- 1First Ward of Neurology Department, Hongqi Hospital Affiliated to Mudanjiang Medical University, Mudanjiang, China
- 2Department of Rehabilitation, Hongqi Hospital Affiliated to Mudanjiang Medical University, Mudanjiang, China
- 3Third Ward of Neurology Department, Hongqi Hospital Affiliated to Mudanjiang Medical University, Mudanjiang, China
- 4Second Ward of Gastroenterology Department, Hongqi Hospital Affiliated to Mudanjiang Medical University, Mudanjiang, China
This study delves into the pivotal role of the gut microbiota and the brain-gut axis in Parkinson’s Disease (PD), a neurodegenerative disorder with significant motor and non-motor implications. It posits that disruptions in gut microbiota—dysbiosis—and alterations in the brain-gut axis contribute to PD’s pathogenesis. Our findings highlight the potential of the gastrointestinal system’s early involvement in PD, suggested by the precedence of gastrointestinal symptoms before motor symptoms emerge. This observation implies a possible gut-originated disease pathway. The analysis demonstrates that dysbiosis in PD patients leads to increased intestinal permeability and systemic inflammation, which in turn exacerbates neuroinflammation and neurodegeneration. Such insights into the interaction between gut microbiota and the brain-gut axis not only elucidate PD’s underlying mechanisms but also pave the way for novel therapeutic interventions. We propose targeted treatment strategies, including dietary modifications and fecal microbiota transplantation, aimed at modulating the gut microbiota. These approaches hold promise for augmenting current PD treatment modalities by alleviating both motor and non-motor symptoms, thereby potentially improving patient quality of life. This research underscores the significance of the gut microbiota in the progression and treatment of PD, advocating for an integrated, multidisciplinary approach to develop personalized, efficacious management strategies for PD patients, combining insights from neurology, microbiology, and nutritional science.
1 Introduction
Parkinson’s disease (PD), a chronic and progressively worsening neurodegenerative disorder, primarily impairs motor functions, manifesting in tremors, stiffness, bradykinesia (a slowness in movement), and instability in posture. Ranking as the second most prevalent neurodegenerative condition after Alzheimer’s disease, PD exerts a profound effect on individuals and communities globally (1). The Parkinson’s Foundation estimates that over 10 million individuals worldwide live with PD, a number projected to increase with the aging of the population (1). Beyond its physical toll, PD imposes significant economic burdens through healthcare and treatment costs (2).
Recent research has highlighted the brain-gut axis as a crucial factor in understanding PD’s pathogenesis and progression (3). This axis is a sophisticated communication network connecting the gastrointestinal (GI) tract’s enteric nervous system (ENS) with the central nervous system (CNS), involving neural, hormonal, and immune pathways (3). Evidence suggests that disruptions in the brain-gut axis may underlie several neurological disorders, including PD (4). Notably, gastrointestinal issues like constipation can precede PD’s hallmark motor symptoms, indicating the gut’s potential early involvement in the disease’s pathology (4).
The gut microbiota, a complex ecosystem of microorganisms in the GI tract, has attracted attention for its role in neurological health and disease (5). These microorganisms influence brain health through various mechanisms, such as immune modulation, neuroactive compound production, and interaction with the GI tract’s mucosal barrier (6). Changes in gut microbiota composition and function have been linked to PD, suggesting a possible contribution of these microorganisms to the disease’s development and progression (7). Through its interaction with the brain-gut axis, the gut microbiota may impact the dopaminergic system and play a part in PD’s neurodegenerative processes (8).
The exploration of the relationship between gut microbiota and the brain-gut axis in PD offers fresh perspectives on the disease’s underlying mechanisms and introduces novel therapeutic targets. Delving into this interplay can broaden our understanding of PD and foster innovative strategies to alleviate its effects, demonstrating the potential of this research domain to revolutionize PD treatment methodologies.
2 Brain-gut axis in PD
The brain-gut axis denotes the sophisticated bidirectional communication network that integrates the CNS with the ENS of the GI tract. This elaborate network comprises neural pathways, including the autonomic nervous system (ANS) and the ENS, alongside immune responses and hormonal signals (9). Dubbed the “second brain,” the ENS hosts millions of neurons that not only function autonomously but also engage in continuous communication with the CNS, thus influencing a broad spectrum of functions from digestion to emotional regulation (10).
Central to the operation of the brain-gut axis is the gut microbiota, an extensive consortium of microorganisms inhabiting the GI tract (11). These microbes play pivotal roles in food digestion, vitamin production, pathogen defense, and immune system modulation (9). Notably, the gut microbiota synthesizes various neuroactive substances, including short-chain fatty acids (SCFAs), neurotransmitters, and metabolic by-products, which can impact brain functions, behaviors, and mood (10). These effects are mediated through both direct and indirect pathways, engaging immune and neural mechanisms, thereby exerting a significant influence on the brain-gut axis.
Disturbances in the brain-gut axis and changes in gut microbiota composition, known as dysbiosis, have been associated with PD pathology (12). Disruptions may increase intestinal permeability, colloquially referred to as “leaky gut,” facilitating the entry of pro-inflammatory agents and pathogens into the bloodstream, which may, in turn, incite inflammatory responses in the CNS (13). Such inflammation is implicated in the neurodegeneration characteristic of PD. Additionally, altered gut microbiota in PD patients tend to produce reduced levels of beneficial SCFAs and increased levels of certain neurotoxic metabolites, thereby linking gut dysbiosis to PD’s pathological landscape (3).
The manifestation of GI symptoms, such as constipation in PD patients, prior to the emergence of motor symptoms underscores the critical role of the brain-gut axis in the disease’s progression (14). The precursory occurrence of GI symptoms suggests the gut as a potential initial site of disease pathology. It is postulated that pathological processes, including the aggregation of alpha-synuclein—a protein intimately associated with PD—may originate in the gut and ascend to the brain via the vagus nerve, a key neural pathway of the brain-gut axis (14).
