- Neurosurgery ICU, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
Background: Patients with acute brain injury (ABI) often exhibit gastrointestinal motility disorder and the administration of sedatives may exacerbate the gastrointestinal dysfunction. This study aims to evaluate the influences of different sedatives on gastric antrum contraction in patients with acute brain injury (ABI).
Methods: A prospective observational study was performed in 37 adult ICU patients with ABI, and 18 adult healthy volunteers were recruited as normal controls. Gastric motility, including frequency (ACF), amplitude (ACA), and motility index (MI), was measured with ultrasound before and after using sedatives, either propofol (Group A), midazolam (Group B), or dexmedetomidine (Group C). The influences of different sedatives on gastric motility were analyzed.
Results: All patients with acute brain injury (n = 37) exhibited decreased ACF and MI compared with those in healthy control (n = 18) (ACF: 2.41 ± 0.89 times/2 min in ABI vs. 4.5 ± 0.39 times/2 min in control, MI: 1.25 ± 0.57 in ABI vs. 3.59 ± 0.24 in control, p = 0.001). All sedatives, either propofol, midazolam, or dexmedetomidine, had inhibited effects on gastric motilities [In Group A (n = 13), 1.14(0.59, 1.44) before vs. 0.84(0.09, 0.83) after, p = 0.002; In group B (n = 12), 1.48(0.73, 1.62) before vs. 0.31(0.04, 0.58) after, p = 0.007; In Group C (n = 12), 2.74(1.70, 3.01) before vs. 1.39(0.70, 2.28)]. However, dexmedetomidine showed significantly less inhibition either on ACA or MI compared with propofol and midazolam (ACA 20.67 ± 33.59% in dexmedetomidine, 51.50 ± 32.83% in propofol, 60.43 ± 22.40% in midazolam, p = 0.002; MI 36.00 ± 34.77% in dexmedetomidine, 60.69 ± 27.49% in propofol, 68.81 ± 20.84% in midazolam, p = 0.012).
Conclusion: Patients with ABI exhibited decreased gastric motility. All sedatives, either propofol, midazolam, or dexmedetomidine, had inhibited effects on gastric motilities. Dexmedetomidine has less inhibitory effects on ACA and MI compared with propofol and midazolam.
Introduction
Patients with acute brain injury (ABI) exhibit gastrointestinal motility disorder (1, 2). The autonomic nerve dysfunction resulting from ABI results in gastrointestinal muscle dysmotility, and the severity is associated with the intracranial pressure (3). On the other hand, ABI can activate the hypothalamic–pituitary–adrenal (HPA) axis and induce a systemic stress response that triggers intestinal smooth muscle inflammation, further leading to the disorder of intestinal smooth muscle contraction (4).
In patients with ABI, sedatives were indicated for controlling anxiety, pain, discomfort, agitation, facilitating mechanical ventilation, and also for “neuro-specific” indications such as reducing cerebral metabolic demands, and enhancing cerebral tolerance to ischemia (5). Sedatives are indispensable therapeutic components in therapeutic measures such as reducing intracranial pressure, maintaining temperature, and controlling seizure (5). However, the effects of sedation on gastric motility in patients with ABI have rarely been well studied.
Gastric antrum ultrasound is an advanced technique developed in recent years (6, 7). Antrum contraction can be observed to evaluate gastric motility directly. It is non-invasive and can be performed at the bedside. In this study, we used gastric antrum ultrasound to assess the effects of different sedatives on gastric motility in patients with ABI and compare their degree of inhibition of gastric antral contractions.
Materials and methods
Study design
A prospective observed study was conducted. The sedative, either propofol (Group A), midazolam (Group B), or dexmedetomidine (Group C), was used according to the patients’s needs, and light sedation was applied. Gastric motility was measured with gastric antrum ultrasound before and after the administration of sedatives. The effects of different sedatives on gastric antrum contraction, which includes frequency (ACF), amplitude (ACA), and motility index (MI), were analyzed and compared. In addition, 18 adult healthy volunteers were recruited as normal controls to compare with patients with ABI.
This research was approved by the Ethics Committee of Clinical Research and Experimental Animals of the First Affiliated Hospital of Sun Yat-sen University (Ethics No. [2018] 161). The institutional review board waived the requirement for informed consent since no intervention was performed and no personally identifiable information appeared.
