- Department of Neurology, First Hospital of Changsha, Changsha, China
Background: Benign Paroxysmal Positional Vertigo (BPPV) is the most common cause of peripheral vertigo, with frequent recurrence, particularly pronounced among middle-aged and elderly populations, significantly affecting patients’ quality of life. This study aimed to identify predictive factors for recurrence in middle-aged and older patients with BPPV and to develop a nomogram prediction model based on these predictors.
Methods: This retrospective study included 582 participants aged ≥45 years who were selected from the electronic medical records system of the First Hospital of Changsha between March 2021 and March 2024. Randomly chosen participants (n = 407, 70%) constituted the training group, whereas the remaining participants (n = 175, 30%) formed the validation group. This study used LASSO binomial regression to select the most predictive variables. A predictor-based nomogram was developed to calculate the risk of BPPV recurrence. The performance of the nomogram was evaluated using the area under the receiver operating characteristic curve (AUC) and calibration curves with 1,000 bootstrap resampling validations. Decision curve analysis (DCA) was conducted to assess the clinical usefulness of the nomogram.
Results: According to findings from least absolute shrinkage and selection operator (LASSO) binomial regression and logistic regression screening, older age, higher levels of uric acid (UA) and homocysteine (HCY), diabetes, migraine, anxiety, and insomnia were identified as independent factors associated with an increased recurrence risk of BPPV. A nomogram model for predicting recurrence risk was developed based on these predictors. The nomogram achieved an AUC (C-statistic) of 0.8974 (95% CI: 0.8603–0.9345) in the training group and 0.8829 (95% CI: 0.8253–0.9406) in the validation group. Calibration curves, after 1,000 bootstrap resamples, demonstrated good agreement between the predicted and actual probabilities in the development and validation cohorts. DCA indicated that the nomogram had clinical utility.
Conclusion: The nomogram model incorporating age, UA, HCY, diabetes, migraine, anxiety status, and insomnia demonstrated a strong predictive capability for estimating the probability of BPPV recurrence in middle-aged and elderly patients. This tool is valuable for identifying individuals at high risk of BPPV recurrence and can aid physicians in making informed treatment decisions aimed at reducing recurrence rates.
Introduction
Benign Paroxysmal Positional Vertigo (BPPV) is an idiopathic vestibular disorder that results from improper activation of the semicircular canal ampullae by free-floating otoconia originating from the utricle (1). This condition is characterized by brief episodic vertigo and nystagmus triggered by changes in head position (2). As one of the most common vestibular disorders, BPPV is the most common peripheral vestibular condition encountered in neurotology clinics, accounting for approximately 20–30% of all vestibular complaints (3, 4), often occurring in middle-aged and elderly patients (2). Following the canalith repositioning maneuver (CRM), which is the preferred treatment for BPPV, over 95% of cases can be successfully resolved (5). However, patients with BPPV frequently experience relapse even after successful treatment, with recurrence rates ranging from 10 to 30% (6, 7), and the likelihood of recurrence over 10 years can be as high as 50% (8). Patients with BPPV are more prone to future ischemic strokes, dementia, and fractures, which significantly diminish their quality of life, particularly among older people (9–11). These complications also increase the burden on patients and the healthcare system.
However, the underlying cause of BPPV recurrence remains unclear. In recent decades, numerous studies have explored the risk factors for BPPV recurrence, including female sex, osteoporosis, vascular risk factors, head trauma, and other potential contributors (3, 7, 12–15). Nevertheless, the identified risk factors varied between studies, and the impact of each risk factor on recurrence differed. Therefore, developing a predictive model that incorporates as many potential risk factors as possible is crucial for assessing the risk of BPPV recurrence in middle-aged and older populations. This approach aids in the early and accurate identification of recurrence risk, allowing for the timely implementation of preventive strategies.
Currently, nomograms serve as tools that integrate various predictors into graphical instruments for statistical models, offering the predicted probability of a clinical event or specific endpoint outcome (16, 17). Therefore, the development of a nomogram can help predict the probability of BPPV recurrence and provide timely, individualized, and comprehensive prevention recommendations. However, effective and sensitive predictive models for BPPV recurrence in middle-aged and elderly individuals are currently lacking.
Therefore, this study aimed to analyze the risk factors of BPPV recurrence in middle-aged and elderly individuals following successful treatment. Additionally, this study aimed to construct a nomogram prediction model to provide patients with optimal and timely clinical decision-making and preventive recommendations.
Methods
Study design and source of data
This retrospective cohort study included 582 patients consecutively diagnosed with BPPV and admitted to the Neurology Department of the First Hospital of Changsha between March 2021 and March 2024. In our study, the licensed neurologist utilized the following approaches to ensure that BPPV was accurately diagnosed: (1) Clinical Criteria: Diagnosis was based on established clinical criteria outlined in the guidelines from the Bárány Society (18). We confirmed the presence of characteristic symptoms, including episodic vertigo triggered by specific head movements, typically lasting less than 1 minute. (2) Clinical Examination: All participants underwent a thorough clinical examination. We specifically performed the Dix-Hallpike maneuver and/or the supine roll test to identify the presence of nystagmus consistent with BPPV. The direction and characteristics of the nystagmus were carefully analyzed. (3) Exclusion of Other Conditions: We excluded other potential causes of vertigo by conducting a comprehensive medical history and physical examination, along with additional tests (e.g., audiometry, MRI) when necessary. And the exclusion criteria were as follows: (1) patients with sudden deafness or other vestibular diseases; (2) individuals with hearing impairment; (3) those who experienced head trauma within the last month; (4) patients with systemic musculoskeletal diseases; (5) patients with severe organic diseases; (6) those with cervical vertigo, brain space-occupying lesions, cerebrovascular malformations, or central vertigo; and (7) individuals with involvement of multiple semicircular canals or superior semicircular canal otolithiasis.
Using the “GetTable_comparet” method in R software, we randomly split the overall dataset into training and validation cohorts in a 7:3 ratio, resulting in 407 patients in the training cohort and 175 patients in the validation cohort. The Ethics Committee of the First Hospital of Changsha approved this study. The study was carried out in accordance with the guidelines of Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) (19). The requirement for informed consent was waived in accordance with national regulations and agency guidelines Owing to the retrospective nature of our study. Patients’ identifying information (e.g., names, addresses, and phone numbers) was removed from the dataset throughout the study to ensure participants’ anonymity and to protect privacy. The data were securely stored using encryption and access controls.
Candidate predictor variables
Based on preliminary observations from clinical practice, references from previous literature (20), and the availability of retrospective study data, we selected potential risk predictors that may influence the recurrence of BPPV. We collected electronic medical record data during the patients’ hospital stays, including demographics, lifestyle factors, comorbidities, clinical symptoms, vestibular function test results, and laboratory findings. Demographic variables included sex, BMI, and age at BPPV onset. Lifestyle factors included smoking habits and alcohol consumption. The medical histories of the participants revealed comorbidities such as hypertension, diabetes, migraine, Meniere’s disease (MD), anxiety, insomnia, and osteoporosis. The laboratory examination variables included hyperlipidemia, thyroid dysfunction, globulin levels, serum calcium ion levels, uric acid (UA), and homocysteine (HCY). The duration of illness in the patients was calculated in days, and we categorized the duration into two groups using a cutoff of 3 days. Additionally, we collected data regarding the season of illness (spring, summer, autumn, and winter) for classification purposes.
We recorded the results of vestibular function tests, including the C-test, cupulolithiasis, and common types of BPPV. The C-test was conducted using VNG (videonystagmography) with the following sequence (21): irrigation of the right ear with air at 24°C, irrigation of the left ear with air at 24°C, irrigation of the right ear with air at 50°C, and irrigation of the left ear with air at 50°C. A positive C-test result was considered when the semicircular canal paresis (CP) value exceeded 25%.
