- Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, Kowloon, Hong Kong SAR, China
Background: Effective post-stroke mobility, recovery, performance, and participation are key goals for stroke survivors. However, these outcomes may be hindered by post-stroke fatigue (PSF), which can affect numerous aspects of post-stroke mobility, recovery, performance, functioning, community participation, and return to work. This review aimed to assess the scientific evidence on the relationship between PSF and mobility function, functional recovery, functional performance, and participation-related outcomes among stroke survivors.
Method: A comprehensive search of Cochrane Central, PubMed, Embase, and Web of Science (WoS) databases was conducted from inception to December 2023. Observational, cross-sectional, and longitudinal studies were included. The methodological quality of the included studies was assessed using the National Institute of Health’s quality assessment tool, while the risk of bias was assessed using the Quality in Prognostic Studies tool. A total of 28 studies (n = 2,495 participants, 1,626 men, mean age ranging from 52.5 ± 9.5 to 71.1 ± 9.9 years) were included. The data analysis was conducted using narrative and quantitative synthesis. Fixed and random effects meta-analyses were conducted to explore the relationships between PSF and relevant outcomes.
Results: Chronic PSF was found to have significant negative correlations with mobility (meta r = −0.106, p < 0.001), balance performance (meta r = −0.172; 95%; p = 0.004), and quality of life (meta r = −0.647; p < 0.001). It also showed significant positive correlations with stroke impairment (meta r = 0.144, p < 0.001) and disability (meta r = 0.480, p < 0.001). Additionally, exertion/acute PSF had significantly negative correlations with walking economy (meta r = −0.627, p < 0.001) and walking endurance (meta r = −0.421, p = 0.022). The certainty of evidence was deemed moderate for these relationships.
Conclusion: Our findings indicate that higher levels of PSF are associated with poorer mobility, balance, and participation, as well as greater disability and stroke impairment. Future studies, especially prospective longitudinal and randomized controlled trials, are warranted to substantiate our findings.
Systematic review registration: PROSPERO, identifier: CRD42023492045.
Introduction
Stroke survivors experience a range of impairments and functional limitations that manifest in various combinations (1). In addition to this, they may experience various post-stroke symptoms, such as fatigue, pain, and spasticity, which often occur concurrently and can significantly influence mobility, motor function, physical function, and activities of daily living (ADLs), adding to the overall burden and hindering recovery (2). Fatigue in stroke survivors can be classified as either chronic or acute (exertion related), with two distinct characteristics (3). Exertion/state fatigue is characterized by its immediate onset and recovery time (3), while chronic fatigue is caused by long periods of accumulation of acute fatigue (4) or the gradual progression of mental fatigue, potentially triggered by daily tasks (4, 5). Post-stroke exertion fatigue is typically experienced after intense physical or mental exertion (5). Additionally, fatigue can be categorized as mental or peripheral (physical), with underlying mechanisms often associated with autonomic diseases (4).
PSF is associated with feelings of mental, physical, and overall exhaustion, with a variation in fatigue levels and activity (6). Its characteristics vary from general fatigue to a certain degree, and it can occur without any specific exertion (7). PSF is a common post-stroke deficit typified by complex multifactorial phenomena (8) and is a frequent, incapacitating health issue due to the complex interactions of numerous factors (9). Fatigue and sleepiness commonly exist together due to lack of sleep and are usually combined under the concept of tiredness by patients. However, they are two separate but interrelated terms (10). The two terms fatigue and sleepy are different, with the suggestion that clinicians and researchers should be cautious when using these terms interchangeably (11). Fatigue is an overwhelming feeling of tiredness, lacking energy, and a sense of exhaustion related to diminished physical and/or cognitive performance, whereas sleepiness is a pervasive phenomenon felt not just as a symptom in various disorders but as a normal state of physiology in most persons during any given 24-h duration (10). Additionally, disorder is inferred both when sleepiness becomes pervasively present or when it is absent, and abnormality is considered when it does not happen when needed or happens at unsuitable periods (10).
Post-stroke fatigue (PSF) is prevalent in stroke survivors (12–15) and affects their daily functioning, and quality of life is a commonly disregarded problem (3). It affects participation, emotions, cognitive performance, and ADLs and can diminish the ability to carry out the expected ADLs (6). PSF, which can have numerous adverse consequences (16), is significantly associated with functional impairments, disability, and diminished quality of life among stroke survivors in several areas (1, 14). It has also been shown to have a negative influence on the survivors’ cardiopulmonary function (13), and fatigue hinders their community integration (12). Thus, fatigue is a major symptom that affects numerous physical functions, such as ADLs and mobility, in stroke survivors.
The effects of fatigue can be lifelong (17, 18) and may impact post-stroke functional recovery and outcomes (2, 18). Moreover, PSF is associated with dual-task performance (19), both cognitive and motor performances (8), lower extremity mobility (20), and lower limb motor tasks, including balance and gait, with more challenges to navigating in complex settings likely to be observed in those with higher fatigue (8). Other studies have found that fatigue is weakly related to post-stroke gait performance (19), inextricably related to affective disorders (21), and also related to poor functional outcomes in young stroke survivors (18).
The summary above shows that most of the studies reporting the relationships or associations between PSF and variables of interest have not expounded the strength, direction, or extent of the relationships. This precludes the drawing of definite conclusions regarding the relationships. Therefore, there is a need to synthesize the scientific evidence of how and to what extent PSF influences the mobility, recovery, performance, and participation of stroke survivors. Such evidence will guide stroke rehabilitation professionals in formulating better rehabilitation strategies by targeting PSF, mobility, recovery, functioning, and participation. Despite the known importance of these outcomes in stroke survivors, to the best of our knowledge, this will be the first systematic review and meta-analysis that determine the scientific evidence on how and to what extent PSF influences these outcomes in stroke survivors.
Methods
This review underwent PROSPERO registration (registration number: CRD42023492045), and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was applied for conducting the review.
Search strategy
The EMBASE, PubMed, PEDro, Cochrane Central Library, and Web of Science online databases have been searched from inception till December 2023. The PICOS approach was applied to form the search terms for searching the databases. All of the databases have been searched in compliance with their unique requirements, and there was a truncation of search terms and utilization of medical subject headings (MeSH) terms where necessary and appropriate. The search process adopted in the databases is in Supplementary material S1. In addition, the reference lists of the retrieved studies and reviews were searched manually. Relevant articles obtained from the general literature search were also involved in the review. The results of the searches were exported to Endnote, duplicate studies were discarded, and the remaining articles were subjected to title and abstract screening, followed by a full-text screening process. The search was conducted independently by a researcher (JSU), and the results were confirmed by two other researchers (TWLW and SSMN).
Eligibility selection criteria
Studies that met the following inclusion criteria were included in the review: studies that (i) included adult stroke survivors; (ii) were published in English; (iii) reported the relationship between PSF and any of the outcomes of interest, namely related to mobility, functional recovery, functional performance and participation, including but not limited to gait, balance, falls, ADLs, quality of life (QOL), international classification of functioning (ICF) domains, self-confidence, motor function and muscular strength; (iv) were cross-sectional (CRS), longitudinal, cohort, observational, prospective or correlational with full texts available. Conference abstracts, review articles, dissertations/theses, and commentaries have been excluded.
Study selection and data extraction
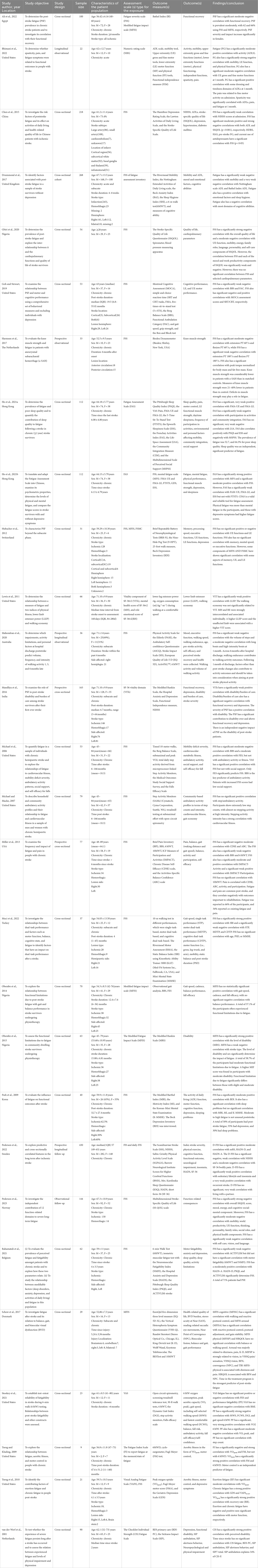
Initially, upon removal of studies that were duplicated, the titles and abstracts of the remaining studies were screened independently by two researchers (JSU and TWLW) against the eligibility criteria. Any differences between the two researchers concerning the involvement of a study were resolved by contacting a third researcher (SSMN) to resolve the differences. During the full-text screening, the full texts of the appropriate studies were accessed, and the data as follows were taken out: study author(s), year of publication and location of study, study objective, study design, sample size, characteristics of the patient population, outcome measures, outcomes assessed, and findings/conclusions. All the extracted data were documented in a Microsoft Excel file. The extracted data and the characteristics of the included studies are presented in Table 1.
Assessment of the methodological quality
The National Institute of Health (NIH) 14-item Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (22) was used to evaluate the internal validity and risks of bias in measurement, information, selection, or confounding in the included studies. This tool also covers aspects such as study design, population, sample size, attrition, blinding, data collection, outcomes, and outcome measures. Each item is rated as ‘Yes’ or ‘No,’ with room for selecting other options such as ‘cannot be determined’ (CD), ‘not reported’ (NR), or ‘not applicable’ (NA). An overall quality appraisal rating of good, fair, or poor is made for each evaluated article (Table 2). The quality assessments were independently performed by two researchers (JSU and TWLW). Any disagreements between the two researchers were resolved by consulting a third researcher (SSMN).
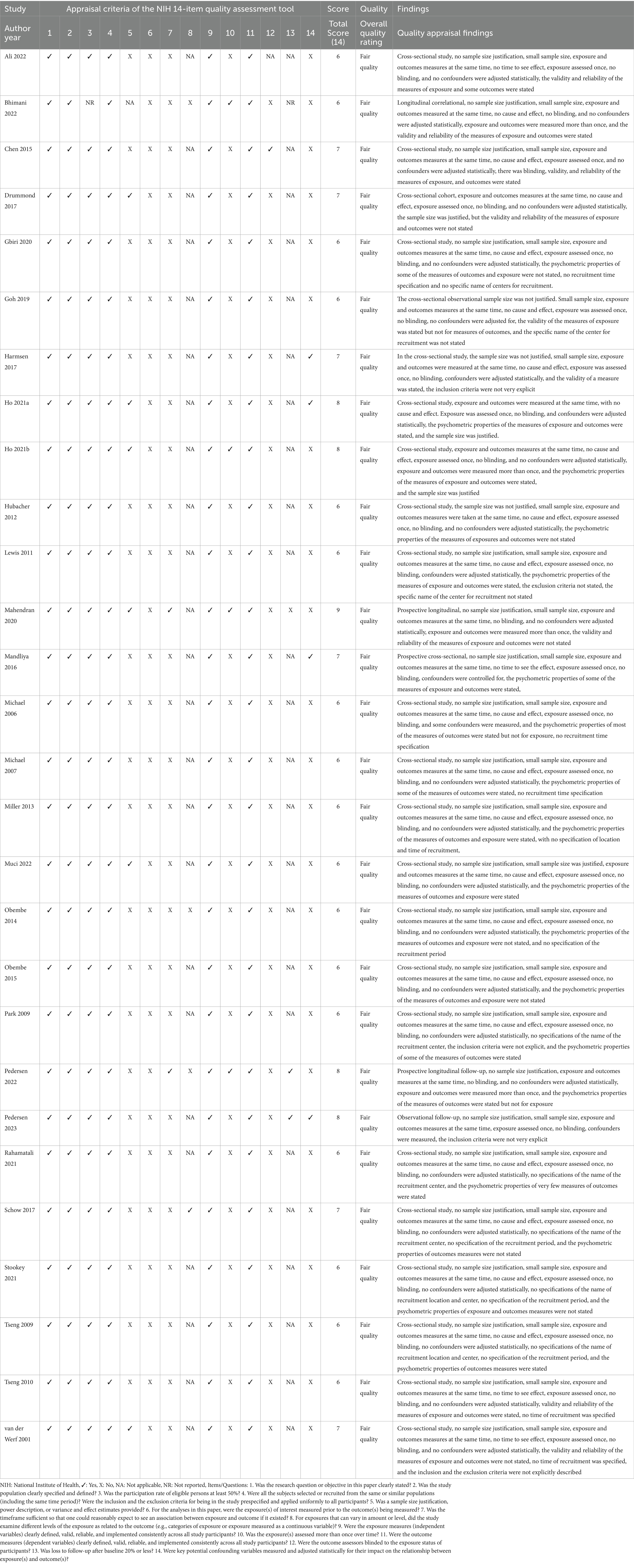
Table 2. Methodological quality of the included studies according to the NIH quality assessment tool for observational cohort and cross-sectional studies.
