
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 10 July 2024
Sec. Neuroinfectious Diseases
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1434046
This article is part of the Research Topic An Update on Neurological Disorders Post COVID-19 Infection Vol 2: cardiovascular effects, neuro-cardiac and neuro-respiratory autonomic dysfunctions View all 11 articles
 Adam Desouky1
Adam Desouky1 Venessa Fuentes1
Venessa Fuentes1 Chhitij Tiwari2
Chhitij Tiwari2 Hikari Usui2
Hikari Usui2 Arthor H. Smith Ayala2
Arthor H. Smith Ayala2 Susan E. Wilson2
Susan E. Wilson2 Monica M. Diaz2*
Monica M. Diaz2*Background: Coronavirus disease 2019 (COVID-19) infection has been associated with severe neurological consequences, including stroke or seizures, and less severe neurological sequelae, including headaches, dizziness, and anosmia. Earlier COVID-19 variants were associated with high morbidity and mortality; however, knowledge of the impact of neurological conditions in the setting of COVID-19 on healthcare outcomes is limited. We sought to determine the impact of acute neurological conditions and acute COVID-19 infection on inpatient hospitalization outcomes.
Methods: This was a retrospective, observational study of adult patients who were admitted to a large academic medical center in the Southeastern US between April 2020 and December 2021 with acute COVID-19 infection and a neurological diagnosis. Patient demographics, medical history, neurological diagnoses, and hospitalization outcomes were obtained from the medical record. Descriptive statistics and unadjusted and adjusted logistic regression analyses were performed.
Results: Of the 1,387 patients included in this study, 27% died and 23% were kept under ventilation during hospitalization. The mean +/− standard deviation (SD) age was 64.6+/−16.9 years, with 52.8% women and 30.1% identifying as Black/African American. The most common neurological conditions included ischemic stroke (35.0%), movement disorder (12.0%), and hemorrhagic stroke (10.7%). In-hospital death was most common among those with epilepsy (p = 0.024), headache (p = 0.026), and dementia (p < 0.0001) compared to individuals without those conditions. Ventilation support was given more commonly to dementia patients (p = 0.020). Age was a significant risk factor for death (p < 0.001) and hospital length of stay (LOS) for ventilation (p < 0.001), but no neurological condition was a significant factor in adjusted logistic regression analyses.
Discussion: Mortality was high in this study, with more than one-quarter of patients dying in the hospital. Death was the most common among those with epilepsy, headache, or dementia, but no neurological condition increased the risk of in-hospital mortality or ventilation. Future studies would determine the long-term neurological sequelae of those discharged from the hospital with COVID-19 and a neurological condition.
The central nervous system (CNS) and the peripheral nervous system (PNS) manifestations are evident in 36–82% of hospitalized adults with acute coronavirus disease 2019 (COVID-19) infection (1). Among patients with the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Omicron variant, the prevalence of the CNS and PNS symptoms was still as high as approximately 40% (2). Neurologic manifestations in the context of SARS-CoV-2 infection typically occur days after the onset of initial characteristic COVID-19 symptoms, including respiratory tract and systemic manifestations (3).
The emergence of neurologic signs and symptoms due to initial SARS-CoV-2 pre-Omicron variants may be attributable to various mechanisms, including direct damage by the virus, cytokine storm, hypercoagulable state, and molecular mimicry (4). It is thought that SARS-CoV-2 original variants may have demonstrated neurotropism (2, 5–7), leading to various neurological conditions observed during the pandemic onset. A recent study found elevated levels of neurofilament light (NfL) and glial fibrillary acidic protein (GFAP) among patients admitted for COVID-19, implying that dysregulations in both innate and adaptive immune responses are contributory to neurologic injury in the setting of COVID-19 (8). In the pre-Omicron era, prior to COVID-19 vaccination becoming widely available, acute encephalopathy, headaches, and stroke have been found to be common neurological syndromes among patients (3, 9).
Although the majority of individuals with acute COVID-19 infection who experience neurologic manifestations survive, the presence of neurologic signs or syndromes, such as acute encephalopathy or stroke, is associated with a higher in-hospital mortality risk (9, 10). Milder neurological symptoms, such as headache or diminished or loss of taste or smell, have been found to have a lower risk of in-hospital mortality (9). Furthermore, COVID-19-infected patients diagnosed after the emergence of the Omicron variant demonstrated lower disease severity and in-hospital mortality rates (11, 12).
