
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 30 October 2024
Sec. Stroke
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1463162
Background and purpose: Physical activity (PA) and sedentary behavior are key targets for secondary stroke prevention, yet their characteristics and contributing factors are not well understood. This study aims to explore PA and sedentary behavior in individuals' post-stroke or transient ischemic attack (TIA) and identify factors linked to low PA (≤5,000 steps/day) and prolonged sedentary time (≥8 h/day).
Methods: A cross-sectional study comparing sensor-derived (activPAL) PA and sedentary time among community-dwelling individuals post stroke or TIA residing in diverse geographical regions of Sweden. Multiple logistic regression models were performed to determine potential factors associated with low PA and prolonged sedentary time.
Results: The study included 101 participants post-stroke (n = 68) and TIA (n = 33), with a mean age of 70.5 years (65% female), mostly with no or mild disability (91%), living in metropolitan (69%) and rural (31%) areas of Sweden. Most participants (72%) had ≥ 8 h of sedentary time per day and 38% performed ≤5,000 steps per day. Using a walking aid (OR = 11.43, p = 0.002) was independently associated with low PA, whereas contextual factors; living alone (OR = 3.49, p = 0.029) and living in metropolitan areas (OR = 2.79, p = 0.036), were associated with prolonged sedentary time.
Discussion and conclusions: In this study encompassing people post stroke or TIA from diverse geographical regions across Sweden, PA was associated with mobility status whereas sedentary behavior was associated with contextual factors. The results also showed a large variation in PA highlighting the need for tailored strategies to promote PA post stroke or TIA.
Stroke is one of the leading causes of mortality and disability globally (1, 2), with mild strokes being the most prevalent type, accounting for about 60% of all strokes (3, 4). People with mild stroke or transient ischemic attack (TIA) may exhibit a significant burden of functional disability and fatigue with an increased risk of mortality remaining years after the onset of stroke or TIA (3, 5). Individuals who suffered a first mild stroke or TIA have a 26% increased risk of recurrent stroke within 5 years (6) and the second stroke is often more fatal and disabling (7). Secondary stroke prevention targets include addressing vascular risk factors, such as blood pressure control, and promoting a healthy lifestyle regarding diet, alcohol consumption, and physical activity (PA) (8).
Physical activity (i.e., any bodily movement produced by skeletal muscles that requires energy expenditure) and sedentary behavior (i.e., any waking activity characterized by an energy expenditure of ≤ 1.5 metabolic equivalents) (9) represent unique aspects of daily life and are important targets for secondary stroke prevention (10). A frequently used proxy for PA is the number of steps taken per day, while sedentary behavior is often measured by the amount of time spent sitting or lying down. It is important to distinguish between the two behaviors as recent evidence has suggested that individuals who meet the recommended guideline for PA, but still accumulate prolonged sedentary time may still face risk to their metabolic health (11). Moreover, despite sedentary behavior posing similar risks to cardiovascular health, most rehabilitation strategies focus on increasing PA levels, with less emphasis on reducing sedentary behavior (12). Physical activity has also been well recognized to be a modifiable risk factor for secondary stroke (13), where engaging in regular PA after a mild stroke or TIA reduces the 5-year risk of stroke-related disability by 44% and recurrence by 48% (5).
The simplest and most popular form of PA is walking, therefore regular ambulation at a certain intensity and duration are common goals for people post stroke or TIA (14). A recent systematic review has shown that people post stroke took on average 5,535 steps per day, well below that measured in healthy participants (8,388 steps per day) (13). In addition, low PA and increased sedentary time have been linked with elevated blood pressure and increased risk of cardiovascular diseases and all-cause mortality (15). For example, a meta-analysis found that individuals sitting for >8 h per day had a 59% higher mortality rate compared to those that were active for about 60–75 min per day of moderate intensity PA (16). A study by English et al. (1) showed that people with mild stroke spend about 75% (10.9 h/day) of their wake hours sitting. Furthermore, individuals post stroke are reported to spend significantly more time sitting (10.9 vs. 8.2 h/day) (1) compared to healthy individuals. While the growing evidence shows that PA significantly benefits overall health and lowers the risk of recurrent stroke, it is crucial to comprehend how individuals post stroke or TIA engage in PA relative to their sedentary behavior.
Previous studies (4, 17) have shown that higher age, balance impairment, low self-efficacy, and symptoms of fatigue, depression and anxiety are associated with low PA post stroke (4, 17). With regards to sedentary behavior, previous studies have shown increased body mass index, use of a walking an aid, higher stroke severity and less degree of independence in activities in daily living to be associated with greater sedentary behavior post stroke (15). It has been recognized that features of the social and physical environment could act as barriers or facilitators to PA (18, 19). However, few studies have has examined how environmental factors affect PA and sedentary time in people post stroke or TIA (20), with most studies focusing on motor and psychosocial determinants (21). Recent studies have highlighted the significance of social support (e.g., living alone) as a facilitator and the physical environment (e.g., living in a rural area as compared to an urban area) as a barrier to engagement in PA post stroke or TIA (21, 22). Additionally, most previous studies examining PA behavior and factors associated with PA post stroke have included community-dwelling people post stroke, with few investigations including those with TIA (23).
