
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Neurol. , 13 August 2024
Sec. Multiple Sclerosis and Neuroimmunology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1437913
 Soon-Tae Lee1
Soon-Tae Lee1 Hesham Abboud2
Hesham Abboud2 Sarosh R. Irani3,4
Sarosh R. Irani3,4 Hideto Nakajima5
Hideto Nakajima5 Amanda L. Piquet6
Amanda L. Piquet6 Sean J. Pittock7
Sean J. Pittock7 E. Ann Yeh8
E. Ann Yeh8 Jiawei Wang9
Jiawei Wang9 Sharmila Rajan10
Sharmila Rajan10 James Overell11
James Overell11 Jillian Smith12
Jillian Smith12 Jane St Lambert12
Jane St Lambert12 Muna El-Khairi12
Muna El-Khairi12 Marina Gafarova11
Marina Gafarova11 Jeffrey M. Gelfand13*
Jeffrey M. Gelfand13*Background: Autoimmune encephalitis (AIE) encompasses a spectrum of rare autoimmune-mediated neurological disorders, which are characterized by brain inflammation and dysfunction. Autoantibodies targeting the N-methyl-D-aspartic acid receptor (NMDAR) and leucine-rich glioma-inactivated 1 (LGI1) are the most common subtypes of antibody-positive AIE. Currently, there are no approved therapies for AIE. Interleukin-6 (IL-6) signaling plays a role in the pathophysiology of AIE. Satralizumab, a humanized, monoclonal recycling antibody that specifically targets the IL-6 receptor and inhibits IL-6 signaling, has demonstrated efficacy and safety in another autoantibody-mediated neuroinflammatory disease, aquaporin-4 immunoglobulin G antibody-positive neuromyelitis optica spectrum disorder, and has the potential to be an evidence-based disease modifying treatment in AIE.
Objectives: CIELO will evaluate the efficacy, safety, pharmacodynamics, and pharmacokinetics of satralizumab compared with placebo in patients with NMDAR-immunoglobulin G antibody-positive (IgG+) or LGI1-IgG+ AIE.
Study design: CIELO (NCT05503264) is a prospective, Phase 3, randomized, double-blind, multicenter, basket study that will enroll approximately 152 participants with NMDAR-IgG+ or LGI1-IgG+ AIE. Prior to enrollment, participants will have received acute first-line therapy. Part 1 of the study will consist of a 52-week primary treatment period, where participants will receive subcutaneous placebo or satralizumab at Weeks 0, 2, 4, and every 4 weeks thereafter. Participants may continue to receive background immunosuppressive therapy, symptomatic treatment, and rescue therapy throughout the study. Following Part 1, participants can enter an optional extension period (Part 2) to continue the randomized, double-blind study drug, start open-label satralizumab, or stop study treatment and continue with follow-up assessments.
Endpoints: The primary efficacy endpoint is the proportion of participants with a ≥1-point improvement in the modified Rankin Scale (mRS) score from study baseline and no use of rescue therapy at Week 24. Secondary efficacy assessments include mRS, Clinical Assessment Scale of Autoimmune Encephalitis (CASE), time to rescue therapy, sustained seizure cessation and no rescue therapy, Montreal Cognitive Assessment, and Rey Auditory Verbal Learning Test (RAVLT) measures. Safety, pharmacokinetics, pharmacodynamics, exploratory efficacy, and biomarker endpoints will be captured.
Conclusion: The innovative basket study design of CIELO offers the opportunity to yield prospective, robust evidence, which may contribute to the development of evidence-based treatment recommendations for satralizumab in AIE.
Autoimmune encephalitis (AIE) encompasses a spectrum of rare, autoimmune-mediated neurological disorders and is characterized by brain inflammation and dysfunction (1–4). People with AIE may experience a wide range of debilitating, and potentially life-threatening symptoms, including cognitive impairment, seizures, memory deficits, psychiatric symptoms, movement disorders, and altered mental status, which may only improve partially with standard immunotherapy and may lead to long-term neurological disability (1, 5–14). Although the closely related group of disorders that encompass AIE have shared overlapping clinical features and neuroimaging findings, they are differentiated by distinct pathophysiological mechanisms, including the presence or absence of neuronal autoantibodies that drive inflammation and synaptic dysfunction in the central nervous system (CNS), as well as characteristic clinical syndromes and epidemiologic characteristics (1–3, 15, 16).
Autoantibodies targeting the N-methyl-D-aspartic acid receptor (NMDAR) and leucine-rich glioma-inactivated 1 (LGI1) comprise the most common subtypes of antibody-positive AIE (2, 17). NMDAR immunoglobulin G antibody-positive (NMDAR-IgG+) AIE commonly affects children and younger adults (2). The primary clinical presentations include psychiatric symptoms, memory loss, seizures, speech disturbance, dyskinesia (typically, orofacial and limb dyskinesia), decreased consciousness, and autonomic dysfunction (2, 11, 18, 19). Up to 40% of patients with NMDAR-IgG+ AIE have an ovarian teratoma and, in these cases, it is thought that germinal centers within the tumor and cervical lymph nodes generate autoantibodies that are misdirected against host antigens in the CNS (20–22). LGI1-IgG+ AIE generally affects middle-aged to older patients, and is associated with frequent faciobrachial dystonic seizures that are characterized by stereotyped dystonic jerks in the face, arm or leg; localization-related seizures; rapidly progressive cognitive impairment with amnesia; and hyponatremia (12, 23–25). AIE may be autoantibody seronegative (26), or may be linked to various other antibodies targeting proteins, such as contactin-associated protein-like 2, γ-aminobutyric acid receptors type A or B, dipeptidyl-peptidase-like protein 6, and glutamic acid decarboxylase 65, each of which is associated with specific phenotypes and different levels of systemic cancer risk (27).
There are currently no approved disease-modifying therapies specifically for AIE, and treatment relies on the off-label use of existing therapies (28–30). Acute immunotherapy regimens commonly include glucocorticoids, intravenous immunoglobulin, plasma exchange therapy, or a combination of these (11, 14, 15, 28–31). In patients with refractory AIE, additional immunosuppressive therapy may involve rituximab, cyclophosphamide, tocilizumab, mycophenolate mofetil, azathioprine, bortezomib, daratumumab, tofacitinib, or low-dose IL-2 (14, 15, 28, 30–43). The current treatment approach for AIE has numerous limitations that highlight the need for higher quality, lower risk of bias evidence, particularly that generated by prospective, multicenter, randomized controlled trials, to guide treatment choices in AIE to address acute and long-term effects (2, 15, 28, 30).
Approximately 45% of patients with NMDAR-IgG+ AIE have moderate functional neurological impairments (modified Rankin scale [mRS] score 2–3) >2 years after disease onset (after the acute disease stage) (44). After almost 5 years following disease onset, 65% of patients with NMDAR-IgG+ AIE have moderate-to-severe cognitive deficits (composite score 2–4 [composite score of cognitive impairment was defined as the number of affected domains on tests of working memory, verbal and visual episodic memory, executive function, and attention]) despite improvements in functional neurological outcomes (44). In LGI1-IgG+ AIE, approximately 12–40% of patients experience relapses even after achieving remission with immunotherapy (45–47). Many patients with NMDAR-IgG+ and LGI1-IgG+ AIE continue to experience long-term impairments, failing to regain their cognitive and functional status (14, 21, 46, 48–54). This is often attributed to delayed treatment and the relatively slow or incomplete response to currently available medications, leading to ineffective control of CNS inflammation (25, 54–56). Consequently, there is an urgent need for prospectively generated high-quality evidence in AIE to guide treatment choices and to inform treatment paradigms to address both the acute and long-term effects of this rare neurological disorder.
Innovations in clinical trial designs have resulted in the development of master protocols to address unmet needs and to answer multiple clinical questions more efficiently (57). One example is the basket study design (57). The basket study design aims to evaluate a single intervention in multiple diseases or disease subtypes (57) and has been used to improve trial efficiency in both oncology (58, 59) and non-malignant conditions (60, 61). Within a basket study, each disease-specific arm (in this case, NMDAR-IgG+ and LGI1-IgG+ AIE) is analyzed independently. Each arm has outcome measures tailored to its specific disease subtype. This results in improved interpretability and reliability of study data, while leveraging the shared infrastructure and efficiencies of the master basket study protocol. AIE is particularly suited to a basket study design, given that the different autoantibody subtypes have distinct epidemiologic characteristics and pathologies while sharing other similarities.
CIELO (NCT05503264) is a Phase 3, randomized, placebo-controlled, basket study of satralizumab, an interleukin-6 (IL-6) receptor (IL-6R) inhibitor, in patients with NMDAR-IgG+ or LGI1-IgG+ AIE. IL-6 is a pro-inflammatory cytokine with pleiotropic functions, such as T-cell polarization toward an inflammatory T-helper 17 phenotype, promotion of survival and functioning of autoantibody-producing plasma cells, and blood–brain barrier disruption (62, 63). In NMDAR-IgG+ and LGI1-IgG+ AIE, processes regulated by IL-6 signaling (e.g., B- and T-cell differentiation, B-cell proliferation, and regulation of the blood–brain barrier) have been suggested to have a pathogenic role (11, 62, 64–67), and IL-6 levels are elevated in the cerebrospinal fluid (CSF) of patients with NMDAR-IgG+ AIE (68–71) and in the serum of patients with LGI1-IgG+ AIE (72). Through IL-6R inhibition, satralizumab has the potential to modulate key upstream immunopathogenic mechanisms in AIE (62, 63, 73). Retrospective studies and a case report of IL-6R inhibition have been described in patients with NMDAR-IgG+ and LGI1-IgG+ AIE, with observed clinical improvements (40, 41, 74).