The characterization of the effect of known neurological conditions on in-hospital outcomes is crucial to target treatments, particularly as COVID-19 evolves over time. In this study, we characterized the risk of neurological conditions on in-hospital mortality and ventilation among patients with acute COVID-19 infection during the pre-Omicron and Omicron waves of the COVID-19 pandemic.
We conducted a retrospective, observational study using electronic medical record review. Patients were identified using the Carolina Data Warehouse service, an inpatient registry of all patients admitted to our hospital, based on inclusion and exclusion criteria. We included all patients who were admitted to the University of North Carolina (UNC) Hospital at Chapel Hill, NC (USA) between 12 April 2020 and 28 December 2021. All patients were adults (18 years of age or older) who had acute COVID-19 infection and a known neurological condition based on ICD-10 diagnosis codes at the time of their hospitalization.
We extracted patient demographics, medical history, neurologic and non-neurological manifestations of COVID-19, and illness courses such as intensive care unit (ICU) admission and hospitalization outcomes, including in-hospital death and ventilation. Vaccination data were also available but were deemed unreliable, thus it was not included in these analyses. If a patient had two separate neurological diagnoses, each diagnosis was considered a separate outcome for the same patient (i.e., epilepsy or seizure and hemorrhagic stroke were counted as two separate outcomes for the same patient).
This study was approved by the UNC internal review board (IRB# 21–2036). Informed consent was not obtained given this was a retrospective chart review, and patients were not contacted.
Descriptive statistics were performed on continuous variables [means and standard deviations (SDs)] and frequencies and percentages on categorical variables. Pearson’s chi-square tests of associations were calculated to compare the proportion of individuals with a neurological condition versus those without a neurological condition. Chi-square tests were adjusted for the number of comparisons performed. We used unadjusted logistic regression analyses to determine prevalence ratios and 95% confidence intervals (95% CI) for all outcome variables (in-hospital death and ventilation). Odds ratios were calculated under binomial distributions using SAS’s PROC GENMOD procedure for logit links. The errors of convergence were not present throughout the length of the study. We also performed multivariable logistic regression analyses that were adjusted for age, sex, race, and hospital length of stay. Statistical significance in this study was set at a p-value of <0.05 a priori. All statistical analyses conducted in this study were performed using SAS Studio 3.84 OnDemand for Academics.
In our study, we included 1,387 adults who met our inclusion criteria. Of these, 370 died during the hospital admission. The average length of stay in the hospital was a median of 7 days (interquartile range [IQR] 4, 13). The most common neurological conditions in the study were as follows: ischemic stroke (485 [35%]), hemorrhagic stroke (149 [10.7%]), and movement disorder (166 [12.0%]), among others (Table 1).
Of all patients in the study, 319 (23%) required the use of bilevel positive airway pressure (BiPAP), or continuous positive airway pressure (CPAP), or both at one or more times during their hospital stay. Individuals with neurological conditions such as epilepsy/seizures, headaches, or dementia were more likely to die in the hospital compared to those with other neurological conditions. Patients with only dementia were more likely to be given ventilation support compared to those without dementia (Table 1).
In unadjusted logistic regression analyses, patients with dementia had a 2.6 times higher risk of dying in the hospital than those without dementia. Patients with either ischemic or hemorrhagic stroke had 1.17 times higher odds of in-hospital death than those without a stroke (p < 0.05). The odds of requiring ventilation were statistically significantly increased among those with movement disorders and hemorrhagic strokes (Table 2).
