- 1Dementia Research Centre, Department of Neurodegenerative Disease, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom
- 2Division of Neurology, Department of Internal Medicine, King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand
- 3Cognitive Clinical and Computational Neuroscience Research Unit, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 4Centre for Preventive Neurology, Wolfson Institute of Population Health, Queen Mary University of London, London, United Kingdom
Background: Inappropriate trusting behaviour may have significant social, financial and other consequences for people living with dementia. However, its clinical associations and predictors have not been clarified. Here we addressed this issue in canonical syndromes of frontotemporal dementia (FTD) and Alzheimer’s disease (AD).
Methods: In 34 patients with AD and 73 with FTD (27 behavioural variant (bv)FTD, 22 semantic variant primary progressive aphasia (svPPA), 24 nonfluent/agrammatic variant (nfv)PPA) we recorded inappropriate trusting and other abnormal socio-emotional behaviours using a semi-structured caregiver survey. Patients were comprehensively characterised using a general cognitive assessment and the Revised Self-Monitoring Scale (RSMS; an informant index of socioemotional awareness).
Results: Inappropriate trusting was more frequent in svPPA (55%) and bvFTD (44%) than nfvPPA (17%) or AD (24%). After adjusting for age, sex, education and Mini-Mental State Examination (MMSE) score, inappropriate trusting was significantly more likely in svPPA (odds ratio 3.61; 95% confidence interval 1.41–8.75) and bvFTD (3.01, 1.23–6.65) than AD. Significant predictors of inappropriate trusting comprised apathy in svPPA, disinhibition and altered pain responsiveness in bvFTD, and lower MMSE and RSMS (self-presentation) scores in AD.
Conclusion: Dementia syndromes vary in prevalence and predictors of abnormal trusting behaviour, with implications for clinical counselling and safeguarding.
Introduction
People living with dementia are at substantial risk from impaired judgment and decision making, including inappropriately placing trust in others. This is particularly pertinent to financial decisions and susceptibility to scams (1, 2). While cognitive impairment per se may lead to inappropriate trusting and impaired scam detection (2, 3), patients with diseases in the frontotemporal dementia (FTD) spectrum may be relatively more vulnerable due to early, prominent changes in socio-emotional behaviour and awareness (1, 4–7). However, the factors that drive abnormal trusting behaviour and how these might vary between canonical dementia syndromes have not been defined.
Here we addressed this issue in patients representing Alzheimer’s disease (AD) and the major behavioural and language-led variant syndromes of FTD (behavioural variant (bv)FTD, semantic variant primary progressive aphasia (svPPA) and nonfluent/agrammatic variant (nfv)PPA). We surveyed patients’ primary caregivers about abnormal trusting and other potentially relevant behavioural changes since illness onset, and assessed the influence of diagnosis, cognitive and behavioural features on the development of inappropriate trusting. Based on clinical experience and previous evidence, we hypothesised that inappropriate trusting behaviour would be more prevalent in FTD syndromes than AD, and would be predicted by abnormal interpretation of socio-emotional signals, impaired governing of own social behaviour, and/or altered responsiveness to aversive consequences. The last is likely to share pathophysiological mechanisms with responsiveness to pain, which is commonly altered in bvFTD and svPPA syndromes and was accordingly used here to index abnormal behavioural sensitivity to negative outcomes more generally (8, 9).
Materials and methods
We studied 107 patients: 34 with typical amnestic AD and 73 with major FTD syndromes (27 bvFTD, 22 svPPA, 24 nfvPPA). All had compatible general neuropsychological, brain MRI and CSF findings, and mild to moderately severe disease (details in Table 1). Each patient had a primary caregiver who could provide reliable information on their premorbid and current behaviour.
