
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 26 July 2024
Sec. Headache and Neurogenic Pain
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1423569
This article is part of the Research Topic Expanding the Paradigm of the Management of Headaches: Integrated Multidisciplinary Perspectives from Bench to Bedside View all 10 articles
 Haibing Xiong1
Haibing Xiong1 Ran Jiang2
Ran Jiang2 Lingzhi Xing3
Lingzhi Xing3 Jiaojiao Zheng4
Jiaojiao Zheng4 Xinhong Tian2
Xinhong Tian2 Jiajie Leng5
Jiajie Leng5 Xin Guo1
Xin Guo1 Shi Zeng1
Shi Zeng1 Haofeng Xiong1
Haofeng Xiong1 Jianhong Huo1
Jianhong Huo1 Letai Li2*
Letai Li2*Background: Previous observational clinical studies and meta-analyses have yielded inconsistent results regarding the relationship between vitamin D and headache, and the causal relationship remains unclear. The aim of this study was to investigate the causal relationship between vitamin D and headache by bidirectional two-sample Mendelian randomisation (MR) analysis.
Methods: The relationship between high levels of vitamin D and headache was investigated by two-sample MR analysis using publicly available genome-wide association study (GWAS) data. The primary method was inverse variance weighting (IVW), and secondary methods were weighted median and MR-Egger methods. No heterogeneity or horizontal multidirectionality was found in the MR results. The robustness and validity of the findings were assessed using the leave-behind method.
Results: A significant causal relationship was found between high vitamin D levels and headache using the IVW method (OR = 0.848; p = 0.007; 95% CI = 0.752–0.956). However, in a reverse analysis, no evidence of a causal relationship between headache and high levels of vitamin D was found using the IVW method (OR = 1.001; p = 0.906; 95% CI = 0.994–1.006). Our MR analyses showed no significant horizontal multidimensionality or heterogeneity (p > 0.05). Sensitivity analyses confirmed that MR estimates were not affected by single nucleotide polymorphisms (SNPs). Confirmation that our results are robust and valid has been obtained by the leave-one-out method.
Conclusion: Our study suggests that high levels of vitamin D prevent the risk of headache. However, there is no evidence of a causal relationship between headache and high levels of vitamin D. Vitamin D may reduce the risk of headache.
Vitamin D is a fat-soluble vitamin that plays several important roles in the body (1). It is mainly synthesised through the skin in the presence of sunlight and can also be ingested through food. Vitamin D promotes the absorption and utilisation of calcium and contributes to normal bone development and maintenance. It plays an important role in immune regulation, cell differentiation and inflammatory responses (2–5). In recent years, studies have increasingly focused on the role of vitamin D in neuropathic pain. Vitamin D receptors are widely distributed in the central and peripheral nervous system, suggesting that vitamin D may play a role in normal nervous system function and nociceptive modulation (6). Several studies have shown an association between vitamin D deficiency and the onset and exacerbation of headache, and that vitamin D supplementation can help alleviate the symptoms of headache (7, 8).
Headache is a common neurological symptom that manifests as pain or discomfort in different areas of the head (9). Its etiology is complex and diverse, including intracranial lesions, vascular abnormalities, infection, inflammation, and metabolic disorders (10). Headache can be divided into two categories: primary and secondary; the former, such as migraine and tension headache, are mostly associated with genetic, endocrine, and environmental factors; the latter is caused by specific diseases, such as brain tumour and cerebrovascular disease. The severity, frequency of attacks and accompanying symptoms of headache vary from person to person and affect the daily life and work of patients (11).
Preliminary studies have investigated the correlation between vitamin D and headache. Some studies have shown that people with lower levels of vitamin D are at greater risk of headaches and have more severe pain (12). In addition, vitamin D supplementation appears to reduce headaches and improve patients’ quality of life (13, 14). Although some relevant studies have shown a correlation between vitamin D and headache, it is difficult to establish a specific causal relationship between the two.
