- Department of Otorhinolaryngology-Head & Neck Surgery, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
Background: Hearing loss (HL) is the third most prevalent condition, significantly affecting individuals and society. Recent research has explored the potential impact of nutrition, particularly caffeine intake, on HL. While some studies focus on coffee, caffeine intake should be assessed across all dietary sources. This study examines the association between dietary caffeine intake and HL.
Methods: Our cross-sectional study included 6,082 participants from the National Health and Nutrition Examination Survey (NHANES). Participants were divided into two groups based on their median caffeine intake: low and high. The study investigated two types of HL: speech-frequency hearing loss (SFHL) and high-frequency hearing loss (HFHL). Binary logistic regression analyzed the correlation between caffeine intake and HL, and a restricted cubic spline (RCS) model assessed potential non-linear associations. Subgroup analyses were also conducted.
Results: High caffeine intake was associated with significantly higher rates of SFHL and HFHL compared to low intake (SFHL: 15.4% vs. 10%, HFHL: 30.5% vs. 20.6%, both p < 0.001). Unadjusted logistic regression showed a higher likelihood of SFHL (OR[95%CI] = 1.65[1.41–1.92]) and HFHL (OR[95%CI] = 1.69[1.50–1.90]) in high caffeine consumers. After adjusting for confounders, high caffeine intake remained significantly associated with SFHL (OR[95%CI] = 1.35[1.09–1.66]) but not HFHL (OR[95%CI] = 1.14[0.96–1.35]). The RCS model indicated a linear increase in the risk of SFHL and HFHL with higher caffeine intake (non-linear p = 0.229 for SFHL, p = 0.894 for HFHL). Subgroup analysis revealed that increased caffeine intake was linked to higher SFHL and HFHL risks in participants under 65 years but not in those 65 years and older (SFHL: p for interaction = 0.002; HFHL: p for interaction <0.001).
Conclusion: Our study indicates a strong correlation between dietary caffeine intake and the risk of HL in American adults, particularly those under 65. High caffeine intake was linked to an increased risk of SFHL, but not HFHL, after adjusting for relevant variables.
1 Introduction
Hearing loss (HL) is a common ailment that can have a significant impact on many aspects of a person’s life if it is not treated or supported in terms of communication needs (1). As per the 2019 Global Burden of Disease (GBD) research conducted by the World Health Organization, HL ranks third globally in terms of causing disability, impacting 157 million individuals. According to projections, 2.45 billion people will have some kind of HL by 2050, and at least 700 million of them would need rehabilitation programs (1, 2).
Pathological hearing loss can result from lesions in the external auditory canal, middle ear, cochlea (which includes hair cells, spiral ganglion cells, and the stria vascularis), and auditory nerve (2–4). Recent research has indicated that systemic inflammation, altered microcirculation, and free radical production may contribute to cochlear injury and subsequent HL (5–8). Moreover, a wide range of factors affect the incidence and severity of HL. The risk and progression of HL have been linked to a number of factors, including exposure to loud noise, socioeconomic difficulties, medical conditions such as the use of ototoxic medications, ear infections, hypertension, and diabetes, genetic predispositions, and hormonal influences, particularly estrogen levels (7, 9–11).
Traditional risk factors help us understand the causes of HL, but they do not fully take into account the complexity of the condition (12). Recently, an increasing number of studies have shown that diet is associated with HL, which can be attributed to specific dietary patterns or specific components of the diet (13–17). And research has demonstrated that diet exerts a dual effect on the risk of HL, with certain dietary components shown to reduce the risk of HL (18–28), while others have been proven to increase the likelihood of developing HL. A higher consumption of n-3 fatty acids (18, 19), oily fish (18–20), magnesium (21), and various vitamins (21–24), as well as antioxidants like β-carotene (21) and moderate alcohol use (25), has been linked to a reduced risk of hearing loss. On the other hand, a diet rich in cholesterol (26), foods with a high glycaemic load (27), and excessive alcohol intake (28) have been shown to be connected with a higher risk of HL.
The most widely used psychostimulant is caffeine (29), which is found naturally in coffee beans, tea leaves, cocoa beans, and kola nuts. It is also added to a variety of meals and drinks. The main food sources are tea, coffee, yerba mate, and caffeinated sodas (especially cola varieties) and energy beverages (30). Caffeine acts as a stimulant to the central nervous system; its biological effects indicate that it affects the peripheral and central auditory systems, including the inner ear directly (31). Despite these links, it’s still unclear how exactly caffeine affects hearing and whether it’s positive or negative (32–34).
There has not been much research done on the relationship between dietary caffeine intake and HL in the general population up until now. Although there is some research on coffee intake and HL, caffeine intake does not depend on coffee alone, but should be considered in the context of caffeine intake from all foods and beverages in the diet (35). In order to close that gap, this study uses information from the National Health and Nutrition Examination Survey (NHANES) to investigate the association between adult population in the United States’ caffeine intake from foods and beverages in the diet and HL.
2 Methods
2.1 Study design and population
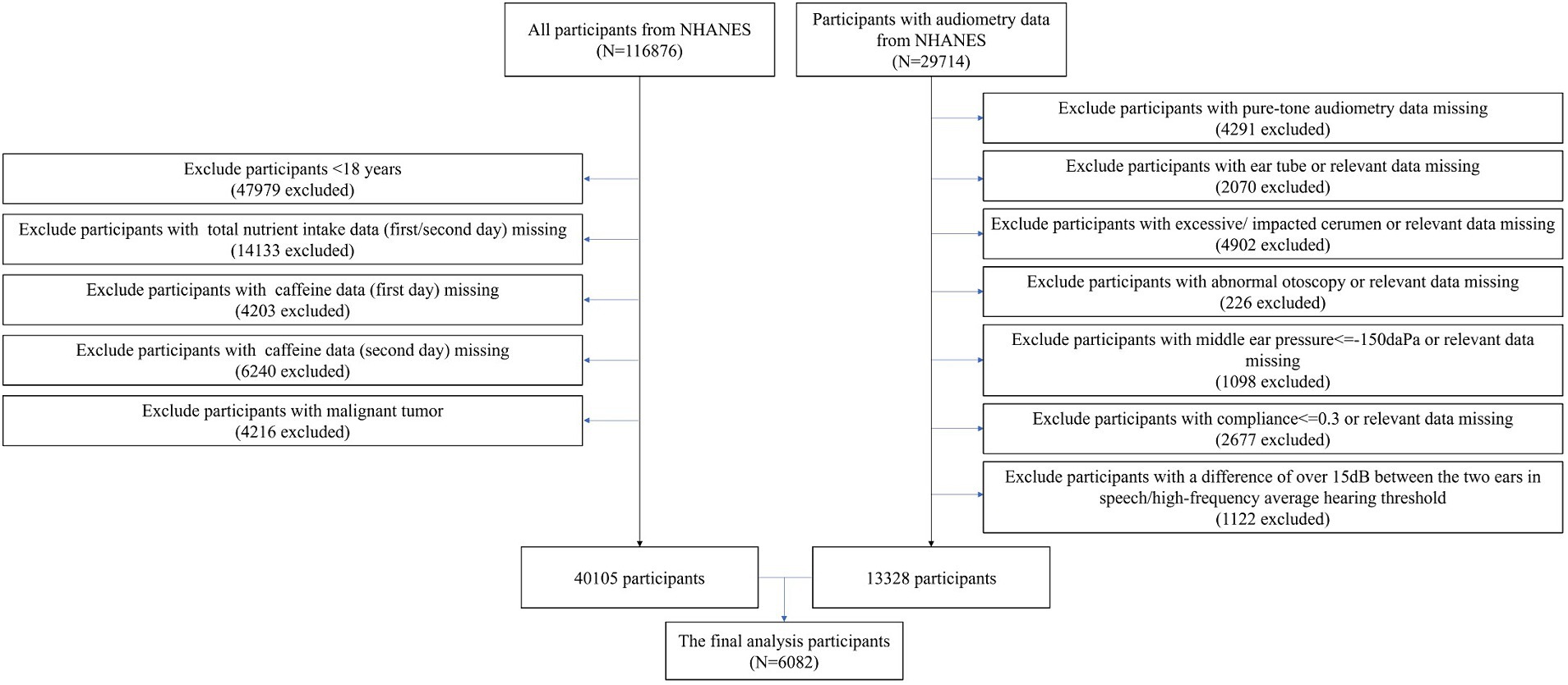
The NHANES survey, which is a cross-sectional study designed to represent the noninstitutionalized U.S. population, was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board.1 All participants provided informed consent. Data collection in NHANES involves detailed in-person interviews conducted at participants’ homes followed by physical examinations and laboratory tests performed at Mobile Examination Centers (MECs). A total of 116,876 participants from NHANES (1999–2020, pre-pandemic) were initially extracted. Among this cohort, 29,714 participants with available audiometric data were included. The study applied several exclusion criteria: (1) participants under 18 years of age; (2) missing data on total nutrient intake or caffeine consumption for either the first or second day; (3) missing pure-tone audiometry data; (4) participants with excessive or impacted cerumen; (5) abnormal otoscopy findings; (6) middle ear pressure below −150 daPa; (7) compliance below 0.3; (8) a difference of over 15 dB between the ears in speech or high-frequency hearing thresholds; and (9) a history of malignant tumors. Given the complexity and vastness of the public database, we processed the data in segments. Initially, we identified 13,328 participants with complete audiometry data after applying the exclusion criteria. Separately, we processed the dietary data and identified 40,105 participants with complete dietary data who also met the exclusion criteria. After applying the criteria to both datasets, a total of 6,082 participants with complete dietary and audiometry data were included in the study, based on data collected between 2003–2012 and 2015–2020 (pre-pandemic). The exclusion of the period from 2013 to 2014 was due to the unavailability of audiometric data (Figure 1).