Patients recruitment
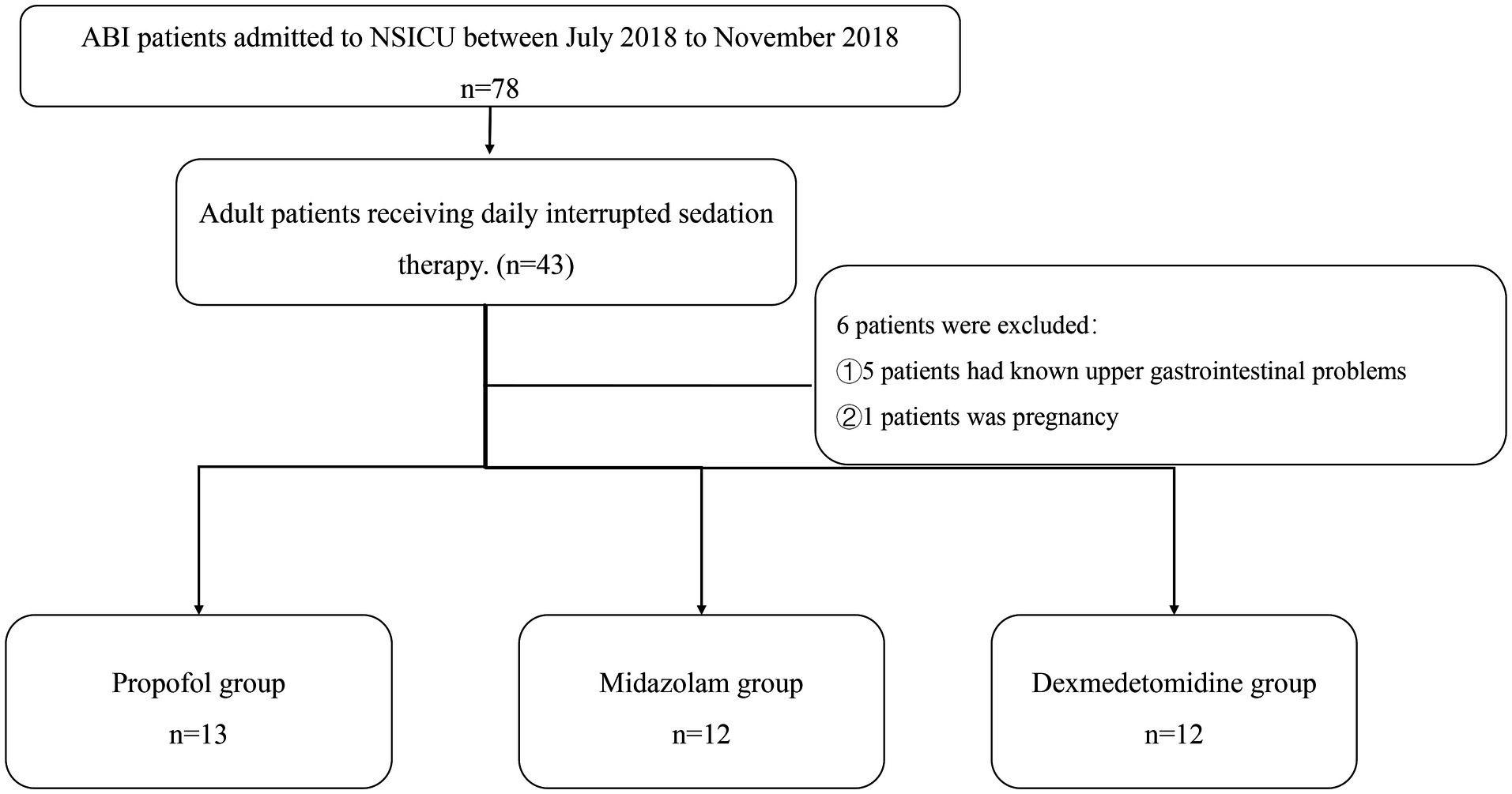
Patients hospitalized in the neurosurgery ICU of the First Affiliated Hospital, Sun Yat-sen University, from July 2018 to November 2018 were recruited (Figure 1).
Inclusion criteria: 18–80 years old. Patients underwent ABI including intracranial hemorrhage, post-selective operation with brain tumors, or traumatic brain injury. Need therapy of short-acting sedatives like midazolam, propofol, or dexmedetomidine, and no oral hypnotic drugs were used.
Exclusion criteria: known upper gastrointestinal anatomical problems. Pregnancy.
Sedation protocol
The Richmond Agitation-Sedation Scale (RASS) was maintained at −2 ~ 1 points, meaning the depth of sedation was maintained at light sedation. A daily wake-up procedure was performed on each patient at 6–9 a.m.
Gastric antrum ultrasound
Gastric antrum ultrasound imaging was performed before and 20 min after sedation. All ultrasonic gastric motility monitoring in this study was completed between 9 and 10 a.m., which is 2 h later after the daily wake-up procedure. Enteral nutrition was performed at 60–80 mL/h through a nasogastric tube. Gastric antrum images were obtained with a 2.5–6 MHz curvilinear probe (SONIMAGE HS1 portable ultrasonic, Konica Minolta). Ultrasound examination was performed at 30-degree head-of-bed elevation and supine position. The antrum was located after identifying the liver’s left anterior lobe, the pancreas’s head, and the abdominal aorta. ACF was measured for 6 min, and an average number of gastric antrum contractions were observed every 2 min. The maximum gastric antral diastolic area (Smax) and minimum contraction area (Smin) were measured thrice. ACA was calculated as follows: ACA = (Smax - Smin)/ Smax. MI was calculated as follows: MI = ACF × ACA. The same experienced sonographer performed all of the scans, as the force of ultrasound probe placement may affect the interpretation of the cross-section.
Bowel sounds auscultation
Bowel sound auscultation was conducted before and 20 min after sedation. Bowel sound auscultation was for 3 min and the frequency of bowel sounds was calculated per minute.