CIELO is the first study of satralizumab, a humanized, immunoglobulin G2 (IgG2), monoclonal recycling antibody against the IL-6R (75), in AIE. Satralizumab provides durable inhibition of IL-6 signaling (76) and is indicated for another autoantibody-mediated neuroimmunological disease, aquaporin-4 immunoglobulin G antibody-positive neuromyelitis optica spectrum disorder, as monotherapy or as an add-on to immunosuppressive therapy (IST) (75, 77–80). As such, satralizumab has the potential to become the first evidence-based IL-6 signaling inhibition treatment in AIE.
Design and execution of studies in AIE and other rare diseases present inherent challenges. These include difficulties related to the heterogeneity of the patient population and symptoms, identification of eligible participants, appropriately powered sample size, use of placebo, availability of rescue therapy, disease outcome definitions, trial duration, and selection of clinically meaningful endpoints (81, 82). Anticipating these challenges ahead of study design can increase the likelihood of the successful generation of robust data in the field. In the last decade, nine randomized clinical trials in AIE have been initiated; six are ongoing and three have been terminated (Table 1) (29, 82–90).
CIELO is a uniquely designed basket study that aims to address the common study design challenges faced in rare disease trials by aiming to balance the interests of patients with the urgent need for scientific advancement in this field. Here, we discuss the crucial trial design and execution decisions, including the learnings from previous and ongoing AIE studies, which may significantly differentiate CIELO from other trials in AIE.
The primary objective of this study is to evaluate the efficacy, safety, pharmacodynamics (PD), and pharmacokinetics (PK) of satralizumab compared with placebo in patients with NMDAR-IgG+ or LGI1-IgG+ AIE in a randomized, double-blind period. The long-term safety and efficacy of satralizumab will be assessed over a treatment exposure period of ≥3 years, during the double-blind and then optional extension period.
CIELO is a Phase 3, randomized, double-blind, placebo-controlled, multicenter, basket study of satralizumab in patients with NMDAR-IgG+ and LGI1-IgG+ AIE (Figure 1). The study consists of two parts. In Part 1, participants will receive placebo or satralizumab over a period of 52 weeks. In Part 2, participants have the option to continue for an extension period, lasting approximately 2 years after the last patient begins Part 2.
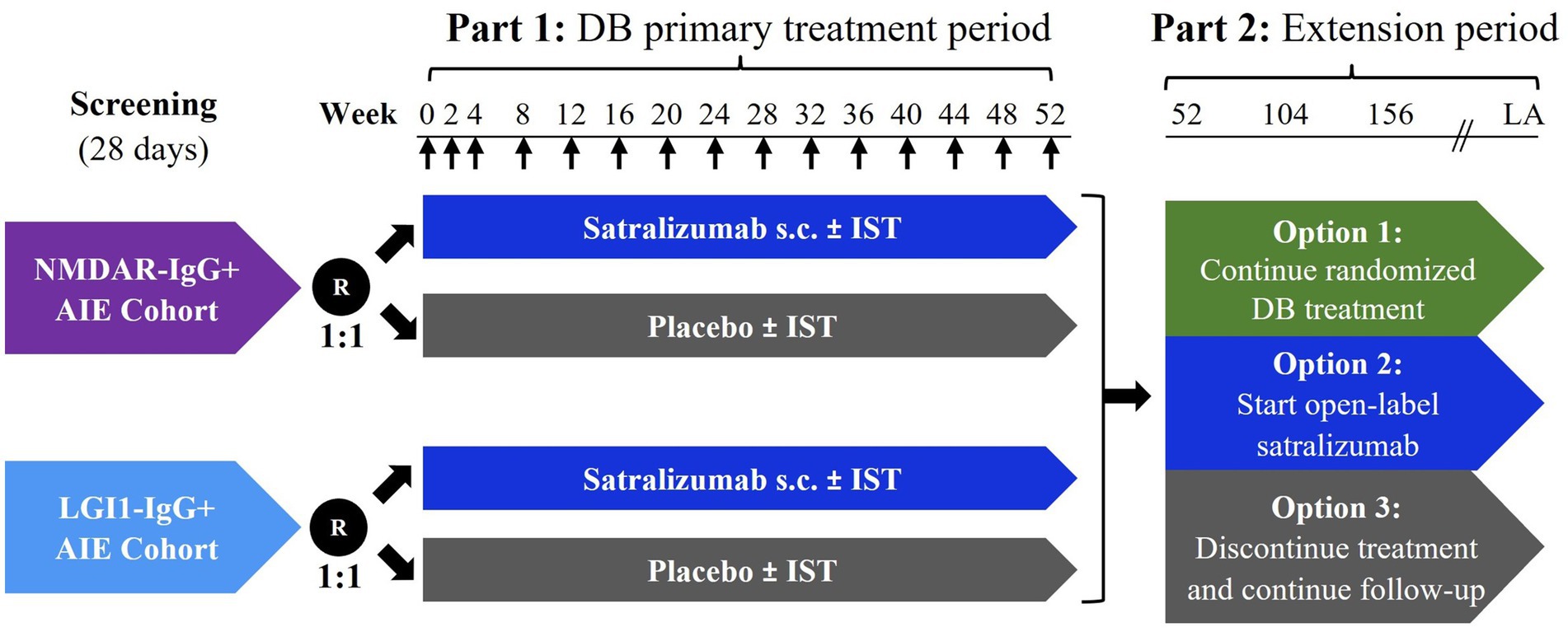
Figure 1. Study schema. ↑ Administration of subcutaneous satralizumab or placebo. Week 0 of Part 2 (extension period) coincides with Week 52 of Part 1 (primary treatment period). Participants who choose to start open-label satralizumab treatment and were treated with active drug in Part 1 will be administered a placebo dose at extension Week 2 to maintain blinding of treatment assignment in Part 1. The duration of Part 2 will be approximately 2 years after the last participant begins Part 2. AIE, autoimmune encephalitis; DB, double-blind; IST, immunosuppressive therapy; LA, last administration; LGI1-IgG+, leucine-rich glioma-inactivated 1 immunoglobulin G antibody-positive; NMDAR-IgG+, N-methyl-D-aspartic acid receptor immunoglobulin G antibody-positive; R, randomization; s.c., subcutaneous.
To avoid recruitment challenges reported by single-center trials in AIE (29, 82), CIELO will be conducted at approximately 85 sites in 15 countries, including countries in Asia, Europe, North America, and South America, maximizing the likelihood of identifying eligible participants and improving potential global generalizability. The study will enroll approximately 152 participants with NMDAR-IgG+ or LGI1-IgG+ AIE.
The basket design offers a unique opportunity to assess whether satralizumab is efficacious in these distinct patient subtypes and to what extent; this will be particularly valuable in AIE, which currently does not have subtype-specific treatment guidelines despite its heterogeneity.
The heterogeneity of AIE is challenging for trial design because it may affect the interpretability and reliability of study data. Regulators have provided guidance about being as specific as possible with disease subtypes and the use of separate studies or arms for different subtypes of AIE. The basket design allows each cohort to have its own placebo arm and be analyzed separately with independent Type I error control at a two-sided 5% significance level.
Given the clear unmet need in both newly diagnosed AIE patients and those with incomplete response to prior immunosuppressive medications, as well as evidence suggesting the potential benefit of IL-6R blockade in both populations (40, 41, 91), participants will be subclassified as “new onset” or “incomplete responders” (Table 2). Although differential efficacy is not expected, this will facilitate the potential identification of any additional findings that may advance the understanding of this complex disease and increase the eligibility and recruitment to provide a balanced representation of the real-world AIE population.
Eligible participants for the NMDAR-IgG+ AIE cohort will be ≥12 years old with a diagnosis of NMDAR-IgG+ AIE according to clinical criteria adapted from Graus et al. (1). In some clinical practice settings, access to NMDAR-IgG assays, and specifically CSF sampling for NMDAR-IgG, can be inconsistent, delayed, or difficult to achieve (9, 92). Hence, this study will include both definite NMDAR-IgG+ AIE (NMDAR-IgG-seropositivity confirmed via a cell-based assay), and probable NMDAR-IgG+ AIE in the population, as defined in Table 3. Criteria for probable NMDAR-IgG+ AIE have been published (1), showing high sensitivity and specificity in adults and children (93–95).
Clinically, adolescents with AIE present similarly to adults and use near-identical treatment regimens (21, 56, 96, 97); therefore, the study aims to recruit adolescent participants in a proportion that is representative of the real-world AIE population. CIELO will investigate a potential long-term therapy for AIE in a pediatric population (Table 1) and has the potential to provide novel insight into the treatment of pediatric AIE. However, there are well-documented increased challenges in the recruitment and retention of adolescent participants to a clinical trial, such as engaging with pediatric specialists, continuity of care, and disruptions to habitual activities (98, 99). Accordingly, this study has identified and included specific sites that routinely care for children, and specific study training materials have been developed with the aim of optimizing recruitment and retention of adolescents.
Eligible participants for the LGI1-IgG+ AIE cohort will be ≥18 years old with a diagnosis of LGI1-IgG+ AIE according to clinical criteria adapted from Graus et al. (1) (Table 3). Given the extremely rare occurrence of LGI1-IgG+ AIE in children (100), no adolescents will be included in this cohort.
Eligible participants must meet the definition of new onset or incomplete responder AIE, as defined in Table 2. The study aims to recruit both populations in representative proportions. Other key inclusion criteria include: (1) the onset of AIE symptoms ≤9 months before randomization, to ensure that patients who have an active state of inflammatory disease are enrolled, as opposed to patients who may have temporal atrophy, encephalomalacia, and long-term sequelae as a consequence of previous AIE episodes, and (2) a stable (≥24 h) mRS score of ≥2 at study baseline (Part 1, Week 0, pre-dose assessments). Patients with mRS scores of ≥2 will be included to ensure representation from patients whose deficits, such as cognitive deficits, persist despite prior treatment and “mild” mRS scores.