2.2 Assessment of caffeine intake
All NHANES participants are eligible for two 24-h dietary recall interviews. The first interview is conducted in-person at the Mobile Examination Center (MEC), and the second is conducted by telephone 3 to 10 days later. Data on caffeine intake (mg/day) is obtained from the files “Dietary Interview – Total Nutrient Intakes, First Day” and “Dietary Interview – Total Nutrient Intakes, Second Day,” which compile nutrients from all foods and beverages reported.
Using information from these interviews, we estimated each participant’s average daily caffeine intake from all caffeine-containing foods and beverages, including coffee, tea, soda (regular, reduced calorie, and diet), energy drinks, and chocolate. The caffeine intake data for this study is the average of the values from the two 24-h recall interviews.
This data is entered into the USDA’s Food and Nutrient Database for Dietary Studies (FNDDS), which provides detailed nutrient information, including caffeine content. The caffeine content for each food and beverage is calculated using standardized data from the FNDDS based on reported intake amounts. The total caffeine intake for each participant is then determined by summing the caffeine content from all reported foods and beverages within the 24-h period.
2.3 Assessment of HL
The “Audiometry” file from the NHANES examination section provided the audiometric data for this study. Audiometric testing was conducted by qualified examiners in sound-isolating chambers using an AD226 audiometer, TDH-39P headphones, and EARTone 3A earphones.
The hearing test procedures included several steps to ensure accuracy and reliability: a pre-exam audiometric questionnaire to identify conditions affecting testing or results, a brief otoscopic exam to detect ear abnormalities, middle ear testing to measure eardrum compliance and identify pathologies, and pure-tone air conduction audiometry to measure hearing sensitivity.
Quality assurance and control were maintained through various measures: daily and start/end of survey site calibration checks, an annual comprehensive National Institute of Standards and Technology (NIST) traceable calibration check, continuous monitoring of environmental noise levels, automatic data upload and review using a computer program to check data consistency and errors, repeated tests to ensure the reliability of audiograms, and professional training and supervision of health technicians by certified audiologists. National Institute for Occupational Safety & Health (NIOSH) consultants also provided annual retraining and protocol updates to maintain high standards of quality and consistency.
Pure-tone air conduction hearing thresholds were measured at frequencies of 0.5, 1, 2, 3, 4, and 6 kHz, with intensity ranges from −10 to 110 dB. High-frequency hearing loss (HFHL) was evaluated using averages at 3, 4, and 6 kHz, while speech-frequency hearing loss (SFHL) was determined using averages at 0.5, 1, 2, and 4 kHz. HL was defined as a threshold of ≥25 dB in either ear for both SFHL and HFHL (35, 36).
2.4 Grouping and outcomes
All participants were divided into low caffeine intake group and high caffeine intake group according to the median caffeine intake level (low caffeine intake: <78 mg/day, n = 3,031; high caffeine intake≥78 mg/day, n = 3,051). SFHL and HFHL were the main outcomes assessed in this investigation.
2.5 Covariates
Information on demographics, education level, ratio of family income to poverty (PIR), smoking status (having smoked at least 100 cigarettes in a lifetime), comorbidities (such as hypertension, diabetes, and hypercholesterolemia), and medication use in the past month (including nonsteroidal anti-inflammatory drugs (NSAIDs), diuretics, and aminoglycosides) was collected through structured questionnaires. Additionally, vital signs, including systolic and diastolic blood pressure, and heart rate, were measured at baseline. Body mass index (BMI) was calculated by dividing weight in kilograms by height in meters squared. The dietary data, encompassing energy, protein, carbohydrate, total sugars, dietary fiber, and total fat, were averaged from two 24-h recall interviews.
2.6 Statistical analyses
We used sampling weights, clusters, and strata in our analysis to account for the intricate, multistage probability sampling architecture of the NHANES. Normal-distribution continuous data were displayed as means ± standard deviations (SD), while skewed-distribution continuous variables were displayed as medians with interquartile ranges (IQR). The reporting of categorical variables was done as counts, or percentages. Depending on the distribution of the data, the Independent T-test and Mann–Whitney U test were employed to analyze continuous variables, while the χ2 test or Fisher’s exact test was used to compare categorical baseline variables. In order to show the distribution of pure-tone audiometry data (averages of left/right hearing thresholds at speech and high frequencies), we also made histograms and classified them according to the amount of caffeine consumed.
Binary logistic regression was used to evaluate the relationship between the levels of caffeine intake and the outcomes (SFHL and HFHL), with odds ratios (ORs) and 95% confidence intervals (CIs) displayed as the outcomes. The correlation between covariates and HL was confirmed through univariate logistic analysis, and variables with p < 0.05 were included in the multivariate logistic regression model. The reference group was the one with low caffeine intake. The methodology required removing one or more variables when there was significant collinearity among them (variance inflation factor [VIF] > 10). However, variables that were essential for improving the model’s relevance or explanatory power were prioritized and retained. Model I was unadjusted. Model II included adjustments for age, sex, race, heart rate, diastolic blood pressure, BMI, education level, and smoking status (at least 100 cigarettes smoked in a lifetime), as well as the use of diuretics, NSAIDs (apart from aspirin), diabetes, protein, carbohydrate, total sugars, and total fat. The variables in the restricted cubic spline (RCS) model matched those in Model II, and it was used to investigate non-linear associations between coffee intake levels and HL. The Akaike Information Criterion (AIC) was used to determine which four knots to use for the RCS model.
The association between caffeine intake levels and HL was examined using subgroup analyses using univariate logistic regression. The p-value for the interaction was computed and displayed in a forest plot. R software version 4.2.1 and Stata version 15.0 were used for the statistical analyses. p-values below 0.05 were regarded as statistically significant.
