- 1Neuroscience Center, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- 2Department of Biostatistics, Epidemiology, and Scientific Computing, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 3Neuroscience Center, King Faisal Specialist Hospital, Alfaisal University, Riyadh, Saudi Arabia
- 4Department of Medicine, University of Alberta, Edmonton, AB, Canada
Background: Little is known about the relationship between lipoprotein (a) [Lp(a)] and cerebral white matter hyperintensities (WMH). The aim of the study was to examine if elevated Lp(a) levels are associated with higher burden of WMH.
Methods: We retrospectively investigated associations between Lp(a) and the burden of WMH among patients with confirmed diagnosis of acute ischemic stroke or transient ischemic attacks. WMH burden was assessed using 3-Tesla brain MRI and graded according to the Fazekas score. Multivariable models were generated to determine the contribution of Lp(a) to the presence and extent of WMH.
Results: One hundred and fifty-three patients were included (mean age, 45.9 years; 35.9% women). When the study population was stratified by Lp(a) level into three categories, low (<75 nmol/L), moderate (75 to <125 nmol/L), and high (≥125 nmol/L), the distribution of the three groups was 60.8, 15.0 and 24.2%, respectively. High Lp(a) Level was associated with higher burden of both periventricular WMH and deep WMH compared to the lower level (odds ratio [OR], 4.4; 95% confidence interval [CI], 1.60–12.07; p = 0.004; and OR, 5.6; CI, 1.69–14.7; p = 0.001, respectively).
Conclusion: We show in this cohort of patients that a higher burden of WMH was observed in patients with higher level of Lp(a). Further studies are needed to confirm this observation and assess whether lowering Lp(a) level may be a potential therapeutic target for mitigating the development of WMH.
Introduction
Lipoprotein a [Lp(a)] is a low-density lipoprotein (LDL) particle (1), consisting of apolipoprotein B100, and glycoprotein apolipoprotein (a) (2) and has emerged as an independent risk factor for atherosclerotic cardiovascular disease (3). Proposed mechanisms include its role in foam cell formation, promotion of cholesterol deposition and increased immunological responses (4).
White matter hyperintensities (WMH) of presumed vascular origin increases with age, hypertension, diabetes and other vascular risk factors (5) and is associated with an increased risk of dementia, stroke, worse post-stroke outcomes and higher mortality. There is limited data regarding the association between Lp(a), WMH, and stroke.
In this study, we report the association between Lp(a) and WMH in patients with acute ischemic stroke or transient ischemic attacks (TIAs).
Methods
Study population
This study was approved by the institutional review board of King Faisal Specialist Hospital and Research Center in Riyadh, Saudi Arabia, number (DNS/952/45). A retrospective analysis was conducted on data obtained from 153 patients admitted to the comprehensive neurovascular unit between January 2023 and March 2024, following a diagnosis of acute ischemic stroke or transient ischemic attacks. LP(a) and high sensitivity C-reactive protein (hsCRP) were measured during their hospital admission. The Lp(a) levels were evaluated as low (<75 nmol/L), moderate (75 to <125 nmol/L), and high (≥125 nmol/L).
MRI acquisition and assessment
Brain MRI scans were acquired using a 3-Tesla MRI scanner (insert scanner model and manufacturer) with a standard head coil. The imaging protocol included T1-weighted, T2-weighted, DWI and FLAIR with slice thickness of 5 mm. MRI images were independently reviewed by two stroke neurologists blinded to the clinical information. Periventricular WMH (PVWMH) and deep WMH (DWMH) burden were assessed separately on FLAIR and T2WI sequences and were graded according to the Fazekas scale into none, mild, moderate, and severe (6).
Statistical analysis
Quantitative variables were summarized as mean (SD) and median (interquartile range) and were compared with one-way ANOVA, whereas qualitative variables were summarized as frequencies and percentages and were compared with Chi square. The effects of the independent variables on Fazekas were investigated using univariate and stepwise multivariable logistic regression. p-value <0.05 was considered significant. Relationships among the different Lp(a) and hsCRP were determined using the Pearson product moment correlation coefficient (r). Stata 18 was used for the analysis.
