- 1Department of Neurology, Tianjin Huanhu Hospital, Tianjin, China
- 2Tianjin Huanhu Hospital, Tianjin, China
Background: Emamectin·chlorfenapyr is a compound comprising chlorfenapyr and emamectin benzoate that is widely used in agriculture. Chlorfenapyr toxicity has been verified in animals; however, its true mechanism and progression in humans remain to be elucidated. Cases of emamectin·chlorfenapyr poisoning are seldom.
Case presentation: We present a case of a 65-year-old female who attempted suicide by consuming 30 g of 9.5% chlorfenapyr and 0.5% emamectin benzoate 14 days before admission to our hospital. Laboratory tests revealed extremely high creatinine kinase levels upon admission. Magnetic resonance imaging revealed diffuse and symmetric T2 hyperintensities in the entire white matter tract of the brain and spinal cord, and cytological smears of the cerebrospinal fluid showed abnormal lymphocyte aggregation. The patient died 19.5 h after admission owing to cardiopulmonary arrest and hyperthermia.
Conclusion: Further research is needed on how to perform flow cytometry in patients with emamectin·chlorfenapyr intoxication, and to elucidate the immunological mechanism underlying the inflammatory response caused by emamectin·chlorfenapyr and provide new insights into antidote development.
Introduction
Emamectin·chlorfenapyr is a formulation that contains 9.5% chlorfenapyr and 0.5% emamectin benzoate (EB). EB (C104H154N2O28) is employed extensively as a broad-spectrum insecticide and pesticide in agriculture (1). It serves as a GABA receptor agonist and modifies the permeability of chloride ions in membranes (2). Chlorfenapyr (C15H11BrClF3N2O), a pyrrole insecticide, is widely used in the cultivation of vegetables and fruits (3). It inhibits the normal oxidative phosphorylation in mitochondria, leading to a decrease in adenosine triphosphate (ATP) and impairing the function of organs that require oxygen (4).
Agriculture serves as a crucial industry pillar in the Asia-Pacific region. The prevalent use of pesticides enhances the accessibility of these toxic substances, which may be ingested either accidentally or intentionally in suicide attempts. Initial clinical symptoms of poisoning are often not immediately apparent. Approximately 7 days post-exposure, toxicity can manifest with delayed onset. Both EB and chlorfenapyr are associated with depression of the nervous system (2). Additionally, common manifestations of chlorfenapyr poisoning include hyperthermia and rhabdomyolysis (5).
Chlorfenapyr exposure, though not necessarily lethal, has resulted in significant mortality in the Asia-Pacific region (4–6) and the United States (7). While the toxicity of chlorfenapyr has been established in animal studies, its exact mechanism and progression in humans remain elusive. This paper describes a fatal incident involving a 65-year-old female who ingested a bottle of emamectin·chlorfenapyr in an attempt to commit suicide. Unlike previous reports on chlorfenapyr and EB poisoning, this case study includes results from cerebrospinal fluid tests and cytological smears.
Case report
A 65-year-old female patient was admitted to our emergency department presenting with urinary incontinence and a 5-day history of progressive weakness and hypoesthesia in the lower extremities. She had a medical history of hypertension, type 2 diabetes mellitus, and depression. 14 days prior to admission, the patient had attempted suicide by ingesting 30 g of 9.5% chlorfenapyr and 0.5% EB.
Electrocardiogram monitoring indicated the following vital signs: body temperature at 37.6°C, heart rate at 73 beats per minute, respiratory rate at 22 breaths per minute, and blood pressure at 140/69 mmHg. Neurological examination revealed motor weakness in both legs (grade 0/grade 0) and diminished response to acupuncture pain below the T8 dermatome. The patient was fully conscious with a Glasgow Coma Scale (GCS) score of E4, V5, M6.