3 Results
3.1 Participant characteristics
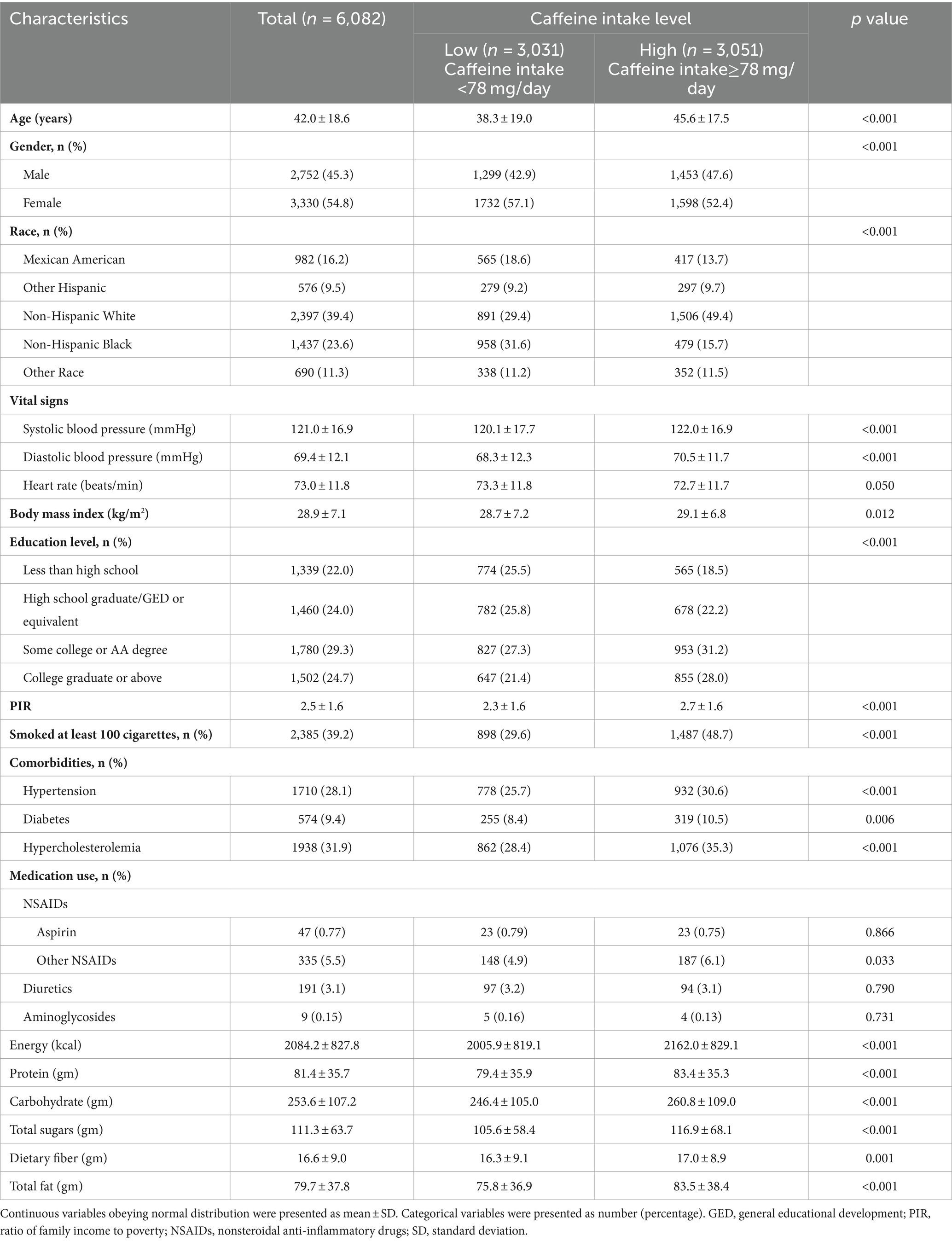
The media of caffeine intake level was 78 mg/day. It was found that the high caffeine intake group was older (p < 0.001), had a significantly higher percentage of males (p < 0.001) and Non-Hispanic Whites (p < 0.001), and exhibited higher systolic (p < 0.001) and diastolic blood pressure (p < 0.001) and higher body mass index (p = 0.012). The high caffeine intake group demonstrated a higher level of educational level, with a greater proportion of individuals being college graduates or above (p < 0.001). The socioeconomic status, as indicated by a higher PIR, was elevated in the high intake group (p < 0.001), as was the prevalence of smoking (p < 0.001), hypertension (p < 0.001), diabetes (p = 0.006), and hypercholesterolemia (p < 0.001). Regarding medication use, consumption of NSAIDs (no aspirin) was significantly higher in the high caffeine group (p = 0.033), with no significant differences noted in the use of aspirin, diuretics, and aminoglycosides. Moreover, the high caffeine intake group exhibited significantly higher intakes of energy (p < 0.001), protein (p < 0.001), carbohydrate (p < 0.001), total sugars (p < 0.001), dietary fiber (p = 0.001), and total fat (p < 0.001) (Table 1).
3.2 Audiometry data of participants
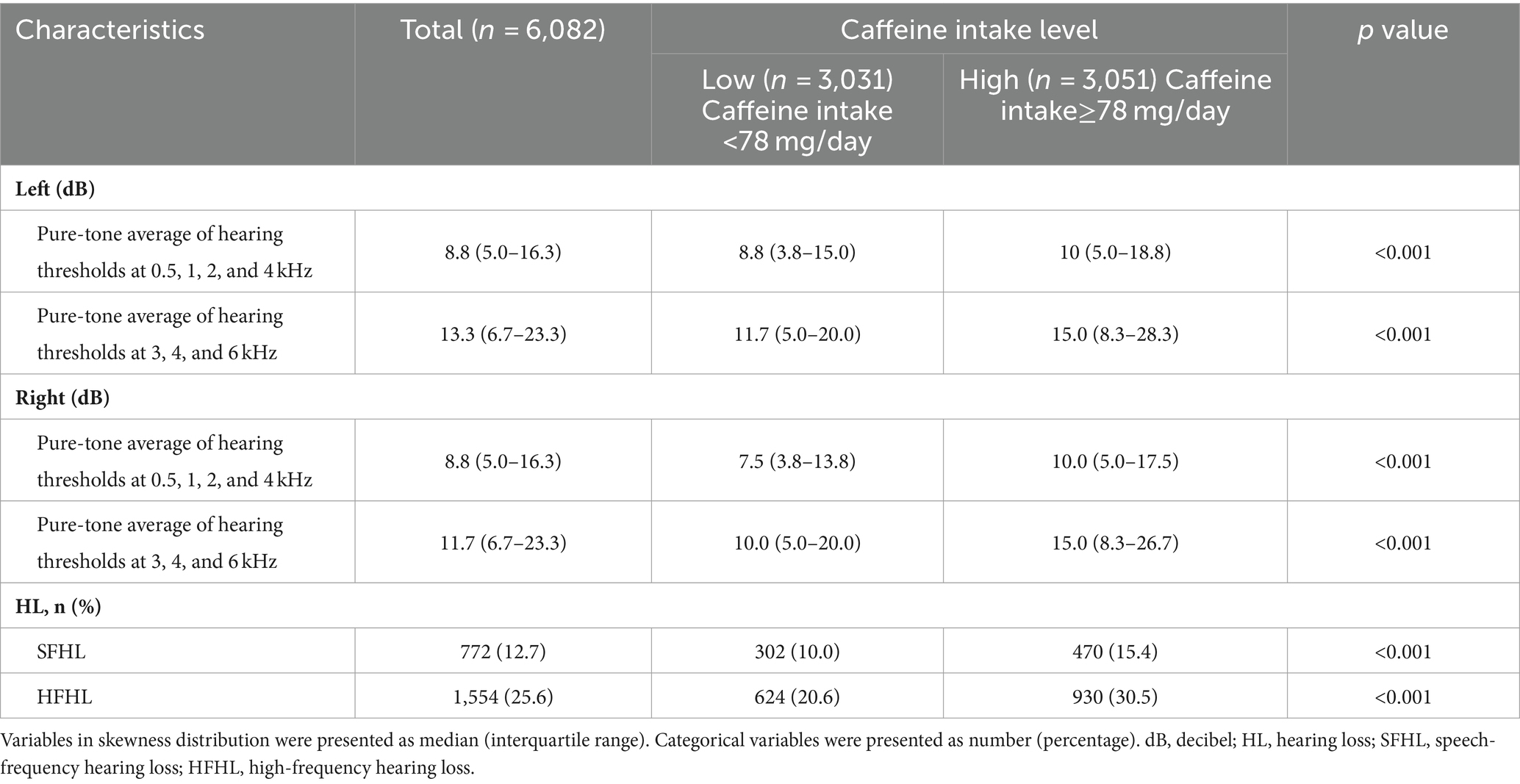
Participants in high caffeine intake group had higher pure-tone average of hearing thresholds at 0.5, 1, 2 and 4 kHz (Left: low caffeine intake group vs. high caffeine intake group: 8.8 [3.8–15.0] dB vs. 10 [5.0–18.8] dB, p < 0.001; Right: low caffeine intake group vs. high caffeine intake group: 7.5 [3.8–13.8] dB vs. 10.0 [5.0–17.5] dB, p < 0.001) and higher pure-tone average of hearing thresholds at 3, 4 and 6 kHz (Left: low caffeine intake group vs. high caffeine intake group: 11.7 [5.0–20.0] dB vs. 15.0 [8.3–28.3] dB, p < 0.001; Right: low caffeine intake group vs. high caffeine intake group: 10.0 [5.0–20.0] dB vs. 15.0 [8.3–26.7] dB, p < 0.001) (Table 2; Figure 2). The incidence of SFHL (low caffeine intake group vs. high caffeine intake group: 302 (10%) vs. 470 (15.4%), p < 0.001) and HFHL (low caffeine intake group vs. high caffeine intake group: 624 (20.6%) vs. 930 (30.5%), p < 0.001) increased significantly in high caffeine intake group (Table 2).
