- 1Department of General Medicine, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Traditional Chinese Medicine), Hangzhou, Zhejiang, China
- 2Department of Orthopaedics, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Traditional Chinese Medicine), Hangzhou, Zhejiang, China
- 3State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
Background: Evidence of an association between maternal use of anti-seizure medication (ASM) during pregnancy and the risk of autism spectrum disorder (ASD) or attention-deficit/hyperactivity disorder (ADHD) in children is conflicting. This systematic review and meta-analysis aimed to summarize the relationship between fetal exposure to ASM and the development of ASD or ADHD in offspring.
Methods: A comprehensive literature search was conducted in PubMed and other databases to identify relevant epidemiological studies published from inception until 1 March 2024.
Results: Seven cohort studies were included in the meta-analysis. The results showed that maternal exposure to ASMs during pregnancy was associated with an increased risk of ASD [odds ratio (OR): 2.1, 95% confidence interval (CI): 1.63–2.71; p < 0.001] in the general population. This association became weaker (ASD: OR: 1.38, 95% CI: 1.11–1.73; p = 0.004) when the reference group was mothers with a psychiatric disorder or epilepsy not treated during pregnancy. Furthermore, an increased risk of ADHD was observed when the study data adjusted for drug indications were pooled (OR: 1.43, 95% CI: 1.07–1.92; p = 0.015). In subgroup analyses based on individual ASM use, only exposure to valproate preconception was significantly associated with an increased risk of ASD or ADHD.
Conclusion: The significant association between maternal ASM use during pregnancy and ASD or ADHD in offspring may be partially explained by the drug indication or driven by valproate.
Introduction
The use of anti-seizure medications (ASMs) has increased among pregnant mothers, with an almost three-fold increase seen in the worldwide during the past 12 years (1, 2). ASMs are used mainly for epilepsy during pregnancy; however, the greater number of prescriptions during pregnancy is driven largely by women with indications other than epilepsy, such as mood disorders or pain (1). Exposure to ASMs may lead to unwanted adverse maternal and neonatal outcomes (3); however, discontinuing the drug before or during pregnancy may lead to worsening or recurrence of the disease and thereby increase maternal mortality (4). Therefore, the decision to use an ASM during pregnancy is complex, and their reproductive safety should be understood.
ASMs theoretically pass through the human placenta and disrupt fetal neurodevelopment by changing the dopaminergic system (5). As dopamine receptors play a role in neurodevelopment (6), there are increasing concerns about adverse neurodevelopmental effects in offspring after prenatal exposure to ASMs. Animal studies have demonstrated that in utero exposure to these agents results in an increased risk of neurodevelopmental disorders, such as autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD), in offspring (7). Accordingly, several epidemiological studies (8–14) have investigated the effect of ASMs on ASD or ADHD risk, but the results were inconsistent. In the earliest cohort study, Christensen et al. (8) observed an increased risk of ASD among offspring exposed to valproate but not among those exposed to other ASMs; however, ASD was reportedly associated with exposure to other ASMs during pregnancy in several other studies (10, 12, 13). Prenatal exposure to ASMs increases the risk of ADHD in offspring; however, the results are inconsistent.
Due to the increased use of ASMs during pregnancy, determining the long-term effects of maternal use of ASMs on the neurodevelopment of offspring, including possible effects on the incidence of ASD or ADHD, is important. To date, no systematic review or meta-analysis has been conducted on this topic, so we conducted the present study to assess the association between maternal ASM exposure during pregnancy and the subsequent risk of neurodevelopmental disorders in offspring.
Methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines.
Search strategy
A literature search was conducted of the PubMed and Embase databases on 1 March 2024, combining the following free-text words: (antiepileptic AND anti-seizure AND anticonvulsant) AND (autism OR autistic OR ASD OR attention-deficit/hyperactivity disorder OR ADHD OR attention-deficit disorder OR hyperkinetic disorder) AND (pregnancy OR mothers OR pregnant OR gestational OR prenatal OR perinatal OR gestation).
Inclusion criteria
We analyzed peer-reviewed epidemiological studies that examined the relationship between maternal exposure to ASMs during pregnancy and the subsequent risk of ASD or ADHD in offspring. A study was eligible if it used a cohort or case–control design, included a non-ASM exposure reference group, reported either the relative risk or hazard ratio or odds ratio (OR), and contained data allowing us to estimate risk. We excluded case reports, case series, animal studies, correspondence, editorials, and reviews.