This study aimed to describe and compare sensor-derived PA levels among people post stroke or TIA with different ambulation profiles and sedentary behavior, and to determine factors associated with low physical activity (≤ 5,000 steps per day) and prolonged sedentary time (≥8 h of sitting time per day). By examining these behaviors separately, this study seeks to inform tailored intervention strategies for promoting PA in people post stroke or TIA.
This cross-sectional study used baseline data from a pilot randomized controlled trial investigating the feasibility of a mobile health program for PA in people post stroke or TIA across Sweden (24). This study was approved by the Swedish Ethical Review Authority (dnr. 2020-05062 and 2021-03622). All study participants provided written informed consent before study participation.
People post stroke or TIA were recruited through advertisements at local or regional (Stockholm) and national patient organizations, on the homepage of Karolinska Institutet and social media. The inclusion criteria were clinical diagnosis of stroke or TIA confirmed through a doctor certificate, between ≥ 3 months and 10 years before study enrolment, with the ability to walk short distances indoors with or without a walking aid. The exclusion criteria were severe health conditions (e.g., cardiac conditions, other neurological diseases, and severe arthritis), significant cognitive impairment, neglect or aphasia compromising the ability to give written consent or use a mobile application to complete digital questionaries in the study.
Participant eligibility screening involved a two-step process. First, interested individuals participated in a telephone interview to assess their eligibility based on stroke/TIA diagnosis, living conditions, ambulation status, and mobile app proficiency. Second, participants were granted access to the STAAR mobile application (Stroke Treatment through Active and Accessible Rehabilitation), which facilitated data collection and administered digital surveys for the study. A video call through the app was conducted to verify participants' ability to use the app, follow instructions, and maintain attention. Final inclusion decisions were based on this screening and a doctor's certificate confirming the diagnosis.
By collecting data remotely without requiring physical visits, data was collected from individuals post stroke or TIA from diverse geographical regions across Sweden. This included administering digital questionnaires through a mobile application and assessment of sensor-derived PA. Data collection was conducted during two periods (September–November 2021 and September–November 2022).
Seven digital questionnaires were administered: (1) Demographics (age, sex, level of education, living situations, geographical location of residency, and stroke or TIA diagnosis), (2) Self-perceived impact of stroke or TIA and stroke recovery assessed using the total sum score of the 8 domains and visual analog scale (VAS; 0 = no recovery to 100 = full recovery) of the Stroke Impact Scale, respectively (25). (3) Balance confidence assessed using the sum score of the Activities-Specific Balance Confidence (ABC) scale (0 = no confidence in balance to 100 = complete confidence in performing the activity) which was specifically developed for application in stroke rehabilitation (26), (4) Self-efficacy for exercises assessed using the Swedish version of Exercise for Self-Efficacy scale (0 = no confidence to 90 = completely confident), which has shown high relative reliability and internal consistency for use in measuring exercise self-efficacy in people with neurological diseases (27), (5) Walking ability assessed using the sum score of the Swedish version of the Generic Walk-12 scale (0 = no walking difficulties to 42 = more walking difficulties) (28), (6) Fatigue was assessed using the Fatigue Severity Scale (FSS), a validated scale with demonstrated reliability in people post stroke. Each item is scored on a 7-point Likert scale, ranging from 1 to 7, and the scores are summed to calculate a total mean score. A score greater than 4 indicates fatigue in stroke patients (29), and (7) Depression, anxiety and stress were assessed using the sum scores pertaining to each of the subscales of the Depression Anxiety Stress Scale (DASS) (30). The DASS is a 21-item questionnaire scored using a 4-point Likert scale (0–3). The scale is divided into three subscales, each containing seven items, with individual scoring systems for depression, anxiety, and stress. Scores range from normal (0–9 for depression, 0–7 for anxiety, and 0–14 for stress) to very serious (≥28 for depression, ≥20 for anxiety, and ≥34 for stress) for each subscale, respectively. The DASS has been recommended as a measure for depression, anxiety and stress in clinical populations due to its strong psychometric properties (30). Additionally, the Modified Rankin Scale was used to assess the degree of disability (31).
Physical activity was measured using the activPAL (PAL Technologies Ltd) activity monitor at a sampling frequency of 10 Hz. The activPAL is a small (43 × 23.5 × 5 mm) triaxial accelerometer and inclinometer worn on the thigh using waterproof tape. ActivPAL is reliable in measuring step count, time spent sitting, standing and walking time in different populations (1, 32, 33) and has been recommended for the measurement of PA and sedentary behavior post stroke (34). The monitor was posted to the participants, and they were instructed to wear the device on the non-hemiparetic leg (or dominant leg if no hemiparesis) for 7 consecutive days (24 h/day) and return the device through prepaid postal service.