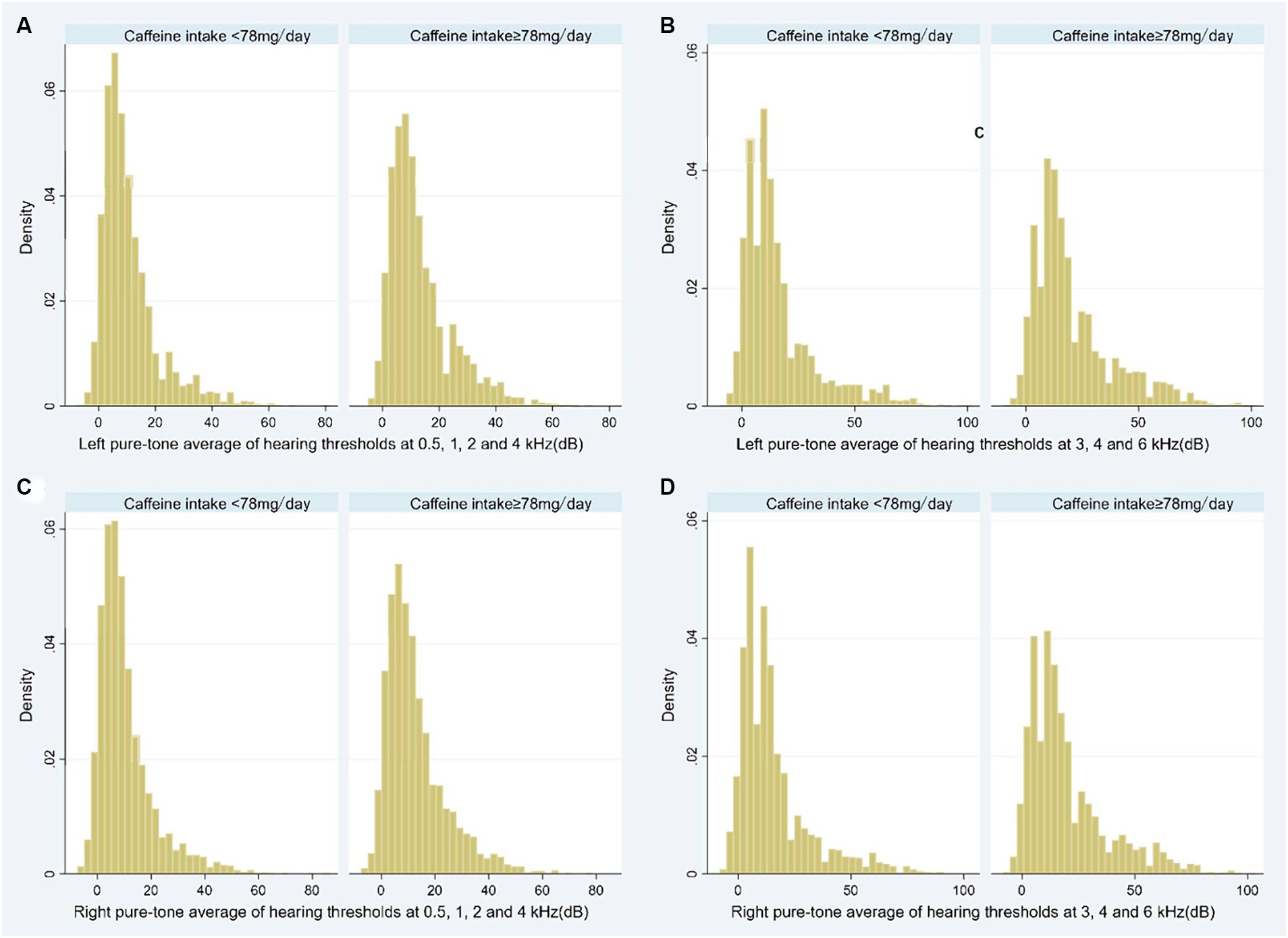
Figure 2. Histogram showing the pure-tone audiometry data of participants stratified by caffeine intake level (A: Left pure-tone average of hearing thresholds at 0.5, 1, 2, and 4 kHz; B: Left pure-tone average of hearing thresholds at 3, 4, and 6 kHz; C: Right pure-tone average of hearing thresholds at 0.5, 1, 2, and 4 kHz; D: Right pure-tone average of hearing thresholds at 3, 4, and 6 kHz). dB, decibel.