After providing informed consent, participants will enter a screening period of up to 28 days. For participants otherwise unable to give consent due to the severity of their disease, informed consent may be given by a legally authorized representative (or equivalent under local law) and participant assent obtained, as per local requirements.
Exclusion criteria were selected to ensure the robustness of NMDAR-IgG+ and LGI1-IgG+ AIE diagnoses, where risk of bias related to concurrent neoplastic conditions or alternative autoantibodies or neuroimmunological conditions is eliminated. These include untreated teratoma or thymoma at study baseline, history of carcinoma or malignancy with recurrence within ≤5 years before screening, known positivity to an intracellular antigen with high cancer association, any other cell-surface neuronal antibodies other than NMDAR-IgG and LGI1-IgG in the absence of NMDAR-IgG or LGI1-IgG positivity, paraneoplastic encephalitis, history of negative anti-NMDAR antibody in CSF using a cell-based assay within 9 months of symptom onset (NMDAR-IgG+ AIE cohort), evidence of diseases that may preclude participation (e.g., central or peripheral nervous system demyelinating disease, alternative cause of associated symptoms, uncontrolled concomitant disease, and infections), and prior treatment with IL-6 inhibitor therapy, anti-CD19 antibody, complement inhibitors, neonatal Fc receptor antagonists, anti-B-lymphocyte stimulator monoclonal antibody, T-cell-depleting therapies, or investigational agents (within 24 weeks prior to screening or within five half-lives of the investigational drug, whichever is longer). To maximize recruitment, participants who fail screening may qualify for rescreening at the investigator’s discretion.
All participants will have started their acute first-line therapy prior to randomization. Participants meeting the definition of incomplete responder may continue to maintain stable doses of background IST throughout the course of the study. To help reduce variability and to facilitate study interpretability, permitted background ISTs are limited to one of either mycophenolate mofetil, intravenous cyclophosphamide, or azathioprine. For incomplete responders who have previously received rituximab, a treatment course must have been initiated ≥2 months prior to screening and the last dose of rituximab must have been administered ≥4 weeks prior to randomization.
After acute first-line therapy, many experts recommend treatment with glucocorticoids, typically at a dose of >20 mg/day. Long-term glucocorticoid use for disease control (usually 3–12 months) is common in AIE, particularly LGI1-IgG+ AIE, and is associated with toxicity, adverse events (AEs), and health risks (28, 101–103). Therefore, participants who receive oral or intravenous glucocorticoids at study baseline are required to taper off using a standard taper schedule, starting 4 weeks after randomization (Week 4), unless they are in a critical-care setting. Because patients with AIE may relapse when reducing their glucocorticoid dose (28, 101), the ability to successfully taper glucocorticoids will be an important outcome of this study.
During Part 1, participants will receive placebo or satralizumab via subcutaneous (SC) injection at Weeks 0, 2, 4, and then every 4 weeks (Q4W) until the end of Part 1. Part 1 consists of a double-blinded treatment period of 52 weeks, with primary and secondary endpoint evaluation at Week 24. The 24 weeks after initiation of satralizumab represent the period after the acute symptomatic first stage, where recovery would be expected to occur, and long-term prognosis would become evident for newly diagnosed patients. Early response to treatment is correlated with overall clinical improvement and long-term functional outcome improvements in NMDAR-IgG+ and LGI1-IgG+ AIE (46, 55). A 24-week period is expected to capture early response to treatment in both the new onset and the incomplete responder populations and determine the need for any additional lines of therapy. However, some deficits, such as cognitive impairment, may persist for more than a year (44, 104). Therefore, a 52-week double-blinded treatment period was selected to characterize the efficacy of satralizumab on longer-term outcomes of cognition, quality of life measures, and regaining of daily functioning.
A common concern reported in AIE trials is the possibility of being randomly assigned to placebo (82), particularly if participants are required to discontinue all prescribed medications. Considering that AIE can have an aggressive course, all participants are permitted to receive medications that are commonly used for the symptomatic management of AIE, as described by Abboud et al. (28). Dose decreases of symptomatic treatments will only be permitted for safety reasons during the first 24 weeks of Part 1. Additionally, participants classified as incomplete responders are permitted to continue receiving the aforementioned ISTs. Rescue therapy with commonly used medications may be administered to participants who do not improve or worsen during the course of the study, in line with current clinical management practice. Introduction of a new IST, dose increases, change of background treatment, or inability to taper off glucocorticoids due to failure to improve or worsening of disease will be treated as a rescue medication event. The increase in glucocorticoid dose or failure to taper off glucocorticoids according to the protocol-directed taper will be regarded as rescue unless prespecified criteria are met.
After completing Part 1 of the study, participants can enter Part 2. The long-term consequences of AIE are thought to be critically influenced by treatment in the acute phase and subsequent months (21, 25, 55, 105). However, the benefit of immunomodulation after acute therapy and the optimal duration of treatment are poorly understood. Hence, Part 2 is key to the provision of longer-term data on the risks and benefits of satralizumab in AIE, as well as the generation of robust data on longer-term outcomes and treatment in AIE.
As a requirement to commit to an extension period at enrollment may negatively influence recruitment and retention of participants, an innovative approach is used for Part 2 that is designed to assist in retaining as many participants as possible in a longer-term assessment. Participants can choose a long-term treatment plan that is most suited to their individual clinical response and circumstance. Participants may choose to (1) continue on randomized treatment, (2) initiate open-label satralizumab (participants will continue to receive satralizumab SC Q4W if they were randomly assigned to satralizumab in Part 1 or receive satralizumab per the dosing regimen in Part 1 if they were randomly assigned to placebo), or (3) stop study treatment and continue with follow-up assessments.
Participants may be tapered off any remaining steroids or ISTs at the discretion of the investigator. This is allowed only after Week 12 of the extension period for participants choosing option 2 to ensure that participants treated with placebo in Part 1 have reached steady-state levels of satralizumab prior to glucocorticoid/IST taper. Satralizumab can be self-administered by participants (or their caregivers) who choose option 1 (from Week 0 of the extension period) and option 2 (from Week 24 of the extension period) on days that do not require additional assessments at the site, if deemed appropriate by the investigator. A telephone interview after each satralizumab dose will confirm treatment adherence and evaluate any changes in health status (e.g., new or worsening neurological symptoms or any possible AEs). The option to stop all study treatment is open to all participants at any time during Part 2. In addition, the option to start open-label satralizumab (option 2) is available to participants who chose to continue their original treatment plan (option 1) at any time.
Part 2 was designed to reflect the real-world choices made after receiving acute therapy for AIE. Incorporating patient preference will hopefully maximize the likelihood of patient participation in the extension period and provide insights into long-term outcomes in AIE.
The choice of appropriate study endpoints has been a significant challenge in AIE trial development (82). For a Phase 3 study, it is important to determine clinically relevant endpoints that have clinical validity and are also sensitive enough to measure the benefit-to-risk profile of an investigational treatment. For AIE, endpoint categories include general measures of disability/functional status, cognitive assessments, and autoimmune encephalitis-focused categorical rating scales. CIELO was designed to use the mRS, a measure that covers the broad range of symptoms that are applicable in both NMDAR-IgG+ and LGI1-IgG+ AIE.
Some previous studies have used the Clinical Assessment Scale in Autoimmune Encephalitis (CASE) scores, clinical worsening (patient or clinician observations, Lawton and Brody Instrumental Activities of Daily Living Scale [IADL]), Texas Functional Living Scale (TFLS), hospitalization (for symptoms of encephalitis), or seizure count measures as the primary endpoint (Table 1) (29, 82, 85, 88, 89); however, these are associated with some limitations. Despite widespread use in AIE clinical trials, CASE has fewer data supporting its applicability for longer-term follow-up and subtler sequelae (53, 106). CASE does not incorporate categories for death, sleep dysfunction, or autonomic dysfunction (13), and there are limited data for its use in pediatric populations (107). As for seizure count, due to inadequate measures of acute seizure reporting available and the fact that not all patients experience seizures, larger sample sizes would be required to demonstrate a clinical effect when measuring clinical worsening or seizure counts as a primary endpoint.
CIELO will use the mRS as the primary outcome measure of both the NMDAR-IgG+ and LGI1-IgG+ AIE basket study arms to evaluate the benefit of satralizumab on clinical status and disability. mRS measures overall disability and has been used in AIE studies for patients with both NMDAR-IgG+ and LGI1-IgG+ subtypes with proven validity and reliability (21, 84, 87, 108, 109) (Table 1). Historically, the mRS has also been used as an outcome measure for infectious encephalitis (110), including as a primary outcome measure in herpes simplex encephalitis studies (111). Available data on the mRS in AIE have been used to help inform the CIELO study design, particularly regarding expected changes in the placebo arm.
As an endpoint that measures disability and patients’ ability to carry out activities of daily living, the mRS may help provide a more comprehensive assessment of therapeutic efficacy than seizure counts or cognitive scoring in patients with NMDAR-IgG+ or LGI1-IgG+ AIE. To enhance the reproducibility and robustness of data, a Structured Interview for the mRS (mRS-SI), adapted from Wilson and Hareendran (112), will supplement the mRS, and the mRS-SI will be tailored to evaluate specific aspects of disability and dependence associated with AIE. A selection of other clinically meaningful patient-reported outcome (PRO), performance-based outcome (PerfO), and clinician-reported outcome endpoints have also been chosen to assess the treatment benefit of satralizumab in CIELO.
