- 1Beijing University of Chinese Medicine, Beijing, China
- 2Department of Brain Disease III, Dongfang Hospital Beijing University of Chinese Medicine, Beijing, China
- 3Shaodong People's Hospital, Hunan, China
- 4Department of Geriatrics, Taizhou Central Hospital (Taizhou University Hospital), Zhejiang, China
- 5Department of Neurology, Peking University People’s Hospital, Beijing, China
Background and purpose: Previous studies have shown that human herpes simplex virus (HSV) infection may be associated with the onset of headache or migraine. We aimed to investigate the association between HSV infection and severe headache or migraine.
Materials and methods: The cross-sectional data on 5,730 participants aged 20–49 years were obtained from the 1999–2004 National Health and Nutrition Examination Survey (NHANES). We used weighted logistic regression analysis to assess the association between HSV infection (HSV-1 gG-1 and HSV-2 gG-2) and severe headache or migraine, and performed subgroup analyses.
Results: Our study found that women, higher education, higher body mass index, better family conditions, smoking and alcohol consumption were all associated with severe headaches or migraines. After adjusting for confounding factors such as sex, age, race, and education, HSV-2 (+) was still significantly associated with severe headache or migraine (OR = 1.22, 95%CI:1.03–1.46, p = 0.0443). In subgroup analyses, we found that participants with HSV-1 (−) and HSV-2 (+) were also significantly associated with severe headache or migraine (OR = 1.41, 95%CI:1.04–1.91, p = 0.0281).
Conclusion: HSV-2 gG-2(+) was significantly associated with severe headache or migraine.
1 Introduction
Epidemiological studies show that 52% of people worldwide suffer from general headache, migraine, tension headache and other headache diseases every year (1). As a neurological disorder with a high risk of disability, migraine can directly affect more than 1 billion people worldwide (2). And migraine is the leading cause of disability in women under the age of 50 years (3). The pathogenesis of headache is complicated and the etiology is varied. Several studies have found that certain infectious inflammatory diseases are associated with an increased risk of migraine (4, 5). Napier et al. found that herpes simplex virus (HSV) infection may be related to the pathogenesis of migraine (6). In addition, research by Meineri et al. found that 42% of patients with new daily persistent headache were infected with HSV (7). However, additional research is needed to establish a more definitive relationship between HSV infection and headache or migraine.
HSV is a widely transmitted virus that is often associated with neurological diseases such as herpes simplex encephalitis (HSE) and neonatal herpes. The trigeminal theory is one of the main theories to explain the pathogenesis of migraine (8). The theory is that the onset of migraine involves the activation of the trigeminal vascular system. Specifically, pain production in migraine is associated with neurons in the trigeminal ganglion, which project onto the meninges and emit central axons to reach trigeminal vascular neurons in the spinal nucleus of the trigeminal nerve, leading to peripheral sensitization of the migraine. The axons of secondary trigeminal vascular neurons further project pain signals to the brain stem, hypothalamus, basal ganglia, and multiple nuclei in the thalamus, forming central sensitization. HSV-1 can be latent in the trigeminal ganglion, and when the body’s immune function is reduced, the virus is activated and retrograde along the central branch of the trigeminal nerve to the base of the brain, causing acute encephalitis, usually limited to the temporal and frontal lobes of the orbital part (9). The pathogenesis of neonatal HSV infection involves the virus entering the host through sensory nerve endings, transporting to the posterior root ganglion along the direction of the inverse nerve axon, and incubating there for life (10). It can be seen that HSV can be latent in ganglia through nerve fibers and cause disease when the virus reactivates. We aimed to investigate the link between HSV infection and severe headache or migraine, and to explore the underlying mechanism.
National Health and Nutrition Examination Survey (NHANES) database provides health information on a large population, including epidemiological data on headaches and HSV infections. We conducted a cross-sectional study using the 1999–2004 NHANES database to explore the association between HSV infection and severe headache or migraine.
2 Methods
2.1 Study population
This study is based on a publicly available NHANES database from 1999 to 2004. The purpose of the NHANES project is to assess the health and nutrition status of deinstitutionalized Americans using stratified multistage probabilistic surveys. NHANES collects demographic and in-depth health information through home visits, screenings, and laboratory tests conducted by mobile screening centers (MECs). Based on available data on HSV and severe headache or migraine, 6,790 participants aged 20–49 years were initially included. After we excluded missing or inactive participants for poverty-to-income ratio (PIR), education level, hypertension, diabetes, the body mass index (BMI), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), cardiovascular events (CVD), stroke, and smoking status, 5,730 participants remained. Due to the lack of data on alcohol consumption, we used the “multiple interpolation method” to interpolate the above missing data (11, 12). Detailed information about participant recruitment is shown in Figure 1.