In a semi-structured survey, caregivers were asked whether there had been increased instances of patients inappropriately trusting other people, such as heightened gullibility, incautiousness or acts of poor judgement, and were invited to provide examples. They were also surveyed about the presence or absence of changes in other socio-emotional behaviours (social disinhibition, obsessionality, apathy, altered pain responsiveness) that we hypothesised might be relevant to inappropriate trusting. Caregivers were asked to assess behavioural changes relative to the patients’ behaviour 10 years previously (an interval predating symptom onset for all patients). Caregivers also completed the Revised Self-Monitoring Scale (RSMS) (10) an index of social impression management and responsiveness to changes in the social environment. The scale has two subscores. The socioemotional expressiveness score (RSMS-EX) measures the ability to understand social cues of others, and the modification of self-presentation score (RSMS-SP) measures the ability to change one’s behaviour when it is not appropriate in a social situation.
Data were analysed using Python (v3.8.5) software and the logistic regression package from scikit-learn 1.2.0 with bootstrapping (10,000 iterations each) for all logistic regression analyses.
Participant groups were compared on demographic, cognitive and behavioural measures using ANOVA and Kruskal-Wallis tests for continuous variables, and chi-square tests and Fisher’s exact tests (when expected counts were small) for categorical variables. Post-hoc pair-wise comparisons were carried out when applicable, with false-discovery-rate correction. For all tests, p < 0.05 was accepted as the threshold for statistical significance.