Physical activity data were downloaded as 15-second epochs, exported as.csv files using the PAL analysis software (v 8.11.8.75). The timestamp data were then used to divide the data into daily segments (midnight to midnight). Participants with ≥3 days and with ≥10 h per day of PA recordings were included in the analysis (35). Physical activity outcomes were average daily steps, walking time, sitting time, number of sit-to-stand transitions, and fragmentation index. The fragmentation index (i.e., number of sitting bouts/total sitting hours) gives insight into whether the participants accumulate their sitting time in many short bouts or a smaller number of longer bouts (36). Since individuals post stroke or TIA may have different physical capacities depending on the level of disability (37, 38), study participants were classified into two PA groups: ≤ 5,000 steps/day and >5,000 steps/day, and two groups based on sedentary behavior: ≥8 h spent sitting/day and < 8 h spent sitting/day (39).
Data were analyzed using IBM SPSS Statistics (v. 24.0) software. Numbers (percentages) and mean (standard deviation) were used to present descriptive data on demographics, disability level, stroke-related variables, balance confidence, self-efficacy for exercise, walking ability, fatigue, symptoms of depression, stress and anxiety, and PA outcomes. Physical activity outcomes were tested for data normality using the Shapiro-Wilk Test and visual inspection of histograms, confirming that the data were normally distributed.
For the first aim, independent t-tests were used to compare the PA outcomes between groups walking ≤ 5,000 steps/day and >5,000 steps/day, and between groups spending ≥8 h per day sedentary and < 8 h per day sedentary.
For the second aim, two multiple logistic regression models were performed. Potential factors associated with low PA (≤ 5,000 steps/day, model 1) and prolonged sedentary time (≥8 h/day, model 2) were first entered one at a time in univariate logistic regression models. Table 1 outlines the factors included and the criteria for categorization used in the logistic regression model. Since there were no reference cut-off points for self-efficacy for exercise and walking ability, we used the median scores. The threshold for including factors in the multivariate logistic analyses was set to an α level P ≤ 0.1. Prior to multivariable analyses, measures of association between covariates were checked by variance inflation statistics and by creating a bivariate matrix for correlations. A variance inflation factor value of < 5 and a Spearman correlation coefficient of < 0.7 was used as a cut-off for the correlation between covariates (3). Factors were subsequently entered into a multivariable logistic regression model with an α level set at P ≤ 0.05 for the identification of independent factors associated with low PA and prolonged sedentary time.
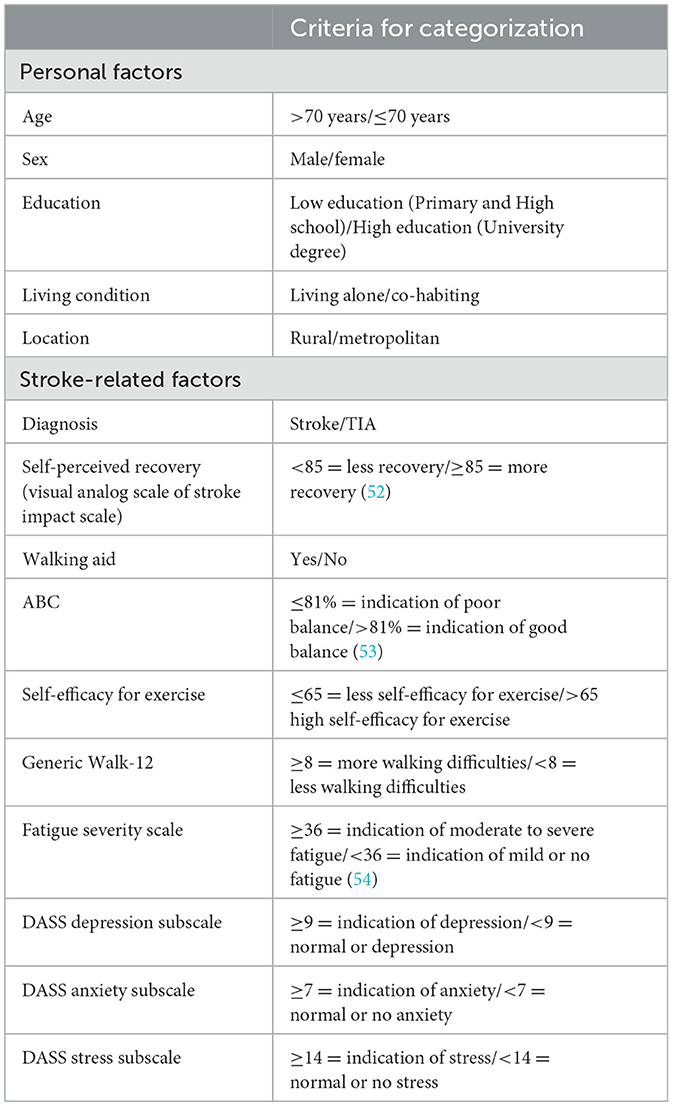
Table 1. Personal and stroke-related factors with the cut-off points used for the logistic regression model.
