
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 18 December 2024
Sec. Endovascular and Interventional Neurology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1496628
Objective: To investigate the dynamic changes in neutrophil–to–lymphocyte ratio (NLR) and its derived indices following mechanical thrombectomy (MT) in patients with acute ischemic stroke (AIS) and evaluate their predictive value for prognosis.
Methods: This single-center retrospective cohort study included AIS patients who underwent MT at Zhongshan Hospital of Xiamen University from January 2018 to February 2024. Peripheral blood samples were collected on admission, day 1, and day 3 after MT to determine the NLR, derived NLR (dNLR), and neutrophil–monocyte–to–lymphocyte ratio (NMLR). The primary endpoint was poor functional outcome at 90 days (modified Rankin scale score 3–6). The secondary endpoints included post-operative hemorrhagic transformation, malignant cerebral edema, in-hospital mortality, and 90-day all-cause mortality. Receiver operating characteristic (ROC) curve analysis was used to evaluate predictive performance, and multivariate logistic regression models were employed to explore the independent associations between inflammatory markers and prognosis.
Results: A total of 423 eligible patients were included. Both groups showed similar dynamic trends in inflammatory markers, peaking on day 1 post-MT and subsequently declining. However, the poor outcome group (n = 255, 60.28%) maintained higher levels on day 3, whereas the good outcome group showed a significant decreasing trend. ROC curve analysis revealed that the NLR (AUC = 0.85, 95% CI: 0.81–0.89), dNLR (AUC = 0.86, 95% CI: 0.82–0.89), and NMLR (AUC = 0.85, 95% CI: 0.81–0.89) on day 3 post-MT had the strongest predictive power for 90-day poor outcomes. After comprehensive adjustment for confounders, these inflammatory markers were independently associated with 90-day poor outcomes: for each unit increase in the NLR, the risk of poor outcome increased by 38% (OR = 1.38, 95% CI: 1.28–1.49, p < 0.001); for dNLR, it increased by 104% (OR = 2.04, 95% CI: 1.73–2.40, p < 0.001); and for NMLR, it increased by 35% (OR = 1.35, 95% CI: 1.26–1.45, p < 0.001).
Conclusion: Inflammatory markers (NLR, dNLR, and NMLR) on day 3 post-MT can serve as independent predictors of prognosis in AIS patients treated with MT. Dynamic monitoring of inflammatory markers may facilitate early risk stratification and guide individualized treatment strategies.
Acute ischemic stroke (AIS) is a leading cause of mortality and disability worldwide. According to statistics from the World Health Organization, approximately 12 million new stroke cases occur globally each year, with ischemic strokes accounting for a staggering 62.4%, resulting in nearly 6 million deaths or severe disabilities (1). Large vessel occlusion (LVO) strokes constitute one-third of AIS cases and often present with significant neurological impairments and poorer prognoses (2).
In recent years, mechanical thrombectomy (MT) has emerged as the standard treatment for LVO patients because of its remarkable clinical benefits (3). Compared with intravenous thrombolysis alone, MT significantly improves functional outcomes and reduces mortality and disability rate (4). However, despite advancements in MT techniques, a considerable proportion of patients still experience unfavorable outcomes. A prospective study revealed that among LVO patients undergoing MT, only 49% reached functional independence (defined as a modified Rankin Scale score [mRS] ≤ 2) at 90 days (5). Poor outcomes not only increase the burden on patients and their families but also place substantial pressure on society and healthcare systems. Therefore, identifying key factors influencing MT outcomes is crucial for early recognition of high-risk patients and optimization of clinical management strategies.
Inflammatory responses play a pivotal role in the occurrence, progression, and prognosis of AIS (6). Ischemic brain injury rapidly activates the innate immune responses, leading to the release and recruitment of inflammatory cells and mediators, which further exacerbates brain tissue damage and disrupts blood–brain barrier (BBB) integrity (7). The neutrophil-to-lymphocyte ratio (NLR), an emerging inflammatory marker in peripheral blood, has been shown to be closely associated with the prognosis of AIS patients (8, 9). However, previous studies have primarily focused on baseline levels or single measurements of the NLR, and a systematic evaluation of its dynamic changes and prognostic value is lacking.
Furthermore, the derived neutrophil-to-lymphocyte ratio (dNLR) and neutrophil-monocyte-to-lymphocyte ratio (NMLR), which are derived indices of the NLR, have demonstrated significant prognostic predictive value in various inflammatory and immune diseases (10, 11) as well as acute myocardial infarction (12). Nevertheless, the clinical utility of these novel inflammatory markers in AIS patients has not been fully validated, and whether their predictive performance is superior to that of the traditional NLR remains to be elucidated.
Given this background, we conducted this retrospective cohort study to investigate the dynamic changes in NLR and its derived indices following MT in AIS patients and evaluate their prognostic predictive value. By systematically assessing the dynamic changes in peripheral immune markers, this study aimed to provide new clinical insights into prognostic assessment for AIS patients and evidence-based guidance for individualized treatment strategies.
This single-center retrospective cohort study consecutively enrolled AIS patients who underwent MT at Zhongshan Hospital of Xiamen University from January 2018 to February 2024. The inclusion criteria were: (1) age ≥ 18 years; (2) met the clinical and imaging diagnostic criteria for AIS established by the World Health Organization, and (3) CT angiography at admission confirmed the presence of intracranial large vessel occlusion, including internal carotid artery, middle cerebral artery (M1 or M2 segment), basilar artery, or posterior cerebral artery (P1 segment). The exclusion criteria were as follows: (1) baseline CT or magnetic resonance imaging indicating intracranial hemorrhage; (2) pre-stroke mRS score ≥ 2; (3) severe systemic diseases such as renal failure, severe liver dysfunction, or malignant tumors; (4) presence of infectious diseases, inflammatory diseases, immune system disorders, or ongoing immunotherapy on admission; (5) comorbidities that may affect inflammatory markers, including tumors, myocardial infarction, trauma, recent surgery, or allergic reactions; (6) lack of complete laboratory data, and (7) lack of follow-up data. During the screening process, 78 patients who did not meet the criteria were excluded, 14 of whom were not included because of incomplete data related to death within 3 days. A total of 423 patients were ultimately included in the analysis.
A standardized electronic data collection form was used to gather patients’ clinical data, including demographic characteristics (age, sex), medical history (hypertension, diabetes, hyperlipidemia, atrial fibrillation, valvular heart disease, coronary artery disease, smoking history, and alcohol consumption history), admission assessments (National Institutes of Health Stroke Scale [NIHSS] score, Glasgow Coma Scale [GCS] score, and blood pressure), vessel occlusion site, stroke etiology, intravenous thrombolysis status, time metrics (onset-to-puncture time, onset-to-recanalization time, and puncture-to-recanalization time), and MT procedural parameters (number of thrombectomy attempts, retrieval technique, and vessel recanalization status).