The primary efficacy endpoint in CIELO is the proportion of participants with a ≥ 1-point improvement from study baseline in the mRS score and no use of rescue therapy at Week 24 (Table 4). A 1-point change in mRS score is considered to be clinically meaningful based on the severity range covered by the scale grade (113, 114) and is used in clinical practice and observational studies to demonstrate a meaningful change in activities of daily living. Because eligible participants for this study will be impaired in carrying out activities at study baseline (as measured by mRS score ≥ 2), a ≥ 1-point improvement, rather than an mRS score of 0–2, which has been defined as a good outcome in some previous studies (21, 108), will be considered to be clinically significant and meaningful (113, 114) in all participants with differing levels of symptomatology and degree of disability.
As AIE can have an aggressive course, rapid symptom resolution is critical in preventing potentially permanent residual long-term morbidity and mortality. The need for rescue or repeated rescue therapy, due to failure to improve or worsening of AIE-related symptoms, is indicative of unsatisfactory outcomes. Therefore, when treated with satralizumab, the lack of need for rescue therapy is a key factor suggesting its treatment benefit. Freedom from rescue therapy use, combined with improvements in disability and dependence on the mRS scale in the primary endpoint, will provide an overall clinically relevant measure of an early and durable response.
Although it was ultimately decided not to incorporate the CASE scale as a primary efficacy endpoint, it is a promising tool for evaluating the severity of AIE. The CASE score is positively associated with the mRS (115) and is more representative of the diverse symptomatology of AIE than the mRS (13). Hence, it will be assessed as a secondary efficacy endpoint (Table 4). Other secondary and exploratory efficacy endpoints will assess long-term neurological PROs and PerfOs using the Montreal Cognitive Assessment (MoCA); Rey Auditory Verbal Learning Test (RAVLT); Beck Depression Inventory, second edition (BDI-II); EuroQoL 5-Dimension 5-Level; and Modified Fatigue Impact Scale (MFIS) scores (Table 4), many of which have been used in studies to assess long-term outcomes in patients with AIE (7, 116–118). Due to the global nature of the study, PRO instruments have been translated into local languages to facilitate their use. Additionally, seizure type, frequency, duration, and severity will be captured using a patient/caregiver completed seizure diary, and electroencephalogram assessments will be conducted at select time points in Part 1 of the study. Together, these assessments will provide a global understanding of disease severity and response to treatment.
Safety endpoints on incidence, seriousness, and severity of AEs, as well as change from study baseline in targeted vital signs and laboratory tests, will be assessed (Table 4). To characterize the efficacy of satralizumab treatment on longer-term outcomes, exploratory efficacy endpoints will evaluate the durability of response to satralizumab, time course of efficacy, and time to disease improvement (Table 4).
Efficacy and safety measures will be collected throughout Part 1 and Part 2 at prespecified timepoints. Further subgroup analyses will be performed for participants with probable NMDAR-IgG+ AIE and for adolescent participants.
To explore the PK, PD, and immunogenicity of satralizumab, serum, plasma, blood, and CSF samples, as appropriate, will be taken prior to each study drug administration at prespecified time points. PK sampling will be used to analyze the impact of a range of covariates on exposure (e.g., sex, race, age, and body weight), and the relationships between exposure and PD, efficacy, immunogenicity, and safety endpoints to support the recommended dose of satralizumab in the NMDAR-IgG+/LGI1-IgG+ AIE population. PD sampling will assess target engagement in response to satralizumab. Immunogenicity sampling will assess antidrug antibodies.
Biomarker analyses will explore whether satralizumab is binding to its intended target (IL-6R) and will help to elucidate the mechanism of action of any underlying treatment effect and disease pathophysiology. These analyses may help to identify biomarkers that are predictive of a response to satralizumab, prognostic biomarkers that are associated with progression to a more severe disease state, and biomarkers associated with susceptibility to developing AEs (that could potentially lead to improved AE monitoring). Exploratory biomarker research may include analysis of NMDAR-IgG and LGI1-IgG, markers of inflammation, immune cell subset activity, CNS damage, and blood samples for flow cytometry of immune cell subsets and T-, B-, and natural killer cells. CSF sampling is included as an optional study activity at prespecified timepoints in Part 1. While lumbar puncture is routine in the diagnostic workup of AIE, research-based lumbar punctures may not be feasible for all patients and may add a potential barrier to recruitment and retention. Therefore, study lumbar punctures have been made optional.
The CIELO study will compare satralizumab with placebo in approximately 152 participants with NMDAR-IgG+ or LGI1-IgG+ AIE, as defined by the study eligibility criteria. The NMDAR-IgG+ and LGI1-IgG+ AIE cohorts will be treated as separate populations and analyzed separately. Each cohort will have independent Type I error control at a two-sided 5% significance level. For adolescent participants, descriptive subgroup analyses will be performed and compared with data collected in adults to check for consistency in any observed treatment effect, and that adolescent data are comparable to the data obtained from the overall population.
Efficacy and safety analyses will be performed in the intent-to-treat and safety populations, respectively. The primary comparison of interest in each cohort is the difference between the placebo and satralizumab groups in the proportion of participants who achieve the primary endpoint, which will be tested using a Cochran–Mantel–Haenszel test by randomization stratification factors (patient population: new onset versus incomplete responders, and region: North America/Europe versus Asia versus rest of world).
Secondary efficacy endpoints will follow a cohort-specific hierarchy, allowing analyses to be tailored to the predominant clinical features and longer-term impairments in the NMDAR-IgG+ and LGI1-IgG+ AIE populations. An independent data monitoring committee will be used for data review during Part 1.
CIELO, a Phase 3, randomized, double-blind, basket study in patients with NMDAR-IgG+ or LGI1-IgG+ AIE, will investigate the efficacy and safety of satralizumab compared with placebo. There is a need for efficacious and well-tolerated AIE therapies that can shorten disease duration, achieve a more complete and longer-lasting response, decrease long-term impacts, and improve quality of life. However, the challenges inherent in designing and executing rare disease clinical trials have impacted the generation of prospective and robust evidence in this condition.
CIELO has been designed to reflect the unmet need of patients with AIE in a real-world clinical setting, using a primary endpoint that is a global measure of neurological function and requires a marked change in clinical status. The study design of CIELO has been informed by the challenges faced in previous and ongoing AIE studies (Table 1) (29, 82, 84–90). Specifically, CIELO will maximize (1) the identification of eligible participants, (2) the inclusion of newly treated patients (new onset) and patients treated with agents beyond first-line therapy (incomplete responder), (3) confidence with a placebo-controlled design, and (4) data generation and interpretability of study results.
CIELO will recruit participants across approximately 85 sites in 15 countries. Initiatives to increase awareness of AIE and the study are also underway, including the development of educational materials for patients and physicians, and establishing referral pathways with antibody-testing laboratories (patients who test positive for NMDAR/LGI1 autoantibodies will receive information on the CIELO study).
Because there is a clear unmet need in both newly treated (new onset) and refractory (incomplete responder) patient populations, the inclusion of both populations should help to shed further light on the utility of satralizumab, allow for a balanced representation of the real-world AIE population, and potentially make the study findings more generalizable to real-world care.
AIE can be an aggressive disease, and the possibility of being randomly assigned to placebo is a common concern reported in AIE trials (82). CIELO was designed toward increasing participant confidence because participants enrolled after receiving standard first-line and/or second-line therapy can choose to continue background ISTs and symptomatic treatments in addition to receiving rescue therapy when needed after enrollment (which reflects AIE management in real-world clinical practice). Furthermore, incorporating patient choice in Part 2 of the study will allow participants to choose a long-term treatment plan that is most suited to their needs and to maximize patient retention.
CIELO has taken the following steps to maximize data generation and interpretability of the study results: (1) the study population is restricted to NMDAR-IgG+ or LGI1-IgG+ subtypes to minimize heterogeneity; (2) the basket study design enables cohort-specific hierarchies to allow analyses to be tailored per NMDAR-IgG+ and LGI1-IgG+ subtype; (3) the mRS score, which has previously been used in AIE, as well as in infectious encephalitis studies, will be the primary endpoint; and (4) the doses of select background medications are kept stable until the primary endpoint timeline.
Although efforts have been taken to maximize the extent of investigations in the CIELO study, there are limitations inherent in the design of every clinical trial. CIELO includes a subset of participants with probable NMDAR-IgG+ AIE, for which consensus criteria (1) have shown high sensitivity and specificity in adults and children (93–95). The inclusion of this subgroup was intended to improve generalizability and practicality, particularly where CSF sampling for NMDAR-IgG may be inconsistent, delayed, or difficult to achieve. The study population with probable NMDAR-IgG+ AIE is expected to be low and sensitivity analyses will be conducted. MRI findings can be heterogenous in NMDAR-IgG+ and LGI1-IgG+ AIE (119, 120), and although exploratory neuroimaging outcomes, including lesional as well as volumetric measures, were seriously considered, research neuroimaging was ultimately not included as a study activity in the final protocol because of the lack of reliable and validated approaches for analyzing neuroimaging data in AIE. There is also emerging literature on sleep disorders in AIE (121, 122), and future trials may consider the inclusion of actigraphy or related measures as an exploratory outcome.
CIELO will investigate the efficacy and safety of IL-6 signaling inhibition with satralizumab, potentially offering a novel approach for the management of AIE and a greater understanding of the disease course. By anticipating the challenges commonly associated with AIE trials, it is hoped that this study will yield prospective, robust evidence that is currently lacking in the field. The successful implementation of this trial will contribute to the development of evidence-based medicine for AIE, ultimately benefiting both patients and their families who are affected by this condition.