Out of the 114 participants included in the study, 13 were excluded from the analysis due to technical problems (i.e., battery failure or data downloading/transferring error) with the activPAL monitor (n = 7) or < 3 valid days of PA data (n = 6). The study characteristics of the included participants (n = 101) are presented in Table 2. Participants were recruited from 20 out of the 21 regions in Sweden. The average age of the participants was 71 years, and the majority had a stroke (n = 68, 67%), experienced mild disability (n = 67, 66%), and lived in a metropolitan area (n = 70, 69%). A minority lived alone (n = 38, 38%) and ambulated with a walking aid (n = 18, 18%).
Figure 1 shows the distribution of the number of daily steps in relation to sedentary time of participants post stroke or TIA. The majority had ≥8 h of sitting/day (n = 73, 72%) and 4 out of 10 (n = 38, 38%) performed ≤ 5,000 steps/day. Thirty-three (33%) participants took ≤ 5,000 steps/day and had a sitting time ≥ 8 h, while 40 (40%) of the participants took >5,000 steps/day and had ≥ 8 h/day of sedentary time.

Figure 1. Relationship between the daily sitting time and steps for each individual post stroke. The dashed lines indicate the cut-point for physically active (>5,000 steps) and prolonged sitting (>8 h). The filled dots refer to individuals post stroke with risk behavior (i.e., ≤5,000 steps/day and ≥8 h of sitting/day) of recurrent stroke and disability in the future.
There was a significant difference in all PA outcomes between the groups walking ≤ 5,000 steps/day and >5,000 steps/day (Table 3). The group spending ≥ 8 hours/day sedentary spent less time walking (76 min vs. 102 min, p = 0.002), more time sitting (609 min vs. 409 min, p < 0.001), and had a lower fragmentation index (5.0 vs. 8.0 bouts per sitting hour, p < 0.001) compared to the group spending < 8 h/day sedentary.
The univariate logistic regression models showed that having a stroke (Odds Ratio (OR) = 2.46, p = 0.057), self-perceived recovery score of < 85 (OR = 2.81, p = 0.020), using a walking aid (OR = 12.6, p < 0.001), ABC score ≤ 81.1% (OR = 4.41, p < 0.001), Generic Walk-12 score ≥8 (OR = 3.39, p = 0.005), DASS depression score ≥9 (OR = 2.59, p = 0.031), and DASS stress score ≥14 (OR = 7.59, p = 0.003) were independently associated with walking ≤5,000 steps/day. The multivariate logistic regression model showed that using a walking aid (OR = 11.4, p = 0.002) was the only factor that remained significantly associated with walking ≤5,000 steps/day (Table 4).

Table 4. Univariate and multivariate logistic regression models for factors predicting physical inactivity and prolonged sitting.
The univariate logistic regression model showed that living alone (OR = 4.13, p = 0.009), living in a metropolitan geographical area (OR = 2.45, p = 0.044), and a Generic Walk-12 score of ≥8 (OR = 2.52, p = 0.043) were associated with spending ≥8 h/day sedentary. The multivariate logistic model showed that living alone (OR = 3.49, p = 0.029) and living in a metropolitan geographical area (OR = 2.79, p = 0.036) remained independently associated with sedentary behavior.
The results showed a large variation in PA and sedentary behavior among people post stroke or TIA across diverse regions in Sweden. About one-third of the participants walked ≤5,000 steps/day and spent ≥ 8 h/day sedentary, indicating a risk behavior of recurrent stroke and disability in the future (5). Furthermore, using a walking aid was associated with low PA post stroke or TIA, whereas contextual factors; living alone and living in metropolitan areas, were independently associated with sedentary time.
Contrary to our results, Miller et al. (21) found living alone to be associated with greater daily step counts post stroke (21). In line with the present findings, previous research has suggested that social support for PA (i.e., family members, partners) (40), comfort levels of caregivers (41) and social roles in the household (41) positively influence the PA, that leads to less sedentary behavior in people post stroke. Furthermore, our results showed that living in a metropolitan area was significantly associated with prolonged sedentary time in people post stroke or TIA. This is consistent with previous findings, which suggest that healthy adults in metropolitan areas, according to self-reported and objective data, spend more time sedentary compared to those in rural areas (42, 43). To our knowledge, no previous research has examined the relationship between physical environment (rural vs. metropolitan) and sedentary behavior in individuals post stroke. Our result highlights the need to further explore the role of the environment for promotion of PA behavior in people post stroke or TIA. Previous studies investigating factors associated with PA levels (e.g., steps/day) identified age, balance, self-efficacy, anxiety, depression, and fatigue as contributing factors post stroke/TIA (4, 17). In relation to sedentary behavior (e.g., sitting time) earlier research has indicated that age, functional independence, stroke severity, and walking speed are contributing factors post stroke/TIA (15, 44, 45). This is consistent with the univariate models in our study. However, in the multivariate model, only the use of a walking aid was significantly associated with low PA post stroke or TIA. The differences in the results could be explained by differences in stroke characteristics and the criteria for categorizing the predictors in the regression models.