Mendelian randomization (MR) analysis is an emerging statistical method that uses genetic variation as an instrumental variable to explore potential causal relationships between exposure factors and disease. Mendelian randomisation analysis provides a classification effect similar to that of a randomised controlled trial (RCT) by randomly classifying genetic alleles during sperm-egg binding (15). However, MRI analyses provide stronger causal inferences than traditional observational studies and are effective in controlling for the effects of confounding factors (16). Furthermore, there is a lack of magnetic resonance studies exploring the potential causal relationship between vitamin D and headache, suggesting that further research is needed in this area.
The aim of this study was to investigate the potential causal relationship between vitamin D and headache. By integrating existing genome-wide association study (GWAS) data, we aimed to assess the effect of vitamin D levels on the risk of headache onset. This will provide a scientific basis for the development of targeted prevention and treatment strategies.
For MR analysis requires a valid instrumental variable (IV) that satisfies three key assumptions to obtain reliable results. Firstly, the IV must be strongly associated with the exposure. Secondly, the IV must be independent of any confounding factors that may affect the exposure and outcome. Finally, the IV must affect the outcome solely through the exposure (17). The study consisted of several core steps, including the use of multiple MR methods (IVW, WM, MR Egger), multiplicity assessment, and heterogeneity and sensitivity analyses. These steps were taken to select genetic IVs associated with exposure and to examine the association between vitamin D levels and headache. To further investigate the causal relationship between vitamin D and headache, a bidirectional two-sample MR study was conducted (see Figure 1). To reduce bias resulting from population stratification and racial differences, we only selected samples from the same racial group. Furthermore, this study followed the latest guidelines for MR in epidemiological studies (STROBE-MR) (18).
The genetic association data for this study were obtained from the IEU Open GWAS database.1 The low level vitamin D dataset (ieu-b-4812) comprises 441,291 participants with a total of 16,668,957 SNPs. The dataset on headaches (finn-b-R18_HEADACHE1) was obtained from the Finngen Consortium and includes 13,345 cases and 172,999 controls.
To fulfill the initial step of the first hypothesis, we identified single nucleotide polymorphisms (SNPs) that were significantly associated with exposure based on stringent criteria (p < 5 × 10–8) and independence (r2 < 0.001, kb = 10,000) in order to select genetic IVs that are strongly associated with exposure. However, in a reverse MR analysis with headache as the exposure, no SNPs significantly associated with exposure at p < 5 × 10–8 were found. The significance level was set at p < 5 × 10–6 for the reverse MR analysis. Furthermore, all SNPs with palindromes and ambiguities were excluded to ensure consistent effect alleles between the exposure and outcome datasets. To evaluate the strength of the instrumental variable (IV), we calculated the F statistic value using the formula F = (N − 2) * R2/(1 − R2) (19). An F value greater than 10 indicates a low risk of weak IV bias and avoids weak instrumental bias (20).
This study used three methods, namely inverse variance weighted (IVW) and MR-Egger, weighted median (WM), to establish the causal relationship between vitamin D and headache. IVW is the dominant method and produces the highest statistical efficacy when all instrumental variables (IVs) are validated tools (20). Criteria for establishing causality include significant results in IVW analyses. The results of WM and MR-Egger analyses should align with those of IVW analyses (21–23).
For sensitivity analyses, we used the MR-Egger intercept to determine the presence of pleiotropy. Intercept values close to 0 and p-values greater than 0.05 indicate no horizontal pleiotropy (24). We then used Cochran’s Q-test to quantify the heterogeneity of the IVW estimates. A p-value greater than 0.05 indicates no heterogeneity (25). The results of the heterogeneity analysis are shown in Table 1. Additionally, we ran the MR-PRESSO test to check for outliers (see Supplementary material). If any outliers were identified, they were removed, and the MR effect was re-evaluated. To ensure the robustness of the results, we also conducted leave-one-out analyses to examine the impact of individual SNPs on the overall causal effect (26). Final funnel plots were used to assess the symmetry of the selected SNPs, while forest plots were used to evaluate the reliability and heterogeneity of chance estimates. Scatter plots were used to visualise the relationship between exposure and outcome. Please refer to the Supplementary material for additional details.
