- Department of Neurology, Acıbadem University School of Medicine, Istanbul, Türkiye
Objective: This real-world study aimed to investigate how onabotulinumtoxinA affects the outcome of migraine, along with accompanying anxiety, depression, and bruxism among a group of patients with chronic migraine (CM) and define predictors of good response.
Methods: Patients diagnosed with CM who received onabotulinumtoxinA were included in this single-center, real-world retrospective cohort study. Monthly headache days (MHDs), monthly migraine days (MMDs), headache intensity (numeric rating scale-NRS) and headache characteristics were evaluated at baseline and 12 weeks post-treatment. Patient-reported outcome measures (PROMs) included Migraine Disability Assessment Scale (MIDAS), Headache Impact Test-6 (HIT-6) scores, 12-item Allodynia Symptom Checklist (ASC-12), Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI). Response to onabotulinumtoxinA (% reduction in MHDs) and treatment-related adverse events (TRAEs) were also evaluated. OnabotulinumA was applied to the masseter muscles in patients complaining of bruxism.
Results: A total of 72 patients (mean ± SD age: 36.3 ± 8.5 years; 91.7% were female) diagnosed with CM were included. OnabotulinumtoxinA revealed significant decrease in median (IQR) MHDs [from 20(15–25) at baseline to 6(4–10), p < 0.001], MMDs [from 9(6–12) to 3(1–6), p < 0.001] and NRS [from 9(8–10) to 7(6–8), p < 0.001], and the MIDAS [from 54(30–81) to 16(7–24), p < 0.001], HIT-6 [from 67(65–69) to 58(54–64), p < 0.001], ASC-12 [from 6(1.5–9) to 2(0–9), p = 0.002], BAI [from 12(6.5–19) to 9(3–17), p < 0.001] and BDI [from 11(6.5–17) to 3(2–7) p < 0.001] scores at 12 weeks post-treatment. Patients complaining of bruxism received onabotulinumtoxinA injections in the first n = 27 (37.5%) and 12. week post-treatment n = 19 (70.4%) periods. Overall, 70.8% of patients responded (≥50% reduction in MHDs), while 29.2% did not (<50% reduction). Both groups showed similar characteristics in demographics, migraine history, baseline PROMs scores, comorbidities, and prior treatments.
Conclusion: OnabotulinumtoxinA is an effective treatment option that rapidly improves migraine outcomes, disability, and impact while also alleviating comorbid depression and/or anxiety. This study’s noteworthy finding is that onabotulinumtoxinA is effective in a majority of CM patients, irrespective of their prior treatment history, migraine characteristics, or concurrent comorbidities. Furthermore, we identified no specific predictors for a favorable response to onabotulinumtoxinA. Applying onabotulinumtoxinA to the masseter muscles can relieve discomfort associated with concurrent bruxism; however, it does not impact migraine outcomes.
Introduction
Migraine, affecting 14% of the global population, is a severe condition leading to marked disability, compromised functionality, diminished quality of life for sufferers, and imposing a considerable socioeconomic load (1–3). Based on the monthly headache days (MHDs), migraine is classified as episodic (EM) and chronic migraine (CM) (4, 5). Patients with EM face the possibility of advancing to CM, while those with CM are at increased risk of migraine disability, impaired quality of life, comorbid medical and psychiatric conditions, and medication-overuse headache (MOH) (5–9). Migraine coexists with psychiatric comorbidities such as anxiety and depression, posing a risk of migraine chronification and poor treatment response (10–12). Sleep bruxism may cause morning headaches with moderate, non-pulsating, and pressure-like symptoms (13) and is found in CM as common as in EM patients but causes more disability (14). Hence, effective migraine management requires prophylactic treatment (15, 16) and addressing comorbidities due to their impact on treatment efficacy and clinical course (10, 17, 18). Despite the availability of several drugs for migraine prevention, studies indicate low adherence to oral migraine prophylactics, with adverse events being the most frequently cited reason for discontinuation, followed by lack of efficacy (19–21). This situation emphasizes the necessity for early, targeted preventive treatments that fulfill both patient and physician expectations with greater specificity and effectiveness (22–24).
Real-world evidence regarding the effectiveness and safety of onabotulinumtoxinA in treating CM is crucial, especially considering the diverse patient population with comorbidities and the complex clinical management strategies not fully represented in randomized controlled trials (RCTs) (25–27). Furthermore, due to the connection between the decrease in headache intensity and disability scales, conducting a thorough headache assessment with patient-reported outcome measures (PROMs), beyond tracking headache days, is advised for a more accurate evaluation of treatment response in chronic migraine. However, only some real-world studies have specifically examined the impact of onabotulinumtoxinA on the qualitative aspects of headache pain in treated individuals (28–30).
Despite being the subject of numerous studies over the past two decades, there still needs to be a consensus on the predictors in chronic migraine patients who respond well to onabotulinumtoxinA. Predictors indicating a favorable response have been suggested to include clinical headache characteristics such as unilateral or ocular location (31–34), level of disability, headache intensity (31), frequency of migraine days per month, and medication overuse (35). Additionally, molecular biomarkers such as elevated serum levels of calcitonin gene-related peptide (CGRP), vasoactive intestinal peptide, and pentraxin-3 have been found to aid in predicting a positive response to onabotulinumtoxinA (36, 37).
This study aims to see the effect of single-session onabotulinumtoxinA on migraine outcomes and accompanying comorbid diseases such as anxiety/depression and to predict which patient profile benefits from onabotulinumtoxinA. Furthermore, we aimed to assess whether implementing an additional injection protocol to alleviate concurrent bruxism in these patients had any impact on migraine recovery.
Methods
Study population
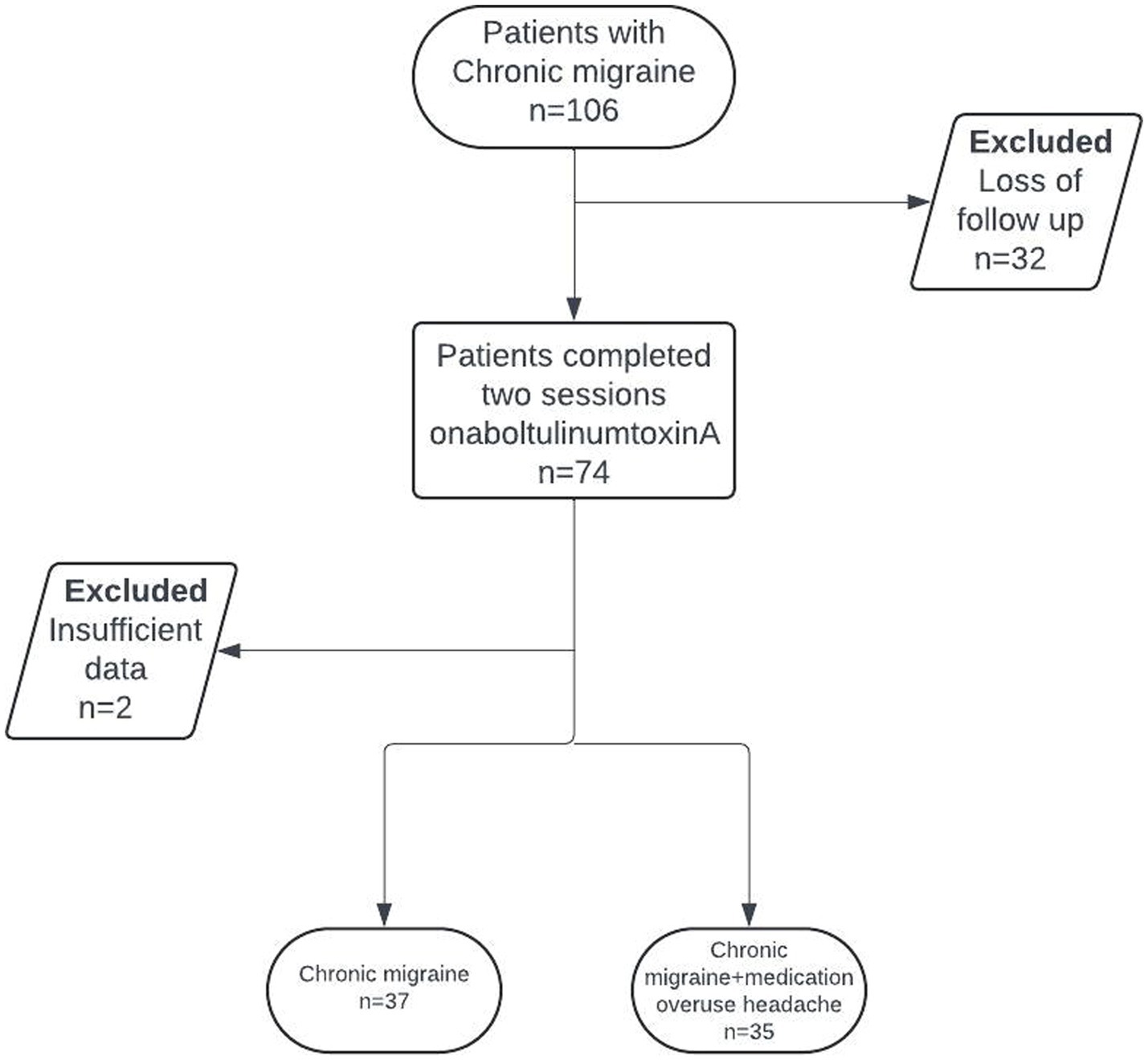
In this retrospective cohort study, patients between 18 and 65 diagnosed CM were included, according to the third edition of the International Classification of Headache Disorders (ICHD-3), who received a single-session onabotulinumtoxinA for migraine prophylaxis and had follow-up at 12 weeks (38). The patient selection for this study is depicted in Figure 1. Throughout the study duration and up to 1 month before enrollment, participants did not receive any other prophylactic migraine treatments, additional antidepressants, nerve blocks, or trigger point injections for migraine or any comorbid conditions. Exclusion criteria included pregnant or breastfeeding women, as well as individuals who had recently initiated a new psychiatric medication or undergone dose adjustments for ongoing psychiatric medication within the 3 months preceding the study enrollment.