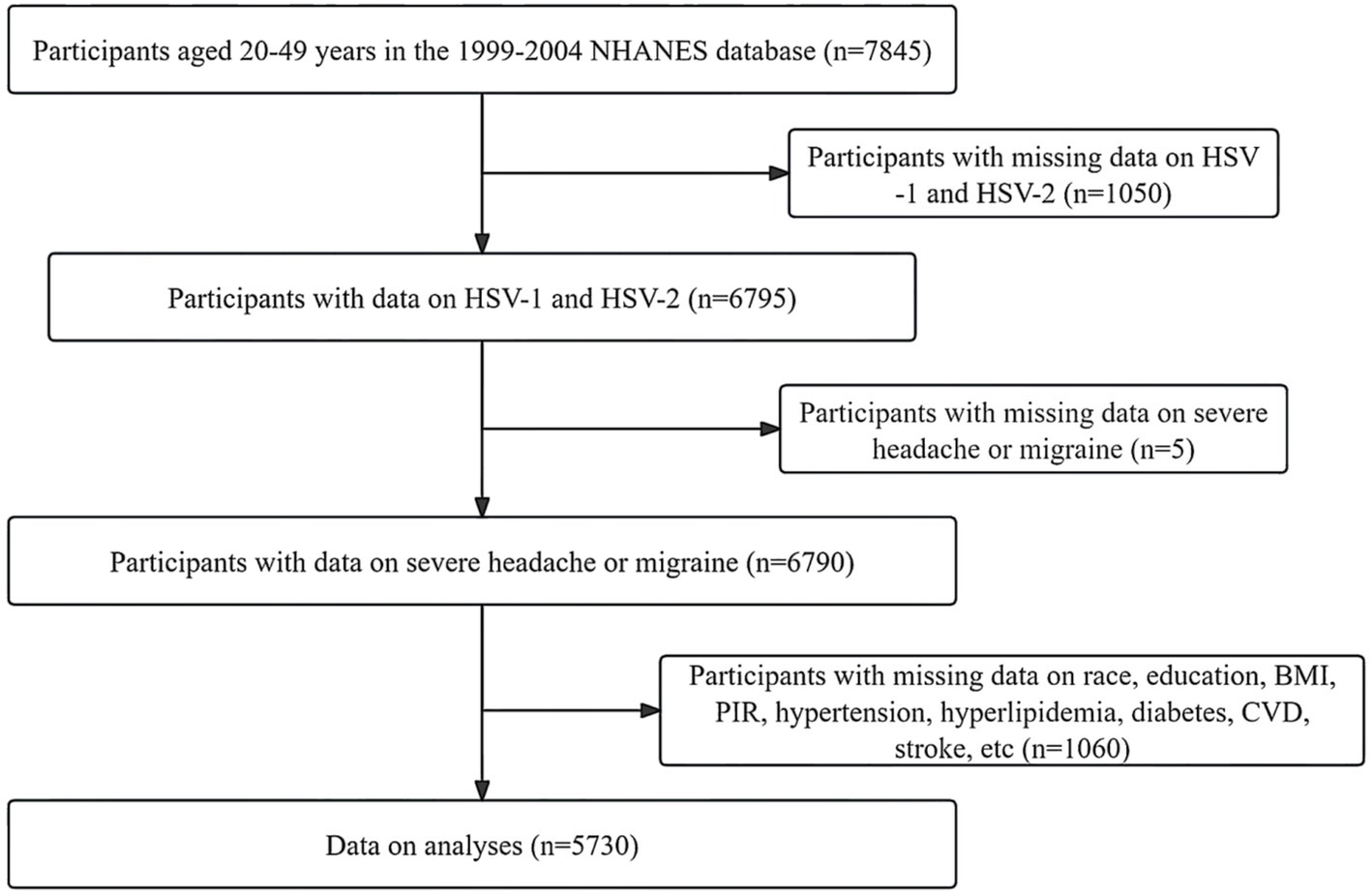
Figure 1. Detailed information about participants recruitment. HSV, human simple virus; BMI, body mass index; CVD, cardiovascular events; PIR, poverty to income ratio.
2.2 HSV infection
According to previous NHANES-based research methods, the determination of HSV infection mainly relies on the detection of HSV-1 and HSV-2 antibodies in participants’ serum by specific glycoproteins G (HSV-1 gG-1 and HSV-2 gG-2). Serum reactive to an immunodot charged with gG-1 indicates the person being tested has HSV-1 infection [HSV-1 (+)]. Serum reactive to an immunodot charged with gG-2 indicates the person being tested has HSV-2 infection [HSV-2 (+)]. According to the HSV infection status of the enrolled participants, they were divided into six types: HSV-1 (+), HSV-2 (+), HSV-1 (−) and HSV-2 (−), HSV-1 (+) and HSV-2 (−), HSV-1 (−) and HSV-2 (+), HSV-1 (+) and HSV-2 (+) (13).
2.3 Definition of severe headache or migraine
Based on the Pain Questionnaire of 1999–2004 NHANES, we defined severe headaches or migraines as: “Have you experienced severe headaches or migraines in the past 3 months?”