Venous blood samples were collected on admission, and on days 1 and 3 post-MT. White blood cell counts and differentials were determined using an automated hematology analyzer. The inflammatory marker calculation formulas were as follows:
NLR = neutrophil count/lymphocyte count.
dNLR = neutrophil count/(white blood cell count – neutrophil count).
NMLR = (monocyte count + neutrophil count)/lymphocyte count.
All relevant data were independently collected and recorded by two trained neurologists following a standardized protocol and cross-checked by other researchers to ensure data accuracy and completeness.
The primary endpoint was poor outcome at 90 days, defined as a mRS score of 3–6. The secondary outcomes included postoperative hemorrhagic transformation (HT), malignant cerebral edema, in-hospital mortality, and 90-day all-cause mortality. Vessel recanalization status was assessed on the basis of immediate post-MT cerebral angiography results using the modified Thrombolysis in Cerebral Infarction (mTICI) scale, with mTICI grades 2b-3 defined as successful recanalization (13). HT was identified as new intracranial hemorrhage detected on postoperative imaging (CT/MRI). Patients routinely underwent follow-up head CT at 24 h post-MT, and additional CT/MRI was performed within 72 h post-MT if neurological deterioration occurred (14). Malignant cerebral edema was defined as significant space-occupying effect (midline shift ≥ 5 mm) in the infarct area on imaging within 72 h post-MT.
All patients underwent follow-up assessments 90 days after stroke onset, which were conducted by specially trained research coordinators through telephone interviews. During the follow-up, we collected information on patients’ functional status and all-cause mortality. For deceased patients, we first obtained preliminary information from the patients’ relatives and then further verified it through death certificates from the primary hospital or the patients’ community hospital to ensure data accuracy and completeness. To ensure the consistency and reliability of the assessments, all follow-up evaluations were performed by rigorously trained personnel. The assessment results were entered in real-time into the National Cerebrovascular Disease Big Data Platform (Stroke Center Construction Information Management System) for centralized data management and analysis.
Statistical analyses were performed using R software (version 4.2.2). The Shapiro–Wilk test was used to assess the normality of continuous variables. Normally distributed continuous variables are expressed as the mean ± standard deviation (mean ± SD), and comparisons between groups were performed using the independent samples t-test. Non-normally distributed continuous variables were expressed as median and interquartile range [median (Q1, Q3)], and the Mann–Whitney U test was used for inter-group comparisons. Categorical variables were expressed as frequencies and percentages [n (%)], and analyzed using Pearson’s chi-square test or Fisher’s exact test.
To evaluate the predictive performance of inflammatory markers, we analyzed the predictive value of the NLR, dNLR, and NMLR at three time points (at admission, 24 h, and 72 h post-MT) for the primary and secondary endpoints. Receiver operating characteristic (ROC) curves were constructed, and the area under the curve (AUC) and its 95% confidence interval were calculated. The optimal cutoff value was determined via Youden’s index (maximum value of sensitivity + specificity – 1), and the corresponding sensitivity, specificity, positive predictive value, and negative predictive value were calculated.
To explore the independent associations between inflammatory markers and 90-day functional outcomes, we constructed three stepwise adjusted logistic regression models: Model A was unadjusted; Model B was adjusted for age, hyperlipidemia, and atrial fibrillation; Model C was further adjusted for the baseline NIHSS score, GCS score, and number of mechanical thrombectomy attempts based on Model B; and the covariates included in the models were based on the results of univariate analysis (p < 0.05). All the statistical tests were two-sided, and p < 0.05 was considered statistically significant.
This study ultimately included 423 AIS patients who underwent MT (Table 1), with a median age of 68.00 years; 66.43% were male. The median NIHSS score on admission was 15, and the median GCS score was 12. Hypertension (68.79%) was the most common risk factor, followed by atrial fibrillation (40.90%), smoking history (34.99%), diabetes (29.79%), and hyperlipidemia (23.88%). Large artery atherosclerosis (52.01%) and cardioembolism (43.26%) were the main stroke etiologies. Most patients had anterior circulation occlusion (84.16%), and 40.90% received intravenous thrombolysis. The median onset-to-puncture time was 378 min, the puncture-to-recanalization time was 98 min, and the onset-to-recanalization time was 499 min. The median number of thrombectomy attempts was 2.00 (IQR, 1.00–2.00), and 38.53% of patients achieved successful recanalization (mTICI score 2b-3).
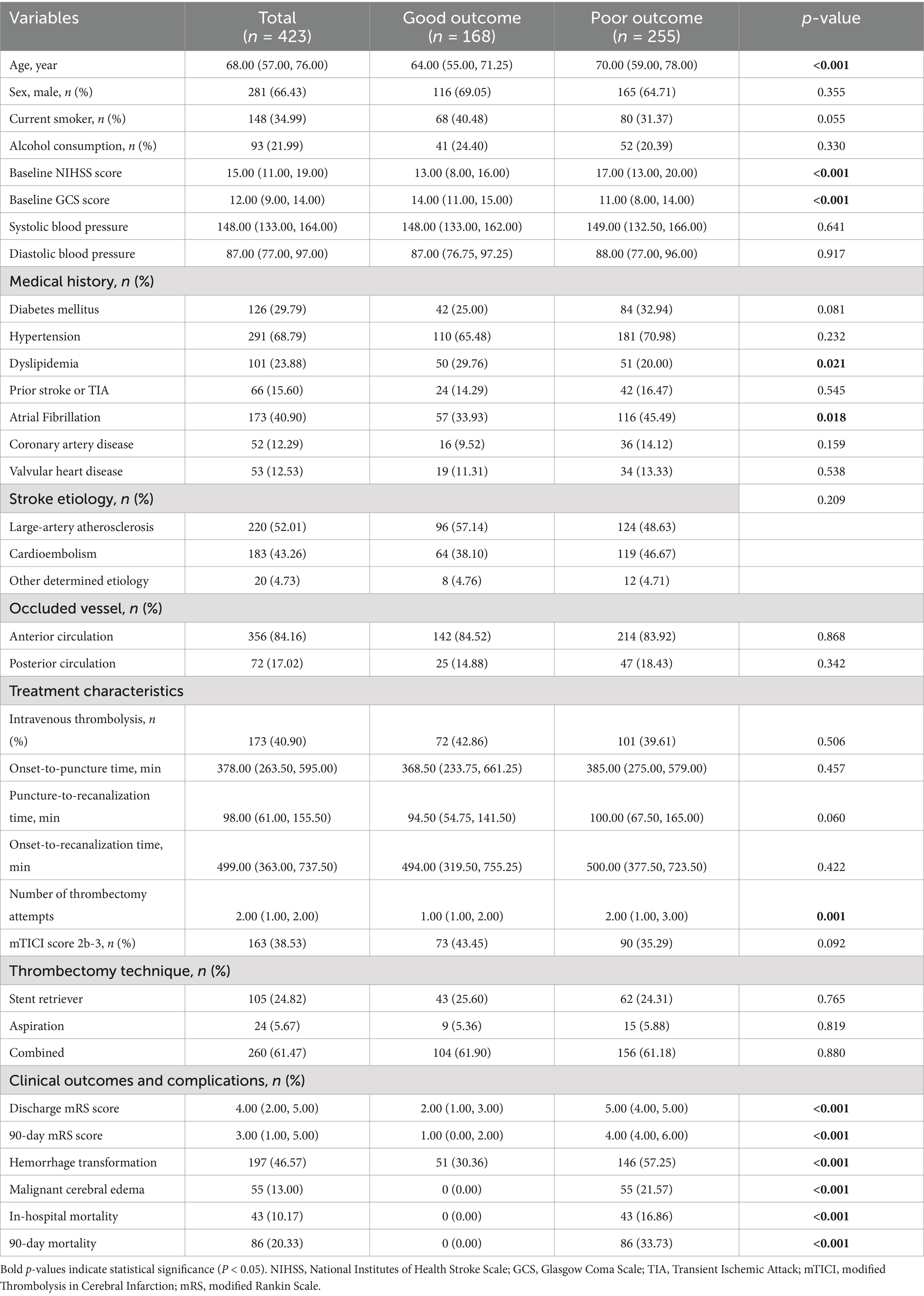
Table 1. Baseline characteristics and clinical features of patients between good and poor outcome groups.