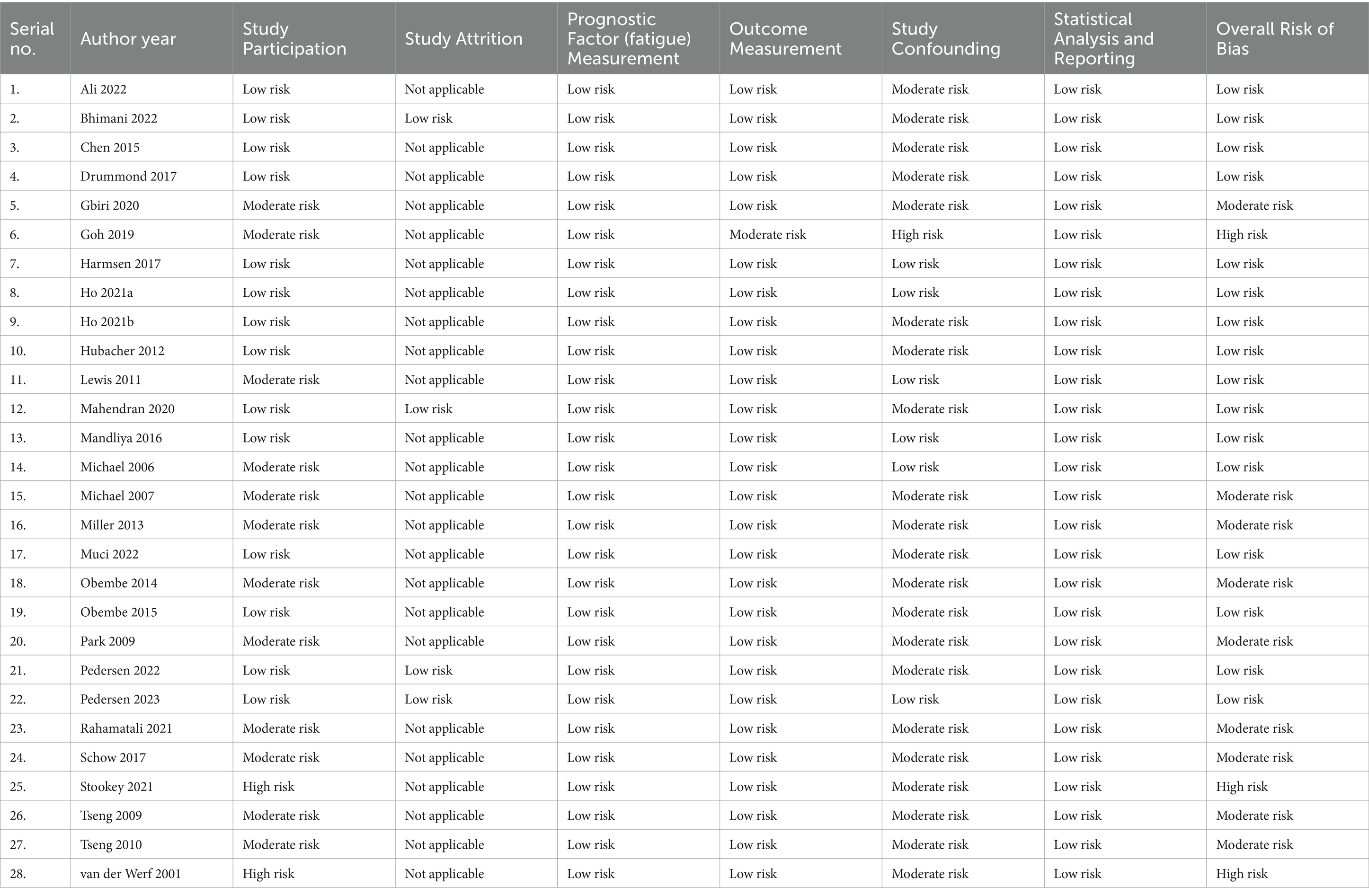
Assessment of risk of bias (ROB)
The ROB of the included studies was evaluated using the Quality in Prognosis Studies (QUIPS) checklist (23). The QUPS checklist assesses ROB across six domains, namely study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting (23). Each of the domains for each study, as well as the overall study, is judged as low, moderate, or high in ROB (24). The results of these evaluations are shown in Table 3. The QUIPS checklist was adapted and used to assess ROB in the context of this review, and the details of the scoring and evaluation method used in the adapted QUIPS checklist are contained in Supplementary material S2. During the evaluation, the domain of study attrition was not scored for most of the studies because it was not applicable to them, being cross-sectional in nature. Two researchers (JSU and TWLW) independently performed the assessments. Any disagreements regarding ROB ratings were resolved by consulting a third researcher (SSMN).
Data analysis and synthesis
Data were analyzed using both qualitative (narrative) and quantitative syntheses. In the qualitative (narrative) synthesis, the characteristics, risk of bias, and methodological quality of the involved studies were summarized. The quantitative synthesis of the relationships between PSF and each of the outcomes of interest (mobility function, functional recovery, functional performance, and participation) involved a comprehensive meta-analysis of the correlation coefficient (r) values and sample sizes (N) of the included studies in the form of meta-correlation.
Synthesis of the evidence profile
The Cochrane GRADE technique was applied to synthesize the evidence and rate the certainty of the evidence to arrive at a definite conclusion.
Results
Identification and selection of eligible studies
The initial search of the online databases and other sources yielded a total of 2,082 relevant articles. Of these, 428 duplicates were removed, and the remaining 1,654 articles were subjected to title and abstract screening. Of these, 1,591 articles were excluded for failing to meet the eligibility requirements. The 63 remaining articles were then subjected to full-text screening against the inclusion and the exclusion criteria. Finally, 28 articles that met the eligibility criteria were included in the review. All of the studies were either cross-sectional (23 studies), longitudinal (3 studies), or observational (2 studies). Figure 1 presents the study identification and selection process in line with PRISMA guidelines.
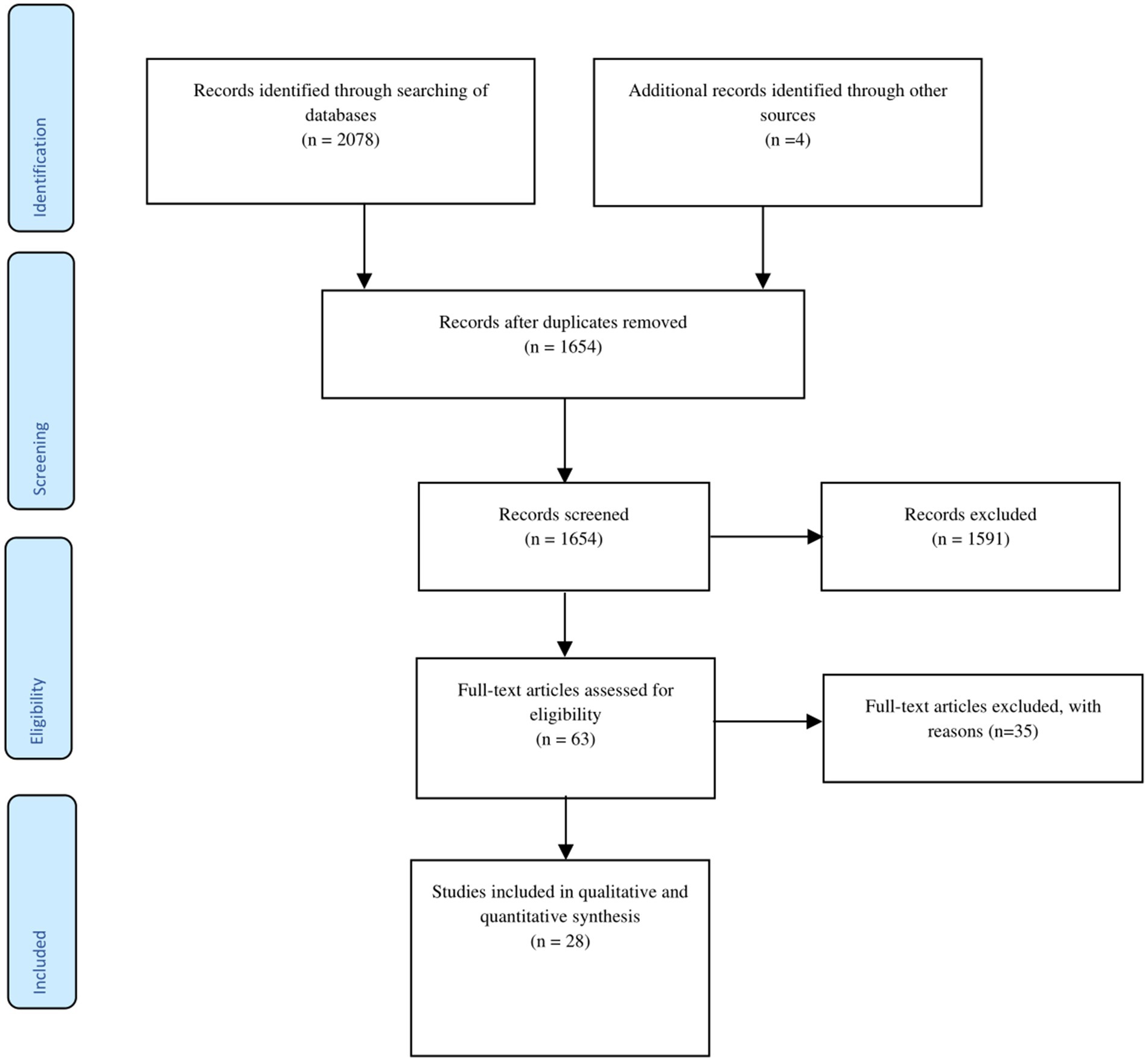
Figure 1. PRISMA flowchart of study selection. This figure illustrates the process of identification and selection of studies in the review.
Description of the included studies
Twenty-eight studies are included in this review, and their detailed characteristics are presented in Table 1. Nine of these studies were conducted in the United States, eight in Europe (two in the Netherlands and one each in the United Kingdom, Switzerland, Norway, Sweden, Belgium, and Denmark), six in Asia (two in Hong Kong and one each in China, Turkey, India, and Korea), four in Africa (three in Nigeria and one in Egypt), and one in Australasia (one in Australia). A total of 2,495 stroke survivors participated in the included studies, of whom 1,626 and 869 participants were male and female, respectively. The age range of the participants was 26–89 years, with their mean age ± standard deviation across the studies ranging from 52.5 ± 9.5 to 71.1 ± 9.9 years.