This study was approved by our university’s Medical Research Ethics Committee (Approval number: 2023-21/726).
Study parameters
Data on patient demographics (age, gender), educational status and employment status, family history for migraine, comorbid diseases, presence of medication overuse headache (MOH) and bruxism, duration of disease, migraine triggers, commonly used analgesics (acute migraine treatment) and previous migraine treatments were recorded at baseline. Migraine outcome, headache characteristics, accompanying symptoms including interictal (between attacks) and ictal (during an attack) photophobia (scored 1: none to 5: extreme disturbance), ictal phonophobia, and ictal osmophobia (yes and no), and PROMs were evaluated at baseline and 12—weeks post-treatment.
Migraine outcome was assessed based on MHDs, MMDs, headache intensity via a numeric rating scale (NRS) (graded 0: no pain to 10: the worst pain imaginable), and the days of analgesics (migraine-specific and non-specific) used in a month. PROMs included Migraine Disability Assessment Scale (MIDAS), Headache Impact Test-6 (HIT-6) scores, 12-item Allodynia Symptom Checklist (ASC-12), Beck Anxiety Inventory (BAI), and Beck Depression Inventory (BDI).
The treatment response to onabotulinumtoxinA was assessed based on the response rate (percentage reduction in monthly headache days), while safety outcomes, including treatment-related adverse events (TRAEs), were evaluated 12 weeks after treatment. Headache relief within 2 h was evaluated using a 5-point Likert scale to assess the response to acute treatment (ranging from 1, indicating never, to 5, indicating always).
MIDAS
The MIDAS is a self-administered 5-item questionnaire used to quantitatively evaluate headache-related disability regarding the number of days in the past 3 months and activity limitations due to migraine. The final total score corresponds to the sum of missed days for the three activities. It is categorized depending on the severity of attacks as little or no disability (scores 0–5), mild disability (scores 6–10), moderate disability (scores 11 to 20) or severe disability (scores ≥ 21) (39, 40).
HIT-6
HIT-6 is a 6-item questionnaire with domains on pain, social functioning, role functioning, vitality, cognitive functioning, and psychological distress. Each item is answered on a 5-point Likert scale (6 = never, 8 = rarely, 10 = sometimes, 11 = very often, 13 = always). The total score ranges between 36 and 78, with higher scores reflecting more significant impact, as categorized into four groups including little or no impact (scores ≤ 49), some impact (scores 50–55), substantial impact (scores 56–59) and, severe impact (scores ≥ 60) (41, 42).
ASC-12
ASC-12 was used to assess allodynia on 12 items during headache; each scored as 0 (not apply to me, never, rarely), 1 (less than half of the time), or 2 (half of the time or more). The total score indicated the allodynia range, as categorized into none (scores 0–2), mild (scores 3–5), moderate (scores 6–8), and severe (scores ≥ 9) (43, 44).
BDI
BDI is a 21-item self-reporting questionnaire for the assessment of the level and changes in the severity of depression over the past 2 weeks based on physical, emotional, cognitive, and motivational symptoms. Each item is scored on a 4-point scale from 0 (no symptom) to 3 (severe symptoms), and the total score achieved by adding the highest ratings for all 21 items ranges from 0 to 63, with higher scores indicating greater symptom severity. Based on the total score, individuals are categorized as severe (scores 30–63), moderate (scores 19–29), mild depression (scores 10–18), and none/minimal depression (scores 0–9) (45, 46).
BAI
This 21-item scale is a self-report measure of anxiety. Each item is scored from 0 (not at all) to 3 (severely—it bothered me a lot), and the total score is calculated by finding the sum of the 21 items and classified as low (scores 0–21), moderate (scores 22–35) and severe anxiety (scores ≥ 36) (47, 48).
OnabotulinumtoxinA injection protocol
Administration of onabotulinumtoxinA was performed as 31 fixed-site, fixed-dose intramuscular injections applied at seven specified head and neck muscle points at baseline and after 12 weeks (2 onabotulinumtoxinA sessions) according to the injection scheme proposed in the PREEMPT studies (49, 50). Additional injection sites involved occipitalis, temporalis, or trapezius muscles using a follow-the-pain strategy. During the 12-week interval, patients were asked to keep a headache diary.
Patients experiencing symptoms of bruxism, including jaw discomfort, nighttime clenching noted by partners, clenching during daytime, and morning headaches without migraine characteristics, were administered onabotulinumtoxinA doses ranging from 10 to 30 IU per masseter muscle. A previous diagnosis by a dentist, physical indications, including hypertrophy of the masseter muscles, linea alba on the cheek mucosa, or signs of pressure on the tongue, were considered supportive markers of bruxism. At the second visit, any reduction in bruxism symptoms was evaluated, and patients who reported improvement received additional injections to the masseter muscles, while those reporting no change did not undergo further treatment.
Treatment response categories
Patients with ≥50% reduction of MHDs from baseline were considered responders, while non-responders were those with <50% reduction of MHDs from baseline. Patients with ≥75% reduction of MHDs from baseline were considered super-responders.
Statistical analysis
Statistical analysis was conducted using MedCalc® Statistical Software version 19.7.2 (MedCalc Software Ltd., Ostend, Belgium; https://www.medcalc.org; 2021). The normality of continuous variables was assessed via Shapiro–Wilk’s test. Descriptive statistics included mean, standard deviation, median, and interquartile range for continuous variables and frequencies (n) and percentages (%) for categorical variables.
For non-normally distributed independent continuous data involving more than two groups, the Kruskal-Wallis Test was employed. For non-normally distributed dependent continuous data comparing two groups, the Wilcoxon Signed Rank Test was used. The Mann–Whitney U Test was applied when comparing two groups with non-normally distributed independent continuous data. A significance level of p < 0.005 was set.
Results
Baseline characteristics and migraine history
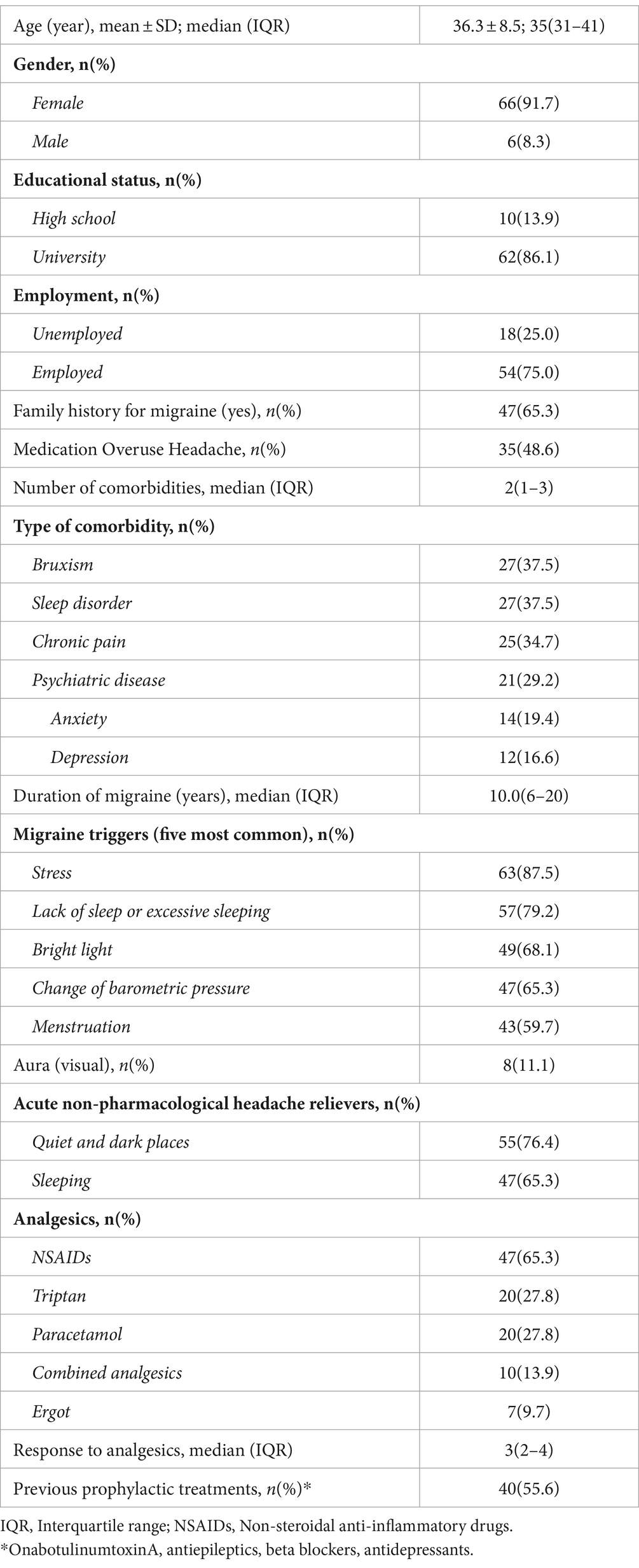
Seventy-four patients out of 106 completed both sessions of onabotulinumtoxinA treatment, while 2 patients were excluded due to insufficient documentation. The mean ± SD age of the 72 patients included was 36.3 ± 8.5 years, with 91.7% of them being female. Most of the patients were university graduates (86.1%), employed (75.0%), and had a migraine family history (65.3%). Thirty-five patients (48.6%) had MOH. Comorbidities included bruxism in 37.5% of patients (n = 27), sleep disorder in 37.5% (n = 27), chronic pain in 34.7% (n = 25), and psychiatric disease in 29.2% (n = 21; anxiety in 19.4% and depression in 16.6%; Table 1).
