
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 24 September 2024
Sec. Applied Neuroimaging
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1439939
This article is part of the Research Topic Risk Factors and Prevention Strategy in Intervertebral Disc Herniation View all 7 articles
 Qin Zhang1,2
Qin Zhang1,2 Hui Ding1*
Hui Ding1*Background: Resting-state functional magnetic resonance imaging (rs-fMRI) reveals diverse neural activity patterns in cervical spondylosis (CS) patients. However, the reported results are inconsistent. Therefore, our objective was to conduct a meta-analysis to synthesize the findings from existing rs-fMRI studies and identify consistent patterns of neural brain activity alterations in patients with CS.
Materials and methods: A systematic search was conducted across PubMed, Web of Knowledge, Embase, Google Scholar, and CNKI for rs-fMRI studies that compared CS patients with healthy controls (HCs), up to January 28, 2024. Significant cluster coordinates were extracted for comprehensive analysis.
Results: We included 16 studies involving 554 CS patients and 488 HCs. CS patients demonstrated decreased brain function in the right superior temporal gyrus and left postcentral gyrus, and increased function in the left superior frontal gyrus. Jackknife sensitivity analysis validated the robustness of these findings, and Egger’s test confirmed the absence of significant publication bias (p > 0.05). Meta-regression showed no significant impact of age or disease duration differences on the results.
Conclusion: This meta-analysis confirms consistent alterations in specific brain regions in CS patients, highlighting the potential of rs-fMRI to refine diagnostic and therapeutic strategies.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024496263.
Cervical Spondylosis (CS) is a prevalent age-related chronic condition characterized primarily by stiffness and pain in the neck and upper back. Due to its high incidence rate, unsatisfactory treatment options, significant healthcare burdens, and impact on quality of life, CS has emerged as a critical public health and societal issue (1). In recent years, the prevalence of CS has been on the rise, becoming a leading cause of non-traumatic spinal cord dysfunction (2). Timely diagnosis and surgical intervention are crucial for alleviating neurological symptoms in CS patients (3). Research shows that chronic pain can lead to the progressive accumulation of brain damage, which may manifest as psychological disorders including depression, anxiety, and sleep disturbances that impact emotional processing. Patients with chronic musculoskeletal pain often experience heightened anxiety, depression, fatigue, and insomnia, which intensify with the severity and multiplicity of pain sites (4). Furthermore, the co-occurrence of anxiety and depression in these patients is linked to significant psychological impairments and reduced quality of life, emphasizing the need to focus on psychological aspects in the management and treatment of chronic pain (5).
Resting state functional magnetic resonance imaging (rs-fMRI) is a commonly used technique to study functional changes in the brains of patients. Analytical methods such as Amplitude of low frequency fluctuation (ALFF) and regional homogeneity (ReHo) are frequently employed. ALFF measures the amplitude of low-frequency fluctuations (0.01–0.08 Hz) in the blood oxygen level dependent (BOLD) signal, providing reliable insights into cortical activity (6). Besides, ReHo assesses the similarity of BOLD signal patterns between a given voxel and its 26 neighboring voxels, offering a different perspective on neural activity (7). ALFF, ReHo, and related algorithms like fractional ALFF (fALFF) play a crucial role in studies with high reliability and specificity on cortical activity, significantly contributing to our understanding of brain function through rs-fMRI technology. Previous studies have indicated functional activity changes in the brains of CS patients in regions such as the superior frontal gyrus, middle frontal gyrus, postcentral gyrus, superior temporal gyrus, insula, sensorimotor area, supplementary motor area, cingulate gyrus, occipital lobe, and precuneus (8–23). However, some studies have reported variability in the functional changes of cortical regions in CS patients, with increased (9, 13), decreased (11, 20), or unchanged activation observed in areas like the precuneus and middle cingulate cortex.
Variability in functional representation across studies may be due to differences in sample sizes, neural activity levels, and imaging parameters. This study uses the Anisotropy Effect Size Signed Differential Mapping (AES-SDM) technique to identify consistent brain function changes and employs a meta-analysis to compare brain region activity between CS patients and healthy controls (HCs), enhancing our understanding of CS’s neurological impact.