People with stroke or TIA in this study who were physically active (i.e., >5,000 steps/day) engaged in a greater number of sit-to-stand transitions and fragmentation index compared to those who walked ≤5,000 steps/day. This is consistent with previous studies comparing the number of steps/day and sedentary time between people post stroke with different physical capacities (35). For example, Fini et al. (35) showed that community ambulators people post stroke with a self-selected gait speed ≥0.8 m/s took more steps/day (469 vs. 4,975) and spent less time sedentary (1,239 vs. 1,105 min) compared to those with a gait speed < 0.8 m/s (35). Therefore, the difference in PA behavior observed between the PA groups in our study could be attributed to the ability to ambulate, which directly influences their capacity to engage in daily PA. The PA level among the group walking ≤5,000 steps/day in our study, averaging 3,150 steps per day, mirrors the level of 3,786 steps/day previously identified as indicative of stroke recurrence risk in individuals with minor stroke (46). This indicates the need to increase PA of individuals with ≤5,000 steps/day to reduce the risk of recurrent stroke. Individuals post-stroke or TIA may exhibit varying PA levels based on disability levels (37, 38). Thus, understanding disparities in PA levels and sedentary behavior among subgroups can pinpoint those at elevated risk. Most existing research primarily focuses on describing PA behaviors post stroke. However, it is crucial to acknowledge that while PA and sedentary behavior are related, they are distinct constructs with different associated risks for cardiovascular disease (47). Specifically, individuals post-stroke who engage in adequate PA may still experience adverse cardiovascular effects if they accumulate prolonged periods of sedentary time. Therefore, rehabilitation strategies should not only promote meeting the recommended PA guidelines for people post stroke/TIA but also promote reducing sedentary behavior (i.e., through regular movements and frequent breaks from sitting), as outlined in the international recommendation for secondary stroke prevention (48).
Previous evidence has suggested that irrespective of their previous PA levels, people who have suffered a mild stroke or TIA make no changes to their PA behavior after the stroke onset (49). Contrary to what previous research has shown (13), the stroke participants in our study had a relatively high PA levels, which is comparable to healthy adults (13). High PA level before stroke onset has been linked to reduced stroke severity and improved long-term functional outcomes (50). However, since we have no data on participants' pre-stroke PA levels, we cannot confirm this. Our results are also in line with previous studies (1) indicating most people post stroke are sedentary. In our study, 72% of the individuals post stroke/TIA had prolonged sedentary hours, with a significant difference between the two sitting groups post stroke regarding sitting time, daily walking time and fragmentation index. Our findings also showed that 40% of participants engaged in PA (>5,000 steps/day) and had extended sedentary hours (≥8 h of sitting/day), highlighting the importance of considering PA and sedentary behavior as distinct concepts.
This was a cross-sectional study and therefore we cannot infer causality between independent factors and PA levels and sedentary behavior post stroke or TIA. Second, people with primarily mild stroke or TIA with the ability to ambulate indoors with or without a walking device were recruited from a clinical trial investigating the feasibility of a mobile health intervention for the promotion of PA post stroke or TIA (24). This inadvertently introduced the risk of selection bias of individuals interested in and aware of PA and health promotion. The age of the study sample was approximately 5 years younger than the population (75 years) and the proportion of women (65%) and those with a university degree (63%) was slightly higher (51). Future studies should aim to include a more diverse study sample post stroke/TIA, particularly regarding a greater representation of men, older adults, and diversity in socioeconomic status. Third, all data, except for the PA measures, were self-reported, which may affect the reliability of the outcomes, particularly in cases of cognitive impairments which is common post stroke. The digital format precludes the inclusion of performance-based clinical tests (e.g., walking, balance, and symptom representations). On the other hand, the digital format facilitated the inclusion of a diverse group of community-dwelling people post stroke or TIA from various geographical regions in Sweden with large variability in PA levels and sedentary behavior. Fourth, the use of cut-points to categorize covariates in regression models may have resulted in the decrease of the sensitivity of the data, while continuous covariates could improve model precision. Finally, possible mediation effects between certain covariates in the logistic models may exist, which may influence the significance of these covariates.
In this study encompassing people post stroke or TIA from diverse geographical regions across Sweden, PA was associated with mobility status whereas sedentary behaviors were associated with contextual factors. The results also showed a large variation in PA and sedentary behavior highlighting the importance of tailored strategies for PA promotion post stroke or TIA.
The datasets presented in this article are not readily available because it contains information that could compromise the privacy of the research participants. Requests to access the datasets should be directed to Research Data Office at the Karolinska Institute (cmRvQGtpLnNl).
The studies involving humans were approved by Swedish Ethical Review Authority (dnr. 2020-05062 and 2021-03622). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SH: Conceptualization, Data curation, Writing – review & editing. DM: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Swedish Research Council, Swedish Research Council for Health, Working Life and Welfare, Center for Innovative Medicine (CIMED), Strategic Research Area Health Care Science at Karolinska Institutet, Sweden's Innovation Agency, the Swedish Stroke Association, and NEURO Sweden.