3.3 Logistic analysis exploring the association between HL and caffeine intake level
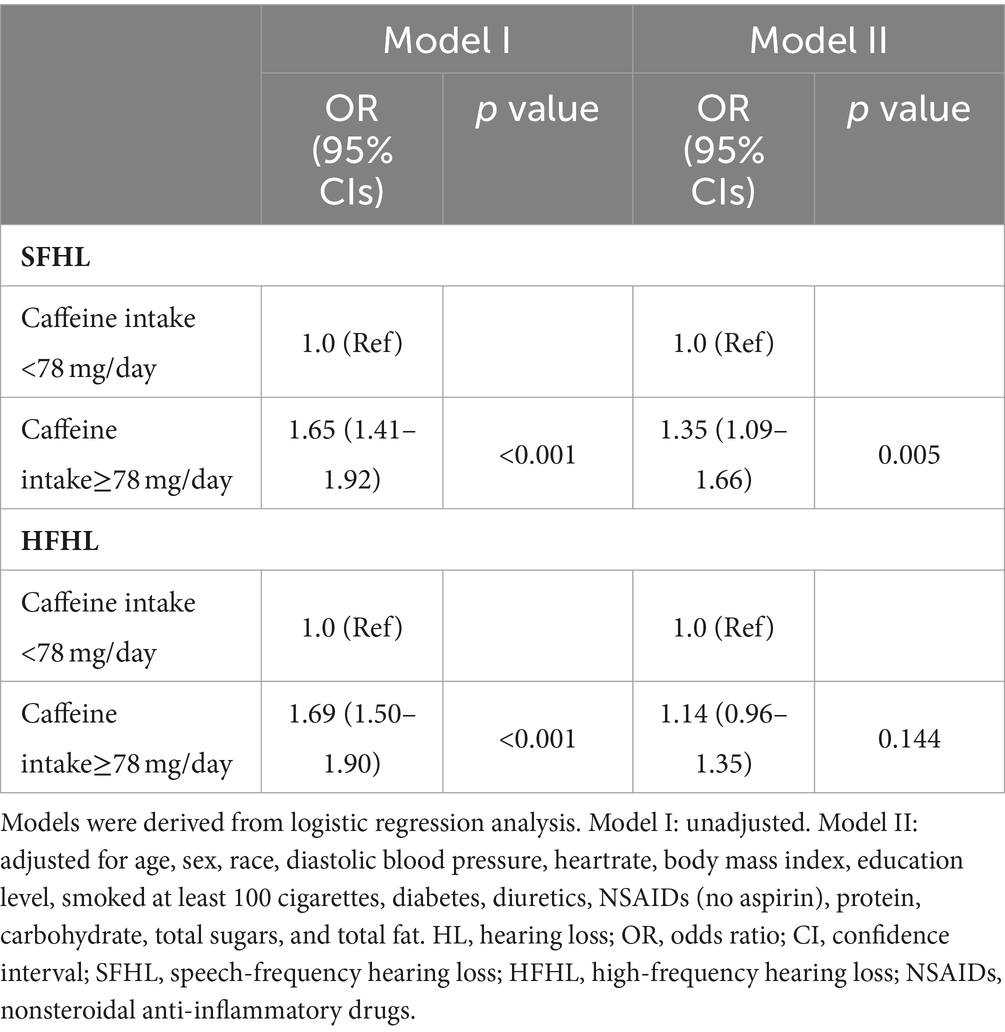
In logistic Model I, no variables were adjusted, the risk of SFHL (OR, 95% CIs: 1.65, 1.41–1.92, p < 0.001) and HFHL (OR, 95% CIs: 1.69, 1.50–1.90, p < 0.001) significantly increased in high caffeine intake group. In multivariable logistic Model II, the risk of SFHL (OR, 95% CIs: 1.35, 1.09–1.66, p = 0.005) still significantly increased in high caffeine intake group. However, the association between caffeine intake and HFHL did not show a statistically significant difference (OR, 95% CIs: 1.14, 0.96–1.35, p = 0.144) (Table 3).
3.4 RCS model showing the association between the caffeine intake level and HL
The RCS model in line with model II showed that the risk of SFHL (non-linear p = 0.229) and HFHL (non-linear p = 0.894) increased as the caffeine intake value increased (Figure 3).
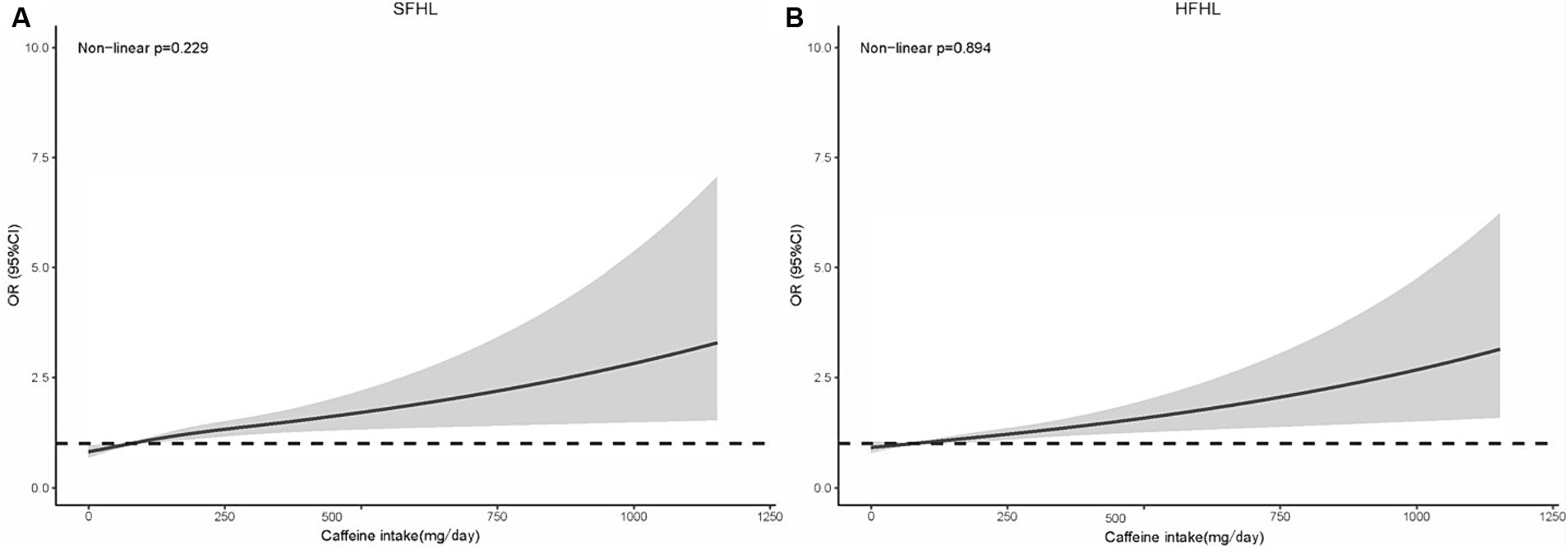
Figure 3. RCS model showing the association between the caffeine intake level and HL (A: SFHL; B: HFHL). RCS, restricted cubic spline; HL, hearing loss; OR, odds ratio; CI, confidence interval; SFHL, speech-frequency hearing loss; HFHL, high-frequency hearing loss.
3.5 Forest plot showing the association between the caffeine intake level and HL in different subgroups
Subgroup analysis confirmed the higher risk of SFHL (p for interaction = 0.002) and HFHL (p for interaction<0.001) with caffeine intake in a subgroup of age < 65, there is no obvious statistical significance in subgroup of age ≥ 65. No obvious interaction was observed in other subgroups (Figure 4).