Odds of inappropriate trusting behaviour in each FTD syndromic group compared to the AD group were assessed using logistic regression models, adjusting for age, sex, years of education and Mini-Mental State Examination (MMSE) score (a surrogate for disease severity). In separate univariate logistic regression models based on 69 patients with complete correlative neuropsychological and behavioural data, we assessed candidate cognitive and behavioural predictors of inappropriate trusting behaviour within different syndromic groups. These candidate predictors comprised MMSE score (overall level of cognitive function), WASI (Wechsler Abbreviated Scale of Intelligence) Matrices subtest score (nonverbal reasoning ability), RSMS-total score, RSMS-EX (socio-emotional expressiveness subscore), RSMS-SP (ability to modify self-presentation subscore) and presence (or absence) of social disinhibition, obsessionality, apathy and altered pain responsiveness.
The study was approved by the University College London institutional ethics committee and all participants gave informed consent in accordance with the Declaration of Helsinki. The data that support the findings of this study are not publicly available (in line with the terms of the original ethics approval) but available on reasonable request from the corresponding author.
Results
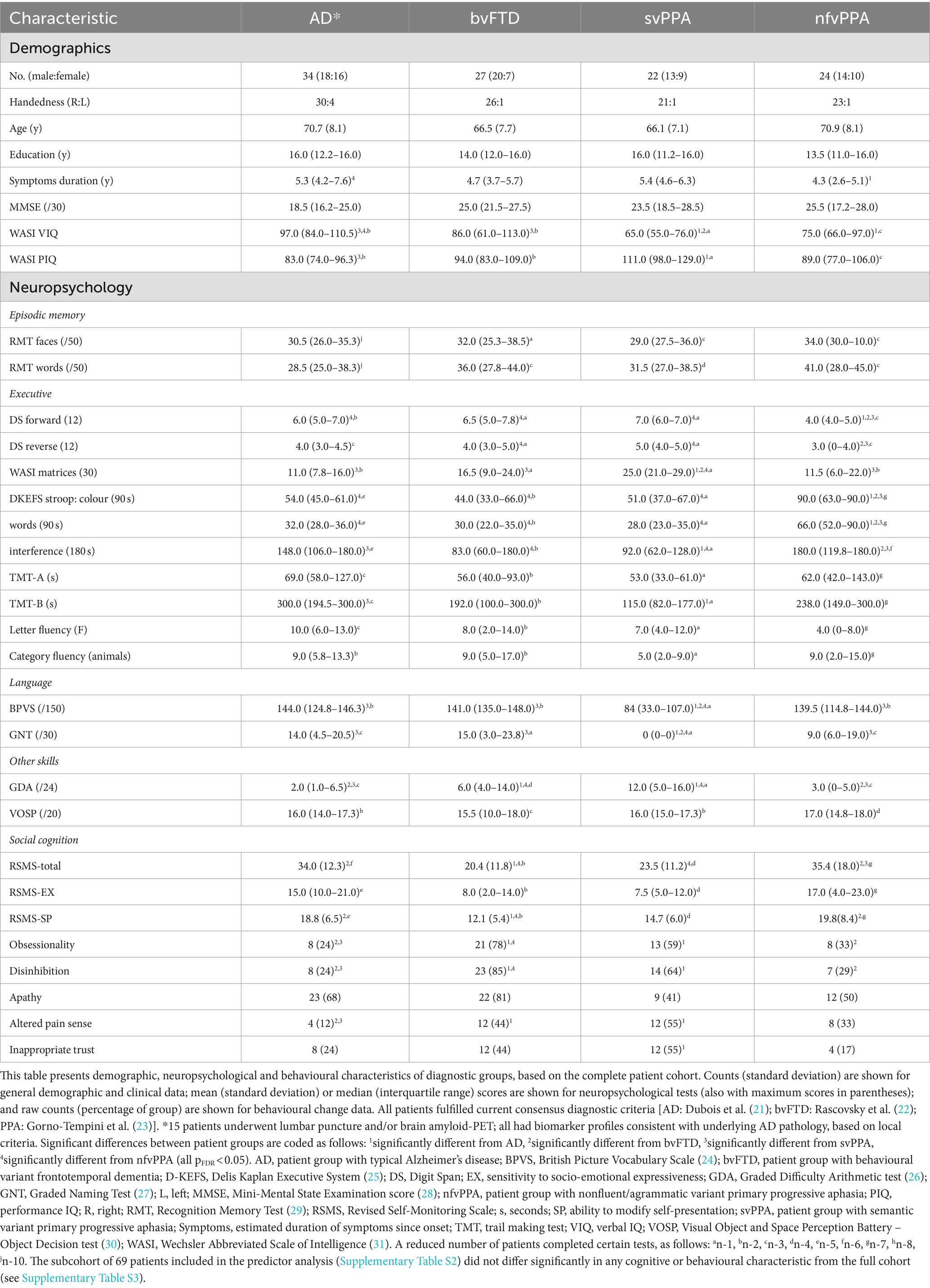
Patient groups were well matched in age, sex and years of education and showed cognitive and behavioural features in keeping with their syndromic diagnoses (Table 1), including lowest RSMS and highest prevalence of social disinhibition, obsessionality and altered pain responsiveness in the bvFTD and svPPA groups.
Across all patient groups, inappropriate trusting behaviour was more prevalent in the svPPA (12/22 cases, 55%) and bvFTD (12/27, 44%) groups than the nfvPPA (4/24, 17%) or AD (8/34, 24%) groups. Risk was most significantly increased with a diagnosis of svPPA (odds ratio 3.61; 95% confidence interval 1.41–8.75) and bvFTD (3.01; 1.23–6.65) relative to AD but did not differ significantly between nfvPPA and AD (1.06; 0.36–2.96) (Figure 1). When invited to describe patients’ inappropriately trusting behaviour, a number of caregivers detailed how they had fallen victim to email and other financial “scams” (examples in Supplementary Table S1).