The authors would like to express their sincere gratitude to the study participants and physiotherapists for their valuable time and efforts.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. English C, Healy GN, Coates A, Lewis L, Olds T, Bernhardt J. Sitting and activity time in people with stroke. Phys Ther. (2016) 96:193–201. doi: 10.2522/ptj.20140522
2. Avan A, Hachinski V. Stroke and dementia, leading causes of neurological disability and death, potential for prevention. Alzheimers Dement. (2021) 17:1072–6. doi: 10.1002/alz.12340
3. Darehed D, Reinholdsson M, Viktorisson A, Abzhandadze T, Sunnerhagen KS. Death and ADL dependency after scoring zero on the NIHSS: a swedish retrospective registry-based study. Neurol Clin Pract. (2023) 13:e200186. doi: 10.1212/CPJ.0000000000200186
4. Hamre C, Fure B, Helbostad JL, Wyller TB, Ihle-Hansen H, Vlachos G, et al. Factors associated with level of physical activity after minor stroke. J Stroke Cerebrovasc Dis. (2021) 30:105628. doi: 10.1016/j.jstrokecerebrovasdis.2021.105628
5. Hobeanu C, Lavallee PC, Charles H, Labreuche J, Albers GW, Caplan LR, et al. Risk of subsequent disabling or fatal stroke in patients with transient ischaemic attack or minor ischaemic stroke: an international, prospective cohort study. Lancet Neurol. (2022) 21:889–98. doi: 10.1016/S1474-4422(22)00302-7
6. Flach C, Muruet W, Wolfe CDA, Bhalla A, Douiri A. Risk and secondary prevention of stroke recurrence: a population-base cohort study. Stroke. (2020) 51:2435–44. doi: 10.1161/STROKEAHA.120.028992
7. Skajaa N, Adelborg K, Horvath-Puho E, Rothman KJ, Henderson VW, Thygesen LC, et al. Risks of stroke recurrence and mortality after first and recurrent strokes in denmark: a nationwide registry study. Neurology. (2022) 98:e329–e42. doi: 10.1212/WNL.0000000000013118
8. Hankey GJ. Secondary stroke prevention. Lancet Neurol. (2014) 13:178–94. doi: 10.1016/S1474-4422(13)70255-2
9. Park JH, Moon JH, Kim HJ, Kong MH, Oh YH. Sedentary lifestyle: overview of updated evidence of potential health risks. Korean J Fam Med. (2020) 41:365–73. doi: 10.4082/kjfm.20.0165
10. Saunders DH, Mead GE, Fitzsimons C, Kelly P, van Wijck F, Verschuren O, et al. Interventions for reducing sedentary behaviour in people with stroke. Cochrane Database Syst Rev. (2021) 6:CD012996. doi: 10.1002/14651858.CD012996.pub2
11. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. (2010) 38:105–13. doi: 10.1097/JES.0b013e3181e373a2
12. Pearson N, Braithwaite RE, Biddle SJH, van Sluijs EMF, Atkin AJ. Associations between sedentary behaviour and physical activity in children and adolescents: a meta-analysis. Obes Rev. (2014) 15:666–75. doi: 10.1111/obr.12188
13. Fini NA, Holland AE, Keating J, Simek J, Bernhardt J. How physically active are people following stroke? Systematic review and quantitative synthesis. Phys Ther. (2017) 97:707–17. doi: 10.1093/ptj/pzx038
14. Handlery R, Regan EW, Stewart JC, Pellegrini C, Monroe C, Hainline G, et al. Predictors of daily steps at 1-year poststroke: a secondary analysis of a randomized controlled trial. Stroke. (2021) 52:1768–77. doi: 10.1161/STROKEAHA.121.034249
15. Hendrickx W, Riveros C, Askim T, Bussmann JBJ, Callisaya ML, Chastin SFM, et al. Identifying factors associated with sedentary time after stroke. Secondary analysis of pooled data from nine primary studies. Top Stroke Rehabil. (2019) 26:327–34. doi: 10.1080/10749357.2019.1601419
16. Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. (2016) 388:1302–10. doi: 10.1016/S0140-6736(16)30370-1
17. Thilarajah S, Mentiplay BF, Bower KJ, Tan D, Pua YH, Williams G, et al. Factors associated with post-stroke physical activity: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2018) 99:1876–89. doi: 10.1016/j.apmr.2017.09.117
18. McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. (2006) 63:1011–22. doi: 10.1016/j.socscimed.2006.03.012
19. Rodríguez DA, Brown AL, Troped PJ. Portable global positioning units to complement accelerometry-based physical activity monitors. Med Sci Sport Exer. (2005) 37:S572–S81. doi: 10.1249/01.mss.0000185297.72328.ce
20. Simpson DB, Breslin M, Cumming T, de Zoete S, Gall SL, Schmidt M, et al. Go Home, Sit Less: the impact of home versus hospital rehabilitation environment on activity levels of stroke survivors. Arch Phys Med Rehabil. (2018) 99:2216–21.e1. doi: 10.1016/j.apmr.2018.04.012