The 90-day follow-up results revealed that 168 (39.72%) patients had good functional outcomes (mRS 0–2), whereas 255 (60.28%) patients had poor functional outcomes (mRS 3–6). Compared with those in the good outcome group, patients in the poor outcome group were older, had higher baseline NIHSS scores, lower baseline GCS scores (all p < 0.001), and required more retrieval attempts (p = 0.001). Patients in the poor outcome group had a lower proportion of hyperlipidemia (p = 0.021) but a higher proportion of atrial fibrillation (p = 0.018). There were no statistically significant differences in the other characteristics between the two groups (all p > 0.05).
The median 90-day mRS score for the entire cohort was 3.00, with 1.00 in the good outcome group and 4.00 in the poor outcome group (p < 0.001). The incidence of hemorrhagic transformation was significantly higher in the poor outcome group (57.25% vs. 30.36%, p < 0.001). Fifty-five (13.00%) patients developed malignant cerebral edema, and 43 (10.17%) patients died during hospitalization. Eighty-six (20.33%) patients died within 90 days.
We evaluated the dynamic changes in the NLR, dNLR, and NMLR at different time points (Figure 1). The results revealed that the levels of inflammatory markers were consistently higher in the poor outcome group than in the good outcome group (all p < 0.001) (Table 2). Further analysis revealed that the median NLR, dNLR, and NMLR in the poor outcome group peaked on day 1 post-MT [NLR: 11.66 (8.18, 17.12) vs. 7.05 (5.05, 10.01); dNLR: 6.86 (4.99, 9.06) vs. 4.66 (3.36, 6.12); NMLR: 12.38 (8.66, 17.88) vs. 7.53 (5.46, 10.63), all p < 0.001]. Although they decreased on day 3 post-MT, the poor outcome group still maintained higher levels [NLR: 10.89 (7.61, 16.08) vs. 5.00 (3.16, 6.58); dNLR: 5.84 (4.35, 7.77) vs. 2.98 (2.16, 3.90); NMLR: 11.64 (8.19, 17.13) vs. 5.46 (3.52, 7.10), all p < 0.001].
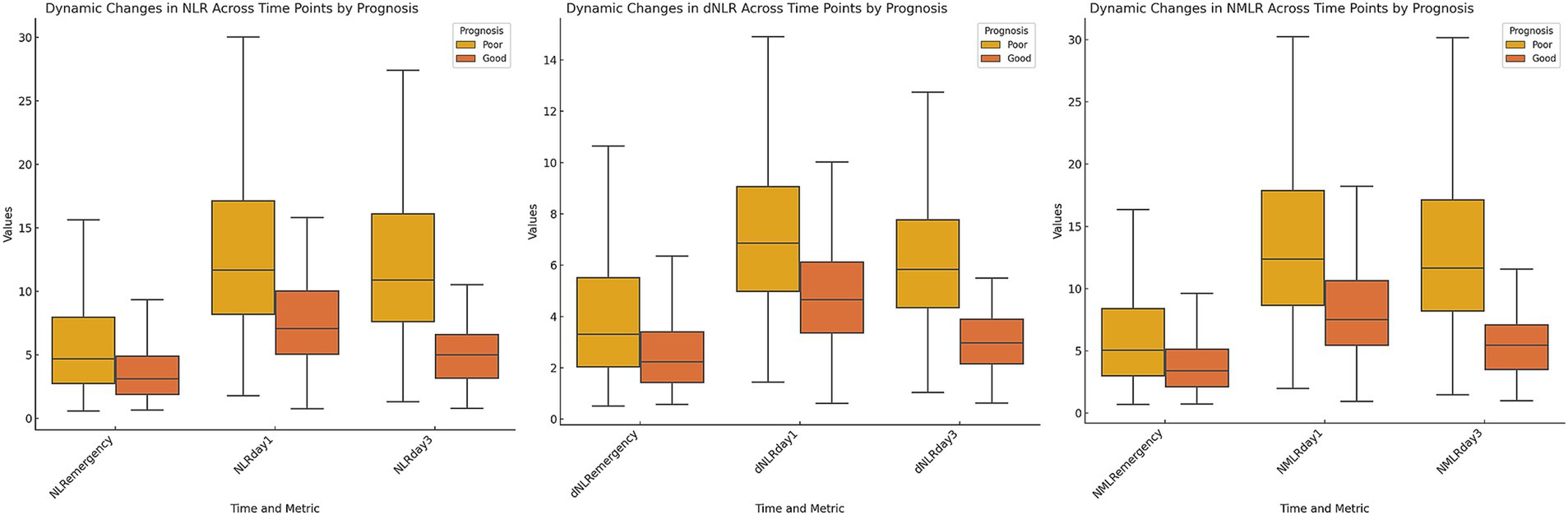
Figure 1. Dynamic changes in inflammatory markers stratified by 90-day poor outcome. Box plots depicting the temporal profiles of neutrophil-to-lymphocyte ratio, derived neutrophil-to-lymphocyte ratio, and neutrophil-monocyte-to-lymphocyte ratio at three time points: on admission, day 1 post-MT, and day 3 post-MT. Patients were stratified by 90-day functional outcome.
