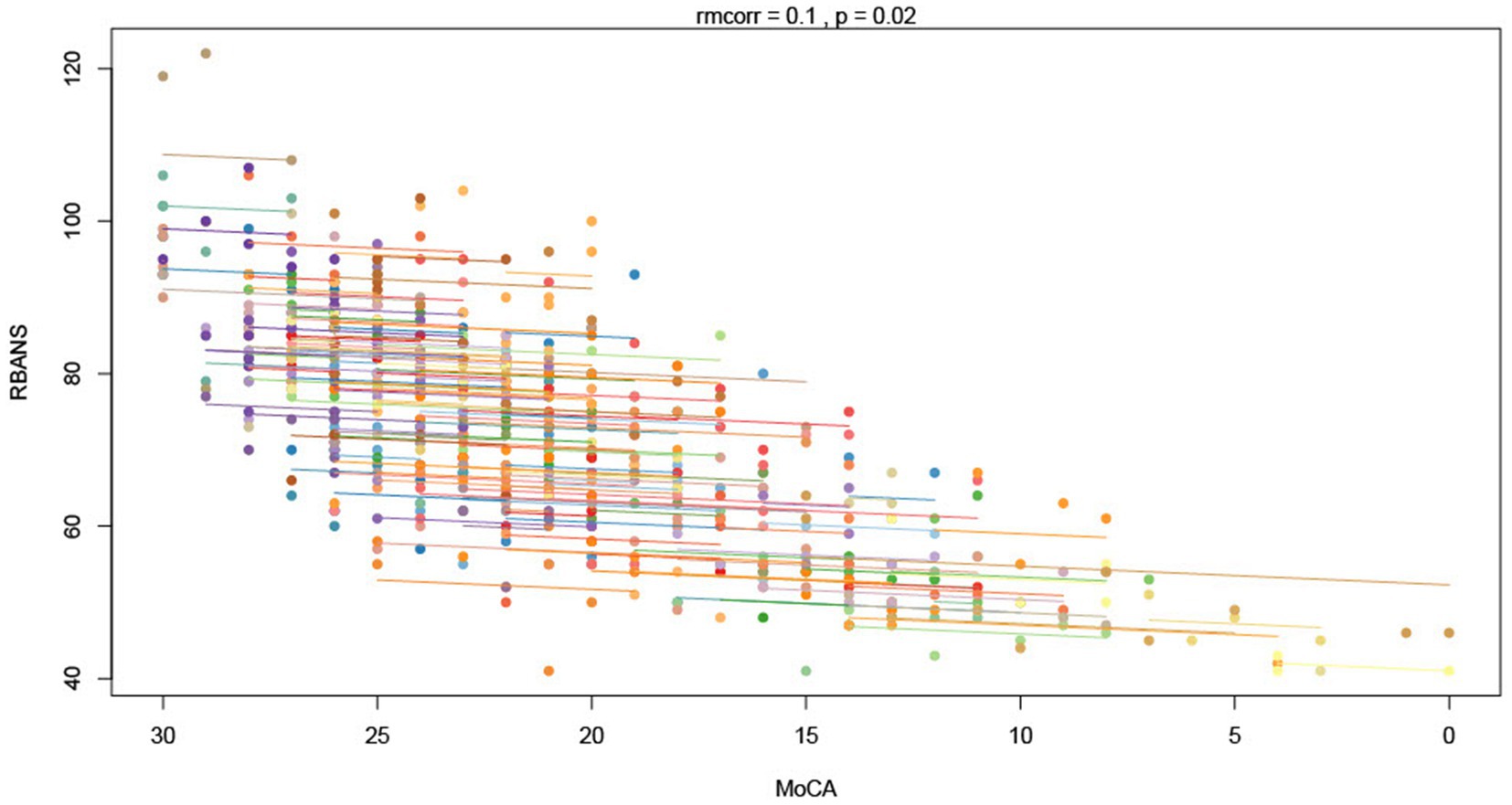
Figure 2. Repeated measures correlation (rmcorr) between the Montreal Cognitive Assessment (MoCA) and the RBANS. Each color indicates a distinct individual. Individual points demonstrate each time point for each individual and the overall slopes demonstrate the trajectory over time.
4 Discussion
The primary aim of our study was to assess the utility of the MoCA as a longitudinal clinical outcome measure in PSP. We found a statistically significant, although small, decline in MoCA scores over a 52-week period in all domains, except for abstraction and recall. Delayed recall improved slightly in participants, likely demonstrating practice effects. Overall, the changes seem to have limited clinical significance. The increase in semantic fluency scores from week 36 to 52 may have also been related to practice effects.