Toxicity screening revealed trace amounts of chlorfenapyr and emamectin benzoate; cholinesterase inhibitors, illicit drugs, and benzodiazepines were not detected. Prior computed tomography of the chest and abdomen conducted in our emergency room showed no significant abnormalities. Laboratory results indicated a markedly elevated creatine kinase level of 2,406 IU/L (normal range: 40–200 IU/L) and mild liver dysfunction, evidenced by an aspartate aminotransferase level of 85 IU/L (normal range: 13–35 IU/L) and an alanine transaminase level of 47 IU/L (normal range: 7–40 IU/L). Routine blood tests disclosed a white blood cell count of 9.93*10^9/L (normal range: 3.5–9.5*10^9/L) and a neutrophil percentage (NEU%) of 82.7% (normal range: 40–75%). The procalcitonin level was slightly abnormal at 0.081 ng/mL (normal range: 0–0.046 ng/mL).
Magnetic resonance imaging (MRI) of the patient’s brain and spine was performed using a 3.0 T unit (MAGNETOM Skyra; Siemens Healthcare GmbH, Erlangen, Germany). The brain MRI exhibited diffuse, bilaterally symmetrical leukoencephalopathy affecting the dentate nucleus of the cerebellum, ventral medulla, bilateral inferior cerebellar peduncles, pons, midbrain, bilateral cerebral peduncles, bilateral corticospinal tracts, corpus callosum, and bilateral parieto-occipital white matter (Figure 1). T2-weighted imaging (T2WI) showed lesions with increased signal intensity (Figure 1A). Diffusion-weighted imaging and an apparent diffusion coefficient map indicated cytotoxic edema and diminished Brownian movement (Figure 1B). The spinal cord was characterized by swollen, hyperintense lesions on T2WI, particularly noted in the cervical spinal cord and conus medullaris (Figure 1C). A lumbar puncture conducted in the emergency room revealed that the cerebrospinal fluid pressure was 240 mmH2O, and the fluid appeared as a yellowish, cloudy liquid containing flocculent material. The total cell count in the cerebrospinal fluid was 6000*10^6/L, with a white blood cell count of 5200*10^6/L and NEU% at 94%. Cerebrospinal fluid analysis yielded the following results: chloride at 114 mmol/L (normal range: 120–132 mmol/L); glucose at 2.37 mmol/L (normal range: 2.5–4.5 mmol/L); lactate at 7.7 mmol/L (normal range: 0.6–2.2 mmol/L); and protein at 1.57 g/L (normal range: 0.15–0.45 g/L). Next-generation sequencing of the cerebrospinal fluid detected no pathogens. Tests for autoimmune encephalitis-related antibodies, demyelinating disease-related antibodies, paraneoplastic nerve syndrome antibodies, cerebrospinal fluid oligoclonal bands, and serum oligoclonal bands were negative. A cytological smear of the cerebrospinal fluid unveiled some lymphocytes clustered around proteinaceous material in a wreath-like configuration (Figures 1D,E).