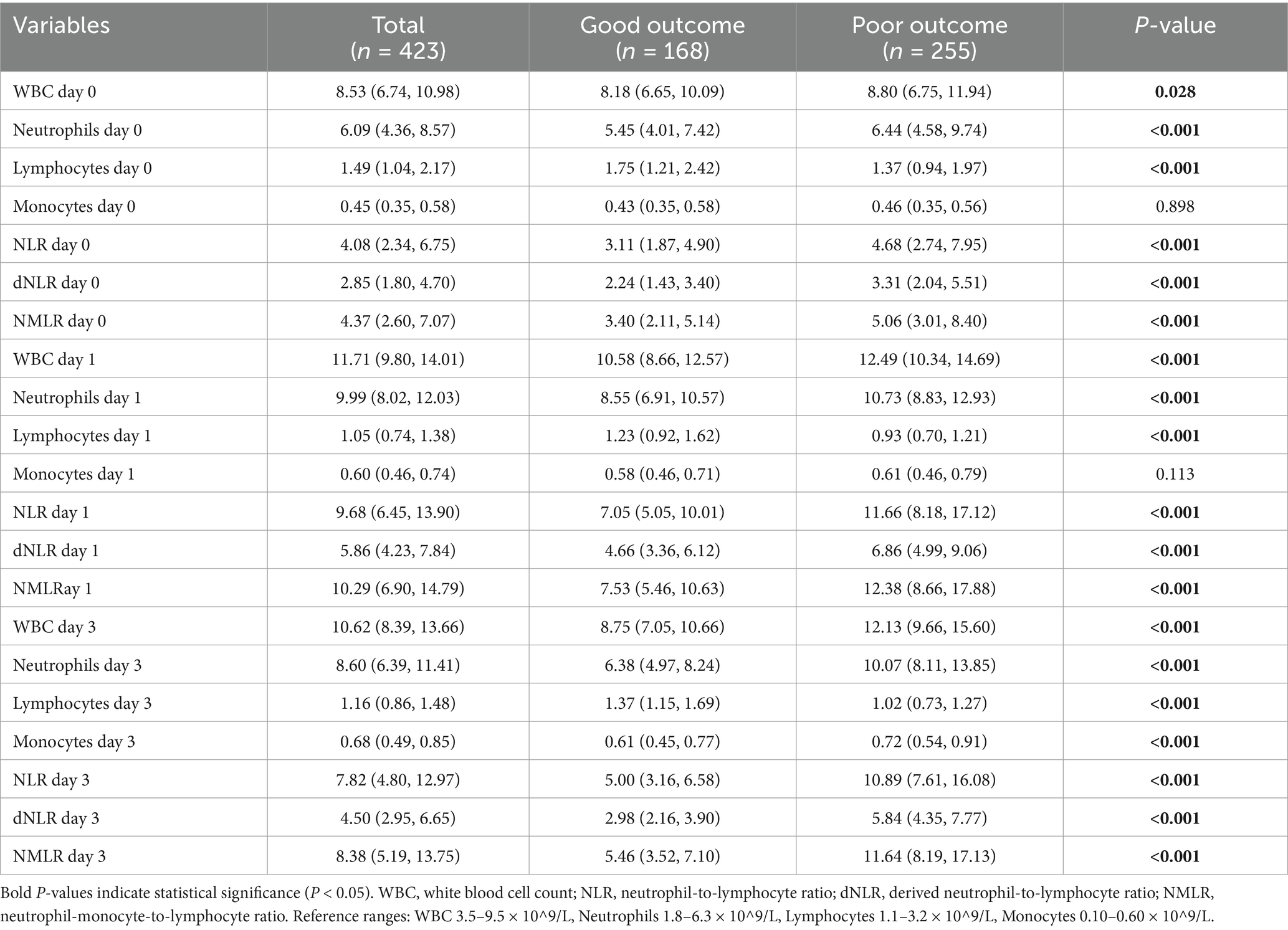
Table 2. Comparison of laboratory parameters at different time points between good and poor outcome groups.
Receiver operating characteristic curve analysis (Table 3 and Figures 2, 3) revealed that the inflammatory markers on day 3 post-MT had the best predictive performance. The NLR on day 3 had an AUC of 0.85 (95% CI: 0.81–0.89) for the prediction of 90-day poor functional outcomes, which was significantly higher than that on day 1 post-MT [0.74 (0.70–0.79)] and on admission [0.65 (0.59–0.70)]. The optimal cut-off value for NLR on day 3 was 7.811, with corresponding accuracy, sensitivity, and specificity of 79, 87, and 75%, respectively. The dNLR and NMLR exhibited similar trends to NLR in predicting 90-day poor outcomes, with their predictive performance on day 3 [dNLR: AUC = 0.86; NMLR: AUC = 0.85] being significantly better than that on day 1 and day 0 (Figure 2).