The time since stroke among the participants ranged from 1 to 166 months, with the mean time since stroke/post-stroke duration across the studies ranging from 5 to 73.56 months. Twenty-three studies recruited chronic stroke survivors, five recruited sub-acute stroke survivors, and three recruited acute stroke survivors. Eleven studies reported stroke types and subtypes, 11 studies reported the affected hemisphere/side, and five studies reported the lesion/infarct location. Regarding the level of fatigue evaluated, 27 studies evaluated chronic fatigue, while three studies evaluated exertion/state fatigue. Sixteen studies used the Fatigue Severity Scale (FSS); six used the Fatigue Impact Scale (FIS) and modified FIS; two studies each used the Fatigue Assessment Scale (FAS), vitality component of the SF36, and the Visual Analogue Scale. One study each used the Numeric Rating Scale (NRS), the Fatigue Scale for Motor and Cognitive Functions (FSMC), the Fatigue Index Scale (FI), and the Checklist Individual Strength (CIS) Fatigue Scale. The outcomes evaluated in the studies included but were not limited to the following domains: lower extremity muscle strength, gait/walking, gait speed, walking endurance, walking/ambulatory activity, neuromuscular fatigability, walking economy, mobility, balance, motor performance, ADLs, physical functioning, activity, stroke severity/impairment, disability, QOL, participation, and self-efficacy/confidence.
Reported measures of fatigue
Chronic and exertion/state fatigue were assessed in 24 and 4 studies, respectively. For the evaluation of chronic fatigue, the FSS was used in 16 studies, the FIS/modified FIS was used in six studies, the FAS was used in two studies, the vitality component of the SF36 (VITs) was used in two studies, and the FSMC, NRS, and CIS were each used in one study. For the evaluation of exertion/state fatigue, the Visual Analogue Scale was used in two studies, and the Fatigue Index (FI) Scale was used in one study.
Reported outcomes
The included studies reported several outcomes. Six studies reported findings on mobility (1, 2, 12, 13, 25), six studies on gait speed (8, 19, 26–29), five studies on walking economy/aerobic capacity (3, 30–32), four studies on walking endurance (14, 26, 27, 32), three studies on walking/ambulatory activity (26, 33, 34), one study on cadence (29), and seven studies on ADLs (2, 12, 14, 15, 25, 35, 36). Furthermore, four studies reported findings on general motor performance (3, 19, 32, 35), five studies on lower extremity motor performance (2, 8, 12, 16, 19), six studies on upper extremity motor performance (2, 8, 12, 16, 17, 19), and one study on physical function (2). In addition, four studies reported findings on balance performance (8, 27, 29, 31), three studies on lower extremity functional muscle strength (8, 12, 16), two studies on knee extensor strength/neuromuscular fatigability (14, 37), and four studies on self-confidence/efficacy (27, 29, 31). Moreover, four studies reported findings on disability (38–41), two studies on activity and participation (12, 27), and three studies on QOL (1, 13, 36). Finally, two studies reported findings on stroke impairment/severity (36, 41).
Reported outcome measures
Outcome measures such as the Bathel Index, the Modified Bathel Index, the 15-item ADL tool, the Lawton ADL scale, the Frenchay Activities Index, and the ACTIVLIM-Stroke Questionnaire were used across studies to evaluate ADLS. The Rivermead Mobility Index, Rivermead Motor Assessment (gross), Life Space Assessment, and 11-item mobility tool were employed among the studies to evaluate mobility function-related outcomes. In the studies, gait speed, walking endurance, and walking economy/aerobic fitness were assessed with a 10-m walk test, a 6-min walk test, and a graded exercise test, respectively. Five-Times-Sit-to-Stand test and dynamometers were used to evaluate lower limb functional muscle strength and knee extensor strength, respectively, in the studies. Across the studies, the Fugl-Meyer Assessment of Motor Recovery, Rivermead Motor Assessment, the Motricity Index, Lower Extremity Motor Function Tool, motor items of the Stroke Impairment Assessment Set, and Nine-Hole Peg test were employed to assess the motor function-related outcomes. Balance performance in the studies was evaluated with the Berg Balance Scale, Kinesthetic Ability Trainer, and Balance Evaluation Systems Test, while the Falls Efficacy Scale, Activity Specific Balance Confidence Scale, and Chronic Disease Self-Efficacy Scale were used to assess the self-efficacy/confidence in the studies. Stroke severity was examined using the National Institute of Health Stroke Scale. The Stroke Specific Quality of Life Questionnaire was used to evaluate the quality of life of stroke survivors in the studies. Among the studies, disability-related outcomes were evaluated with outcome measures such as the Modified Ranking Scale, Functional Independence Measure, and the Sickness Impact Scale. Participation-related outcomes were assessed using the Community Integration Measure and ICF Measure of Participation and Activities in the studies.
Study quality assessment
Using the NIH 14-item quality assessment tool, all of the included studies were rated to have fair quality. The majority of the studies were deficient in elements related to items 5 (sample size justification), 6 (measuring of exposure prior to the outcome), 7 (sufficient time to see the effect of exposure on outcome), 10 (assessment of exposure more than once), 12 (blinding of outcome assessors), and 14 (confounders measurement and adjustment). Elements related to item 13 (follow-up assessment) were not applicable to most of the studies due to them being cross-sectional; similarly, elements in item 8 (exposure evaluation in relation to outcome) were not applicable to many of the studies because of the evaluation tools for the exposure used in the studies. All of the studies reported and scored items 1 (research question or objective), 2 (study population), 3 (participation rate), 4 (selection of subjects), 9 (exposure measures), and 11 (outcome measures), except one study that did not report item 3. Only four studies (12, 16, 19, 25) justified the sample sizes used, and most of the studies (19) had small sample sizes.
All except two studies evaluated the exposure and outcomes once and simultaneously, without having cause and effect. The two studies (33, 41) that evaluated more than once were longitudinal. Only one study (36) blinded the assessors, and only four studies (1, 8, 12, 37) adjusted for confounders. Almost all of the studies clearly defined the exposure and outcome measures for various outcomes. A total of 10 studies (13, 17, 25, 26, 28, 29, 33, 38, 39, 41) did not report the psychometric properties of the exposure or outcome measures. Nine studies (3, 13, 26–29, 32, 34, 39) did not specify the recruitment period of the participants, and eight studies (8, 13, 14, 26–28, 30, 32) did not specify the recruitment Centre. The inclusion criteria were not explicitly stated in two studies (37, 39), and the exclusion criteria were also not explicitly stated in two studies (30, 39). The study quality assessment and quality appraisal findings are presented in Table 2.
ROB
With regard to ROB, of the 28 included studies, 16 studies were judged to have a low ROB (1, 2, 12, 15–17, 19, 25, 30, 31, 33, 36–38, 40, 41), nine had a moderate ROB (3, 13, 14, 27–29, 32, 34, 35), and three studies had a high ROB (8, 26, 39). In the domains of prognostic factor measurement, outcome measurement, and statistical analysis and reporting, all of the included studies had a low ROB, except one study that had a moderate ROB (8). In the domain of study confounding, all of the studies had a moderate ROB, except six studies that had a low ROB (1, 16, 30, 31, 37, 40) and one study that had a high ROB (8). The domain of study attrition was not applicable to most of the studies except three studies that had a low ROB in this domain (1, 33, 41) due to their longitudinal design. In the domain of study participation, most of the studies had a low or moderate ROB, except two studies that had a high ROB (26, 39). Details of the ROB assessment results are provided in Table 3.
Analysis of the results
The main findings of the studies were categorized into four broad outcomes domains: mobility function-related outcomes (mobility, gait, walking economy/aerobic fitness, walking endurance, and walking/ambulatory activity), functional recovery-related outcomes (ADLs, motor performance, physical function, activity, stroke severity/impairments, and lower extremity functional muscle strength), functional performance-related outcomes (balance and self-confidence/efficacy), and participation-related outcomes (disability, participation, and QOL). We evaluated the relationship of PSF with each individual outcome within these four broad domains.
PSF and mobility function-related outcomes
Relationship between PSF and mobility function
PSF and mobility (narrative synthesis): Six studies reported a relationship between mobility and PSF, which was negative in all of these studies. This negative relationship was significantly strong in one study (2), significantly moderate in two studies (1, 13), significantly weak in two studies (19, 25), and non-significantly very weak in one study (12). PSF and mobility (quantitative synthesis): A statistically significant weak negative relationship (Figure 2A) was found between PSF and mobility in both fixed-effects (FE) (meta r = −0.211; p = <0.001) and random-effects (RE) meta-analyses (meta r = −0.266; p = 0.005), with statistically significant heterogeneity observed between the studies (Q = 7.8781; p = 0.0486; I2 = 61.92%; 95% CI for I2 = 0.00 to 87.22). Evidence profile synthesis: Using the GRADE approach, as the included studies had a low ROB and fair methodological qualities, with a certain level of heterogeneity, the certainty of evidence was considered moderate for a weak negative correlation between PSF and mobility.
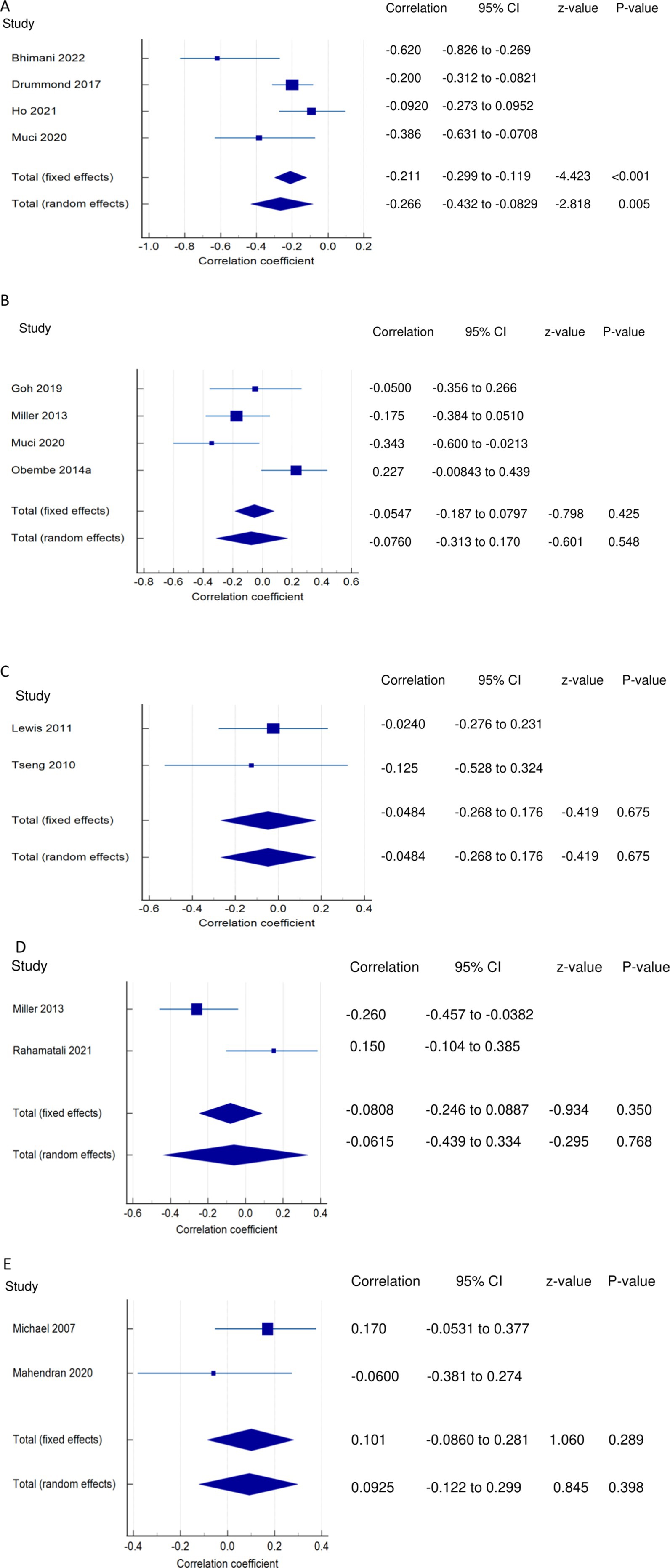
Figure 2. Relationship of PSF with mobility function related outcomes. This figure illustrates the relationship of PSF with (A) mobility; (B) gait speed; (C) walking economy; (D) walking endurance; (E) walking activity. The boxes represent point estimate and the size for each individual study, the horizontal lines represent the 95% CI and its boundaries for each individual study result, whereas the diamond shapes represent the pooled result estimate (summary of the meta-analysis) showing the overall relationship estimate for both fixed and random effects.
