
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 06 June 2024
Sec. Stroke
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1413632
 Hiroyuki Sakata1,2*
Hiroyuki Sakata1,2* Atsushi Kanoke1
Atsushi Kanoke1 Hiroki Uchida1
Hiroki Uchida1 Shinya Haryu2
Shinya Haryu2 Shunsuke Omodaka3
Shunsuke Omodaka3 Naoto Kimura4
Naoto Kimura4 Masahiro Yoshida5
Masahiro Yoshida5 Kuniyasu Niizuma3,6,7,8
Kuniyasu Niizuma3,6,7,8 Teiji Tominaga3,8
Teiji Tominaga3,8 Hidenori Endo3,8*
Hidenori Endo3,8*Introduction: Clazosentan, a selective endothelin receptor subtype A antagonist, reduces vasospasm-related morbidity and all-cause mortality following aneurysmal subarachnoid hemorrhage (SAH) in the Japanese population, as demonstrated by a recent randomized phase 3 trial. However, evidence to suggest clazosentan should be prioritized over the current standard of care to prevent cerebral vasospasm is still lacking. Therefore, we investigated the efficacy and safety of clazosentan in comparison with conventional postoperative management in real-world clinical practice.
Methods: We conducted a single-center, retrospective, observational cohort study using prospectively collected data from consecutive patients with aneurysmal SAH. After clazosentan was approved for use in Japan, the conventional postoperative management protocol, composed of intravenous fasudil chloride and oral cilostazol (control group, April 2021 to March 2022), was changed to the clazosentan protocol (clazosentan group, April 2022 to March 2023). The primary endpoint was the incidence of vasospasm-related symptomatic infarction. The secondary endpoints were favorable functional outcomes (modified Rankin scale score < 3) at discharge, angiographic vasospasm, and the need for rescue therapy for delayed cerebral ischemia.
Results: The analysis included 100 and 81 patients in the control and clazosentan groups, respectively. The incidence of vasospasm-related symptomatic infarction was significantly lower in the clazosentan group than in the control group (6.2% vs. 16%, p = 0.032). Multiple logistic analyses demonstrated that the use of clazosentan was independently associated with fewer incidence of vasospasm-related symptomatic infarct (23.8% vs. 47.5%, odds ratio 0.34 [0.12–0.97], p = 0.032). Clazosentan was significantly associated with favorable outcomes at discharge (79% vs. 66%, p = 0.037). Moreover, both the incidence of angiographic vasospasm (25.9% vs. 44%, p = 0.013) and the need for rescue therapy (16.1% vs. 34%, p = 0.006) was lower in the clazosentan group. The occurrence of pulmonary edema was significantly higher with clazosentan use (19.8% vs. 5%, p = 0.002), which did not result in morbidity.
Conclusion: A postoperative management protocol centering on clazosentan was associated with the reduced vasospasm-related symptomatic infarction and improved clinical outcomes compared to the conventional management protocol in Japanese clinical practice. Clazosentan might be a promising treatment option for counteracting cerebral vasospasm after aneurysmal SAH.
Aneurysmal subarachnoid hemorrhage (SAH), caused by the rupture of an intracranial aneurysm, is associated with substantial morbidity and mortality. One of the most important complications is cerebral vasospasm, which causes delayed cerebral ischemia (DCI) in approximately 40% of aneurysmal SAH cases (1). Moreover, half of patients with cerebral vasospasm exhibit cerebral infarction, seriously decreasing the patients’ functionality and quality of life.
Clazosentan, a selective endothelin receptor subtype A antagonist, has been found to inhibit endothelin-mediated cerebral vasospasm (2–5). Recent randomized phase 3 trials conducted in Japan have demonstrated that clazosentan significantly reduces the combined incidence of vasospasm-related morbidity and all-cause mortality in patients with aneurysmal SAH who were in World Federation of Neurosurgical Societies (WFNS) grades I–IV after clipping and coil embolization (6), which led to the approval of clazosentan by the Pharmaceuticals and Medical Devices Agency in Japan (7). However, this phase 3 trial did not compare clazosentan and previously approved treatments such as fasudil hydrochloride, which has been widely used in Japan based on the Japan Stroke Society Guideline (8, 9). Therefore, it is unclear if the effect of clazosentan to prevent vasospasm is superior to the conventional standard of care.
This retrospective analysis aimed to investigate the efficacy and safety of clazosentan in real-world clinical practice by comparing it with conventional postoperative management.
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Review Board of Kohnan Hospital (approval number: 2023-0118-03). Reporting followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) criteria (10). The requirement for informed consent was waived because the analysis used anonymous clinical data obtained after each patient agreed to the treatment and provided written consent. We used the opt-out method to obtain approval for this study using a poster approved by the Institutional Review Board.