The study is being performed in compliance with the International Conference on Harmonization, in accordance with Good Clinical Practice guidelines, and in line with the principles of the Declaration of Helsinki and applicable local, regional, and national laws. All participants are required to provide written informed consent before any study-related procedures, including screening evaluations, are performed. Institutional review board approval will be obtained at each participating center before activating each study site.
The study was prospectively registered on ClinicalTrials.gov prior to enrolling the first participant (ClinicalTrials.gov identifier: NCT05503264). Results of this study will be reported periodically, as data become available, at international congresses where AIE is of interest to the audience and published in international peer-reviewed journals.
S-TL: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization. HA: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization. SI: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization. HN: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization. AP: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization. SP: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization. EY: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization. JW: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization. SR: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization, Methodology, Visualization. JO: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization, Methodology, Visualization. JS: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization, Methodology, Visualization. JSL: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization, Methodology, Visualization. ME-K: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization, Methodology, Visualization. MG: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization, Methodology, Visualization. JG: Writing – original draft, Writing – review & editing, Investigation, Resources, Conceptualization.
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by F. Hoffmann-La Roche Ltd.
S-TL reports advisory roles for Roche/Genentech, Biofire Diagnostics, GC Pharma, Celltrion, and Advanced Neural Technologies. HA is a consultant and speaker for Biogen, Genentech, BMS, Horizon, and Alexion. HA has served as a consultant and/or advisory board member for Cycle Pharma, Alpine Pharma, and Axonics. HA received honoraria from Neurology Live. HA receives research support from Genentech, BMS, Novartis, Sanofi, UCB, and the Guthy-Jackson Charitable Foundation. HA receives royalties from UpToDate. HA serves as an Assistant Editor for Neurology Journal. SI has received honoraria/research support from UCB, Immunovant, Roche, Janssen, Cerebral Therapeutics, ADC Therapeutics, Brain, CSL Behring, and ONO Pharma. SI receives licensed royalties on patent application WO/2010/046716 entitled ‘Neurological Autoimmune Disorders,’ and has filed two other patents entitled “Diagnostic method and therapy” (WO2019211633 and US-2021-0071249-A1; PCT application WO202189788A1) and “Biomarkers” (PCT/GB2022/050614 and WO202189788A1). HN reports being a consultant/advisor for F. Hoffmann-La Roche Ltd. and has received speaker honoraria from Alexion, Biogen, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Kirin, and Takeda. AP reports research grants from the University of Colorado, Rocky Mountain MS Center, and the Foundation for Sarcoidosis; consulting fees from Genentech/Roche, UCB, EMD Serono, and Alexion; and honoraria from MedLink and publication royalties from Springer as co-editor of a medical textbook. AP serves as an unpaid member of the Medical Advisory Board for the Autoimmune Encephalitis Alliance. SP reports grants, personal fees, and nonfinancial support from Alexion Pharmaceuticals; grants, personal fees, and nonfinancial support from MedImmune, Viela Bio, and Horizon; grants from Adimune; grants, personal fees, and nonfinancial support from Genentech and Roche, and personal fees for consulting from UCB, Astellas, and Arialys. SP has two patents issued (8889102; application 12-678350; Neuromyelitis Optica Autoantibodies as a Marker for Neoplasia; and 9891219B2; application 12-573942; Methods for Treating Neuromyelitis Optica [NMO] by Administration of Eculizumab to an Individual That is Aquaporin-4 [AQP4]-IgG Autoantibody-Positive), for which he has received royalties. SP also has patents pending for IgGs to the following proteins as biomarkers of autoimmune neurological disorders: septin-5, kelch-like protein 11, GFAP, PDE10A, and MAP1B. SP is an employee of Mayo Clinic, which offers commercial NMDAR and LGI1-IgG testing. SP receives no royalties from the sale of tests done at the neuroimmunology laboratory at Mayo Clinic. EY has received research funding from NMSS, CMSC, CIHR, NIH, OIRM, SCN, CBMH Chase an Idea, SickKids Foundation, Rare Diseases Foundation, MS Scientific Foundation, McLaughlin Centre, Leong Center, and Peterson Foundation, and investigator-initiated research funding from Biogen. EY has served on a scientific advisory board for F. Hoffmann-La Roche and a data safety monitoring board for TG Therapeutics and has received speaker honoraria from Biogen, JHU, Saudi Epilepsy Society, NYU, MS-ATL, ACRS, PRIME, and CNPS. JW serves as a trial steering committee member for the CIELO study. JO and MG are employees and shareholders of F. Hoffmann-La Roche Ltd. JS, JSL, and ME-K are employees of Roche Products Ltd. and shareholders of F. Hoffmann-La Roche Ltd. SR is an employee and shareholder of Genentech, Inc. JG receives research support to his institution from F. Hoffmann-La Roche Ltd. for clinical trials and serves as chair of the Trial Steering Committee for the CIELO study. JG has also received research support to his institution for clinical trials from Vigil Neurosciences; and received personal fees for consulting for Arialys and Ventyx Bio. The authors declare that this study received funding from F. Hoffmann-La Roche. The sponsor had the following involvement in the study: study design, collection, analysis and the decision to submit it for publication. Medical writing assistance was provided by Reece Bracewell, MBiolSci and Nicole Ellman, PhD, of Apothecom, London, UK, and was funded by F. Hoffmann-La Roche Ltd. All authors contributed to the conceptualisation, drafting, revising, and critical reviewing of the manuscript for intellectual content, and approved the final version of this manuscript to be published.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Graus, F, Titulaer, MJ, Balu, R, Benseler, S, Bien, CG, Cellucci, T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
2. Dalmau, J, and Graus, F. Antibody-mediated encephalitis. N Engl J Med. (2018) 378:840–51. doi: 10.1056/NEJMra1708712
3. Kelley, BP, Patel, SC, Marin, HL, Corrigan, JJ, Mitsias, PD, and Griffith, B. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR Am J Neuroradiol. (2017) 38:1070–8. doi: 10.3174/ajnr.A5086
4. Venkatesan, A, Tunkel, AR, Bloch, KC, Lauring, AS, Sejvar, J, Bitnun, A, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. (2013) 57:1114–28. doi: 10.1093/cid/cit458
5. Blum, RA, Tomlinson, AR, Jetté, N, Kwon, CS, Easton, A, and Yeshokumar, AK. Assessment of long-term psychosocial outcomes in anti-NMDA receptor encephalitis. Epilepsy Behav. (2020) 108:107088. doi: 10.1016/j.yebeh.2020.107088
6. Broadley, J, Seneviratne, U, Beech, P, Buzzard, K, Butzkueven, H, O'Brien, T, et al. Prognosis in autoimmune encephalitis: database. Data Brief. (2018) 21:2694–703. doi: 10.1016/j.dib.2018.11.020
7. Diaz-Arias, LA, Yeshokumar, AK, Glassberg, B, Sumowski, JF, Easton, A, Probasco, JC, et al. Fatigue in survivors of autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1064. doi: 10.1212/NXI.0000000000001064
8. Ghimire, P, Khanal, UP, Gajurel, BP, Karn, R, Rajbhandari, R, Paudel, S, et al. Anti-LGI1, Anti-GABABR, and Anti-CASPR2 Encephalitides in Asia: a systematic review. Brain Behav. (2020) 10:e01793. doi: 10.1002/brb3.1793
9. Guasp, M, Modena, Y, Armangue, T, Dalmau, J, and Graus, F. Clinical features of seronegative, but CSF antibody-positive, anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e659. doi: 10.1212/NXI.0000000000000659
10. Huang, Q, Xie, Y, Hu, Z, and Tang, X. Anti-N-methyl-D-aspartate receptor encephalitis: a review of pathogenic mechanisms, treatment, prognosis. Brain Res. (2020) 1727:146549. doi: 10.1016/j.brainres.2019.146549
11. Leypoldt, F, Armangue, T, and Dalmau, J. Autoimmune encephalopathies. Ann N Y Acad Sci. (2015) 1338:94–114. doi: 10.1111/nyas.12553
12. Shao, X, Fan, S, Luo, H, Wong, TY, Zhang, W, Guan, H, et al. Brain magnetic resonance imaging characteristics of anti-leucine-rich glioma-inactivated 1 encephalitis and their clinical relevance: a single-center study in China. Front Neurol. (2020) 11:618109. doi: 10.3389/fneur.2020.618109
13. Abboud, H, Briggs, F, Buerki, R, Elkasaby, M, BacaVaca, GF, Fotedar, N, et al. Residual symptoms and long-term outcomes after all-cause autoimmune encephalitis in adults. J Neurol Sci. (2022) 434:120124. doi: 10.1016/j.jns.2021.120124
14. Shin, YW, Lee, ST, Park, KI, Jung, KH, Jung, KY, Lee, SK, et al. Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord. (2018) 11:1756285617722347. doi: 10.1177/1756285617722347
15. Abboud, H, Probasco, JC, Irani, S, Ances, B, Benavides, DR, Bradshaw, M, et al. Autoimmune encephalitis: proposed best practice recommendations for diagnosis and acute management. J Neurol Neurosurg Psychiatry. (2021) 92:757–68. doi: 10.1136/jnnp-2020-325300