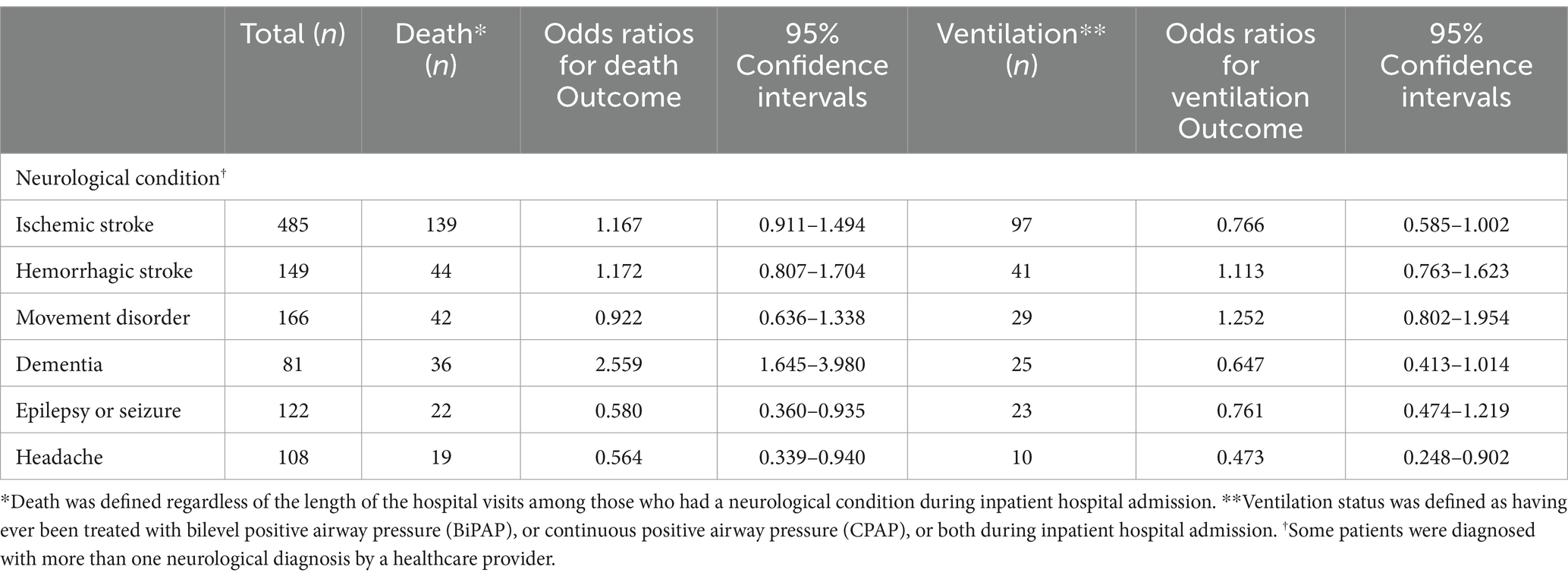
Table 2. Unadjusted odds ratios (ORs) of death or ventilation among patients with acute COVID-19 infection by neurological condition (N = 1,387).
Adjusting for age, sex, race, and hospital length of stay, we found that no neurological condition increased the risk of in-hospital mortality or ventilation. Older age statistically significantly increased the risk of in-hospital death, while the longer hospital length of stay increased the risk of needing ventilation (Table 3).
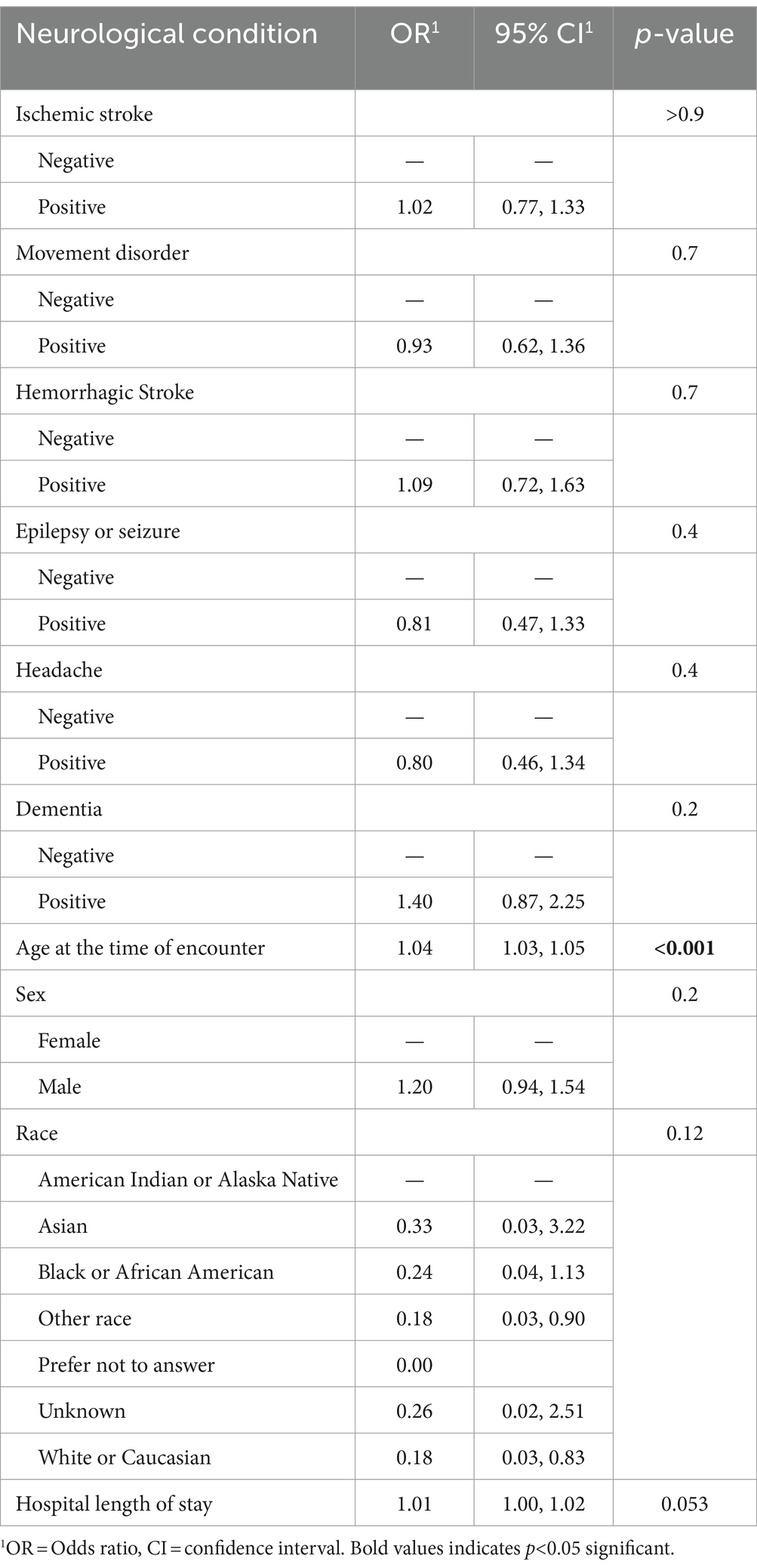
Table 3. Multivariable analyses of effect the of neurological conditions among patients with acute COVID-19 infection on in-hospital death as an outcome (N = 1,387).
In our study among patients with a neurological condition diagnosed with acute COVID-19 during the pre-Omicron and Omicron waves of the pandemic, we found that no neurological condition increased the risk of in-hospital death after adjustment for age, sex, race, and hospital length of stay. However, in-hospital death was more common among those with epilepsy/seizures, headaches, or dementia compared to those without neurological conditions. Our results highlight that these diagnoses may be more associated with in-hospital death. Moreover, we found that epilepsy/seizure or headache diagnosis had a lower risk of in-hospital death. Older age and hospital length of stay may have more influence on in-hospital outcomes than neurological conditions (Table 4).