The repeated measures correlations between MoCA scores and those of the RBANS and PSPRS revealed only weak associations over time. Interestingly, a slightly stronger correlation was identified between the MoCA and PSPRS than between the MoCA and the RBANS. This suggests that the MoCA may reflect other deficits of PSP, as opposed to providing a pure assessment of cognition/cognitive domains; this has been observed in other cognitive outcome measures, including the RBANS (28).
In particular, the visuospatial domain of the MoCA requires ocular motor and motor function for the drawing tasks. Previous research by Jaegar et al. has attempted to address this by developing a cognitive composite battery for PSP that accounts for the confounding effects of motor impairment (29). The lack of correlation with the mentation domain of the PSPRS suggests that the mentation domain may be limited in its ability to capture cognitive impairment in PSP across multiple time points. It may also be due to the fact that the mentation domain of the PSPRS captures aspects of mood and behavior, which the MoCA does not.
Our study had several limitations. First, we chose the RBANS as the comparator standard for cognitive function and decline because few cognitive instruments have been validated in PSP (30). However, there are limitations to the RBANS as well, such as the length of administration and limited sensitivity. Second, the inherent limitations of the rmcorr function in R in handling missing data restricted our analysis to participants with no missed visits, and our findings should be interpreted within the context of the limited dataset. Third, using the same version of the MoCA every time likely resulted in a practice effect that contributed to the small decrease in scores seen over time (and the improved delayed recall and semantic fluency tests at the end of the study). Lastly, while the LMM analysis did account for missing data under the MAR assumption, it is likely that the missing data may not have been random (i.e., participants with steeper cognitive decline being more likely to drop out of the study), which could contribute to the surprising apparent lack of decline in the MoCA over time. Examining only participants who discontinued the study before week 52, the mean MoCA score at baseline was 20.8 (n = 19) which is slightly lower than the average of the other participants. While the sample size is limited, this suggests that missingness was not completely at random.
Overall, our results are in line with a previous observational cohort study including all PSP variants that found an annual rate of progression of −0.9 points in the MoCA score (SD 3.7, n = 117). In the RS subgroup, the rate was −0.8 (SD 3.0, n = 57) (23). While the MoCA was able to capture the cognitive impairment that is common in PSP (average baseline MoCA scores were < 26), the changes observed over the one-year period were surprisingly small in magnitude, showing a lack of sensitivity in capturing the cognitive decline associated with PSP as revealed by the RBANS. These preliminary findings show that the MoCA is insensitive to the natural progression of the disease and cast doubt on its use as a cognitive outcome measure in PSP. However, this does not completely rule out the possibility of the MoCA showing improvement over time in response to a symptomatic treatment. Altogether, this suggests that the MoCA suggests that should only be used to screen for cognitive impairment. These findings underscore the need for more specific and sensitive instruments to comprehensively and conveniently evaluate cognitive function over time in PSP.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://portal.rdca.c-path.org/.
Ethics statement
The studies involving humans were approved by the Massachusetts General Brigham Human Research Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
VI: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Methodology. CI: Writing – review & editing, Conceptualization. CS: Writing – review & editing. JI: Writing – review & editing, Methodology. MD: Writing – review & editing. DG: Writing – review & editing, Methodology, Supervision. EB: Writing – review & editing. TX: Writing – review & editing. AP: Writing – review & editing. LM-K: Writing – review & editing. IG-C: Writing – review & editing. MT: Writing – review & editing. AL: Writing – review & editing. MS: Writing – review & editing. AB: Writing – review & editing. LG: Writing – review & editing. A-MW: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We wish to thank the participants and care partners who gave their time and effort to participate in the phase 2 trial of gosuranemab study, and the clinical investigators and research staff who contributed to the collection of data used in this study.