Relationship between PSF and gait/walking function
PSF and gait (narrative synthesis): Five studies showed a negative relationship between PSF and walking speed, which was significantly strong (26), weak (19), and non-significantly weak (29) each in one study and very weak in two studies (8, 27). In another study, gait speed was significantly related to cognitive fatigue but not to physical and overall fatigue (28). PSF and gait speed (quantitative synthesis):
Both FE and RE meta-analysis results revealed a statistically non-significant and very weak negative relationship (Figure 2B) between PSF and gait speed (p > 0.05), with statistically significant heterogeneity observed among the studies (Q = 9.6913; p = 0.0214; I2 = 69.04%). Evidence profile synthesis: Using the GRADE approach, as half of the included studies had a low ROB and fair methodological qualities, with certain imprecision and substantial heterogeneity, the certainty of the evidence was considered low for the lack of a significant correlation between PSF and gait speed. Additionally, the dynamic gait index had a significantly strong negative relationship with performance fatigability (26).
PSF and walking economy/aerobic fitness (narrative synthesis): The studies revealed a negative relationship between PSF and walking economy/aerobic fitness. The relationship was not significant for chronic fatigue (3, 30, 31) but was significant and strong (32) and significant and moderate for exertion/state fatigue (3). PSF and walking economy/aerobic fitness (quantitative synthesis): Both FE and RE meta-analysis results showed a statistically non-significant and very weak negative relationship (Figure 2C) between PSF and walking economy (p > 0.05), and there was no significant heterogeneity among the studies (Q = 0.1414; p = 0.7069; I2 = 0.00%). Evidence profile synthesis: Using the GRADE approach, as few of the included studies had a low or moderate ROB and fair methodological qualities, with no heterogeneity and certain imprecision, the certainty of evidence was considered low for the lack of significant correlation between PSF and walking economy.
PSF and walking endurance (narrative synthesis): Some of the included studies evaluated the relationship between walking endurance and chronic fatigue and/or exertion/acute fatigue. Chronic fatigue was reported to have a significant, weak negative relationship (27) and a non-significant, very weak positive relationship (14) with walking endurance. Exertion/state fatigue had non-significant, strong (32) and weak (26) negative relationships with walking endurance. PSF and walking endurance (quantitative synthesis): Both FE and RE meta-analysis results revealed a statistically non-significant and very weak negative relationship (Figure 2D) between PSF and walking endurance (p > 0.05), with statistically significant heterogeneity observed among the studies (Q = 5.7151; p = 0.0168; I2 = 82.50%). Evidence profile synthesis: Using the GRADE approach, as few of the included studies had a moderate ROB and fair methodological qualities, with considerable heterogeneity and certain imprecision, the certainty of evidence was considered very low for the of a significant correlation between PSF and walking endurance.
PSF and walking/ambulatory activity (narrative synthesis): The studies showed opposing evidence on the relationship between PSF and walking/ambulatory activity. One study showed a non-significant, moderate (26) and very weak (33) negative relationship between PSF and walking/ambulatory activity, while another study (34) showed a non-significant, very weak positive relationship between PSF and walking/ambulatory activity. In another study, PSF had a significantly weak negative relationship with functional ambulation (8). PSF and walking activity (quantitative synthesis): Both the FE and RE meta-analysis results showed a statistically non-significant, very weak positive relationship (Figure 2E) between PSF and walking/ambulatory activity (p > 0.05), with no significant heterogeneity observed among the studies (Q = 1.2357; p = 0.2663; I2 = 19.07%). Evidence profile synthesis: Using the GRADE approach, as few of the included studies had a low and moderate ROB and fair methodological qualities, with no heterogeneity and certain imprecision, the certainty of evidence was considered low for the lack of a significant correlation between PSF and walking activity.
PSF and functional recovery-related outcomes
Relationships of PSF with ADLs
PSF and ADLs (narrative synthesis): Most studies revealed a negative relationship between PSF and ADL. The inverse relationship is significantly moderate (15), weak (12, 14), and very weak (25). On the other hand, the correlation was found to be non-significantly very weak negative (35) and significantly moderate positive (2, 36). PSF and ADLs (quantitative synthesis): Both the FE and RE meta-analysis results revealed a statistically non-significant and very weak negative relationship (Figure 3A) between PSF and ADLs (p > 0.05), with statistically significant heterogeneity observed among the studies (Q = 117.5112; p < 0.0001; I2 = 94.89%). Evidence profile synthesis: Using the GRADE approach, as the majority of included studies had a low ROB and fair methodological qualities, with considerable heterogeneity and certain imprecision, the certainty of the evidence was considered very low for the lack of a significant correlation between PSF and ADLs.
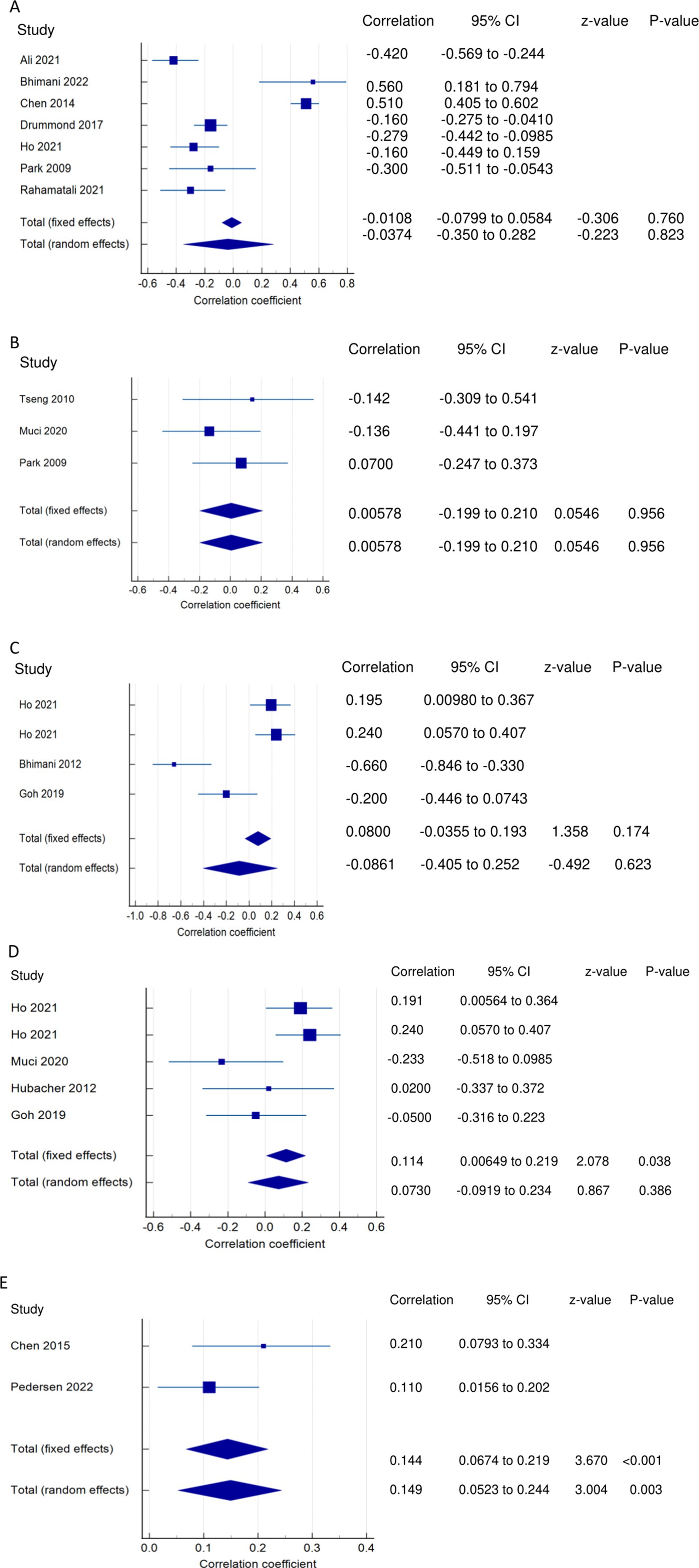
Figure 3. Relationship of PSF with functional recovery related outcomes. This figure illustrates the relationship of PSF with (A) ADLs; (B) general motor performance; (C) lower extremity motor performance; (D) upper extremity motor performance; (E) stroke impairment/severity. The boxes represent point estimate and the size for each individual study, the horizontal lines represent the 95% CI and its boundaries for each individual study result, whereas the diamond shapes represent the pooled result estimate (summary of the meta-analysis) showing the overall relationship estimate for both fixed and random effects.
Relationships of PSF with motor performance
PSF and general motor performance (narrative synthesis): Some studies reported a non-significant, very weak positive (3, 35) and negative (19) relationship between chronic PSF and motor performance. However, a significantly strong negative relationship was demonstrated between exertion/state fatigue and motor function (32). PSF and general motor performance (quantitative synthesis): Both the FE and RE meta-analysis results showed a statistically non-significant and very weak positive relationship (Figure 3B) between PSF and general motor performance (p > 0.05), with no significant heterogeneity observed among the studies (Q = 1.1836; p = 0.5533; I2 = 0.00%). Evidence profile synthesis: Using the GRADE approach, as few included studies had a moderate ROB and fair methodological qualities, with no heterogeneity and certain imprecision, the certainty of the evidence was considered low for the lack of significant correlation between PSF and general motor performance.
PSF and lower extremity motor performance (LEMP) (narrative synthesis): Most of the studies showed a negative relationship between PSF and LEMP, with the correlations being significant and strong (2), non-significant and weak (8) or non-significant and very weak (19). One study reported a significant weak (16) and very weak (12) positive relationship between PSF and LEMP. PSF and lower extremity motor performance (LEMP) (quantitative synthesis): The FE and RE meta-analysis results revealed statistically non-significant, very weak positive and weak negative relationships, respectively (Figure 3C) between PSF and LEMP (p > 0.05), but there was statistically significant heterogeneity among the studies (Q = 22.9362; p = <0.0001; I2 = 86.92%). Evidence profile synthesis: Using the GRADE approach, as the majority of included studies had a low ROB and fair methodological qualities, with considerable heterogeneity, the certainty of the evidence was considered low for the lack of a significant correlation between PSF and LEMP.
PSF and upper extremity motor performance (UEMP) (narrative synthesis): Three studies revealed a negative relationship between PSF and UEMP, with the correlations being significant and moderate (2), non-significant and weak (19), or non-significant and very weak (8). Three other studies showed a positive relationship between PSF and UEMP, with the relationships being significant and weak (16), significant and very weak (12), or non-significant and very weak (17). PSF and upper extremity motor performance (UEMP) (quantitative synthesis): A statistically significant, very weak positive relationship (Figure 3D) was found between PSF and UEMP in the FE meta-analysis (meta r = 0.114; p = 0.038) and RE meta-analysis (meta r = 0.0730; p = 0.386), with no significant heterogeneity among the studies (Q = 8.3411; p = 0.0799; I2 = 52.04%; 95% CI for I2 = 0.00 to 82.38). Evidence profile synthesis: Using the GRADE approach, as the majority of the included studies had a low ROB and fair methodological qualities, with no significant heterogeneity and certain imprecision, the certainty of evidence was considered moderate for a significant, very weak positive correlation between PSF and UEMP.
Relationships of PSF with stroke severity/impairment
PSF and stroke severity/impairment (SI) (narrative synthesis): One study each showed a significant weak positive (36) or very weak positive (41) relationship between PSF and stroke severity/impairment. PSF and stroke severity/impairment (SI) (quantitative synthesis): The meta-analysis results revealed statistically significant very weak positive (FE, meta r = 0.144; p < 0.001) and (RE, meta r = 0.149; p = 0.003) relationships (Figure 3E), between PSF and SI, with no significant heterogeneity among the studies (Q = 1.5090; p = 0.2193; I2 = 33.73%; 95% CI for I2 = 0.00 to 0.00). Evidence profile synthesis: Using the GRADE approach, as few of the included studies had a low ROB and fair methodological qualities, with no heterogeneity, the certainty of the evidence was considered moderate for a significant, very weak positive correlation between PSF and stroke severity/impairment.
Relationships of PSF with physical function and activity
PSF and Physical function (PF) (narrative synthesis): PSF had a significantly strong negative relationship with PF (2).