This review was registered with PROSPERO (ID: CRD42024496263). A systematic search for relevant studies was conducted in the PubMed, Web of Knowledge, Embase, Google, and CNKI databases up to January 28, 2024. Keywords included (“cervical spondylopathy” OR “cervical spondylosis” OR “spondylosis” OR “cervical spondylotic”) AND (“regional homogeneity” OR “ReHo” OR “amplitude of low-frequency fluctuation” OR “ALFF” OR “fALFF”) AND (“magnetic resonance” OR “MRI” OR “functional MRI” OR “fMRI”). Manual screening of all potentially related results was also conducted.
Studies were considered eligible based on the following criteria: (1) Clear diagnosis of CS via MRI; (2) absence of pain in other body parts; and (3) reporting of whole-brain results in stereotactic space (MNI or Talairach coordinates) for ALFF, fALFF, dALFF, PerAF, ReHo. Exclusion criteria included: (1) Abstracts, case reports, systematic reviews, meta-analyses; (2) Intervention studies; (3) Studies using only region of interest (ROI) or seed voxel-based analyses; (4) Studies not adhering to CS diagnostic criteria or presenting significant data heterogeneity; (5) Studies without reported coordinates. Two physicians with at least attending doctor qualifications independently conducted the literature search and reached consensus on the results. Study selection was conducted in accordance with the PRISMA guidelines (24) (Table 1).
The anisotropy effect size signed differential mapping (AES-SDM) software was used to analyze ReHo/zReHo & ALFF/dALFF/zALFF differences between CS patients and HCs (25). Peak coordinates in Talairach space were converted to MNI space.1 If results were presented as z-values, they were converted to t-values for use in the analysis.2 AES-SDM reconstructed effect size and statistical parameter maps of increased and decreased brain region activation from individual studies. The Monte Carlo random effects model used in AES-SDM integrated these statistical maps with a significance threshold set at: FWHM = 20 mm, uncorrected voxel p < 0.005, and cluster extent ≥10 voxels (26). Cluster coordinate reconstruction involved converting peak t-values to Hedges’ g, followed by application of a Gaussian kernel for nonuniform smoothing of adjacent peak coordinate voxels. Volume rendering of cortical clusters with significant differences was performed in MNI standard space using Mango software.
Egger’s regression test, integrated within the AES-SDM software, was employed to evaluate the effect sizes of peak voxels within significant clusters. Heterogeneity among studies was calculated to assess brain regions contributing to heterogeneity. Jackknife sensitivity analysis was performed to evaluate the robustness of the results by repeating the meta-analysis 16 times, excluding one of the 16 studies in each iteration (27). Funnel plots and meta-regression analyses were created using the peak coordinates of significant cortical activation clusters to assess potential publication bias and the impact of study-level covariates on the observed effects.
A total of 165 articles were retrieved through the search, among which 16 met the inclusion criteria for the meta-analysis (Figure 1). No additional relevant studies were identified from the references of the selected articles. Various resting-state analysis methods were used across the studies, such as ReHo/zReHo and ALFF/fALFF/dALFF/zALFF. Two papers, Chen (12) and Kuang (16), utilized ReHo and ALFF, zReHo and zALFF methods, respectively, and were considered as separate studies due to the different analytical approaches. Consequently, 16 articles comprising 18 studies were included in the meta-analysis (8–23), involving a total of 554 CS patients and 488 HCs. No statistically significant differences were observed in age and gender between CS patients and HCs (p > 0.05; Table 1).
The results of the AES-SDM analysis are summarized in Table 2. The meta-analysis comparing CS patients with HCs revealed decreased brain function in CS patients in the right superior temporal gyrus and left postcentral gyrus, and increased function in the left superior frontal gyrus (Figure 2). Egger’s test results for the right superior temporal gyrus (Bias 1.19, Z 1.00, P 0.318), left postcentral gyrus (Bias-0.02, Z-0.02, P 0.988), and left superior frontal gyrus (Bias 0.77, Z 0.73, P 0.467) confirmed no significant heterogeneity among the studies (Table 2). Jackknife sensitivity analysis showed consistent results, with the findings for the right superior temporal gyrus replicated in 13 of 16 iterations; for the left postcentral gyrus in 14 of 16; and for the left superior frontal gyrus in 14 of 16 iterations (Table 3). Meta-regression analysis indicated no significant impact from differences in age and gender between groups.