Elucidating the complex interplay within the brain-gut axis and the influence of gut microbiota on this axis sheds light on PD’s pathogenesis and heralds novel therapeutic interventions targeting gut microbiota to bolster the health of the brain-gut axis. This nuanced understanding fosters a deeper comprehension of PD and invites innovative approaches to mitigate its impact.
3 Gut microbiota’s role in PD
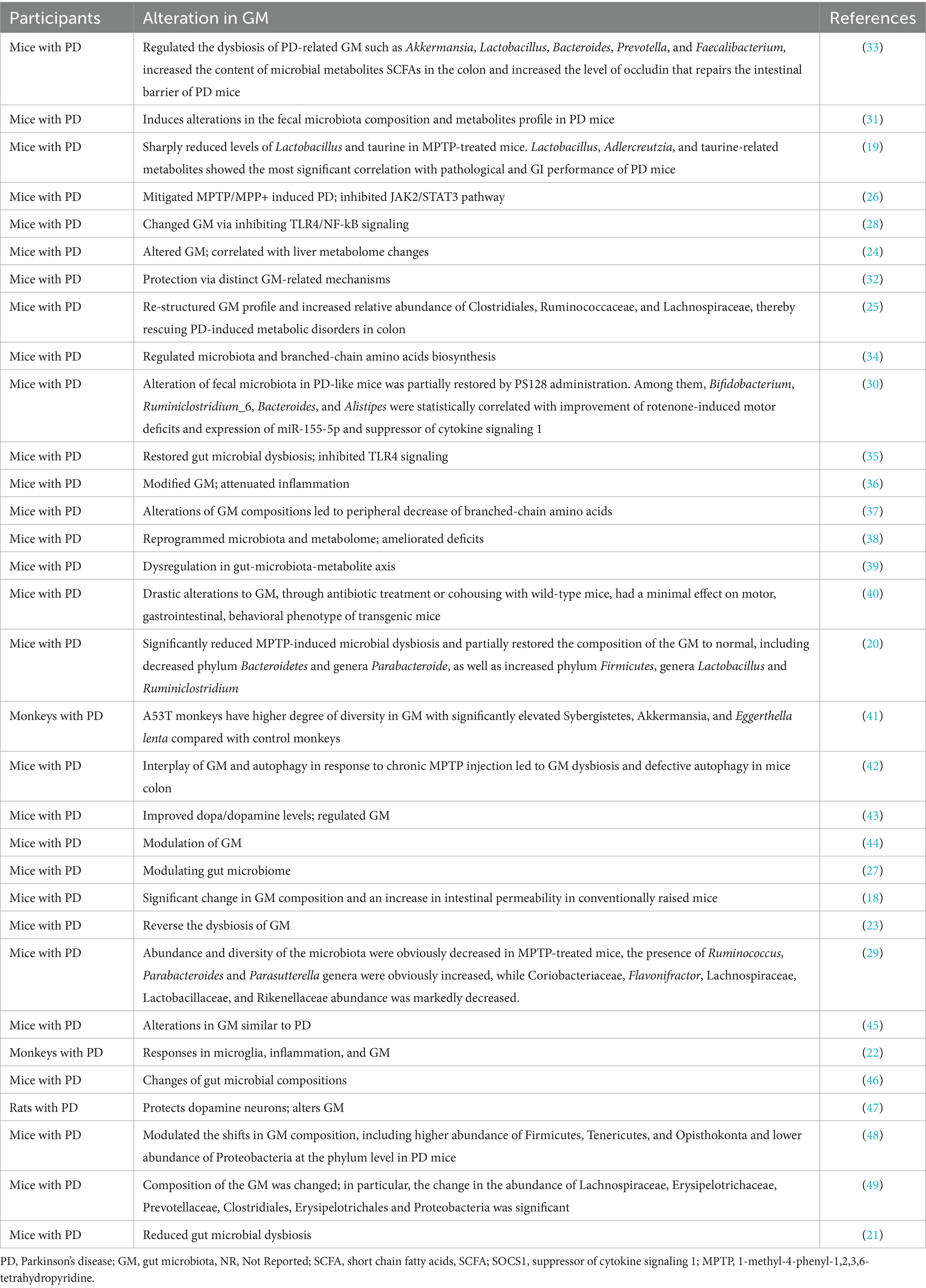
The gut microbiota, comprising trillions of microorganisms such as bacteria, viruses, fungi, and protozoa within the human GI tract, plays an indispensable role in maintaining human health. This intricate microbial ecosystem is pivotal for digestion, nutrient absorption, immune system modulation, and the biosynthesis of vital vitamins. Moreover, the gut microbiota engages in a dynamic interaction with the host’s CNS via the brain-gut axis, influencing both brain function and behavior (10). This interaction highlights the gut microbiota’s critical role in neurological wellness and disorders, including PD.
Observations in PD have revealed notable alterations in both the composition and functionality of the gut microbiota. Studies indicate a diminution in the levels of beneficial bacteria, particularly those that produce SCFAs such as butyrate (7). These beneficial microbes are essential for sustaining gut integrity, modulating inflammation, and bolstering neuronal health (7). In contrast, there is an upsurge in bacteria linked to pro-inflammatory states in PD patients, resulting in a microbial imbalance known as dysbiosis.
The repercussions of gut microbiota dysbiosis on the progression of PD are complex and multifaceted (13). Dysbiosis may lead to increased intestinal permeability, colloquially termed “leaky gut,” enabling the translocation of toxins, microbial by-products, and pro-inflammatory agents into the bloodstream (13). This phenomenon may initiate systemic and neuroinflammation, contributing to the neurodegeneration characteristic of PD (3). Furthermore, certain microbial metabolites could directly affect the aggregation of alpha-synuclein, a protein critically implicated in PD pathology (15). The pathological accumulation of alpha-synuclein within the enteric nervous system might traverse neural pathways to the CNS, potentially marking a pathway for PD’s onset and progression initiating in the gut (15).
Additionally, the gut microbiota can influence the pharmacokinetics and efficacy of PD medications, thereby impacting therapeutic outcomes (16). For example, specific bacterial strains are capable of metabolizing levodopa, the cornerstone of PD treatment, potentially diminishing its bioavailability and therapeutic effect.