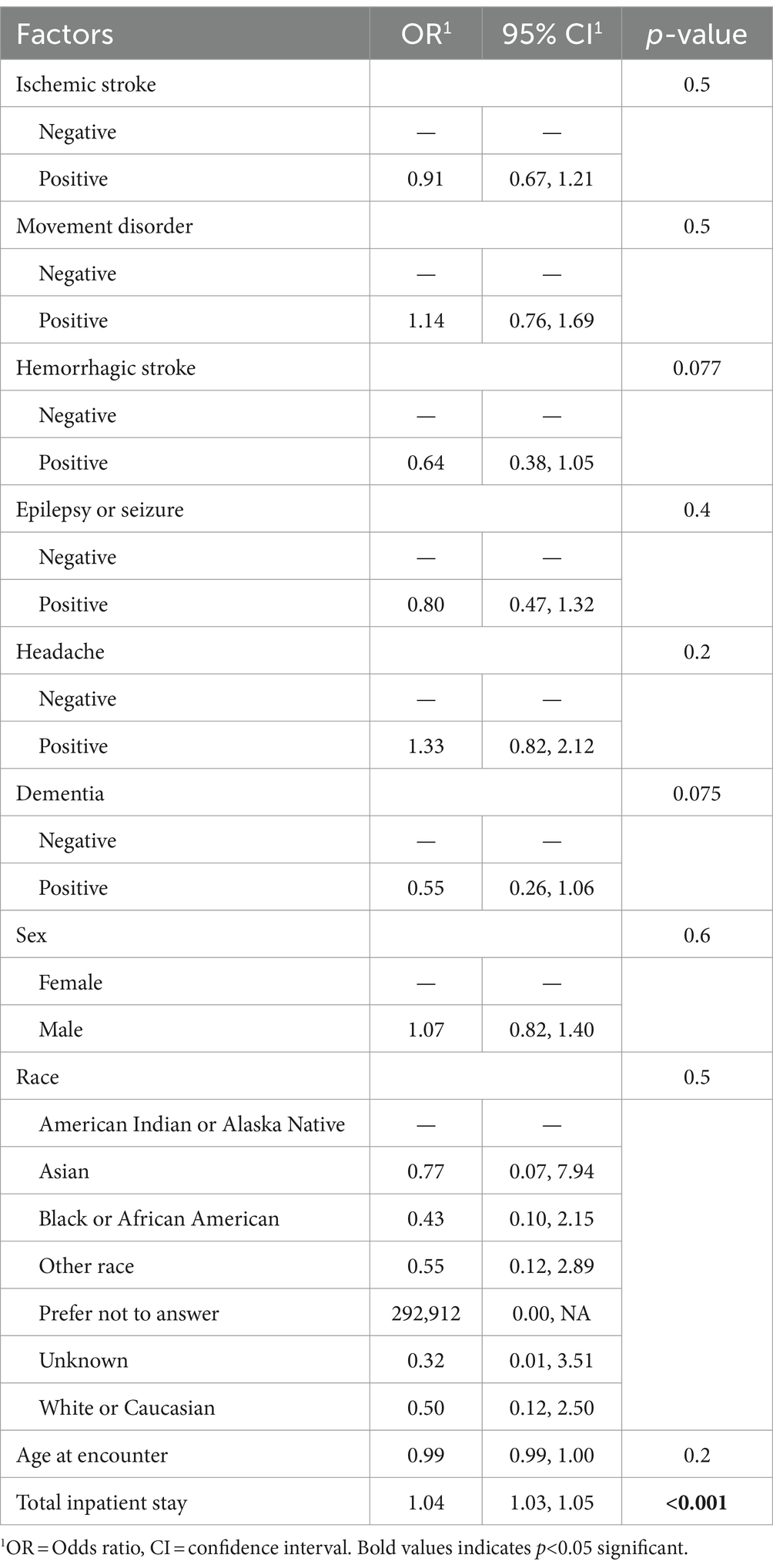
Table 4. Multivariable analyses of the effect of neurological conditions among patients with acute COVID-19 infection on ventilation as an outcome (N = 1,387).
Sociodemographic factors are known to be associated with in-hospital outcomes in the setting of COVID-19 infection. Although initial studies showed that individuals of older age, male sex, and white race were at higher risk of neurologic manifestations and a worse prognosis (9, 13, 14), younger age and female sex have also been associated with an increased likelihood of neurological manifestations among patients infected with the Omicron variant (2). Another study demonstrated worse cognitive performance 6 months post-hospitalization due to COVID-19 among individuals identifying as Black (15). In our study, we found that older age increased the risk of in-hospital death, while longer hospital LOS increased the risk of ventilation, but no neurological condition increased the risk of these outcomes after adjustment for demographics and LOS.