2.4 Other covariates
The selection of covariates is based on existing literature and biological factors (13–15). Demographic characteristics include age, sex, education level, race or ethnicity, PIR, smoking status, alcohol consumption, cardiovascular events, stroke, etc. The mean systolic blood pressure (SBP), mean diastolic blood pressure (DBP), and BMI were selected for each participant during the physical examination. In laboratory tests, TC, HDL-C and glycosylated hemoglobin (HBA1c) were selected. We divided education levels into three groups: below high school, high school, and above high school. Race and ethnicity were grouped into five categories: Mexican American, non-Hispanic black, non-Hispanic white, other Hispanic, and other racial groups (14, 15). We classified household income based on PIR as low (PIR ≤ 1.3), medium (1.3 < PIR < 1.85), and high (PIR ≥ 1.85) (15). The smoking status of our participants was divided into three groups: non-smokers, current smokers, and former smokers. Participants who smoked <100 cigarettes in their lifetime were defined as never smoking. We defined previous smoking as having smoked >100 cigarettes in a lifetime, but no longer smoking. Current smoking was defined as smoking >100 cigarettes sometimes or daily in a lifetime. BMI was divided into three subgroups: BMI < 25 kg/m2, 25 kg/m2 ≤ BMI < 30 kg/m2, and BMI ≥ 30 kg/m2 (13). In addition, we defined drinking as “Had at least 12 alcohol drinks per year.” Diabetes is defined as having been told by a doctor that they have diabetes, or having an HbA1c > 6.5%. Hypertension is defined as having been told by a doctor that he or she has high blood pressure, or that SBP ≥ 140 mmHg, or DBP ≥ 90 mmHg. Hyperlipidemia is defined as TC > 5.18 mmol/L, or HDL-C < 1.04 mmol/L in men and < 1.3 mmol/L in women. CVD includes self-reported coronary atherosclerotic heart disease, congestive heart failure, heart attack, and angina pectoris.
2.5 Statistical analyses
We calculated and combined the weights for 1999–2002 and 2003–2004 according to the NHANES analysis Guide. Categorical data is expressed as frequency and weighted percentage, while continuous variables are expressed as mean (standard deviation, SD). For baseline features, we used t-tests or non-parametric tests to analyze the statistical difference of continuous variables, and chi-square tests to analyze the statistical difference of categorical variables. We used weighted univariate and multivariate logistic regression analysis to study the relationship between different HSV infection status and migraine. The regression model was tested by