Data extraction and quality assessment
Two reviewers independently extracted the data from the studies using an Excel spreadsheet, and any discrepancies were resolved by a third reviewer. The following data were collected from each study: first author, publication year, study location, study period, data source, information source for ASM exposure, exposure time, outcome measures, and statistical adjustments. Risk of bias and quality were assessed using the Newcastle-Ottawa scale (NOS), which was developed to assess the quality of epidemiological studies (15). This scale scores studies on three dimensions with nine questions relevant to research quality: the selection and comparability of the study groups and the relevance to the exposure (case–control) or outcome (cohort) of interest; all questions were scored as 0 or 1, and studies with scores ≥ 7 were considered to be of high quality.
Outcome measurements
The primary outcome was the risk of ASD or ADHD with ASM use during pregnancy compared with no treatment in the general population. To rule out the potential effect of confounding by indication (bipolar disorder or epilepsy), the risk was assessed by comparing mothers with a clinical indication for taking an ASM to mothers with a clinical indication for not taking an ASM. To identify potential sources of heterogeneity, subgroup analyses were stratified according to the individual ASM.
Statistical analysis
STATA 12.0 software (StataCorp LP, College Station, TX, United States) was utilized for all statistical analyses. Between-study heterogeneity was assessed using the χ2 test and I2 statistic; we considered I2 > 50% or p < 0.05 for the Q-statistic to indicate high heterogeneity (16). Pooled ORs with 95% confidence intervals (CIs) for dichotomous data were calculated using random or fixed-effects models according to the heterogeneity of the studies. We used a random effects model because its assumptions account for high heterogeneity among studies; otherwise, a fixed effects model was used (17). Publication bias was evaluated by Begg’s funnel plot (18). The funnel plot was not used to assess publication bias in analyses of <10 studies (19). We considered p < 0.05 to indicate a significant difference.
Results
Search results
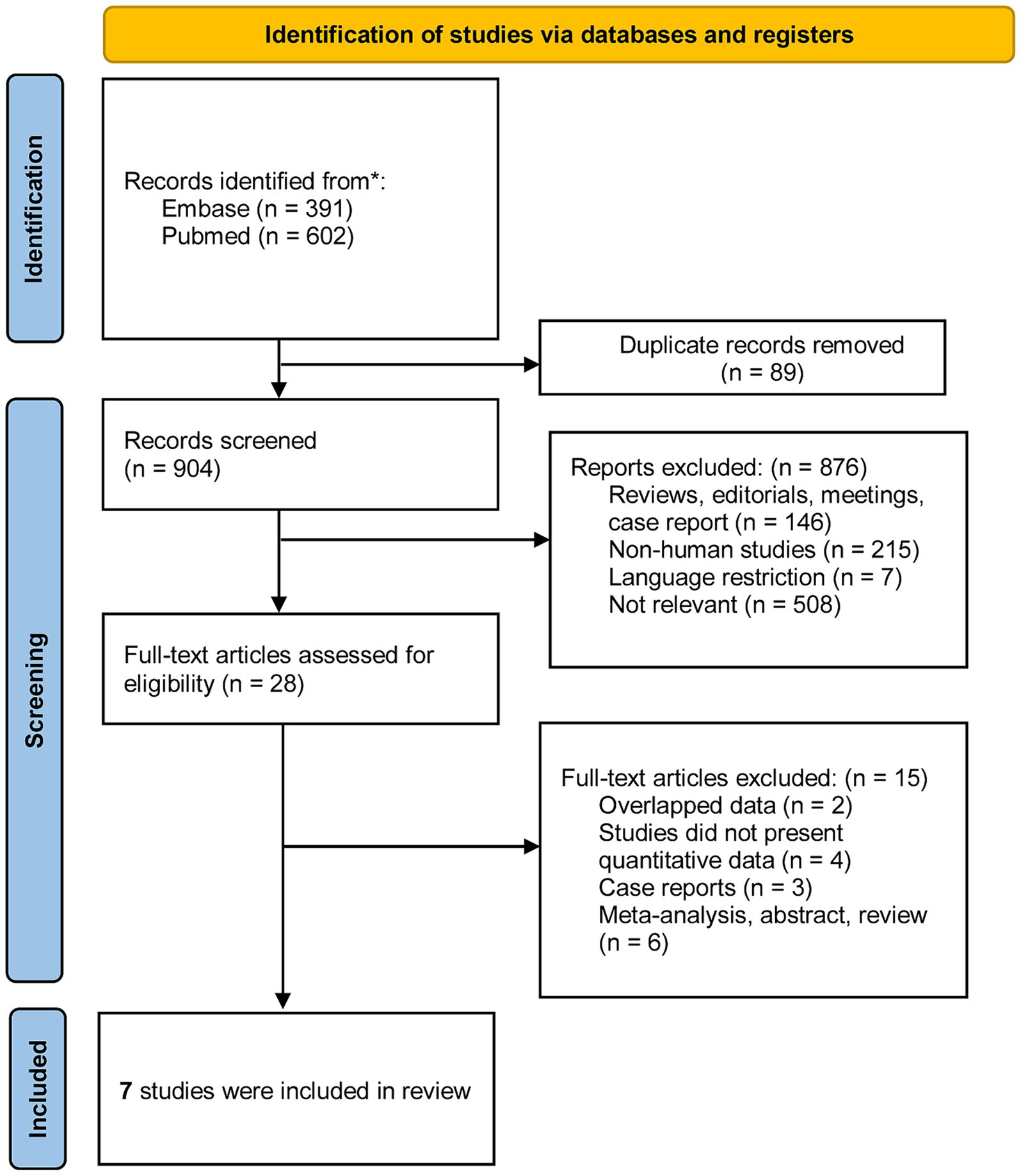
The study selection process is shown in the PRISMA flow diagram (Figure 1). The initial search resulted in 993 citations, of which 89 were duplicates discarded before screening. A total of 876 articles were removed due to irrelevance to the research question, and 28 studies were included in the full-text review stage. Ultimately, seven publications fulfilled the eligibility criteria and were included in the present review.