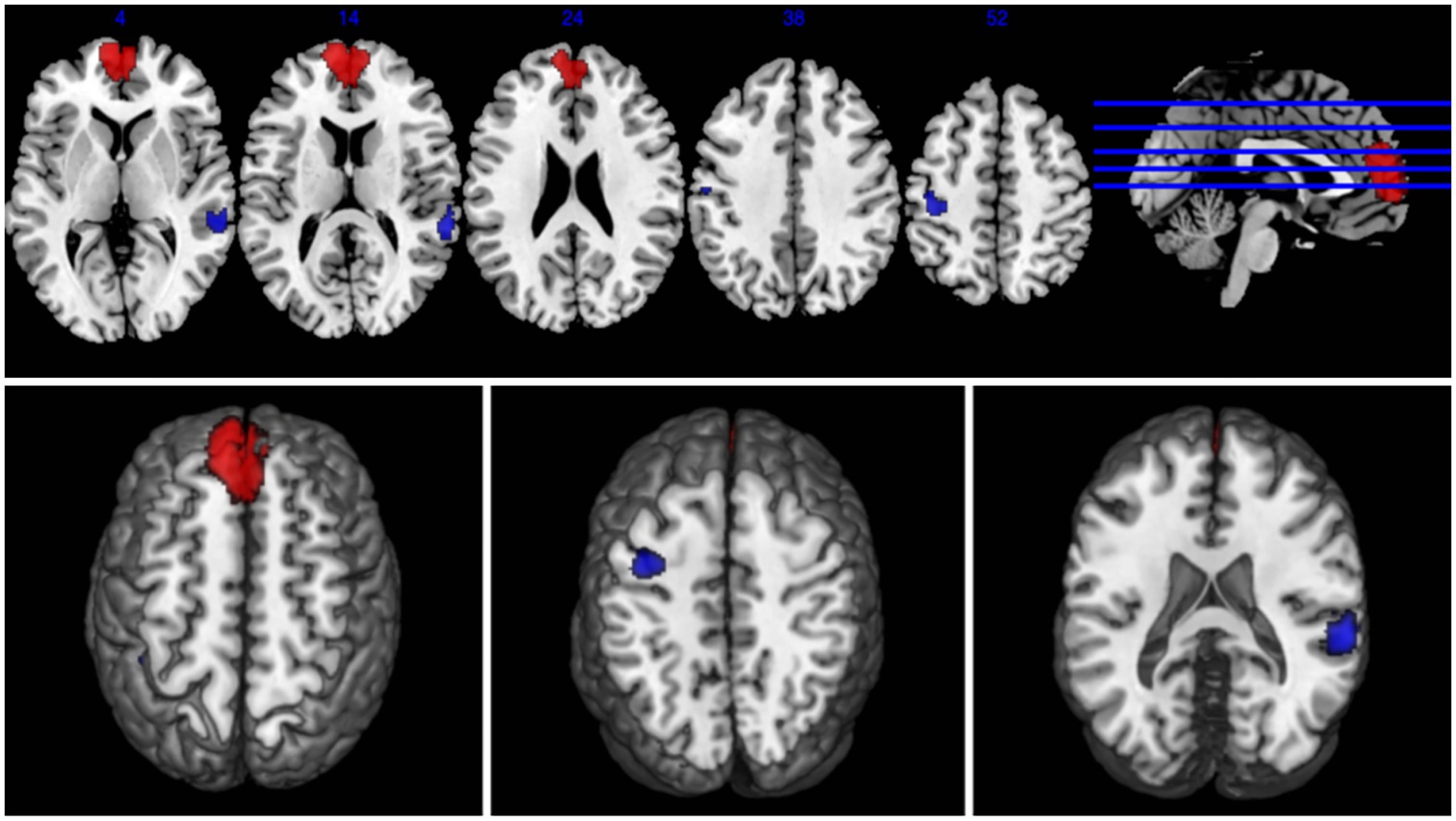
Figure 2. Abnormal regions identified in an SDM-Meta analysis of neuroimaging studies in cervical spondylosis (CS). Regions showing increased activation in CS patients compared to healthy controls (HCs) are highlighted in red, while regions with decreased activation are highlighted in blue.