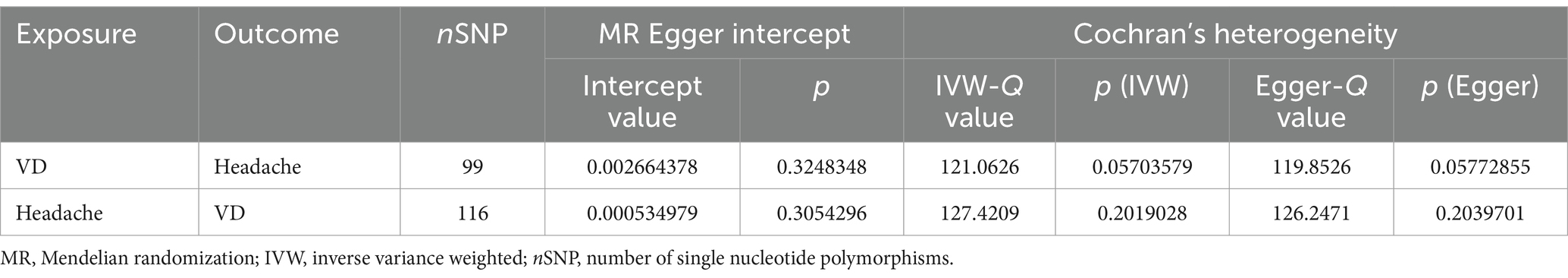
Table 1. The heterogeneity and sensitivity of omega-3/omega-6 fatty acids and cerebrovascular disease after removal unqualified IVs.
The methodology used in the inverse MR analysis was the same as described above, which involved using SNPs associated with headache to investigate the causal effect of headache and vitamin D. The results of the inverse MR analysis were presented in RStudio. The entire analysis was conducted in R Studio (version 4.3.0) using the “TwoSampleMR” and “MRPRESSO” software packages.
Finally, we included 101 and 121 SNPs in the exposure and outcome datasets, respectively. Two and five SNP deleted due to palindromes, respectively. As shown in Table 2, the results of IVW analysis indicated that high-level vitamin D were significantly associated with an decreased risk of headache (OR = 0.848; p = 0.007; 95% CI = 0.752–0.956). This finding was also supported by WM and MR Egger. The forest plots of SNP effect sizes for each phenotype in the forward analyses are presented in Figure 2. No horizontal pleiotropy was observed for any of the phenotypes (MR Egger intercept, p > 0.05). After removing the palindromic SNPs, there was no heterogeneity observed among the exposure-associated SNPs. The MR-Egger intercept test results indicated no outliers or evidence of horizontal pleiotropy. Additionally, leave-one-out analyses revealed that no single SNP had a potential effect on MR estimates. The funnel plots were essentially symmetrical in both the forward and reverse MR analyses, suggesting no directional horizontal pleiotropy in the selected variables. The Supplementary material display scatter plots, funnel plots, and leave-one-out methods for forward analyses, which can demonstrate the absence of outliers that may affect causality.