Characteristics of the included studies
Table 1 summarizes the nine studies considered in this review. The studies included 10,370,996 mothers from three different continents: five from Europe, one from North America, and one study from Asia. The sample size of the studies ranged from 38,661 to 4,494,926, and the publication year ranged from 2013 to 2024. Four studies evaluated these associations in mothers with epilepsy or the general population; two studies only assessed these associations in mothers with epilepsy; and the remaining study only evaluated these associations in mothers with bipolar disorder. There was heterogeneity in the type of ASM used among the studies; the ASMs included valproate, oxcarbazepine, lamotrigine, clonazepam, carbamazepine, oxcarbazepine, levetiracetam and topiramate. All studies scored > 7 on the NOS scale, indicating high overall quality (Supplementary Table S1).
Meta-analysis
Associations between ASM use and ASD and ADHD in the general population
Three studies with nine estimates reported the risk of ASD in relation to ASM exposure during pregnancy in the general population; the combined OR of the ASD risk was 2.1 (95% CI: 1.63–2.71; I2 = 76.6%, p < 0.001; Figure 2A).
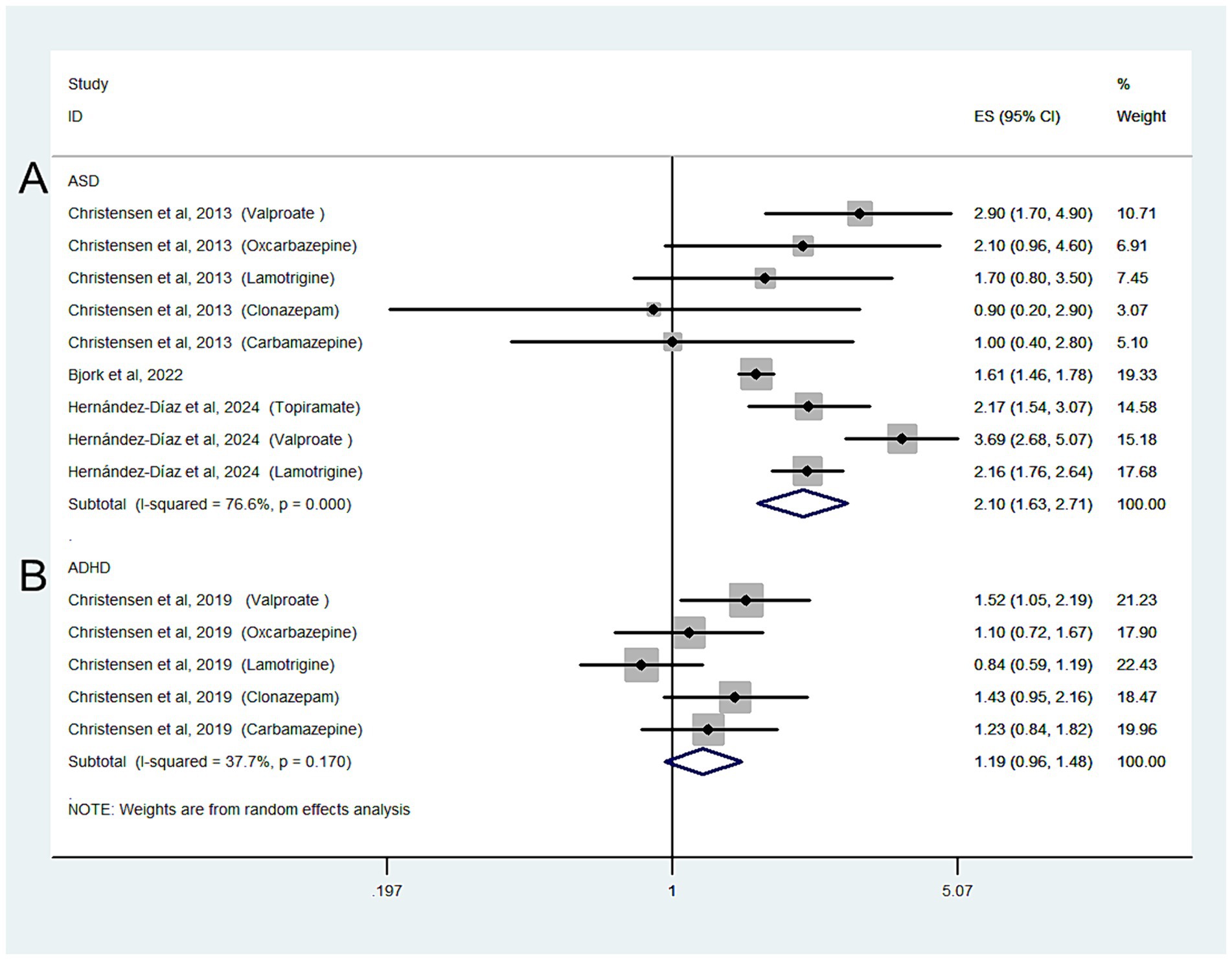
Figure 2. Forest plot of the overall risk of ASD (A) or ADHD (B) in relation to ASM exposure during pregnancy in general population.