This study aimed to predict whether patients will experience recurrence after undergoing CRM. We have dedicated staff who follow up with patients diagnosed with BPPV who have received CRM, using methods such as telephone follow-ups, outpatient visits, and readmission assessments. Patients who were readmitted or return for outpatient care due to BPPV symptoms more than 2 weeks after successful repositioning, as well as those identified as having BPPV after being treated for dizziness at other hospitals after 2 weeks based on our telephone follow-ups, were uniformly classified as having recurrent BPPV (6). In this study, we excluded patients who had a history of head trauma or whiplash injuries, those with incomplete clinical histories, and individuals diagnosed with “persistent” Benign Paroxysmal Positional Vertigo (BPPV). “Persistent” BPPV is defined as the absence of symptom remission or nystagmus after 2 weeks or following five repositioning maneuvers (6).
Statistical analysis
Statistical methods were used to evaluate differences between the training and validation cohorts, including (1) normality tests, such as skewness and kurtosis tests for continuous variables; (2) the Mann–Whitney U-test for continuous variables that did not exhibit a normal distribution, with median and interquartile range (IQR) values reported; and (3) the chi-square test for categorical variables, presented as percentages.
Subsequent analyses were conducted using the R software (version 4.4.0) with various packages, including rms, car, glmnet, pROC, regplot, and rmda. First, to avoid collinearity among the included covariates and to identify the optimal predictive risk factors, we screened the potential risk factors for recurrent BPPV using LASSO regression. LASSO binomial regression is a statistical technique that helps identify key predictors for a binary outcome by selectively including variables while minimizing complexity. It adds a penalty to the regression to prevent overfitting, making the model more reliable and easier to interpret. This method helps avoid collinearity among covariates and selects optimal predictive risk factors (22). Variables selected by LASSO regression were included in multivariate logistic regression analysis to develop a nomogram prediction model. Receiver-operating characteristic (ROC) curves were used to assess the sensitivity and specificity of the nomogram (23). Calibration curve and decision curve analyses (DCA) were used to evaluate the predictive performance of the nomogram. Decision curve analysis (DCA) was conducted to assess the clinical utility of the nomogram and calculate the net benefits across various threshold probabilities (24). We employed the 1,000-bootstrap resampling validation method for internal validation to enhance the accuracy and reliability of the model. For validation, the ROC curve, calibration curve, and decision curve analyses (DCA) were performed using the same methods as described previously. Statistical significance was set at p < 0.05, indicating statistical significance.
Results
A total of 645 patients diagnosed with BPPV met the initial inclusion criteria. After reviewing medical records and conducting telephone interviews, we excluded 21 patients aged <45 years, six patients with severe cognitive impairment who could not cooperate in conversations, and 36 patients who were lost to follow-up or had missing data. Finally, 582 eligible participants were included in the analysis, with 407 and 175 participants in the training and validation cohorts, respectively (Figure 1).
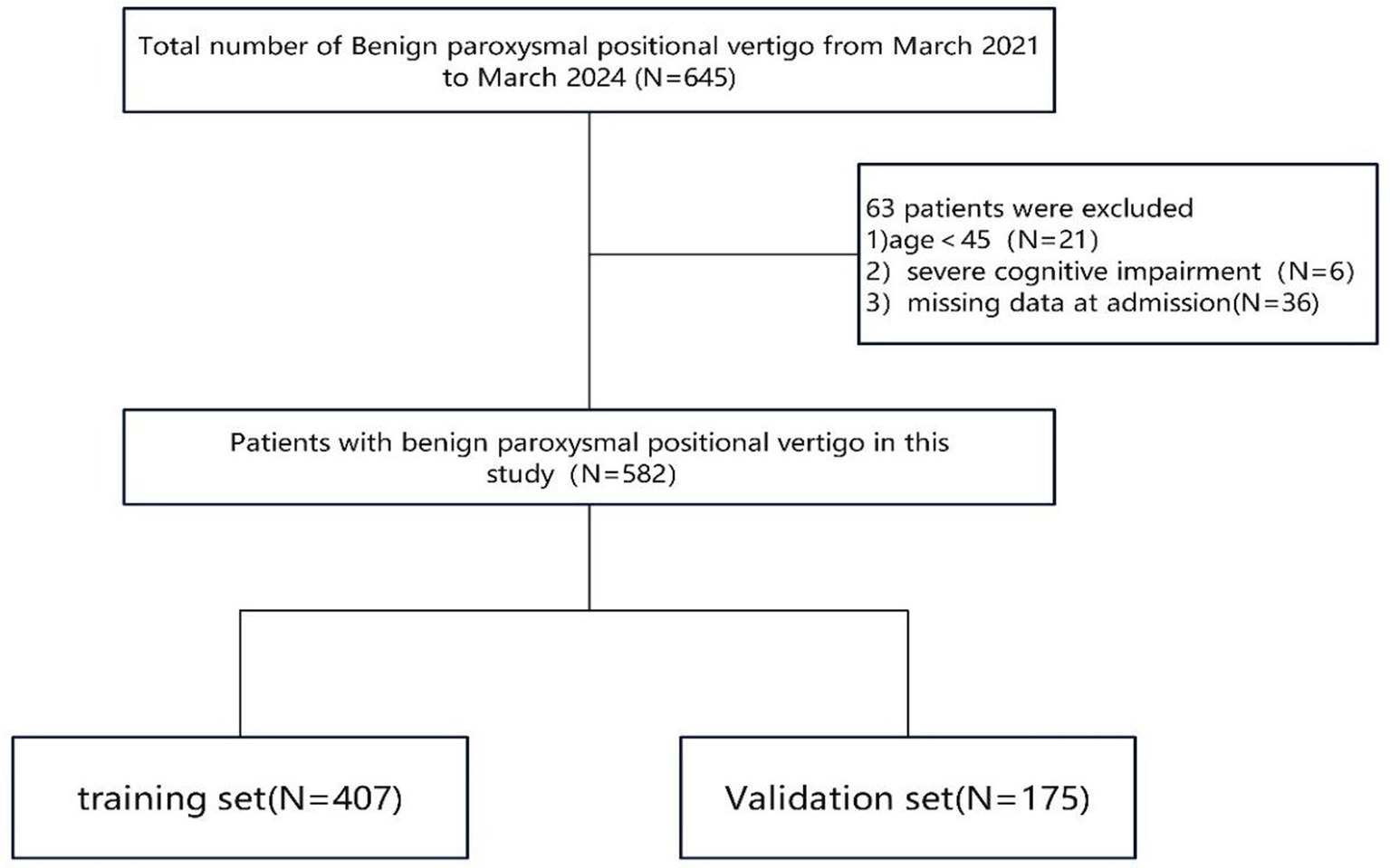
Figure 1. The flow chart of patient recruitment concisely outlines the sequential application of inclusion and exclusion criteria, ultimately defining the final study cohort.