Results
One hundred and fifty-three patients were included (mean age, 45.9 years; 35.9% women). There was no significant difference in the demographics as shown in Table 1. The etiology of stroke was distributed as follows, large artery atherosclerosis accounted for 35%, small vessel disease comprised 34%, cardioembolic strokes were 9.8%, strokes of other determined etiology were 6.5%, and strokes of undetermined etiology made up 15%. The mean NIHSS score was 9. Patients stratified by Lp(a) into three categories, low (<75 nmol/L), moderate (75 to <125 nmol/L) and high (≥125 nmol/L). The distribution of Lp(a) in the three groups was 60.8, 15.0 and 24.2%, respectively. The hsCRP levels and Fazekas scores (periventricular and deep) were significantly higher with increased Lp(a) (p = 0.015. and p = <0.001 respectively).
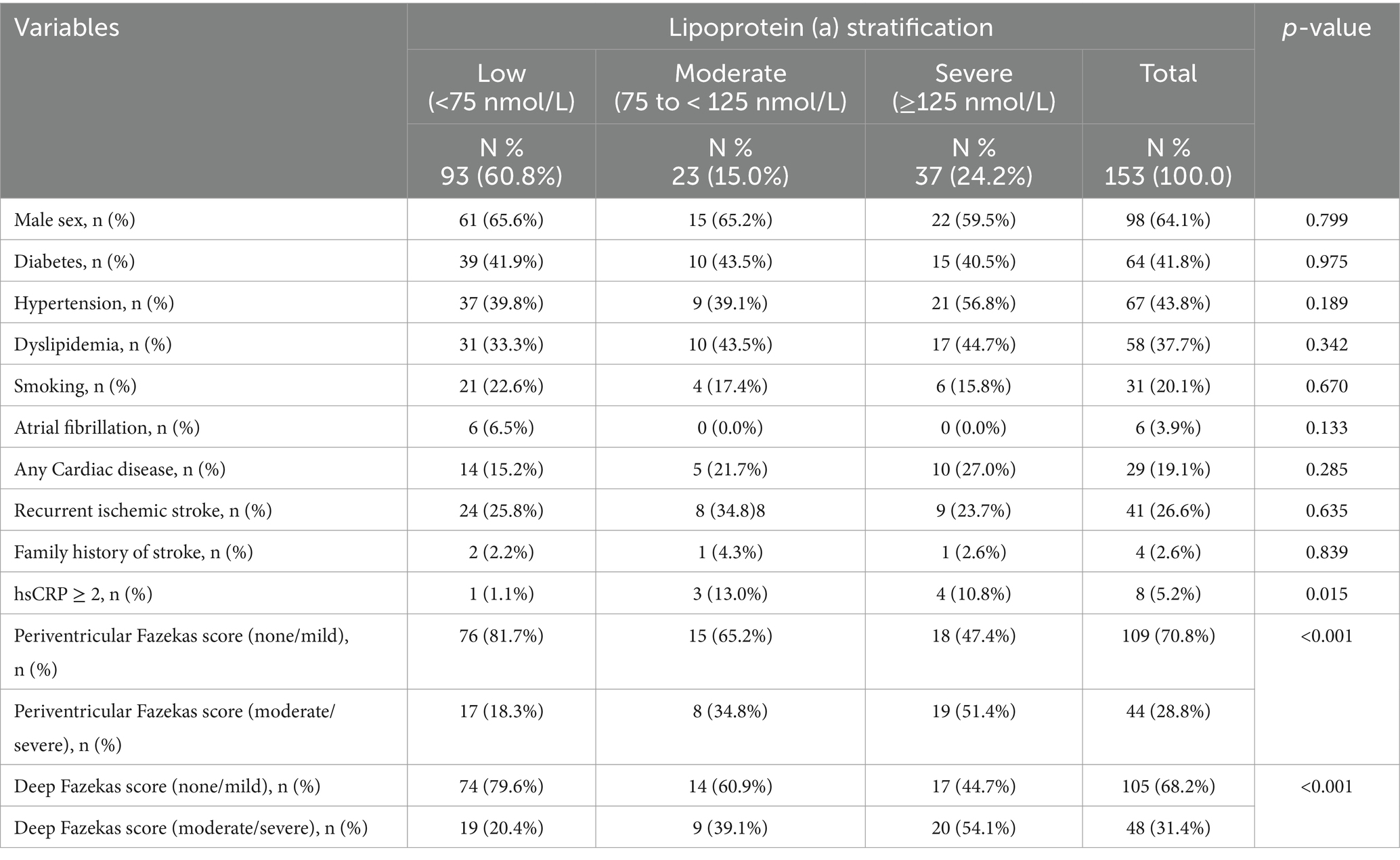
Table 1. Baseline characteristics of the study cohort stratified according to the lipoprotein a levels.