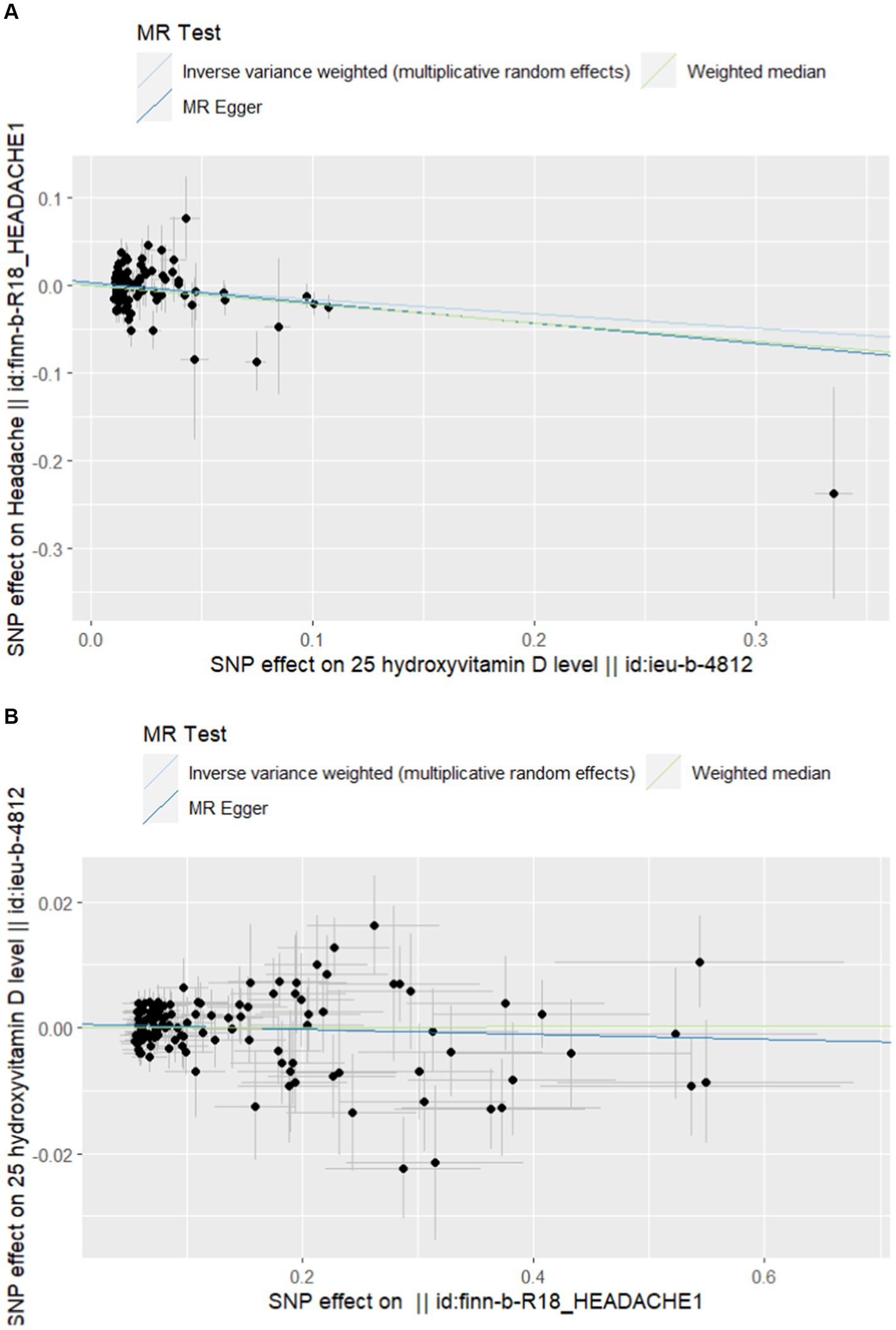
Figure 2. (A) Scatterplot of forward Mendelian randomization analysis. (B) Scatter plot of reverse Mendelian randomization analysis.
In the reverse MR analysis, none of the causal relationships between headache and vitamin D were significant when headache was considered as exposure (OR = 1.001; p = 0.906; 95% CI = 0.994–1.006). Table 2 and Figure 2 provided further details. The statistical analysis indicated that there was no significant causal relationship between headache and vitamin D. The Supplementary material include scatterplots, funnel plots, and leave-one-out methods for inverse analyses.
This is the first study to explore a bidirectional causal relationship between vitamin D and headache using MR analysis. The results of the MR analysis showed that high levels of vitamin D significantly decreased the risk of headache. Furthermore, reverse MR studies did not find any evidence of a causal relationship between genetically predicted headache and vitamin D levels.