Basic characteristics
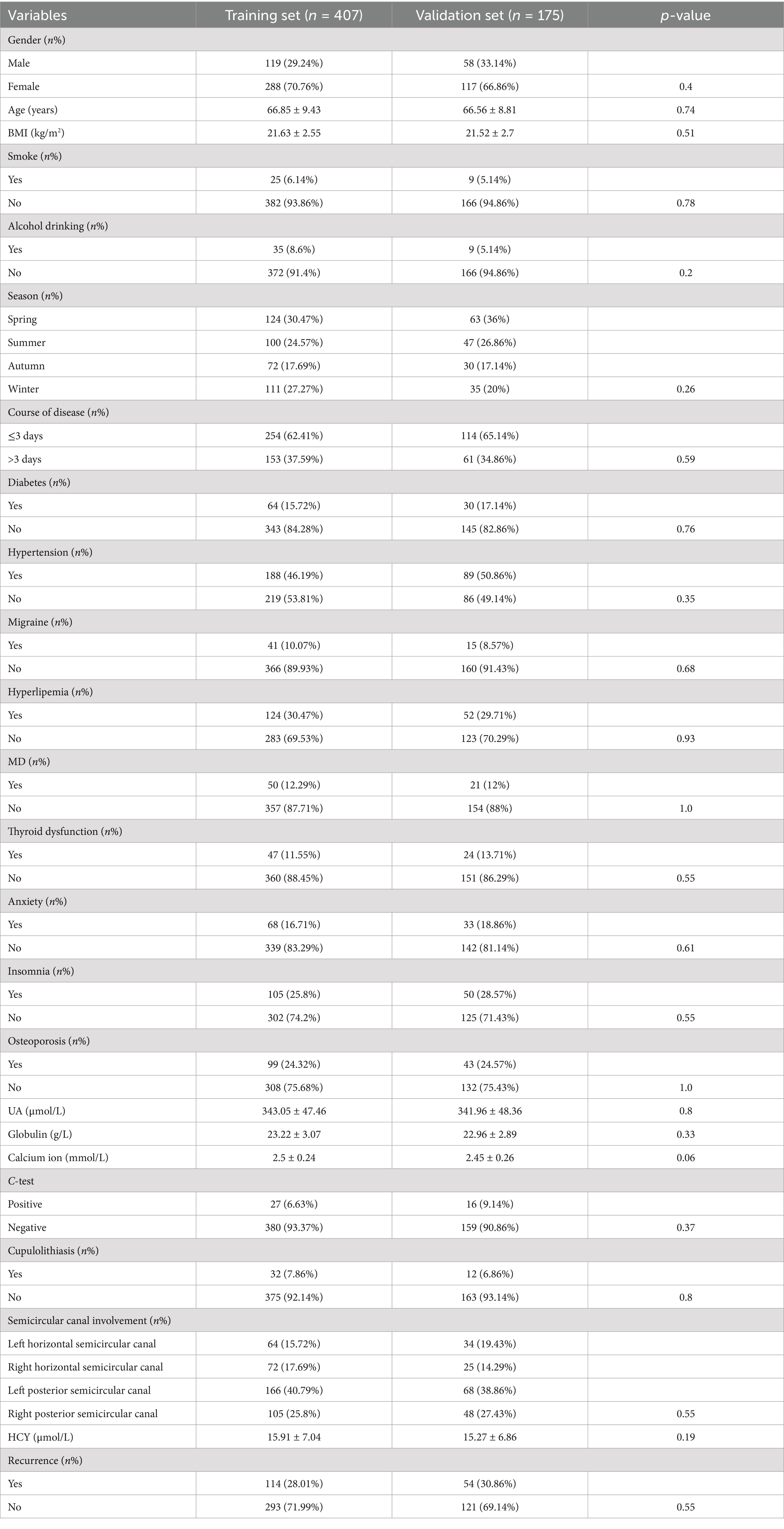
Table 1 summarizes the baseline characteristics of the training and validation datasets used in this study. The training set comprised 407 middle-aged and elderly patients, whereas the validation set included 175 similar patients. Importantly, there were no statistically significant differences between the training and validation groups for all characteristics (p > 0.05), indicating that the grouping of all BPPV patients was reasonable.
Selection of variables and construction of the nomogram
Variable selection was performed using LASSO regression, as illustrated in Figure 2. Seven variables were significantly associated with BPPV recurrence (p < 0.05). These variables include age (years), diabetes (Yes), migraine (Yes), anxiety (Yes), insomnia (Yes), UA (μmol/L), and HCY (μmol/L). A nomogram was constructed using these significant factors, as shown in Figure 3. Detailed information on the relationship between these variables and BPPV recurrence is provided in Table 2.
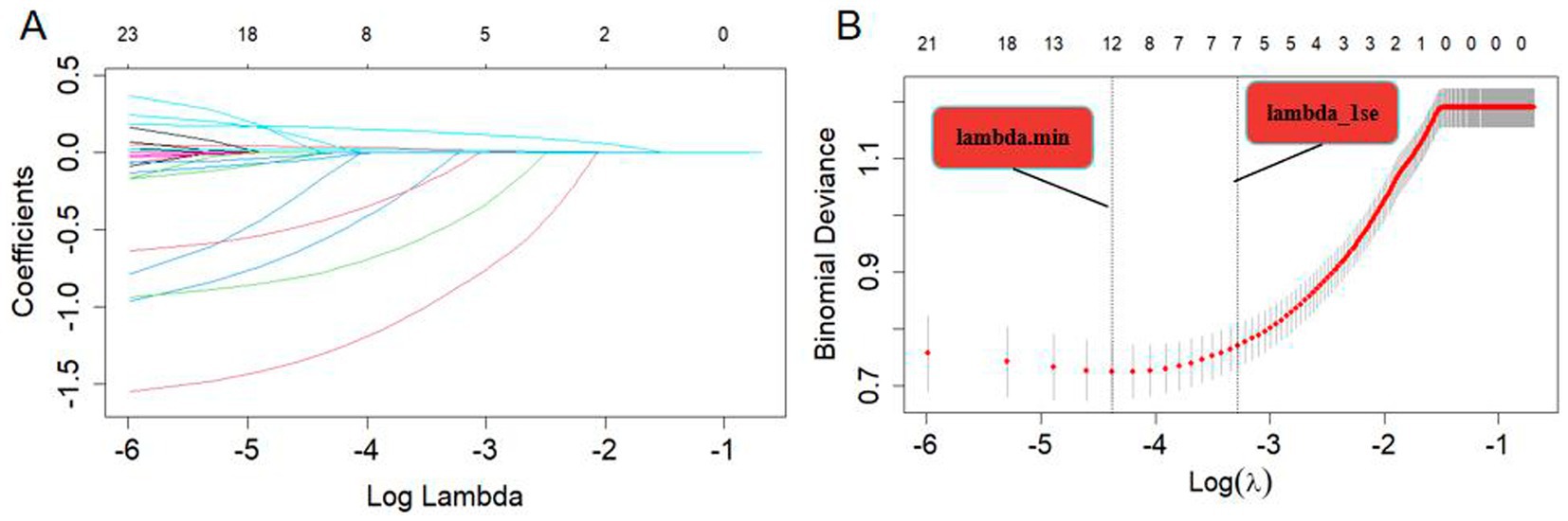
Figure 2. (A) LASSO coefficient profiles of the 23 risk factors. (B) Risk factors were selected using LASSO regression analysis. The two dotted lines indicate the optimal scores according to the minimum criteria (including age, smoking, Alcohol drinking, diabetes, hypertension, migraine, hyperlipidemia, anxiety, insomnia, osteoporosis, UA, and HCY at minimum criteria; age, diabetes, migraine, anxiety, insomnia, UA, and HCY at 1-se criteria).