Other clinical data collection
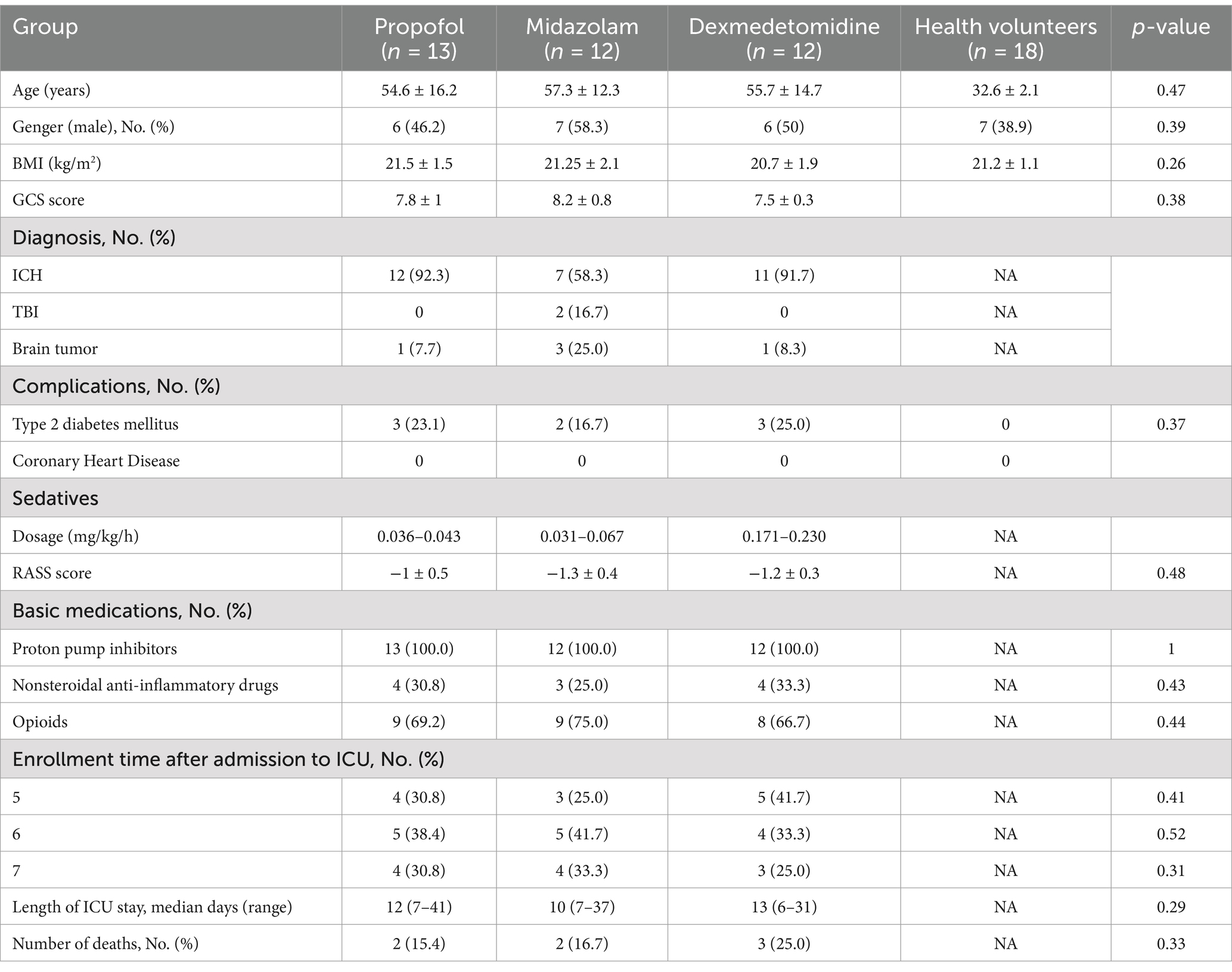
The following clinical data were collected: age, gender, disease diagnosis, complications such as diabetes and coronary heart disease, body mass index (BMI), GCS score, and the utilization of basic medications such as proton pump inhibitors, or nonsteroidal anti-inflammatory drugs, or opioids, enrollment time after admission to ICU, length of ICU stay, and death.
Statistical analysis
The SPSS software (version 23.0) was used for the statistical tests. Normally distributed data were expressed as mean ± standard deviation, non-normal distribution data median (interquartile ranges, IQR). The paired student’s test or the paired rank-sum test was adopted to analyze the difference before and after the sedative therapy. The least significant difference t-test (LSD-t) or Bonferroni method was applied for multiple comparisons. p < 0.05 was considered to be statistically significant.
Results
Baseline characteristics
This study included 37 patients. Among them, 30 cases were with intracranial hemorrhage, 5 cases were with brain tumor, and 2 cases were with traumatic brain injury. In addition, 18 healthy volunteers were recruited for this study to compare gastric motility in patients with ABI. The sedative dosage was recorded. In this study, the dosage of propofol (n = 13) was 0.036–0.043 mg/kg/h, the dosage of midazolam (n = 12) was 0.031–0.067 mg/kg/h, and the dosage of dexmedetomidine (n = 12) was 0.171–0.230 mg/kg/h. It is shown in Table 1.