Univariate logistic regression analysis revealed that high Lp(a) level was significantly associated with higher score of PVWMH (OR = 4.7, 95% CI:2.05–10.84, p < 0.001) and DWMH (OR = 4.6, 95% CI:2.01–10.40, p = <0.001), hsCRP level ≥ 2 was associated with higher Fazekas scores (OR = 4.5, 95% CI: 1.03–19.85, p < 0.045) (Figures 1A,B).

Figure 1. Forest plots showing the results of univariate and multivariable logistic regression analysis for WMH burden. (A,B) Forest plots for univariate analysis for periventricular WMH and deep WMH, respectively. (C,D) Forest plots for multivariate analysis for periventricular WMH and deep WMH, respectively.
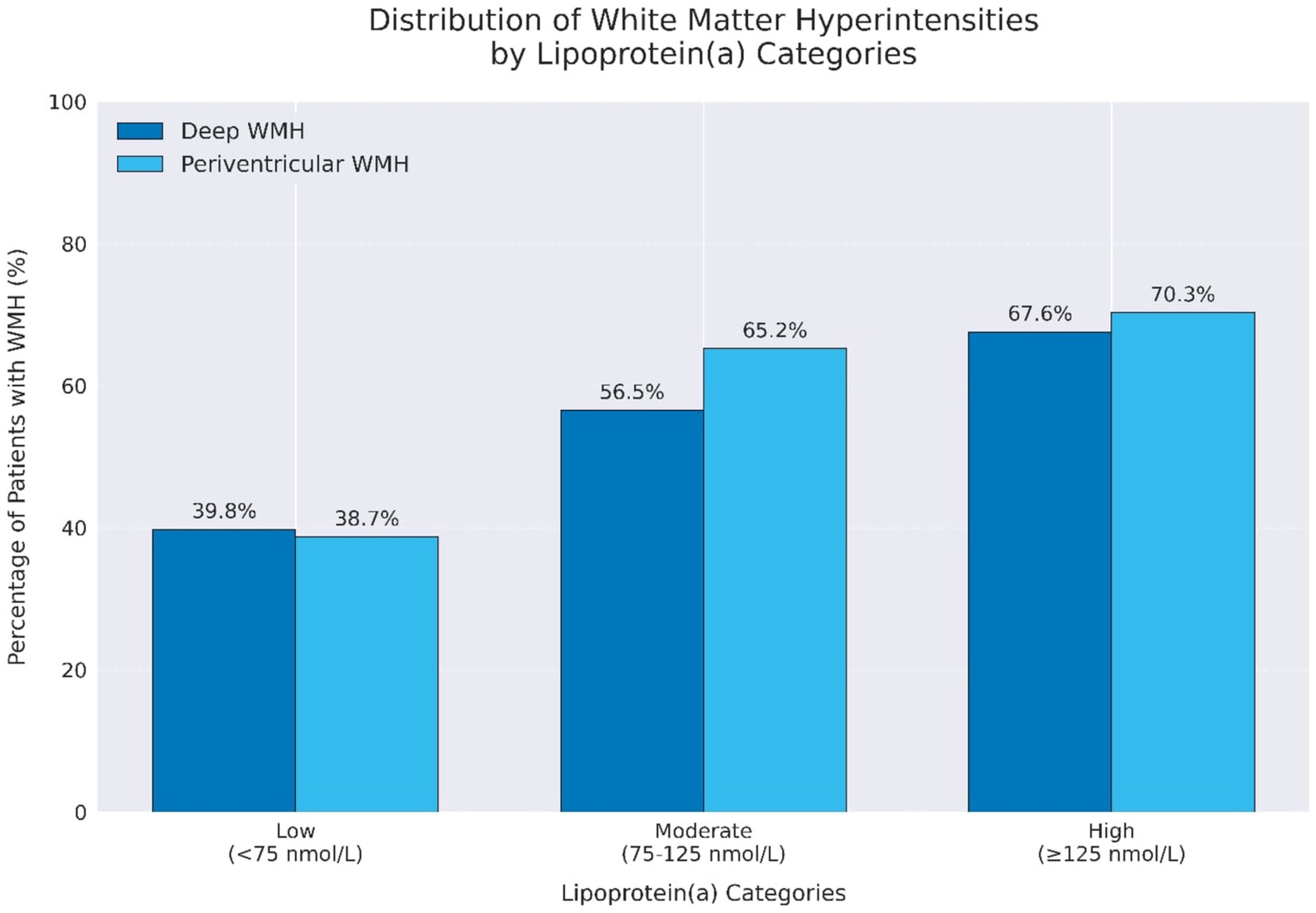
Stepwise multivariable logistic regression analysis showed that high Lp(a) remained a significant predictor of higher Fazekas scores for both PVWMH (OR = 4.4, 95% CI: 1.6–12.07, p = 0.004) and DWMH (OR = 5.4, 95% CI: 1.96–14.70, p = 0.001) (Figures 1C,D). The odds ratio for moderate and high Fazekas score for PVWMH and DWMH are illustrated in Forest plots (Figures 1A,B, 2). There was an increase in the percentage of WMH with moderate and high scores as Lp(a), increasing from 18.3% in the low group to 52.6% in the high group for PVWMH scores and from 20.4 to 55.3% for DWMT scores (Figure 2). We observed low correlations between the Lp(a) level and hsCRP (r = 0.369) (Supplementary Figure S1).
Discussion
Numerous studies have investigated the relationship between high Lp(a) and cardiovascular disease and stroke (7). Lp(a) is a risk factor for stroke (8) and is associated with an increased risk of recurrent vascular events in patients with acute stroke (9). Our study adds two important observations between Lp(a) and the burden of WMH. Firstly, we found a positive association between increasing Lp(a) levels and higher burden of WMH. After excluding the six patients with atrial fibrillation from the low LP(a) group, the analysis still shows a statistically significant association between LP(a) levels and the presence of WMH (p = 0.0108). Secondly, similar to reports in cardiac diseases, we observed an association between the impact of Lp(a) and the hsCRP level.