Stress (87.5%), lack of sleep/excessive sleeping (79.2%), and bright lights (68.1%) were the most commonly reported migraine triggers, while quiet and dark places (76.4%) and sleeping (65.3%) were the most common acute non-pharmacological -headache relievers (Table 1).
Non-steroidal anti-inflammatory drugs (NSAIDs, 65.3%) were the most commonly used analgesics, while the response to acute treatment was noted as median (IQR) 3(2–4). The percentage of patients with prior migraine prophylaxis experience was 55.6% (Table 1).
Migraine outcome
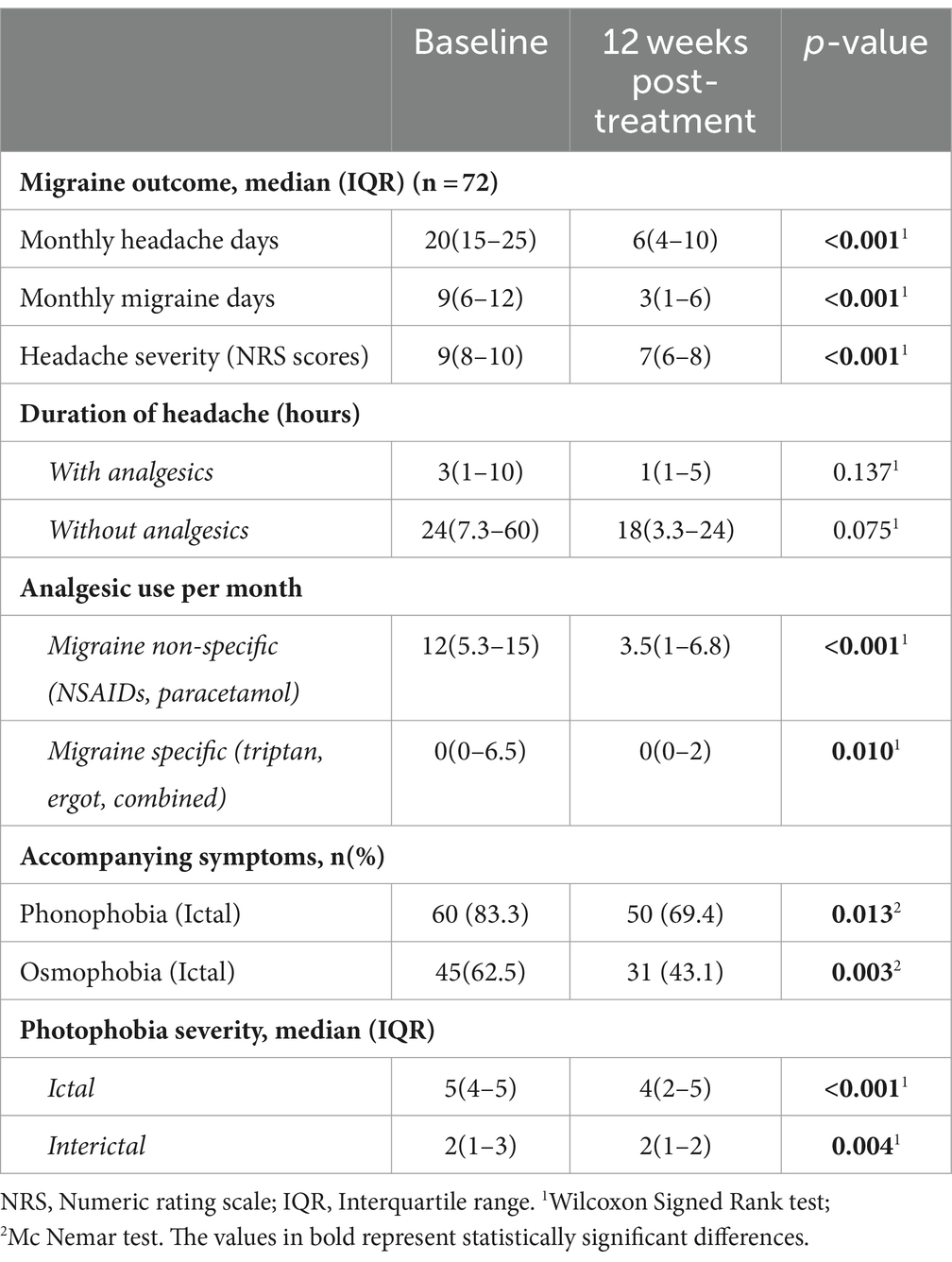
OnabotulinumtoxinA revealed a significant decrease in median (IQR) MHDs (from 20(15–25) at baseline to 6(4–10), p < 0.001), MMDs (from 9(6–12) to 3(1–6), p < 0.001), headache severity NRS scores (from 9(8–10) to 7(6–8), p < 0.001) and migraine-specific (from 0(0–6.5) to 0(0–2), p = 0.010) and migraine non-specific (from 12(5.3–15) to 3.5(1–6.8), p < 0.001) analgesic use, at 12 weeks post-treatment (Table 2; Figure 2). Additionally, the number of patients overusing medications decreased from n = 35 (47.3%) to n = 11 (14.9%).

Figure 2. Migraine outcome in terms of monthly headache days, monthly migraine days, headache severity and monthly days of analgesic use after single-session onabotulinumtoxinA (at baseline vs. 12 weeks post-treatment).
Overall, 70.8% were considered responders (≥50% reduction of MHDs), and 29.2% of patients were non-responders (<50% reduction of MHDs). Also, 34.7% were super-responders (>75% reduction in MHDs).
Accompanying features
A substantial decrease was noted in the rate of ictal phonophobia (from 83.3% to 69.4%, p = 0.013) and osmophobia (from 62.5% to 43.1%, p = 0.003) as well as in the severity of both ictal (from 5(4–5) to 4(2–5), p < 0.001) and interictal (from 2(1–3) to 2(1–2), p = 0.004) photophobia at 12 weeks post-treatment (Table 2).
Patient-reported outcome measures
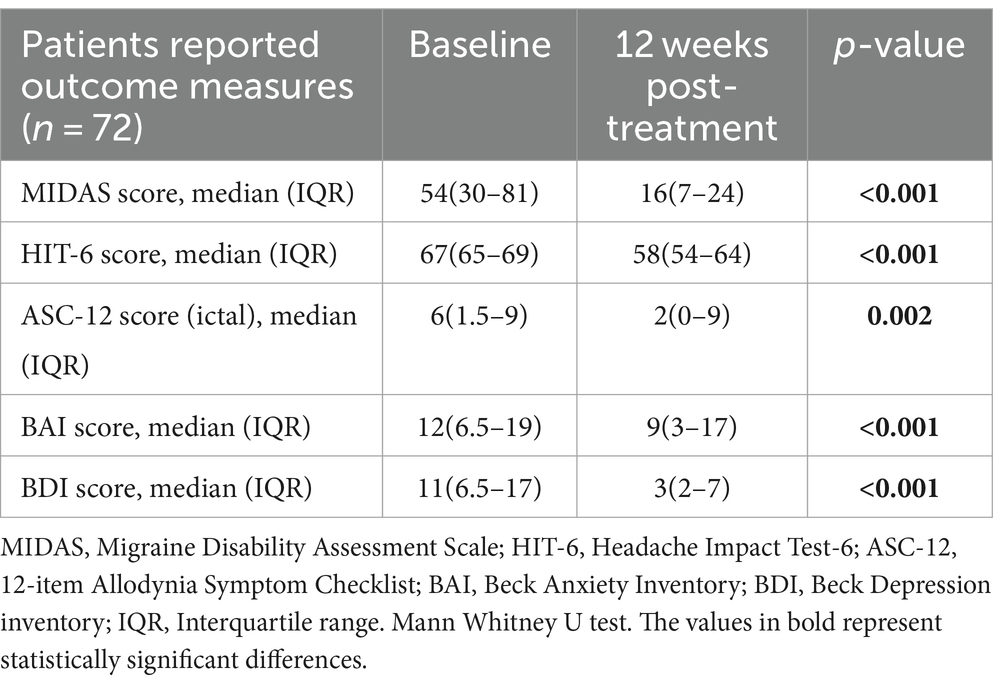
The median(IQR) MIDAS scores (from 54(30–81) at baseline to 16(7–24) at 12 weeks, p < 0.001), HIT-6 scores (from 67(65–69) to 58(54–64), p < 0.001) and ASC-12 scores (from 6(1.5–9) to 2(0–9), p = 0.002) were significantly improved at 12 weeks post-treatment when compared to baseline scores (Table 3; Figure 3).