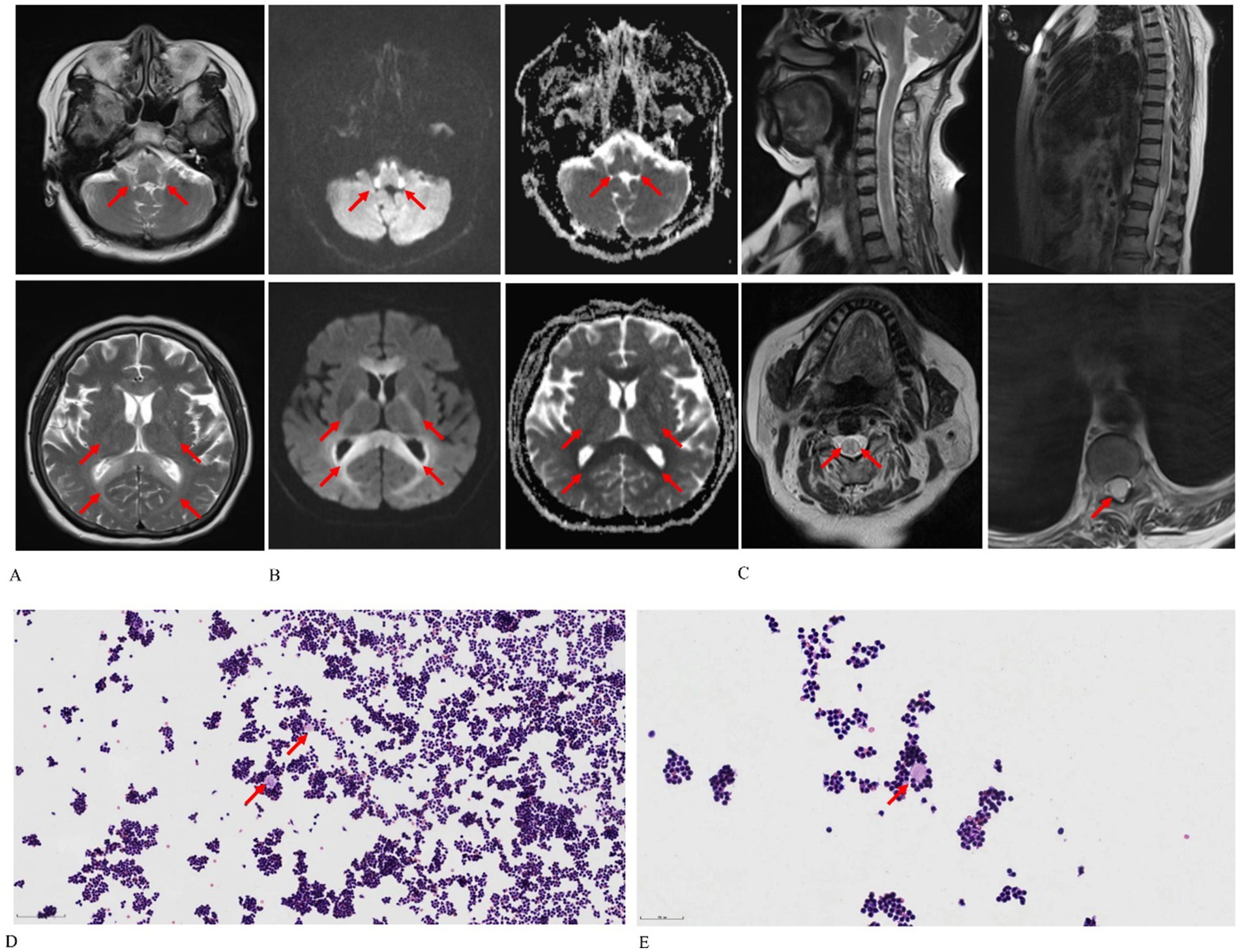
Figure 1. Chlorfenapyr and emamectin benzoate-induced leukoencephalomyelopathy in a 65-year-old female patient. (A) Axial T2-weighted images reveal diffuse and bilaterally symmetrical leukoencephalopathy affecting the dentate nucleus of the cerebellum, ventral medulla, bilateral inferior cerebellar peduncles, pons, midbrain, bilateral cerebral peduncles, bilateral corticospinal tracts, corpus callosum, and bilateral parieto-occipital white matter. (B) Axial diffusion-weighted imaging and apparent diffusion coefficient maps demonstrate cytotoxic edema and reduced Brownian motion. (C) Sagittal T2-weighted spine images display swelling and hyperintensity throughout the spinal cord, particularly in the cervical region and conus medullaris. (D,E) Cytological smears of cerebrospinal fluid [optical microscope: Hematoxylin–eosin stain, 100× (D), 200× (E)] show lymphocytes clustering around protein-like material in a wreath-like formation, as indicated by the red arrow.
Based on the imaging and laboratory findings, toxic leukoencephalomyelopathy was suspected. The patient’s condition deteriorated on the second day, and hyperthermia ensued 16 h post-admission, with body temperatures rising to 39–40°C. Antipyretic medications proved ineffective. The Glasgow Coma Scale (GCS) was assessed at E3, V3, M5, indicating a progressive deterioration in consciousness. 18 h after admission, the patient developed tachycardia (heart rate of 110 beats/min) and tachypnea (respiratory rate of 30 breaths/min) with shallow breathing. Consequently, steroid pulse therapy was initiated. The patient’s percutaneous oxygen saturation fluctuated between 80 and 90%, and a Venturi mask delivering 10 L/min was employed to enhance oxygenation. 19 h post-admission, arterial blood gas analysis revealed hypoxemia (pH 7.31; partial pressure of CO2 50 mmHg; partial pressure of O2 44 mmHg; HCO3 26.5 mmol/L). The patient then entered a deep coma, reflected by a GCS score of E1, V1, M1. Immediate transfer to the intensive care unit was arranged, where tracheal intubation was performed. Unfortunately, the patient succumbed to cardiopulmonary arrest 19.5 h after admission, following the family’s issuance of do-not-resuscitate orders.