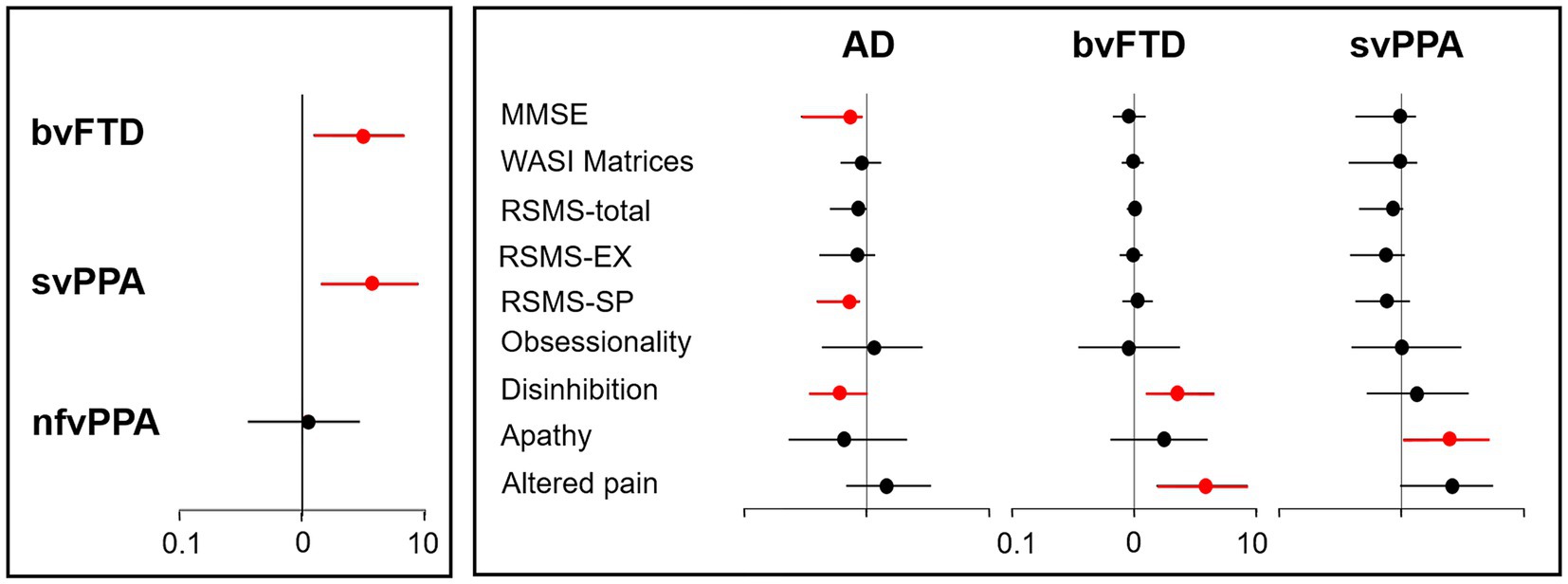
Figure 1. Risk factors for inappropriate trusting behaviour in dementia syndromes. The figure is a graphical representation of diagnostic, cognitive and behavioural risk factors for inappropriate trusting behaviour in patients with dementia (see Supplementary Table S2). Odds ratios (with 95% confidence intervals) are shown, plotted on a log-10 scale; significantly increased or reduced odds are depicted in red. The left panel displays the risk associated with a diagnosis of each canonical frontotemporal dementia syndrome relative to Alzheimer’s disease (adjusted for age, sex, years of education, and Mini-Mental State Examination score; see text). The right panel displays the risk associated with key cognitive and behavioural factors within each diagnostic group (excluding the nfvPPA group, as only four patients with this diagnosis showed inappropriate trusting). AD, patient group with Alzheimer’s disease; bvFTD, patient group with behavioural variant frontotemporal dementia; EX, sensitivity to socio-emotional expressiveness; MMSE nfvPPA, patient group with non-fluent/agrammatic primary progressive aphasia; MMSE, Mini-Mental State Examination score; RSMS, Revised Self-Monitoring Scale; SP, ability to modify self-presentation; svPPA, patient group with semantic variant primary progressive aphasia; WASI, Wechsler Abbreviated Scale of Intelligence.
Significant predictors of inappropriate trusting behaviour (after adjusting for age, sex and years of education) comprised apathy (odds ratio 2.48; 95% confidence interval 1.03–5.14) and a trend for higher altered pain responsiveness (2.59; 0.97–5.58) in the svPPA group; disinhibition (2.24; 1.25–4.50) and altered pain responsiveness (3.83; 1.52–8.41) in the bvFTD group; and lower MMSE (0.71; 0.29–0.92) and RSMS-SP scores (0.71; 0.39–0.88) and absence of disinhibition (0.60; 0.34–0.99) in the AD group (Figure 1; Supplementary Table S2).