Gastric motility in patients with ABI
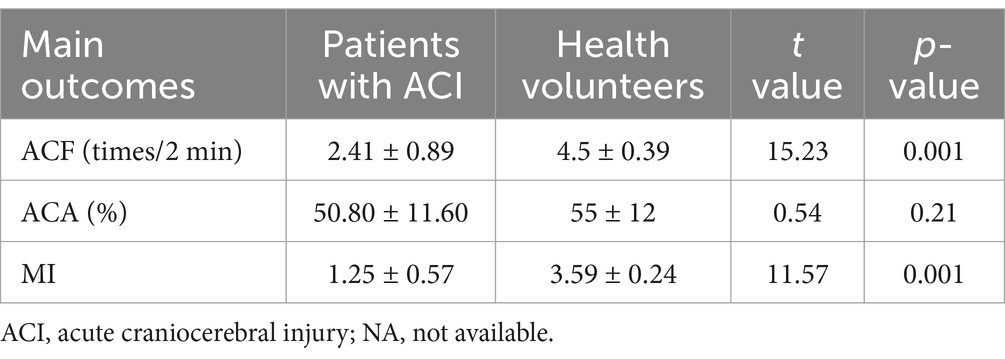
In this study, patients with ABI showed lower ACF (2.41 ± 0.89 times/2 min) and MI (1.25 ± 0.57) than health volunteers (ACF: 4.5 ± 0.39 times/2 min, p = 0.001; MI: 3.59 ± 0.24, p = 0.001). There was no significant inhibition of ACA (50.80 ± 11.60%), as shown in Table 2.
Influences of propofol on gastric antrum contraction and bowel sounds
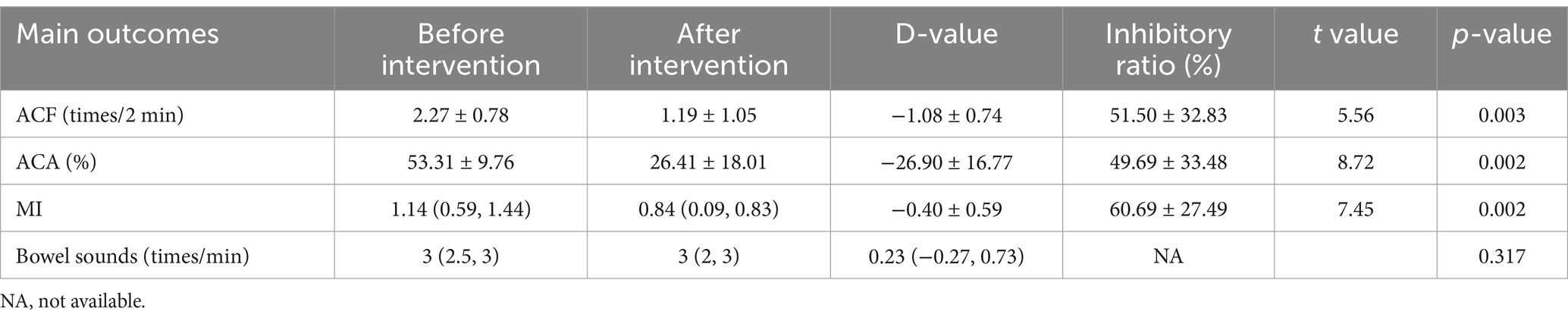
In patients receiving propofol (n = 13), the ACA decreased significantly (53.31 ± 9.76% before vs. 26.41 ± 18.01% after, p = 0.002), ACF decreased significantly (2.27 ± 0.78 times/2 min before vs. 1.08 ± 0.74 times/2 min after, p = 0.002), and MI decreased significantly (1.14(0.59, 1.44) before vs. 0.84(0.09, 0.83) after, p = 0.002). The bowel sounds had no significant change after using propofol (3 (2.5, 3) times/min before vs. 3 (2, 3) times/min after, p = 0.317). It is shown in Table 3.
Influences of midazolam on the contraction of the gastric antrum and bowel sounds
In patients receiving midazolam (n = 12), the ACA decreased significantly (53.35 ± 13.87% before vs. 21.04 ± 12.47% after, p = 0.002), ACF decreased significantly (2.23 ± 1.04 times/2 min before vs. 1.32 ± 1.04 times/2 min after, p = 0.007), MI decreased significantly (1.48 (0.73, 1.62) before vs. 0.31 (0.04, 0.58) after, p = 0.007). The bowel sounds decreased after using midazolam (3 (2.25, 3) before vs. 2 (2, 3) after, p = 0.034). It is shown in Table 4.