When we grouped the studies by ASM type, significant associations were observed for mothers using valproate (OR: 2.29, 95% CI: 1.7–3.07; p < 0.001; I2 = 0%), topiramate (OR: 1.11, 95% CI: 0.84–1.47; p < 0.001; I2 = 0%), and oxcarbazepine (OR: 1.92, 95% CI: 1.37–2.69; p < 0.001; I2 = 0%), but not for those using lamotrigine (OR: 1.59, 95% CI: 0.95–2.6; p = 0.077; I2 = 89.2%), carbamazepine (OR: 1.32, 95% CI: 0.99–1.77; p = 0.062; I2 = 0%), or clonazepam (OR: 1.38, 95% CI: 0.94–2.01; p = 0.098; I2 = 0%).
One study with five estimates reported the risk of ADHD according to ASM exposure during pregnancy in the general population; the overall OR of ADHD was 1.19 (95% CI: 0.96–1.48; I2 = 37.7%, p = 0.118; Figure 2B).
Associations of ASM use with ASD and ADHD among mothers with a clinical indication
Six studies with 13 estimates reported the risk of ASD according to ASM exposure during pregnancy in mothers with a clinical indication; the overall OR of ASD was 1.38 (95% CI: 1.11–1.73; I2 = 74.8%, p = 0.004; Figure 3A). We also performed a post hoc sensitivity analysis to evaluate the impact of including studies from the same database (Bjørk and Dreier et al.’s studies). Including only one study from the same database from the main analysis for the risk of ASD had no significant effect on the pooled OR.
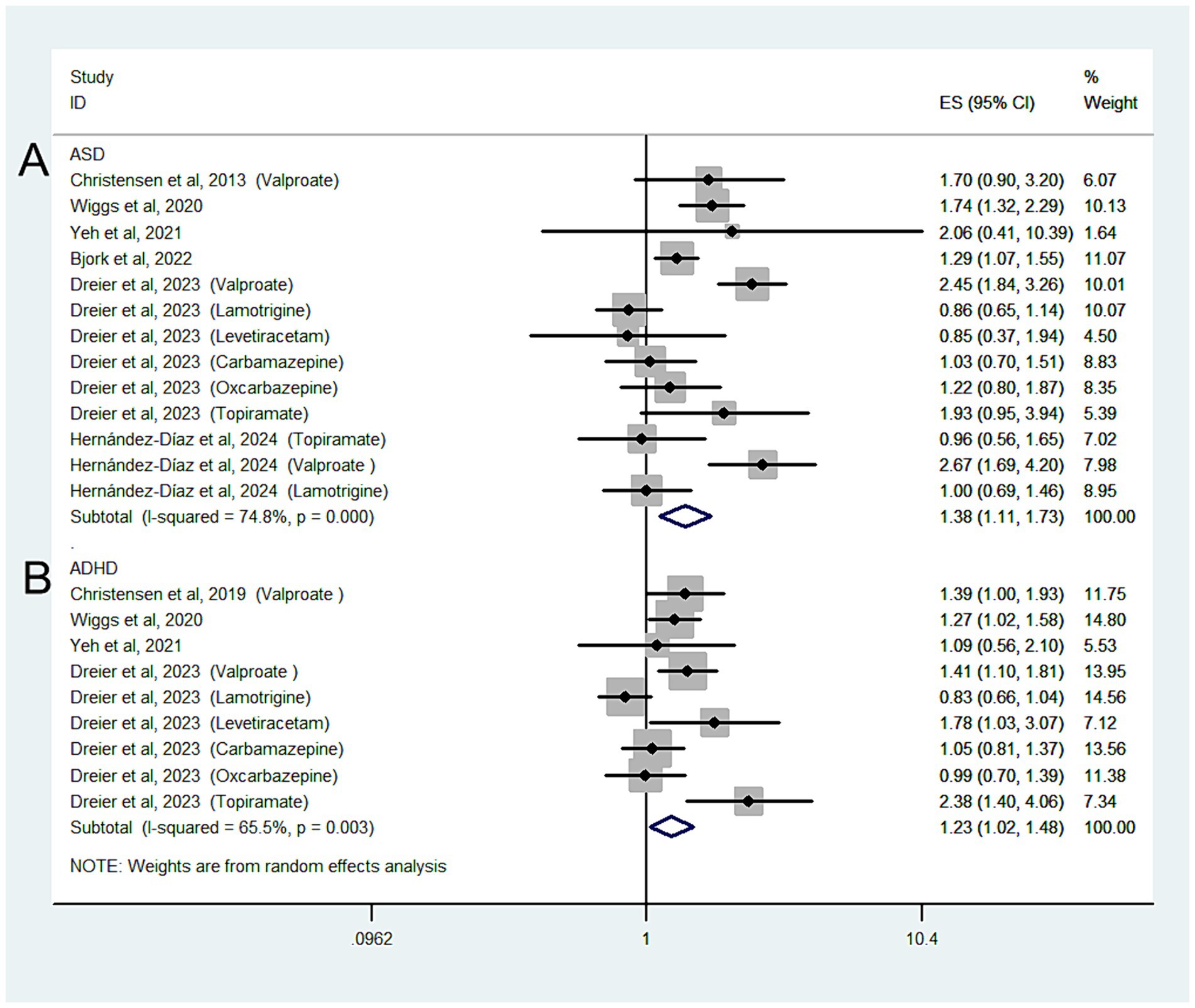
Figure 3. Forest plot of the overall risk of ASD (A) or ADHD (B) in relation to ASM exposure during pregnancy among mothers with a clinical indication.
When our analysis was limited to studies that only included mothers with epilepsy, the overall OR of ASD was 1.37 (95% CI: 1.1–1.73; p = 0.006; I2 = 76.8%).