PSF and activity (narrative synthesis): PSF had a significant, moderately positive relationship with activity (27).
Relationships of PSF with functional muscle strength
PSF and lower extremity functional muscle strength (LEFMS) (narrative synthesis): One study and two studies reported non-significant weak (8) and very weak (12, 16) positive relationships, respectively, between PSF and LEFMS. Lower extremity functional muscle strength (LEFMS) (quantitative synthesis): Both the FE and RE meta-analysis results revealed a statistically significant, very weak positive relationship (Figure 4A) between PSF and LEFMS (meta r = 0.130; p = 0.039), with no significant heterogeneity among the studies (Q = 0.3291; p = 0.8483; I2 = 0.00%; 95% CI for I2 = 0.00 to 79.61). Evidence profile synthesis: Using the GRADE approach, as the majority of the included studies had a low ROB and fair methodological qualities, with no heterogeneity, the certainty of evidence was considered moderate for a significant, very weak correlation between PSF and LEFMS.
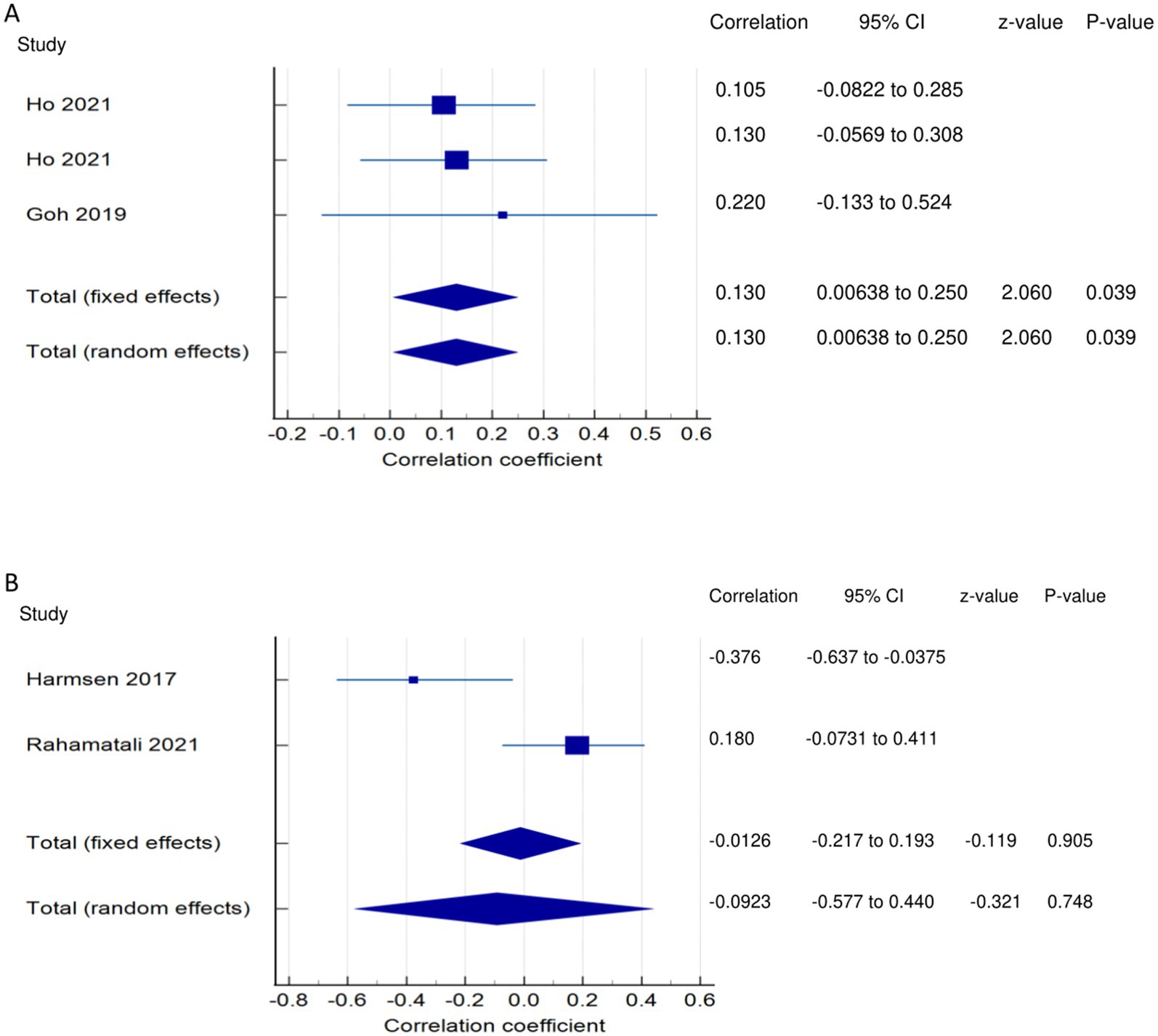
Figure 4. Relationship of PSF with functional recovery related outcomes. This figure illustrates the relationship of PSF with (A) lower extremity functional muscle strength; (B) knee extensor strength. The boxes represent point estimate and the size for each individual study, the horizontal lines represent the 95% CI and its boundaries for each individual study result, whereas the diamond shapes represent the pooled result estimate (summary of the meta-analysis) showing the overall relationship estimate for both fixed and random effects.
PSF and knee extensor strength (KES)/neuromuscular fatigability (narrative synthesis): Two studies showed opposing findings regarding the correlation between PSF and KES. One study showed a significantly weak negative relationship (37) and a non-significant, very weak positive relationship between PSF and KES (14). PSF and knee extensor strength/neuromuscular fatigability (KES) (quantitative synthesis): Both the FE and RE meta-analysis results revealed a statistically non-significant, very weak negative relationship (Figure 4B) between PSF and KES (p > 0.05), with statistically significant heterogeneity among the studies (Q = 6.6298; p < 0.0100; I2 = 84.92%). Evidence profile synthesis: Using the GRADE approach, as few of the included studies had a low or moderate ROB and fair methodological qualities, with considerable heterogeneity and certain imprecision, the certainty of evidence was considered very low for the lack of significant correlation between PSF and KES.
PSF and functional performance-related outcomes
PSF and balance performance (narrative synthesis): The majority of the studies showed a negative relationship between PSF and balance, with the correlations being significant and moderate (31), significant and weak (8, 27), or non-significant very weak (29). One study reported a significant correlation between PSF and balance performance (28), and another showed a significantly strong positive relationship with static balance (lower scores demonstrate better balance) (19). PSF and balance performance (quantitative synthesis): A statistically significant, very weak negative relationship (Figure 5A) was found between PSF and balance performance in the FE meta-analysis (meta r = −0.172; 95%; p = 0.004), with a non-significant, very weak negative relationship in the RE meta-analysis (p > 0.05). There was statistically significant heterogeneity among the studies (Q = 34.2972; p < 0.0001; I2 = 88.34%; 95% CI for I2 = 75.38 to 94.48). Evidence profile synthesis: Using the GRADE approach, as half of the included studies had a low ROB and fair methodological qualities, with considerable heterogeneity and certain imprecision, the certainty of the evidence was considered low for a significant, very weak negative correlation between PSF and balance.
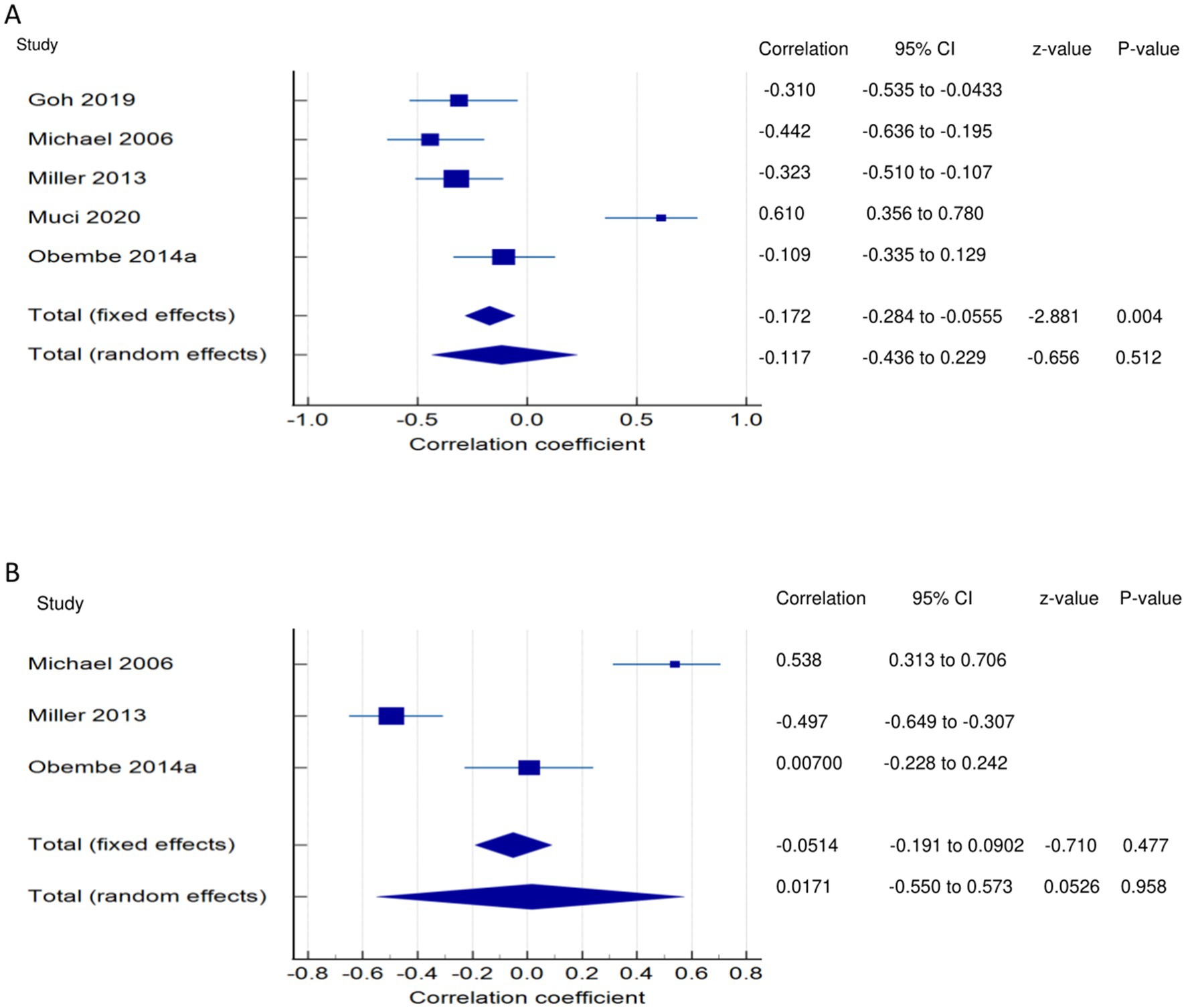
Figure 5. Relationship of PSF with functional performance related outcomes. This figure illustrates the relationship of PSF with (A) balance; (B) self-efficacy/confidence. The boxes represent point estimate and the size for each individual study, the horizontal lines represent the 95% Cl and its boundaries for each individual study result, whereas the diamond shapes represent the pooled result estimate (summary of the meta-analysis) showing the overall relationship estimate for both fixed and random effects.
PSF and self-confidence/efficacy (narrative synthesis): One study each showed a significant moderate negative (27) and positive (31) relationship between PSF and self-confidence/efficacy. However, in one study (29), the correlation was non-significant and very weakly positive. PSF and self-confidence/efficacy (quantitative synthesis): The FE and RE meta-analysis results revealed statistically non-significant, very weak negative and positive relationships, respectively, (Figure 5B) between PSF and self-confidence/efficacy (p > 0.05). There was statistically significant heterogeneity among the studies (Q = 39.5841; p < 0.0001; I2 = 94.95%). Evidence profile synthesis: Using the GRADE approach, as most of the included studies had a low ROB and fair methodological qualities with considerable heterogeneity and certain imprecision, the certainty of the evidence was considered low for the lack of a correlation between PSF and self-efficacy.