In essence, the gut microbiota occupies a central role in the realm of PD research and therapeutic development. The discernible shifts in the gut microbiota associated with PD highlight the therapeutic potential of targeting these microbial communities to modulate the disease trajectory. Ongoing research aiming to elucidate the intricate interactions between gut microbiota and PD holds the promise of unveiling innovative therapeutic avenues. These future strategies may leverage the gut’s complex microbial ecosystem to decelerate or even alter PD’s progression, offering new hope for affected individuals.
4 Mechanisms of gut microbiota influence on PD
The influence of gut microbiota on PD unfolds through a spectrum of mechanisms spanning visceral sensory pathways, endocrine pathways, and immune pathways (10, 11, 13, 17–61). This multifaceted interplay between the GI tract and the brain elucidates how variations in gut microbiota composition can play a pivotal role in PD’s pathogenesis.
Visceral sensory pathways: The ENS, dubbed the “second brain,” comprises an extensive network of neurons that line the GI tract, facilitating crucial gut-brain communication. The gut microbiota impacts the ENS and, by extension, communicates with the CNS via visceral sensory pathways, with the vagus nerve serving as a direct conduit between the gut and the brain (13, 18–61). Emerging research posits that pathogenic variants of alpha-synuclein—a hallmark protein in PD—may originate within the gut and ascend to the brain through the vagus nerve, implicating this route in the disease’s early development and progression (13, 22, 32, 41, 46, 49, 58–60). Additionally, the gut microbiota’s role in neurotransmitter production, such as serotonin and dopamine, which are integral to mood regulation and motor control, respectively, further underscores its potential impact on PD symptomatology (13, 20, 21, 25, 31, 32, 35, 38, 39, 42, 43, 45, 48, 50, 53, 61).
Endocrine pathways: The gut microbiota exerts a substantial influence on host metabolism and endocrine functions through the production of metabolites like SCFAs—butyrate, propionate, and acetate. These metabolites possess the ability to traverse the blood–brain barrier, modulating brain functions including neural activity, neuroinflammation, and neuroprotection (22, 23, 26, 27, 29, 31–37, 39, 42, 43, 45, 46, 48, 51–53, 55, 57–60). In PD, alterations in the production of gut microbiota-derived metabolites may compromise neuronal health and foster neurodegeneration (19, 20, 22, 24–26, 28, 30–32, 35–44, 46–54, 56–61). The microbiota’s capacity to regulate the secretion of gut hormones such as ghrelin and glucagon-like peptide-1 (GLP-1), both noted for their neuroprotective properties, may also be pivotal in modulating PD’s progression (23, 27, 31, 33, 35, 39, 53).
Immune pathways: The gut microbiota is instrumental in shaping the host’s immune system development and functionality (13, 18, 19, 21–23, 25–29, 31–38, 40–42, 44–61). Dysbiosis, defined as an imbalance in gut microbiota, can precipitate a skewed immune response characterized by heightened production of pro-inflammatory cytokines and activation of microglia, the CNS’s innate immune cells (13, 21–23, 27, 28, 32, 34–36, 39, 41, 44, 46–48, 50, 52–60). Such systemic inflammation is believed to intensify neuroinflammation, thereby exacerbating PD’s neurodegenerative trajectory (13, 21–23, 27, 28, 32, 34–36, 39, 41, 44, 46–48, 50, 52–54, 56–60). Notably, certain gut bacterial strains are known to induce regulatory T cells (Tregs) production, pivotal in dampening inflammatory responses (21, 23, 27, 32, 35, 39, 53). A disruption in this delicate balance within the gut microbiota may undermine these regulatory mechanisms, propelling unchecked inflammation (21, 23, 27, 32, 35, 39, 53).
In essence, gut microbiota’s contribution to PD spans several intricate mechanisms, including the modulation of visceral sensory and endocrine pathways, along with the orchestration of immune responses (13, 18–61). A deeper comprehension of these mechanisms not only illuminates PD’s underlying pathophysiology but also heralds novel gut microbiota-targeted therapeutic strategies, potentially curbing PD’s progression.
5 Gut microbiota in PD treatment
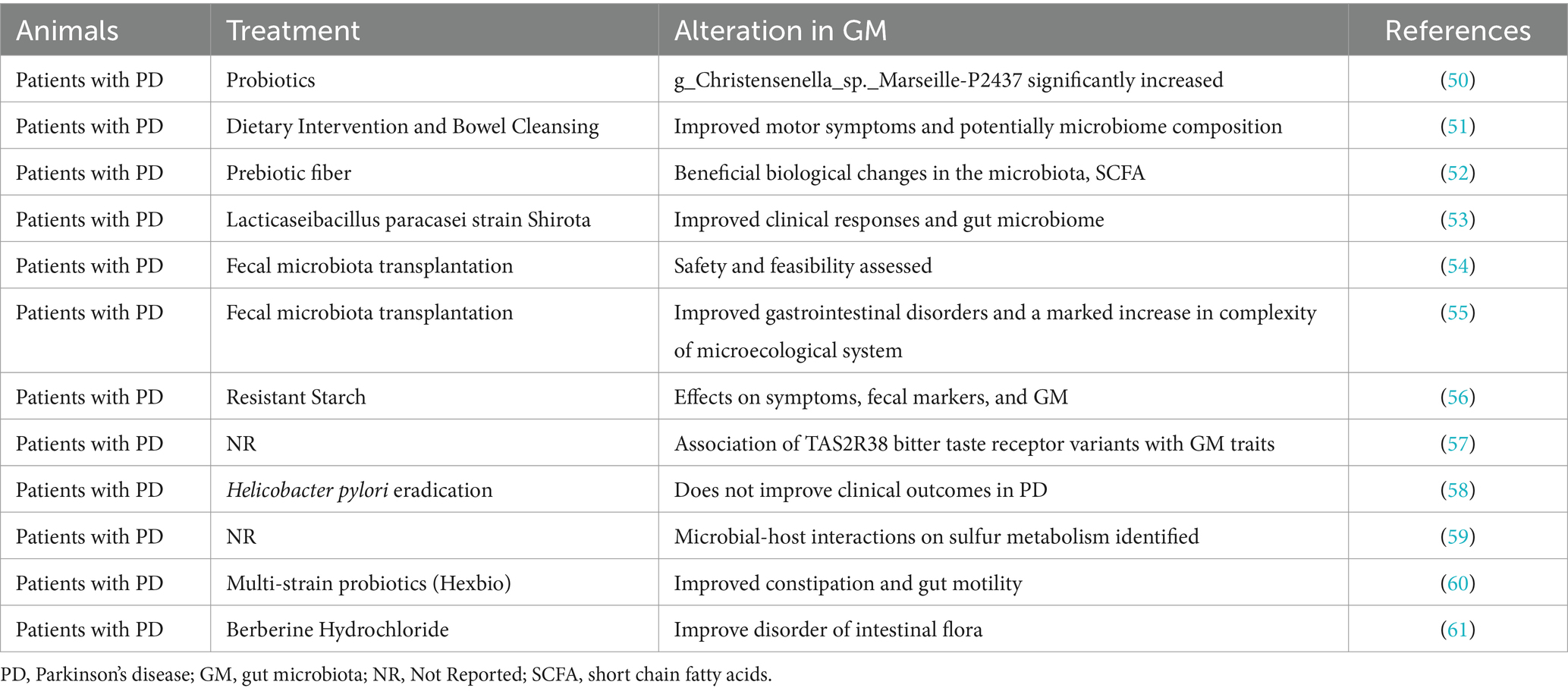
The therapeutic paradigm for PD is broadening to encompass strategies that specifically target the gut microbiota, acknowledging its substantial influence on the disease’s pathophysiology (13, 18–61). This approach includes dietary modifications, fecal microbiota transplantation (FMT), and an understanding of the interactions between gut microbiota and PD medications as promising pathways to enhance patient care (Tables 1, 2).