Conflict of interest
Unrelated to this study CS has provided scientific advisory for SwanBio Therapeutics and received research funding from Sanofi-Genzyme for a study of video oculography in late-onset GM2 gangliosidosis. His institution has received financial support from Sanofi-Genzyme, SwanBio therapeutics and Encora Therapeutics and previously from Biogen and Biohaven, for the conduct of clinical trials. He has received honoraria from the American Academy of Neurology and The International Parkinson and Movement Disorders Society. He has received grant support from the National Institutes of Health K23 NS118045. Unrelated to this study MD has funding from NIH NINDS K23NS121402 and has served as a consultant for Synergic Medical Technologies and Cognito Therapeutics. EB receives research funding from the NIA (K99AG073453) and Lewy Body Dementia Association. Unrelated to this study AP is supported by NIH/NINDS U01 NS102035 and NIH/NIA R44 AG080861. IG-C is funded by the Clinical Research Training Scholarship in FTD from the Holloway Family Fund of The Association for Frontotemporal Degeneration, the Bob Burros Family Memorial Fund of the American Brain Foundation, and the American Academy of Neurology. Unrelated to this study MT has participated in clinical trials funded by Janssen, Biogen, Avanex, Green Valley, UCB, Novo Nordisk, GSK, BMS, and Passage Bio, and has served as a consultant for Eisai, Lilly, and Novo Nordisk. Unrelated to this study AL has served as an advisor for AbbVie, Amylyx, Aprinoia, Biogen, BioAdvance, Biohaven, BioVie, BlueRock, BMS, Denali, Janssen, Lilly, Pharma 2B, Sun Pharma, and UCB; received honoraria from Sun Pharma, AbbVie and Sunovion; received grants from Canadian Institutes of Health Research, Edmond J Safra Philanthropic Foundation, Krembil Brain Institute, Michael J. Fox Foundation, Parkinson Foundation, Parkinson Canada, and The Rossy Foundation; is serving as an expert witness in litigation related to paraquat and Parkinson’s disease, received publishing royalties from Elsevier, Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press. Unrelated to this study MS has served as investigator on clinical trials sponsored by Sage Therapeutics, Biogen Inc., and BIA R&D. LG: Consultancies: AI Therapeutics, Amylyx, Apellis, Aprinoia, Ferrer, IQVIA, UCB, Woolsey. Advisory Boards: Amylyx, CurePSP (unpaid), Roche, Rossy Centre, Springer. Royalties: Rutgers University Press for single-author text, A Clinician’s Guide to PSP (2019). Copyright material licensing fees (via Rutgers University). Travel expenses: CurePSP. AW has research funding from NIA/NIH 1R01AG085029 and R44AG080861, from the Parkinson’s Foundation, has participated in clinical trials funded by Roche/Genentech, Biogen/Denali, Bial, Amylyx Pharmaceuticals, Ferrer, Ono Pharmaceuticals, and received consultant payments from Accordant, CVS/Caremark, Genentech, Amylyx Pharmaceuticals and Ono Pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1501206/full#supplementary-material
References
1. Dickson, DW, Ahmed, Z, Algom, AA, Tsuboi, Y, and Josephs, KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol. (2010) 23:394–400. doi: 10.1097/WCO.0b013e32833be924
2. Golbe, LI. Progressive supranuclear palsy. Semin Neurol. (2014) 34:151–9. doi: 10.1055/s-0034-1381736
3. Randolph, C, Tierney, MC, Mohr, E, and Chase, TN. The repeatable battery for the assessment of neuro-psychological status (RBANS): preliminary clinical validity*. J Clin Exp Neuropsychol. (1997) 20:310–9. doi: 10.1076/jcen.20.3.310.823
4. Duff, K, McDermott, D, Luong, D, Randolph, C, and Boxer, AL. Cognitive deficits in progressive supranuclear palsy on the repeatable battery for the assessment of neuropsychological status. J Clin Exp Neuropsychol. (2019) 41:469–75. doi: 10.1080/13803395.2019.1572073
5. Duff, K, Randolph, C, and Boxer, AL. Cognitive decline on the repeatable battery for the assessment of neuropsychological status in progressive supranuclear palsy. Clin Neuropsychol. (2020) 34:529–40. doi: 10.1080/13854046.2019.1670865
6. Nasreddine, ZS, Phillips, NA, Bédirian, V, Charbonneau, S, Whitehead, V, Collin, I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
7. Santangelo, G, Cuoco, S, Pellecchia, MT, Erro, R, Barone, P, and Picillo, M. Comparative cognitive and neuropsychiatric profiles between Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. J Neurol. (2018) 265:2602–13. doi: 10.1007/s00415-018-9038-x