PSF and participation-related outcomes
PSF and disability (narrative synthesis): Two studies showed a significantly strong positive relationship (38, 39), and one each showed a moderate positive (41) and weak positive (40) relationship between PSF and disability. PSF and disability (quantitative synthesis): A statistically significant moderate positive relationship (Figure 6) was found between PSF and disability in the FE meta-analysis (meta r = 0.480; p < 0.001) and the RE meta-analysis (meta r = 0.508; p < 0.001), with statistically significant heterogeneity among the studies (Q = 16.3379; p = 0.0010; I2 = 81.64%; 95% CI for I2 = 52.31 to 92.93). Evidence profile synthesis: Using the GRADE approach, as most of the included studies had a low ROB and fair methodological qualities, with considerable heterogeneity, the certainty of the evidence was considered moderate for a significant moderate positive correlation between PSF and disability.
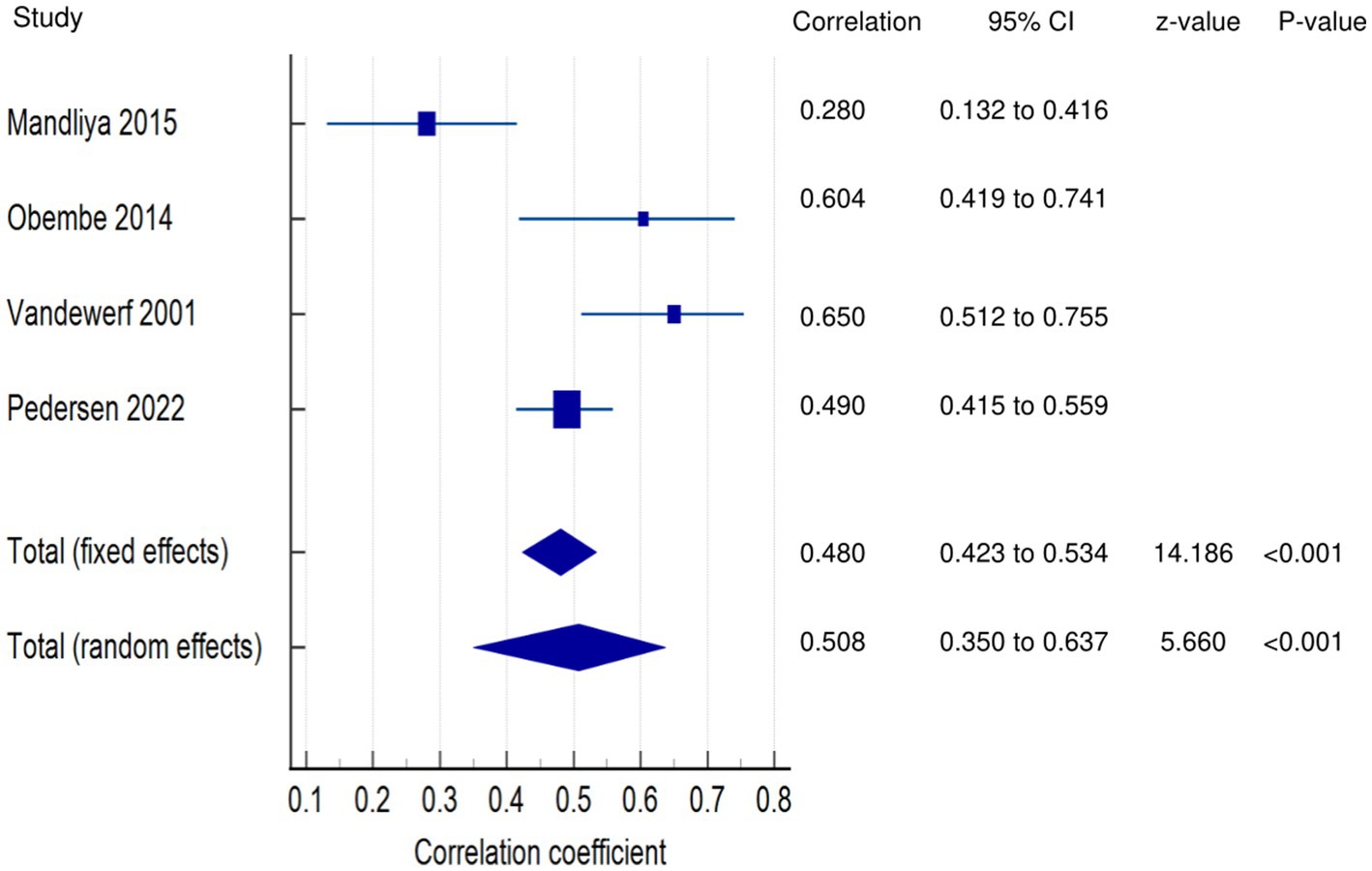
Figure 6. Relationship of PSF with participation related outcomes. This figure illustrates the relationship of PSF with disability. The boxes represent point estimate and the size for each individual study, the horizontal lines represent the 95% CI and its boundaries for each individual study result, whereas the diamond shapes represent the pooled result estimate (summary of the meta-analysis) showing the overall relationship estimate for both fixed and random effects.
PSF and participation (narrative synthesis): In one study each, PSF was found to have a significant moderate positive relationship with activity (27), a significant weak relationship with participation (27), and a significant negative, weak relationship with community integration/participation (12). PSF and Participation (quantitative synthesis): Both the FE and RE meta-analysis results showed statistically non-significant, very weak negative and positive relationships, respectively (Figure 7A) between PSF and participation (p > 0.05), with statistically significant heterogeneity among the studies (Q = 15.0937; p = 0.0001; I2 = 93.37%). Evidence profile synthesis: Using the GRADE approach, as few of the included studies had a low or moderate ROB and fair methodological qualities, with considerable heterogeneity, the certainty of the evidence was considered low for the lack of a correlation between PSF and participation.
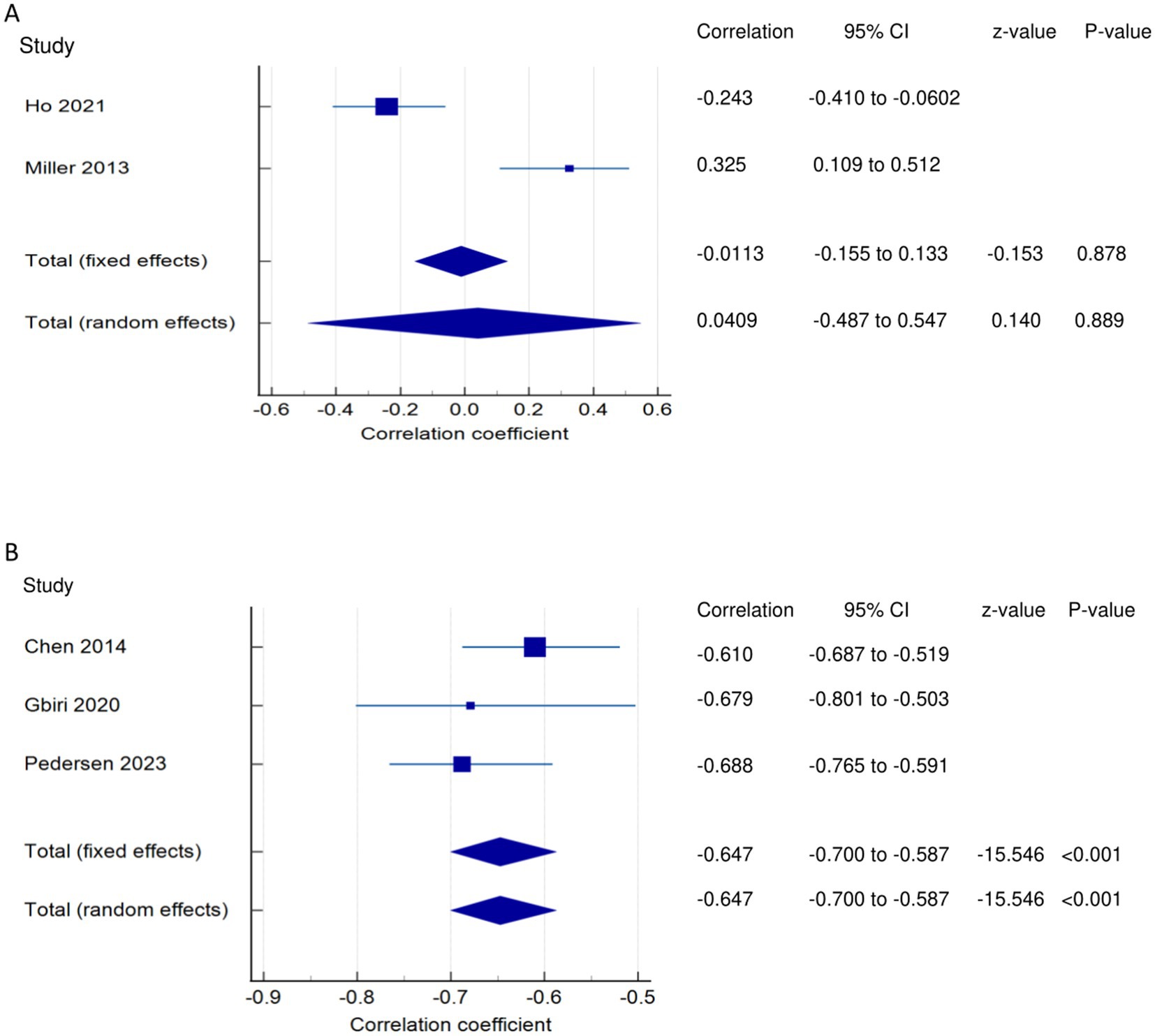
Figure 7. Relationship of PSF with participation related outcomes. This figure illustrates the relationship of PSF with (A) participation; (B) quality of life. The boxes represent point estimate and the size for each individual study, the horizontal lines represent the 95% CI and its boundaries for each individual study result, whereas the diamond shapes represent the pooled result estimate (summary of the meta-analysis) showing the overall relationship estimate for both fixed and random effects.
PSF and QOL (narrative synthesis): Three studies showed a significantly strong negative relationship between PSF and QOL (1, 13, 36). PSF and QOL (quantitative synthesis): Both FE and RE meta-analysis results revealed a statistically and significantly strong negative relationship (Figure 7B) between PSF and QOL (meta r = −0.647; p < 0.001), with no significant heterogeneity among the studies (Q = 1.7443; p = 0.4180; I2 = 0.00%; 95% CI for I2 = 0.00 to 96.15). Evidence profile synthesis: Using the GRADE approach, as most of the included studies had a low ROB and fair methodological qualities with no heterogeneity, the certainty of the evidence was considered moderate for a significantly strong negative correlation between PSF and QOL.
Exertion/acute fatigue
Relationship between exertion/acute fatigue and walking economy
Walking economy and PSF (exertion/acute fatigue): Both the FE and RE meta-analysis results of both FE and RE revealed a statistically and significantly strong negative relationship (Figure 8A) between PSF (exertion/acute) and walking economy (meta r = −0.627; p < 0.001), with no significant heterogeneity among the studies (Q = 0.3599; p = 0.5486; I2 = 0.00%; 95% CI for I2 = 0.00 to 0.00). Evidence profile synthesis: Using the GRADE approach, as few of the included studies had a moderate ROB and fair methodological qualities, with no heterogeneity and certain imprecision, the certainty of the evidence was considered low for a significantly strong negative correlation between PSF (exertion) and walking economy.