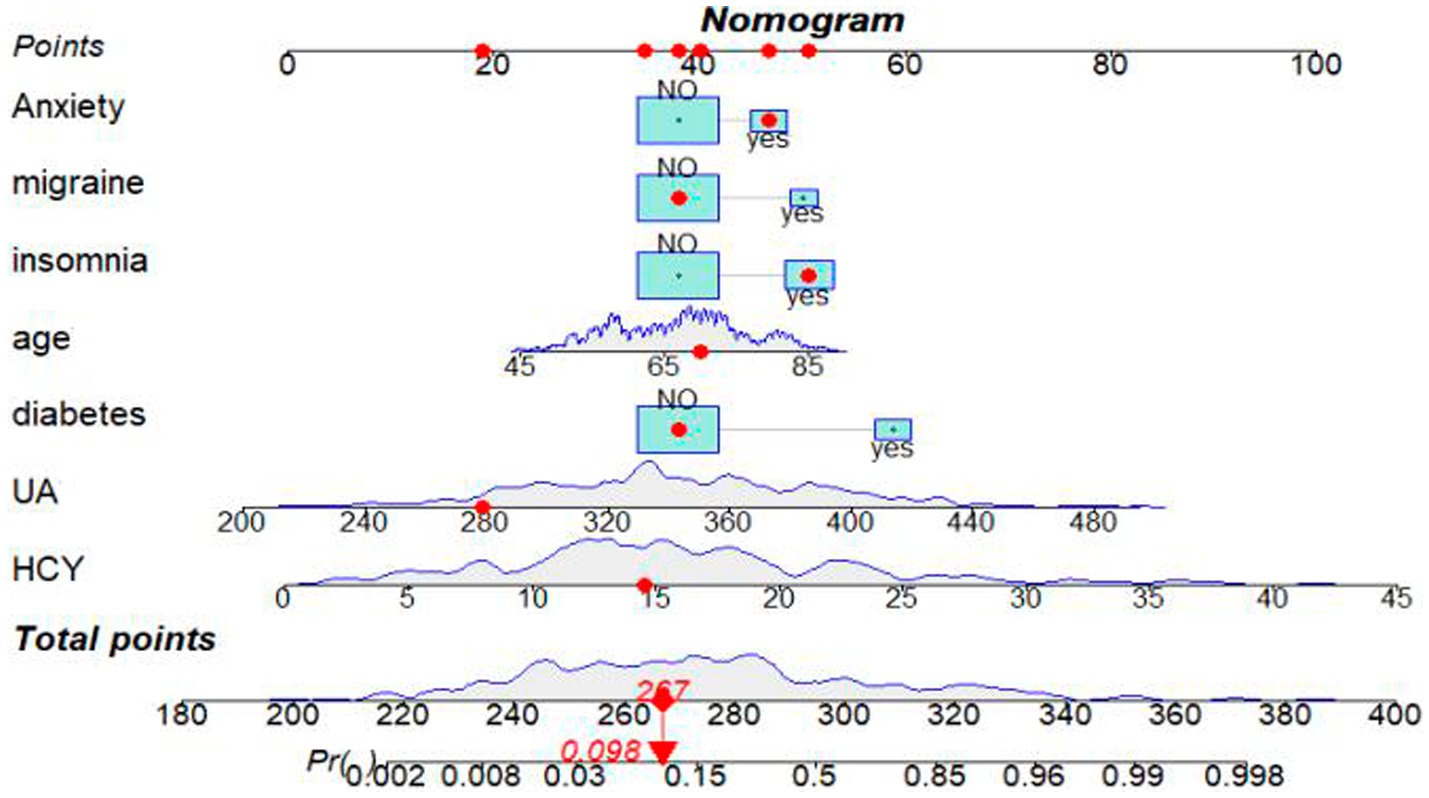
Figure 3. Nomogram for predicting the probability of BPPV recurrence after CRM. A red dot on the nomogram represents the specific characteristics of a patient.In this example, a 70-year-old individual with a history of insomnia and anxiety, but no migraines or diabetes, has a UA level of 279 μmol/L and a HCY level of 14.57 μmol/L. The calculated sum of these specific points is 267, which corresponds to a position on the total point line. From this point, a solid line is drawn vertically down to the survival axis, indicating a recurrence probability of BPPV of 9.8% for this patient.
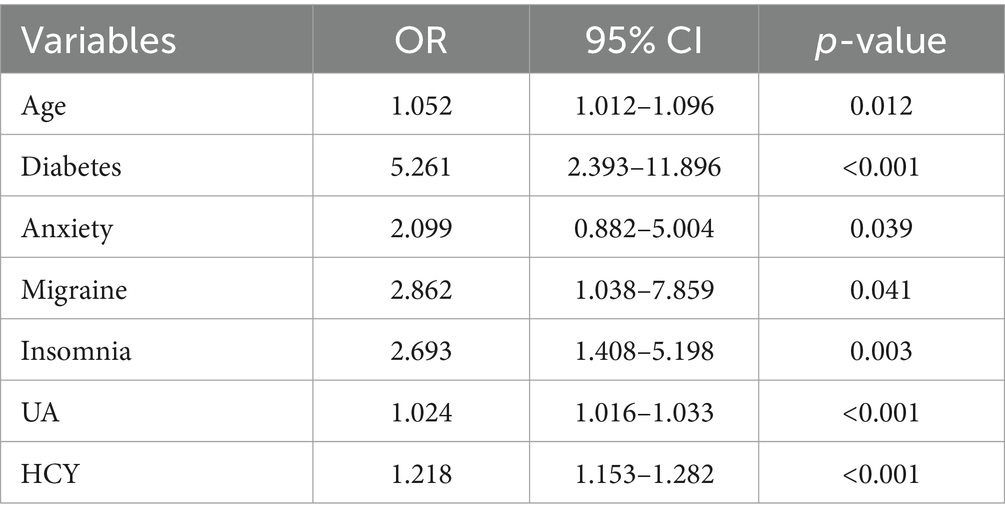
Table 2. Identification of independent risk factors for the recurrence of Benign Paroxysmal Positional Vertigo in middle-aged and older populations using multivariable logistic regression: findings from the training dataset.
Evaluation and validation of the nomogram
The nomogram was validated using AUC-ROC. In the training set (Figure 4A), the AUC-ROC was 0.8974 (95% CI: 0.8603–0.9345), whereas in the validation set (Figure 4B), it was 0.8829 (95% CI: 0.8253–0.9406).
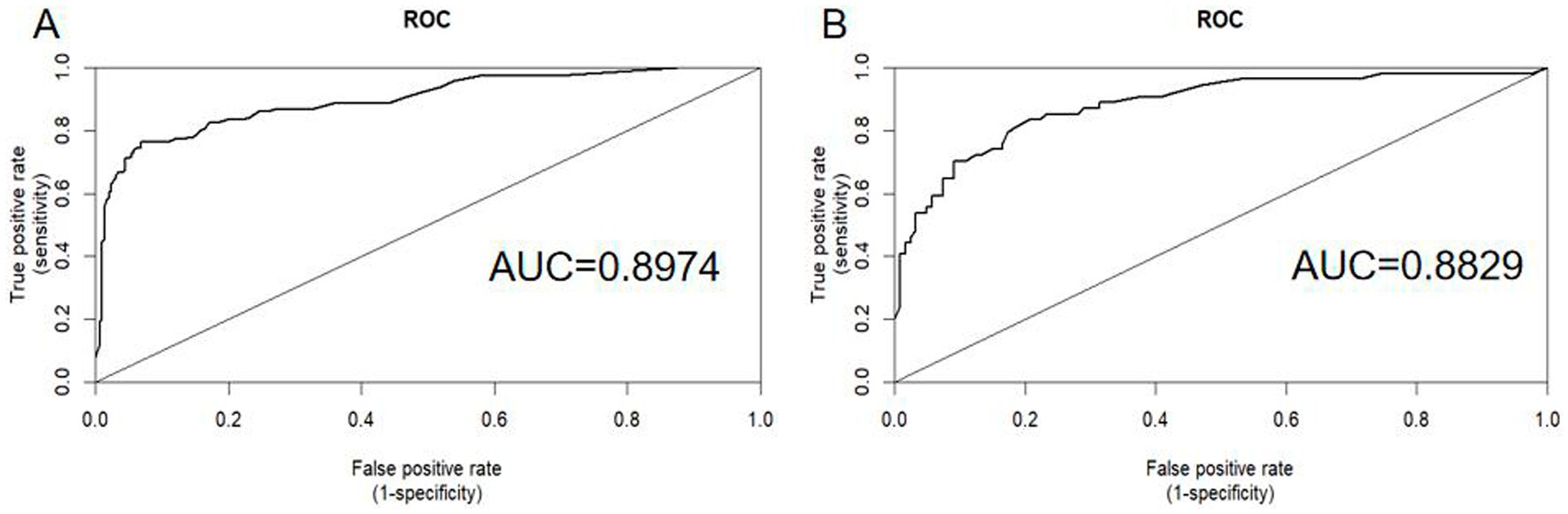
Figure 4. ROC curves were generated to assess the predictive performance of the nomogram for the recurrence probability of BPPV following CRM. Panel A displays the ROC curve for the training set, whereas Panel B shows the ROC curve for the validation set. AUC, area under the ROC curve; ROC, receiver operating characteristic.
The nomogram model was calibrated using calibration plots. These plots demonstrated excellent agreement between the predicted and observed outcomes for the training (Figure 5A) and validation sets (Figure 5B).