This study employs AES-SDM analysis to integrate brain functional activation maps, confirming notable changes in brain function in CS patients compared to HCs. Specifically, we observed decreased activation in the right superior temporal gyrus and the left postcentral gyrus, and increased activation in the left superior frontal gyrus.
The right superior temporal gyrus, part of the Wernicke’s area involved in auditory and language processing and a key structure in social cognitive functions, showed decreased brain activity in this study. This region also acts as a cortical center for vestibular functions (28). Yu et al. (11) reported that the cognitive levels maintained in CS patients might be a result of compensatory mechanisms, which, upon failure, could lead to significant cognitive decline.
Furthermore, Song et al. (29) suggested that changes in gray matter volume in regions such as the left precentral gyrus, medial prefrontal cortex, supplementary motor area, superior temporal gyrus, parietal lobe, occipital lobe, and postcentral gyrus may indicate Wallerian degeneration, which occurs distal to damage in the corticospinal tract. Adaptive changes in sensory and motor functions have been confirmed in animal models with hemisected spinal cords (30). Wrigley et al. (31) also found significant reductions in gray matter volume in the left precentral gyrus, bilateral superior temporal gyri, insula, hypothalamus, medial prefrontal cortex, and anterior cingulate cortex in patients with spinal cord injury compared to healthy individuals. CS damages afferent or efferent fibers, such as those in the corticospinal tract, disrupting intact motor-related neural reflex-feedback control systems. Brain regions involved in motor activity, including the premotor and supplementary motor areas, play crucial roles in the preparation and execution of movement (32). Therefore, this study posits that the decreased brain function activity in the superior temporal gyrus in CS patients affects the intention, purpose, and rough planning of the motor area during movement execution, which may explain the fine motor abnormalities seen in CS patients.
The left postcentral gyrus, part of the primary sensory cortex, plays a crucial role in the pathological process of CS, integrating and executing various motor information and participating in the perception of touch, pressure, temperature, and pain (33–35). The observed decrease in brain function activity in the left postcentral gyrus suggests cortical reorganization in the cortical functional area (i.e., postcentral gyrus) following damage to the corticospinal tract nerve fibers, compensating for functional deficits in CS patients. Recent studies have also identified functional, structural, and metabolic changes in the postcentral gyrus of CS patients, considered manifestations of sensorimotor cortex plasticity, revealing dynamic adjustments by the brain in response to secondary damage during chronic spinal cord injury progression (36, 37). Moreover, previous literature indicates the postcentral gyrus’s association with chronic pain (38, 39). The regulation of external stimuli or neurofeedback can directly impact chronic pain, highlighting the significance of the sensorimotor cortex in the pain process. Our findings of decreased brain function activity in the left postcentral gyrus suggest that CS-related chronic neck and shoulder pain affects the functional expression of the somatosensory cortex, leading to weakened sensory abilities and long-term behavioral impacts. Thus, it can be inferred that the decrease in brain function activity may signify the activation of brain functions in the postcentral gyrus, thereby influencing pain perception.
The prefrontal cortex, encompassing areas such as the medial superior frontal gyrus and the orbitofrontal cortex, is responsible for a wide array of advanced cognitive functions and emotional regulation. It plays a crucial role in modulating the brain’s perception of and response to internal and external environmental changes, executive functions, cognitive control, language processing, and the retrieval of episodic memory. Additionally, it coordinates with other brain regions to maintain the fundamental activities of the brain in a resting state (40–42). A study by Wang et al. (43) found that, compared to the control group, patients with mild, moderate, and severe CS showed lower ALFF values in the left medial superior frontal gyrus. This suggests that the reduction in ALFF in the left medial superior frontal gyrus might be an underlying neuroimaging phenotype of CS, underscoring the significant role of the medial prefrontal cortex in regulating the dynamic interaction of emotional and cognitive signals in the brain (44). This revelation provides insights into the neurobiological basis of CS and highlights the importance of understanding changes in brain activity associated with CS. However, the impact of emotional states on the pre-motor behavioral responses related to ALFF changes warrants further investigation beyond the scope of the current study.