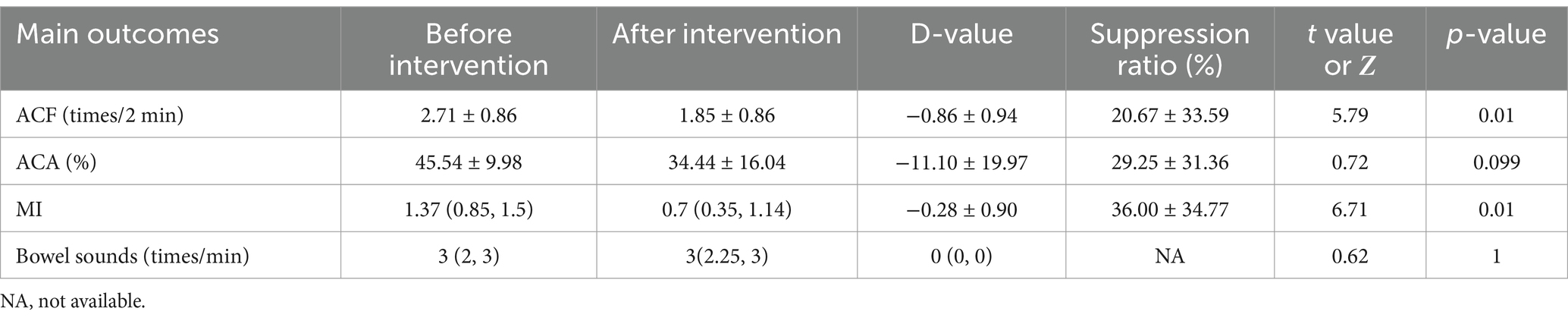
Influences of dexmedetomidine on the contraction of the gastric antrum and bowel sounds
In patients receiving dexmedetomidine (n = 12), ACF decreased significantly (2.71 ± 0.86 times/2 min before vs. 1.85 ± 0.86 times/2 min after, p = 0.01), MI decreased significantly (1.37 (0.85, 1.5) before vs. 0.9 (0.35, 1.14) after, p = 0.01), and no significant difference in ACA (45.54 ± 9.98% before vs. 34.44 ± 16.04 after, p = 0.099) and bowel sounds (3 (2, 3) before vs. 3 (2.25, 3) after, p = 1). It is shown in Table 5.
Comparison of influences of midazolam, propofol, and dexmedetomidine on gastric antrum contraction
Dexmedetomidine showed less inhibitory effects on ACA compared with midazolam and propofol. D-value of ACA was −11.10 ± 19.96% in dexmedetomidine, −32.21 ± 14.03% in midazolam, −26.90 ± 16.77% in propofol, p = 0.003. ACA suppression ratio was 20.67 ± 33.59% in dexmedetomidine, 60.43 ± 22.40% in midazolam, 51.50 ± 32.83% in propofol, p = 0.002 (Figures 2A,D).
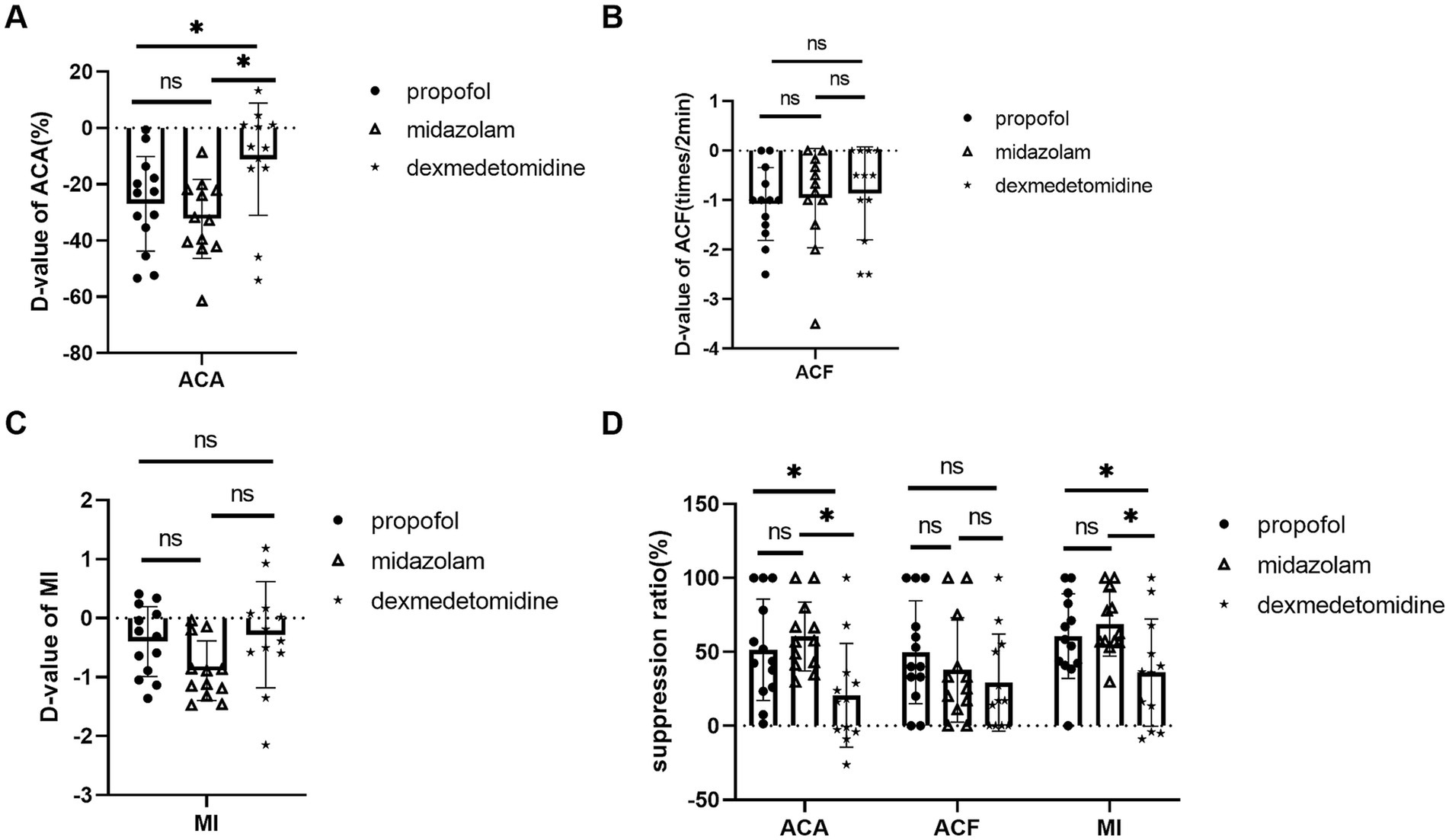
Figure 2. Comparison of midazolam, propofol, and dexmedetomidine effects on ACA, ACF, and MI. (A) D-value of ACA of 3 groups. (B) D-value of ACF of 3 groups. (C) D-value of MI of 3 groups. (D) The three groups’ suppression ratios of ACA, ACF, and MI. Ns: no significant difference. *p ≤ 0.05 between groups.
Dexmedetomidine also showed less inhibitory effects on MI. MI suppression ratio was 36.00 ± 34.77% in dexmedetomidine, 68.81 ± 20.84% in midazolam, 60.69 ± 27.49% in propof ol, p = 0.012. D-value of MI was −0.28 ± 0.90 in dexmedetomidine, −0.89 ± 0.51 in midazolam, −0.4 ± 0.59 in propofol, p = 0.066 (Figures 2C,D).
There was no statistical difference in ACF. D-value of ACF was −1.08 ± 0.74 times/2 min in propofol, −0.96 ± 1.00 times/2 min in midazolam, 0.86 ± 0.94 times/2 min in dexmedetomidine, p = 0.811. ACF suppression ratio 49.74 ± 34.81% in propofol, 37.87 ± 35.24% in midazolam, and 29.0 ± 32.8% in dexmedetomidine, p = 0.518 (Figures 2B,D).
Discussion