When we grouped the studies by ASM type, significant associations were observed in mothers using valproate (OR: 2.38, 95% CI: 2.01–2.81; p < 0.001; I2 = 0%) but not in those using lamotrigine (OR: 0.87, 95% CI: 0.73–1.04; p = 0.123; I2 = 0%), carbamazepine (OR: 1.09, 95% CI: 0.87–1.37; p = 0.448; I2 = 0%), oxcarbazepine (OR: 1.27, 95% CI: 0.93–1.74; p = 0.14; I2 = 0%), levetiracetam (OR: 0.96, 95% CI: 0.54–1.68; p = 0.877; I2 = 0%), or topiramate (OR: 1.66, 95% CI: 0.87–3.18; p = 0.123; I2 = 66.2%).
Four studies with nine estimates reported the risk of ADHD in relation to ASM exposure during pregnancy in mothers with a clinical indication; the overall OR of the ADHD risk was 1.23 (95% CI: 1.02–1.48; I2 = 65.5%, p = 0.026).
When the analysis was limited to studies that only included mothers with epilepsy, the overall OR of ADHD was 1.25 (95% CI: 1.02–1.52; p = 0.029; I2 = 69.8%; Figure 3B).
When we grouped the studies by ASM type, significant associations were observed for mothers using valproate (OR: 1.49, 95% CI: 1.26–1.77; p < 0.001; I2 = 0%) but not for those using lamotrigine (OR: 0.87, 95% CI: 0.71–1.06; p = 0.166; I2 = 0%) or carbamazepine (OR: 1.11, 95% CI: 0.93–1.34; p = 0.246; I2 = 0%).
Discussion
There has been increasing interest in the association between the use of ASMs during pregnancy and neurodevelopmental disorders in offspring. The findings of our study suggest that maternal exposure to ASMs during pregnancy was associated with a modestly increased risk of ASD or ADHD in offspring, even after controlling for potential confounders by indication. However, subgroup analyses based on individual ASMs found that only valproate was associated with an increased risk of ASD or ADHD, suggesting that the overall association may be driven by valproate use alone.