Additionally, research by Koenigs M et al. indicates that activity in the medial superior frontal gyrus is associated with the experience of negative emotions, playing a foundational role in the processing of negative emotions (45). In adolescent females with severe depression who have attempted suicide, an increase in ALFF in the right superior frontal gyrus has been observed (46, 47). Furthermore, changes in ALFF in regions including the right superior frontal gyrus are induced following electroconvulsive therapy in adolescents with depression, suggesting the right superior frontal gyrus as a potential neurobiological marker for clinical treatment (48). Therefore, a deeper understanding of the function of the medial superior frontal gyrus is crucial for exploring the pathogenesis and treatment approaches for diseases like CS. Future research will further elucidate the role of the medial prefrontal cortex in emotional and cognitive domains, offering more effective strategies for the diagnosis and treatment of neurological diseases.
Overall, this study demonstrates an increase in brain function activity in the left superior frontal gyrus in CS patients, providing further evidence of compensatory mechanisms distal to spinal structural damage in CS patients. It is reasonable to believe that the increased functional activity in the left superior frontal gyrus may play a significant role in neural plasticity. This might also explain a mechanism in some CS patients who, despite having clear evidence of neck compression and significant degenerative demyelination, are able to function normally with minimal or mild neurological deficits.
This meta-analysis has several limitations that warrant cautious interpretation of the results. First, the number of studies included in the meta-analysis is relatively small, which may affect the generalizability and robustness of the findings. Secondly, our reliance on peak coordinates from published data may not comprehensively represent the spatial extent of brain activity alterations, introducing potential selection bias. Variability in imaging protocols and analysis methods across studies might influence the results, despite rigorous inclusion criteria and statistical approaches. Finally, some of the included studies involved patients who had received pharmacological treatment prior to MRI scanning.
This study, utilizing SDM-Meta analysis, identified decreased brain function in the right superior temporal gyrus and left postcentral gyrus, and increased function in the left superior frontal gyrus in patients with CS. These findings contribute to our understanding of the changes in brain regions associated with CS as reported in previous literature and provide a basis for further investigation into the central mechanisms of CS. Future research is needed to determine how these localized changes in neural activity can be applied to the diagnosis of the disease, monitoring disease progression, and developing potential therapeutic interventions for patients with CS.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
QZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. HD: Conceptualization, Data curation, Formal analysis, Software, Writing – review & editing, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
QZ expresses her gratitude to Mr. Hou Yongzhe for his unwavering encouragement and support, as well as his selfless assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Baime, MJ. In chronic nonspecific neck pain, adding Alexander technique lessons or acupuncture to usual care improved pain. Ann Intern Med. (2016) 164:JC29. doi: 10.7326/ACPJC-2016-164-6-029
2. de Luca, K, Tavares, P, Yang, H, Hurwitz, EL, Green, BN, Dale, H, et al. Spinal pain, chronic health conditions and health behaviors: data from the 2016–2018 National Health Interview Survey. Int J Environ Res Public Health. (2023) 20:5369. doi: 10.3390/ijerph20075369
3. Williams, TL, Joseph, C, Nilsson-Wikmar, L, and Phillips, J. Exploration of the experiences of persons in the traumatic spinal cord injury population in relation to chronic pain management. Int J Environ Res Public Health. (2022) 20:77. doi: 10.3390/ijerph20010077
4. Bäckryd, E, and Alföldi, P. Chronic pain and its relationship with anxiety and depression. Lakartidningen. (2023) 120:23010–08.