The clinical assessment of gastric motility has relied on clinical symptoms such as bloating and vomiting, which are subjective and untimely. Some studies used electrogastrogram to assess gastric motility and gastric emptying (8, 9). However, the electrogastrogram can only represent gastric electrical activity and does not reflect effective gastric contraction. In this study, gastric motility was measured using bedside gastric antrum ultrasound. The gastric antral ultrasound can quantify the contraction amplitude and frequency of the antrum (10). Also, it is noninvasive, easy to operate, and can be performed at the bedside to observe the dynamic effect of clinical treatments. We found that ACF and MI deteriorated in patients with ABI. The finding is consistent with previous studies, which showed gastric motility disorders are severe (11, 12).
Light sedation has an influence on gastric motility in ABI patients
Sedation is a common treatment for brain injury. Previous studies that explored the effects of sedation on gastric motility in patients with ABI have focused on deep sedation (13, 14). However, light sedation is the current trend in ICU sedation management (5, 15). This study found that light sedation can also reduce gastric motility in patients with ABI.
Weaker gastric motility inhibitory effect of dexmedetomidine
This study also compared the inhibitory effects of different sedatives on gastric motility. We found dexmedetomidine had less inhibitory effects on gastric motility than propofol and midazolam. Midazolam, a short-acting benzodiazepine, acts directly on the γ-aminobutyric ABId receptor (16). Propofol acts on the subtype A of the γ-aminobutyric ABId receptor (17). Different from propofol or midazolam, dexmedetomidine is a highly selective α2 agonist, having mild sedative, mild analgesic, and antisympathetic properties (18). Dexmedetomidine showed a less inhibitory effect than midazolam and propofol, possibly due to its lighter sedation effect and sympathoexcitatory depressant function (19). In addition, dexmedetomidine has the ability to attenuate intestinal ischemia–reperfusion injury, inhibit the inflammatory response, and ameliorate the stress response (20). This could also rationalize the weaker inhibition of gastric antral contraction by dexmedetomidine.