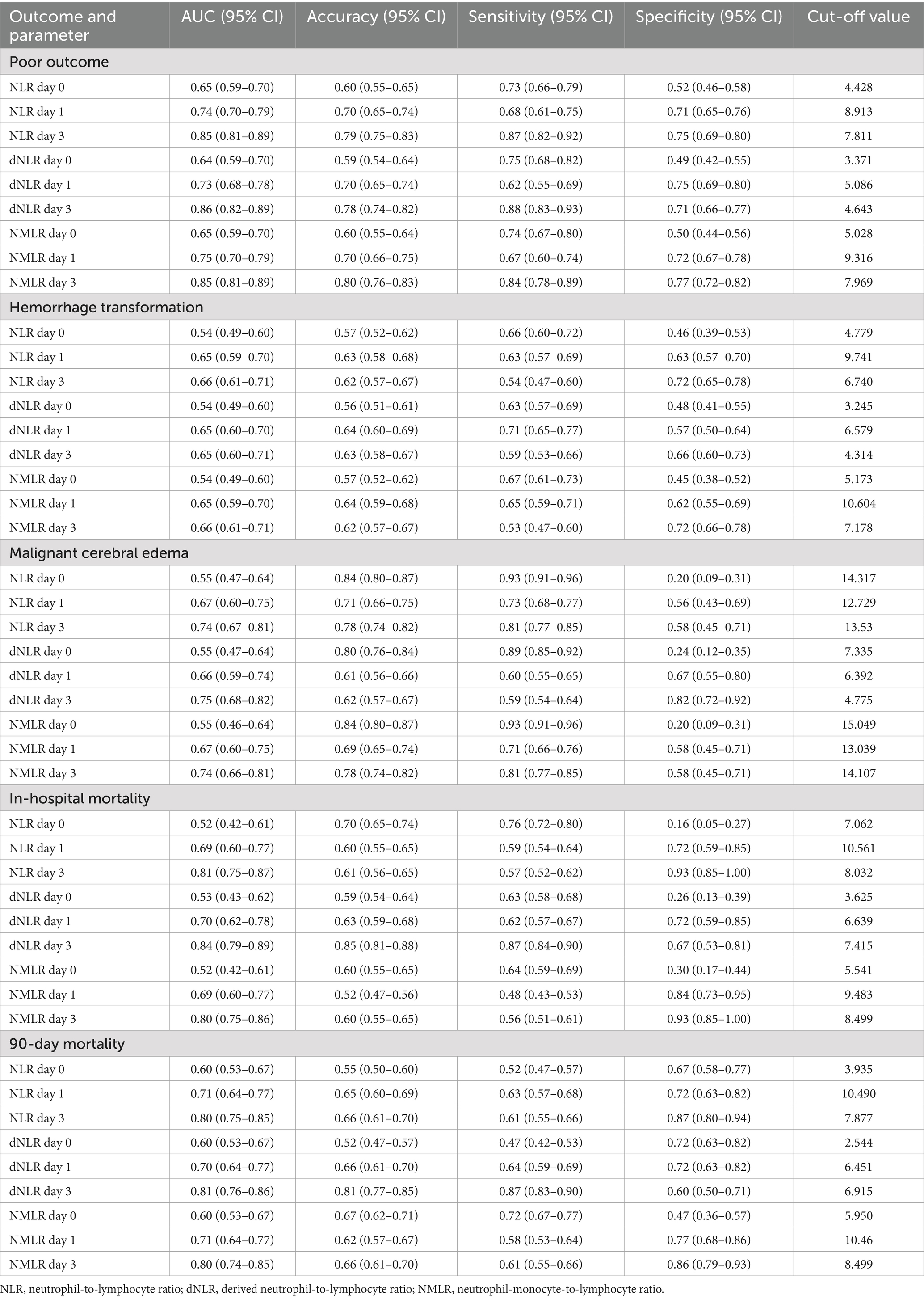
Table 3. Comparison of laboratory parameters at different time points between good and poor outcome groups.

Figure 2. Receiver operating characteristic curves for inflammatory markers in predicting 90-day poor functional outcome.
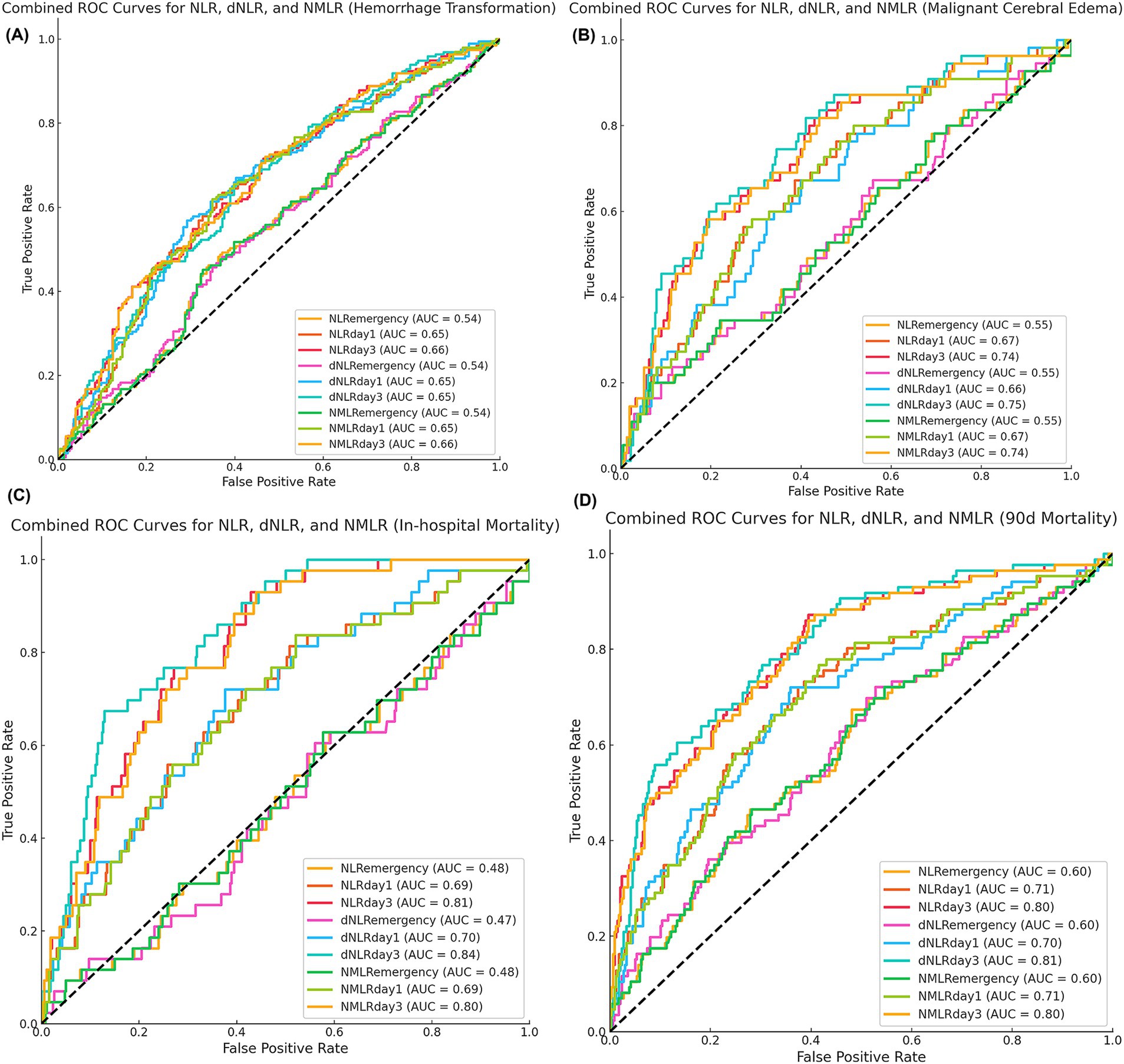
Figure 3. Predictive performance of inflammatory markers for secondary outcomes. Time-specific receiver-operating-characteristic curves for (A) hemorrhagic transformation, (B) malignant cerebral edema, (C) in-hospital mortality, and (D) 90-day all-cause mortality. Values in parentheses indicate area under the curve.