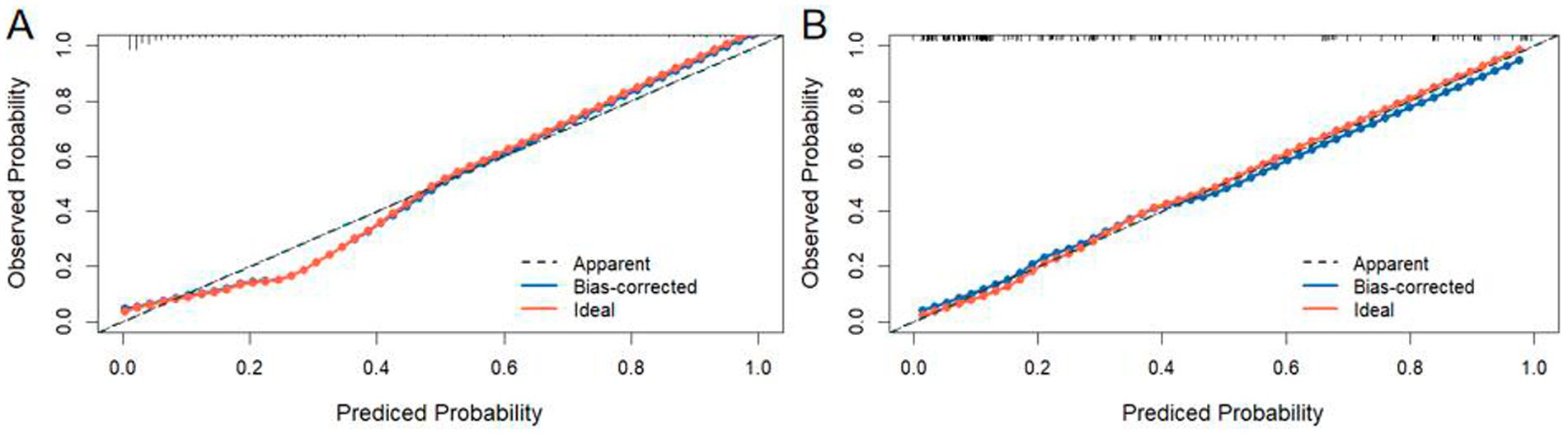
Figure 5. Calibration curves of the nomogram were plotted separately for the training set (A) and the validation set (B).
DCA was used to evaluate the clinical utility of the nomogram (Figures 6A,B). The results confirmed the robust clinical applicability of the nomogram for predicting the probability of BPPV recurrence, as evidenced by the wide and practical range of threshold probabilities across the training and validation sets.
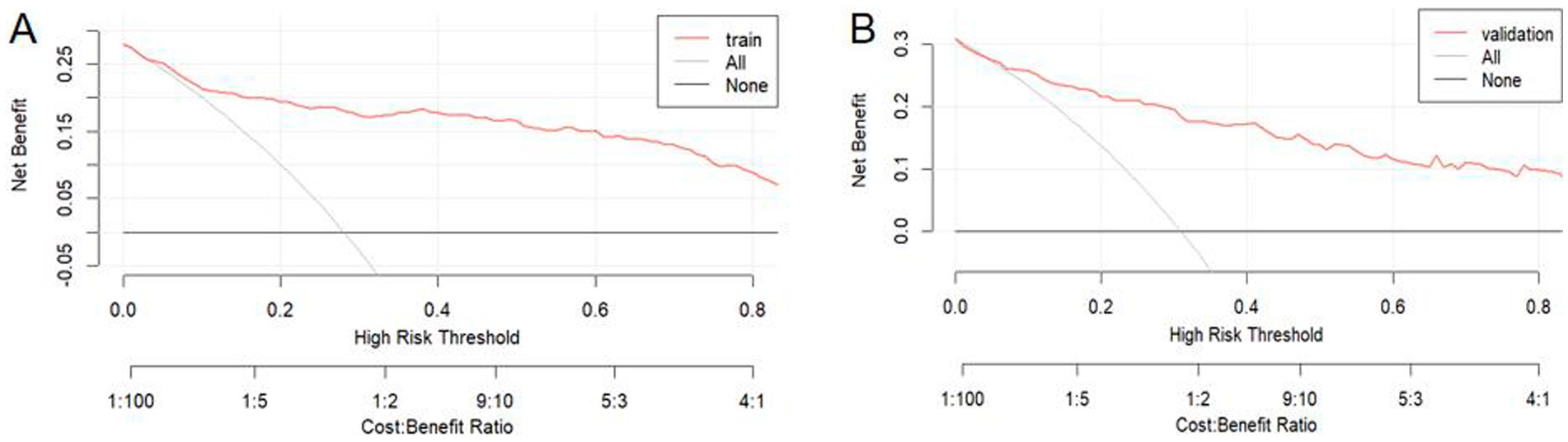
Figure 6. Decision curve analysis was performed for the training set (A) and the validation set (B). In these plots, a horizontal line signifies that no patients are predicted positive, resulting in a net benefit of zero. Conversely, an oblique line indicates that all patients are predicted positive. A backslash with a negative slope represents the net benefit.
Discussion
Currently, no predictive model has been identified for BPPV recurrence. Therefore, developing a model for predicting BPPV recurrence is essential. This study is the first to create a nomogram for predicting the risk of BPPV recurrence. In this study, we developed a straightforward, valid, and clinically useful model for predicting the probability of BPPV recurrence in middle-aged and older patients. The AUC for the training and validation groups were 0.8974 and 0.8829, respectively, indicating that the nomogram-predictive model demonstrated good accuracy and stability. This nomogram model incorporates easily obtainable risk factors, including age, diabetes, migraine, anxiety, insomnia, UA, and HCY. All of these variables can be readily collected during the hospital stay of patients with BPPV. Using this model, clinicians can quickly calculate recurrence risk in middle-aged and elderly patients with BPPV. Patients at high risk of recurrence may benefit from more preventive interventions and early treatment.
Nomograms have emerged as advanced and straightforward prediction tools. They offer a visual representation of a statistical predictive model that generates the numerical probability of a clinical event, making them more accurate than conventional methods that use odds ratios (ORs) (25). This nomogram combines seven variables and assigns appropriate weights to each variable based on its prognostic value. It provides a personalized and highly accurate estimation of the risk of BPPV recurrence, making it easy to use. Thus, physicians can assess a patient’s high risk of BPPV recurrence, recommend lifestyle and behavioral changes, actively manage risk factors, and implement appropriate treatment measures to reduce the likelihood of recurrence.
Our recurrence rate of 28.9% was consistent with that reported in the literature, which ranges from 7 to 50% (3, 6, 7). Previous studies have identified several risk factors for BPPV recurrence. Two recent population-based retrospective cohort studies have indicated that patients with anxiety have a higher risk of developing BPPV compared to those without anxiety (26, 27). The higher risk of recurrence could be owing to the notably reduced efficacy of initial CRM treatment in patients with BPPV and anxiety, resulting in a higher risk of recurrence several months after treatment (28). Recovery from balance disorders, including BPPV, involves habituation and relearning. This process involves various structures, mechanisms, and activities, such as neurophysiological adaptation, desensitization to dizziness sensations, and restoration of automatic perception and control of orientation, all of which are influenced by psychological factors (29). Therefore, anxiety can affect the recovery process from BPPV, leading to prolonged vertigo or dizziness (29).
Furthermore, BPPV symptoms are often unpredictable and uncontrollable, leading to uncertainty and fear of experiencing new symptoms (30). Yardley and colleagues (31) outlined three distinct clusters of concerns among patients with vertigo: fear of losing control, worry about serious illnesses, and anticipation of severe vertigo attacks. Hence, we hypothesized that psychological conditions could complicate and disrupt habituation and coping processes, potentially heightening vertigo symptoms through amplified autonomic responses (29).
Our study found that insomnia (OR = 2.693; p = 0.003) was associated with an increased risk of BPPV recurrence, which is consistent with the findings of Wang Y (32). Previous studies have also suggested that insomnia could lead to BPPV by triggering neuroendocrine dysfunction, marked by elevated cortisol levels and increased sympathetic nervous system activity (33–35), along with inflammatory activation of vestibular neurons (33, 36).