5. Quidé, Y, Norman-Nott, N, Hesam-Shariati, N, McAuley, JH, and Gustin, SM. Depressive symptoms moderate functional connectivity within the emotional brain in chronic pain. Bjpsych Open. (2023) 9:e80. doi: 10.1192/bjo.2023.61
6. Zhang, W, and Bijsterbosch, J. Functional MRI analysis[M]//advances in magnetic resonance technology and applications. Academic Press. (2021) 4:247–59. doi: 10.1016/B978-0-12-822479-3.00028-2
7. Buchbinder, BR. Functional magnetic resonance imaging. Handb Clin Neurol. (2016) 135:61–92. doi: 10.1016/B978-0-444-53485-9.00004-0
8. Zhou, F, Gong, H, Liu, X, Wu, L, Luk, KDK, and Hu, Y. Increased low-frequency oscillation amplitude of sensorimotor cortex associated with the severity of structural impairment in cervical myelopathy. PLoS One. (2014) 9:e104442. doi: 10.1371/journal.pone.0104442
9. Tan, Y, Zhou, F, Wu, L, Liu, Z, Zeng, X, Gong, H, et al. Alteration of regional homogeneity within the sensorimotor network after spinal cord decompression in cervical spondylotic myelopathy: a resting-state fMRI study. Biomed Res Int. (2015) 2015:1–6. doi: 10.1155/2015/647958
10. YM, T, FQ, Z, and LC, H. Alteration of Cerebral Regional Homogeneity in Cervical Spondylotic Myelopathy:A Resting State Functional Magnetic Resonance Imaging Study. J Clinical Radiol. (2015) 34:1544–8. doi: 10.13437/j.cnki.jcr.2015.10.003
11. Yu, C, Ji, T, Song, H, Li, B, Han, Q, Li, L, et al. Abnormality of spontaneous brain activities in patients with chronic neck and shoulder pain: a resting-state fMRI study. J Int Med Res. (2017) 45:182–92. doi: 10.1177/0300060516679345
12. Chen, J, Wang, Z, Tu, Y, Liu, X, Jorgenson, K, Ye, G, et al. Regional homogeneity and multivariate pattern analysis of cervical spondylosis neck pain and the modulation effect of treatment. Front Neurosci. (2018) 12:900. doi: 10.3389/fnins.2018.00900
13. Chen, Z, Wang, Q, Liang, M, Zhao, R, Zhu, J, Xiong, W, et al. Visual cortex neural activity alteration in cervical spondylotic myelopathy patients: a resting-state fMRI study. Neuroradiology. (2018) 60:921–32. doi: 10.1007/s00234-018-2061-x
14. Yk, X, Jl, P, and Li, B. Regional homogeneity of brain in patients suffering from chronic neck and shoulder pain caused by cervical spondylotic radiculopathy:a resting-state fMRI study. Radiologic Prac. (2018) 33:549–54. doi: 10.13609/j.cnki.1000-0313.2018.06.001
15. Cl, Z, Ym, T, and Lc, H. Frequency-dependent alterations in fractional amplitude of low-frequency fluctuations in cervical Spondylotic myelopathy:resting-state fMRI study. J Clinical Radiol. (2019) 4:578–82. doi: 10.13437/j.cnki.jcr.2019.04.002
16. Kuang, C, and Zha, Y. Abnormal intrinsic functional activity in patients with cervical spondylotic myelopathy: a resting-state fMRI study. Neuropsychiatr Dis Treat. (2019) 15:2371–83. doi: 10.2147/NDT.S209952
17. Yue, X, and Du, Y. Altered intrinsic brain activity and regional cerebral blood flow in patients with chronic neck and shoulder pain. Pol J Radiol. (2020) 85:e155:155–62. doi: 10.5114/pjr.2020.94063
18. Zc, G, Jj, W, and Sun, H. Changes of intrinsic brain activity in patients with cervical spondylotic myelopathy based on fMRI classification. J Tongji University(Med Sci). (2021) 42:785–90. doi: 10.13437/j.cnki.jcr.2021.05.004
19. Kf, W, Liu, M, He, LC, et al. A preliminary MRI study of brain function changes in patients with cervical Spondylotic myelopathy. J Clinical Radiol. (2021) 40:850–4.