For predicting hemorrhagic transformation, the performance of the NLR, dNLR, and NMLR was relatively weak (Figure 3A). Among them, the predictive value on day 3 was the highest [NLR: AUC = 0.66; dNLR: AUC = 0.65; NMLR: AUC = 0.66]. In predicting malignant cerebral edema, NLR, dNLR, and NMLR on day 3 had comparable performance [NLR: AUC = 0.74; dNLR: AUC = 0.75; NMLR: AUC = 0.74] (Figure 3B). The NLR, dNLR, and NMLR had higher predictive values for in-hospital mortality and 90-day mortality [NLR: AUC = 0.81 vs. AUC = 0.80; dNLR: AUC = 0.84 vs. AUC = 0.81; NMLR: AUC = 0.80 vs. AUC = 0.80] (Figures 3C,D).
Moreover, we observed an increasing trend in the predictive ability of inflammatory markers over time. The markers on admission usually performed the worst, while the markers on day 3 post-MT performed the best in predicting most outcomes. This trend was evident for all three inflammatory markers (NLR, dNLR, and NMLR).
To further explore the associations between the NLR and its derived indices at different time points with patients’ 90-day functional outcomes, we constructed three stepwise adjusted multivariable logistic regression models (Table 4). In the unadjusted crude model (Model A), the NLR, dNLR, and NMLR at all time points were significantly associated with 90-day poor outcomes (all p < 0.001). For each unit increase in the NLR, dNLR, and NMLR, the risk of 90-day poor outcomes increased by 41, 116, and 38%, respectively [NLR d0: OR = 1.41, 95% CI: 1.31–1.52; dNLR d0: OR = 2.16, 95% CI: 1.84–2.53; NMLR d0: OR = 1.38, 95% CI: 1.29–1.48].
After further adjusting for age, hyperlipidemia, and atrial fibrillation (Model B), these associations persisted, and the effect sizes remained essentially unchanged. In the final model (Model C), we further adjusted for baseline NIHSS score, GCS score, and the number of thrombectomy attempts. The results indicated that all inflammatory markers still maintained significant associations with 90-day poor outcomes (both p < 0.001). The NLR, dNLR, and NMLR all retained strong independent predictive power, with each unit increase associated with a 38% (OR = 1.38, 95% CI: 1.28–1.49), 104% (OR = 2.04, 95% CI: 1.73–2.40), and 35% (OR = 1.35, 95% CI: 1.26–1.45) increased risk of poor outcomes, respectively. Furthermore, for all three inflammatory markers (NLR, dNLR, and NMLR), their ability to predict 90-day poor outcomes showed an increasing trend over time.
This study systematically evaluated the dynamic changes in the NLR, dNLR, and NMLR and their prognostic predictive value in AIS patients after MT. The findings revealed that: (1) the NLR, dNLR, and NMLR in both groups peaked on day 1 post-MT and gradually declined, but the poor outcome group maintained higher levels, while the good outcome group showed a significant decreasing trend. (2) The NLR, dNLR, and NMLR on day 3 post-MT had stronger predictive power for 90-day prognosis compared to those on admission and day 1 post-MT. (3) Regarding secondary outcomes, the NLR, NMLR, and dNLR on day 3 post-MT had good predictive value for in-hospital mortality and 90-day all-cause mortality, but their predictive performance for hemorrhagic transformation and malignant cerebral edema was relatively weak. (4) After comprehensive adjustment for confounding factors, there was still a significant independent association between these inflammatory markers and 90-day poor outcomes.
Our study results are consistent with previous research findings. In a study of 204 AIS patients, Qian et al. (8) reported a similar dynamic change pattern in NLR: it gradually increased after onset, peaked at 24 h, and began to decline at 72 h. They also confirmed that the 72-h NLR was the optimal time point for predicting poor prognosis. Chen et al. (9) demonstrated that the 24-h NLR had stronger predictive value for 90-day functional outcomes and mortality than did baseline levels, but unfortunately, their study did not extend to the 72-h time point. The dynamic changes pattern in inflammatory markers may reflect the migration patterns of neutrophils after cerebral ischemia. Previous studies have shown that AIS can rapidly trigger a strong inflammatory cascade reaction. Ischemic brain tissue releases large amounts of chemokines and cytokines within hours of injury, leading to the recruitment and migration of peripheral circulating neutrophils to the ischemic region (15–17). The number of circulating neutrophils begins to increase 6 h after onset, reaches a peak within 24–48 h, and then gradually decreases after 72 h (18, 19). This time course is highly consistent with the dynamic changes in NLR observed in our study.
Notably, the poor outcome group maintained higher inflammatory levels on day 3 post-MT, while the good outcome group showed a significant decreasing trend. These finding suggest that patients with poor prognoses may have persistent inflammatory responses and immune imbalance. As a marker of systemic inflammatory response, an elevated NLR reflects the dual effects of innate immune activation (neutrophil increase) and adaptive immune suppression (lymphocyte decrease). Previous studies have shown that neutrophils can participate in the pathological process of ischemic brain injury through multiple mechanisms (20, 21). First, neutrophils are the main source of matrix metalloproteinase-9 (MMP-9). MMP-9 can directly act on the tight junction proteins between vascular endothelial cells, thereby opening the BBB on the luminal side of blood vessels or acting on the vascular basement membrane through endocytosis, thus exacerbating the neuroinflammatory response (22). Second, neutrophils release various cytotoxic factors, such as reactive oxygen species, myeloperoxidase, proteases, and inflammatory mediators (23). The combined action of these factors leads to damage to the neurovascular unit and expansion of brain tissue injury. In addition, neutrophils also promote microthrombus formation through interactions with platelets and coagulation factors, and may interfere with the recovery of local cerebral blood flow by adhering to the microvasculature (24).
Conversely, lymphocyte count changes reflect the body’s stress state and immune function. Lymphocytopenia suggests the occurrence of glucocorticoid-mediated stress responses and sympathetic nervous system activation, which may exacerbate ischemic injury (25). Different lymphocyte subsets play complex regulatory roles in the progression of ischemic stroke: γδ T cells and CD8+ T cells aggravate tissue damage by releasing pro-inflammatory factors, while regulatory T cells exert neuroprotective effects by suppressing inflammatory responses and maintaining immune homeostasis (26–28).