The relationship between vitamin D and headaches remains inconclusive, despite numerous studies exploring the topic. A case-control study conducted in Egypt found a significant vitamin D deficiency in migraine patients, which can significantly impact the character, duration, frequency, and severity of headache attacks (27). Similarly, a study in Turkish children also found a possible link between vitamin D deficiency and headaches (28). A cross-sectional descriptive study conducted in Norway confirmed that patients with headaches had lower average vitamin D levels compared to patients with other types of pain symptoms. Specifically, patients with headaches had a higher incidence of vitamin D deficiency (29). Quintero-Fabián et al. (30) found that vitamin D acts as an immunomodulatory hormone to reduce neuroinflammation and prevent headaches as a neurological disorder. Recent literature suggests that migraine sufferers may have a vitamin D deficiency, and taking vitamin D alongside conventional medication may reduce the frequency of migraine attacks (31). However, further verification of these results by other methods is necessary.
It is important to note that not all research findings support a link between vitamin D and headache. A randomized controlled trial conducted in Norway found that the use of vitamin D supplements did not have a significant effect on the occurrence and extent of pain or headache (32). Furthermore, a recent meta-analysis did not find any relationship between cluster headache and the three single nucleotide polymorphisms of the vitamin D receptor gene (33). The researchers also noted the lack of articles exploring the relationship between vitamin D and headaches. The relationship between vitamin D and headaches remains a controversial issue for objective reasons.
The varying results could be attributed to the fact that the majority of the studies were observational or meta-analyses and lacked the support of prospective randomized studies. Observational studies have inherent limitations, such as methodological flaws, selection bias, and insufficient adjustment for confounders, which make it difficult to establish a clear causal link. Mendelian randomization is a research methodology that can reveal the causal relationship between exposure and outcome by using genetic variation as an instrumental variable. This approach avoids the influence of non-heritable environmental factors. The study found a significant causal relationship between vitamin D and headache using two-sample MR analysis. A reverse MR study further confirmed this finding’s robustness. Our study did not find significant levels of pleiotropy or heterogeneity, which increases the credibility and reliability of our findings.
However, it is worth noting that MR studies have limitations. Firstly, existing databases lack data on different levels of vitamin D, making it difficult to explore the specific association between vitamin D levels and headache. Secondly, as our GWAS data is primarily derived from European populations, our findings may exhibit some racial or geographic bias and require further validation in other ethnic groups. It is expected that more high-quality studies and data will be published in the future to provide additional insight into the relationship between vitamin D and headache. Further research and technological advances may reveal the exact link between the two, leading to new ideas and approaches for preventing, testing, and treating headache.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
HiX: Funding acquisition, Investigation, Writing – original draft, Writing – review & editing, Methodology. RJ: Software, Writing – review & editing. LX: Investigation, Software, Writing – original draft, Writing – review & editing. JZ: Software, Writing – original draft, Writing – review & editing. XT: Investigation, Writing – original draft. JL: Methodology, Writing – original draft. XG: Data curation, Writing – original draft. SZ: Methodology, Writing – original draft. HoX: Formal analysis, Writing – original draft. JH: Writing – original draft. LL: Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing, Investigation.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Chongqing Science and Health Joint Medical Research Project (2021MSXM261).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1423569/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | (A) Forward Mendelian randomized leave-one graph. (B) Reverse Mendelian randomized leave-one graph.
SUPPLEMENTARY FIGURE S2 | (A) Funnel plot for forward Mendelian randomization. (B) Funnel plot for reverse Mendelian randomization.
SUPPLEMENTARY FIGURE S3 | (A) Forward Mendelian randomization of forest graphs. (B) Reverse Mendelian randomization of forest graphs.