20. Fan, N, Zhao, B, Liu, LY, Yang, WZ, Chen, X, and Lu, ZB. Dynamic and static amplitude of low-frequency fluctuation is a potential biomarker for predicting prognosis of degenerative cervical myelopathy patients: a preliminary resting-state fMRI study. Front Neurol. (2022) 13:829714. doi: 10.3389/fneur.2022.829714
21. Bai, L, Zhang, L, Chen, Y, Li, Y, Ma, D, Li, W, et al. Middle cingulate cortex function contributes to response to non-steroidal anti-inflammatory drug in cervical spondylosis patients: a preliminary resting-state fMRI study. Neuroradiology. (2022) 64:1401–10. doi: 10.1007/s00234-022-02964-3
22. Zhao, R, Guo, X, Wang, Y, Song, YC, Su, Q, Sun, HR, et al. Functional MRI evidence for primary motor cortex plasticity contributes to the disease’s severity and prognosis of cervical spondylotic myelopathy patients. Eur Radiol. (2022) 32:3693–704. doi: 10.1007/s00330-021-08488-3
23. Su, Q, Li, J, Chu, X, and Zhao, R. Preoperative pain hypersensitivity is associated with axial pain after posterior cervical spinal surgeries in degenerative cervical myelopathy patients: a preliminary resting-state fMRI study. Insights Imag. (2023) 14:16. doi: 10.1186/s13244-022-01332-2
24. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGPRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
25. Radua, J, Rubia, K, and Canales-Rodriguez, EJ. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psychol. (2014) 5:13. doi: 10.3389/fpsyt.2014.00013
26. Nortje, G, Stein, DJ, Radua, J, Mataix-Cols, D, and Horn, N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J Affect Disord. (2013) 150:192–200. doi: 10.1016/j.jad.2013.05.034
27. Radua, J, Van Den Heuvel, OA, and Surguladze, SA. Metaanalytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. (2010) 67:701–11. doi: 10.1001/archgenpsychiatry.2010.70
28. Dziobek, I, Preissler, S, and Grozdanovic, Z. Neuronal correlates of altered empathy and social cognition in borderline personality disorder. NeuroImage. (2011) 57:539–48. doi: 10.1016/j.neuroimage.2011.05.005
29. Song, F, Yin, DZ, and Hu, YS. The study of mechanism on the completely paralyzed hands of chronic stroke patients by voxel-based morphometry. Chinese J Rehabil Med. (2013) 28:604–10.
30. Frigon, A, Barriere, G, and Leblond, H. Asymmetric changes in cuta-neous reflexes after a partial spinal lesion and retention following spinalization during locomotion in the cat[J]. J Neurophysiol. (2009) 102:2667–80. doi: 10.1152/jn.00572.2009
31. Wrigley, PJ, Gustin, SM, and Macey, PM. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex. (2009) 19:224–32. doi: 10.1093/cercor/bhn072
32. MH, W, YH, Z, and JC, L. Functional magnetic resonance imaging of whole brain related to motor preparation and execution. National Med J China. (2007) 87:971–4.