Discussion
Our findings show that inappropriate trusting presents a significant issue in people living with both AD and FTD, and that patients with bvFTD and svPPA are at highest risk of developing this behaviour, in line with the greater prominence of socio-emotional behavioural deficits in these FTD syndromes (5, 7, 10). Predictors of abnormal trusting here varied between dementia syndromes. In keeping with previous evidence (3), overall level of cognitive impairment and inability to monitor one’s own social conduct were predictors in AD. Disinhibition and abnormal responsiveness to aversive consequences (here indexed as pain) predicted inappropriate trusting behaviour in bvFTD, while apathy was a predictor in svPPA. These profiles suggest different candidate neural mechanisms for abnormal trusting linked to particular socio-emotional behavioural abnormalities in these diseases, in line with previous work (6–8, 10, 11).
Complex behavioural changes are multi-dimensional (8). Apathy in svPPA might promote inappropriate trusting and financial vulnerability by impairing initiative, motivation and autonomous goal-setting (12, 13). However, disinhibition in bvFTD here tended to promote inappropriate trusting behaviour but in AD was relatively ‘protective’. We do not have details about how these behavioural complexes presented in our AD and FTD patients – and further, they were indexed by caregiver report. Whereas disinhibition in FTD tends to manifest as over-familiarity and lack of awareness of social cues, disinhibition and other forms of social inappropriateness in AD may be more associated with irritability, anxiety, social withdrawal, less compliance with social suggestions and wariness of novelty (14, 15). Absence of disinhibition in our AD group may have been a risk factor for inappropriate trusting behaviour if (in AD) disinhibition promotes irritability toward potential scammers, reduced social compliance and wariness of others’ suggestions. Further, the presence versus absence of disinhibition in AD and FTD may be differentially associated with other cognitive capacities (such as emotional sensitivity and decision making) that were not directly captured here.
Altered pain responsiveness might signify a more general problem with physiological anticipation, homeostasis and autonomic coding of potentially salient events, rewards and/or punishments, corroborating previous evidence in FTD syndromes (8, 9, 16, 17). Moreover, the association between pain responsiveness and decision making might be underpinned by overlapping neuroanatomical correlates involving the brain’s salience and homeostatic networks (9, 18). Blunted sensitivity to diverse kinds of negative consequences could tend to promote recurrent risk taking behaviours, reduced apprehensiveness of potentially harmful consequences and gullibility in ambiguous interpersonal exchanges. Pathological gambling can be a significant issue in FTD (19) and it is noteworthy that many caregivers here recorded substantial daily-life impacts of patients’ inappropriate trusting behaviour, notably financial exploitation (see Supplementary Table S1).
Our findings illuminate socio-emotional behavioural changes that promote vulnerability to poor financial and other decision making linked to misplaced trusting in people with major dementias. The findings add to existing evidence that links reduced cognition (1, 6), accumulation of neurodegenerative pathology (3) and cerebrovascular insults (20) to impaired decision making and financial vulnerability. This is a significant clinical issue that warrants greater awareness and understanding by clinicians, health policy makers, safeguarding authorities, financial regulators and especially, people living with dementia and their caregivers. Potential vulnerability to financial and other scams should be considered in all cognitively impaired people: indeed, even in the “lower risk” syndromic groups here (AD, nfvPPA), a substantial minority of patients had exhibited misplaced trust. However, our findings may help prioritise clinical counselling and financial safeguarding discussions where the syndromic diagnosis (bvFTD, svPPA) or behavioural profile places the patient at particularly high risk of exploitation.