In our primary analyses, these associations were explored in the general population, and an almost two-fold increased risk of ASD was observed. ASMs are widely used to treat epilepsy and psychiatric disorders and have been associated with neurodevelopmental disorders in offspring (20, 21). Thus, enrolling healthy pregnant women unexposed to ASMs as a reference group may have overestimated this association. The ideal control for confounding by indication is a reference group of women with epilepsy or a psychiatric disorder who did not take an ASM during pregnancy. In our secondary analysis, these associations were explored among mothers with a clinical indication, and the risk of ASD was reduced 1.38-fold. When we further limited our analysis to pregnant mothers with epilepsy, the combined OR of ASD was 1.37. Although we reported inconsistent results for ADHD risk, these findings may have been limited by the small number of studies included in our analysis.
Animal studies have demonstrated different neurodevelopmental effects of prenatal individual ASM exposure (22). Several systematic reviews have also summarized the evidence regarding the neurobehavioral teratogenicity of intrauterine ASM exposure (23–25). Most clinical data are derived from case reports and small case series studies (26), and findings for different types of ASM have been inconsistent. Drawing conclusions from epidemiological studies has been hampered by differences in methodology and limited sample sizes. In primary analyses, three types of ASM were associated with an increased risk of ASD; however, these findings could not be replicated in a secondary analysis, in which only exposure to valproate during pregnancy was associated with an increased risk of ASD or ADHD in offspring. The precise biological mechanisms underlying the relationship are not entirely clear. Valproate is a short-chain low-molecular-weight fatty acid that quickly crosses the placental and blood–brain barriers (27). Valproate disrupts the metabolism of glutamate and neuronal plasticity in the fetal brain, thus triggering a neurodevelopmental disorder (28). In addition, valproate is a histone deacetylation histone deacetylase inhibitor, which affects gene replication and transcription (29). Results from a recent animal model study suggest that maternal exposure to valproate promotes replication and transcription of autism-associated genes, leading to autism-like behaviors (30). Finally, exposure to maternal valproate has been linked to congenital malformations (31), which are associated with an increased risk of neurodevelopmental disorders (32).
This meta-analysis is the first to provide an overall estimate of the effect of maternal ASM use during pregnancy on ASD and ADHD risk in offspring. The strength of our meta-analysis lies in the exclusive use of cohort studies, which are less prone to bias when assessing drug exposure during pregnancy. Second, the overall sample of all studies for the meta-analysis comprised >10 million pregnancy episodes across nine different countries, which makes the findings more generalizable and robust. Third, all of the studies were considered of high quality, which may minimize the bias of the review. Fourth, the associations were investigated in subgroup analyses based on ASM indication and type.
However, our review also had several limitations. The most important limitation of our meta-analysis was the significant heterogeneity among the studies, although in the subgroup analysis based on individual ASM, heterogeneity was reduced to 0%. Second, due to the inherent nature of observational studies, those included in this meta-analysis were prone to various types of confounding. We conducted analyses by pooling the adjusted data. Third, the etiology of epilepsy contributing to different ASM choice may affect the contributors toward ASD or ADHD, which may be an important confounding factor. Fourth, there were limited data in the studies on the dose or use of combination ASM agents; therefore, we could confirm that any exposure parameter was associated with neurodevelopmental outcome. Fifth, due to the small number of included studies, we could not evaluated the interactions between different ASM contribute to the incidence of autism or ADHD; however, the results of subgroup analyses based on individual ASM were consistent with the findings of two included studies (9, 12), which evaluated the risk of ADHD or ASD in the offspring of women who used antiepileptic drugs in monotherapy during pregnancy.