Headache, dizziness, nausea, vomiting, confusion, anosmia, ageusia, and myalgia are among the most commonly reported neurologic symptoms experienced by COVID-19-infected individuals in both pre-Omicron and Omicron variants (2, 3, 10, 16, 17). Among younger individuals, a rise in neurologic symptoms, such as altered mental status and seizures, was also observed when the Omicron variant emerged (18, 19). This is consistent with the findings of another study, which stratified subjects into pre-Omicron and post-Omicron surge groups and demonstrated that encephalopathy is the most common neurologic diagnosis among both variants (11). The frequency of stroke and seizure was higher among post-Omicron surge patients compared to pre-Omicron patients (11). Our study was conducted during the pre-Omicron and Omicron waves and demonstrated that approximately 30% of patients with a stroke died in the hospital. Interestingly, we found that having a headache diagnosis lowered the risk of in-hospital death in multivariable analyses. One study found that those with pain syndromes also had a lower risk of in-hospital death compared to those without pain. One possible reason is that those who had worse COVID-19 severity (i.e., respiratory distress) may suffer from pain perception, and therefore, headaches may not be reported by those with worse COVID-19 severity (20).
Moreover, we found that having a diagnosis of epilepsy/seizure or headache carried a lower risk of death in multivariable analyses. This is different from prior studies that found an increased risk of death among people with epilepsy and COVID-19 infection compared to people without COVID-19 (a hazard ratio of 2.15 [95% CI 1.78–2.59]) (21). However, two other studies did not demonstrate an increased risk of in-hospital death among people with epilepsy and COVID-19 infection (22, 23). It is possible that in our studies, the small number of death events among the epilepsy group (n = 22) may not have been powered enough to detect an increased risk of death. Further studies would require chart review to determine reasons why particular patient characteristics among the group of patients with epilepsy may have been protective against death.
The course of disease among SARS-CoV-2-infected individuals appears to be largely influenced by pre-existing neurological disorders during the pre-Omicron era. For instance, individuals with pre-existing cerebrovascular disease tend to have worse outcomes such as lack of clinical improvement, development of acute respiratory distress syndrome (ARDS), need for ICU treatment, and symptom remission (14, 24, 25). Patients hospitalized with acute COVID-19 infection and known neurodegenerative diseases, such as dementia, parkinsonism, or multiple sclerosis, experienced altered mental status more often than those without neurodegenerative diseases (26). Although patients with neurodegenerative diseases typically demonstrate higher COVID-19 mortality rates due to older age (5), a comparison between matched groups of COVID-19 hospitalized patients with and without neurodegenerative diseases showed no significant difference in mortality, hospital length of stay, and ventilation when controlling for code status (26). In our study, we did not have access to code status, which may have influenced our results. Similar to prior findings, after controlling for age, those with dementia, as well as other neurological conditions, did not have an increased risk of in-hospital mortality or ventilation.
Our study has some limitations. First, we neither controlled for the length of time with a neurological condition nor did we have information on COVID-19 severity, neurological disease severity, or staging, given the diagnoses were based on ICD-10 codes. Patients who were more likely to have had a neurological condition for longer may be at higher risk of poor in-hospital outcomes. We were unable to distinguish the timing of the neurological condition, given the nature of the dataset; therefore, those who were on ventilation may have had an increase in the incidence of a neurological condition during their hospital stay. Third, those who were in the hospital for a longer LOS may be more likely to have poor outcomes, and those with a code status of do-not-resuscitate may not have been given ventilation support. We did not have access to coding status data, and vaccination status data were inconsistent; therefore, the ventilation and mortality outcome findings may have been influenced by data that we were unable to capture. We did not have vaccination data, but future research would account for the effects of vaccination status, mortality, and ventilation. Finally, this was a large retrospective study based on ICD-10 codes from a registry of all patients who were admitted to our hospital; therefore, some diagnoses might have been missed if coded differently.
Among patients with acute COVID-19 infection and a neurological condition, both mortality and ventilation were high, each at approximately one-quarter. Death was the most common among those with epilepsy, headaches, or dementia, but no neurological condition increased the risk of in-hospital mortality or ventilation. Future studies would determine the long-term neurological sequelae of those discharged from the hospital with COVID-19 and a neurological condition. It would also determine how the severity or etiology of neurological illness (i.e., sub-types of dementia) could impact outcomes of COVID-19 infection and how thrombolysis among patients with ischemic stroke and acute COVID-19 infection may affect outcomes. Our study highlights that those with a neurological condition should be monitored closely for adverse outcomes during their hospital stay if diagnosed with a COVID-19 infection.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by University of North Carolina at Chapel Hill IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This was a retrospective study using chart review. Our IRB accepted a HIPAA waiver and did not require informed consent for this study.