1. Delrue, C, and Speeckaert, MM. Vitamin D and vitamin D-binding protein in health and disease. Int J Mol Sci. (2023) 24:4642. doi: 10.3390/ijms24054642
2. Bouillon, R, Marcocci, C, Carmeliet, G, Bikle, D, White, JH, Dawson-Hughes, B, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. (2019) 40:1109–51. doi: 10.1210/er.2018-00126
3. Ismailova, A, and White, JH. Vitamin D, infections and immunity. Rev Endocr Metab Disord. (2022) 23:265–77. doi: 10.1007/s11154-021-09679-5
4. Mailhot, G, and White, JH. Vitamin D and immunity in infants and children. Nutrients. (2020) 12:1233. doi: 10.3390/nu12051233
5. Carlberg, C, and Muñoz, A. An update on vitamin D signaling and cancer. Semin Cancer Biol. (2022) 79:217–30. doi: 10.1016/j.semcancer.2020.05.018
6. Habib, AM, Nagi, K, Thillaiappan, NB, Sukumaran, V, and Akhtar, S. Vitamin D and its potential interplay with pain signaling pathways. Front Immunol. (2020) 11:820. doi: 10.3389/fimmu.2020.00820
7. Nowaczewska, M, Wiciński, M, Osiński, S, and Kaźmierczak, H. The role of vitamin D in primary headache-from potential mechanism to treatment. Nutrients. (2020) 12:243. doi: 10.3390/nu12010243
8. Dell’Isola, GB, Tulli, E, Sica, R, Vinti, V, Mencaroni, E, Di Cara, G, et al. The vitamin D role in preventing primary headache in adult and pediatric population. J Clin Med. (2021) 10:5983. doi: 10.3390/jcm10245983
9. Souza, WPO, Sousa-Santos, PM, and Silva-Néto, RP. A historical review of headaches: who first described them and when did this occur? Headache. (2020) 60:1535–41. doi: 10.1111/head.13906
10. Rizzoli, P, and Mullally, WJ. Headache. Am J Med. (2018) 131:17–24. doi: 10.1016/j.amjmed.2017.09.005
12. Prakash, S, Mehta, NC, Dabhi, AS, Lakhani, O, Khilari, M, and Shah, ND. The prevalence of headache may be related with the latitude: a possible role of vitamin D insufficiency? J Headache Pain. (2010) 11:301–7. doi: 10.1007/s10194-010-0223-2
13. Al-Nimer, MS . Vitamin D: is it a primary hormone targeting the migraine headache or just as adjunct therapy? Neurosciences. (2017) 22:69. doi: 10.17712/nsj.2017.1.20160561
14. Yang, Y, Zhang, HL, and Wu, J. Is headache related with vitamin D insufficiency? J Headache Pain. (2010) 11:369–71. doi: 10.1007/s10194-010-0235-y
15. Bai, R, Ren, L, Guo, J, Xian, N, Luo, R, Chang, Y, et al. The causal relationship between pure hypercholesterolemia and psoriasis: a bidirectional, two-sample Mendelian randomization study. Skin Res Technol. (2023) 29:e13533. doi: 10.1111/srt.13533
16. Du, L, Wang, B, Wen, J, and Zhang, N. Examining the causal association between psoriasis and bladder cancer: a two-sample Mendelian randomization analysis. Skin Res Technol. (2024) 30:e13663. doi: 10.1111/srt.13663
17. Skrivankova, VW, Richmond, RC, Woolf, BAR, Davies, NM, Swanson, SA, TJ, VW, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. (2021):n2233:375. doi: 10.1136/bmj.n2233
18. Pierce, BL, Ahsan, H, and Vanderweele, TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
19. Zhang, Y, Xiong, Y, Shen, S, Yang, J, Wang, W, Wu, T, et al. Causal association between tea consumption and kidney function: a Mendelian randomization study. Front Nutr. (2022) 9:801591. doi: 10.3389/fnut.2022.801591
20. Burgess, S, Davey, SG, Davies, NM, Dudbridge, F, Gill, D, Glymour, MM, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. (2019) 4:186. doi: 10.12688/wellcomeopenres.15555.1
21. Chen, X, Hong, X, Gao, W, Luo, S, Cai, J, Liu, G, et al. Causal relationship between physical activity, leisure sedentary behaviors and COVID-19 risk: a Mendelian randomization study. J Transl Med. (2022) 20:216. doi: 10.1186/s12967-022-03407-6