This study has several limitations that should inform future work. Assessment of inappropriate trusting and other socio-emotional behaviours was based on caregiver reports, in a relatively small patient cohort. Future studies should focus on creating more objective methods for assessing these behaviours, alongside decision making capacity and financial vulnerability in people with dementia, and should assess prospectively the specific daily life impacts of inappropriate trusting and other risky behaviours on financial and social functioning, well-being and care burden. Inappropriate trust is a highly complex psychological construct: functional neuroimaging techniques such as fMRI would further understanding by elucidating underlying neural mechanisms, and clarifying how these differ (or converge) between dementia syndromes. Additionally, more detailed stratification of the neuropsychological, behavioural and neural predictors of misplaced trusting in larger and more diverse neurodegenerative disease cohorts as well as in cognitively well older people would allow development of bespoke clinical counselling and safeguarding strategies. It will also be important to establish in longitudinal studies when and how potential vulnerabilities develop over the course of the illness. As a first step, the present findings should prompt clinicians to enquire about inappropriate trusting and vulnerability to scams in all people living with dementia, with a particularly high index of suspicion in bvFTD and svPPA, and in the setting of other socio-emotional behavioural changes.
Data availability statement
The datasets presented in this article are not readily available because our ethical approvals and clinical confidentiality considerations specifically preclude public archiving of participant data. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by University College London institutional ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
AC: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. DP: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. CA: Data curation, Writing – original draft, Writing – review & editing. JJ: Data curation, Methodology, Writing – original draft, Writing – review & editing. EB: Data curation, Methodology, Writing – original draft, Writing – review & editing. LR: Data curation, Methodology, Writing – original draft, Writing – review & editing. CH: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. CM: Data curation, Methodology, Writing – original draft, Writing – review & editing. JR: Conceptualization, Data curation, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing. JW: Conceptualization, Data curation, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Dementia Research Centre is supported by Alzheimer’s Research UK, Brain Research UK, and the Wolfson Foundation. This work was supported by the Alzheimer’s Society, Alzheimer’s Research UK and the National Institute for Health Research University College London Hospitals Biomedical Research Centre. JJ was supported by an Association of British Neurologists Clinical Research Training Fellowship. EB was supported by a Brain Research UK PhD Studentship. CH has been supported by an RNID-Dunhill Medical Trust Pauline Ashley Fellowship (PA23_Hardy). CM is supported by a grant from Bart’s Charity. JR is supported by a Medical Research Council Clinician Scientist Fellowship (MR/M008525/1), the Miriam Marks Brain Research UK Senior Fellowship and the NIHR Rare Disease Translational Research Collaboration (BRC149/NS/MH). JW receives grant support from Alzheimer’s Research UK, the Alzheimer’s Society and the National Brain Appeal (Frontotemporal Dementia Research Studentship in Memory of David Blechner). This research was funded in part by UKRI. For the purpose of Open Access, the authors have applied a Creative Commons Attribution (CC BY) public copyright licence to any author accepted manuscript version arising from this submission. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Acknowledgments