We conducted a retrospective observational cohort study using prospectively collected data from consecutive patients with aneurysmal SAH at our institute between April 2021 and March 2023. After the approval of clazosentan in Japan in April 2022, the conventional postoperative management protocol (control group, April 2021, and March 2022) was reformed to a new protocol using clazosentan (clazosentan group, April 2022 to March 2023) (Figure 1). The inclusion criteria were as follows: (1) patients aged 20 and over, (2) spontaneous aneurysmal SAH (WFNS grades I-V) and (3) surgical clipping or endovascular coiling performed within 72 h after the onset of SAH. The exclusion criteria were as follows: (1) Fisher group 1 on preoperative CT, (2) surgical intervention beyond 72 h after the onset of SAH, (3) preexisting cerebral vasospasm on preoperative angiography, (4) significant complication during the procedure, including new large cerebral infarct and new significant neurosurgical deficits lasting 12 h or more after the procedure, and (4) contraindication to clazosentan (pregnant, severe liver dysfunction, and prolonged QT interval) after April 2022.
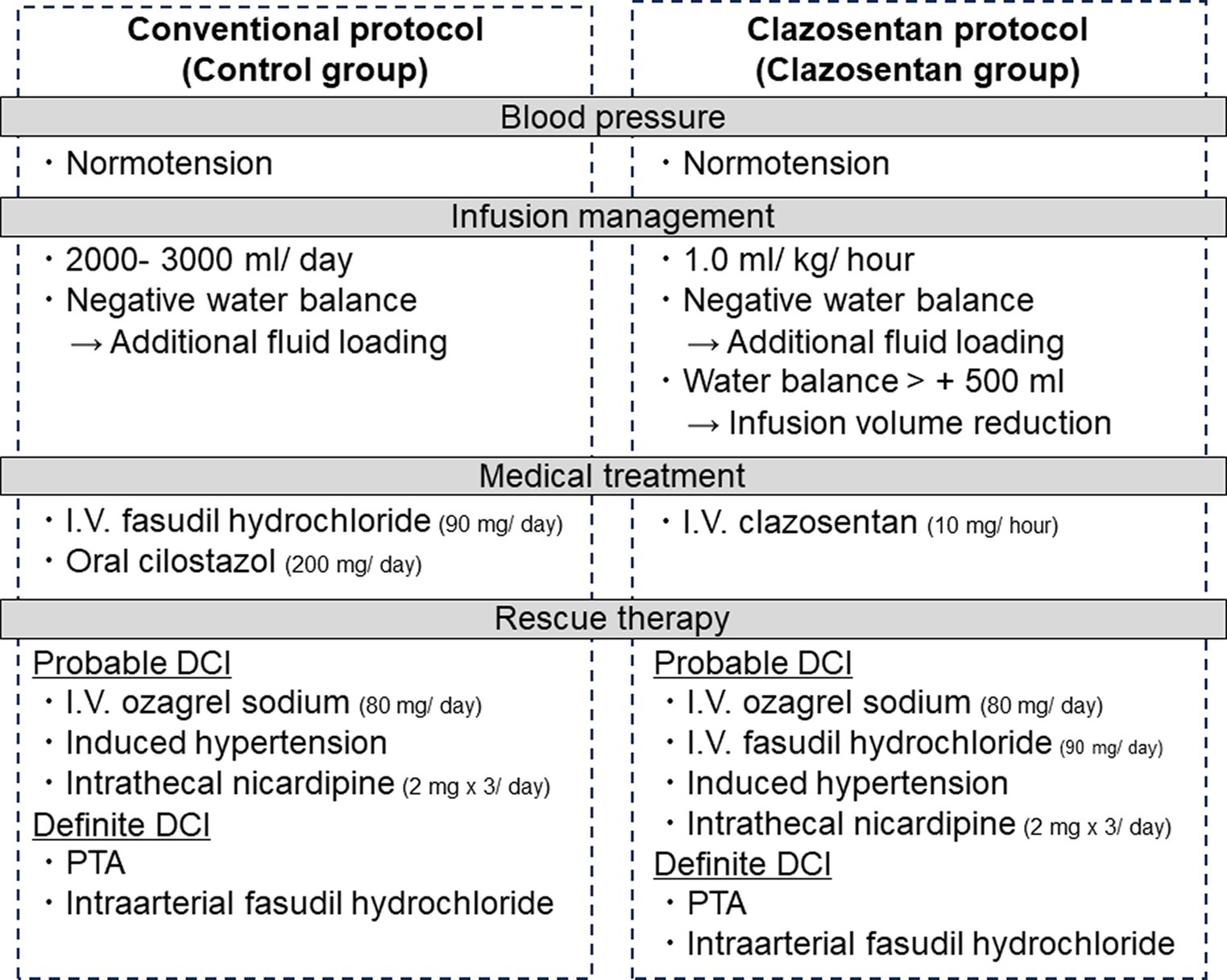
Figure 1. Conventional (control group, April 2021 to March 2022) and clazosentan postoperative management protocols (clazosentan group, April 2022 to March 2023).