gradually adjusting for potential confounding factors (Models 1–3). Model 1 is a crude model without adjusting any covariates. Model 2 was adjusted for sex, age, race, education level, PIR, and BMI. Model 3 was based on model 2, and then adjusted for confounding factors such as smoking status, alcohol consumption, hypertension, hyperlipidemia, diabetes, CVD and stroke. p < 0.05 was statistically significant. This study is based on the statistical analysis of DecisionLink.1.0.1
3 Results
3.1 Baseline characteristics
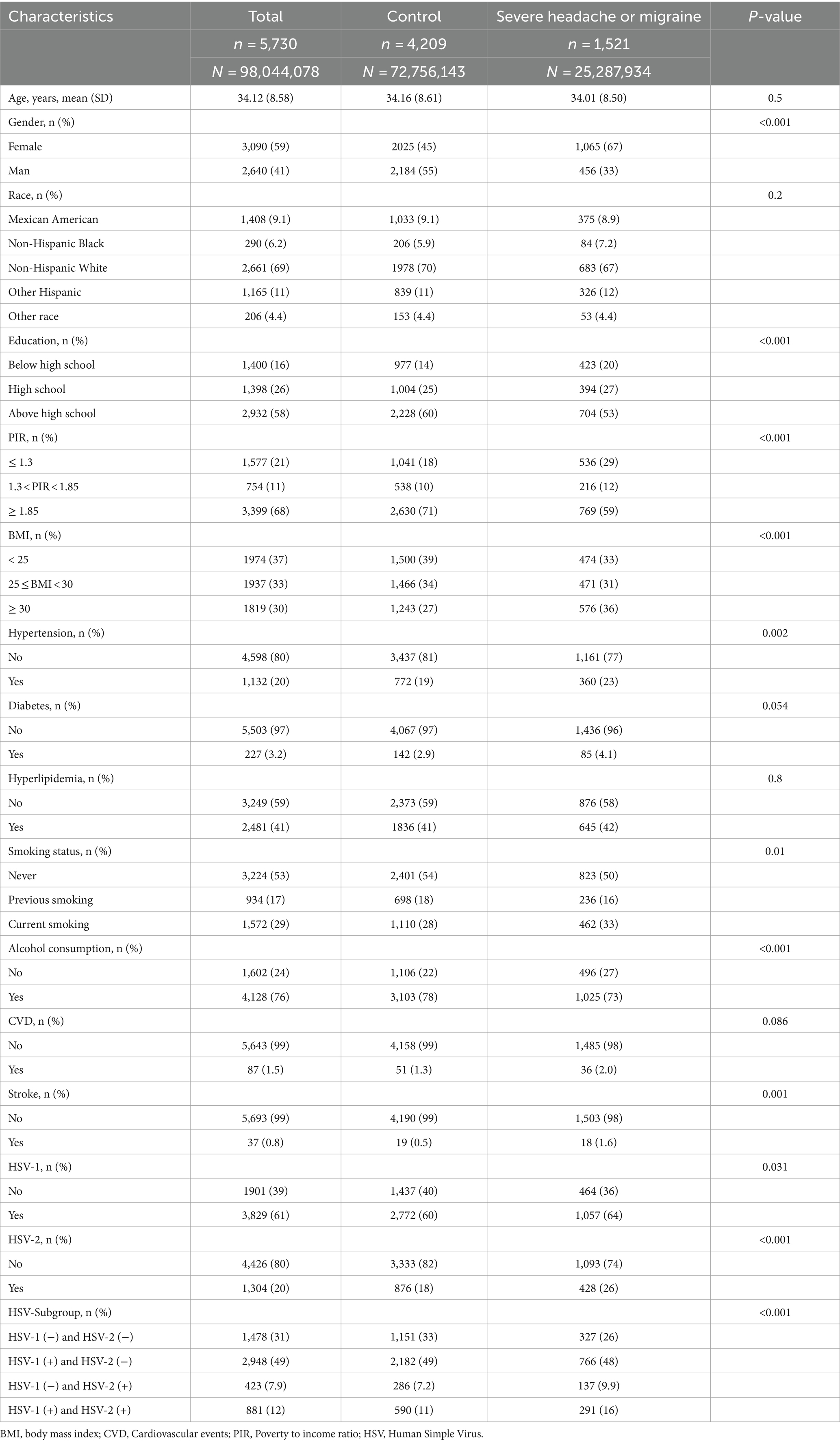
The baseline information of all participants in this study is shown in Table 1. The study included 5,730 participants aged 20–49 years, of whom 1,521 had severe headaches or migraines. The study found that participants with severe headache or migraine were more likely to be female, and participants had higher levels of education, income, and BMI. In addition, the two groups of participants had significant statistical differences in hypertension, smoking status, alcohol consumption, stroke, and HSV infection. The above results were shown in the Table 1.
3.2 Association between HSV infection and severe headache or migraine
In Model 1 (crude model), we found that HSV-1 (+) participants had an 18% higher risk of severe headache or migraine compared to HSV-1 (−) participants, and HSV-2 (+) participants had a 57% higher risk of severe headache or migraine compared to HSV-2 (−) participants. After adjusting for confounding factors such as sex, age, race, education, and BMI, HSV-2(+) was still significantly associated with severe headache or migraine [(Model 2: OR 1.25, 95%CI:1.04–1.51, p-value: 0.0202); (Model 3: OR 1.22, 95%CI:1.03–1.46, p-value: 0.0443)]. We divided the participants into four different subgroups based on HSV-1 and HSV-2 infection, and set the HSV-1 (−) and HSV-2 (−) subgroups as the reference group. In Model 1, we found that participants in the HSV-1 (+) and HSV-2 (−) groups, HSV-1 (−) and HSV-2 (+) groups, and HSV-1 (+) and HSV-2 (+) groups had a 22, 72, and 82% higher risk of severe headache or migraine compared to the reference group, respectively. However, after adjusting for confounding factors, only the HSV-1 (−) and HSV-2 (+) groups were still significantly associated with severe headache or migraine [(Model 2: OR 1.42, 95%CI:1.05–1.92, p-value: 0.0231); (Model 3: OR 1.41, 95%CI:1.04–1.91, p-value: 0.0281)]. All of the above results were shown in Table 2.
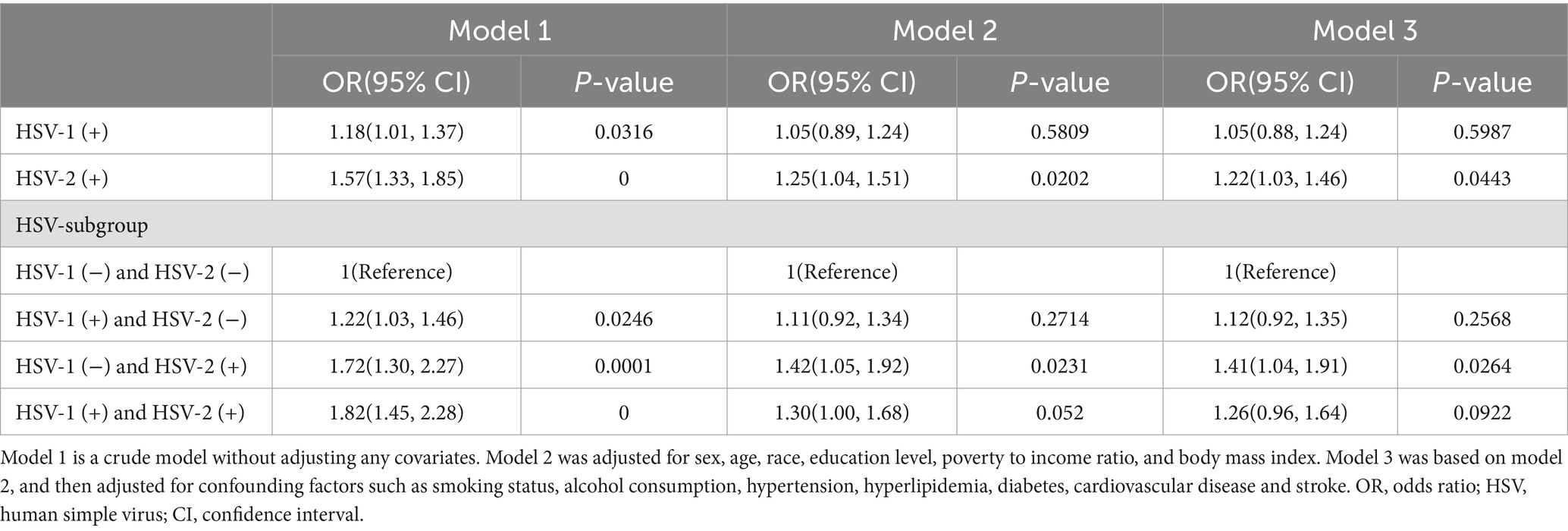
Table 2. Association of HSV infection with severe headache or migraine in participants of the 1999–2004 NHANES survey.