AD: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. VF: Data curation, Writing – original draft, Writing – review & editing. CT: Formal analysis, Writing – original draft, Writing – review & editing. HU: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. AS: Data curation, Writing – original draft, Writing – review & editing. SW: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. MD: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to acknowledge Samuel Hawke, BS, and Bahjat F Qaqish, PhD, from the Department of Biostatistics, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, USA. We would also like to acknowledge the Carolina Data Warehouse for providing us with this data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fink, EL, Robertson, CL, Wainwright, MS, Roa, JD, Lovett, ME, Stulce, C, et al. Prevalence and risk factors of neurologic manifestations in hospitalized children diagnosed with acute SARS-CoV-2 or MIS-C. Pediatr Neurol. (2022) 128:33–44. doi: 10.1016/j.pediatrneurol.2021.12.010
2. Shen, X, Wang, P, Shen, J, Jiang, Y, Wu, L, Nie, X, et al. Neurological manifestations of hospitalized patients with mild to moderate infection with SARS-CoV-2 omicron variant in Shanghai. China J Infect Public Health. (2023) 16:155–62. doi: 10.1016/j.jiph.2022.12.005
3. Meppiel, E, Peiffer-Smadja, N, Maury, A, Bekri, I, Delorme, C, Desestret, V, et al. Neurologic manifestations associated with COVID-19: a multicentre registry. Clin Microbiol Infect. (2021) 27:458–66. doi: 10.1016/j.cmi.2020.11.005
4. Fotuhi, M, Mian, A, Meysami, S, and Raji, CA. Neurobiology of COVID-19. J Alzheimers Dis. (2020) 76:3–19. doi: 10.3233/JAD-200581
5. Fu, Y-W, Xu, H-S, and Liu, S-J. COVID-19 and neurodegenerative diseases. Eur Rev Med Pharmacol Sci. (2022) 26:4535–44. doi: 10.26355/eurrev_202206_29093
6. Hingorani, KS, Bhadola, S, and Cervantes-Arslanian, AM. COVID-19 and the brain. Trends Cardiovasc Med. (2022) 32:323–30. doi: 10.1016/j.tcm.2022.04.004
7. Zhang, P-P, He, Z-C, Yao, X-H, Tang, R, Ma, J, Luo, T, et al. COVID-19-associated monocytic encephalitis (CAME): histological and proteomic evidence from autopsy. Signal Transduct Target Ther. (2023) 8:24. doi: 10.1038/s41392-022-01291-6
8. Needham, EJ, Ren, AL, Digby, RJ, Norton, EJ, Ebrahimi, S, Outtrim, JG, et al. Brain injury in COVID-19 is associated with dysregulated innate and adaptive immune responses. Brain J Neurol. (2022) 145:4097–107. doi: 10.1093/brain/awac321
9. Chou, SH-Y, Beghi, E, Helbok, R, Moro, E, Sampson, J, Altamirano, V, et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19—a report for the GCS-NeuroCOVID consortium and the ENERGY consortium. JAMA Netw Open. (2021) 4:e2112131. doi: 10.1001/jamanetworkopen.2021.12131
10. Collantes, MEV, Espiritu, AI, Sy, MCC, Anlacan, VMM, and Jamora, RDG. Neurological manifestations in COVID-19 infection: a systematic review and Meta-analysis. Can J Neurol Sci J Can Sci Neurol. (2021) 48:66–76. doi: 10.1017/cjn.2020.146
11. Kim, CY, Sardar, Z, Ayele, BA, Fleck-Derderian, S, Barrett, CE, Sun, Y, et al. Neurological diagnoses in hospitalized COVID-19 patients during the B.1.1.529 surge. Ann Clin Transl Neurol. (2023) 10:1433–41. doi: 10.1002/acn3.51833
12. Taquet, M, Sillett, R, Zhu, L, Mendel, J, Camplisson, I, Dercon, Q, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. (2022) 9:815–27. doi: 10.1016/S2215-0366(22)00260-7
13. Wang, D, Hu, B, Hu, C, Zhu, F, Liu, X, Zhang, J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