Midazolam caused a decrease in bowel sounds
This study also observed bowel sounds to evaluate intestinal motility. We found that midazolam suppresses bowel sounds, but propofol and dexmedetomidine had no significant inhibition of bowel sounds. However, there are no studies to explore the effects of sedative medications on bowel sounds in patients with ABI. We conjecture that this might be related to the influence of sedatives on gastrointestinal hormones. Narchi et al. (21) found that sedatives, including midazolam and propofol, affect gastrin secretion. Early animal experiments revealed that midazolam intervention could cause a decrease in the secretion of motilin (22, 23). Xu et al. (24) also found that propofol can increase the levels of gastrin and vasoactive intestinal peptide in patients undergoing gastrointestinal endoscopy. Moreover, in a clinical study of open surgery for colon cancer, patients undergoing intravenous anesthesia involving dexmedetomidine had a quicker recovery of gastrointestinal motility, and the levels of prokinetic gastrointestinal hormones such as motilin and gastrin in the plasma increased (25). Thus, It could be explained by the different mechanisms of midazolam, propofol, and dexmedetomidine affecting gastrointestinal motility and bowel sound.
Limitations and strengths
This is a prospective observational study. There are some limitations in this study. First, the clinical application of basic drugs such as opioids (12), proton pump inhibitors, and non-steroidal anti-inflammatory drugs (26) may influence gastric motility. However, the before-and-after comparison was employed in this study, which mitigated the errors brought by other therapies. Second, in this study, radioactive nuclides, gastrointestinal electrical examination, and capsule endoscopy were not utilized for the detection of gastrointestinal motility because these methods are not suitable for before-and-after comparison of short-acting sedatives. By contrast, gastric antrum ultrasound can be operated non-invasively and enables dynamic re-examination. Nevertheless, further studies were needed to explore the specific mechanisms of different sedatives affecting gastric motility.
Conclusion
Patients with ABI exhibited decreased gastric motility. All sedatives, either propofol, midazolam, or dexmedetomidine, had inhibited effects on gastric motilities. Dexmedetomidine has less inhibitory effects on ACA and MI compared with propofol and midazolam.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Clinical Research and Experimental Animals of the First Affiliated Hospital of Sun Yat-sen University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because no intervention was performed and no personally identifiable information appeared.
Author contributions
MM: Formal analysis, Writing – original draft. MY: Methodology, Supervision, Writing – review & editing. JL: Methodology, Writing – review & editing. CQ: Investigation, Writing – review & editing. YW: Conceptualization, Writing – review & editing. YL: Data curation, Writing – review & editing. LS: Validation, Writing – review & editing. LW: Writing – review & editing. BO: Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. De Rosa, S, Battaglini, D, Llompart-Pou, JA, and Godoy, DA. Ten good reasons to consider gastrointestinal function after acute brain injury. J Clin Monit Comput. (2024) 38:355–62. doi: 10.1007/s10877-023-01050-0
2. Sharkey, KA, and Mawe, GM. The enteric nervous system. Physiol Rev. (2023) 103:1487–564. doi: 10.1152/physrev.00018.2022
3. Battaglini, D, De Rosa, S, and Godoy, DA. Crosstalk between the nervous system and systemic organs in acute brain injury. Neurocrit Care. (2024) 40:337–48. doi: 10.1007/s12028-023-01725-1
4. Olsen, AB, Hetz, RA, Xue, H, Aroom, KR, Bhattarai, D, Johnson, E, et al. Effects of traumatic brain injury on intestinal contractility. Neurogastroenterol Motil. (2013) 25:593–e463. doi: 10.1111/nmo.12121
5. Oddo, M, Crippa, IA, Mehta, S, Menon, D, Payen, JF, Taccone, FS, et al. Optimizing sedation in patients with acute brain injury. Crit Care. (2016) 20:128. doi: 10.1186/s13054-016-1294-5
6. Steinsvik, EK, Sangnes, DA, Søfteland, E, Biermann, M, Assmus, J, Dimcevski, G, et al. Gastric function in diabetic gastroparesis assessed by ultrasound and scintigraphy. Neurogastroenterol Motil. (2022) 34:e14235. doi: 10.1111/nmo.14235
7. Perlas, A, Arzola, C, and Van de Putte, P. Point-of-care gastric ultrasound and aspiration risk assessment: a narrative review. Can J Anaesth. (2018) 65:437–48. doi: 10.1007/s12630-017-1031-9
8. Bor, C, Bordin, D, Demirag, K, and Uyar, M. The effect of brain death and coma on gastric myoelectrical activity. Turk J Gastroenterol. (2016) 27:216–20. doi: 10.5152/tjg.2016.16019