21. Miller A, Pohlig RT, Reisman DS. Social and physical environmental factors in daily stepping activity in those with chronic stroke. Top Stroke Rehabil. (2021) 28:161–9. doi: 10.1080/10749357.2020.1803571
22. Miller A, Pohlig RT, Reisman DS. Relationships among environmental variables, physical capacity, balance self-efficacy, and real-world walking activity post-stroke. Neurorehabil Neural Repair. (2022) 36:535–44. doi: 10.1177/15459683221115409
23. Sammut M, Fini N, Haracz K, Nilsson M, English C, Janssen H. Increasing time spent engaging in moderate-to-vigorous physical activity by community-dwelling adults following a transient ischemic attack or non-disabling stroke: a systematic review. Disabil Rehabil. (2022) 44:337–52. doi: 10.1080/09638288.2020.1768599
24. Thurston C, Bezuidenhout L, Humphries S, Johansson S, von Koch L, Hager CK, et al. Mobile health to promote physical activity in people post stroke or transient ischemic attack - study protocol for a feasibility randomised controlled trial. BMC Neurol. (2023) 23:124. doi: 10.1186/s12883-023-03163-0
25. Lin KC, Fu T, Wu CY, Hsieh YW, Chen CL, Lee PC. Psychometric comparisons of the Stroke Impact Scale 3.0 and Stroke-Specific Quality of Life Scale. Qual Life Res. (2010) 19:435–43. doi: 10.1007/s11136-010-9597-5
26. Botner EM, Miller WC, Eng JJ. Measurement properties of the Activities-specific Balance Confidence Scale among individuals with stroke. Disabil Rehabil. (2005) 27:156–63. doi: 10.1080/09638280400008982
27. Ahlstrom I, Hellstrom K, Emtner M, Anens E. Reliability of the Swedish version of the Exercise Self-Efficacy Scale (S-ESES): a test-retest study in adults with neurological disease. Physiother Theory Pract. (2015) 31:194–9. doi: 10.3109/09593985.2014.982776
28. Bladh S, Nilsson MH, Hariz GM, Westergren A, Hobart J, Hagell P. Psychometric performance of a generic walking scale (Walk-12G) in multiple sclerosis and Parkinson's disease. J Neurol. (2012) 259:729–38. doi: 10.1007/s00415-011-6254-z
29. Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep. (2008) 31:1601–7. doi: 10.1093/sleep/31.11.1601
30. Brown TA, Chorpita BF, Korotitsch W, Barlow DH. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav Res Ther. (1997) 35:79–89. doi: 10.1016/S0005-7967(96)00068-X
31. Wilson JTL, Hareendran A, Grant M, Baird T, Schulz UGR, Muir KW, et al. Improving the assessment of outcomes in stroke - Use of a structured interview to assign grades on the modified Rankin Scale. Stroke. (2002) 33:2243–6. doi: 10.1161/01.STR.0000027437.22450.BD
32. Blackwood J, Suzuki R, Webster N, Karczewski H, Ziccardi T, Shah S. Use of activPAL to measure physical activity in community-dwelling older adults: a systematic review. Arch Rehabil Res Clin Transl. (2022) 4:100190. doi: 10.1016/j.arrct.2022.100190
33. Paul L, Brewster S, Wyke S, Gill JM, Alexander G, Dybus A, et al. Physical activity profiles and sedentary behaviour in people following stroke: a cross-sectional study. Disabil Rehabil. (2016) 38:362–7. doi: 10.3109/09638288.2015.1041615
34. Fini NA, Simpson D, Moore SA, Mahendran N, Eng JJ, Borschmann K, et al. How should we measure physical activity after stroke? An international consensus. Int J Stroke. (2023) 18:1132–42. doi: 10.1177/17474930231184108
35. Fini NA, Bernhardt J, Holland AE. Low gait speed is associated with low physical activity and high sedentary time following stroke. Disabil Rehabil. (2021) 43:2001–8. doi: 10.1080/09638288.2019.1691273
36. Alghaeed Z, Reilly JJ, Chastin SF, Martin A, Davies G, Paton JY. The influence of minimum sitting period of the ActivPAL on the measurement of breaks in sitting in young children. PLoS ONE. (2013) 8:e71854. doi: 10.1371/journal.pone.0071854
37. Roos MA, Rudolph KS, Reisman DS. The structure of walking activity in people after stroke compared with older adults without disability: a cross-sectional study. Phys Ther. (2012) 92:1141–7. doi: 10.2522/ptj.20120034
38. Fulk GD, Lopez-Meyer P, Sazonov ES. Characterizing walking activity in people with stroke. In: 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE (2011). p. 5211–4. doi: 10.1109/IEMBS.2011.6091289
39. Mackie P, Weerasekara I, Crowfoot G, Janssen H, Holliday E, Dunstan D, et al. What is the effect of interrupting prolonged sitting with frequent bouts of physical activity or standing on first or recurrent stroke risk factors? A scoping review. PLoS ONE. (2019) 14:e0217981. doi: 10.1371/journal.pone.0217981