In conclusion, our results suggest that the link between prenatal ASM exposure and ASD or ADHD risk in children was attributable to the indication, rather than there being a causal relation. However, maternal use of valproate during pregnancy was associated with a significantly increased risk of ASD or ADHD, even compared to mothers with bipolar disorder or epilepsy who were not exposed to ASMs in utero.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
S-CX: Writing – review & editing, Methodology, Conceptualization. YZ: Writing – review & editing, Resources, Project administration, Investigation. H-YJ: Writing – review & editing, Visualization, Investigation, Formal analysis. JT: Writing – review & editing, Writing – original draft, Validation, Supervision, Funding acquisition, Formal analysis.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the High Quality Development Boost Program of Zhejiang Provincial Hospital of Traditional Chinese Medicine (2D02311), Zhejiang Province Traditional Chinese Medicine Science and Technology Project (2024ZL404), and Heritage Program of Zhejiang Provincial Prestigious Traditional Chinese Medicine Doctors (GZS2021021). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1440145/full#supplementary-material
References
1. Madley-Dowd, P, Rast, J, Ahlqvist, VH, Zhong, C, Martin, FZ, Davies, NM, et al. Trends and patterns of antiseizure medication prescribing during pregnancy between 1995 and 2018 in the United Kingdom: a cohort study. BJOG. (2024) 131:15–25. doi: 10.1111/1471-0528.17573
2. Vajda, F, O'Brien, T, Graham, J, Hitchcock, A, Perucca, P, Lander, C, et al. Changes over 24 years in a pregnancy register—teratogenicity and epileptic seizure control. Epilepsy Behav. (2023) 148:109482. doi: 10.1016/j.yebeh.2023.109482
3. Fantaneanu, TA, Thornton, HF, Zhang, T, Bercovici, E, Hrazdil, C, Ikeda, KM, et al. Real-world practices for the care of women with epilepsy during pregnancy: a Canadian perspective. Epilepsy Behav. (2023) 148:109468. doi: 10.1016/j.yebeh.2023.109468
4. Dreier, JW, and Christensen, J. Antiseizure medication and early pregnancy loss: should we be worried? J Neurol Neurosurg Psychiatry. (2024):jnnp-2024-333620. doi: 10.1136/jnnp-2024-333620
5. Castro, PA, Pinto-Borguero, I, Yévenes, GE, Moraga-Cid, G, and Fuentealba, J. Antiseizure medication in early nervous system development. Ion channels and synaptic proteins as principal targets. Front Pharmacol. (2022) 13:948412. doi: 10.3389/fphar.2022.948412
6. Jung, JW, Kim, YJ, Choi, JS, Goto, Y, and Lee, YA. Dopamine and serotonin alterations by Hizikia fusiformis extracts under in vitro cortical primary neuronal cell cultures. Nutr Res Pract. (2023) 17:408–20. doi: 10.4162/nrp.2023.17.3.408
7. Ornoy, A, Echefu, B, and Becker, M. Valproic acid in pregnancy revisited: neurobehavioral, biochemical and molecular changes affecting the embryo and fetus in humans and in animals: a narrative review. Int J Mol Sci. (2023) 25:390. doi: 10.3390/ijms25010390
8. Christensen, J, Grønborg, TK, Sørensen, MJ, Schendel, D, Parner, ET, Pedersen, LH, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. (2013) 309:1696–703. doi: 10.1001/jama.2013.2270
9. Christensen, J, Pedersen, L, Sun, Y, Dreier, JW, Brikell, I, and Dalsgaard, S. Association of Prenatal Exposure to valproate and other antiepileptic drugs with risk for attention-deficit/hyperactivity disorder in offspring. JAMA Netw Open. (2019) 2:6606. doi: 10.1001/jamanetworkopen.2018.6606
10. Wiggs, KK, Rickert, ME, Sujan, AC, Quinn, PD, Larsson, H, Lichtenstein, P, et al. Antiseizure medication use during pregnancy and risk of ASD and ADHD in children. Neurology. (2020) 95:e3232–40. doi: 10.1212/WNL.0000000000010993
11. Yeh, T-C, Bai, Y-M, Hsu, J-W, Huang, K-L, Tsai, S-J, Chu, H-T, et al. Bipolar women's antepartum psychotropic exposure and offspring risk of attention-deficit/hyperactivity disorder and autism spectrum disorder. J Affect Disord. (2021) 295:1407–14. doi: 10.1016/j.jad.2021.09.016
12. Bjørk, M-H, Zoega, H, Leinonen, MK, Cohen, JM, Dreier, JW, Furu, K, et al. Association of Prenatal Exposure to Antiseizure medication with risk of autism and intellectual disability. JAMA Neurol. (2022) 79:672–81. doi: 10.1001/jamaneurol.2022.1269
13. Dreier, JW, Bjørk, M-H, Alvestad, S, Gissler, M, Igland, J, Leinonen, MK, et al. Prenatal exposure to Antiseizure medication and incidence of childhood-and adolescence-onset psychiatric disorders. JAMA Neurol. (2023) 80:568–77. doi: 10.1001/jamaneurol.2023.0674