9. Lu, CL, Shan, DE, Chen, CY, Luo, JC, Chang, FY, Lee, SD, et al. Impaired gastric myoelectrical activity in patients with Parkinson's disease and effect of levodopa treatment. Dig Dis Sci. (2004) 49:744–9. doi: 10.1023/b:ddas.0000030083.50003.07
10. Ebara, R, Ishida, S, Miyagawa, T, and Imai, Y. Effects of peristaltic amplitude and frequency on gastric emptying and mixing: a simulation study. J R Soc Interface. (2023) 20:20220780. doi: 10.1098/rsif.2022.0780
11. Ott, L, Young, B, Phillips, R, McClain, C, Adams, L, Dempsey, R, et al. Altered gastric emptying in the head-injured patient: relationship to feeding intolerance. J Neurosurg. (1991) 74:738–42. doi: 10.3171/jns.1991.74.5.0738
12. Kunovac, F, Cicvaric, A, Robba, C, Turk, T, Muzevic, D, Kralik, K, et al. Gastrointestinal motility disorders correlate with intracranial bleeding, opioid use, and brainstem edema in neurosurgical patients. Neurocrit Care. (2023) 39:368–77. doi: 10.1007/s12028-023-01678-5
13. Walldén, J, Thörn, SE, Lövqvist, A, Wattwil, L, and Wattwil, M. The effect of anesthetic technique on early postoperative gastric emptying: comparison of propofol-remifentanil and opioid-free sevoflurane anesthesia. J Anesth. (2006) 20:261–7. doi: 10.1007/s00540-006-0436-3
14. Nguyen, NQ, Chapman, MJ, Fraser, RJ, Bryant, LK, Burgstad, C, Ching, K, et al. The effects of sedation on gastric emptying and intra-gastric meal distribution in critical illness. Intensive Care Med. (2008) 34:454–60. doi: 10.1007/s00134-007-0942-2
15. Mehta, S, Spies, C, and Shehabi, Y. Ten tips for ICU sedation. Intensive Care Med. (2018) 44:1141–3. doi: 10.1007/s00134-017-4992-9
16. Inada, T, Asai, T, Yamada, M, and Shingu, K. Propofol and midazolam inhibit gastric emptying and gastrointestinal transit in mice. Anesth Analg. (2004) 99:1102–6. doi: 10.1213/01.Ane.0000130852.53082.D5
17. Friedberg, BL. Hypnotic doses of propofol block ketamine-induced hallucinations. Plast Reconstr Surg. (1993) 91:196–7. doi: 10.1097/00006534-199301000-00047
18. Gregersen, H, Kraglund, K, Rittig, S, and Tøttrup, A. The effect of a new selective alpha 2-adrenoreceptor antagonist, idazoxan, and the agonist, clonidine, on fasting antroduodenal motility in healthy volunteers. Aliment Pharmacol Ther. (1989) 3:435–43. doi: 10.1111/j.1365-2036.1989.tb00234.x
19. Wang, K, Wu, M, Xu, J, Wu, C, Zhang, B, Wang, G, et al. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta-analysis. Br J Anaesth. (2019) 123:777–94. doi: 10.1016/j.bja.2019.07.027
20. Jiang, L, Hu, M, Lu, Y, Cao, Y, Chang, Y, and Dai, Z. The protective effects of dexmedetomidine on ischemic brain injury: a meta-analysis. J Clin Anesth. (2017) 40:25–32. doi: 10.1016/j.jclinane.2017.04.003
21. Narchi, P, Benhamou, D, Elhaddoury, M, Locatelli, C, and Fernandez, H. Interactions of pre-operative erythromycin administration with general anaesthesia. Can J Anaesth. (1993) 40:444–7. doi: 10.1007/bf03009515
22. Cui, GL, Sandvik, AK, Munkvold, B, and Waldum, HL. Effects of anaesthetic agents on gastrin-stimulated and histamine-stimulated gastric acid secretion in the totally isolated vascularly perfused rat stomach. Scand J Gastroenterol. (2002) 37:750–3. doi: 10.1080/00365520213249
23. Norlén, P, Kitano, M, Lindström, E, and Håkanson, R. Anaesthetic agents inhibit gastrin-stimulated but not basal histamine release from rat stomach ECL cells. Br J Pharmacol. (2000) 130:725–30. doi: 10.1038/sj.bjp.0703347
24. Xu, BB, Zhao, XL, and Xu, GP. Clinical study of anesthetization by dezocine combined with propofol for indolent colonoscopy. World J Gastroenterol. (2016) 22:5609. doi: 10.3748/wjg.v22.i24.5609
25. Ou, C, Kang, S, Xue, R, Lai, J, and Zhang, Y. Effect of Dexmedetomidine-assisted intravenous anesthesia on gastrointestinal motility in Colon Cancer patients after open colectomy. Front Surg. (2022) 9:842776. doi: 10.3389/fsurg.2022.842776
Keywords: acute brain injury, sedatives, ultrasonography, gastric motility, propofol, midazolam, dexmedetomidine
Citation: Mei M, Yao M, Li J, Qiu C, Wang Y, Li Y, Shi L, Wang L and Ouyang B (2024) Influences of different sedatives on gastric antrum contraction in patients with acute brain injury. Front. Neurol. 15:1492604. doi: 10.3389/fneur.2024.1492604
Edited by:
Linlin Zhang, Capital Medical University, ChinaReviewed by:
Wang Cuixue, Capital Medical University, ChinaShuya Wang, Capital Medical University, China
Copyright © 2024 Mei, Yao, Li, Qiu, Wang, Li, Shi, Wang and Ouyang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Ouyang, b3V5YkBtYWlsLnN5c3UuZWR1LmNu
†These authors have contributed equally to this work
 Meihua Mei
Meihua Mei Mingli Yao†
Mingli Yao† Chunfang Qiu
Chunfang Qiu Lei Shi
Lei Shi Bin Ouyang
Bin Ouyang