The potential mechanisms underlying the association between Lp(a) and cerebral WMH are likely multifactorial, including enhanced inflammation, accelerated atherosclerotic plaque formation and embolization, leading to cerebral microvascular occlusion and ischemic injury (10). The increase in WMH with higher Lp(a) observed in our study suggests that microvascular damage precedes the onset of symptomatic stroke. The higher levels of hsCRP observed in the patients with higher Lp(a) might point to inflammation contributing to the WMH.
Limitation of the study is that recruiting patients in the acute stroke phase may introduce selection bias, as it excludes patients with different types of strokes who do not present to the hospital under a similar setting. Further limitation is that this is a cohort study, and we cannot draw conclusions regarding causality. The relationship between higher Lp(a) and high hsCRP is intriguing. However, our sample size was small and requires confirmation from prospective studies whether this may have a synergistic impact on WMH.
In conclusion, to our knowledge, this is the first stroke cohort study using 3-Tesla MRI to systematically assess the burden of periventricular and deep WMH stratified by Lp(a) levels. We show a higher burden of WMH in patients with higher level of Lp(a). Further studies are needed to confirm this observation and assess whether lowering Lp(a) level may be a potential therapeutic target for mitigating the development of WMH.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the King Faisal Specialist Hospital Ethical Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FA: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GM: Data curation, Formal analysis, Software, Writing – review & editing. SB: Data curation, Writing – review & editing. MAlq: Conceptualization, Data curation, Writing – review & editing. HB: Conceptualization, Investigation, Writing – review & editing. AmA: Conceptualization, Methodology, Writing – review & editing. AbA: Writing – review & editing. MAlZ: Writing – review & editing. AdA: Writing – review & editing. AS: Writing – original draft. FA-A: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be considered as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1476005/full#supplementary-material
Abbreviations
WMH, white matter hyperintensities; PVWMH, periventricular white matter hyperintensities; DWMH, deep white matter hyperintensities; Lp(a), lipoprotein (a); hsCRP, high sensitivity C-reactive protein; FLAIR, fluid attenuated inversion recovery; T2WI, T2 weighted image.