4 Discussion
Our study found that being female, higher education, higher BMI, better home conditions, smoking and alcohol consumption were all associated with severe headache or migraine. The difference in the reported incidence of migraine was greatest between the sexes (women vs. men: 17% vs. 8.6%) (1). It may be related to changes in women’s estrogen levels and greater social pressure (16). A higher BMI is a known risk factor for migraine. Obesity can cause migraines through neuropeptides, inflammatory mediators, adipokines, gut microbiota, and changes in eating behavior and lifestyle (17, 18). Alcohol consumption, caffeine intake and smoking are the most common diet-related triggers for increased frequency of migraine attacks (19). In addition, participants with more education and better home conditions may have faced higher expectations and stress, as well as more environmental stimuli, which are likely to trigger migraines.
This study also found that the headache group and the control group had significant statistical differences in hypertension and stroke. Previous studies have shown that people with migraine have a significantly increased risk of hypertension (20, 21). Genome-wide association studies (GWAS) has found that migraine and blood pressure share common genetic loci, and cross-trait association analysis have revealed potential common biological mechanisms between migraine and blood pressure regulation, such as vascular development, endothelial function, and neurogenic inflammation (22). In addition, there is a lot of evidence that migraines increase the risk of stroke (23). Peng et al. found that patients with migraine had a 24% higher risk of ischemic stroke compared to the control group (24). In Lee’s study, patients with migraine had an 18% higher risk of ischemic stroke (25). The pathological mechanism of migraine leading to stroke may be related to cortical diffusion inhibition, endothelial dysfunction, microembolism, coagulation dysfunction, etc. (26, 27).
At present, there are few studies on HSV infection and migraine. Napier et al. reported that after 1 year of using famciclovir, patients’ migraine symptoms were significantly reduced (6). In their retrospective clinical study, Meineri et al. found that 42% of patients with new daily persistent headaches had recently been infected with HSV (7). Our findings suggest that HSV infection is positively associated with severe headache or migraine, especially HSV-2 infection. In subgroup analyses, we also found that participants with HSV-1 (+) and HSV-2 (+), HSV-1 (−) and HSV-2 (+) had a 50 and 60% higher risk of severe headaches or migraines than participants with HSV-1 (+) and HSV-2 (−), respectively. In addition, after we adjusted for potential confounding factors, only participants with HSV-2 (+), participants with HSV-1 (−) and HSV-2 (+) were also significantly associated with severe headaches or migraines. Both HSV-1 and HSV-2 are known to be latent in the trigeminal ganglion (28, 29). The trigeminal ganglion is a major component of the trigeminal nerve, which plays an important role in the pathogenesis of migraine. The trigeminal ganglion contains neurons that transmit sensations such as pain, touch, and pressure. KCNK18, a migraine susceptibility gene, encodes neuronal potassium channels that are highly enriched in the trigeminal ganglion (30). Previous studies have shown that dominant negative mutations in this gene cause a loss of channel conductance, leading to familial migraine (31, 32). However, it is unclear whether HSV can cause migraines by causing mutations in the KCNK18 gene. HSV infection is closely related to neuroinflammation. When the virus is activated, host immune cells such as microglia and astrocytes are activated, releasing inflammatory mediators such as cytokines and chemokines, exacerbating neuroinflammation (33). Neuroinflammation plays an important role in the pathophysiology of migraine. For example, the activation of inflammatory bodies can lead to the production of inflammatory cytokines, which stimulate trigeminal neurons and cause migraines (34). In the pathophysiology of migraine, neurogenic inflammation and neuroinflammation can affect the dural vessels, trigeminal nerve endings, trigeminal ganglion, trigeminal caudal nucleus, central trigeminal nerve pain processing structures (35). HSV infection may lead to migraine or headache by causing neuroinflammation, but the exact pathophysiological mechanism needs to be further explored.