Older age is a well-recognized risk factor for BPPV recurrence and has been consistently noted in most studies of BPPV. As age progresses, demineralization occurs, leading to the breakdown, fragmentation, and detachment of utricular otoconia, which in turn causes BPPV (37). Another possible reason is that vestibular function deteriorates with age, leading to an abnormal dynamic balance between the production and absorption of otoliths. Additionally, after repositioning, otolith fragments may remain in the ear, further delaying the adaptation of the central system (38). Furthermore, the increased recurrence rate in elderly patients may be attributed to decreased daily activity levels, restricted mobility, fatigue, and a higher incidence of falls (39–41). However, whether age is an independent prognostic factor remains controversial. Some studies (42, 43) have found that BPPV recurrence is not associated with age, whereas others (15, 39) indicate that the recurrence rate of BPPV increases with advancing age. Large-scale studies have demonstrated that patients aged over 40 (44) or 50 years (40), particularly those in their sixth (7) or seventh decade of life (4), are more likely to experience recurrence. Piccioti et al. (15) demonstrated that individuals older than 65 years have a 1.6 times higher risk of BPPV recurrence than those younger than 65 years. They also highlighted that the presence of comorbidities, such as hypertension, diabetes, and vascular diseases, might contribute to increased recurrence rates among elderly patients.
Migraine is strongly associated with various forms of vertigo, including BPPV. A recent survey indicated that BPPV is twice as common in individuals with migraines (45). Additionally, a recent meta-analysis of the existing literature identified migraine as a factor that predisposes individuals to BPPV recurrences (46). Our study also found that migraine (OR, 2.862; p = 0.041) was associated with an increased risk of BPPV recurrence. The pathophysiological link between migraines and BPPV is not fully understood. Previous studies have confirmed that repeated vasospasms can affect the microvasculature of the inner ear, leading to vascular damage and, consequently, the recurrence of BPPV (47, 48). Furthermore, vasospasm-induced suppression of the inner ear microvasculature may result in cochlear symptoms, such as hearing disturbances and vestibular symptoms (49).
This study found that diabetes played a significant role among the factors contributing to BPPV recurrence (OR = 5.261; p < 0.001). This conclusion is consistent with those of many previous studies (50–52). Chronic hyperglycemia can lead to microvascular damage and histopathological changes in peripheral neuropathy, which negatively impacts the blood supply to the inner ear via terminal branches, thereby affecting the vestibular function of patients. Additionally, glucose can enter the endolymph, altering the pH and changing the solubility of otoconia, leading to abnormal metabolism and detachment of utricular otoconia, which in turn results in disease recurrence (4, 53). However, other studies have found no association between diabetes and the recurrence of BPPV (28, 41, 54). Therefore, further studies are required to determine the effects of diabetes on BPPV recurrence.
This study also identified UA as an independent risk factor for BPPV recurrence, which is consistent with the conclusions of a previous meta-analysis (55). UA has unique oxidative and antioxidative properties and possibly acts as a novel inflammatory factor involved in oxidative stress, inflammation, and metabolic processes. Elevated serum uric acid levels may trigger inflammation in the otoconial gelatinous matrix, release inflammatory mediators, induce the production of reactive oxygen species (ROS), and damage the vascular system. These processes, in turn, impair endothelial function and blood supply to the inner ear, leading to BPPV recurrence (55, 56). In addition, research indicates that higher levels of uric acid entering the lymphatic fluid can lower the pH of the endolymph, thereby preventing the dissolution of otoconia fragments, which in turn may trigger BPPV (57). However, this study also indicated that HCY could increase the risk of BPPV recurrence. As an intermediate product of methionine metabolism, HCY primarily damages the vascular endothelium and promotes the participation of vascular smooth muscle cells in coagulation, leading to microcirculatory disorders of the inner ear. High HCY levels can induce atherosclerosis and stenosis in the arteries supplying blood to the inner ear (58, 59), resulting in inner ear ischemia, thereby increasing the risk of BPPV.
It is noteworthy that previous studies (20, 38) have indicated that hypertension, hyperlipidemia and stroke are risk factors for BPPV, while this study confirms that diabetes, hyperuricemia, and hyperhomocysteinemia are also risk factors for the recurrence of BPPV. The mechanisms by which these factors lead to BPPV onset are similar to those of hyperlipidemia, hypertension, and stroke, as they cause narrowing and spasm of cerebral arteries, resulting in vestibular dysfunction that is sensitive to ischemia, ultimately leading to abnormal otolith metabolism and the detachment of otoliths. The differences in study results may be attributed to interactions among sample characteristics, regional characteristics, potential risk factors, and the accessibility of medical resources. Therefore, future research needs to expand the sample size and conduct prospective studies to validate these findings.
Certainly, this study has several limitations that need to be taken into account. First, our data were gathered retrospectively from a single center potentially restricting the statistical robustness of the findings. Additionally, owing to the retrospective design of the study, some admission data were not available. For example, previous study (60) indicated that low vitamin D levels may increase the risk of BPPV recurrence, potentially due to its effect on calcium metabolism, which can impair the synthesis and function of otoconia composed of calcium carbonate, leading to their dislodgement and reformation of BPPV. However, as this study is retrospective, more than half of the patients did not undergo vitamin D level assessment, resulting in significant missing values. Therefore, we chose not to include this variable in the analysis, which constitutes a limitation of this study. This limitation highlights the need for future research to incorporate vitamin D assessment in prospective studies to better understand its role in BPPV recurrence. Second, our model has not been tested on external cohorts. It is essential to evaluate the utility of our new nomogram in future prospective studies and validate it through multicenter investigations. Third, we should consider the probability that a bias in our work may have resulted from the fact that we excluded participants who we were unable to contact by phone, possibly without recurrence, leading to an overestimation of the value. The accuracy of some of our results may be limited by significant heterogeneity or the limited number of included studies. Therefore, further research is needed to confirm these results. Finally, our nomogram was developed using only seven available predictors. Further investigations are required to determine whether expanding the number of variables enhances the nomogram. However, increasing the complexity of a nomogram can reduce its clinical utility. Despite these limitations, we successfully identified seven prognostic factors for BPPV recurrence in the middle-aged and older populations. In future, the findings of this study could serve as a reference for predicting the risk of BPPV recurrence across all age groups.
Conclusion
This study developed a nomogram incorporating demographic traits, vascular risk factors, emotional aspects, and lifestyle behaviors to predict the risk of BPPV recurrence in middle-aged and older adults. Validation demonstrated the accurate and stable predictive performance of the nomogram. This tool aids physicians in making informed treatment decisions to minimize BPPV recurrence.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the First Hospital of Changsha. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the requirement for informed consent was waived in accordance with national regulations and agency guidelines owing to the retrospective nature of our study.