Discussion
Reports on the full spectrum of manifestations of emamectin-chlorfenapyr poisoning in humans are scarce. A review of the literature was conducted to identify characteristics of such poisoning, yielding a total of 17 reported cases of emamectin-chlorfenapyr poisoning. The details are presented in Table 1. Among these cases, the mean age of the patients was 46.11 ± 15.82 years, and the average hyperthermal temperature was 40.08 ± 1.46°C. Only three cases in which the patient survived have been documented, resulting in a mortality rate of 84.21%. More than half of the patients died in hospital within 1 day of admission.
Mammalian species exhibit low sensitivity to EB due to the chemical’s minimal affinity for gamma-aminobutyric acid and the relative impermeability of the blood–brain barrier (2). Chlorfenapyr, classified as moderately hazardous, disrupts the conversion of adenosine diphosphate to ATP by targeting mitochondria, thereby halting ATP production and leading to energy depletion, cellular dysfunction, and death (5). Emamectin·chlorfenapyr, a pesticide combination, is formulated to delay resistance development in insect pests and enhance synergistic effects. EB synergistically contributes to oxidative phosphorylation decoupling induced by chlorfenapyr, leading to cell death (8). This mechanism likely underlies the predominant features of emamectin·chlorfenapyr toxicity, which include decreased consciousness, respiratory failure, rhabdomyolysis, and demyelination of the brain and spinal cord, targeting organs with high energy demands.
In previous studies on chlorfenapyr poisoning, the clinical trajectory typically exhibits a latent period of up to 14 days, succeeded by a rapid progression to death (4, 9, 10). Kang et al. (10) postulated that the incubation period for chlorfenapyr toxicity correlates with the time needed for the pro-insecticide to convert into the active toxicant. Similarly, our patient underwent a latent phase of approximately 9 days, during which she displayed no discernible symptoms until the onset of paraplegia. Furthermore, the exact lethal dosage of chlorfenapyr or emamectin·chlorfenapyr remains undetermined, owing to variations in medical settings and individual biochemical characteristics. In a documented survival case (11) following chlorfenapyr ingestion, the patient, who ingested about 10 mL, did not show hyperintensity in the central nervous system and received immediate treatment through early gastrointestinal decontamination, including gastric lavage and whole-bowel irrigation. Conversely, our patient experienced a concealed progression of the condition, with severe damage to her brain and spinal cord upon hospital admission, thus missing the critical window for effective treatment. EB may also contribute synergistically to the lethality of this process.
Other typical clinical manifestations of chlorfenapyr poisoning observed in our patient include rhabdomyolysis and hyperthermia. Delayed hyperthermia, a common observation in cases of chlorfenapyr intoxication, was also noted. In a case report from China, similar to ours, tracheal intubation appeared to hasten clinical decline (12), This deterioration may be associated with pulmonary infections and respiratory muscle failure following tracheal intubation, although the exact mechanisms are yet to be elucidated. Rhabdomyolysis and hyperthermia are also indicative of malignant hyperthermia, a rare condition triggered by certain anesthetics. In this patient, no anesthetics or medications known to induce malignant hyperthermia were administered. It is hypothesized that the lipophilic nature of chlorfenapyr leads to its accumulation in striated muscles, uncoupling oxidative phosphorylation in mitochondria, disrupting ATP synthesis, and inducing cell death, which elevates creatine kinase levels. Consequently, this triggers non-shivering thermogenesis in brown adipose tissue, culminating in hyperthermia (10).