In Model 1 (crude model), HSV-1 (+) is associated with headaches or migraines. However, after adjusting for potential confounding factors, HSV-1 (+) was not significantly associated with headaches or migraines compared to HSV-2 (+). Both HSV-1 and HSV-2 can cause oral and genital herpes (36, 37). In general, the symptoms of genital herpes are usually more severe than those of oral cold sores. Clinical symptoms of primary genital herpes often include headache, fever, muscle pain, etc. (37). In the past, it was thought that 90% of genital herpes was caused by HSV-2 infection, but with the change of sexual behavior, the proportion of HSV-1 infection caused by oral genital sexual behavior has increased significantly (10% ~ 40%) (38). After genital infection, T cells control the colonization of sensory ganglia by HSV-2 less efficiently than HSV-1 (39). The recurrence frequency and aggressiveness of HSV-2 are also higher than that of HSV-1 (40). HSV-2 is the main cause of recurrent genital herpes infections. Its frequent recurrence, may bring lasting pain to the patient, greater psychological pressure, and thus more likely to induce migraine. Studies have shown that HSV-2 can promote neurite growth by enhancing the activity of nerve growth factor (41, 42). Abnormal growth or regeneration of neurites is associated with neuroinflammation, neurotransmitter release, or abnormal excitability of the nervous system. These may all be related to an overreaction of the central nervous system to pain signals. In addition, although nerve growth factor can improve nerve regeneration, it can also accelerate neuropathic pain (43, 44). Reactivation of HSV-2 may lead to sacral root neuritis. Neuroinflammation caused by sacral root neuritis may spread to the head via nerve pathways, causing headaches. In addition, sacral radicular neuritis can cause chronic pain and may produce significant stress and anxiety, which are also predisposing factors for migraines. Neuroinflammation caused by sacral root neuritis may spread to the head via nerve pathways, causing headaches. In addition, sacral radicular neuritis can cause chronic pain and may produce significant stress and anxiety, which are also predisposing factors for migraines.
There are some limitations to our study. First, although our study accounted for a number of potential confounding factors, we cannot rule out the influence of other factors on the results, such as the overall health of the patients, co-existing infections, or environmental influences. Second, although we found an association between HSV infection and migraine or headache, we cannot be sure whether there is a causal relationship. Third, our study population was participants aged 20–49 years in the United States, and the results may not be applicable to all populations, or other ethnicities.
5 Conclusion
HSV-2 gG-2(+) was significantly associated with severe headache or migraine.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
TZ: Writing – review & editing, Writing – original draft, Supervision, Software, Data curation, Conceptualization. LJ: Writing – original draft, Software, Methodology, Formal analysis. GL: Writing – original draft, Software, Methodology, Data curation. NZ: Writing – original draft, Supervision, Software, Methodology. BY: Writing – original draft, Software, Methodology. SD: Writing – original draft, Software, Investigation, Conceptualization. GW: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition, Conceptualization. ZL: Writing – review & editing, Writing – original draft, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 52073310), Peking University People’s Hospital Talent Introduction Scientific Research Launch Fund (No. 2022-T-02), and the Key Research Projects of Beijing University of Chinese Medicine in 2024 (No. 2024-JYB-JBZD-005).
Acknowledgments
We are grateful to the NHANES database, the FinnGen database, and the GWAS Catalog for supplying data on summary statistics for our study. We are also grateful to all the researchers who shared these data and the study participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Stovner, LJ, Hagen, K, Linde, M, and Steiner, TJ. The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain. (2022) 23:34. doi: 10.1186/s10194-022-01402-2
3. Steiner, TJ, Stovner, LJ, Jensen, R, Uluduz, D, and Katsarava, Z. Lifting the burden: the global campaign against headache. Migraine remains second among the world's causes of disability, and first among young women: findings from GBD2019. J Headache Pain. (2020) 21. doi: 10.1186/s10194-020-01208-0