Dietary interventions targeting gut microbiota: The diet significantly impacts the composition and functionality of the gut microbiota. The incorporation of probiotics—beneficial live microorganisms—has been recognized for its positive influence on gut health. In the context of PD, probiotics may mitigate gastrointestinal symptoms such as constipation and could potentially decelerate the disease’s progression by modulating inflammatory responses and exerting neuroprotective effects (23, 31, 33–35, 38, 42, 44, 45, 48, 50, 53, 54, 60, 61). Similarly, the ketogenic diet, characterized by its high fat and low carbohydrate content, is posited to offer benefits to PD patients by providing an alternative energy source for brain cells and potentially reshaping the gut microbiota composition, thus mitigating inflammation and oxidative stress associated with PD pathology (20, 22, 32, 37, 39, 40, 43, 46, 51).
FMT: FMT, the process of transferring fecal matter from a healthy donor to a patient’s gastrointestinal tract, aims to re-establish a balanced microbiome (25, 29, 34, 35, 44, 45, 54–61). Initially utilized for Clostridium difficile infections, FMT is now being explored as a viable treatment option for PD (34, 35, 45, 54, 57, 60, 61). By directly modifying the gut microbiota, FMT seeks to rectify the dysbiosis prevalent in PD patients, thereby possibly reducing systemic and neuroinflammation while providing neuroprotective advantages (25, 29, 34, 35, 44, 45, 54–61). Despite its potential, the efficacy and safety of FMT for PD treatment require more extensive clinical investigation (25, 29, 34, 35, 44, 45, 54–61).
Interplay between gut microbiota and PD medications: The gut microbiota significantly affects the effectiveness and potential toxicity of PD medications (20–61). Certain gut bacteria have the ability to metabolize levodopa, the cornerstone treatment for PD, thereby influencing its bioavailability and therapeutic impact (23, 25, 27–29, 31–33, 35–37, 39, 42, 44, 46, 49–51, 53–61). Conversely, PD medications might alter the gut microbiota’s composition, influencing the disease’s trajectory and gastrointestinal manifestations (20, 22, 24, 26, 30, 34, 38, 40, 41, 43, 45, 47, 48, 52). Appreciating this reciprocal relationship is essential for refining PD management strategies, suggesting that gut microbiota modulation could amplify medication efficacy and minimize negative side effects (20–61).
In summary, focusing on gut microbiota presents an innovative treatment avenue for PD, with dietary adjustments, FMT, and an insightful understanding of the microbiota-medication interaction holding promise for advancing patient outcomes (20–61). Continued research in this domain is anticipated to integrate these strategies into personalized PD management plans, fostering improved quality of life and offering potential for disease modification in PD patients.
6 Behavioral and cognitive effects of gut microbiota in PD
The complex interplay between the gut microbiota and PD notably affects both behavioral and cognitive aspects of the condition, extending the impact of the microbiota beyond merely influencing motor symptoms (3, 6, 7, 9, 12, 13, 16–61). Current research underscores the link between gut microbiota and PD’s non-motor symptoms, such as depression and anxiety, alongside cognitive decline and dementia associated with the disease.
Association with Non-motor Symptoms: Non-motor symptoms, including depression and anxiety, significantly detract from the quality of life for individuals with PD and often manifest prior to the disease’s motor symptoms. This timing suggests a potential connection to early pathological changes within PD (13, 18, 20, 21, 24, 26, 28, 31, 33, 34, 36, 39, 42, 43, 45, 47, 50, 51, 53, 55, 58, 59, 61). The gut-brain axis mediates the gut microbiota’s effect on mood and behavior, with specific bacterial strains producing neurotransmitters like serotonin and dopamine, crucial for mood regulation (9, 13, 16, 18, 20, 22, 25, 27, 30, 32, 35, 37, 43, 45, 50, 52, 56, 58). Dysbiosis, a disruption in the balance of gut microbiota, has been linked to mood disorders within the PD population. For example, a decrease in beneficial bacteria, particularly those producing SCFAs, may increase gut permeability and systemic inflammation, leading to neuroinflammation implicated in the onset of depression and anxiety in PD (6, 13, 14, 16, 18, 20, 23, 25, 27, 29, 31, 34, 36, 39, 41, 43, 46, 48, 50, 53, 56, 59).