14. Herman, C, Mayer, K, and Sarwal, A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology. (2020) 95:77–84. doi: 10.1212/WNL.0000000000009673
15. Valdes, E, Fuchs, B, Morrison, C, Charvet, L, Lewis, A, Thawani, S, et al. Demographic and social determinants of cognitive dysfunction following hospitalization for COVID-19. J Neurol Sci. (2022) 438:120146. doi: 10.1016/j.jns.2022.120146
16. Beretta, S, Cristillo, V, Camera, G, Morotti Colleoni, C, Pellitteri, G, Viti, B, et al. Incidence and long-term functional outcome of neurologic disorders in hospitalized COVID-19 patients infected with pre-omicron variants. Neurology. (2023) 101:e892–903. doi: 10.1212/WNL.0000000000207534
17. Bhola, S, Trisal, J, Thakur, V, Kaur, P, Kulshrestha, S, Bhatia, SK, et al. Neurological toll of COVID-19. Neurol Sci. (2022) 43:2171–86. doi: 10.1007/s10072-022-05875-6
18. Dimopoulou, D, Dasoula, F, Liaska, M, Eleftheriou, I, Maritsi, D, Tsolia, M, et al. Rise of neurologic manifestations during SARS-CoV-2 omicron wave in children with COVID-19. Pediatr Infect Dis J. (2023) 42:e128–9. doi: 10.1097/INF.0000000000003826
19. Sahin, A, Karadag-Oncel, E, Buyuksen, O, Ekemen-Keles, Y, Ustundag, G, Elvan-Tuz, A, et al. The diversity in the clinical features of children hospitalized with COVID-19 during the nonvariant, alpha (B.1.1.7), Delta (B.1.617.2), and omicron (B.1.1.529) variant periods of SARS CoV-2: caution for neurological symptoms in omicron variant. J Med Virol. (2023) 95:e28628. doi: 10.1002/jmv.28628
20. Knox, N, Lee, C-S, Moon, JY, and Cohen, SP. Pain manifestations of COVID-19 and their association with mortality: a Multicenter prospective observational study. Mayo Clin Proc. (2021) 96:943–51. doi: 10.1016/j.mayocp.2020.12.014
21. Strafford, H, Hollinghurst, J, Lacey, AS, Akbari, A, Watkins, A, Paterson, J, et al. Epilepsy and the risk of COVID-19-related hospitalization and death: a population study. Epilepsia. (2024) 65:1383–93. doi: 10.1111/epi.17910
22. Muccioli, L, Zenesini, C, Taruffi, L, Licchetta, L, Mostacci, B, Di Vito, L, et al. Risk of hospitalization and death for COVID-19 in persons with epilepsy over a 20-month period: the EpiLink Bologna cohort. Italy Epilepsia. (2022) 63:2279–89. doi: 10.1111/epi.17356
23. Mbizvo, GK, Schnier, C, Ramsay, J, Duncan, SE, and Chin, RF. Epilepsy-related mortality during the COVID-19 pandemic: a nationwide study of routine Scottish data. Seizure. (2023) 110:160–8. doi: 10.1016/j.seizure.2023.06.010
24. Fang, X, Li, S, Yu, H, Wang, P, Zhang, Y, Chen, Z, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging. (2020) 12:12493–503. doi: 10.18632/aging.103579
25. Mo, P, Xing, Y, Xiao, Y, Deng, L, Zhao, Q, Wang, H, et al. Clinical characteristics of refractory coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. (2021) 73:e4208–13. doi: 10.1093/cid/ciaa270
Keywords: COVID-19, SARS-CoV-2, neurological, in-hospital mortality, ventilation
Citation: Desouky A, Fuentes V, Tiwari C, Usui H, Smith Ayala AH, Wilson SE and Diaz MM (2024) Hospital outcomes of acute COVID-19 infection among patients with neurological conditions: a single-center study. Front. Neurol. 15:1434046. doi: 10.3389/fneur.2024.1434046
Received: 17 May 2024; Accepted: 25 June 2024;
Published: 10 July 2024.
Edited by:
Beatrice Paradiso, University of Milan, ItalyReviewed by:
George D. Vavougios, University of Cyprus, CyprusCopyright © 2024 Desouky, Fuentes, Tiwari, Usui, Smith Ayala, Wilson and Diaz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monica M. Diaz, TW9uaWNhLmRpYXpAbmV1cm9sb2d5LnVuYy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.