16. Dalmau, J, and Rosenfeld, MR. Autoimmune encephalitis update. Neuro Oncol. (2014) 16:771–8. doi: 10.1093/neuonc/nou030
17. Goodfellow, JA, and Mackay, GA. Autoimmune encephalitis. J R Coll Physicians Edinb. (2019) 49:287–94. doi: 10.4997/jrcpe.2019.407
18. Abbatemarco, JR, Yan, C, Kunchok, A, and Rae-Grant, A. Antibody-mediated autoimmune encephalitis: a practical approach. Cleve Clin J Med. (2021) 88:459–71. doi: 10.3949/ccjm.88a.20122
19. Yan, L, Zhang, S, Huang, X, Tang, Y, and Wu, J. Clinical study of autonomic dysfunction in patients with anti-NMDA receptor encephalitis. Front Neurol. (2021) 12:609750. doi: 10.3389/fneur.2021.609750
20. Al-Diwani, A, Theorell, J, Damato, V, Bull, J, McGlashan, N, Green, E, et al. Cervical lymph nodes and ovarian teratomas as germinal centres in NMDA receptor-antibody encephalitis. Brain. (2022) 145:2742–54. doi: 10.1093/brain/awac088
21. Titulaer, MJ, McCracken, L, Gabilondo, I, Armangue, T, Glaser, C, Iizuka, T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. (2013) 12:157–65. doi: 10.1016/S1474-4422(12)70310-1
22. Wu, CY, Wu, JD, and Chen, CC. The association of ovarian teratoma and anti-N-methyl-D-aspartate receptor encephalitis: an updated integrative review. Int J Mol Sci. (2021) 22:e659. doi: 10.3390/ijms222010911
23. Irani, SR, Alexander, S, Waters, P, Kleopa, KA, Pettingill, P, Zuliani, L, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. (2010) 133:2734–48. doi: 10.1093/brain/awq213
24. Ramberger, M, Berretta, A, Tan, JMM, Sun, B, Michael, S, Yeo, T, et al. Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms. Brain. (2020) 143:1731–45. doi: 10.1093/brain/awaa104
25. Thompson, J, Bi, M, Murchison, AG, Makuch, M, Bien, CG, Chu, K, et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain. (2018) 141:348–56. doi: 10.1093/brain/awx323
26. Lee, WJ, Lee, HS, Kim, DY, Lee, HS, Moon, J, Park, KI, et al. Seronegative autoimmune encephalitis: clinical characteristics and factors associated with outcomes. Brain. (2022) 145:3509–21. doi: 10.1093/brain/awac166
27. Graus, F, Vogrig, A, Muñiz-Castrillo, S, Antoine, JG, Desestret, V, Dubey, D, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1014. doi: 10.1212/nxi.0000000000001014
28. Abboud, H, Probasco, J, Irani, SR, Ances, B, Benavides, DR, Bradshaw, M, et al. Autoimmune encephalitis: proposed recommendations for symptomatic and long-term management. J Neurol Neurosurg Psychiatry. (2021) 92:897–907. doi: 10.1136/jnnp-2020-325302
29. Dubey, D, Britton, J, McKeon, A, Gadoth, A, Zekeridou, A, Lopez Chiriboga, SA, et al. Randomized placebo-controlled trial of intravenous immunoglobulin in autoimmune LGI1/CASPR2 epilepsy. Ann Neurol. (2020) 87:313–23. doi: 10.1002/ana.25655
30. Hermetter, C, Fazekas, F, and Hochmeister, S. Systematic review: syndromes, early diagnosis, and treatment in autoimmune encephalitis. Front Neurol. (2018) 9:706. doi: 10.3389/fneur.2018.00706
31. Lancaster, E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol. (2016) 12:1–13. doi: 10.3988/jcn.2016.12.1.1
32. Scheibe, F, Ostendorf, L, Prüss, H, Radbruch, H, Aschman, T, Hoffmann, S, et al. Daratumumab for treatment-refractory antibody-mediated diseases in neurology. Eur J Neurol. (2022) 29:1847–54. doi: 10.1111/ene.15266
33. Scheibe, F, Ostendorf, L, Reincke, SM, Prüss, H, von Brünneck, AC, Köhnlein, M, et al. Daratumumab treatment for therapy-refractory anti-Caspr2 encephalitis. J Neurol. (2020) 267:317–23. doi: 10.1007/s00415-019-09585-6
34. Scheibe, F, Prüss, H, Mengel, AM, Kohler, S, Nümann, A, Köhnlein, M, et al. Bortezomib for treatment of therapy-refractory anti-NMDA receptor encephalitis. Neurology. (2017) 88:366–70. doi: 10.1212/wnl.0000000000003536
35. Behrendt, V, Krogias, C, Reinacher-Schick, A, Gold, R, and Kleiter, I. Bortezomib treatment for patients with anti-N-methyl-D-aspartate receptor encephalitis. JAMA Neurol. (2016) 73:1251–3. doi: 10.1001/jamaneurol.2016.2588
36. Keddie, S, Crisp, SJ, Blackaby, J, Cox, A, Coles, A, Hart, M, et al. Plasma cell depletion with bortezomib in the treatment of refractory N-methyl-D-aspartate (NMDA) receptor antibody encephalitis. Rational developments in neuroimmunological treatment. Eur J Neurol. (2018) 25:1384–8. doi: 10.1111/ene.13759
37. Zhang, XT, Wang, CJ, Wang, BJ, and Guo, SG. The short-term efficacy of combined treatments targeting B cell and plasma cell in severe and refractory anti-N-methyl-D-aspartate receptor encephalitis: two case reports. CNS Neurosci Ther. (2019) 25:151–3. doi: 10.1111/cns.13078
38. Wang, T, Wang, B, Zeng, Z, Li, H, Zhang, F, Ruan, X, et al. Efficacy and safety of bortezomib in rituximab-resistant anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis as well as the clinical characteristics: an observational study. J Neuroimmunol. (2021) 354:577527. doi: 10.1016/j.jneuroim.2021.577527
39. Ratuszny, D, Skripuletz, T, Wegner, F, Groß, M, Falk, C, Jacobs, R, et al. Case report: daratumumab in a patient with severe refractory anti-NMDA receptor encephalitis. Front Neurol. (2020) 11:602102. doi: 10.3389/fneur.2020.602102
40. Lee, WJ, Lee, ST, Moon, J, Sunwoo, JS, Byun, JI, Lim, JA, et al. Tocilizumab in autoimmune encephalitis refractory to rituximab: an institutional cohort study. Neurotherapeutics. (2016) 13:824–32. doi: 10.1007/s13311-016-0442-6
41. Lee, WJ, Lee, ST, Shin, YW, Lee, HS, Shin, HR, Kim, DY, et al. Teratoma removal, steroid, IVIG, rituximab and tocilizumab (T-Sirt) in anti-NMDAR encephalitis. Neurotherapeutics. (2021) 18:474–87. doi: 10.1007/s13311-020-00921-7
42. Lim, JA, Lee, ST, Moon, J, Jun, JS, Park, BS, Byun, JI, et al. New feasible treatment for refractory autoimmune encephalitis: low-dose Interleukin-2. J Neuroimmunol. (2016) 299:107–11. doi: 10.1016/j.jneuroim.2016.09.001
43. Jang, Y, Lee, WJ, Lee, HS, Chu, K, Lee, SK, and Lee, ST. Tofacitinib treatment for refractory autoimmune encephalitis. Epilepsia. (2021) 62:e53–9. doi: 10.1111/epi.16848
44. Heine, J, Kopp, UA, Klag, J, Ploner, CJ, Prüss, H, and Finke, C. Long-term cognitive outcome in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. (2021) 90:949–61. doi: 10.1002/ana.26241
45. Zhong, R, Chen, Q, Zhang, X, Zhang, H, and Lin, W. Relapses of anti-NMDAR, anti-GABABR, and anti-LGI1 encephalitis: a retrospective cohort study. Front Immunol. (2022) 13:918396. doi: 10.3389/fimmu.2022.918396
46. van Sonderen, A, Thijs, RD, Coenders, EC, Jiskoot, LC, Sanchez, E, de Bruijn, MA, et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology. (2016) 87:1449–56. doi: 10.1212/wnl.0000000000003173
47. Smith, KM, Dubey, D, Liebo, GB, Flanagan, EP, and Britton, JW. Clinical course and features of seizures associated with LGI1-antibody encephalitis. Neurology. (2021) 97:e1141–9. doi: 10.1212/wnl.0000000000012465
48. Hang, HL, Zhang, JH, Chen, DW, Lu, J, and Shi, JP. Clinical characteristics of cognitive impairment and 1-year outcome in patients with anti-LGI1 antibody encephalitis. Front Neurol. (2020) 11:852. doi: 10.3389/fneur.2020.00852
49. McKeon, GL, Robinson, GA, Ryan, AE, Blum, S, Gillis, D, Finke, C, et al. Cognitive outcomes following anti-N-methyl-D-aspartate receptor encephalitis: a systematic review. J Clin Exp Neuropsychol. (2018) 40:234–52. doi: 10.1080/13803395.2017.1329408
50. Rodriguez, A, Klein, CJ, Sechi, E, Alden, E, Basso, MR, Pudumjee, S, et al. LGI1 antibody encephalitis: acute treatment comparisons and outcome. J Neurol Neurosurg Psychiatry. (2022) 93:309–15. doi: 10.1136/jnnp-2021-327302
51. Finke, C, Kopp, UA, Pruss, H, Dalmau, J, Wandinger, KP, and Ploner, CJ. Cognitive deficits following anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. (2012) 83:195–8. doi: 10.1136/jnnp-2011-300411
52. Gibson, LL, McKeever, A, Coutinho, E, Finke, C, and Pollak, TA. Cognitive impact of neuronal antibodies: encephalitis and beyond. Transl Psychiatry. (2020) 10:304. doi: 10.1038/s41398-020-00989-x
53. Aboseif, A, Li, Y, Amin, M, Lapin, B, Milinovich, A, Abbatemarco, JR, et al. Clinical determinants of longitudinal disability in LGI1-IgG autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. (2024) 11:e200178. doi: 10.1212/nxi.0000000000200178
54. Titulaer, MJ, McCracken, L, Gabilondo, I, Iizuka, T, Kawachi, I, Bataller, L, et al. Late-onset anti-NMDA receptor encephalitis. Neurology. (2013) 81:1058–63. doi: 10.1212/WNL.0b013e3182a4a49c
55. Balu, R, McCracken, L, Lancaster, E, Graus, F, Dalmau, J, and Titulaer, MJ. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology. (2019) 92:e244–52. doi: 10.1212/WNL.0000000000006783
56. Nosadini, M, Thomas, T, Eyre, M, Anlar, B, Armangue, T, Benseler, SM, et al. International consensus recommendations for the treatment of pediatric NMDAR antibody encephalitis. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1052. doi: 10.1212/NXI.0000000000001052
57. Woodcock, J, and LaVange, LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. (2017) 377:62–70. doi: 10.1056/NEJMra1510062
58. Heinrich, MC, Joensuu, H, Demetri, GD, Corless, CL, Apperley, J, Fletcher, JA, et al. Phase II, open-label study evaluating the activity of imatinib in treating life-threatening malignancies known to be associated with imatinib-sensitive tyrosine kinases. Clin Cancer Res. (2008) 14:2717–25. doi: 10.1158/1078-0432.ccr-07-4575
59. Hyman, DM, Puzanov, I, Subbiah, V, Faris, JE, Chau, I, Blay, JY, et al. Vemurafenib in multiple nonmelanoma cancers with Braf V600 mutations. N Engl J Med. (2015) 373:726–36. doi: 10.1056/NEJMoa1502309
60. Studies in Progress. Antibacterial leadership resistance group. Available at: http://arlg.org/studies-in-progress (Accessed October 2023).