As a novel inflammatory marker, NMLR provides a more comprehensive assessment of the inflammatory state by integrating the monocyte count (29). Studies have shown that AIS patients commonly exhibit elevated neutrophil and monocyte counts accompanied by decreased lymphocyte counts. Among them, monocytes can activate platelets to form platelet–monocyte aggregates, promoting the release of inflammatory and vasoactive substances and affecting hemodynamics, thereby exacerbating ischemic brain injury (23). This viewpoint is supported by Dragu et al.’s (30) study, which revealed that an elevated baseline monocyte count was associated with mortality risk in patients with acute myocardial infarction. Although theoretically integrating monocyte indicators may improve predictive ability, in our study, the predictive performance of the NMLR was comparable to that of the NLR and dNLR. Additionally, Liu et al. (26) confirmed that the dNLR could independently predict prognosis in patients with coronary heart disease after percutaneous coronary intervention, and our study further validated the application value of the dNLR in prognostic assessment after MT. However, we noted that there were no significant differences in predictive value among the NLR, dNLR, and NMLR.
On the basis of the key role of inflammatory responses in stroke prognosis revealed in our study, therapeutic interventions targeting immune-inflammatory reactions may provide new directions for improving patient outcomes. Although related research has made some progress, clinical translation still faces challenges. Currently, studies on the ability of fingolimod combined with alteplase to improve neurological deficits by regulating circulating lymphocyte levels have shown potential value but still require validation in large-scale randomized controlled trials (31). In basic research, dextromethorphan has exhibited significant anti-inflammatory effects by inhibiting the expression of TNF-α, iNOS, IL-1β, and COX-2, whereas edaravone alleviates secondary neurological injury by scavenging oxygen free radicals (32, 33). These research advances suggest that multitarget intervention strategies targeting inflammatory responses may open new avenues for the treatment of ischemic stroke, but their clinical application value still needs further exploration. Therefore, in combination with our study results, for patients with persistently high inflammatory markers on day 3, treatment regimens targeting immune–inflammatory responses may help improve patient prognosis, but this hypothesis still needs to be confirmed through large-scale, prospective clinical studies.
This study has several limitations that need to be noted. First, as a single-center retrospective study, the generalizability of the results may be limited. In particular, the inclusion criteria requiring complete day 3 laboratory examination data may lead to selection bias, as some patients with early death or extremely severe clinical conditions may be excluded from the analysis. Second, although we identified the optimal predictive thresholds for inflammatory markers, the external validation and clinical application value of these cutoff values remain to be confirmed. Moreover, this study was unable to provide specific recommendations for individualized treatment strategies for patients with different inflammatory levels.
This study confirmed that inflammatory markers (NLR, dNLR, and NMLR) on day 3 post-MT can serve as independent predictors of prognosis in AIS patients treated with MT, especially those with strong predictive value for poor 90-day functional outcomes, in-hospital mortality, and 90-day mortality. Notably, patients with a poor prognosis maintained increased inflammatory levels on day 3 post-MT, whereas patients with a good prognosis presented a significant decreasing trend. This dynamic change pattern may provide a new perspective for clinical risk assessment. These findings emphasize the importance of dynamic monitoring of inflammatory markers after MT and may offer new strategic evidence for early risk stratification and individualized treatment plan formulation in AIS patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of the Affiliated Zhongshan Hospital of Xiamen University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
WG: Conceptualization, Data curation, Formal analysis, Writing – original draft. AA: Conceptualization, Data curation, Formal analysis, Writing – original draft. LY: Methodology, Visualization, Writing – original draft. RH: Supervision, Validation, Visualization, Writing – original draft. BL: Methodology, Resources, Supervision, Validation, Writing – original draft. YL: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft. HL: Methodology, Visualization, Writing – review & editing. RZ: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Xiamen (3502Z20227270) and the Fujian Provincial Clinical Key Specialty Construction Project (050172).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Lakomkin, N, Dhamoon, M, Carroll, K, Singh, IP, Tuhrim, S, Lee, J, et al. Prevalence of large vessel occlusion in patients presenting with acute ischemic stroke: a 10-year systematic review of the literature. J Neurointerv Surg. (2019) 11:241–5. doi: 10.1136/neurintsurg-2018-014239
3. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
4. Goyal, M, Menon, BK, van Zwam, WH, Dippel, DW, Mitchell, PJ, Demchuk, AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
5. Anadani, M, Orabi, MY, Alawieh, A, Goyal, N, Alexandrov, AV, Petersen, N, et al. Blood pressure and outcome after mechanical Thrombectomy with successful revascularization. Stroke. (2019) 50:2448–54. doi: 10.1161/STROKEAHA.118.024687
6. Kim, JY, Kawabori, M, and Yenari, MA. Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Curr Med Chem. (2014) 21:2076–97. doi: 10.2174/0929867321666131228205146
7. Shi, K, Tian, DC, Li, ZG, Ducruet, AF, Lawton, MT, and Shi, FD. Global brain inflammation in stroke. Lancet Neurol. (2019) 18:1058–66. doi: 10.1016/S1474-4422(19)30078-X
8. Qian, K, Hu, J, Wang, C, Xu, C, Chen, Y, Feng, Q, et al. Dynamic change of neutrophil-to-lymphocyte ratio and its predictive value of prognosis in acute ischemic stroke. Brain Behav. (2024) 14:e3616. doi: 10.1002/brb3.3616
9. Chen, S, Cheng, J, Ye, Q, Ye, Z, Zhang, Y, Liu, Y, et al. Day 1 neutrophil-to-lymphocyte ratio (NLR) predicts stroke outcome after intravenous thrombolysis and mechanical thrombectomy. Front Neurol. (2022) 13:941251. doi: 10.3389/fneur.2022.941251