4. Huang, YK, Yang, LC, Wang, YH, and Chang, YC. Increased risk of migraine in patients with chronic periodontitis: a population-based cohort study. Int J Environ Res Public Health. (2021) 18:1921. doi: 10.3390/ijerph18041921
5. Mohammed, MMA, Almayeef, D, Abbas, D, Ali, M, Haissam, M, Mabrook, R, et al. The association between periodontal disease and chronic migraine: a systematic review. Int Dent J. (2023) 73:481–8. doi: 10.1016/j.identj.2023.04.007
6. Napier, BL, Morimoto, M, and Napier, E. Migraine headache treated with Famciclovir and celecoxib: a case report. Perm J. (2018) 22:17–020. doi: 10.7812/TPP/17-020
7. Meineri, P, Torre, E, Rota, E, and Grasso, E. New daily persistent headache: clinical and serological characteristics in a retrospective study. Neurol Sci. (2004) 25:S281–2. doi: 10.1007/s10072-004-0310-8
8. Iyengar, S, Johnson, KW, Ossipov, MH, and Aurora, SK. CGRP and the trigeminal system in migraine. Headache. (2019) 59:659–81. doi: 10.1111/head.13529
9. Bradshaw, MJ, and Venkatesan, A. Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics. (2016) 13:493–508. doi: 10.1007/s13311-016-0433-7
10. Pinninti, SG, and Kimberlin, DW. Neonatal herpes simplex virus infections. Semin Perinatol. (2018) 42:168–75. doi: 10.1053/j.semperi.2018.02.004
11. Zembic, A, Eckel, N, Stefan, N, Baudry, J, and Schulze, MB. An empirically derived definition of metabolically healthy obesity based on risk of cardiovascular and total mortality. JAMA Netw Open. (2021) 4:e218505. doi: 10.1001/jamanetworkopen.2021.8505
12. Zhang, Q, Xiao, S, Jiao, X, and Shen, Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001-2018. Cardiovasc Diabetol. (2023) 22:279. doi: 10.1186/s12933-023-02030-z
13. Song, Y, Liu, N, and Gao, L. Association between human herpes simplex virus and periodontitis: results from the continuous National Health and Nutrition Examination Survey 2009-2014. BMC Oral Health. (2023) 23:675. doi: 10.1186/s12903-023-03416-x
14. Wang, K, Mao, Y, Lu, M, Ding, Y, Li, Z, Li, Y, et al. Association between migraine and cardiovascular disease: a cross-sectional study. Front Cardiovasc Med. (2022) 9:1044465. doi: 10.3389/fcvm.2022.1044465
15. Liu, X, Liu, R, Liu, W, Hua, R, and Xu, H. Association between oxidative balance score and self-reported severe headache or migraine based on NHANES 1999 to 2004 data: a cross-sectional study. Heliyon. (2024) 10:e27426. doi: 10.1016/j.heliyon.2024.e27426
16. Maasumi, K, Tepper, SJ, and Kriegler, JS. Menstrual migraine and treatment options: review. Headache. (2017) 57:194–208. doi: 10.1111/head.12978
17. Jahromi, SR, Martami, F, Morad Soltani, K, and Togha, M. Migraine and obesity: what is the real direction of their association? Expert Rev Neurother. (2023) 23:75–84. doi: 10.1080/14737175.2023.2173575
18. Peterlin, BL, Rapoport, AM, and Kurth, T. Migraine and obesity: epidemiology, mechanisms, and implications. Headache. (2010) 50:631–48. doi: 10.1111/j.1526-4610.2009.01554.x
19. Hindiyeh, NA, Zhang, N, Farrar, M, Banerjee, P, Lombard, L, and Aurora, SK. The role of diet and nutrition in migraine triggers and treatment: a systematic literature review. Headache. (2020) 60:1300–16. doi: 10.1111/head.13836
20. MacDonald, CJ, el Fatouhi, D, Madika, AL, Fagherazzi, G, Kurth, T, Severi, G, et al. Association of Migraine with Incident Hypertension after Menopause: a longitudinal cohort study. Neurology. (2021) 97:e34–41. doi: 10.1212/WNL.0000000000011986
21. Zhang, J, Mao, Y, Li, Y, Zhao, K, Xie, Q, Wang, K, et al. Association between migraine or severe headache and hypertension among US adults: a cross-sectional study. Nutr Metab Cardiovasc Dis. (2023) 33:350–8. doi: 10.1016/j.numecd.2022.11.014
22. Guo, Y, Rist, PM, Daghlas, I, Giulianini, F, The International Headache Genetics ConsortiumGormley, P, et al. A genome-wide cross-phenotype meta-analysis of the association of blood pressure with migraine. Nat Commun. (2020) 11:3368. doi: 10.1038/s41467-020-17002-0
23. Øie, LR, Kurth, T, Gulati, S, and Dodick, DW. Migraine and risk of stroke. J Neurol Neurosurg Psychiatry. (2020) 91:593–604. doi: 10.1136/jnnp-2018-318254
24. Peng, KP, Chen, YT, Fuh, JL, Tang, CH, and Wang, SJ. Migraine and incidence of ischemic stroke: a nationwide population-based study. Cephalalgia. (2017) 37:327–35. doi: 10.1177/0333102416642602
25. Lee, SY, Lim, JS, Oh, DJ, Kong, IG, and Choi, HG. Risk of ischaemic stroke in patients with migraine: a longitudinal follow-up study using a national sample cohort in South Korea. BMJ Open. (2019) 9:e027701. doi: 10.1136/bmjopen-2018-027701