40. Damush TM, Plue L, Bakas T, Schmid A, Williams LS. Barriers and facilitators to exercise among stroke survivors. Rehabil Nurs. (2007) 32:253–60.62. doi: 10.1002/j.2048-7940.2007.tb00183.x
41. Outermans J, Pool J, van de Port I, Bakers J, Wittink H. What's keeping people after stroke from walking outdoors to become physically active? A qualitative study, using an integrated biomedical and behavioral theory of functioning and disability. BMC Neurol. (2016) 16:137. doi: 10.1186/s12883-016-0656-6
42. Robertson MC, Song J, Taylor WC, Durand CP, Basen-Engquist KM. Urban-rural differences in aerobic physical activity, muscle strengthening exercise, and screen-time sedentary behavior. J Rural Health. (2018) 34:401–10. doi: 10.1111/jrh.12295
43. Castrillon CIM, Beckenkamp PR, Ferreira ML, Michell JA, de Aguiar Mendes VA, Luscombe GM, et al. Are people in the bush really physically active? A systematic review and meta-analysis of physical activity and sedentary behaviour in rural Australians populations. J Glob Health. (2020) 10:010410. doi: 10.7189/jogh.10.010410
44. Tieges Z, Mead G, Allerhand M, Duncan F, van Wijck F, Fitzsimons C, et al. Sedentary behavior in the first year after stroke: a longitudinal cohort study with objective measures. Arch Phys Med Rehabil. (2015) 96:15–23. doi: 10.1016/j.apmr.2014.08.015
45. English C, Healy GN, Coates A, Lewis LK, Olds T, Bernhardt J. Sitting time and physical activity after stroke: physical ability is only part of the story. Top Stroke Rehabil. (2016) 23:36–42. doi: 10.1179/1945511915Y.0000000009
46. Ushio M, Kanaoka M, Kinoshita Y, Maeno S, Fujita K. Moderate-to-vigorous physical activity and the risk of stroke recurrence in patients with a history of minor ischemic stroke in Japan: a retrospective analysis. Top Stroke Rehabil. (2018) 25:591–8. doi: 10.1080/10749357.2018.1507309
47. Collins AM, Molina-Hidalgo C, Aghjayan SL, Fanning J, Erlenbach ED, Gothe NP, et al. Differentiating the influence of sedentary behavior and physical activity on brain health in late adulthood. Exp Gerontol. (2023) 180:112246. doi: 10.1016/j.exger.2023.112246
48. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. (2021) 52:e364–467. doi: 10.1161/STR.0000000000000375
49. Sammut M, Haracz K, English C, Shakespeare D, Crowfoot G, Nilsson M, et al. Participants' perspective of engaging in a gym-based health service delivered secondary stroke prevention program after TIA or mild stroke. Int J Environ Res Public Health. (2021) 18:11448. doi: 10.3390/ijerph182111448
50. Tanaka H, Kitamura G, Tamura M, Nankaku M, Taniguchi M, Kikuchi T, et al. Pre-stroke physical activity is associated with post-stroke physical activity and sedentary behavior in the acute phase. Sci Rep. (2023) 13:21298. doi: 10.1038/s41598-023-48232-z
51. Riksstroke: The Swedish Stroke Register. Quality of Swedish Stroke Care (2022). Available at: https://www.riksstroke.org/eng (accessed October 9, 2024).
52. Flink M, Lindblom S, von Koch L, Carlsson AC, Ytterberg C. Health literacy is associated with less depression symptoms, higher perceived recovery, higher perceived participation, and walking ability one year after stroke - a cross-sectional study. Top Stroke Rehabil. (2023) 30:865–71. doi: 10.1080/10749357.2023.2178133
53. Beninato M, Portney LG, Sullivan PE. Using the International Classification of Functioning, Disability and Health as a framework to examine the association between falls and clinical assessment tools in people with stroke. Phys Ther. (2009) 89:816–25. doi: 10.2522/ptj.20080160
Keywords: stroke, transient ischemic attack, physical activity, sedentary behavior, environmental factors
Citation: Bezuidenhout L, Humphries S and Moulaee Conradsson D (2024) Physical activity and influencing factors in people post stroke or transient ischemic attack across diverse regions in Sweden. Front. Neurol. 15:1463162. doi: 10.3389/fneur.2024.1463162
Received: 11 July 2024; Accepted: 14 October 2024;
Published: 30 October 2024.
Edited by:
Anna Bersano, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Anna Danielsson, University of Gothenburg, SwedenCopyright © 2024 Bezuidenhout, Humphries and Moulaee Conradsson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucian Bezuidenhout, bHVjaWFuLmJlenVpZGVuaG91dEBraS5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.