10. Pang, Y, Shao, H, Yang, Z, Fan, L, Liu, W, Shi, J, et al. The (neutrophils + monocyte)/lymphocyte ratio is an independent prognostic factor for progression-free survival in newly diagnosed multiple myeloma patients treated with BCD regimen. Front Oncol. (2020) 10:1617. doi: 10.3389/fonc.2020.01617
11. Yan, XN, Jin, JL, Zhang, M, Hong, LF, Guo, YL, Wu, NQ, et al. Differential leukocyte counts and cardiovascular mortality in very old patients with acute myocardial infarction: a Chinese cohort study. BMC Cardiovasc Disord. (2020) 20:465. doi: 10.1186/s12872-020-01743-3
12. Wang, Y, Yuan, M, Ma, Y, Shao, C, Wang, Y, Qi, M, et al. The admission (neutrophil+monocyte)/lymphocyte ratio is an independent predictor for in-hospital mortality in patients with acute myocardial infarction. Front Cardiovasc Med. (2022) 9:870176. doi: 10.3389/fcvm.2022.870176
13. Zaidat, OO, Yoo, AJ, Khatri, P, Tomsick, TA, von Kummer, R, Saver, JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. (2013) 44:2650–63. doi: 10.1161/STROKEAHA.113.001972
14. von Kummer, R, Broderick, JP, Campbell, BC, Demchuk, A, Goyal, M, Hill, MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. (2015) 46:2981–6. doi: 10.1161/STROKEAHA.115.010049
15. Albaqami, FF, Abdel-Rahman, RF, Althurwi, HN, Alharthy, KM, Soliman, GA, Aljarba, TM, et al. Targeting inflammation and oxidative stress for protection against ischemic brain injury in rats using cupressuflavone. Saudi Pharm J. (2024) 32:101933. doi: 10.1016/j.jsps.2023.101933
16. Bui, TA, Jickling, GC, and Winship, IR. Neutrophil dynamics and inflammaging in acute ischemic stroke: a transcriptomic review. Front Aging Neurosci. (2022) 14:1041333. doi: 10.3389/fnagi.2022.1041333
17. Otxoa-de-Amezaga, A, Miró-Mur, F, Pedragosa, J, Gallizioli, M, Justicia, C, Gaja-Capdevila, N, et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol. (2019) 137:321–41. doi: 10.1007/s00401-018-1954-4
18. Ross, AM, Hurn, P, Perrin, N, Wood, L, Carlini, W, and Potempa, K. Evidence of the peripheral inflammatory response in patients with transient ischemic attack. J Stroke Cerebrovasc Dis. (2007) 16:203–7. doi: 10.1016/j.jstrokecerebrovasdis.2007.05.002
19. Tu, XK, Yang, WZ, Shi, SS, Wang, CH, Zhang, GL, Ni, TR, et al. Spatio-temporal distribution of inflammatory reaction and expression of TLR2/4 signaling pathway in rat brain following permanent focal cerebral ischemia. Neurochem Res. (2010) 35:1147–55. doi: 10.1007/s11064-010-0167-6
20. Jickling, GC, Liu, D, Ander, BP, Stamova, B, Zhan, X, and Sharp, FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. (2015) 35:888–901. doi: 10.1038/jcbfm.2015.45
21. Tang, N, Gong, XR, Huang, H, and Meng, Q. Activated neutrophil-derived exosomes contribute to blood-brain barrier damage and hemorrhagic transformation after cerebral ischemia/reperfusion. Brain Res. (2023) 1810:148374. doi: 10.1016/j.brainres.2023.148374
22. Jickling, GC, Liu, D, Stamova, B, Ander, BP, Zhan, X, Lu, A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. (2014) 34:185–99. doi: 10.1038/jcbfm.2013.203
23. Ray, MJ, Walters, DL, Bett, JN, Cameron, J, Wood, P, and Aroney, CN. Platelet-monocyte aggregates predict troponin rise after percutaneous coronary intervention and are inhibited by Abciximab. Int J Cardiol. (2005) 101:249–55. doi: 10.1016/j.ijcard.2004.03.033
24. Yu, S, Arima, H, Bertmar, C, Clarke, S, Herkes, G, and Krause, M. Neutrophil to lymphocyte ratio and early clinical outcomes in patients with acute ischemic stroke. J Neurol Sci. (2018) 387:115–8. doi: 10.1016/j.jns.2018.02.002
25. Acanfora, D, Gheorghiade, M, Trojano, L, Furgi, G, Pasini, E, Picone, C, et al. Relative lymphocyte count: a prognostic indicator of mortality in elderly patients with congestive heart failure. Am Heart J. (2001) 142:167–73. doi: 10.1067/mhj.2001.115792
26. Liu, GQ, Zhang, WJ, Shangguan, JH, Zhu, XD, Wang, W, Guo, QQ, et al. Association of Derived Neutrophil-to-Lymphocyte Ratio with Prognosis of coronary heart disease after PCI. Front Cardiovasc Med. (2021) 8:705862. doi: 10.3389/fcvm.2021.705862
27. Maida, CD, Norrito, RL, Daidone, M, Tuttolomondo, A, and Pinto, A. Neuroinflammatory mechanisms in ischemic stroke: focus on cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci. (2020) 21:6454. doi: 10.3390/ijms21186454
28. Sharma, D, Spring, KJ, and Bhaskar, SMM. Neutrophil-lymphocyte ratio in acute ischemic stroke: immunopathology, management, and prognosis. Acta Neurol Scand. (2021) 144:486–99. doi: 10.1111/ane.13493
29. Liao, R, Peng, C, Li, M, Li, DW, Jiang, N, Li, PZ, et al. Comparison and validation of the prognostic value of preoperative systemic immune cells in hepatocellular carcinoma after curative hepatectomy. Cancer Med. (2018) 7:1170–82. doi: 10.1002/cam4.1424
30. Dragu, R, Huri, S, Zukermann, R, Suleiman, M, Mutlak, D, Agmon, Y, et al. Predictive value of white blood cell subtypes for long-term outcome following myocardial infarction. Atherosclerosis. (2008) 196:405–12. doi: 10.1016/j.atherosclerosis.2006.11.022
31. Zhu, Z, Fu, Y, Tian, D, Sun, N, Han, W, Chang, G, et al. Combination of the immune modulator Fingolimod with Alteplase in acute ischemic stroke: a pilot trial. Circulation. (2015) 132:1104–12. doi: 10.1161/CIRCULATIONAHA.115.016371
32. Xu, J, Wang, A, Meng, X, Yalkun, G, Xu, A, Gao, Z, et al. Edaravone dexborneol versus edaravone alone for the treatment of acute ischemic stroke: a phase III, randomized, double-blind, Comparative Trial. Stroke. (2021) 52:772–80. doi: 10.1161/STROKEAHA.120.031197
Keywords: acute ischemic stroke, mechanical thrombectomy, inflammatory markers, neutrophil-to-lymphocyte ratio, derived neutrophil-to-lymphocyte ratio, neutrophil-monocyte-to-lymphocyte ratio, prognosis
Citation: Gao W, Annadurdyyev A, Yu L, Huang R, Liu B, Lin Y, Li H and Zhu R (2024) Day 3 neutrophil-to-lymphocyte ratio and its derived indices predict 90-day poor outcomes following mechanical thrombectomy in acute ischemic stroke patients. Front. Neurol. 15:1496628. doi: 10.3389/fneur.2024.1496628
Received: 15 September 2024; Accepted: 10 December 2024;
Published: 18 December 2024.
Edited by:
Bharathi Dasan Jagadeesan, University of Minnesota Twin Cities, United StatesReviewed by:
Archana Hinduja, The Ohio State University, United StatesCopyright © 2024 Gao, Annadurdyyev, Yu, Huang, Liu, Lin, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renjing Zhu, emh1cmVuamluZ0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.