26. Zhang, Y, Parikh, A, and Qian, S. Migraine and stroke. Stroke Vasc Neurol. (2017) 2:160–7. doi: 10.1136/svn-2017-000077
27. Sacco, S, Harriott, AM, Ayata, C, Ornello, R, Bagur, R, Jimenez-Ruiz, A, et al. Microembolism and other links between migraine and stroke: clinical and pathophysiologic update. Neurology. (2023) 100:716–26. doi: 10.1212/WNL.0000000000201699
28. Held, K, and Derfuss, T. Control of HSV-1 latency in human trigeminal ganglia--current overview. J Neurovirol. (2011) 17:518–27. doi: 10.1007/s13365-011-0063-0
29. Bertke, AS, Ma, A, Margolis, MS, and Margolis, TP. Different mechanisms regulate productive herpes simplex virus 1 (HSV-1) and HSV-2 infections in adult trigeminal neurons. J Virol. (2013) 87:6512–6. doi: 10.1128/JVI.00383-13
30. LaPaglia, DM, Sapio, MR, Burbelo, PD, Thierry-Mieg, J, Thierry-Mieg, D, Raithel, SJ, et al. RNA-Seq investigations of human post-mortem trigeminal ganglia. Cephalalgia. (2018) 38:912–32. doi: 10.1177/0333102417720216
31. Lafrenière, RG, Cader, MZ, Poulin, JF, Andres-Enguix, I, Simoneau, M, Gupta, N, et al. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat Med. (2010) 16:1157–60. doi: 10.1038/nm.2216
32. Andres-Enguix, I, Shang, L, Stansfeld, PJ, Morahan, JM, Sansom, MSP, Lafrenière, RG, et al. Functional analysis of missense variants in the TRESK (KCNK18) K channel. Sci Rep. (2012) 2:237. doi: 10.1038/srep00237
33. Hussain, MS, Gupta, G, Samuel, VP, Almalki, WH, Kazmi, I, Alzarea, SI, et al. Immunopathology of herpes simplex virus-associated neuroinflammation: unveiling the mysteries. Rev Med Virol. (2024) 34:e2491. doi: 10.1002/rmv.2491
34. Kursun, O, Yemisci, M, van den Maagdenberg, AMJM, and Karatas, H. Migraine and neuroinflammation: the inflammasome perspective. J Headache Pain. (2021) 22:55. doi: 10.1186/s10194-021-01271-1
35. Biscetti, L, Cresta, E, Cupini, LM, Calabresi, P, and Sarchielli, P. The putative role of neuroinflammation in the complex pathophysiology of migraine: from bench to bedside. Neurobiol Dis. (2023) 180:106072. doi: 10.1016/j.nbd.2023.106072
36. Aggarwal, R, Bansal, D, and Naru, J HSV-1 as well as HSV-2 is frequent in oral mucosal lesions of children on chemotherapy. Support Care Cancer. (2014) 22:1773–1779. doi: 10.1007/s00520-014-2152-0
37. Gupta, R, Warren, T, and Wald, A. Genital herpes. Lancet. (2007) 370:2127–2137. doi: 10.1016/S0140-6736(07)61908-4
38. Van Wagoner, N, Qushair, F, and Johnston, C. Genital Herpes Infection: Progress and Problems. Infect Dis Clin North Am. (2023) 37:351–367. doi:doi: 10.1016/j.idc.2023.02.011
39. Lee, AG, Scott, JM, and Fabbrizi, MR. T cell response kinetics determines neuroinfection outcomes during murine HSV infection. JCI. Insight. (2020) (5):e134258. doi: 10.1172/jci.insight.134258
40. Zhu, S, and Viejo-Borbolla, A. Pathogenesis and virulence of herpes simplex virus. Virulence. (2021) 12:2670–2702. doi: 10.1080/21505594.2021.1982373
41. Cabrera, JR, Viejo-Borbolla, A, Martinez-Martín, N, Blanco, S, Wandosell, F, and Alcamí, A. Secreted herpes simplex virus-2 glycoprotein G modifies NGF-TrkA signaling to attract free nerve endings to the site of infection. PLoS Pathog. (2015) 11:e1004571. doi: 10.1371/journal.ppat.1004571
42. Kropp, KA, López-Muñoz, AD, and Ritter, B Herpes Simplex Virus 2 Counteracts Neurite Outgrowth Repulsion during Infection in a Nerve Growth Factor-Dependent Manner. J Virol. (2020) 94:e01370. doi: 10.1128/JVI.01370-20
43. Wei, J, Su, W, and Zhao, Y Maresin 1 promotes nerve regeneration and alleviates neuropathic pain after nerve injury. J Neuroinflammation. (2022) 19:32. doi: 10.1186/s12974-022-02405-1
Keywords: herpes simplex virus, severe headache, migraine, cross-sectional study, NHANES
Citation: Zheng T, Jiang L, Li G, Zeng N, Yu B, Duan S, Wang G and Liu Z (2024) Association between human herpes simplex virus and severe headache or migraine among aged 20–49 years: a cross-sectional study. Front. Neurol. 15:1476863. doi: 10.3389/fneur.2024.1476863
Edited by:
Catherine Stika, Northwestern University, United StatesReviewed by:
Andreia Costa, Centro Hospitalar Universitário de São João (CHUSJ), PortugalXianpeng Xu, Hospital of Chengdu University of Traditional Chinese Medicine, China
Copyright © 2024 Zheng, Jiang, Li, Zeng, Yu, Duan, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zunjing Liu, bGl1enVuamluZ0Bwa3UuZWR1LmNu; Gesheng Wang, d2FuZ2dlc2hlbmdAYnVjbS5lZHUuY24=; Shaojie Duan, MTc4MjgwMjE3MUBxcS5jb20=
†These authors have contributed equally to this work
 Tao Zheng
Tao Zheng Li Jiang1,2†
Li Jiang1,2† Guanglu Li
Guanglu Li Shaojie Duan
Shaojie Duan