Few cases of chlorfenapyr intoxication in humans that include integrated radiological data have been reported. Tharaknath et al. (9) reported a case of chlorfenapyr poisoning with diffuse and bilateral hyperintensity in the white matter of the brain and spinal cord on T2WI MRI; their case was very similar to ours. Baek et al. (6) reported a case of survival after chlorfenapyr intoxication and found reversible signal changes in the white matter tracts throughout the brain, brainstem, and spinal cord after 75 days of follow-up visits. Other similar radiological features reported by Kang et al. (10) also indicated a low white matter density in the whole brain tissue on computed tomography scans. In a previous animal experiment involving rats with chlorfenapyr poisoning, vacuolar myelopathy and mild myelin sheath swelling were found in a variety of structures ranging from the subcortical areas to the brainstem and spinal cord in neurohistopathological examinations (13); this is consistent with MRI findings in clinical case reports. However, the pathophysiology of the susceptibility of the white matter tracts alone is unknown. We presume that chlorfenapyr tends to invade and accumulate in the myelin sheath owing to its weak lipophilic acid features, and causes myelin disintegration, the presence of which is shown on MRI. In addition, heat-regulating centers in the hypothalamus are believed to be involved in chlorfenapyr cases, causing hyperthermia (13).
A detailed interpretation of why the cerebrospinal fluid had a count of a white blood cell count of 5200*10^6/L and NEU% of 94% could be assumed that toxicant destroyed blood–brain barrier and raised immunologic derangement, which recruiting inflammatory cells. Decidedly few case reports that present cerebrospinal fluid consequences have been published. Baek et al. (6) reported that the cerebrospinal fluid test results of a patient with diffuse abnormal signals in the brain and spinal cord were normal. However, using next-generation sequencing and cytometric bead array, we confirmed that the cerebrospinal fluid of our patient showed a secondary inflammatory process with no pathogenic microorganisms or related antibodies. In the cytological smear of the cerebrospinal fluid, we observed that some lymphocytes surrounded the protein-like material and lined up in a wreath-like pattern. We speculated that the protein-like material may be a type of myelin tissue that has been shed and that contains abundant tralopyril, which is a metabolized form from chlorfenapyr (14). In addition, chlorfenapyr could activate some lymphocyte subsets, which, in turn, could gather around the exogenous substance. Only one case report of methadone intoxication may provide some evidential support of our speculations. Repple et al. (15) performed flow cytometry on the cerebrospinal fluid of methadone-intoxicated patients and found cell composition alterations that were characterized by the transformation of monocytes from the classical (CD14+CD16−) to the nonclassical (CD14+CD16+) and a switch from CD56bright nonkilling cells to CD56dim nonkilling cells. Notably, CD14+CD16+ monocytes and CD56dim nonkilling cells are pro-inflammatory cells that may be involved in delayed encephalopathy (16). These findings from Repple et al. may explain the lymphocyte aggregation observed in the current case, which provides the first reported cerebrospinal fluid findings of chlorfenapyr intoxication.
Pesticide poisoning is a significant global public health issue, resulting in approximately 110,000–168,000 deaths annually, predominantly in Asia. The World Health Organization (WHO) reported that accidental poisonings led to around 350,000 deaths in 2000. A further estimate indicates that in 2012, unintentional poisoning claimed the lives of 193,000 people globally, with 84 percent of these fatalities occurring in low- and middle-income countries (17). Developing nations experience higher rates of suicide via poisoning, notably through pesticides. Conversely, in high-income countries, substances used in suicide attempts include psychotropic drugs, painkillers, antihistamines, antidepressants, psychoactive medications, and sedative hypnotics. It raises concerns that suicide remains an increasingly prevalent “illness.” Pesticide poisoning represents merely one method of suicide; thus, focusing on prevention is substantially more critical than merely addressing the effects. Health systems must continue to provide special attention to the mental health of their citizens.
Limitations in this case reports include that: (1) The precise dosage of emamectin·chlorfenapyr is still unclear as described with 30 g. The reason goes that the pesticide were produced by unqualified manufacturer in Chinese mainland, and the manufacturer did not have production permit, so we could not know the specific dosage form. (2) Chlorfenapyr is metabolized to tralopyril in the body and persists for a prolonged period, but the blood concentration levels observed for tralopyril is unreviewable for the interpretation that blood concentration levels were very low at the time when testing, there is no idea about the accurate information. Although there are deficiencies on clinical observations, the diagnosis is still can be done by comprehensive assessment.