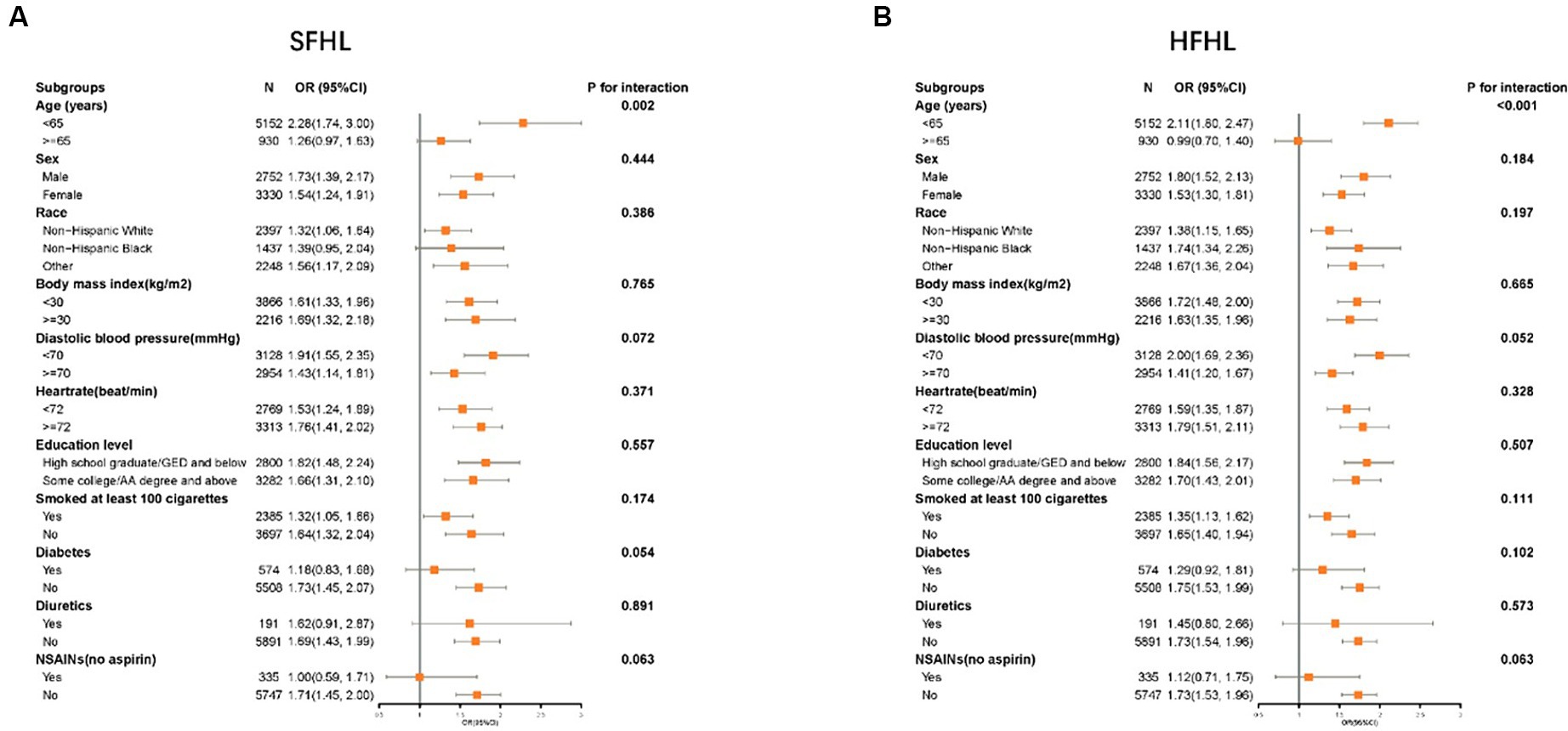
Figure 4. Forest plot showing the association between the caffeine intake level and HL in different subgroups (A: SFHL; B: HFHL). SFHL: speech-frequency hearing loss; HFHL: high-frequency hearing loss; OR: odds ratio; CI: confidence interval; NSAIDs: nonsteroidal anti-inflammatory drugs; HL: hearing loss.
4 Discussion
This study focused on American adults from the NHANES dataset, exploring the association between caffeine intake levels and HL. The major findings were as follows: (1) High caffeine intake was significantly associated with the elevated risk of SFHL even after adjusting for possible confounding risk factors, yet showed no significant link with HFHL; (2) The RCS model showed that the risk of SFH and HFHL increased as the caffeine intake value increased and there was a linear relationship (non-linear p > 0.05); and (3) Significant interaction effect was observed in subgroup of age.
HL, the third leading cause of disability, affected 157 million individuals globally in 2019, and projections indicated that by 2050, 2.45 billion people will experience HL (1, 37). The large and rapidly growing population necessitates our attention to the issue of HL. It is crucial to identify relevant risk factors and implement corresponding preventive measures. However, traditional risk factors such as aging, genetic mutations, noise exposure, and ototoxic side effects from certain medications only account for a portion of the causes of HL (4, 12). Recently, there has been increasing focus on non-traditional risk factors, including lifestyle and dietary habits (14, 38).
Caffeine, widely recognized for its stimulating effects on the central nervous system (CNS), is one of the most consumed psychoactive substances globally (29, 39). And previous studies indicated that it played a role in the peripheral and central auditory system, while also directly affecting the inner ear (31–34). Caffeine has been shown to impact hearing negatively through its antagonistic effects on adenosine receptors, particularly following acoustic trauma (40). It impedes cochlear blood reperfusion, increases oxidative stress, exacerbates cochlear hair cells’ apoptosis via calcium accumulation (40–43), and heightens noise-induced cochlear hypoperfusion and ischemia by promoting the reduction of cerebral blood flow and arteriole diameter (44, 45). Moreover, caffeine elicits an acute auditory response to noise by affecting corticosterone levels and triggers autophagy and apoptosis in cochlear hair cells through SGK1/HIF-1α pathway (46–48). These actions collectively suggest caffeine’s potential to interfere with hearing recovery processes and contribute to auditory system disorders. The aforementioned mechanisms may explain the correlation between dietary intake of caffeine and HL.
Currently, no studies have specifically identified a relationship between dietary intake of caffeine and hearing loss (HL). Previous research has explored urinary caffeine metabolites and found no association with hearing threshold shifts in US adults (49). Several studies have investigated the relationship between coffee consumption and HL, but they have yet to reach a consensus (35, 50–52). A cross-sectional analysis including 1894 adult individuals showed that coffee consumption was related to higher prevalence of HL (35). In a population-based cohort study involving 36,923 participants, it was discovered that coffee consumption correlated with a reduced risk of developing disabling hearing impairment among men, while no significant association was observed for women. This discrepancy was attributed to differences between men and women in brain biochemistry, cochlear structure, the progression of age-related hearing loss, estrogen levels, and antioxidant responses. Additionally, obese men, who had higher levels of inflammation and oxidative stress, experienced more pronounced protective effects from coffee (50). Similarly, a study based on a national population-based survey revealed that regular coffee drinkers experienced a 50–70% reduction in hearing loss compared to infrequent consumers, suggesting a relationship that appears to be dependent on dosage (51). The variation in research outcomes may be attributed to differences in the ethnic backgrounds of the study populations (Americans, British, Koreans). Additionally, it was possible that the complex composition of coffee, which includes proteins, fats, tannins, caffeine, minerals, and other trace ingredients, contributes to varying outcomes, as the proportional composition of these components differs across regions (52).
Therefore, our study, focused on the American population and analyzing solely the caffeine content, concluded that high dietary intake of caffeine was associated with an increased risk of HL. However, as this investigation is a cross-sectional study, it cannot establish causality between these variables. Large-scale, prospective studies are required to confirm this conclusion. Furthermore, our study revealed that in the multivariate logistic regression, there was no statistically significant difference in the correlation between dietary caffeine intake and HFHL. However, upon reanalysis, when covariates other than age were included, a significant correlation between dietary caffeine intake and HFHL was observed (OR, 95% CIs: 1.61, 1.42–1.83, p < 0.001). Age-related HL develops from the aging process’s cumulative effects on the auditory system, characterized by a gradual, bilateral, and symmetrical decrease in hearing sensitivity, especially at higher frequencies (53). Age-related HL affects high frequencies initially and then progresses to lower frequencies over time (54). This pattern suggests that HFHL is often the first to be affected as individuals age. These could explain why, in a multiple regression analysis, after adding age as a covariate, the correlation between caffeine intake and SFHL remained statistically significant, while the correlation with HFHL no longer did. In subgroup analysis, we uncovered an interesting phenomenon: there was a significant correlation between caffeine intake and HL in the subgroup under 65 years of age, but no significant correlation was found in the subgroup aged 65 and older. This indicated that within the older population, HL was predominantly influenced by age, with the contribution of dietary factors becoming less significant. In our research, the prevalence of SFHL and HFHL among individuals under the age of 65 was documented at 4.8 and 15.1%, respectively. Such elevated incidence rates highlighted the critical need for intervention. Consequently, for individuals younger than 65, particularly those exposed to risk factors for HL like noise, modifying dietary habits to decrease caffeine consumption was considered crucial.