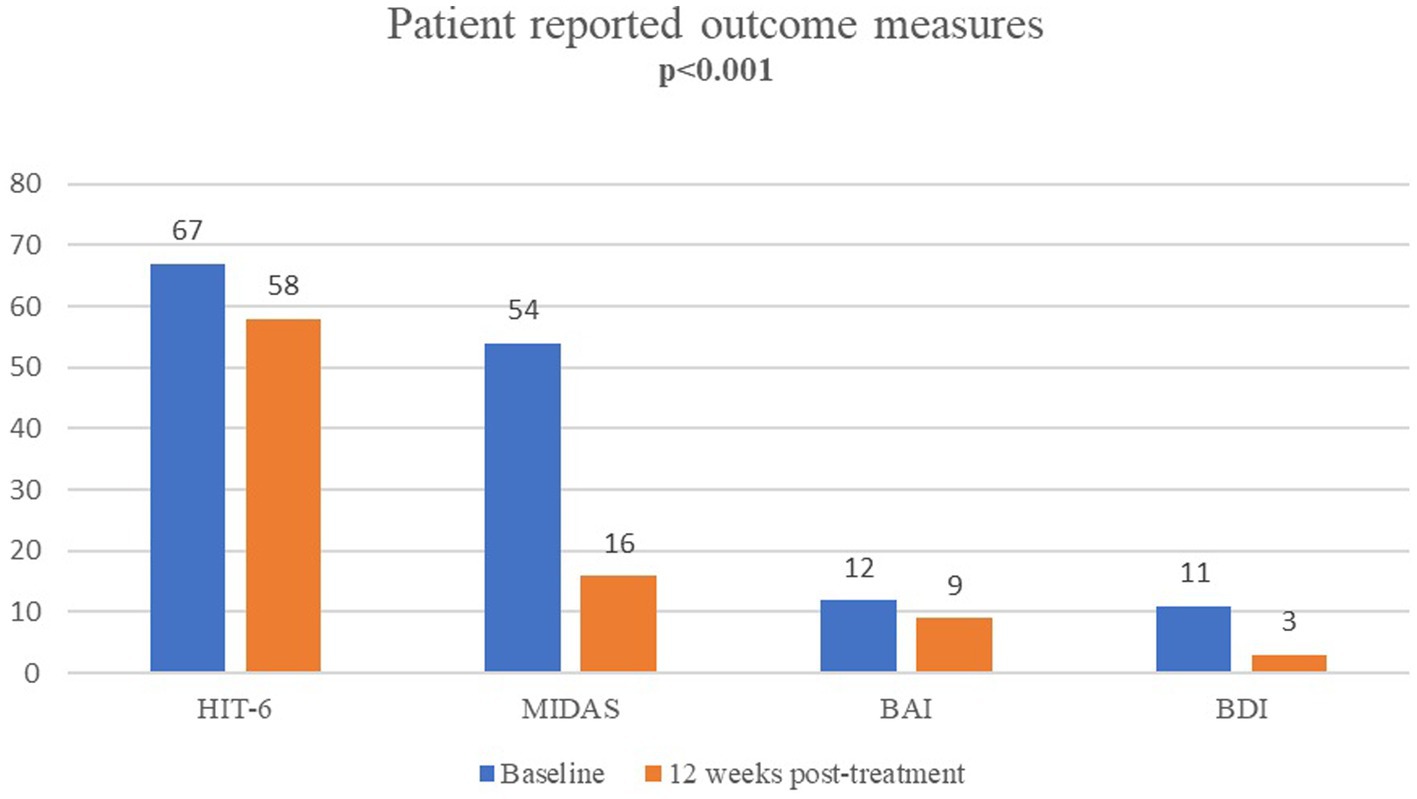
Figure 3. Patient-reported outcomes for migraine disability (MIDAS), impact (HIT-6), anxiety (BAI) and depression (BDI) after single-session onabotulinumtoxinA (at baseline vs. 12 weeks post-treatment).
Median (IQR) BAI (from 12(6.5–19) to 9(3–17) p < 0.001) and BDI (from 11(6.5–17) to 3(2–7) p < 0.001) scores were significantly improved at 12 weeks post-treatment (Table 3; Figure 3).
Masseter muscle injection and re-injection rate
At the initial visit, onabotulinumtoxinA was administered to 27(37.5%) patients with bruxism symptoms or findings, with a median dose of 30 IU. Of these patients, 19 (70.4%) who reported symptom improvement following the initial application received a re-injection during the second visit, with a mean dose of 40 IU.
Baseline characteristics between responders and non-responders
The responder and non-responder groups were homogenous in terms of patient demographics, migraine history, headache characteristics, and accompanying symptoms, MOH, baseline scores for PROMs (MIDAS, HIT-6, ASC-12, BAI, and BDI), and the number of comorbidities and number of previous treatments (Table 4).

Table 4. Baseline characteristics in responder (≥50% reduction in MHDs) and non-responder (<50% reduction in MHDs) groups.
No notable distinctions were observed in the baseline BAI and BDI scores, and no alterations from baseline for MIDAS, HIT-6, and ASC-12 among individuals with a prior treatment history (Table 5).
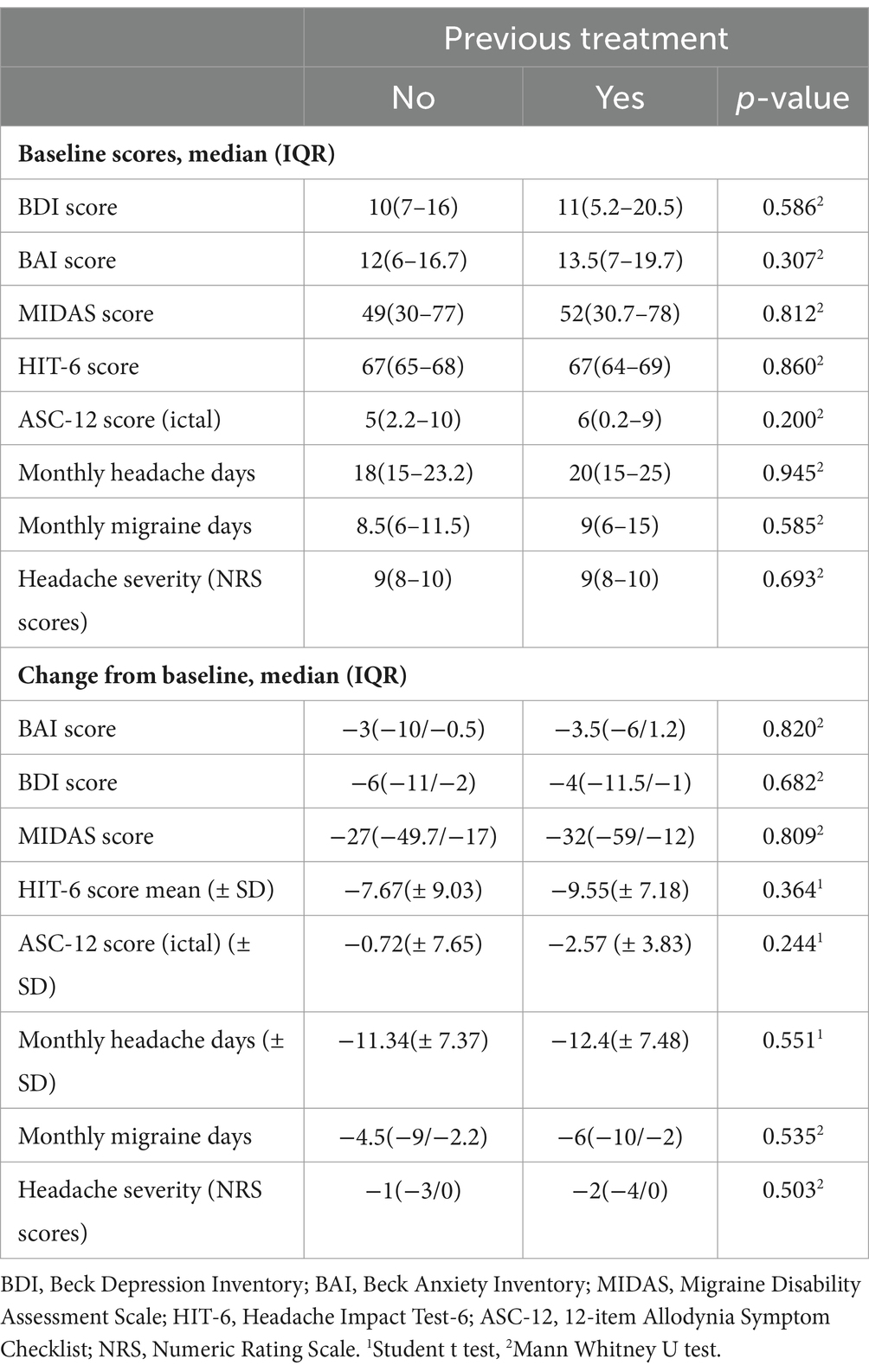
Table 5. Comparison of patient-reported outcome measures between previously treated and untreated patients.
Safety outcome
The TRAEs included headache in 9(12.5%) patients and pain at the injection site in 5(6.9%) patients, followed by neck pain, back pain, ptosis, and neck weakness (each in 1[1.4%] patient).
Discussion
This real-world observational study in CM indicated the association of single-session onabotulinumtoxinA treatment with an improved migraine outcome (MHDs, MMDs, and analgesic use) and reduced migraine disability and impact. Furthermore, improvements were noted in headache intensity and the alleviation of associated symptoms, including allodynia, photophobia, osmophobia, and phonophobia, alongside a reduction in comorbid anxiety and depression.