Author contributions
BT: Formal analysis, Investigation, Methodology, Software, Supervision, Writing – original draft. CZ: Conceptualization, Data curation, Writing – original draft. DW: Data curation, Writing – original draft. ML: Data curation, Writing – original draft. YH: Data curation, Writing – original draft. YX: Data curation, Writing – original draft. XY: Conceptualization, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fife, TD, Iverson, DJ, Lempert, T, Furman, JM, Baloh, RW, Tusa, RJ, et al. Practice parameter: therapies for benign paroxysmal positional vertigo (an evidence-based review): report of the quality standards subcommittee of the american academy of neurology. Neurology. (2008) 70:2067–74. doi: 10.1212/01.wnl.0000313378.77444.ac
2. von Brevern, M, Radtke, A, Lezius, F, Feldmann, M, Ziese, T, Lempert, T, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. (2007) 78:710–5. doi: 10.1136/jnnp.2006.100420
3. De Stefano, A, Dispenza, F, Suarez, H, Perez-Fernandez, N, Manrique-Huarte, R, Ban, JH, et al. A multicenter observational study on the role of comorbidities in the recurrent episodes of benign paroxysmal positional vertigo. Auris Nasus Larynx. (2014) 41:31–6. doi: 10.1016/j.anl.2013.07.007
4. Sreenivas, V, Sima, NH, and Philip, S. The role of comorbidities in benign paroxysmal positional vertigo. Ear Nose Throat J. (2021) 100:NP225–30. doi: 10.1177/0145561319878546
5. Dorigueto, RS, Mazzetti, KR, Gabilan, YP, and Ganança, FF. Benign paroxysmal positional vertigo recurrence and persistence. Braz J Otorhinolaryngol. (2009) 75:565–72. doi: 10.1016/s1808-8694(15)30497-3
6. Choi, SJ, Lee, JB, Lim, HJ, Park, HY, Park, K, In, SM, et al. Clinical features of recurrent or persistent benign paroxysmal positional vertigo. Otolaryngology--head and neck surgery: official journal of American Academy of otolaryngology-head and neck. Surgery. (2012) 147:919–24. doi: 10.1177/0194599812454642
7. Brandt, T, Huppert, D, Hecht, J, Karch, C, and Strupp, M. Benign paroxysmal positioning vertigo: a long-term follow-up (6-17 years) of 125 patients. Acta Otolaryngol. (2006) 126:160–3. doi: 10.1080/00016480500280140
8. Pérez, P, Franco, V, Cuesta, P, Aldama, P, Alvarez, MJ, and Méndez, JC. Recurrence of benign paroxysmal positional vertigo. Otol Neurotol. (2012) 33:437–43. doi: 10.1097/MAO.0b013e3182487f78
9. Kao, CL, Cheng, YY, Leu, HB, Chen, TJ, Ma, HI, Chen, JW, et al. Increased risk of ischemic stroke in patients with benign paroxysmal positional vertigo: a 9-year follow-up nationwide population study in Taiwan. Front Aging Neurosci. (2014) 6:108. doi: 10.3389/fnagi.2014.00108
10. Lo, MH, Lin, CL, Chuang, E, Chuang, TY, and Kao, CH. Association of dementia in patients with benign paroxysmal positional vertigo. Acta Neurol Scand. (2017) 135:197–203. doi: 10.1111/ane.12581
11. Liao, WL, Chang, TP, Chen, HJ, and Kao, CH. Benign paroxysmal positional vertigo is associated with an increased risk of fracture: a population-based cohort study. J Orthop Sports Phys Ther. (2015) 45:406–12. doi: 10.2519/jospt.2015.5707
12. Yamanaka, T, Shirota, S, Sawai, Y, Murai, T, Fujita, N, and Hosoi, H. Osteoporosis as a risk factor for the recurrence of benign paroxysmal positional vertigo. Laryngoscope. (2013) 123:2813–6. doi: 10.1002/lary.24099
13. Chen, J, Zhang, S, Cui, K, and Liu, C. Risk factors for benign paroxysmal positional vertigo recurrence: a systematic review and meta-analysis. J Neurol. (2021) 268:4117–27. doi: 10.1007/s00415-020-10175-0
14. Tan, J, Deng, Y, Zhang, T, and Wang, M. Clinical characteristics and treatment outcomes for benign paroxysmal positional vertigo comorbid with hypertension. Acta Otolaryngol. (2017) 137:482–4. doi: 10.1080/00016489.2016.1247985
15. Picciotti, PM, Lucidi, D, De Corso, E, Meucci, D, Sergi, B, and Paludetti, G. Comorbidities and recurrence of benign paroxysmal positional vertigo: personal experience. Int J Audiol. (2016) 55:279–84. doi: 10.3109/14992027.2016.1143981
16. Cahlon, O, Brennan, MF, Jia, X, Qin, LX, Singer, S, and Alektiar, KM. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb-sparing surgery without adjuvant radiation. Ann Surg. (2012) 255:343–7. doi: 10.1097/SLA.0b013e3182367aa7
17. Makkouk, A, Sundaram, V, Chester, C, Chang, S, Colevas, AD, Sunwoo, JB, et al. Characterizing cd137 upregulation on nk cells in patients receiving monoclonal antibody therapy. Ann Oncol. (2017) 28:415–20. doi: 10.1093/annonc/mdw570
18. von Brevern, M, Bertholon, P, Brandt, T, Fife, T, Imai, T, Nuti, D, et al. Benign paroxysmal positional vertigo: diagnostic criteria. J Vestib Res. (2015) 25:105–17. doi: 10.3233/VES-150553
19. Collins, GS, Reitsma, JB, Altman, DG, and Moons, KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (tripod): the tripod statement. Eur Urol. (2015) 67:1142–51. doi: 10.1016/j.eururo.2014.11.025
20. Cao, W, Geng, Y, Chang, J, and Li, F. Risk factors for benign paroxysmal positional vertigo and construction of a nomogram predictive model. Am J Transl Res. (2024) 16:2435–44. doi: 10.62347/DHAJ4799
21. Kitahara, T, Ota, I, Horinaka, A, Ohyama, H, Sakagami, M, Ito, T, et al. Idiopathic benign paroxysmal positional vertigo with persistent vertigo/dizziness sensation is associated with latent canal paresis, endolymphatic hydrops, and osteoporosis. Auris Nasus Larynx. (2019) 46:27–33. doi: 10.1016/j.anl.2018.05.010
22. Sauerbrei, W, Royston, P, and Binder, H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. (2007) 26:5512–28. doi: 10.1002/sim.3148
23. Heagerty, PJ, Lumley, T, and Pepe, MS. Time-dependent roc curves for censored survival data and a diagnostic marker. Biometrics. (2000) 56:337–44. doi: 10.1111/j.0006-341X.2000.00337.x
24. Vickers, AJ, Cronin, AM, Elkin, EB, and Gonen, M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. (2008) 8:53. doi: 10.1186/1472-6947-8-53
25. Balachandran, VP, Gonen, M, Smith, JJ, and DeMatteo, RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16:e173–80. doi: 10.1016/S1470-2045(14)71116-7
26. Chen, ZJ, Chang, CH, Hu, LY, Tu, MS, Lu, T, Chen, PM, et al. Increased risk of benign paroxysmal positional vertigo in patients with anxiety disorders: a nationwide population-based retrospective cohort study. BMC Psychiatry. (2016) 16:238. doi: 10.1186/s12888-016-0950-2
27. Hsu, CL, Tsai, SJ, Shen, CC, Lu, T, Hung, YM, and Hu, LY. Risk of benign paroxysmal positional vertigo in patients with depressive disorders: a nationwide population-based cohort study. BMJ Open. (2019) 9:e026936. doi: 10.1136/bmjopen-2018-026936
28. Wei, W, Sayyid, ZN, Ma, X, Wang, T, and Dong, Y. Presence of anxiety and depression symptoms affects the first time treatment efficacy and recurrence of benign paroxysmal positional vertigo. Front Neurol. (2018) 9:178. doi: 10.3389/fneur.2018.00178
29. Yardley, L, and Redfern, MS. Psychological factors influencing recovery from balance disorders. J Anxiety Disord. (2001) 15:107–19. doi: 10.1016/S0887-6185(00)00045-1
30. Zhu, C, Li, Y, Ju, Y, and Zhao, X. Dizziness handicap and anxiety depression among patients with benign paroxysmal positional vertigo and vestibular migraine. Medicine. (2020) 99:e23752. doi: 10.1097/MD.0000000000023752