We are grateful to all patients and caregivers for their participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1433135/full#supplementary-material
References
1. Spreng, RNP, Karlawish, JM, and Marson, DCM. Cognitive, social, and neural determinants of diminished decision-making and financial exploitation risk in aging and dementia: a review and new model. J Elder Abuse Negl. (2016) 28:320–44. doi: 10.1080/08946566.2016.1237918
2. Fenton, L, Weissberger, GH, Boyle, PA, Mosqueda, L, Yassine, HN, Nguyen, AL, et al. Cognitive and neuroimaging correlates of financial exploitation vulnerability in older adults without dementia: implications for early detection of Alzheimer's disease. Neurosci Biobehav Rev. (2022) 140:104773. doi: 10.1016/j.neubiorev.2022.104773
3. Boyle, PA, Yu, L, Schneider, JA, Wilson, RS, and Bennett, DA. Scam awareness related to incident Alzheimer dementia and mild cognitive impairment: a prospective cohort study. Ann Intern Med. (2019) 170:702–9. doi: 10.7326/M18-2711
4. Gill, S, Blair, M, Kershaw, M, Jesso, S, Mac Kinley, J, Coleman, K, et al. Financial capacity in frontotemporal dementia and related presentations. J Neurol. (2019) 266:1698–707. doi: 10.1007/s00415-019-09317-w
5. Pressman, PS, and Miller, BL. Diagnosis and management of behavioral variant frontotemporal dementia. Biol Psychiatry. (2014) 75:574–81. doi: 10.1016/j.biopsych.2013.11.006
6. Wong, S, Irish, M, O'Callaghan, C, Kumfor, F, Savage, G, Hodges, JR, et al. Should I trust you? Learning and memory of social interactions in dementia. Neuropsychologia. (2017) 104:157–67. doi: 10.1016/j.neuropsychologia.2017.08.016
7. Fittipaldi, S, Ibanez, A, Baez, S, Manes, F, Sedeno, L, and Garcia, AM. More than words: social cognition across variants of primary progressive aphasia. Neurosci Biobehav Rev. (2019) 100:263–84. doi: 10.1016/j.neubiorev.2019.02.020
8. Chiong, W, Wood, KA, Beagle, AJ, Hsu, M, Kayser, AS, Miller, BL, et al. Neuroeconomic dissociation of semantic dementia and behavioural variant frontotemporal dementia. Brain. (2016) 139:578–87. doi: 10.1093/brain/awv344
9. Fletcher, PD, Downey, LE, Golden, HL, Clark, CN, Slattery, CF, Paterson, RW, et al. Pain and temperature processing in dementia: a clinical and neuroanatomical analysis. Brain. (2015) 138:3360–72. doi: 10.1093/brain/awv276
10. Franklin, HD, Russell, LL, Peakman, G, Greaves, CV, Bocchetta, M, Nicholas, J, et al. The revised self-monitoring scale detects early impairment of social cognition in genetic frontotemporal dementia within the GENFI cohort. Alzheimers Res Ther. (2021) 13:127. doi: 10.1186/s13195-021-00865-w
11. Martinez, M, Multani, N, Anor, CJ, Misquitta, K, Tang-Wai, DF, Keren, R, et al. Emotion detection deficits and decreased empathy in patients with Alzheimer's disease and Parkinson's disease affect caregiver mood and burden. Front Aging Neurosci. (2018) 10:120. doi: 10.3389/fnagi.2018.00120
12. Husain, M, and Roiser, JP. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci. (2018) 19:470–84. doi: 10.1038/s41583-018-0029-9
13. Giannouli, V, and Tsolaki, M. Is depression or apathy playing a key role in predicting financial capacity in Parkinson's disease with dementia and frontotemporal dementia? Brain Sci. (2021) 11:785. doi: 10.3390/brainsci11060785
14. Craig, KJ, Hietanen, H, Markova, IS, and Berrios, GE. The irritability questionnaire: a new scale for the measurement of irritability. Psychiatry Res. (2008) 159:367–75. doi: 10.1016/j.psychres.2007.03.002
15. St-Georges, MA, Wang, L, Chapleau, M, Migliaccio, R, Carrier, T, and Montembeault, M. Social cognition and behavioral changes in patients with posterior cortical atrophy. J Neurol. (2024) 271:1439–50. doi: 10.1007/s00415-023-12089-z