Cognitive impacts and PD-related dementia: Cognitive impairment, including dementia, poses a significant challenge in PD, potentially progressing as the disease advances (14, 15, 17, 23, 30, 41, 44, 48, 52). The gut microbiota’s role in cognitive health is increasingly acknowledged, with evidence suggesting that dysbiosis may underlie the cognitive decline observed in PD (3, 4, 7, 8, 11, 12, 16, 18, 20, 24, 26, 27, 29, 31, 33, 35, 37, 39, 42, 43, 45, 47, 49, 50, 53, 55, 57, 60). Certain bacteria influence the levels of amyloid and tau proteins, associated with neurodegenerative conditions such as Alzheimer’s disease and PD-related dementia (9, 19, 22, 28, 34, 38, 46, 51, 54, 58, 61). Dysbiosis-induced inflammation could intensify neurodegeneration, hastening cognitive deterioration (5, 6, 10, 13, 21, 25, 32, 36, 40, 56, 59). Additionally, neurotoxic metabolites produced by specific pathogenic bacteria might directly affect brain function, implicating gut microbiota in PD’s cognitive deficits (2, 54).
The modulation of gut microbiota represents a viable therapeutic avenue to address PD-related dementia and cognitive impairments (1, 38, 52, 55). Interventions such as probiotics, prebiotics, and dietary adjustments targeting gut health may help manage PD’s cognitive symptoms (26, 30, 35, 41, 47, 60). However, the relationship between gut microbiota and cognitive function in PD involves complex, multifactorial mechanisms that necessitate further research for a comprehensive understanding and clinical application.
In summary, the influence of gut microbiota on PD’s behavioral and cognitive symptoms emphasizes the need for a holistic disease management approach (18, 34, 43, 50, 57). Integrating strategies to improve gut health could enhance current treatments, offering new methods to mitigate non-motor symptoms and cognitive decline (16, 24, 31, 53, 58). With ongoing advances in this research area, modulating gut microbiota may emerge as a crucial component of PD management, striving to elevate patient outcomes and life quality.
7 Future directions in research and treatment
As the nexus between gut microbiota and PD gains empirical support, specific domains have emerged, delineating where future research could significantly enhance our comprehension of PD and foster the development of novel, tailored therapeutic approaches.
Current gaps in understanding: A fundamental obstacle in current research is the partial grasp of the interplay between gut microbiota and genetic and environmental determinants in affecting PD’s onset and course (3, 12, 14–61). While disparities in the gut microbiome between PD patients and healthy individuals have been documented, the exact causal connections and the mechanisms driving these differences remain elusive. The contribution of particular microbial strains or their by-products to PD’s neuropathological traits, such as alpha-synuclein misfolding and aggregation, is not fully elucidated (12, 14–61). Present studies, predominantly animal-based or observational in humans, hint at associations without establishing causality. There is a pressing need for robust, longitudinal human studies to demystify these complex interactions.
Potential for personalized gut microbiota-based therapies: The individual variability in gut microbiota composition hints at the efficacy of personalized modulation strategies in PD management (18, 20–61). Such tailored interventions could encompass targeted probiotics to rebalance gut flora, prebiotics to foster beneficial microbes, or FMT from healthy donors (18, 20–61). Moreover, crafting microbiota-focused diets or utilizing specific microbial derivatives as therapeutic agents holds promise. Realizing the potential of these personalized treatments necessitates further research to pinpoint the optimal microbial strains and metabolites for PD patients and to assess the safety profile of such microbiota-based interventions (20–61).
The importance of further research into the brain-gut-microbiota axis: Delving into the brain-gut-microbiota axis’s intricate dynamics is vital for unveiling novel PD treatments (4, 6, 9–13, 17, 23, 26–61). This exploration should aim to clarify how gut microbiota modulates neuroinflammation, alpha-synuclein aggregation, and the blood–brain barrier’s integrity. Investigating gut microbiota’s impact on the pharmacodynamics of PD medications could refine therapeutic protocols (16, 25, 32, 38). Leveraging advanced genomics, proteomics, metabolomics, and bioinformatics tools could facilitate a granular analysis of the brain-gut-microbiota axis, setting the stage for PD treatment breakthroughs (9, 17, 23, 25, 31, 38, 39, 41, 43, 47, 49, 52, 59).
In summary, research linking gut microbiota with PD treatment is poised for significant breakthroughs, yet it demands a dedicated endeavor to bridge existing knowledge gaps. Emphasizing personalized microbiota-based therapies and a deeper investigation of the brain-gut-microbiota axis heralds a promising horizon for PD research and care, potentially transforming the lives of those afflicted with PD.
8 Summary
The burgeoning body of research accentuates the pivotal role of the gut microbiota and the brain-gut axis in both the pathogenesis and the therapeutic management of PD. This complex interaction sheds light on the disease from a perspective that extends beyond its conventional neurological confines, spotlighting gut microbiota as a viable target for innovative therapeutic approaches. Adjusting the gut microbiota through dietary measures, probiotics, FMT, and other targeted treatments emerges as a promising strategy to augment current PD therapies, potentially mitigating both motor and the frequently incapacitating non-motor symptoms.
There exists an urgent necessity to elevate awareness and propel research into comprehensive treatments leveraging the gut microbiota’s capabilities for PD care. Such endeavors demand a collaborative, interdisciplinary methodology, merging expertise across neurology, microbiology, nutrition, among other pertinent disciplines, to craft efficacious, tailored treatment modalities. As our comprehension of the brain-gut-microbiota nexus deepens, there is optimism for forging ahead with groundbreaking therapies that could significantly enhance PD patients’ life quality, steering them toward a more optimistic future. This rallying cry highlights the critical need for continued investigation and resource allocation in this dynamic and hopeful sector of PD research and therapeutic development.