14. Hernández-Díaz, S, Straub, L, Bateman, BT, Zhu, Y, Mogun, H, Wisner, KL, et al. Risk of autism after prenatal Topiramate, valproate, or lamotrigine exposure. N Engl J Med. (2024) 390:1069–79. doi: 10.1056/NEJMoa2309359
15. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
16. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
17. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
18. Egger, M, Smith, GD, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
19. Lau, J, Ioannidis, JP, Terrin, N, Schmid, CH, and Olkin, I. The case of the misleading funnel plot. BMJ. (2006) 333:597–600. doi: 10.1136/bmj.333.7568.597
20. Morales, DR, Slattery, J, Evans, S, and Kurz, X. Antidepressant use during pregnancy and risk of autism spectrum disorder and attention deficit hyperactivity disorder: systematic review of observational studies and methodological considerations. BMC Med. (2018) 16:6. doi: 10.1186/s12916-017-0993-3
21. Mazzone, PP, Hogg, KM, Weir, CJ, Stephen, J, Bhattacharya, S, Richer, S, et al. Comparison of neurodevelopmental, educational and adult socioeconomic outcomes in offspring of women with and without epilepsy: a systematic review and meta-analysis. Seizure. (2024) 117:213–21. doi: 10.1016/j.seizure.2024.02.014
22. Ikonomidou, C, and Turski, L. Antiepileptic drugs and brain development. Epilepsy Res. (2010) 88:11–22. doi: 10.1016/j.eplepsyres.2009.09.019
23. Peron, A, Picot, C, Jurek, L, Nourredine, M, Ripoche, E, Ajiji, P, et al. Neurodevelopmental outcomes after prenatal exposure to lamotrigine monotherapy in women with epilepsy: a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2024) 24:103. doi: 10.1186/s12884-023-06242-9
24. Honybun, E, Cockle, E, Malpas, CB, O'Brien, TJ, Vajda, FJ, Perucca, P, et al. Neurodevelopmental and functional outcomes following in utero exposure to Antiseizure medication: a systematic review. Neurology. (2024) 102:e209175. doi: 10.1212/WNL.0000000000209175
25. Vaccaro, C, Shakeri, A, Czaplinski, E, and Eltonsy, S. New-generation antiepileptic drugs during pregnancy and the risk of attention-deficit hyperactivity disorder: a scoping review. J Popul Therapeut Clin Pharmacol. (2020) 27:e1–e18. doi: 10.15586/jptcp.v27i4.722
26. Bromley, RL, Mawer, GE, Briggs, M, Cheyne, C, Clayton-Smith, J, García-Fiñana, M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. (2013) 84:637–43. doi: 10.1136/jnnp-2012-304270
27. Chiu, CT, Wang, Z, Hunsberger, JG, and Chuang, DM. Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev. (2013) 65:105–42. doi: 10.1124/pr.111.005512
28. Traetta, ME, Codagnone, MG, Uccelli, NA, Ramos, AJ, Zárate, S, and Reinés, A. Hippocampal neurons isolated from rats subjected to the valproic acid model mimic in vivo synaptic pattern: evidence of neuronal priming during early development in autism spectrum disorders. Mol Autism. (2021) 12:23. doi: 10.1186/s13229-021-00428-8
29. Kalinin, AA, Hou, X, Ade, AS, Fon, GV, Meixner, W, Higgins, GA, et al. Valproic acid-induced changes of 4D nuclear morphology in astrocyte cells. Mol Biol Cell. (2021) 32:1624–33. doi: 10.1091/mbc.E20-08-0502
30. Guerra, M, Medici, V, Weatheritt, R, Corvino, V, Palacios, D, Geloso, MC, et al. Fetal exposure to valproic acid dysregulates the expression of autism-linked genes in the developing cerebellum. Transl Psychiatry. (2023) 13:114. doi: 10.1038/s41398-023-02391-9
31. Battino, D, Tomson, T, Bonizzoni, E, Craig, J, Perucca, E, Sabers, A, et al. Risk of major congenital malformations and exposure to Antiseizure medication monotherapy. JAMA Neurol. (2024) 81:481–9. doi: 10.1001/jamaneurol.2024.0258
32. Sood, E, Newburger, JW, Anixt, JS, Cassidy, AR, Jackson, JL, Jonas, RA, et al. Neurodevelopmental outcomes for individuals with congenital heart disease: updates in neuroprotection, risk-stratification, evaluation, and management: a scientific statement from the American Heart Association. Circulation. (2024) 149:e997–e1022. doi: 10.1161/CIR.0000000000001211
Keywords: mother, anticonvulsant, anti-epileptic, autistic, attention
Citation: Xu S-C, Zhong Y, Jiang H-Y and Tang J (2024) Exposure to anti-seizure medication during pregnancy and the risk of autism and ADHD in offspring: a systematic review and meta-analysis. Front. Neurol. 15:1440145. doi: 10.3389/fneur.2024.1440145
Edited by:
Venus Tang, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Natela Okujava, Tbilisi State Medical University, GeorgiaMaggie LoYee Yau, Prince of Wales Hospital, China
Copyright © 2024 Xu, Zhong, Jiang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Tang, dGFuZ2p1bnpqdGNtQDEyNi5jb20=
 Shan-Chun Xu1
Shan-Chun Xu1 Jun Tang
Jun Tang