Conclusion
We present a fatal case of leukoencephalomyelopathy induced by emamectin·chlorfenapyr with delayed hyperthermia and rapid deterioration. The latent period from emamectin·chlorfenapyr exposure to paraplegia onset was less than 9 days, and MRI revealed bilateral, symmetric, and diffuse T2 hyperintensity of the entire white matter tract in the brain and spinal cord. In the cytological smear of the cerebrospinal fluid, we observed that some of the lymphocytes gathered in a wreath-like pattern surrounding a protein-like material; this is the first report of cerebrospinal fluid microscopic characteristics in emamectin·chlorfenapyr intoxication. Further research is needed to clarify how to perform flow cytometry on patients with emamectin·chlorfenapyr intoxication, to elucidate the immunological mechanism underlying the inflammatory process caused by emamectin·chlorfenapyr, and to provide new insights into antidote development.
Author contributions
XL: Software, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Investigation, Resources. YY: Conceptualization, Investigation, Writing – review & editing. YZ: Investigation, Supervision, Writing – review & editing. XZ: Validation, Visualization, Writing – review & editing. NZ: Conceptualization, Supervision, Visualization, Writing – review & editing. WY: Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the China Association Against Epilepsy Research Fund (No. CJ-2022-021), and the Tianjin Key Medical Discipline (Specialty) Construction Project (No. TJYXZDXK-052B).
Acknowledgments
This article was submitted as a preprint to Research Square as Li et al. (23).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Campbell, WC, Fisher, MH, Stapley, EO, Albers-Schönberg, G, and Jacob, TA. Ivermectin: a potent new antiparasitic agent. Science. (1983) 221:823–8. doi: 10.1126/science.6308762
2. Yen, TH, and Lin, JL. Acute poisoning with emamectin benzoate. J Toxicol Clin Toxicol. (2004) 42:657–61. doi: 10.1081/CLT-200026968
3. Raghavendra, K, Barik, TK, Sharma, P, Bhatt, RM, Srivastava, HC, Sreehari, U, et al. Chlorfenapyr: a new insecticide with novel mode of action can control pyrethroid resistant malaria vectors. Malar J. (2011) 10:16. doi: 10.1186/1475-2875-10-16
4. Choi, UT, Kang, GH, Jang, YS, Ahn, HC, Seo, JY, and Sohn, YD. Fatality from acute chlorfenapyr poisoning. Clin Toxicol. (2010) 48:458–9. doi: 10.3109/15563651003750074
5. Zhang, S, Deng, Y, and Gao, Y. Malignant hyperthermia-like syndrome in acute chlorfenapyr poisoning - a case report. Heliyon. (2022) 8:e10051. doi: 10.1016/j.heliyon.2022.e10051
6. Baek, BH, Kim, SK, Yoon, W, Heo, TW, Lee, YY, and Kang, HK. Chlorfenapyr-induced toxic leukoencephalopathy with radiologic reversibility: a case report and literature review. Korean J Radiol. (2016) 17:277–80. doi: 10.3348/kjr.2016.17.2.277
7. Chomin, J, Heuser, W, Nogar, J, Ramnarine, M, Stripp, R, and Sud, P. Delayed hyperthermia from chlorfenapyr overdose. Am J Emerg Med. (2018) 36:e1–e2129.e2. doi: 10.1016/j.ajem.2018.05.035
8. Liao, GH, Yu, HY, Bao, LJ, and Cheng, B. Two cases of acute chlorfenapyr poisoning and literature review. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. (2022) 40:212–6. doi: 10.3760/cma.j.cn121094-20210721-00366
9. Tharaknath, VR, Prabhakar, YV, Kumar, KS, and Babu, NK. Clinical and radiological findings in chlorfenapyr poisoning. Ann Indian Acad Neurol. (2013) 16:252–4. doi: 10.4103/0972-2327.112486
10. Kang, C, Kim, DH, Kim, SC, and Kim, DS. A patient fatality following the ingestion of a small amount of chlorfenapyr. J Emerg Trauma Shock. (2014) 7:239–41. doi: 10.4103/0974-2700.136874