Author contributions
XJ: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. QW: Conceptualization, Data curation, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. ML: Conceptualization, Data curation, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. J-yD: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dorsey, ER, and Bloem, BR. The Parkinson pandemic—a call to action. JAMA Neurol. (2018) 75:9–10. doi: 10.1001/jamaneurol.2017.3299
2. GBD 2016 Parkinson's Disease Collaborators. Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2018. (2018) 17:939–53. doi: 10.1016/S1474-4422(18)30295-3
3. Houser, MC, and Tansey, MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson's disease pathogenesis? NPJ Parkinsons Dis. (2017) 3:3. doi: 10.1038/s41531-016-0002-0
4. Mulak, A, and Bonaz, B. Brain-gut-microbiota axis in Parkinson's disease. World J Gastroenterol. (2015) 21:10609–20. doi: 10.3748/wjg.v21.i37.10609
5. Cenit, MC, Sanz, Y, and Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol. (2017) 23:5486–98. doi: 10.3748/wjg.v23.i30.5486
6. Keshavarzian, A, Green, SJ, Engen, PA, Voigt, RM, Naqib, A, Forsyth, CB, et al. Colonic bacterial composition in Parkinson's disease. Mov Disord. (2015) 30:1351–60. doi: 10.1002/mds.26307
7. Bedarf, JR, Hildebrand, F, Coelho, LP, Sunagawa, S, Bahram, M, Goeser, F, et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson's disease patients. Genome Med. (2017) 9:39. doi: 10.1186/s13073-017-0428-y
8. Petrov, VA, Saltykova, IV, Zhukova, IA, Alifirova, VM, Zhukova, NG, Dorofeeva, YB, et al. Analysis of gut microbiota in patients with Parkinson's disease. Bull Exp Biol Med. (2017) 162:734–7. doi: 10.1007/s10517-017-3700-7
9. Mayer, EA, Knight, R, Mazmanian, SK, Cryan, JF, and Tillisch, K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. (2014) 34:15490–6. doi: 10.1523/JNEUROSCI.3299-14.2014
10. Cryan, JF, and O'Mahony, SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. (2011) 23:187–92. doi: 10.1111/j.1365-2982.2010.01664.x
11. Sharon, G, Sampson, TR, and Geschwind, DH. The central nervous system and the gut microbiome. Cell. (2016) 167:915–32. doi: 10.1016/j.cell.2016.10.027
12. Scheperjans, F, Aho, V, Pereira, PAB, Koskinen, K, Paulin, L, Pekkonen, E, et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord. (2015) 30:350–8. doi: 10.1002/mds.26069
13. Sampson, TR, Debelius, JW, Thron, T, Janssen, S, Shastri, GG, Ilhan, ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. (2016) 167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018
14. Braak, H, de Vos, RA, Bohl, J, and del Tredici, K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett. (2006) 396:67–72. doi: 10.1016/j.neulet.2005.11.012
15. Stokholm, MG, Danielsen, EH, Hamilton-Dutoit, SJ, and Borghammer, P. Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol. (2016) 79:940–9. doi: 10.1002/ana.24648
16. Maini Rekdal, V, Bess, EN, Bisanz, JE, Turnbaugh, PJ, and Balskus, EP. Discovery and inhibition of an interspecies gut bacterial pathway for levodopa metabolism. Science. (2019) 364:eaau6323. doi: 10.1126/science.aau6323
17. Borre, YE, Moloney, RD, Clarke, G, Dinan, TG, and Cryan, JF. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol. (2014) 817:373–403. doi: 10.1007/978-1-4939-0897-4_17
18. Bhattarai, Y, Si, J, Pu, M, Ross, OA, McLean, PJ, Till, L, et al. Role of gut microbiota in regulating gastrointestinal dysfunction and motor symptoms in a mouse model of Parkinson's disease. Gut Microbes. (2021) 13:1866974. doi: 10.1080/19490976.2020.1866974
19. Cui, C, Song, H, Han, Y, Yu, H, Li, H, Yang, Y, et al. Gut microbiota-associated taurine metabolism dysregulation in a mouse model of Parkinson’s disease. mSphere. (2023) 8:e0043123. doi: 10.1128/msphere.00431-23
20. Wang, N, Feng, BN, Hu, B, Cheng, YL, Guo, YH, and Qian, H. Neuroprotection of chicoric acid in a mouse model of Parkinson's disease involves gut microbiota and TLR4 signaling pathway. Food Funct. (2022) 13:2019–32. doi: 10.1039/D1FO02216D
21. Sun, MF, Zhu, YL, Zhou, ZL, Jia, XB, Xu, YD, Yang, Q, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav Immun. (2018) 70:48–60. doi: 10.1016/j.bbi.2018.02.005
22. Joers, V, Masilamoni, G, Kempf, D, Weiss, AR, Rotterman, TM, Murray, B, et al. Microglia, inflammation and gut microbiota responses in a progressive monkey model of Parkinson's disease: a case series. Neurobiol Dis. (2020) 144:105027. doi: 10.1016/j.nbd.2020.105027
23. Sun, J, Li, H, Jin, Y, Yu, J, Mao, S, Su, KP, et al. Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson's disease via gut microbiota-GLP-1 pathway. Brain Behav Immun. (2021) 91:703–15. doi: 10.1016/j.bbi.2020.10.014
24. Hu, J, Li, P, Zhao, H, Ji, P, Yang, Y, Ma, J, et al. Alterations of gut microbiota and its correlation with the liver metabolome in the process of ameliorating Parkinson's disease with Buyang Huanwu decoction. J Ethnopharmacol. (2024) 318:116893. doi: 10.1016/j.jep.2023.116893