Various pathways likely mediate the beneficial effects of onabotulinumtoxinA. It inhibits the release of pro-inflammatory and excitatory neurotransmitters such as substance P and CGRP from c-fibers endings, reducing peripheral sensitization. Additionally, it decreases the insertion of pain-sensitive ion channels into synaptic membranes, lowering sensory neuron excitability and inhibiting central sensitization involved in migraine pathophysiology (51–53).
The pivotal PREEMPT (Phase 3 Research Evaluating Migraine Prophylaxis Therapy) randomized controlled trials (RCTs) confirmed the efficacy of onabotulinumtoxinA in reducing MHDs and monthly migraine days (MMDs) with favorable safety and tolerability in CM patients (49, 50, 54, 55). A systematic review of real-world studies and RCTs related to the use of onabotulinumtoxinA in CM patients (26), found that the 28-day post-treatment change in MHDs (range, −7.4 to −14.7) (56–62) and MMDs (range, −9.4 to 11.9) (60, 61) aligned with the data from PREEMPT trials (−8.2 and −8.4, respectively) (49, 54, 55). Similarly, a meta-analysis of 44 real-world studies (25) indicated that outcomes at approximately 24 weeks and 52 weeks were broadly consistent with PREEMPT trials (54, 55) in terms of reducing MHDs, days of analgesics intake per month, and HIT-6 score (25).
Hence, our findings support the previous studies in the real-life setting confirming the benefits of onabotulinumtoxinA demonstrated in the RCTs and open-label studies of onabotulinumtoxinA, which involved several clinical parameters (i.e., monthly days of headache, acute medication intake, pain intensity, and migraine-related disability) (25, 26, 49, 50, 56–63). The changes in median MHDs (from 20 to 6), MMDs (from 9 to 3), headache intensity (from 9 to 7), MIDAS scores (from 54.0 to 16.0) and HIT-6 scores (from 67 to 58) at 12 weeks post-treatment in our cohort are in line with the previous onabotulinumtoxinA studies, while the onabotulinumtoxinA response rate was 70.8% (34.7% were super-responders) indicating the real-world effectiveness of onabotulinumtoxinA starting from the first session.
The reduction in median MIDAS scores and HIT-6 scores from baseline to 12 weeks post-treatment in our patients highlights the association of a single-session onabotulinumtoxinA treatment with significant enhancement of migraine disability (from severe to moderate disability status) and impact (from severe to substantial impact status). When combined with improvements in accompanying symptoms like photophobia, phonophobia, and osmophobia, onabotulinumtoxinA emerges as a comprehensive agent in migraine prophylaxis. Similarly, studies noted significant improvements in the HIT-6 and the MIDAS scores after 2 to 4 sessions of onabotulinumtoxinA (64, 65).
Nearly 30% of our patients were experiencing comorbid psychiatric disorders, with 19.4% having anxiety and 16.6% having depression. This aligns with the known association of CM with depression (up to 47%) and anxiety (up to 58%), and a fivefold increased risk of developing major depression compared to the general population (26, 66, 67). The prompt onset of improvement in our cohort’s BDI, BAI, and ASC-12 scores is crucial, given that depression, anxiety, and allodynia are suggested risk factors for migraine chronification, reduced treatment response, diminished quality of life, and heightened overall disease burden (8, 58, 68–70). Notably, findings from the real-world COMPEL study revealed that CM patients receiving 2 years of onabotulinumtoxinA experienced enhanced depressive symptoms even in the non-responders without a satisfactory reduction in headache days, emphasizing the likelihood of the direct effect of onabotulinumtoxinA on depression and anxiety (26, 71, 72).
Numerous studies investigate predictive markers for the effectiveness of onabotulinumtoxinA in chronic migraine patients. The presence of pericranial muscle tenderness was stated as a predictor for a higher probability for a good response to onabotulinumtoxinA in CM (33, 73). There are conflicting views on whether a good response to triptans could predict future outcomes. Some argue in favor of this notion (74), while others oppose it (75). While alleviation of CM after onabotulinumtoxinA decreases over 30 years of disease duration (35, 76), treatment within the first year after the diagnosis of CM may increase the chance for a better response (76).
In our cohort, both responders (70.8%) and non-responders (29.2%) exhibited similar baseline headache characteristics, comorbid diseases, days of analgesic use per month, migraine duration in years, and scores on patient-reported outcome measures (PROMs) like MIDAS, HIT-6, BDI, and BAI. Despite over half of the patients having received prior migraine prophylaxis there was no significant difference observed between previously treated and treatment-naive patients or between responders and non-responders to previous treatments and abortive medication in terms of MHDs and MMDs following onabotulinumtoxinA injection. In a study of 212 patients with CM and high-frequency episodic migraine receiving onabotulinumtoxinA no anamnestic characteristics differentiated responders from non-responders in the CM group (77). Similarly, a study by Pagola et al. failed to identify any clinical feature in patients with refractory migraine that predicts a favorable response to onabotulinumtoxinA treatment (78).
While onabotulinumtoxinA is usually initiated after the failure of at least three prior prophylactic agents in CM patients, by the current guideline recommendations (79–81), its efficacy is considered to be more significant when administered earlier in the course of CM (82, 83). Besides, a negative correlation was noted between the reduction in pain intensity and the number of previous drug treatments before the onset of onabotulinumtoxinA (82, 83). OnabotulinumtoxinA is widely regarded as an effective treatment for different types of migraine, including CM in patients with prior treatment failures, MOH, and comorbid depression and/or anxiety. It could be considered a first-line therapy for CM (26, 84). In our cohort, the one-third reduction in MOH rates at the second visit underscores the efficacy of onabotulinumtoxinA as a treatment option for this headache type, emphasizing the importance of early recognition of migraine chronification for timely initiation of effective prophylactic therapy and potentially better outcomes (85–87).
In our study, each patient was asked whether they experienced symptoms of teeth grinding and if they felt any discomfort associated with it. Additionally, a short examination included checking for linea alba at the buccal mucosa, masseter muscle hypertrophy and indentations along the tongue due to chronic repeated pressure. The first session of onabotulinumtoxinA injection was applied to the masseter muscles in 27 patients (37.5%). A majority (70.4%) of these gave positive feedback regarding decreased bruxism symptoms after 12 weeks, prompting a second injection of onabotulinumtoxinA. Despite significantly higher baseline BAI scores in patients with comorbid bruxism, the effectiveness of onabotulinumtoxinA on migraine outcomes was similar in patients with and without bruxism. In a double-blind, placebo-controlled study, participants experienced the largest reduction in the bruxism index at 4 weeks post-injection of onabotuliunmtoxinA compared with placebo, especially when injections were administered together to the temporal (each 15 IU) and masseter (each 30 IU) muscles. Furthermore, near our findings, 77% of participants asked for reinjection after 12 weeks (88). A meta-analysis indicated that injecting onabotuliunmtoxinA into the masseter, temporalis, and pterygoid muscles led to greater pain reduction than targeting only the masseter and temporalis muscles, after 6 months (89). A study demonstrated that onabotulinumtoxinA injections effectively lowered pain scores in the masseter muscles compared to conventional bruxism treatments (90), establishing it as a safe treatment option for bruxism with potential to prevent dental complications (91–93). OnabotulinumtoxinA demonstrated a positive impact on sleep quality in CM patients who did not exhibit negative emotional states (94). Employing masseter muscle onabotulinumtoxinA injections in patients with comorbid bruxism could offer additional value by alleviating bruxism as comorbidity alongside chronic migraine (93, 95, 96).
The safety profile and tolerability of onabotulinumtoxinA are considered excellent with rare, mild, and self-limiting TRAEs that rapidly and spontaneously resolve without complications (26, 97). Consistent with our findings, in a meta-analysis of RCTs on the safety of onabotulinumtoxinA, neck pain, musculoskeletal pain, muscular weakness, migraine, eyelid ptosis, blurred vision, and injection site pain were found to be the most common TRAEs, which were also mild-to-moderate in severity and resolved without sequelae (97).
Certain limitations to this study should be considered. First, despite providing data on real-life clinical practice, the potential lack of generalizability is an important limitation due to the single-center retrospective design of the study. Second, our findings provide data on single-session onabotulinumtoxinA therapy with likely changes in outcome measures and response rates in the consequent sessions.
Third, while polysomnography is the gold standard for diagnosing sleep bruxism, the diagnosis is often based on medical or dental history (98). Furthermore, the absence of data regarding the efficacy of masseter injections in treating bruxism within our cohort is an additional limitation. Also, enhancing our methodology by including the assessment of phonophobia and osmophobia during the interictal period could have provided further valuable information about the interictal burden in migraine in our cohort.