11. Ku, JE, Joo, YS, You, JS, Chung, SP, and Lee, HS. A case of survival after chlorfenapyr intoxication with acute pancreatitis. Clin Exp Emerg Med. (2015) 2:63–6. doi: 10.15441/ceem.15.004
12. Gong, Y, Meng, QB, Liu, L, An, YQ, Zhang, R, Sun, YQ, et al. Vigilance against a highly lethal insecticide chlorfenapyr poisoning (report of 4 cases and literature review). Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. (2021) 39:689–93. doi: 10.3760/cma.j.cn121094-20210513-00251
13. Chien, SC, Chien, SC, and Su, YJ. A fatal case of chlorfenapyr poisoning and a review of the literature. J Int Med Res. (2022) 50:3000605221121965. doi: 10.1177/03000605221121965
14. Yu, G, Kan, B, Li, W, and Jian, X. Tralopyril poisoning due to respiratory exposure. Clin Toxicol. (2024) 62:472–5. doi: 10.1080/15563650.2024.2370319
15. Repple, J, Haessner, S, Johnen, A, Landmeyer, NC, Schulte-Mecklenbeck, A, Pawlitzki, M, et al. Intravenous methadone causes acute toxic and delayed inflammatory encephalopathy with persistent neurocognitive impairments. BMC Neurol. (2021) 21:85. doi: 10.1186/s12883-021-02108-9
16. Ziegler-Heitbrock, L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. (2007) 81:584–92. doi: 10.1189/jlb.0806510
17. Albano, GD, Malta, G, La Spina, C, Rifiorito, A, Provenzano, V, Triolo, V, et al. Toxicological findings of self-poisoning suicidal deaths: a systematic review by countries. Toxics. (2022) 10:654. doi: 10.3390/toxics10110654
18. Kwon, JS, Kim, HY, Han, HJ, Kim, JY, and Park, JH. A case of Chlorfenapyr intoxication with central nervous system involvement. J Clin Toxicol. (2012) 2:147. doi: 10.4172/2161-0495.1000147
19. Lee, J, Lee, JH, Baek, JM, Lee, DS, Park, IY, Won, JM, et al. Toxicity from intra-abdominal injection of chlorfenapyr. Case Rep Emerg Med. (2013) 2013:425179. doi: 10.1155/2013/425179
20. Park, SJ, Jung, JU, Kang, YK, Chun, BY, and Son, BJ. Toxic optic neuropathy caused by Chlorfenapyr poisoning. J Korean Ophthalmol Soc. (2018) 59:1097–102. doi: 10.3341/jkos.2018.59.11.1097
21. Han, SK, Yeom, SR, Lee, S-H, Park, SC, Kim, H-B, and Cho, YM. A fatal case of chlorfenapyr poisoning following dermal exposure. Hong Kong J Emerg Med. (2018) 26:375–8. doi: 10.1177/1024907918782065
22. Luo, ZH, Chen, YQ, Lin, JR, and Jiang, WZ. A case report of death from acute emamectin·chlorfenapyr poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. (2020) 38:534–5. doi: 10.3760/cma.j.cn121094-20190805-00328
23. Li, X., Yang, Y., Zhang, Y., Zhang, X., Zhao, N., and Yue, W. (2023). Emamectin·chlorfenapyr-induced fatal leukoencephalomyelopathy with delayed Hyperthermia. Research square [Preprint]. Available at: https://www.researchsquare.com/article/rs-3517798/v1 (Accessed November 13, 2023).
Keywords: emamectin·chlorfenapyr, leukoencephalomyelopathy, hyperthermia, lymphocyte, fatal outcome
Citation: Li X, Yang Y, Zhang Y, Zhang X, Zhao N and Yue W (2024) Emamectin·chlorfenapyr-induced fatal leukoencephalomyelopathy with delayed hyperthermia: insecticide endanger public safety. Front. Neurol. 15:1449728. doi: 10.3389/fneur.2024.1449728
Edited by:
Luis Rafael Moscote-Salazar, Colombian Clinical Research Group in Neurocritical Care, ColombiaReviewed by:
Luis Alberto Camputaro, Specialized Institute “Hospital El Salvador”, El SalvadorYu-Jang Su, Mackay Memorial Hospital, Taiwan
Guangcai Yu, Shandong University, China
Copyright © 2024 Li, Yang, Zhang, Zhang, Zhao and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Yue, eXVlX3dlaTg1OEAyMWNuLmNvbQ==
 Xun Li
Xun Li Yun Yang1
Yun Yang1 Yajing Zhang
Yajing Zhang Wei Yue
Wei Yue