References
1. Liu, Y, Yuan, C, Chen, X, Fang, X, Hao, J, Zhou, M, et al. Association of Plasma Lipids with white matter Hyperintensities in patients with acute ischemic stroke. Int J Gen Med. (2023) 16:5405–15. doi: 10.2147/IJGM.S440655
2. Pan, Y, Cai, X, Jing, J, Wang, S, Meng, X, Mei, L, et al. Differential associations of lipoprotein(a) level with cerebral large artery and small vessel diseases. Stroke Vasc Neurol. (2022) 7:534–40. doi: 10.1136/svn-2022-001625
3. Berman, AN, Biery, DW, Besser, SA, Singh, A, Shiyovich, A, Weber, BN, et al. Lipoprotein(a) and major adverse cardiovascular events in patients with or without baseline atherosclerotic cardiovascular disease. J Am Coll Cardiol. (2024) 83:873–86. doi: 10.1016/j.jacc.2023.12.031
4. Kamstrup, PR. Lipoprotein(a) and cardiovascular disease. Clin Chem. (2021) 67:154–66. doi: 10.1093/clinchem/hvaa247
5. Wardlaw, JM, Smith, EE, Biessels, GJ, Cordonnier, C, Fazekas, F, Frayne, R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. (2013) 12:822–38. doi: 10.1016/S1474-4422(13)70124-8
6. Fazekas, F, Chawluk, JB, Alavi, A, Hurtig, HI, and Zimmerman, RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. (1987) 149:351–6. doi: 10.2214/ajr.149.2.351
7. Nissen, SE, Wolski, K, Cho, L, Nicholls, SJ, Kastelein, BG, et al. Lipoprotein(a) levels in a global population with established atherosclerotic cardiovascular disease. Open Heart. (2022) 9:e002060. doi: 10.1136/openhrt-2022-002060
8. Arora, P, Kalra, R, Callas, PW, Alexander, KS, Zakai, NA, Wadley, V, et al. Lipoprotein(a) and risk of ischemic stroke in the REGARDS study. Arterioscler Thromb Vasc Biol. (2019) 39:810–8. doi: 10.1161/ATVBAHA.118.311857
9. Lange, KS, Nave, AH, Liman, TG, Grittner, U, Endres, M, and Ebinger, M. Lipoprotein (a) levels and recurrent vascular events after first ischemic stroke. Stroke. (2017) 48:36–42. doi: 10.1161/STROKEAHA.116.014436
Keywords: cerebrovascular disease, stroke, white matter disease, genetics, lipoprotein (a), C-reactive protein
Citation: Alotaibi FF, Mohamed G, Bakry SS, Alqahtani M, BinAmir H, AlKawi A, Alreshaid AA, AlZawahmaha M, Alhazzani A, Shuaib A and Al-Ajlan FS (2024) Association of white matter hyperintensities with lipoprotein (a) levels: insights from a cohort study. Front. Neurol. 15:1476005. doi: 10.3389/fneur.2024.1476005
Edited by:
Nicola Rifino, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Ming-Chun Yu, Creighton University, United StatesAmrou Sarraj, University Hospitals of Cleveland, United States
Copyright © 2024 Alotaibi, Mohamed, Bakry, Alqahtani, BinAmir, AlKawi, Alreshaid, AlZawahmaha, Alhazzani, Shuaib and Al-Ajlan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahad S. Al-Ajlan, ZmFsYWpsYW45N0BrZnNocmMuZWR1LnNh
 Fawaz F. Alotaibi
Fawaz F. Alotaibi Gamal Mohamed2
Gamal Mohamed2 Hussain BinAmir
Hussain BinAmir Ammar AlKawi
Ammar AlKawi Ashfaq Shuaib
Ashfaq Shuaib Fahad S. Al-Ajlan
Fahad S. Al-Ajlan