25. Yang, C, Wang, W, Deng, P, Wang, X, Zhu, L, Zhao, L, et al. Fibroblast growth factor 21 ameliorates behavior deficits in Parkinson's disease mouse model via modulating gut microbiota and metabolic homeostasis. CNS Neurosci Ther. (2023) 29:3815–28. doi: 10.1111/cns.14302
26. Ji, LL, Huang, TT, Mao, LL, Xu, YF, Chen, WY, Wang, WW, et al. The gut microbiota metabolite butyrate mitigates MPTP/MPP(+) -induced Parkinson's disease by inhibiting the JAK2/STAT3 signaling pathway. Kaohsiung J Med Sci. (2023) 39:1002–10. doi: 10.1002/kjm2.12745
27. Hou, YF, Shan, C, Zhuang, SY, Zhuang, QQ, Ghosh, A, Zhu, KC, et al. Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson's disease. Microbiome. (2021) 9:34. doi: 10.1186/s40168-020-00988-6
28. He, ZQ, Huan, PF, Wang, L, and He, JC. Compound Dihuang granule changes gut microbiota of MPTP-induced Parkinson's disease mice via inhibiting TLR4/NF-kappa B signaling. Neurochem Res. (2023) 48:3610–24. doi: 10.1007/s11064-023-04004-9
29. Zhu, Y, Huan, F, Wang, J, Xie, X, Yu, G, Wang, X, et al. 1-Methyl-4-phenyl-1, 2,3,6-tetrahydropyridine induced Parkinson's disease in mouse: potential association between neurotransmitter disturbance and gut microbiota Dysbiosis. ACS Chem Neurosci. (2020) 11:3366–76. doi: 10.1021/acschemneuro.0c00475
30. Lee, YZ, Cheng, SH, Chang, MY, Lin, YF, Wu, CC, and Tsai, YC. Neuroprotective effects of Lactobacillus plantarum PS128 in a mouse model of Parkinson's disease: the role of gut microbiota and Micro RNAs. Int J Mol Sci. (2023) 24:6794. doi: 10.3390/ijms24076794
31. Meng, T, Zhang, Y, Huang, J, Pandey, V, Fu, S, and Ma, S. Rubusoside mitigates neuroinflammation and cellular apoptosis in Parkinson's disease, and alters gut microbiota and metabolite composition. Phytomedicine. (2024) 124:155309. doi: 10.1016/j.phymed.2023.155309
32. Cao, X, du, ZR, Liu, X, Wang, X, Li, C, Zhou, SN, et al. Low and high doses of oral maslinic acid protect against Parkinson's disease via distinct gut microbiota-related mechanisms. Biomed Pharmacother. (2023) 165:115100. doi: 10.1016/j.biopha.2023.115100
33. Gan, QX, Peng, MY, Wei, HB, Chen, LL, Chen, XY, Li, ZH, et al. Gastrodia elata polysaccharide alleviates Parkinson's disease via inhibiting apoptotic and inflammatory signaling pathways and modulating the gut microbiota. Food Funct. (2024) 15:2920–38. doi: 10.1039/D3FO05169B
34. Chu, C, Yu, L, Li, Y, Guo, H, Zhai, Q, Chen, W, et al. Lactobacillus plantarum CCFM405 against rotenone-induced Parkinson's disease mice via regulating gut microbiota and branched-chain amino acids biosynthesis. Nutrients. (2023) 15:1737. doi: 10.3390/nu15071737
35. Guo, TT, Zhang, Z, Sun, Y, Zhu, RY, Wang, FX, Ma, LJ, et al. Neuroprotective effects of sodium butyrate by restoring gut microbiota and inhibiting TLR4 signaling in mice with MPTP-induced Parkinson's disease. Nutrients. (2023) 15:930. doi: 10.3390/nu15040930
36. Hong, CT, Chan, L, Chen, KY, Lee, HH, Huang, LK, Yang, YCSH, et al. Rifaximin modifies gut microbiota and attenuates inflammation in Parkinson's disease: preclinical and clinical studies. Cells. (2022) 11:3468. doi: 10.3390/cells11213468
37. Yan, Z, Yang, F, Sun, L, Yu, J, Sun, L, Si, Y, et al. Role of gut microbiota-derived branched-chain amino acids in the pathogenesis of Parkinson's disease: An animal study. Brain Behav Immun. (2022) 106:307–21. doi: 10.1016/j.bbi.2022.09.009
38. Cui, C, Han, Y, Li, H, Yu, H, Zhang, B, and Li, G. Curcumin-driven reprogramming of the gut microbiota and metabolome ameliorates motor deficits and neuroinflammation in a mouse model of Parkinson's disease. Front Cell Infect Microbiol. (2022) 12:887407. doi: 10.3389/fcimb.2022.887407
39. Yan, Z, Li, R, Shi, W, and Yao, L. Role of the gut-microbiota-metabolite axis in the rotenone model of early-stage Parkinson's disease. Metab Brain Dis. (2022) 37:2511–20. doi: 10.1007/s11011-022-01004-6
40. Radisavljevic, N, Cirstea, M, Bauer, K, Lo, C, Metcalfe-Roach, A, Bozorgmehr, T, et al. Effects of gut microbiota alterations on motor, gastrointestinal, and behavioral phenotype in a mouse model of Parkinson's disease. J Parkinsons Dis. (2022) 12:1479–95. doi: 10.3233/JPD-223165
41. Yan, Y, Ren, S, Duan, Y, Lu, C, Niu, Y, Wang, Z, et al. Gut microbiota and metabolites of alpha-synuclein transgenic monkey models with early stage of Parkinson's disease. NPJ Biofilms Microbiomes. (2021) 7:69. doi: 10.1038/s41522-021-00242-3
42. Liu, X, du, ZR, Wang, X, Luk, KH, Chan, CH, Cao, X, et al. Colonic dopaminergic neurons changed reversely with those in the midbrain via gut microbiota-mediated autophagy in a chronic Parkinson's disease mice model. Front Aging Neurosci. (2021) 13:649627. doi: 10.3389/fnagi.2021.649627
43. Wang, Y, Tong, Q, Ma, SR, Zhao, ZX, Pan, LB, Cong, L, et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson's disease by regulating gut microbiota. Signal Transduct Target Ther. (2021) 6:77. doi: 10.1038/s41392-020-00456-5
44. Han, QQ, Fu, Y, le, JM, Pilot, A, Cheng, S, Chen, PQ, et al. Electroacupuncture may alleviate behavioral defects via modulation of gut microbiota in a mouse model of Parkinson's disease. Acupunct Med. (2021) 39:501–11. doi: 10.1177/0964528421990658