8. Fiorenzato, E, Weis, L, Falup-Pecurariu, C, Diaconu, S, Siri, C, Reali, E, et al. Montreal cognitive assessment (MoCA) and Mini-mental state examination (MMSE) performance in progressive supranuclear palsy and multiple system atrophy. J Neural Transm. (2016) 123:1435–42. doi: 10.1007/s00702-016-1589-3
9. Jia, P, Zhang, J, Han, J, and Ji, Y. Clinical outcomes and cognitive impairments between progressive supranuclear palsy and multiple system atrophy. Brain Behav. (2022) 12:e2827. doi: 10.1002/brb3.2827
10. Hoops, S, Nazem, S, Siderowf, AD, Duda, JE, Xie, SX, Stern, MB, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. (2009) 73:1738–45. doi: 10.1212/WNL.0b013e3181c34b47
11. Salvadori, E, Poggesi, A, Pracucci, G, Chiti, A, Ciolli, L, Del Bene, A, et al. Longitudinal changes in MoCA performances in patients with mild cognitive impairment and small vessel disease. Results from the VMCI-Tuscany study. Cereb Circ Cogn Behav. (2021) 2:100008. doi: 10.1016/j.cccb.2021.100008
12. Bernier, PJ, Gourdeau, C, Carmichael, PH, Beauchemin, JP, Voyer, P, Hudon, C, et al. It’s all about cognitive trajectory: accuracy of the cognitive charts–MoCA in normal aging, MCI, and dementia. J Am Geriatr Soc. (2023) 71:214–20. doi: 10.1111/jgs.18029
13. Lindvall, E, Abzhandadze, T, Quinn, TJ, Sunnerhagen, KS, and Lundström, E. Is the difference real, is the difference relevant: the minimal detectable and clinically important changes in the Montreal cognitive assessment. Cereb Circ Cogn Behav. (2024) 6:100222. doi: 10.1016/j.cccb.2024.100222
14. Buvarp, D, Rafsten, L, Abzhandadze, T, and Sunnerhagen, KS. A prospective cohort study on longitudinal trajectories of cognitive function after stroke. Sci Rep. (2021) 11:17271. doi: 10.1038/s41598-021-96347-y
15. Marzolini, S, Oh, P, McIlroy, W, and Brooks, D. The effects of an aerobic and resistance exercise training program on cognition following stroke. Neurorehabil Neural Repair. (2013) 27:392–402. doi: 10.1177/1545968312465192
16. Aiello, EN, Solca, F, Torre, S, Colombo, E, Maranzano, A, De Lorenzo, A, et al. Longitudinal feasibility of the Montreal cognitive assessment (MoCA) in non-demented ALS patients. Eur Neurol. (2024) 87:79–83. doi: 10.1159/000538828
17. Aiello, EN, Solca, F, Torre, S, Carelli, L, Ferrucci, R, Priori, A, et al. Diagnostics and clinical usability of the Montreal cognitive assessment (MoCA) in amyotrophic lateral sclerosis. Front Psychol. (2022) 13:1012632. doi: 10.3389/fpsyg.2022.1012632
18. Lessig, S, Nie, D, Xu, R, and Corey-Bloom, J. Changes on brief cognitive instruments over time in Parkinson’s disease. Move. Dis. (2012) 27:1125–8. doi: 10.1002/mds.25070
19. Kim, HM, Nazor, C, Zabetian, CP, Quinn, JF, Chung, KA, Hiller, AL, et al. Prediction of cognitive progression in Parkinson’s disease using three cognitive screening measures. Clin Park Relat Disord. (2019) 1:91–7. doi: 10.1016/j.prdoa.2019.08.006
20. Biundo, R, Weis, L, Bostantjopoulou, S, Stefanova, E, Falup-Pecurariu, C, Kramberger, MG, et al. MMSE and MoCA in Parkinson’s disease and dementia with Lewy bodies: a multicenter 1-year follow-up study. J Neural Transm. (2016) 123:431–8. doi: 10.1007/s00702-016-1517-6
21. Pourzinal, D, Lawson, RA, Yarnall, AJ, Williams-Gray, CH, Barker, RA, Yang, J, et al. Profiling people with Parkinson’s disease at risk of cognitive decline: insights from PPMI and ICICLE-PD data. Diag Assess Dis Monit. (2024) 16:e12625. doi: 10.1002/dad2.12625
22. Faust-Socher, A, Duff-Canning, S, Grabovsky, A, Armstrong, MJ, Rothberg, B, Eslinger, PJ, et al. Responsiveness to change of the Montreal cognitive assessment, Mini-mental state examination, and SCOPA-cog in non-demented patients with Parkinson’s disease. Dement Geriatr Cogn Disord. (2019) 47:187–97. doi: 10.1159/000496454
23. Street, D, Jabbari, E, Costantini, A, Jones, PS, Holland, N, Rittman, T, et al. Progression of atypical parkinsonian syndromes: PROSPECT-M-UK study implications for clinical trials. Brain. (2023) 146:3232–42. doi: 10.1093/brain/awad105
24. Dam, T, Boxer, AL, Golbe, LI, Höglinger, GU, Morris, HR, Litvan, I, et al. Safety and efficacy of anti-tau monoclonal antibody gosuranemab in progressive supranuclear palsy: a phase 2, randomized, placebo-controlled trial. Nat Med. (2021) 27:1451–7. doi: 10.1038/s41591-021-01455-x