16. Perry, DC, Datta, S, Sturm, VE, Wood, KA, Zakrzewski, J, Seeley, WW, et al. Reward deficits in behavioural variant frontotemporal dementia include insensitivity to negative stimuli. Brain. (2017) 140:3346–56. doi: 10.1093/brain/awx259
17. Hoefer, M, Allison, SC, Schauer, GF, Neuhaus, JM, Hall, J, Dang, JN, et al. Fear conditioning in frontotemporal lobar degeneration and Alzheimer's disease. Brain. (2008) 131:1646–57. doi: 10.1093/brain/awn082
18. Samanez-Larkin, GR, and Knutson, B. Decision making in the ageing brain: changes in affective and motivational circuits. Nat Rev Neurosci. (2015) 16:278–89. doi: 10.1038/nrn3917
19. Manes, FF, Torralva, T, Roca, M, Gleichgerrcht, E, Bekinschtein, TA, and Hodges, JR. Frontotemporal dementia presenting as pathological gambling. Nat Rev Neurol. (2010) 6:347–52. doi: 10.1038/nrneurol.2010.34
20. Kapasi, A, Schneider, JA, Yu, L, Lamar, M, Bennett, DA, and Boyle, PA. Association of Stroke and Cerebrovascular Pathologies with Scam Susceptibility in older adults. JAMA Neurol. (2023) 80:49–57. doi: 10.1001/jamaneurol.2022.3711
21. Dubois, B, Feldman, HH, Jacova, C, Hampel, H, Molinuevo, JL, Blennow, K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. (2014) 13:614–29. doi: 10.1016/S1474-4422(14)70090-0
22. Rascovsky, K, Hodges, JR, Knopman, D, Mendez, MF, Kramer, JH, Neuhaus, J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. (2011) 134:2456–77. doi: 10.1093/brain/awr179
23. Gorno-Tempini, ML, Hillis, AE, Weintraub, S, Kertesz, A, Mendez, M, Cappa, SF, et al. Classification of primary progressive aphasia and its variants. Neurology. (2011) 76:1006–14. doi: 10.1212/WNL.0b013e31821103e6
24. Dunn, L, Dunn, L, Whetton, C, and Plntlllie, D. The British picture vocabulary scales. Windsor: NFER-Nelson (1982).
25. Delis, DC, Kaplan, E, and Kramer, JH. Delis-Kaplan executive function system: Technical manual. San Antonio, TX: Harcourt Assessment Company (2001).
26. Jackson, M, and Warrington, EK. Arithmetic skills in patients with unilateral cerebral lesions. Cortex. (1986) 22:611–620. doi: 10.1016/s0010-9452(86)80020-x
27. McKenna, P, and Warrington, EK. Testing for nominal dysphasia. J Neurol Neurosurg Psychiatry. (1980) 43:781–788. doi: 10.1136/jnnp.43.9.781
28. Folstein, MF, Folstein, SE, and McHugh, PR. “Mini-mental state,” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–198. doi: 10.1016/0022-3956(75)90026-6
30. Warrington, EK, and James, M. Thames Valley Test Company. Bury St Edmunds: The visual object and space perception battery (1991).
Keywords: trust, Alzheimer’s disease, frontotemporal dementia, primary progressive aphasia, dementia
Citation: Chokesuwattanaskul A, Penn D, Albero C, Johnson JCS, Benhamou E, Russell LL, Hardy CJD, Marshall CR, Rohrer JD and Warren JD (2024) Inappropriate trusting behaviour in dementia. Front. Neurol. 15:1433135. doi: 10.3389/fneur.2024.1433135
Edited by:
Robert Fleischmann, Independent Researcher, Greifswald, GermanyReviewed by:
Nahid Olfati, University of California, San Diego, United StatesStephen Macfarlane, HammondCare, Australia
Copyright © 2024 Chokesuwattanaskul, Penn, Albero, Johnson, Benhamou, Russell, Hardy, Marshall, Rohrer and Warren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jason D. Warren, amFzb24ud2FycmVuQHVjbC5hYy51aw==
†ORCID: Jeremy C. S. Johnson, https://orcid.org/0000-0002-4265-7997
 Anthipa Chokesuwattanaskul
Anthipa Chokesuwattanaskul Dexter Penn
Dexter Penn Claudia Albero
Claudia Albero Jeremy C. S. Johnson
Jeremy C. S. Johnson Elia Benhamou
Elia Benhamou Lucy L. Russell
Lucy L. Russell Chris J. D. Hardy
Chris J. D. Hardy Charles R. Marshall
Charles R. Marshall Jonathan D. Rohrer
Jonathan D. Rohrer Jason D. Warren
Jason D. Warren