61. Park, JJH, Siden, E, Zoratti, MJ, Dron, L, Harari, O, Singer, J, et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials. (2019) 20:572. doi: 10.1186/s13063-019-3664-1
62. Kimura, A, and Kishimoto, T. Il-6: regulator of Treg/Th17 balance. Eur J Immunol. (2010) 40:1830–5. doi: 10.1002/eji.201040391
63. Takeshita, Y, Fujikawa, S, Serizawa, K, Fujisawa, M, Matsuo, K, Nemoto, J, et al. New Bbb model reveals that Il-6 blockade suppressed the Bbb disorder, preventing onset of Nmosd. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1076. doi: 10.1212/nxi.0000000000001076
64. Armangue, T, Spatola, M, Vlagea, A, Mattozzi, S, Carceles-Cordon, M, Martinez-Heras, E, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. (2018) 17:760–72. doi: 10.1016/S1474-4422(18)30244-8
65. Ding, YW, Pan, SY, Xie, W, Shen, HY, and Wang, HH. Elevated soluble Fas and Fasl in cerebrospinal fluid and serum of patients with anti-N-methyl-D-aspartate receptor encephalitis. Front Neurol. (2018) 9:904. doi: 10.3389/fneur.2018.00904
66. Martinez-Hernandez, E, Horvath, J, Shiloh-Malawsky, Y, Sangha, N, Martinez-Lage, M, and Dalmau, J. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. (2011) 77:589–93. doi: 10.1212/WNL.0b013e318228c136
67. Helmstaedter, C, Hansen, N, Leelaarporn, P, Schwing, K, Oender, D, Widman, G, et al. Specific B- and T-cell populations are associated with cognition in patients with epilepsy and antibody positive and negative suspected limbic encephalitis. J Neurol. (2021) 268:455–66. doi: 10.1007/s00415-020-10158-1
68. Ma, Y, Wang, J, Guo, S, Meng, Z, Ren, Y, Xie, Y, et al. Cytokine/chemokine levels in the CSF and serum of anti-NMDAR encephalitis: a systematic review and meta-analysis. Front Immunol. (2022) 13:1064007. doi: 10.3389/fimmu.2022.1064007
69. Byun, JI, Lee, ST, Moon, J, Jung, KH, Sunwoo, JS, Lim, JA, et al. Distinct intrathecal Interleukin-17/Interleukin-6 activation in anti-N-methyl-D-aspartate receptor encephalitis. J Neuroimmunol. (2016) 297:141–7. doi: 10.1016/j.jneuroim.2016.05.023
70. Kothur, K, Wienholt, L, Mohammad, SS, Tantsis, EM, Pillai, S, Britton, PN, et al. Utility of CSF cytokine/chemokines as markers of active intrathecal inflammation: comparison of demyelinating, anti-NMDAR and enteroviral encephalitis. PLoS One. (2016) 11:e0161656. doi: 10.1371/journal.pone.0161656
71. Chen, J, Ding, Y, Zheng, D, Wang, Z, Pan, S, Ji, T, et al. Elevation of Ykl-40 in the CSF of anti-NMDAR encephalitis patients is associated with poor prognosis. Front Neurol. (2018) 9:727. doi: 10.3389/fneur.2018.00727
72. Borko, T, Mizenko, C, Kammeyer, R, Ritchie, A, Selva, S, Barrera, B, et al. Biomarkers of neuronal and glial injury in leucine-rich glioma inactivated-1 (LGI1) autoimmune encephalitis patients: a pilot study (P5-5.013). Neurology. (2023) 100:4925. doi: 10.1212/WNL.0000000000204310
73. Kishimoto, T, Kang, S, and Tanaka, T. IL-6: a new era for the treatment of autoimmune inflammatory diseases. Tokyo: Springer Japan (2015).
74. Jang, S, Kim, SY, Kim, WJ, Chae, JH, Kim, KJ, and Lim, BC. A case of pediatric anti-leucine-rich glioma inactivated 1 encephalitis with faciobrachial dystonic seizure. Brain Dev. (2023) 45:348–53. doi: 10.1016/j.braindev.2023.02.003
75. US Food and Drug Administration. Enspryng prescribing information. (2020). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761149s000lbl.pdf (Accessed September 2023).
76. Lennon-Chrimes, S, Baumann, HS, Klingelschmitt, G, Kou, X, Anania, VG, Ito, H, et al. Characterisation of the PK and PD of satralizumab, a recycling antibody, to support Q4W dosing in patients with NMOSD (1483). Neurology. (2020) 94:1483. doi: 10.1212/WNL.94.15_supplement.1483
77. Traboulsee, A, Greenberg, BM, Bennett, JL, Szczechowski, L, Fox, E, Shkrobot, S, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. (2020) 19:402–12. doi: 10.1016/s1474-4422(20)30078-8
78. Kleiter, I, Traboulsee, A, Palace, J, Yamamura, T, Fujihara, K, Saiz, A, et al. Long-term efficacy of satralizumab in Aqp4-IgG-seropositive neuromyelitis optica spectrum disorder from SAkuraSky and SAkuraStar. Neurol Neuroimmunol Neuroinflamm. (2023) 10:e200071. doi: 10.1212/nxi.0000000000200071
79. Yamamura, T, Kleiter, I, Fujihara, K, Palace, J, Greenberg, B, Zakrzewska-Pniewska, B, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med. (2019) 381:2114–24. doi: 10.1056/NEJMoa1901747
80. Yamamura, T, Weinshenker, B, Yeaman, MR, De Seze, J, Patti, F, Lobo, P, et al. Long-term safety of satralizumab in Neuromyelitis Optica Spectrum Disorder (NMOSD) from SAkuraSky and SAkuraStar. Mult Scler Relat Disord. (2022) 66:104025. doi: 10.1016/j.msard.2022.104025
81. Pizzamiglio, C, Vernon, HJ, Hanna, MG, and Pitceathly, RDS. Designing clinical trials for rare diseases: unique challenges and opportunities. Nat Rev Methods Primers. (2022) 2:s43586-022-00100-2. doi: 10.1038/s43586-022-00100-2
82. Blackburn, KM, Denney, DA, Hopkins, SC, and Vernino, SA. Low recruitment in a double-blind, placebo-controlled trial of ocrelizumab for autoimmune encephalitis: a case series and review of lessons learned. Neurol Ther. (2022) 11:893–903. doi: 10.1007/s40120-022-00327-x
83. Nct05503264. Available at: https://classic.clinicaltrials.gov/ct2/show/nct05503264 (Accessed September 2023).
84. Nct04372615. Available at: https://classic.clinicaltrials.gov/ct2/show/nct04372615 (Accessed September 2023).
85. Nct04875975. Available at: https://classic.clinicaltrials.gov/ct2/show/nct04875975 (Accessed September 2023).
86. Lennox, B, Yeeles, K, Jones, PB, Zandi, M, Joyce, E, Yu, LM, et al. Intravenous immunoglobulin and rituximab versus placebo treatment of antibody-associated psychosis: study protocol of a randomised phase Iia double-blinded placebo-controlled trial (SINAPPS2). Trials. (2019) 20:331. doi: 10.1186/s13063-019-3336-1
87. Wickel, J, Chung, HY, Platzer, S, Lehmann, T, Pruss, H, Leypoldt, F, et al. Generate-boost: study protocol for a prospective, multicenter, randomized controlled, double-blinded Phase II trial to evaluate efficacy and safety of bortezomib in patients with severe autoimmune encephalitis. Trials. (2020) 21:625. doi: 10.1186/s13063-020-04516-7