31. Yardley, L, Luxon, LM, and Haacke, NP. A longitudinal study of symptoms, anxiety and subjective well-being in patients with vertigo. Clin Otolaryngol Allied Sci. (1994) 19:109–16. doi: 10.1111/j.1365-2273.1994.tb01192.x
32. Wang, Y, Xia, F, Wang, W, and Hu, W. Assessment of sleep quality in benign paroxysmal positional vertigo recurrence. Int J Neurosci. (2018) 128:1143–9. doi: 10.1080/00207454.2018.1486835
33. Shih, CP, Wang, CH, Chung, CH, Lin, HC, Chen, HC, Lee, JC, et al. Increased risk of benign paroxysmal positional vertigo in patients with non-apnea sleep disorders: a nationwide, population-based cohort study. J Clin Sleep Med. (2018) 14:2021–9. doi: 10.5664/jcsm.7528
34. Besnard, S, Tighilet, B, Chabbert, C, Hitier, M, Toulouse, J, Le Gall, A, et al. The balance of sleep: role of the vestibular sensory system. Sleep Med Rev. (2018) 42:220–8. doi: 10.1016/j.smrv.2018.09.001
35. Qian, S, Wang, Y, and Zhang, X. Inhibiting histamine signaling ameliorates vertigo induced by sleep deprivation. J Mol Neurosci. (2019) 67:411–7. doi: 10.1007/s12031-018-1244-6
36. Irwin, MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. (2015) 66:143–72. doi: 10.1146/annurev-psych-010213-115205
37. Agrawal, Y, Carey, JP, Della Santina, CC, Schubert, MC, and Minor, LB. Disorders of balance and vestibular function in us adults: data from the national health and nutrition examination survey, 2001-2004. Arch Intern Med. (2009) 169:938–44. doi: 10.1001/archinternmed.2009.66
38. Zhou, C, Ma, C, Li, Y, Zhou, X, Shui, L, and Han, C. Risk factors and a nomogram model for residual symptoms of cured benign paroxysmal positional vertigo. J Int Adv Otol. (2023) 19:523–8. doi: 10.5152/iao.2023.231127
39. Korres, S, Balatsouras, DG, and Ferekidis, E. Prognosis of patients with benign paroxysmal positional vertigo treated with repositioning manoeuvres. J Laryngol Otol. (2006) 120:528–33. doi: 10.1017/S0022215106000958
40. Babac, S, Djeric, D, Petrovic-Lazic, M, Arsovic, N, and Mikic, A. Why do treatment failure and recurrences of benign paroxysmal positional vertigo occur? Otology & neurotology: official publication of the American Otological Society. Otol Neurotol. (2014) 35:1105–10. doi: 10.1097/MAO.0000000000000417
41. Zhu, CT, Zhao, XQ, Ju, Y, Wang, Y, Chen, MM, and Cui, Y. Clinical characteristics and risk factors for the recurrence of benign paroxysmal positional vertigo. Front Neurol. (2019) 10:1190. doi: 10.3389/fneur.2019.01190
42. Kansu, L, Avci, S, Yilmaz, I, and Ozluoglu, LN. Long-term follow-up of patients with posterior canal benign paroxysmal positional vertigo. Acta Otolaryngol. (2010) 130:1009–12. doi: 10.3109/00016481003629333
43. Faralli, M, Ricci, G, Molini, E, Bressi, T, Simoncelli, C, and Frenguelli, A. Paroxysmal positional vertigo: the role of age as a prognostic factor. Acta Otorhinolaryngol Ital. (2006) 26:25–31.
44. Rashad, UM. Long-term follow up after epley's manoeuvre in patients with benign paroxysmal positional vertigo. J Laryngol Otol. (2009) 123:69–74. doi: 10.1017/S0022215108002430
45. Chu, CH, Liu, CJ, Lin, LY, Chen, TJ, and Wang, SJ. Migraine is associated with an increased risk for benign paroxysmal positional vertigo: a nationwide population-based study. J Headache Pain. (2015) 16:62. doi: 10.1186/s10194-015-0547-z
46. Chen, J, Zhao, W, Yue, X, and Zhang, P. Risk factors for the occurrence of benign paroxysmal positional vertigo: a systematic review and meta-analysis. Front Neurol. (2020) 11:506. doi: 10.3389/fneur.2020.00506
47. Ishiyama, A, Jacobson, KM, and Baloh, RW. Migraine and benign positional vertigo. Ann Otol Rhinol Laryngol. (2000) 109:377–80. doi: 10.1177/000348940010900407
48. Bruss, D, Abouzari, M, Sarna, B, Goshtasbi, K, Lee, A, Birkenbeuel, J, et al. Migraine features in patients with recurrent benign paroxysmal positional vertigo. Otol Neurotol. (2021) 42:461–5. doi: 10.1097/MAO.0000000000002976
49. Evans, RW, and Ishiyama, G. Migraine with transient unilateral hearing loss and tinnitus. Headache. (2009) 49:756–8. doi: 10.1111/j.1526-4610.2008.01075.x
50. Webster, G, Sens, PM, Salmito, MC, Cavalcante, JD, Santos, PR, Silva, AL, et al. Hyperinsulinemia and hyperglycemia: risk factors for recurrence of benign paroxysmal positional vertigo. Braz J Otorhinolaryngol. (2015) 81:347–51. doi: 10.1016/j.bjorl.2014.09.008
51. Angeli, RD, Lavinsky, L, and Dolganov, A. Alterations in cochlear function during induced acute hyperinsulinemia in an animal model. Braz J Otorhinolaryngol. (2009) 75:760–4. doi: 10.1016/s1808-8694(15)30530-9
52. Yoda, S, Cureoglu, S, Yildirim-Baylan, M, Morita, N, Fukushima, H, Harada, T, et al. Association between type 1 diabetes mellitus and deposits in the semicircular canals. Otolaryngology--head and neck surgery: official journal of American Academy of otolaryngology-head and neck. Surgery. (2011) 145:458–62. doi: 10.1177/0194599811407610
53. Messina, A, Casani, AP, Manfrin, M, and Guidetti, G. Italian survey on benign paroxysmal positional vertigo. Acta Otorhinolaryngol Ital. (2017) 37:328–35. doi: 10.14639/0392-100X-1121
54. Luryi, AL, Lawrence, J, Bojrab, DI, LaRouere, M, Babu, S, Zappia, J, et al. Recurrence in benign paroxysmal positional vertigo: a large, single-institution study. Otol Neurotol. (2018) 39:622–7. doi: 10.1097/MAO.0000000000001800
55. Yang, X, Yang, B, Wu, M, Wang, F, Huang, X, Li, K, et al. Association between serum uric acid levels and benign paroxysmal positional vertigo: a systematic review and meta-analysis of observational studies. Front Neurol. (2019) 10:91. doi: 10.3389/fneur.2019.00091
56. Ko, J, Kang, HJ, Kim, DA, Kim, MJ, Ryu, ES, Lee, S, et al. Uric acid induced the phenotype transition of vascular endothelial cells via induction of oxidative stress and glycocalyx shedding. FASEB J. (2019) 33:13334–45. doi: 10.1096/fj.201901148R
57. Walther, LE, Blödow, A, Buder, J, and Kniep, R. Principles of calcite dissolution in human and artificial otoconia. PLoS One. (2014) 9:e102516. doi: 10.1371/journal.pone.0102516
58. Faralli, M, Lapenna, R, Giommetti, G, Pellegrino, C, and Ricci, G. Residual dizziness after the first bppv episode: role of otolithic function and of a delayed diagnosis. Eur Arch Otorhinolaryngol. (2016) 273:3157–65. doi: 10.1007/s00405-016-3947-z
59. Zhang, H, Wang, B, Ye, Y, Chen, W, and Song, X. A ratiometric fluorescent probe for simultaneous detection of cys/hcy and gsh. Org Biomol Chem. (2019) 17:9631–5. doi: 10.1039/C9OB01960J
Keywords: risk factors, Benign Paroxysmal Positional Vertigo, nomogram, recurrence, BPPV
Citation: Tang B, Zhang C, Wang D, Luo M, He Y, Xiong Y and Yu X (2024) Development and verification of a nomogram for recurrence risk of Benign Paroxysmal Positional Vertigo in middle-aged and older populations. Front. Neurol. 15:1483233. doi: 10.3389/fneur.2024.1483233
Edited by:
Faisal Karmali, Harvard Medical School, United StatesReviewed by:
Andrea Migliorelli, University Hospital of Ferrara, ItalyAnand K. Bery, The Johns Hopkins Hospital, United States
Copyright © 2024 Tang, Zhang, Wang, Luo, He, Xiong and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojun Yu, eGlhb2p1bnl1NkAxNjMuY29t
 Bo Tang
Bo Tang Chuang Zhang
Chuang Zhang Xiaojun Yu
Xiaojun Yu