25. Molenberghs, G, and Verbeke, G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer New York (2000). 63–153.
26. Golbe, LI, and Ohman-Strickland, PA. A clinical rating scale for progressive supranuclear palsy. Brain. (2007) 130:1552–65. doi: 10.1093/brain/awm032
27. Bakdash, JZ, and Marusich, LR. Repeated measures correlation. Front Psychol. (2017) 8:456. doi: 10.3389/fpsyg.2017.00456
28. Seemiller, J, Morrow, C, Hinkle, JT, Perepezko, K, Kamath, V, Pontone, GM, et al. Impact of acute dopamine replacement on cognitive function in Parkinson’s disease. Mov Disord Clin Pract. (2024) 11:534–42. doi: 10.1002/mdc3.14017
29. Jaeger, J, Yang, L, Li, Y, Castrillo-Viguera, C, Haeberlein, SB, Dam, T, et al. Development of a cognitive composite for measuring change in progressive supranuclear palsy. Parkinsonism Relat Disord. (2021) 92:94–100. doi: 10.1016/j.parkreldis.2021.10.007
Keywords: MoCA = Montreal Cognitive Assessment, PSP, cognitive outcome measure, fluency, progressive supranucelar palsy
Citation: Ibrahim V, Isroff C, Stephen CD, Iyer J, Dale ML, Gunzler DA, Bayram E, Xie T, Pantelyat A, Montaser-Kouhsari L, Garcia-Cordero I, Tartaglia MC, Lang AE, Swan M, Boxer AL, Golbe LI and Wills A-M (2024) Montreal cognitive assessment as a cognitive outcome measure in progressive supranuclear palsy. Front. Neurol. 15:1501206. doi: 10.3389/fneur.2024.1501206
Edited by:
Ji Hyun Ko, University of Manitoba, CanadaReviewed by:
Luisa Sambati, University of Bologna, ItalyAlexandra Economou, National and Kapodistrian University of Athens, Greece
Copyright © 2024 Ibrahim, Isroff, Stephen, Iyer, Dale, Gunzler, Bayram, Xie, Pantelyat, Montaser-Kouhsari, Garcia-Cordero, Tartaglia, Lang, Swan, Boxer, Golbe and Wills. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anne-Marie Wills, YXdpbGxzQG1naC5oYXJ2YXJkLmVkdQ==
†ORCID: Vanessa Ibrahim, orcid.org/0009-0009-0354-6221
Catherine Isroff, orcid.org/0009-0003-2535-776X
Christopher D. Stephen, orcid.org/0000-0002-4727-192X
Jay Iyer, orcid.org/0000-0002-1519-3752
Marian L. Dale, orcid.org/0000-0003-1010-982X
Ece Bayram, orcid.org/0000-0002-6875-4242
Tao Xie, orcid.org/0000-0001-9551-6613
Alex Pantelyat, orcid.org/0000-0002-6427-7485
Leila Montaser Kouhsari, orcid.org/0000-0003-4058-1607
Indira Garcia-Cordero, orcid.org/0000-0001-9739-5018
Maria Carmela Tartaglia, orcid.org/0000-0002-5944-8497
Matthew Swan, orcid.org/0000-0003-0516-4680
Lawrence I. Golbe, orcid.org/0000-0002-4373-3365
Anne-Marie Wills, orcid.org/0000-0002-0901-4711
 Vanessa Ibrahim
Vanessa Ibrahim Catherine Isroff1†
Catherine Isroff1† Christopher D. Stephen
Christopher D. Stephen Marian L. Dale
Marian L. Dale Ece Bayram
Ece Bayram Alex Pantelyat
Alex Pantelyat Maria Carmela Tartaglia
Maria Carmela Tartaglia Matthew Swan
Matthew Swan Lawrence I. Golbe
Lawrence I. Golbe Anne-Marie Wills
Anne-Marie Wills