In conclusion, this real-world study revealed that onabotulinumtoxinA therapy was an effective treatment option with favorable safety profile in patients with CM, enabling rapid-onset improvements in migraine outcome (MHDs, MMDs, headache intensity, and analgesic use), migraine disability and impact, regardless of previous migraine prophylaxis history. Besides, the benefits of onabotulinumtoxinA were not limited to migraine outcomes but also involved a decrease in accompanying symptoms and the amelioration of comorbid depression and/or anxiety, allodynia, and possibly bruxism. Further real-world studies with longer follow-up in migraineurs, particularly in those with comorbidities, are needed to understand better the extent, durability, and predictors of response to onabotulinumtoxinA and to optimize its positioning within the current migraine prophylaxis practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Research Ethics Committee of Acıbadem University (Approval number: 2023-21/726). Written informed consent from the patients/participants or patients/participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
EI: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. TE: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. PY: Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Puledda, F, Silva, EM, Suwanlaong, K, and Goadsby, PJ. Migraine: from pathophysiology to treatment. J Neurol. (2023) 270:3654–66. doi: 10.1007/s00415-023-11706-1
2. Steiner, TJ, Stovner, LJ, Vos, T, Jensen, R, and Katsarava, Z. Migraine is first cause of disability in under 50s: will health politicians now take notice? J Headache Pain. (2018) 19:17. doi: 10.1186/s10194-018-0846-2
3. Stovner, LJ, Hagen, K, Linde, M, and Steiner, TJ. The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain. (2022) 23:34. doi: 10.1186/s10194-022-01402-2
4. Lipton, RB, and Silberstein, SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. (2015) 55:103–22. doi: 10.1111/head.12505_2
5. Torres-Ferrús, M, Quintana, M, Fernandez-Morales, J, Alvarez-Sabin, J, and Pozo-Rosich, P. When does chronic migraine strike? A clinical comparison of migraine according to the headache days suffered per month. Cephalalgia. (2017) 37:104–13. doi: 10.1177/0333102416636055
6. Irimia, P, Carmona-Abellán, M, and Martínez-Vila, E. Chronic migraine: a therapeutic challenge for clinicians. Expert Opin Emerg Drugs. (2012) 17:445–7. doi: 10.1517/14728214.2012.726612
7. Ford, JH, Jackson, J, Milligan, G, Cotton, S, Ahl, J, and Aurora, SK. A real-world analysis of migraine: a cross-sectional study of disease burden and treatment patterns. Headache. (2017) 57:1532–44. doi: 10.1111/head.13202
8. Bigal, ME, and Lipton, RB. Migraine chronification. Curr Neurol Neurosci Rep. (2011) 11:139–48. doi: 10.1007/s11910-010-0175-6
9. Buse, DC, Manack, A, Serrano, D, Turkel, C, and Lipton, RB. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry. (2010) 81:428–32. doi: 10.1136/jnnp.2009.192492
10. Giannini, G, Cevoli, S, Sambati, L, and Cortelli, P. Migraine: risk factor and comorbidity. Neurol Sci. (2012) 33:37–41. doi: 10.1007/s10072-012-1029-6
11. Chu, HT, Liang, CS, Lee, JT, Yeh, TC, Lee, MS, Sung, YF, et al. Associations between depression/anxiety and headache frequency in Migraineurs: a cross-sectional study. Headache. (2018) 58:407–15. doi: 10.1111/head.13215
12. Alwhaibi, M, and Alhawassi, TM. Humanistic and economic burden of depression and anxiety among adults with migraine: a systematic review. Depress Anxiety. (2020) 37:1146–59. doi: 10.1002/da.23063
13. Vieira, KRM, Folchini, CM, Heyde, MDVD, Stuginski-Barbosa, J, Kowacs, PA, and Piovesan, EJ. Wake-up headache is associated with sleep bruxism. Headache. (2020) 60:974–80. doi: 10.1111/head.13816
14. Godk, KS, Dos, SML, Utiumi, MAT, Küster, JGB, Canalli Filho, LC, Kotsifas, NJE, et al. Association between sleep and awake bruxism in patients with migraine. Headache Med. (2021) 12:35–43. doi: 10.48208/HeadacheMed.2021.7
15. Ashina, M, Buse, DC, Ashina, H, Pozo-Rosich, P, Peres, MFP, Lee, MJ, et al. Migraine: integrated approaches to clinical management and emerging treatments. Lancet. (2021) 397:1505–18. doi: 10.1016/S0140-6736(20)32342-4
16. Lee, MJ, Al-Karagholi, MA, and Reuter, U. New migraine prophylactic drugs: current evidence and practical suggestions for non-responders to prior therapy. Cephalalgia. (2023) 43:3331024221146315. doi: 10.1177/03331024221146315
17. Bigal, ME, Serrano, D, Reed, M, and Lipton, RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. (2008) 71:559–66. doi: 10.1212/01.wnl.0000323925.29520.e7
18. Ziplow, J. The importance of studying comorbidities in migraine. Headache. (2021) 61:697. doi: 10.1111/head.14113
19. Blumenfeld, AM, Bloudek, LM, Becker, WJ, Buse, DC, Varon, SF, Maglinte, GA, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. (2013) 53:644–55. doi: 10.1111/head.12055
20. Vécsei, L, Majláth, Z, Szok, D, Csáti, A, and Tajti, J. Drug safety and tolerability in prophylactic migraine treatment. Expert Opin Drug Saf. (2015) 14:667–81. doi: 10.1517/14740338.2015.1014797
21. Berger, A, Bloudek, LM, Varon, SF, and Oster, G. Adherence with migraine prophylaxis in clinical practice. Pain Pract. (2012) 12:541–9. doi: 10.1111/j.1533-2500.2012.00530.x
22. Delussi, M, Vecchio, E, Libro, G, Quitadamo, S, and de Tommaso, M. Failure of preventive treatments in migraine: an observational retrospective study in a tertiary headache center. BMC Neurol. (2020) 20:256. doi: 10.1186/s12883-020-01839-5
23. Ailani, J, Winner, P, Hartry, A, Brevig, T, Bøg, M, Lassen, AB, et al. Patient preference for early onset of efficacy of preventive migraine treatments. Headache. (2022) 62:374–82. doi: 10.1111/head.14255
24. Dodick, DW, Turkel, CC, DeGryse, RE, Diener, HC, Lipton, RB, Aurora, SK, et al. Assessing clinically meaningful treatment effects in controlled trials: chronic migraine as an example. J Pain. (2015) 16:164–75. doi: 10.1016/j.jpain.2014.11.004
25. Lanteri-Minet, M, Ducros, A, Francois, C, Olewinska, E, Nikodem, M, and Dupont-Benjamin, L. Effectiveness of onabotulinumtoxinA (BOTOX®) for the preventive treatment of chronic migraine: a meta-analysis on 10 years of real-world data. Cephalalgia. (2022) 42:1543–64. doi: 10.1177/03331024221123058
26. Domitrz, I, Sławek, J, Słowik, A, Boczarska-Jedynak, M, Stępień, A, Rejdak, K, et al. Onabotulinumtoxin a (ONA-BoNT/a) in the treatment of chronic migraine. Neurol Neurochir Pol. (2022) 56:39–47. doi: 10.5603/PJNNS.a2021.0061
27. The American Headache Society. The American headache society position statement on integrating new migraine treatments into clinical practice. Headache. (2019) 59:1–18. doi: 10.1111/head.13456
28. Vernieri, F, Paolucci, M, Altamura, C, Pasqualetti, P, Mastrangelo, V, Pierangeli, G, et al. Onabotulinumtoxin-a in chronic migraine: should timing and definition of non-responder status be revised? Suggestions from a real-life Italian multicenter experience. Headache. (2019) 59:1300–9. doi: 10.1111/head.13617
29. Altamura, C, Cevoli, S, Brunelli, N, Aurilia, C, Fofi, L, Egeo, G, et al. GARLIT study group collaborators. When should we consider chronic patients as non-responders to monoclonal antibodies targeting the CGRP pathway? J Neurol. (2022) 269:1032–4. doi: 10.1007/s00415-021-10772-7
30. Altamura, C, Brunelli, N, Viticchi, G, Salvemini, S, Cecchi, G, Marcosano, M, et al. Quantitative and qualitative pain evaluation in response to OnabotulinumtoxinA for chronic migraine: an observational real-life study. Toxins. (2023) 15:284. doi: 10.3390/toxins15040284
31. Domínguez, C, Pozo-Rosich, P, Torres-Ferrús, M, Hernández-Beltrán, N, Jurado-Cobo, C, González-Oria, C, et al. OnabotulinumtoxinA in chronic migraine: predictors of response. A prospective multicentre descriptive study. Eur J Neurol. (2018) 25:411–6. doi: 10.1111/ene.13523
32. Lin, KH, Chen, SP, Fuh, JL, Wang, YF, and Wang, SJ. Efficacy, safety, and predictors of response to botulinum toxin type a in refractory chronic migraine: a retrospective study. J Chin Med Assoc. (2014) 77:10–5. doi: 10.1016/j.jcma.2013.09.006
33. Mathew, NT, Kailasam, J, and Meadors, L. Predictors of response to botulinum toxin type a (BoNTA) in chronic daily headache. Headache. (2008) 48:194–200. doi: 10.1111/j.1526-4610.2007.00914.x
34. Jakubowski, M, McAllister, PJ, Bajwa, ZH, Ward, TN, Smith, P, and Burstein, R. Exploding vs. imploding headache in migraine prophylaxis with botulinum toxin a. Pain. (2006) 125:286–95. doi: 10.1016/j.pain.2006.09.012
35. Alpuente, A, Gallardo, VJ, Torres-Ferrús, M, Álvarez-Sabin, J, and Pozo-Rosich, P. Short and mid-term predictors of response to OnabotulinumtoxinA: real-life experience observational study. Headache. (2020) 60:677–85. doi: 10.1111/head.13765
36. Cernuda-Morollón, E, Martínez-Camblor, P, Ramón, C, Larrosa, D, Serrano-Pertierra, E, and Pascual, J. CGRP, and VIP levels as predictors of efficacy of on botulinum toxin type a in chronic migraine. Headache. (2014) 54:987–95. doi: 10.1111/head.12372
37. Domínguez, C, Vieites-Prado, A, Pérez-Mato, M, Sobrino, T, Rodríguez-Osorio, X, López, A, et al. CGRP and PTX3 as predictors of efficacy of Onabotulinumtoxin type a in chronic migraine: an observational study. Headache. (2018) 58:78–87. doi: 10.1111/head.13211
38. Headache classification Committee of the International Headache Society (IHS) The international classification of headache disorders, 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
39. Stewart, WF, Lipton, RB, Dowson, AJ, and Sawyer, J. Development and testing of the migraine disability assessment (MIDAS) questionnaire to assess headache-related disability. Neurology. (2001) 56:S20–8. doi: 10.1212/wnl.56.suppl_1.s20
40. Ertaş, M, Siva, A, Dalkara, T, Uzuner, N, Dora, B, İnan, L, et al. Validity and reliability of the Turkish migraine disability assessment (MIDAS) questionnaire. Headache. (2004) 44:786–93. doi: 10.1111/j.1526-4610.2004.04146.x
41. Bayliss, MS, Dewey, JE, Dunlap, I, Batenhorst, AS, Cady, R, Diamond, ML, et al. A study of the feasibility of internet administration of a computerized health survey: the headache impact test (HIT). Qual Life Res. (2003) 12:953–61. doi: 10.1023/a:1026167214355
42. Dikmen, PY, Bozdağ, M, Güneş, M, Koşak, S, Taşdelen, B, Uluduz, D, et al. Reliability and validity of Turkish version of headache impact test (HIT-6) in patients with migraine. Noro Psikiyatr Ars. (2020) 58:300–7. doi: 10.29399/npa.24956
43. Jakubowski, M, Silberstein, S, Ashkenazi, A, and Burstein, R. Can allodynic migraine patients be identified interictally using a questionnaire? Neurology. (2005) 65:1419–22. doi: 10.1212/01.wnl.0000183358.53939.38
44. Yalin, OÖ, Uludüz, D, Sungur, MA, Sart, H, and Özge, A. Identification of Allodynic migraine patients with the Turkish version of the allodynia symptom checklist: reliability and consistency study. Noro Psikiyatr Ars. (2017) 54:260–6. doi: 10.5152/npa.2016.15953
45. Beck, AT, Ward, CH, Mendelson, M, Mock, J, and Erbaugh, J. An inventory for measuring depression. Arch Gen Psychiatry. (1961) 4:561–71. doi: 10.1001/archpsyc.1961.01710120031004
46. Hisli, N. Reliability and validity of Beck depression inventory among university students. Psikoloji Dergisi. (1989) 7:3–19.