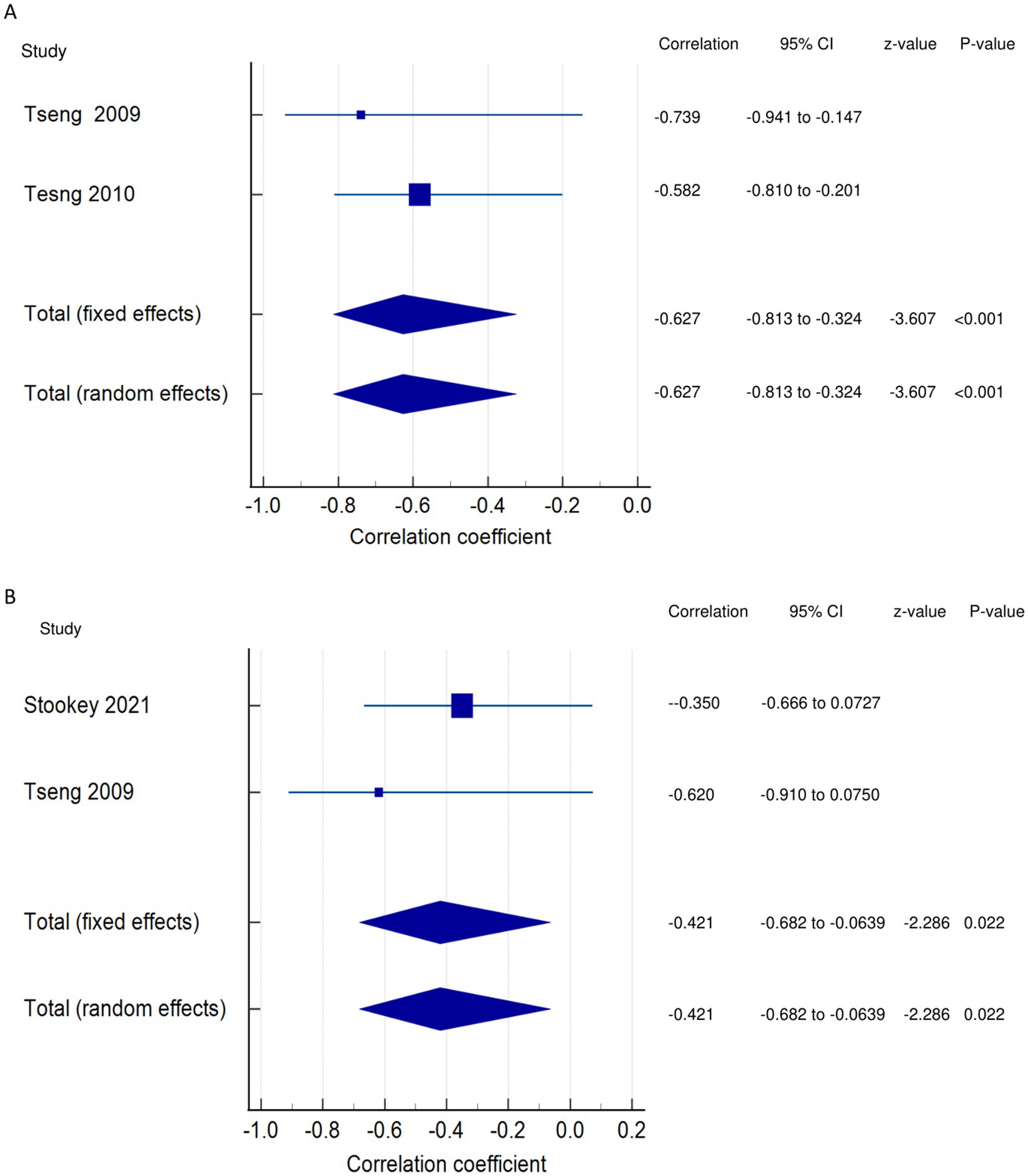
Figure 8. Relationship of exertion/acute fatigue with walking. This figure illustrates the relationship of exertion/acute fatigue with (A) walking economy; (B) walking endurance. The boxes represent point estimate and the size for each individual study, the horizontal lines represent the 95% CI and its boundaries for each individual study result, whereas the diamond shapes represent the pooled result estimate (summary of the meta-analysis) showing the overall relationship estimate for both fixed and random effects.
Relationship between exertion/acute fatigue and walking endurance
Walking endurance and PSF (exertion/acute fatigue): Both the FE and RE meta-analysis results showed a statistically significant moderate negative relationship (Figure 8B) between PSF (exertion/acute) and walking endurance (meta r = −0.421; p = 0.022), with no significant heterogeneity among the studies (Q = 0.5967; p = 0.4398; I2 = 0.00%; 95% CI for I2 = 0.00 to 0.00). Evidence profile synthesis: Using the GRADE approach, as few of the included studies had a moderate or high ROB and fair methodological qualities, with no heterogeneity and certain imprecision, the certainty of evidence was considered low for a significant moderate negative correlation between PSF (exertion) and walking endurance.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to establish the scientific evidence on the relationships of PSF with mobility, recovery, performance, and participation of stroke survivors. Studies have shown that components of mobility-related function, including mobility, gait speed, walking economy, and functional ambulation, have significant negative relationships with PSF, with correlation strengths ranging from strong to moderate to weak. However, walking activity has no significant correlation with PSF. In the meta-analysis, only mobility showed statistically significant weak negative relationships with PSF, with moderate to low certainty of evidence. This suggests that greater PSF is associated with less mobility. These findings are supported by a previous study that reported mobility deficit severity to be related to fatigue, with a consequent reduction in home and community ambulatory activity in stroke survivors (31). The weak correlations obtained with some variables in our meta-analysis may be due to a lack of control of some confounding variables in some of the included studies or to the small number of studies evaluating those variables. Thus, future correlation studies should adjust for confounding factors to verify the existing correlations, and RCTs to involve training in relation to PSF are warranted. It is vital to differentiate between walking economy/aerobic fitness, walking endurance, and walking activity. Aerobic fitness has to do with submaximal and peak VO2 during walking exercise testing (3, 30), walking endurance has to do with distance covered while walking during a 6-min walk test (27), whereas walking activity involves total stride count or number of steps during walking (26, 31, 33, 34).
Our results revealed that components of functional recovery, such as ADLs, motor performance, physical function, and KES, had significant negative relationships with PSF. However, stroke severity/impairments were positively correlated with PSF, and lower extremity functional muscle strength showed no correlation with PSF. The strengths of the correlations ranged from strong and moderate to weak and very weak. The meta-analysis showed that stroke impairment had significant, very weak positive correlations with PSF, with moderate certainty of evidence. This suggests that greater stroke impairment is associated with greater PSF. This finding is supported by other findings that functional limitations because of fatigue commonly occur in stroke survivors (38) and that stroke survivors, in turn, tend to attribute more functional limitations to their fatigue (42).
Some of the included studies revealed contradictory relationships, in terms of the direction (negative, positive) and significance (significant, non-significant), between some recovery/function variables and PSF. Such discrepancies may be attributable to the lack of control over factors influencing the study outcomes, the use of different outcome measures, and the nature of the recruited stroke population, among others. This indicates the need for further studies to explore the relationship between PSF and such variables while controlling for confounding factors.
Some studies also reported that components of functional performance, such as balance performance and self-confidence, had a significant negative relationship with PSF. The meta-analysis findings revealed that balance performance had significant, very weak negative correlations with PSF, with low certainty of evidence. This suggests that greater PSF is associated with reduced balance performance and self-efficacy/confidence. This result is backed by the finding that the severity of fatigue in stroke survivors is significantly associated with Berg balance scale scores and self-efficacy (31). Although the direction of the correlation is majorly negative and the strength of the correlation ranges from moderate to weak, a few studies reported contradictory findings of positive correlations between PSF and balance performance and self-efficacy/confidence. This discrepancy may be attributable to the study settings, outcome measures used, and methodological limitations, especially those related to confounder adjustments and study selection. Thus, further studies are warranted to confirm our results.
Studies on disability have reported a significant positive relationship (strong, moderate, and weak) between disability and PSF. In the meta-analysis, disability showed a significant moderate positive correlation with PSF, with a moderate certainty of evidence. This suggests that a greater PSF is associated with greater post-stroke disability. This is expected, as PSF has been reported to be substantially related to post-stroke disability (39, 40). The findings of the studies are uniform in terms of the correlation direction, which supports the relationship. Hence, future RCTs aimed at reducing disability and its impact on PSF in stroke survivors are warranted.
The included studies demonstrated a significant negative relationship (strong and weak) between PSF and participation and QOL. In the meta-analysis, PSF was found to have a non-significant, very weak negative correlation with participation (low certainty of the evidence) and a significantly strong negative correlation with QOL (moderate certainty of the evidence). In the studies, the correlation direction was reported to be negative. These findings suggest that greater PSF is associated with reduced post-stroke participation and QOL. This result is supported by a previous finding that fatigue adversely affects QOL, rehabilitation outcomes, social participation, functional independence, and return to work among stroke survivors (43). Longitudinal studies are recommended to conduct follow-ups and determine how PSF influences post-stroke participation and QOL.
The included studies demonstrated significantly strong negative correlations of exertion/acute fatigue with walking activity and general motor performance. In the meta-analysis, exertion/acute fatigue showed a significantly strong negative correlation (low certainty of evidence) with walking economy and a significant moderate negative correlation (low certainty of evidence) with walking endurance. This suggests that greater exertion/acute PSF is associated with reduced post-stroke mobility. This is supported by a previous finding that mobility deficit is strongly associated with post-stroke fatigability, with compromised mobility and functional limitations increasing the incidence of fatigue (26). We found that few studies have reported on the impact of exertion/acute fatigue; hence, further studies are warranted to explore the effects of exertion/acute fatigue on the outcomes of interest in stroke survivors. Additionally, few studies have reported correlations with the components of fatigue; hence, future studies are required to assess the relationships of components of fatigue, such as physical and mental/cognitive fatigue, with the outcomes of interest. The review findings are crucial, and the implication is that since PSF has been found to be associated with reduced mobility, performance, and participation and increased impairment and disability in stroke, it is, therefore, crucial to have in place interventions and strategies targeted at reducing PSF in stroke survivors. Although fatigue differs from sleep, numerous subjective measures of sleep were found to correlate with fatigue, with sleep quality contributing to fatigue in chronic stroke survivors a year or more post-stroke (12). Additionally, this sleep quality was found to correlate positively with fatigue and independently predict it post-stroke, with poor sleep quality likely resulting in post-stroke daytime sleepiness (12). However, sleep quality was reported to likely be a target element in interventions for improving fatigue (12). Thus, it may be possible that interventions aimed at enhancing sleep quality post-stroke may also affect PSF.
Strengths and limitations
The main strengths of this review are the literature search conducted in important databases for articles, the use of recommended tools for the assessment of methodological quality and ROB, adherence to PRISMA guidelines for conducting and reporting the review, and the use of Cochrane GRADE criteria for evidence synthesis. All the tools and guidelines/criteria facilitated clear evidence synthesis and the drawing of meaningful conclusions.
The review has some limitations. Only articles published in English were used, which may have resulted in selection bias. Many of the included studies were cross-sectional in nature. Thus, we could not establish a cause-and-effect relationship among the variables. Furthermore, the low certainty of evidence obtained for some correlations possibly signifies that the relationships may go in either positive or negative directions. Hence, some of the findings should be interpreted with caution, considering these limitations.
The main limitations of the included studies should also be taken into account. Some of the studies had small samples, which may limit the generalization of the findings, and many studies did not control or adjust for confounders, which may have affected their outcomes. Furthermore, the use of self-reported measures for fatigue in most of the studies might have affected the reliability of the evaluations. Additionally, some studies had limitations in sample selection and study participation. The majority of the studies did not categorize or report specific correlations with fatigue components such as physical and mental/cognitive components.
Conclusion
This review and meta-analysis found that chronic PSF had significant negative correlations with mobility (moderate certainty of evidence), balance performance (low certainty of evidence), and quality of life (moderate certainty of evidence). It was also found to have a significant negative relationship with functional motor recovery in the narrative synthesis, though this was not supported by the meta-analysis. Additionally, chronic PSF revealed significant positive correlations with stroke severity/impairment (moderate certainty of evidence) and disability (moderate certainty of evidence).
For exertion/acute fatigue, our results revealed significant negative correlations of PSF with walking economy and walking endurance, both with low certainty of evidence. The narrative synthesis identified revealed significant negative correlations between exertion/acute fatigue and walking activity, as well as general motor performance.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Data will be provided on reasonable request. Requests to access these datasets should be directed to c2hhbWF5Lm5nQHBvbHl1LmVkdS5oaw==.