22. Venkatesh, SS, Ferreira, T, Benonisdottir, S, Rahmioglu, N, Becker, CM, Granne, I, et al. Obesity and risk of female reproductive conditions: a Mendelian randomisation study. PLoS Med. (2022) 19:e1003679. doi: 10.1371/journal.pmed.1003679
23. Cheng, F, Luk, AO, Shi, M, Huang, C, Jiang, G, Yang, A, et al. Shortened leukocyte telomere length is associated with glycemic progression in type 2 diabetes: a prospective and Mendelian randomization analysis. Diabetes Care. (2022) 45:701–9. doi: 10.2337/dc21-1609
24. Bowden, J, Del, GMF, Minelli, C, Zhao, Q, Lawlor, DA, Sheehan, NA, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. (2019) 48:728–42. doi: 10.1093/ije/dyy258
25. Zhou, H, Zhang, Y, Liu, J, Yang, Y, Fang, W, Hong, S, et al. Education and lung cancer: a Mendelian randomization study. Int J Epidemiol. (2019) 48:743–50. doi: 10.1093/ije/dyz121
26. Liu, H, Song, X, Li, C, and Zhang, H. The causal relationship between psoriasis and artificial joint re-operation after arthroplasty: a two-sample Mendelian randomization study. Skin Res Technol. (2024) 30:e13560. doi: 10.1111/srt.13560
27. Hussein, M, Fathy, W, and Abd Elkareem, RM. The potential role of serum vitamin D level in migraine headache: a case-control study. J Pain Res. (2019) 12:2529–36. doi: 10.2147/JPR.S216314
28. Donmez, A, Orun, E, and Sonmez, FM. Vitamin D status in children with headache: a case-control study. Clin Nutr ESPEN. (2018) 23:222–7. doi: 10.1016/j.clnesp.2017.09.010
29. Knutsen, KV, Brekke, M, Gjelstad, S, and Lagerløv, P. Vitamin D status in patients with musculoskeletal pain, fatigue and headache: a cross-sectional descriptive study in a multi-ethnic general practice in Norway. Scand J Prim Health Care. (2010) 28:166–71. doi: 10.3109/02813432.2010.505407
30. Quintero-Fabián, S, Bandala, C, Pichardo-Macías, LA, Contreras-García, IJ, Gómez-Manzo, S, Hernández-Ochoa, B, et al. Vitamin D and its possible relationship to neuroprotection in COVID-19: evidence in the literature. Curr Top Med Chem. (2022) 22:1346–68. doi: 10.2174/1568026622666220401140737
31. Ghorbani, Z, Togha, M, Rafiee, P, Ahmadi, ZS, Rasekh Magham, R, Haghighi, S, et al. Vitamin D in migraine headache: a comprehensive review on literature. Neurol Sci. (2019) 40:2459–77. doi: 10.1007/s10072-019-04021-z
32. Knutsen, KV, Madar, AA, Brekke, M, Meyer, HE, Natvig, B, Mdala, I, et al. Effect of vitamin D on musculoskeletal pain and headache: a randomized, double-blind, placebo-controlled trial among adult ethnic minorities in Norway. Pain. (2014) 155:2591–8. doi: 10.1016/j.pain.2014.09.024
Keywords: vitamin D, headache, Mendelian randomisation study, single nucleic acid polymorphism, prevention
Citation: Xiong H, Jiang R, Xing L, Zheng J, Tian X, Leng J, Guo X, Zeng S, Xiong H, Huo J and Li L (2024) New evidence that vitamin D prevents headache: a bidirectional two-sample Mendelian randomization analysis. Front. Neurol. 15:1423569. doi: 10.3389/fneur.2024.1423569
Received: 26 April 2024; Accepted: 11 July 2024;
Published: 26 July 2024.
Edited by:
Wensheng Qu, Huazhong University of Science and Technology, ChinaReviewed by:
Ying Tan, Guizhou Provincial People’s Hospital, ChinaCopyright © 2024 Xiong, Jiang, Xing, Zheng, Tian, Leng, Guo, Zeng, Xiong, Huo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Letai Li, 2021220596@stu.cqmu.edu.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.