The data for our study were obtained from the NHANES, a long-standing program in the USA characterized by its rigorous and mature survey methodology. This foundation enhanced the reliability of our findings. Moreover, the intricate design of NHANES, which includes complex, multistage, probability sampling, necessitated the use of sampling weights in our statistical analyses. Such measures were crucial in mitigating biases associated with such data, further bolstering the credibility of our results. This study also presented a number of limitations: (1) Due to the inherent nature of an observational cross-sectional study, this research is only capable of exploring correlations and cannot establish definitive causality. This constitutes one of the study’s limitations, necessitating large-scale prospective studies to confirm the reliability of the findings. (2) The acquisition of data on caffeine intake was performed via questionnaire surveys, potentially leading to recall bias among participants when documenting their consumption of caffeine-containing foods and beverages. This presents a challenge in accurately capturing detailed information on caffeine intake levels. Therefore, the discrepancy between the assessed exposure and the true exposure to caffeine intake cannot be entirely eradicated. (3) Differences in metabolism between individuals can also potentially impact the relationship between caffeine intake and HL.
5 Conclusion
The risk of SFHL and HFHL significantly increased in high caffeine intake group. But after adjusting for confounding variables, high caffeine intake was still associated with the elevated risk of SFHL, yet showed no significant link with HFHL. The RCS model showed that the risk of SFHL and HFHL increased positively and linearly with caffeine intake. A significant interaction was observed in the age subgroup. The results of this study underscore the importance of dietary caffeine intake in the management of HL, particularly in individuals younger than 65 years. However, large-scale prospective studies are needed to confirm the reliability of these findings.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: the National Health and Nutrition Examination Survey (NHANES) database (https://www.cdc.gov/nchs/nhanes/index.htm).
Ethics statement
The studies involving humans were approved by the Ethics Committee of Beijing Chaoyang Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
FX: Writing – original draft, Methodology. YR: Writing – review & editing, Formal analysis.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Collaborators, GBDHL . Hearing loss prevalence and years lived with disability, 1990–2019: findings from the global burden of disease study 2019. Lancet. (2021) 397:996–1009. doi: 10.1016/S0140-6736(21)00516-X
2. Nelson, EG, and Hinojosa, R. Presbycusis: a human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. Laryngoscope. (2006) 116:1–12. doi: 10.1097/01.mlg.0000236089.44566.62
3. Schuknecht, HF, and Gacek, MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. (1993) 102:1–16. doi: 10.1177/00034894931020S101
4. Cunningham, LL, and Tucci, DL. Hearing loss in adults. N Engl J Med. (2017) 377:2465–73. doi: 10.1056/NEJMra1616601
5. Seidman, MD . Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope. (2000) 110:727–38. doi: 10.1097/00005537-200005000-00003
6. Seidman, MD, Ahmad, N, Joshi, D, Seidman, J, Thawani, S, and Quirk, WS. Age-related hearing loss and its association with reactive oxygen species and mitochondrial DNA damage. Acta Otolaryngol Suppl. (2004) 124:16–24. doi: 10.1080/03655230410017823
7. Yamasoba, T, Lin, FR, Someya, S, Kashio, A, Sakamoto, T, and Kondo, K. Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear Res. (2013) 303:30–8. doi: 10.1016/j.heares.2013.01.021
8. Watson, N, Ding, B, Zhu, X, and Frisina, RD. Chronic inflammation – inflammaging – in the ageing cochlea: a novel target for future presbycusis therapy. Ageing Res Rev. (2017) 40:142–8. doi: 10.1016/j.arr.2017.10.002
9. Hultcrantz, M, Simonoska, R, and Stenberg, AE. Estrogen and hearing: a summary of recent investigations. Acta Otolaryngol. (2006) 126:10–4. doi: 10.1080/00016480510038617
10. Ohlemiller, KK . Mechanisms and genes in human strial presbycusis from animal models. Brain Res. (2009) 1277:70–83. doi: 10.1016/j.brainres.2009.02.079
11. Uchida, Y, Sugiura, S, Ando, F, Nakashima, T, and Shimokata, H. Diabetes reduces auditory sensitivity in middle-aged listeners more than in elderly listeners: a population- based study of age-related hearing loss. Med Sci Monit. (2010) 16:PH63-8.
12. Nieman, CL, and Oh, ES. Hearing loss. Ann Intern Med. (2020) 173:ITC81–96. doi: 10.7326/AITC202012010
13. Choi, JE, Ahn, J, and Moon, IJ. Associations between age-related hearing loss and DietaryAssessment using data from Korean National Health andNutrition examination survey. Nutrients. (2021) 13:1230. doi: 10.3390/nu13041230
14. Gallagher, NE, Patterson, CC, Neville, CE, Yarnell, J, Ben-Shlomo, Y, Fehily, A, et al. Dietary patterns and hearing loss in older men enrolled in the Caerphilly study. Br J Nutr. (2019) 121:877–86. doi: 10.1017/S0007114519000175
15. Wei, X . Dietary magnesium and calcium intake is associated with lower risk of hearing loss in older adults: a cross-sectional study of NHANES. Front Nutr. (2023) 10:1101764. doi: 10.3389/fnut.2023.1101764
16. Gopinath, B, McMahon, CM, Lewis, JR, Bondonno, NP, Bondonno, CP, Burlutsky, G, et al. Associations between intake of dietary flavonoids and 10-year incidence of age-related hearing loss. Nutrients. (2020) 12:3297. doi: 10.3390/nu12113297
17. Kim, TS, and Chung, JW. Associations of dietary riboflavin, niacin, and retinol with age-related hearing loss: an analysis of Korean National Health and nutrition examination survey data. Nutrients. (2019) 11:896. doi: 10.3390/nu11040896
18. Gopinath, B, Flood, VM, Rochtchina, E, McMahon, CM, and Mitchell, P. Consumption of omega-3 fatty acids and fish and risk of age-related hearing loss. Am J Clin Nutr. (2010) 92:416–21. doi: 10.3945/ajcn.2010.29370
19. Curhan, SG, Eavey, RD, Wang, M, Rimm, EB, and Curhan, GC. Fish and fatty acid consumption and the risk of hearing loss in women. Am J Clin Nutr. (2014) 100:1371–7. doi: 10.3945/ajcn.114.091819
20. Rosenhall, U, Idrizbegovic, E, Hederstierna, C, and Rothenberg, E. Dietary habits and hearing. Int J Audiol. (2015) 54:S53–6. doi: 10.3109/14992027.2014.972524
21. Choi, YH, Miller, JM, Tucker, KL, Hu, H, and Park, SK. Antioxidant vitamins and magnesium and the risk of hearing loss in the US general population. Am J Clin Nutr. (2014) 99:148–55. doi: 10.3945/ajcn.113.068437
22. Gopinath, B, Flood, VM, McMahon, CM, Burlutsky, G, Spankovich, C, Hood, LJ, et al. Dietary antioxidant intake is associated with the prevalence but not incidence of age-related hearing loss. J Nutr Health Aging. (2011) 15:896–900. doi: 10.1007/s12603-011-0119-0
23. Lasisi, AO, Fehintola, FA, and Yusuf, OB. Age-related hearing loss, vitamin B12, and folate in the elderly. Otolaryngol Head Neck Surg. (2010) 143:826–30. doi: 10.1016/j.otohns.2010.08.031
24. Peneau, S, Jeandel, C, Dejardin, P, Andreeva, VA, Hercberg, S, Galan, P, et al. Intake of specific nutrients and foods and hearing level measured 13 years later. Br J Nutr. (2013) 109:2079–88. doi: 10.1017/S0007114512004291
25. Popelka, MM, Cruickshanks, KJ, Wiley, TL, Tweed, TS, Klein, BE, Klein, R, et al. Moderate alcohol consumption and hearing loss: a protective effect. J Am Geriatr Soc. (2000) 48:1273–8. doi: 10.1111/j.1532-5415.2000.tb02601.x
26. Gopinath, B, Flood, VM, Teber, E, McMahon, CM, and Mitchell, P. Dietary intake of cholesterol is positively associated and use of cholesterol-lowering medication is negatively associated with prevalent age-related hearing loss. J Nutr. (2011) 141:1355–61. doi: 10.3945/jn.111.138610
27. Gopinath, B, Flood, VM, McMahon, CM, Burlutsky, G, Brand-Miller, J, and Mitchell, P. Dietary glycemic load is a predictor of age-related hearing loss in older adults. J Nutr. (2010) 140:2207–12. doi: 10.3945/jn.110.128462
28. Rosenhall, U, Sixt, E, Sundh, V, and Svanborg, A. Correlations between presbyacusis and extrinsic noxious factors. Audiology. (1993) 32:234–43. doi: 10.3109/00206099309072939
29. Fredholm, BB, Battig, K, Holmen, J, Nehlig, A, and Zvartau, EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. (1999) 51:83–133.