Author contributions
JU: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. TW: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. SN: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the General Research Fund (15101023), Research Grant Council, Hong Kong SAR Government, awarded to Prof. Shamay Ng and her team.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1420443/full#supplementary-material
References
1. Pedersen, SG, Løkholm, M, Friborg, O, Halvorsen, MB, Kirkevold, M, Heiberg, G, et al. Visual problems are associated with long-term fatigue after a stroke. J Rehabil Med. (2023) 55:jrm00374. doi: 10.2340/jrm.v55.4813
2. Bhimani, R, Chappuis, D, Mathiason, MA, and Anderson, LC. Spasticity, pain, and fatigue: are they associated with functional outcomes in people with stroke? Rehabil Nurs J. (2022) 47:60–71. doi: 10.1097/RNJ.0000000000000357
3. Tseng, BY, Billinger, SA, Gajewski, BJ, and Kluding, PM. Exertion fatigue and chronic fatigue are two distinct constructs in people post-stroke. Stroke. (2010) 41:2908–12. doi: 10.1161/STROKEAHA.110.596064
4. Mizuno, K, Tanaka, M, Yamaguti, K, Kajimoto, O, Kuratsune, H, and Watanabe, Y. Mental fatigue caused by prolonged cognitive load associated with sympathetic hyperactivity. Behav Brain Funct. (2011) 7:17–7. doi: 10.1186/1744-9081-7-17
5. Paciaroni, M, and Acciarresi, M. Poststroke Fatigue. Stroke. (2019) 50:1927–33. doi: 10.1161/STROKEAHA.119.023552
6. Skogestad, IJ, Kirkevold, M, Larsson, P, Borge, CR, Indredavik, B, Gay, CL, et al. Post-stroke fatigue: an exploratory study with patients and health professionals to develop a patient-reported outcome measure. J Patient-Rep Outcomes. (2021) 5:1–11. doi: 10.1186/s41687-021-00307-z
7. Lerdal, A, Bakken, LN, Kouwenhoven, SE, Pedersen, G, Kirkevold, M, Finset, A, et al. Poststroke fatigue—a review. J Pain Symptom Manag. (2009) 38:928–49. doi: 10.1016/j.jpainsymman.2009.04.028
8. Goh, H-T, and Stewart, JC. Post-stroke fatigue is related to motor and cognitive performance: a secondary analysis. J Neurol Phys Ther. (2019) 43:233–9. doi: 10.1097/NPT.0000000000000290
9. Ponchel, A, Bombois, S, Bordet, R, and Hénon, H. Factors associated with poststroke fatigue: a systematic review. Stroke Res Treat. (2015) 2015:1–11. doi: 10.1155/2015/347920
10. Shen, J, Barbera, J, and Shapiro, CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. (2006) 10:63–76. doi: 10.1016/j.smrv.2005.05.004
11. Long, H, Scott, H, and Lack, L. Sleepy, tired, drowsy, and fatigue have different meanings for a university student sample. J Clin Sleep Med. (2022) 18:1235–41. doi: 10.5664/jcsm.9780
12. Ho, LYW, Lai, CKY, and Ng, SSM. Contribution of sleep quality to fatigue following a stroke: a cross-sectional study. BMC Neurol. (2021) 21:1–9. doi: 10.1186/s12883-021-02174-z
13. Gbiri, CAO, Aweto, HA, Mogaji, EO, and Usman, JS. Post-stroke fatigue, cardiopulmonary function and quality of life of stroke survivors. Int J Med Med Sci. (2020)
14. Rahamatali, M, De Bont, N, Valet, M, Halkin, V, Hanson, P, Deltombe, T, et al. Post-stroke fatigue: how it relates to motor fatigability and other modifiable factors in people with chronic stroke. Acta Neurol Belg. (2021) 121:181–9. doi: 10.1007/s13760-020-01453-9
15. Ali, AS, Farid, DS, Shendy, WS, Mourad, HS, Ahmed, KT, and Ahmed, WEH. Post stroke fatigue prevalence and its correlation to the functional recovery. Clin Schizophr Relat Psychoses. (2022) 16:1–5. doi: 10.3371/CSRP.AADF.0228222
16. Ho, LY, Lai, CK, and Ng, SS. Measuring fatigue following stroke: the Chinese version of the fatigue assessment scale. Disabil Rehabil. (2021) 43:3234–41. doi: 10.1080/09638288.2020.1730455
17. Hubacher, M, Calabrese, P, Bassetti, C, Carota, A, Stöcklin, M, and Penner, I-K. Assessment of post-stroke fatigue: the fatigue scale for motor and cognitive functions. Eur Neurol. (2012) 67:377–84. doi: 10.1159/000336736
18. Maaijwee, NA, Arntz, RM, Rutten-Jacobs, LC, Schaapsmeerders, P, Schoonderwaldt, HC, van Dijk, EJ, et al. Post-stroke fatigue and its association with poor functional outcome after stroke in young adults. J Neurol Neurosurg Psychiatry. (2015) 86:1120–6. doi: 10.1136/jnnp-2014-308784
19. Muci, B, Keser, I, Meric, A, and Karatas, GK. What are the factors affecting dual-task gait performance in people after stroke? Physiother Theory Pract. (2022) 38:621–8. doi: 10.1080/09593985.2020.1777603
20. MacIntosh, BJ, Edwards, JD, Kang, M, Cogo-Moreira, H, Chen, JL, Mochizuki, G, et al. Post-stroke fatigue and depressive symptoms are differentially related to mobility and cognitive performance. Front Aging Neurosci. (2017) 9:343. doi: 10.3389/fnagi.2017.00343
21. Watanabe, K, Sasaki, AT, Tajima, K, Mizuseki, K, Mizuno, K, and Watanabe, Y. Mental fatigue is linked with attentional bias for sad stimuli. Sci Rep. (2019) 9:8797. doi: 10.1038/s41598-019-45428-0
22. National Heart LaBI . Study quality assessment tools: National Heart, Lung and Blood Institute; (2021) Available at: http://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
23. Hayden, JA, van der Windt, DA, Cartwright, JL, Côté, P, and Bombardier, C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
24. Grooten, WJA, Tseli, E, Äng, BO, Boersma, K, Stålnacke, B-M, Gerdle, B, et al. Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using QUIPS—aspects of interrater agreement. Diagn Progn Res. (2019) 3:1–11. doi: 10.1186/s41512-019-0050-0
25. Drummond, A, Hawkins, L, Sprigg, N, Ward, NS, Mistri, A, Tyrrell, P, et al. The Nottingham fatigue after stroke (NotFAST) study: factors associated with severity of fatigue in stroke patients without depression. Clin Rehabil. (2017) 31:1406–15. doi: 10.1177/0269215517695857
26. Stookey, AD, Macko, RF, Ivey, FM, and Katzel, LI. Evaluating test-retest reliability of fatigability in chronic stroke. J Stroke Cerebrovasc Dis. (2021) 30:105895. doi: 10.1016/j.jstrokecerebrovasdis.2021.105895
27. Miller, KK, Combs, SA, Van Puymbroeck, M, Altenburger, PA, Kean, J, Dierks, TA, et al. Fatigue and pain: relationships with physical performance and patient beliefs after stroke. Top Stroke Rehabil. (2013) 20:347–55. doi: 10.1310/tsr2004-347
28. Schow, T, Teasdale, TW, Quas, KJ, and Rasmussen, MA. Problems with balance and binocular visual dysfunction are associated with post-stroke fatigue. Top Stroke Rehabil. (2017) 24:41–9. doi: 10.1080/10749357.2016.1188475
29. Obembe, AO, Olalemi, AE, and Loto, BO. Fatigue impact, gait and balance performance in chronic stroke survivors. Physiother Pract Res. (2014) 35:49–54. doi: 10.3233/PPR-130029
30. Lewis, SJ, Barugh, AJ, Greig, CA, Saunders, DH, Fitzsimons, C, Dinan-Young, S, et al. Is fatigue after stroke associated with physical deconditioning? A cross-sectional study in ambulatory stroke survivors. Arch Phys Med Rehabil. (2011) 92:295–8. doi: 10.1016/j.apmr.2010.10.030
31. Michael, KM, Allen, JK, and Macko, RF. Fatigue after stroke: relationship to mobility, fitness, ambulatory activity, social support, and falls efficacy. Rehabil Nurs. (2006) 31:210–7. doi: 10.1002/j.2048-7940.2006.tb00137.x
32. Tseng, BY, and Kluding, P. The relationship between fatigue, aerobic fitness, and motor control in people with chronic stroke: a pilot study. J Geriatr Phys Ther. (2009) 32:97–102. doi: 10.1519/00139143-200932030-00003
33. Mahendran, N, Kuys, SS, and Brauer, SG. Which impairments, activity limitations and personal factors at hospital discharge predict walking activity across the first 6 months poststroke? Disabil Rehabil. (2020) 42:763–9. doi: 10.1080/09638288.2018.1508513
34. Michael, K, and Macko, RF. Ambulatory activity intensity profiles, fitness, and fatigue in chronic stroke. Top Stroke Rehabil. (2007) 14:5–12. doi: 10.1310/tsr1402-5
35. Park, JY, Chun, MH, Kang, SH, Lee, JA, Kim, BR, and Shin, MJ. Functional outcome in poststroke patients with or without fatigue. Am J Phys Med Rehabil. (2009) 88:554–8. doi: 10.1097/PHM.0b013e3181a0dae0
36. Chen, Y-K, Qu, J-F, Xiao, W-M, Li, W-Y, Weng, H-Y, Li, W, et al. Poststroke fatigue: risk factors and its effect on functional status and health-related quality of life. Int J Stroke. (2015) 10:506–12. doi: 10.1111/ijs.12409
37. Harmsen, WJ, Ribbers, GM, Zegers, B, Sneekes, EM, Praet, SF, Heijenbrok-Kal, MH, et al. Impaired muscle strength may contribute to fatigue in patients with aneurysmal subarachnoid hemorrhage. Int J Rehabil Res. (2017) 40:29–36. doi: 10.1097/MRR.0000000000000197
38. Obembe, AO, Olaogun, MM, and Olalemi, AE. Functional limitations due to fatigue among independently ambulant stroke survivors in Osun, South-Western Nigeria. Physiother Res Int. (2015) 20:54–9. doi: 10.1002/pri.1596
39. van der Werf, SP, van den Broek, HL, Anten, HW, and Bleijenberg, G. Experience of severe fatigue long after stroke and its relation to depressive symptoms and disease characteristics. Eur Neurol. (2001) 45:28–33. doi: 10.1159/000052085
40. Mandliya, A, Das, A, Unnikrishnan, J, Amal, M, Sarma, PS, and Sylaja, P. Post-stroke fatigue is an independent predictor of post-stroke disability and burden of care: a path analysis study. Top Stroke Rehabil. (2016) 23:1–7. doi: 10.1080/10749357.2015.1110273
41. Pedersen, A, Almkvist, E, Holmegaard, L, Lagging, C, Redfors, P, Blomstrand, C, et al. Fatigue 7 years post-stroke: predictors and correlated features. Acta Neurol Scand. (2022) 146:295–303. doi: 10.1111/ane.13665
42. Ingles, JL, Eskes, GA, and Phillips, SJ. Fatigue after stroke. Arch Phys Med Rehabil. (1999) 80:173–8. doi: 10.1016/S0003-9993(99)90116-8
Keywords: stroke, post-stroke fatigue, mobility, recovery, performance, walking, motor function, participation
Citation: Usman JS, Wong TWL and Ng SSM (2024) Relationships of post-stroke fatigue with mobility, recovery, performance, and participation-related outcomes: a systematic review and meta-analysis. Front. Neurol. 15:1420443. doi: 10.3389/fneur.2024.1420443
Edited by:
Victor W. Mark, University of Alabama at Birmingham, United StatesReviewed by:
Dawn M. Nilsen, Columbia University, United StatesCatherine Duclos, Montreal University, Canada
Copyright © 2024 Usman, Wong and Ng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shamay S. M. Ng, c2hhbWF5Lm5nQHBvbHl1LmVkdS5oaw==
 Jibrin S. Usman
Jibrin S. Usman Thomson W. L. Wong
Thomson W. L. Wong Shamay S. M. Ng
Shamay S. M. Ng