88. Nct05177939. Available at: https://classic.clinicaltrials.gov/ct2/show/nct05177939 (Accessed September 2023).
89. Nct02697292. Available at: https://classic.clinicaltrials.gov/ct2/show/ (Accessed September 2023).
90. Nct03542279. Available at: https://classic.clinicaltrials.gov/ct2/show/nct03542279 (Accessed September 2023).
91. Randell, RL, Adams, AV, and Van Mater, H. Tocilizumab in refractory autoimmune encephalitis: a series of pediatric cases. Pediatr Neurol. (2018) 86:66–8. doi: 10.1016/j.pediatrneurol.2018.07.016
92. Gresa-Arribas, N, Titulaer, MJ, Torrents, A, Aguilar, E, McCracken, L, Leypoldt, F, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. (2014) 13:167–77. doi: 10.1016/s1474-4422(13)70282-5
93. Kaneko, A, Kaneko, J, Tominaga, N, Kanazawa, N, Hattori, K, Ugawa, Y, et al. Pitfalls in clinical diagnosis of anti-NMDA receptor encephalitis. J Neurol. (2018) 265:586–96. doi: 10.1007/s00415-018-8749-3
94. Ho, ACC, Mohammad, SS, Pillai, SC, Tantsis, E, Jones, H, Ho, R, et al. High sensitivity and specificity in proposed clinical diagnostic criteria for anti-N-methyl-D-aspartate receptor encephalitis. Dev Med Child Neurol. (2017) 59:1256–60. doi: 10.1111/dmcn.13579
95. Nishida, H, Kohyama, K, Kumada, S, Takanashi, JI, Okumura, A, Horino, A, et al. Evaluation of the diagnostic criteria for anti-NMDA receptor encephalitis in Japanese children. Neurology. (2021) 96:e2070–7. doi: 10.1212/wnl.0000000000011789
96. Bien, CG, and Bien, CI. Autoimmune encephalitis in children and adolescents. Neurol Res Pract. (2020) 2:4. doi: 10.1186/s42466-019-0047-8
97. Florance, NR, Davis, RL, Lam, C, Szperka, C, Zhou, L, Ahmad, S, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. (2009) 66:11–8. doi: 10.1002/ana.21756
98. Knox, CA, and Burkhart, PV. Issues related to children participating in clinical research. J Pediatr Nurs. (2007) 22:310–8. doi: 10.1016/j.pedn.2007.02.004
99. Watson, SE, Smith, P, Snowden, J, Vaughn, V, Cottrell, L, Madden, CA, et al. Facilitators and barriers to pediatric clinical trial recruitment and retention in rural and community settings: a scoping review of the literature. Clin Transl Sci. (2022) 15:838–53. doi: 10.1111/cts.13220
100. López-Chiriboga, AS, Klein, C, Zekeridou, A, McKeon, A, Dubey, D, Flanagan, EP, et al. LGI1 and CASPR2 neurological autoimmunity in children. Ann Neurol. (2018) 84:473–80. doi: 10.1002/ana.25310
101. Irani, SR, Stagg, CJ, Schott, JM, Rosenthal, CR, Schneider, SA, Pettingill, P, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain. (2013) 136:3151–62. doi: 10.1093/brain/awt212
102. Li, D, Huang, T, Zhang, F, Zhang, X, Dou, J, Wang, C, et al. Long-term efficacy and safety of different corticosteroid courses plus mycophenolate mofetil for autoimmune encephalitis with neuronal surface antibodies without tumor. Front Immunol. (2023) 14:1195172. doi: 10.3389/fimmu.2023.1195172
103. Galati, A, Brown, ES, Bove, R, Vaidya, A, and Gelfand, J. Glucocorticoids for therapeutic immunosuppression: clinical pearls for the practicing neurologist. J Neurol Sci. (2021) 430:120004. doi: 10.1016/j.jns.2021.120004
104. Binks, SNM, Veldsman, M, Easton, A, Leite, MI, Okai, D, Husain, M, et al. Residual fatigue and cognitive deficits in patients after leucine-rich glioma-inactivated 1 antibody encephalitis. JAMA Neurol. (2021) 78:617–9. doi: 10.1001/jamaneurol.2021.0477
105. Nosadini, M, Mohammad, SS, Ramanathan, S, Brilot, F, and Dale, RC. Immune therapy in autoimmune encephalitis: a systematic review. Expert Rev Neurother. (2015) 15:1391–419. doi: 10.1586/14737175.2015.1115720
106. Brenner, J, Olijslagers, SHC, Crijnen, YS, de Vries, JM, Mandarakas, MR, and Titulaer, MJ. Clinical outcome assessments in encephalitis. Neurol Neuroimmunol Neuroinflamm. (2024) 11:e200168. doi: 10.1212/nxi.0000000000200168
107. Panda, PK, Sharawat, IK, Ramachandran, A, Elwadhi, A, Tomar, A, Bhardwaj, S, et al. Validity and prognostic utility of clinical assessment scale for autoimmune encephalitis (CASE) score in children with autoimmune encephalitis. Brain Dev. (2023) 45:8–15. doi: 10.1016/j.braindev.2022.09.009
108. Gadoth, A, Pittock, SJ, Dubey, D, McKeon, A, Britton, JW, Schmeling, JE, et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG-positive patients. Ann Neurol. (2017) 82:79–92. doi: 10.1002/ana.24979
109. Wilson, JT, Hareendran, A, Hendry, A, Potter, J, Bone, I, and Muir, KW. Reliability of the modified Rankin scale across multiple raters: benefits of a structured interview. Stroke. (2005) 36:777–81. doi: 10.1161/01.STR.0000157596.13234.95
110. Dubey, D, Pittock, SJ, Kelly, CR, McKeon, A, Lopez-Chiriboga, AS, Lennon, VA, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. (2018) 83:166–77. doi: 10.1002/ana.25131
111. Abbuehl, LS, Hofmann, E, Hakim, A, and Dietmann, A. Can we forecast poor outcome in herpes simplex and varicella zoster encephalitis? A narrative review. Front Neurol. (2023) 14:1130090. doi: 10.3389/fneur.2023.1130090
112. Wilson, L, and Hareendran, A. Stuctured interview for the modified Rankin scale. Questionnaire and guidelines. (2002). Available at: https://toneurologiaufpr.files.wordpress.com/2013/04/modified-rankin-scale-structured-interview.pdf.
113. Banks, JL, and Marotta, CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. (2007) 38:1091–6. doi: 10.1161/01.STR.0000258355.23810.c6
114. Harrison, JK, McArthur, KS, and Quinn, TJ. Assessment scales in stroke: clinimetric and clinical considerations. Clin Interv Aging. (2013) 8:201–11. doi: 10.2147/CIA.S32405
115. Cai, MT, Lai, QL, Zheng, Y, Fang, GL, Qiao, S, Shen, CH, et al. Validation of the clinical assessment scale for autoimmune encephalitis: a multicenter study. Neurol Ther. (2021) 10:985–1000. doi: 10.1007/s40120-021-00278-9
116. de Bruijn, M, Aarsen, FK, van Oosterhout, MP, van der Knoop, MM, Catsman-Berrevoets, CE, Schreurs, MWJ, et al. Long-term neuropsychological outcome following pediatric anti-NMDAR encephalitis. Neurology. (2018) 90:e1997–2005. doi: 10.1212/WNL.0000000000005605
117. Hebert, J, Day, GS, Steriade, C, Wennberg, RA, and Tang-Wai, DF. Long-term cognitive outcomes in patients with autoimmune encephalitis. Can J Neurol Sci. (2018) 45:540–4. doi: 10.1017/cjn.2018.33
118. Kvam, KA, Stahl, JP, Chow, FC, Soldatos, A, Tattevin, P, Sejvar, J, et al. Outcome and sequelae of autoimmune encephalitis. J Clin Neurol. (2024) 20:3–22. doi: 10.3988/jcn.2023.0242
119. Huang, EY, Gao, H, and Zhong, N. Heterogeneity of clinical features, EEG and brain imaging findings in anti-leucine-rich glioma-inactivated protein 1 autoimmune encephalitis: a retrospective case series study and review of the literature. Acta Epileptol. (2023) 5:21. doi: 10.1186/s42494-023-00132-5
120. Bacchi, S, Franke, K, Wewegama, D, Needham, E, Patel, S, and Menon, D. Magnetic resonance imaging and positron emission tomography in anti-NMDA receptor encephalitis: a systematic review. J Clin Neurosci. (2018) 52:54–9. doi: 10.1016/j.jocn.2018.03.026
121. Muñoz-Lopetegi, A, Graus, F, Dalmau, J, and Santamaria, J. Sleep disorders in autoimmune encephalitis. Lancet Neurol. (2020) 19:1010–22. doi: 10.1016/s1474-4422(20)30341-0
Keywords: autoimmune encephalitis (AIE), clinical trial, leucine-rich glioma-inactivated 1 (LGI1), N-methyl-D-aspartic acid receptor (NMDAR), satralizumab
Citation: Lee S-T, Abboud H, Irani SR, Nakajima H, Piquet AL, Pittock SJ, Yeh EA, Wang J, Rajan S, Overell J, Smith J, St Lambert J, El-Khairi M, Gafarova M and Gelfand JM (2024) Innovation and optimization in autoimmune encephalitis trials: the design and rationale for the Phase 3, randomized study of satralizumab in patients with NMDAR-IgG-antibody-positive or LGI1-IgG-antibody-positive autoimmune encephalitis (CIELO). Front. Neurol. 15:1437913. doi: 10.3389/fneur.2024.1437913
Received: 24 May 2024; Accepted: 15 July 2024;
Published: 13 August 2024.
Edited by:
Philipp Albrecht, Heinrich Heine University of Düsseldorf, GermanyReviewed by:
Frank Leypoldt, University Medical Center Schleswig-Holstein, GermanyCopyright © 2024 Lee, Abboud, Irani, Nakajima, Piquet, Pittock, Yeh, Wang, Rajan, Overell, Smith, St Lambert, El-Khairi, Gafarova and Gelfand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeffrey M. Gelfand, SmVmZnJleS5HZWxmYW5kQHVjc2YuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.