47. Beck, AT, Epstein, N, Brown, G, and Steer, RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. (1988) 56:893–7. doi: 10.1037/0022-006X.56.6.893
48. Ulusoy, M, Sahin, NH, and Erkmen, H. Turkish version of the Beck anxiety inventory: psychometric properties. J Cogn Psychother. (1998) 12:163.
49. Aurora, SK, Dodick, DW, Turkel, CC, DeGryse, RE, Silberstein, SD, Lipton, RB, et al. PREEMPT 1 chronic migraine study group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. (2010) 30:793–803. doi: 10.1177/0333102410364676
50. Aurora, SK, Dodick, DW, Diener, HC, DeGryse, RE, Turkel, CC, Lipton, RB, et al. OnabotulinumtoxinA for chronic migraine: efficacy, safety, and tolerability in patients who received all five treatment cycles in the PREEMPT clinical program. Acta Neurol Scand. (2014) 129:61–70. doi: 10.1111/ane.12171
51. Burstein, R, Blumenfeld, AM, Silberstein, SD, Manack Adams, A, and Brin, MF. Mechanism of action of OnabotulinumtoxinA in chronic migraine: a narrative review. Headache. (2020) 60:1259–72. doi: 10.1111/head.13849
52. Cernuda-Morollón, E, Ramón, C, Martínez-Camblor, P, Serrano-Pertierra, E, Larrosa, D, and Pascual, J. OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain. (2015) 156:820–4. doi: 10.1097/j.pain.0000000000000119
53. Meng, J, Ovsepian, SV, Wang, J, Pickering, M, Sasse, A, Aoki, KR, et al. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. (2009) 29:4981–92. doi: 10.1523/JNEUROSCI.5490-08.2009
54. Diener, HC, Dodick, DW, Aurora, SK, Turkel, CC, DeGryse, RE, Lipton, RB, et al. PREEMPT 2 chronic migraine study group. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. (2010) 30:804–14. doi: 10.1177/0333102410364677
55. Dodick, DW, Turkel, CC, DeGryse, RE, Aurora, SK, Silberstein, SD, and Lipton, RB. Et al; PREEMPT chronic migraine study group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. (2010) 50:921–36. doi: 10.1111/j.1526-4610.2010.01678.x
56. Ahmed, F, Gaul, C, García-Moncó, JC, Sommer, K, Martelletti, P, Principal, REPOSE, et al. An open-label prospective study of the real-life use of onabotulinumtoxinA for the treatment of chronic migraine: the REPOSE study. J Headache Pain. (2019):26. doi: 10.1186/s10194-019-0976-1
57. Vikelis, M, Argyriou, AA, Dermitzakis, EV, Spingos, KC, and Mitsikostas, DD. Onabotulinumtoxin-a treatment in Greek patients with chronic migraine. J Headache Pain. (2016) 17:84. doi: 10.1186/s10194-016-0676-z
58. Yalinay Dikmen, P, Kosak, S, Ilgaz Aydinlar, E, and Sagduyu, KA. A single-center retrospective study of onabotulinumtoxinA for treatment of 245 chronic migraine patients: survey results of a real-world experience. Acta Neurol Belg. (2018) 118:475–84. doi: 10.1007/s13760-018-0978-9
59. Blumenfeld, AM, Stark, RJ, Freeman, MC, Orejudos, A, and Manack, AA. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. (2018) 19:13. doi: 10.1186/s10194-018-0840-8
60. Negro, A, Curto, M, Lionetto, L, and Martelletti, P. A two years open-label prospective study of OnabotulinumtoxinA 195 U in medication overuse headache: a real-world experience. J Headache Pain. (2015) 17:1. doi: 10.1186/s10194-016-0591-3
61. Stark, C, Stark, R, Limberg, N, Rodrigues, J, Cordato, D, Schwartz, R, et al. Real-world effectiveness of onabotulinumtoxinA treatment for the prevention of headaches in adults with chronic migraine in Australia: a retrospective study. J Headache Pain. (2019) 20:81. doi: 10.1186/s10194-019-1030-z
62. Santoro, A, Copetti, M, Miscio, AM, Leone, MA, and Fontana, A. Chronic migraine long-term regular treatment with onabotulinumtoxinA: a retrospective real-life observational study up to 4 years of therapy. Neurol Sci. (2020) 41:1809–20. doi: 10.1007/s10072-020-04283-y
63. Ornello, R, Ahmed, F, Negro, A, Miscio, AM, Santoro, A, Alpuente, A, et al. Early management of OnabotulinumtoxinA treatment in chronic migraine: insights from a real-life European multicenter study. Pain Ther. (2021) 10:637–50. doi: 10.1007/s40122-021-00253-0
64. Boudreau, GP, Grosberg, BM, McAllister, PJ, Lipton, RB, and Buse, DC. Prophylactic onabotulinumtoxinA in patients with chronic migraine and comorbid depression: an open-label, multicenter, pilot study of efficacy, safety and effect on headache-related disability, depression, and anxiety. Int J Gen Med. (2015) 8:79–86. doi: 10.2147/IJGM.S70456
65. Demiryurek, BE, Ertem, DH, Tekin, A, Ceylan, M, Aras, YG, and Gungen, BD. Effects of onabotulinumtoxinA treatment on efficacy, depression, anxiety, and disability in Turkish patients with chronic migraine. Neurol Sci. (2016) 37:1779–84. doi: 10.1007/s10072-016-2665-z
66. Minen, MT, Begasse De Dhaem, O, Kroon Van Diest, A, Powers, S, Schwedt, TJ, Lipton, R, et al. Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry. (2016) 87:741–9. doi: 10.1136/jnnp-2015-312233
67. Breslau, N, Lipton, RB, Stewart, WF, Schultz, LR, and Welch, KM. Comorbidity of migraine and depression: investigating potential etiology and prognosis. Neurology. (2003) 60:1308–12. doi: 10.1212/01.wnl.0000058907.41080.54
68. Tietjen, GE, Brandes, JL, Peterlin, BL, Eloff, A, Dafer, RM, Stein, MR, et al. Childhood maltreatment and migraine (part II). Emotional abuse as a risk factor for headache chronification. Headache. (2010) 50:32–41. doi: 10.1111/j.1526-4610.2009.01557.x
69. Louter, MA, Bosker, JE, van Oosterhout, WP, van Zwet, EW, Zitman, FG, Ferrari, MD, et al. Cutaneous allodynia as a predictor of migraine chronification. Brain. (2013) 136:3489–96. doi: 10.1093/brain/awt251
70. Bigal, ME, Ashina, S, Burstein, R, Reed, ML, Buse, D, and Serrano, D. Et al; AMPP group. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. (2008) 70:1525–33. doi: 10.1212/01.wnl.0000310645.31020.b1
71. Blumenfeld, AM, Tepper, SJ, Robbins, LD, Manack Adams, A, Buse, DC, Orejudos, A, et al. Effects of onabotulinumtoxinA treatment for chronic migraine on common comorbidities including depression and anxiety. J Neurol Neurosurg Psychiatry. (2019) 90:353–60. doi: 10.1136/jnnp-2018-319290
72. Affatato, O, Moulin, TC, Pisanu, C, Babasieva, VS, Russo, M, Aydinlar, EI, et al. High efficacy of onabotulinumtoxinA treatment in patients with comorbid migraine and depression: a meta-analysis. J Transl Med. (2021) 19:133. doi: 10.1186/s12967-021-02801-w
73. Sandrini, G, Perrotta, A, Tassorelli, C, Torelli, P, Brighina, F, Sances, G, et al. Botulinum toxin type-a in the prophylactic treatment of medication-overuse headache: a multicenter, double-blind, randomized, placebo-controlled, parallel group study. J Headache Pain. (2011) 12:427–33. doi: 10.1007/s10194-011-0339-z