45. Li, W, Zhao, Q, Wang, J, Wang, Y, and Wen, T. Dcf1 deletion presents alterations in gut microbiota of mice similar to Parkinson's disease. Biochem Biophys Res Commun. (2020) 529:1137–44. doi: 10.1016/j.bbrc.2020.06.150
46. Dong, XL, Wang, X, Liu, F, Liu, X, du, ZR, Li, RW, et al. Polymannuronic acid prevents dopaminergic neuronal loss via brain-gut-microbiota axis in Parkinson's disease model. Int J Biol Macromol. (2020) 164:994–1005. doi: 10.1016/j.ijbiomac.2020.07.180
47. Koutzoumis, DN, Vergara, M, Pino, J, Buddendorff, J, Khoshbouei, H, Mandel, RJ, et al. Alterations of the gut microbiota with antibiotics protects dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson's disease. Exp Neurol. (2020) 325:113159. doi: 10.1016/j.expneurol.2019.113159
48. Zhou, ZL, Jia, XB, Sun, MF, Zhu, YL, Qiao, CM, Zhang, BP, et al. Neuroprotection of fasting mimicking diet on MPTP-induced Parkinson's disease mice via gut microbiota and metabolites. Neurotherapeutics. (2019) 16:741–60. doi: 10.1007/s13311-019-00719-2
49. Lai, F, Jiang, R, Xie, W, Liu, X, Tang, Y, Xiao, H, et al. Intestinal pathology and gut microbiota alterations in a Methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. Neurochem Res. (2018) 43:1986–99. doi: 10.1007/s11064-018-2620-x
50. du, Y, Li, Y, Xu, X, Li, R, Zhang, M, Cui, Y, et al. Probiotics for constipation and gut microbiota in Parkinson's disease. Parkinsonism Relat Disord. (2022) 103:92–7. doi: 10.1016/j.parkreldis.2022.08.022
51. Hegelmaier, T, Lebbing, M, Duscha, A, Tomaske, L, Tönges, L, Holm, JB, et al. Interventional influence of the intestinal microbiome through dietary intervention and bowel cleansing might improve motor symptoms in Parkinson's disease. Cells. (2020) 9:376. doi: 10.3390/cells9020376
52. Hall, DA, Voigt, RM, Cantu-Jungles, TM, Hamaker, B, Engen, PA, Shaikh, M, et al. An open label, non-randomized study assessing a prebiotic fiber intervention in a small cohort of Parkinson's disease participants. Nat Commun. (2023) 14:926. doi: 10.1038/s41467-023-36497-x
53. Yang, X, He, X, Xu, S, Zhang, Y, Mo, C, Lai, Y, et al. Effect of Lacticaseibacillus paracasei strain Shirota supplementation on clinical responses and gut microbiome in Parkinson's disease. Food Funct. (2023) 14:6828–39. doi: 10.1039/D3FO00728F
54. Vendrik, KE, Chernova, VO, Kuijper, EJ, Terveer, EM, van Hilten, J, Contarino, MF, et al. Safety and feasibility of faecal microbiota transplantation for patients with Parkinson's disease: a protocol for a self-controlled interventional donor-FMT pilot study. BMJ Open. (2023) 13:e071766. doi: 10.1136/bmjopen-2023-071766
55. Cheng, Y, Tan, G, Zhu, Q, Wang, C, Ruan, G, Ying, S, et al. Efficacy of fecal microbiota transplantation in patients with Parkinson's disease: clinical trial results from a randomized, placebo-controlled design. Gut Microbes. (2023) 15:2284247. doi: 10.1080/19490976.2023.2284247
56. Becker, A, Schmartz, GP, Gröger, L, Grammes, N, Galata, V, Philippeit, H, et al. Effects of resistant starch on symptoms, fecal markers, and gut microbiota in Parkinson's disease-the RESISTA-PD trial. Genomics Proteomics Bioinfo. (2022) 20:274–87. doi: 10.1016/j.gpb.2021.08.009
57. Vascellari, S, Melis, M, Cossu, G, Melis, M, Serra, A, Palmas, V, et al. Genetic variants of TAS2R38 bitter taste receptor associate with distinct gut microbiota traits in Parkinson's disease: a pilot study. Int J Biol Macromol. (2020) 165:665–74. doi: 10.1016/j.ijbiomac.2020.09.056
58. Tan, AH, Lim, SY, Mahadeva, S, Loke, MF, Tan, JY, Ang, BH, et al. Helicobacter pylori eradication in Parkinson's disease: a randomized placebo-controlled trial. Mov Disord. (2020) 35:2250–60. doi: 10.1002/mds.28248
59. Hertel, J, Harms, AC, Heinken, A, Baldini, F, Thinnes, CC, Glaab, E, et al. Integrated analyses of microbiome and longitudinal metabolome data reveal microbial-host interactions on sulfur metabolism in Parkinson's disease. Cell Rep. (2019) 29:1767–1777.e8. doi: 10.1016/j.celrep.2019.10.035
60. Ibrahim, A, Ali, RAR, Manaf, MRA, Ahmad, N, Tajurruddin, FW, Qin, WZ, et al. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson's disease: a randomised controlled trial. PLoS One. (2020) 15:e0244680. doi: 10.1371/journal.pone.0244680
Keywords: Parkinson’s disease, gut microbiota, brain-gut axis, dietary modifications, fecal microbiota transplantation
Citation: Jia X, Wang Q, Liu M and Ding J-y (2024) The interplay between gut microbiota and the brain-gut axis in Parkinson’s disease treatment. Front. Neurol. 15:1415463. doi: 10.3389/fneur.2024.1415463
Edited by:
Sheila Pirooznia, National Institutes of Health (NIH), United StatesReviewed by:
Joyce Lee-Iannotti, Banner - University Medical Center Phoenix, Banner Health, United StatesCopyright © 2024 Jia, Wang, Liu and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia-yuan Ding, SmlhLXl1YW5kaW5nQG91dGxvb2suY29t
 Xi Jia1
Xi Jia1 Jia-yuan Ding
Jia-yuan Ding