30. Zucconi, S, Volpato, C, Adinolfi, F, Gandini, E, Gentile, E, Loi, A, et al. Gathering consumption data on specific consumer groups of energy drinks. EFSA Support Publ. (2013) 10:394E. doi: 10.2903/sp.efsa.2013.EN-394
31. Tavanai, E, Farahani, S, Ghahraman, MA, Soleimanian, S, and Jalaie, S. Effects of caffeine on auditory- and vestibular-evoked potentials in healthy individuals: a double-blind placebo-controlled study. J Audiol Otol. (2020) 24:10–6. doi: 10.7874/jao.2019.00227
32. Dixit, A, Vaney, N, and Tandon, OP. Effect of caffeine on central auditory pathways: an evoked potential study. Hear Res. (2006) 220:61–6. doi: 10.1016/j.heares.2006.06.017
33. Yamamoto, T, Kakehata, S, Yamada, T, Saito, T, Saito, H, and Akaike, N. Caffeine rapidly decreases potassium conductance of dissociated outer hair cells of guinea-pig cochlea. Brain Res. (1995) 677:89–96. doi: 10.1016/0006-8993(95)00121-6
34. Ghahraman, MA, Farahani, S, and Tavanai, E. A comprehensive review of the effects of caffeine on the auditory and vestibular systems. Nutr Neurosci. (2022) 25:2181–94. doi: 10.1080/1028415X.2021.1918984
35. Wu, S, Zhu, S, Mo, F, Yuan, X, Zheng, Q, Bai, Y, et al. Association of coffee consumption with the prevalence of hearing loss in US adults, NHANES 2003–2006. Public Health Nutr. (2023) 26:2322–32. doi: 10.1017/S1368980023001271
36. Tu, Y, Fan, G, Wu, N, Wu, H, and Xiao, H. Association of plasma lead, cadmium and selenium levels with hearing loss in adults: National Health and nutrition examination survey (NHANES) 2011–2012. Br J Nutr. (2022) 128:1100–7. doi: 10.1017/S0007114521004335
37. Chadha, S, Kamenov, K, and Cieza, A. The world report on hearing, 2021. Bull World Health Organ. (2021) 99:242–242A. doi: 10.2471/BLT.21.285643
38. Yevenes-Briones, H, Caballero, FF, Banegas, JR, Rodriguez-Artalejo, F, and Lopez-Garcia, E. Association of Lifestyle Behaviors with Hearing Loss: the UK biobank cohort study. Mayo Clin Proc. (2022) 97:2040–9. doi: 10.1016/j.mayocp.2022.03.029
39. Fiani, B, Zhu, L, Musch, BL, Briceno, S, Andel, R, Sadeq, N, et al. The neurophysiology of caffeine as a central nervous system stimulant and the resultant effects on cognitive function. Cureus. (2021) 13:e15032. doi: 10.7759/cureus.15032
40. Zawawi, F, Bezdjian, A, Mujica-Mota, M, Rappaport, J, and Daniel, SJ. Association of Caffeine and Hearing Recovery after Acoustic Overstimulation Events in a Guinea pig model. JAMA Otolaryngol Head Neck Surg. (2016) 142:383–8. doi: 10.1001/jamaoto.2015.3938
41. Mujica-Mota, MA, Gasbarrino, K, Rappaport, JM, Shapiro, RS, and Daniel, SJ. The effect of caffeine on hearing in a guinea pig model of acoustic trauma. Am J Otolaryngol. (2014) 35:99–105. doi: 10.1016/j.amjoto.2013.11.009
42. Sheth, S, Sheehan, K, Dhukhwa, A, Al Aameri, RFH, Mamillapalli, C, Mukherjea, D, et al. Oral Administration of Caffeine Exacerbates Cisplatin-Induced Hearing Loss. Sci Rep. (2019) 9:9571. doi: 10.1038/s41598-019-45964-9
43. Seidman, MD, Quirk, WS, and Shirwany, NA. Mechanisms of alterations in the microcirculation of the cochlea. Ann N Y Acad Sci. (1999) 884:226–32. doi: 10.1111/j.1749-6632.1999.tb08644.x
44. Le Prell, CG, Yamashita, D, Minami, SB, Yamasoba, T, and Miller, JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res. (2007) 226:22–43. doi: 10.1016/j.heares.2006.10.006
45. Li, W, Zhao, L, Jiang, S, and Gu, R. Effects of high intensity impulse noise on ionic concentrations in cochlear endolymph of the guinea pig. Chin Med J. (1997) 110:883–6.
47. Prasher, D . Is there evidence that environmental noise is immunotoxic? Noise Health. (2009) 11:151–5. doi: 10.4103/1463-1741.53361
48. Tang, X, Sun, Y, Xu, C, Guo, X, Sun, J, Pan, C, et al. Caffeine induces autophagy and apoptosis in auditory hair cells via the SGK1/HIF-1alpha pathway. Front Cell Dev Biol. (2021) 9:751012. doi: 10.3389/fcell.2021.751012
49. Long, L, and Tang, Y. Urine caffeine metabolites and hearing threshold shifts in US adults: a cross-sectional study [J]. Sci Rep. (2021) 11:21631. doi: 10.1038/s41598-021-01094-9
50. Machado-Fragua, MD, Struijk, EA, Yevenes-Briones, H, Caballero, FF, Rodriguez-Artalejo, F, and Lopez-Garcia, E. Coffee consumption and risk of hearing impairment in men and women. Clin Nutr. (2021) 40:3429–35. doi: 10.1016/j.clnu.2020.11.022
51. Lee, SY, Jung, G, Jang, MJ, Suh, MW, Lee, JH, Oh, SH, et al. Association of Coffee Consumption with hearing and tinnitus based on a National Population-Based Survey. Nutrients. (2018) 10:1429. doi: 10.3390/nu10101429
52. Saud, S, and Salamatullah, AM. Relationship between the chemical composition and the biological functions of coffee. Molecules. (2021) 26:7634. doi: 10.3390/molecules26247634
53. Bowl, MR, and Dawson, SJ. Age-related hearing loss. Cold Spring Harb Perspect Med. (2019) 9:a033217. doi: 10.1101/cshperspect.a033217
Keywords: hearing loss, dietary, caffeine intake, speech-frequency hearing loss, high-frequency hearing loss, NHANES
Citation: Xia F and Ren Y (2024) Association between caffeine intake from foods and beverages in the diet and hearing loss in United States adults. Front. Neurol. 15:1436238. doi: 10.3389/fneur.2024.1436238
Edited by:
Andrés Soto-Varela, Complejo Hospitalario Universitario de Santiago, SpainReviewed by:
Liu Chunyan, Epilepsy Society, United KingdomAna Margarida Amorim, Coimbra Hospital and University Center, Portugal
Copyright © 2024 Xia and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Xia, eGlhZmVpQG1haWwuY2NtdS5lZHUuY24=
 Fei Xia
Fei Xia Yuanyuan Ren
Yuanyuan Ren