74. Lovati, C, Giani, L, Mariotti, D, Alessandro, C, Tabaee Damavandi, P, Mariani, C, et al. May migraine attack response to triptans be a predictor of the efficacy of Onabotulinum toxin-a prophylaxis? Neurol Sci. (2018) 39:153–4. doi: 10.1007/s10072-018-3388-0
75. Eren, OE, Gaul, C, Peikert, A, Gendolla, A, Ruscheweyh, R, and Straube, A. Triptan efficacy does not predict onabotulinumtoxinA efficacy but improves with onabotulinumtoxinA response in chronic migraine patients. Sci Rep. (2020) 10:11382. doi: 10.1038/s41598-020-68149-1
76. Eross, EJ, Gladstone, JP, Lewis, S, Rogers, R, and Dodick, DW. Duration of migraine is a predictor for response to botulinum toxin type a. Headache. (2005) 45:308–14. doi: 10.1111/j.1526-4610.2005.05067.x
77. Martinelli, D, Pocora, MM, De Icco, R, Allena, M, Vaghi, G, Sances, G, et al. Searching for the predictors of response to BoNT-A in migraine using machine learning approaches. Toxins. (2023) 15:364. doi: 10.3390/toxins15060364
78. Pagola, I, Esteve-Belloch, P, Palma, JA, Luquin, MR, Riverol, M, Martinez-Vila, E, et al. Factores predictores de respuesta al tratamiento con onabotulinumtoxina a en la migraña refractaria [predictive factors of the response to treatment with onabotulinumtoxinA in refractory migraine]. Rev Neurol. (2014) 58:241–6. doi: 10.33588/rn.5806.2013407
79. Simpson, DM, Hallett, M, Ashman, EJ, Comella, CL, Green, MW, Gronseth, GS, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the guideline development Subcommittee of the American Academy of neurology. Neurology. (2016) 86:1818–26. doi: 10.1212/WNL.0000000000002560
80. Bendtsen, L, Sacco, S, Ashina, M, Mitsikostas, D, Ahmed, F, Pozo-Rosich, P, et al. Guideline on the use of onabotulinumtoxinA in chronic migraine: a consensus statement from the European headache federation. J Headache Pain. (2018) 19:91. doi: 10.1186/s10194-018-0921-8
81. Kępczyńska, K, and Domitrz, I. Botulinum toxin-a current place in the treatment of chronic migraine and other primary headaches. Toxins. (2022) 14:619. doi: 10.3390/toxins14090619
82. Tassorelli, C, Aguggia, M, De Tommaso, M, Geppetti, P, Grazzi, L, Pini, LA, et al. Onabotulinumtoxin a for the management of chronic migraine in current clinical practice: results of a survey of sixty-three Italian headache centers. J Headache Pain. (2017) 18:66. doi: 10.1186/s10194-017-0773-7
83. Castrillo Sanz, A, Morollón Sánchez-Mateos, N, Simonet Hernández, C, Fernández Rodríguez, B, Cerdán Santacruz, D, Mendoza Rodríguez, A, et al. Experience with botulinum toxin in chronic migraine. Neurologia. (2018) 33:499–504. doi: 10.1016/j.nrl.2016.09.004
84. Shaterian, N, Shaterian, N, Ghanaatpisheh, A, Abbasi, F, Daniali, S, Jahromi, MJ, et al. Botox (OnabotulinumtoxinA) for treatment of migraine symptoms: a systematic review. Pain Res Manag. (2022) 2022:3284446. doi: 10.1155/2022/3284446
85. Ornello, R, Guerzoni, S, Baraldi, C, Evangelista, L, Frattale, I, Marini, C, et al. Sustained response to onabotulinumtoxin a in patients with chronic migraine: real-life data. J Headache Pain. (2020) 21:40. doi: 10.1186/s10194-020-01113-6
86. Agostoni, EC, Barbanti, P, Calabresi, P, Colombo, B, Cortelli, P, Frediani, F, et al. Italian chronic migraine group. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain. (2019) 2019:92. doi: 10.1186/s10194-019-1038-4
87. Tassorelli, C, Tedeschi, G, Sarchielli, P, Pini, LA, Grazzi, L, Geppetti, P, et al. Optimizing the long-term management of chronic migraine with onabotulinumtoxinA in real life. Expert Rev Neurother. (2018) 18:167–76. doi: 10.1080/14737175.2018.1419867
88. Cruse, B, Dharmadasa, T, White, E, Hollis, C, Evans, A, Sharmin, S, et al. Efficacy of botulinum toxin type a in the targeted treatment of sleep bruxism: a double-blind, randomised, placebo-controlled, cross-over study. BMJ Neurol Open. (2022) 4:e000328. doi: 10.1136/bmjno-2022-000328
89. Li, K, Tan, K, Yacovelli, A, and Bi, WG. Effect of botulinum toxin type a on muscular temporomandibular disorder: a systematic review and meta-analysis of randomized controlled trials. J Oral Rehabil. (2024) 51:886–97. doi: 10.1111/joor.13648
90. Al-Wayli, H. Treatment of chronic pain associated with nocturnal bruxism with botulinum toxin. A prospective and randomized clinical study. J Clin Exp Dent. (2017) 9:e112–7. doi: 10.4317/jced.53084
91. Long, H, Liao, Z, Wang, Y, Liao, L, and Lai, W. Efficacy of botulinum toxins on bruxism: an evidence-based review. Int Dent J. (2012) 62:1–5. doi: 10.1111/j.1875-595X.2011.00085.x
92. Tan, EK, and Jankovic, J. Treating severe bruxism with botulinum toxin. J Am Dent Assoc. (2000) 131:211–6. doi: 10.14219/jada.archive.2000.0149
93. Ondo, WG, Simmons, JH, Shahid, MH, Hashem, V, Hunter, C, and Jankovic, J. Onabotulinum toxin-a injections for sleep bruxism: a double-blind, placebo-controlled study. Neurology. (2018) 90:e559–64. doi: 10.1212/WNL.0000000000004951
94. Aydinlar, EI, Dikmen, PY, Kosak, S, and Kocaman, AS. OnabotulinumtoxinA effectiveness on chronic migraine, negative emotional states and sleep quality: a single-center prospective cohort study. J Headache Pain. (2017) 18:23. doi: 10.1186/s10194-017-0723-4
95. Fernández-Núñez, T, Amghar-Maach, S, and Gay-Escoda, C. Efficacy of botulinum toxin in the treatment of bruxism: systematic review. Med Oral Patol Oral Cir Bucal. (2019) 24:e416–24. doi: 10.4317/medoral.22923
96. Baraldi, C, Lo Castro, F, Ornello, R, Sacco, S, Pani, L, and Guerzoni, S. OnabotulinumtoxinA: still the present for chronic migraine. Toxins. (2023) 15:59. doi: 10.3390/toxins15010059
97. Corasaniti, MT, Bagetta, G, Nicotera, P, Tarsitano, A, Tonin, P, Sandrini, G, et al. Safety of Onabotulinumtoxin a in chronic migraine: a systematic review and Meta-analysis of randomized clinical trials. Toxins. (2023) 15:332. doi: 10.3390/toxins15050332
Keywords: chronic migraine, onabotulinumtoxinA, outcome, anxiety, depression, bruxism, efficacy, predictors
Citation: Ilgaz Aydinlar E, Erdogan Soyukibar T and Yalinay Dikmen P (2024) The effectiveness and predictors influencing the outcome of onabotulinumtoxinA treatment in chronic migraine: understanding from diverse patient profiles in a single session. Front. Neurol. 15:1417303. doi: 10.3389/fneur.2024.1417303
Edited by:
Sabina Cevoli, IRCCS Institute of Neurological Sciences of Bologna (ISNB), ItalyReviewed by:
Roberta Messina, Vita-Salute San Raffaele University, ItalyArife Çimen Atalar, İstanbul Kanuni Sultan Süleyman Eğitim ve Araştırma Hastanesi, Türkiye
Copyright © 2024 Ilgaz Aydinlar, Erdogan Soyukibar and Yalinay Dikmen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elif Ilgaz Aydinlar, ZWxpZi5heWRpbmxhckBhY2liYWRlbS5lZHUudHI=
 Elif Ilgaz Aydinlar
Elif Ilgaz Aydinlar Tuba Erdogan Soyukibar
Tuba Erdogan Soyukibar