33. Qiu, T, Zhang, Y, Tang, X, Liu, X, Wang, Y, Zhou, C, et al. Precentral degeneration and cerebellar compensation in amyotrophic lateralsclerosis:a multi modal MRl analysis. Hum Brain Mapp. (2019) 40:3464–74. doi: 10.1002/hbm.24609
34. Ploner, M, Schmitz, F, Freund, HJ, and Schnitzler, A. Differential organi-zation of touch and pain in human primary somatosensory cortex. J Neurophysiol. (2000) 83:1770–6. doi: 10.1152/jn.2000.83.3.1770
35. Vartiainen, N, Kirveskari, E, Kallio-Laine, K, Kalso, E, and Forss, N. Cortical reorgani-zation in primary somatosensory cortex in patients with unilateral chronic pain. J Pain. (2009) 10:854–9. doi: 10.1016/j.jpain.2009.02.006
36. Takenaka, S, Kan, S, Seymour, B, Makino, T, Sakai, Y, Kushioka, J, et al. Resting-state amplitude of low-frequency fluctuation is a potentially useful prognostic functional biomarker in cervical myelopathy. Clin Orthop Relat Res. (2020) 478:1667–80. doi: 10.1097/CORR.0000000000001157
37. Eisdorfer, JT, Sobotka-Briner, H, Schramfield, S, Moukarzel, G, Chen, J, Campion, TJ, et al. Chemogenetic modulation of sensory afferents induces locomotor changes and plasticity after spinal cord injury. Front Mol Neurosci. (2022) 15:872634. doi: 10.3389/fnmol.2022.872634
38. Neumann, L, Wulms, N, Witte, V, Spisak, T, Zunhammer, M, Bingel, U, et al. Network properties and regional brain morphology of the insular cortex correlate with individual pain thresholds. Hum Brain Mapp. (2021) 42:4896–908. doi: 10.1002/hbm.25588
39. Saway, BF, Webb, T, Weber, A, Triano, M, Barley, J, Spampinato, M, et al. Functional MRI-guided motor cortex and deep brain stimulation for intractable facial pain: a novel, personalized approach in 1 patient. Oper Neurosurg (Hagerstown). (2023) 24:103–10. doi: 10.1227/ons.0000000000000440
40. Dargembeau, A, Ruby, P, and Collette, F. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and P erspective taking. J Cogn Neurosci. (2007) 19:935–44. doi: 10.1162/jocn.2007.19.6.935
41. Funahashi, S, and Andreau, JM. Prefrontal cortex and neural mechanisms of executive function. J Physiol Paris. (2013) 107:471–82. doi: 10.1016/j.jphysparis.2013.05.001
42. Ricciardi, E, Bonino, D, Gentili, C, Sani, L, Pietrini, P, and Vecchi, T. Neural correlates of spatial working memory in humans: a functional magnetic resonance imaging study comparing visual and tactile processes. Neuroscience. (2006) 139:339–49. doi: 10.1016/j.neuroscience.2005.08.045
43. Wang, S, Sun, J, Han, D, Fan, J, Yu MM, Y, Yang MM, H, et al. Magnetic resonance imaging-CCCFLS scoring system: toward predicting clinical symptoms and C5 paralysis. Global Spine J. (2023):219256822311706. doi: 10.1177/21925682231170607
44. Zald, DH, Mattson, DL, and Pardo, IV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc Natl Acad Sci USA. (2002) 99:2450–4. doi: 10.1073/pnas.042457199
45. Koenigs, M, and Grafman, J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. IJJ Behav Brain Res. (2009) 201:239–43. doi: 10.1016/j.bbr.2009.03.004
46. Liu, M, Huang, Y, Li, X, Liu, Y, Yu, R, Long, Y, et al. Aberrant frontolimbic circuit in female depressed adolescents with and without suicidal attempts: a resting-state functional magnetic resonance imaging study. Front Psychol. (2022) 13:1007144. doi: 10.3389/fpsyt.2022.1007144
47. Wang, XY, Tan, H, Li, X, Dai, LQ, Zhang, ZW, Lv, FJ, et al. Resting-state functional magnetic resonance imaging-based identification of altered brain the fractional amplitude of low frequency fluctuation in adolescent major depressive disorder patients undergoing electroconvulsive therapy. Front Psychol. (2022) 13:972968. doi: 10.3389/fpsyt.2022.972968
Keywords: cervical spondylosis, neuroimaging, rs-fMRI, AES-SDM, meta-analysis
Citation: Zhang Q and Ding H (2024) Meta-analysis of resting-state fMRI in cervical spondylosis patients using AES-SDM. Front. Neurol. 15:1439939. doi: 10.3389/fneur.2024.1439939
Received: 31 May 2024; Accepted: 21 August 2024;
Published: 24 September 2024.
Edited by:
Houqing Long, The First Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Xiaofei Hu, Army Medical University, ChinaCopyright © 2024 Zhang and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Ding, ODU3NzQ3NDM4QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.