Figure 1 details our postoperative management protocol before (control group) and after (clazosentan group) clazosentan approval. All patients with aneurysmal SAH were cared for in an intensive care unit specialized in the care of neurosurgical patients after securing the ruptured aneurysm. In the control group, all patients received intravenous injections of fasudil hydrochloride (a Rho kinase inhibitor, 30 mg three times a day for 14 days) and oral or enteral cilostazol (a selective inhibitor of phosphodiesterase type III, 200 mg a day for 14 days) from the next day after aneurysmal obliteration to prevent DCI unless contraindicated. In contrast, in the calazosentan group, all patients were treated with continuous intravenous administration of clazosentan (10 mg/h) from postoperative day (POD) 1 to day 15 of SAH onset. Neither fasudil hydrochloride nor cilostazol were prophylactically administered in the clazosentan group. Levetiracetam (an inhibitor of presynaptic Ca2+ channels, 1,000 mg a day for 14 days) was used in both groups to prevent perioperative epilepsy. All patients were kept normovolemic and normotensive, although the patients in the clazosentan group were managed with less extracellular fluid (1 mL/kg/h) to avoid fluid retention, which is reported to be one of the major side effects of clazosentan (11). If the daily fluid balance was negative, additional extracellular fluid loading was delivered during this period. If the water balance exceeded 500 mL/12 h, reduction of fluid volume and intravenous injection of furosemide (loop diuretics, 10 mg) was considered only in the clazosentan group. Enteral feeding or oral intake was initiated on POD1.
We diagnosed “probable DCI,” if the patient had DCI-suspicious clinical symptoms (i.e., minor consciousness disturbance) in addition to the finding of cerebral vasospasm (<50% diameter reduction in intracranial arteries on magnetic resonance (MR) angiography). “Definitive DCI” was diagnosed according to the definition by Vergouwen et al. (12). After “probable DCI” diagnosis, we started continuous intravenous administration (80 mg/day) of ozagrel sodium (a selective inhibitor of TXA2 synthase) and induced hypertension and/or intrathecal nicardipine (2 mg three times a day) to counteract DCI in the control group. In the clazosentan group, intravenous injections of fasudil hydrochloride (30 mg three times) and/or intravenous administration of ozagrel sodium were considered in cases of “probable DCI.” After a Definitive DCI” diagnosis, intra-arterial infusion of fasudil hydrochloride (chemical angioplasty) and/or percutaneous transluminal angioplasty were performed as rescue therapies in both groups.
Independent neurosurgeons (A.K. and H.U.) blinded to the clinical information reviewed the consecutive imaging studies. All patients underwent digital subtraction angiography (DSA) and MR angiography preoperatively, followed by MR angiography on approximately POD 7 and 14 and neurological deterioration. Moderate to severe vasospasm was diagnosed when DSA (≥34%) or MR angiography (≥50%) demonstrated segmental or diffuse narrowing of the vessel diameter of a cerebral artery compared to the initial angiography. The proximal arteries represent the internal carotid artery, A1, M1, vertebral artery, basilar artery, and P1. Vasospasm-related infarction was defined as a newly developed cerebral infarct in the territory of the cerebral artery, associated with moderate-to-severe vasospasm.
The primary efficacy endpoint was the proportion of patients with vasospasm-related symptomatic infarcts following aneurysmal SAH. The secondary efficacy endpoints included the incidence of moderate to severe angiographic vasospasm within 14 days post-aneurysmal SAH as well as clinical outcomes assessed by modified Rankin scale (mRS), dichotomized as good (<3) and poor (≥3) outcomes at discharge. In addition, the proportion of patients who received rescue therapy for DCI was evaluated. The safety endpoints included death within 12 weeks after aneurysmal SAH and the incidence of symptomatic pulmonary edema defined by chest radiography.
Data are expressed as medians (interquartile range) for continuous variables and numbers (%) for categorical variables. Mann–Whitney U test was used for continuous variables, and Fisher’s exact test was used for categorical variables. The median value of each continuous variable was used as the cut-off value for multivariate analysis. As for vasospasm-related symptomatic infarct, multivariate logistic regression analysis was first adjusting for sex and age as underlying confounding factors. Next, we also added analysis in a model that additionally corrected for the WFNS grade or Fisher group associated with infarction. As for pulmonary edema, multivariate logistic regression analysis was adjusting for sex and age as underlying confounding factors. Statistical significance was set at p < 0.05. Statistical analyses were performed using JMP Pro version 16 (SAS Institute Inc.).
Of the 206 patients with aneurysmal SAH who underwent surgical intervention at our institute in the respective time, 181 were included in the present study (control group, n = 100; clazosentan group, n = 81) (Figure 2). Clazosentan was prematurely discontinued in two patients in the clazosentan group because of excessive fluid retention (n = 1) and hypotension (n = 1). The patient characteristics are presented in Table 1. The baseline patient characteristics were comparable between the control and clazosentan groups. Two-thirds of the patients were female, and the majority (80%) had aneurysms in the anterior circulation. More than 60% of the patients had WFNS grades I or II, and most (>80%) had thick and diffuse clots.
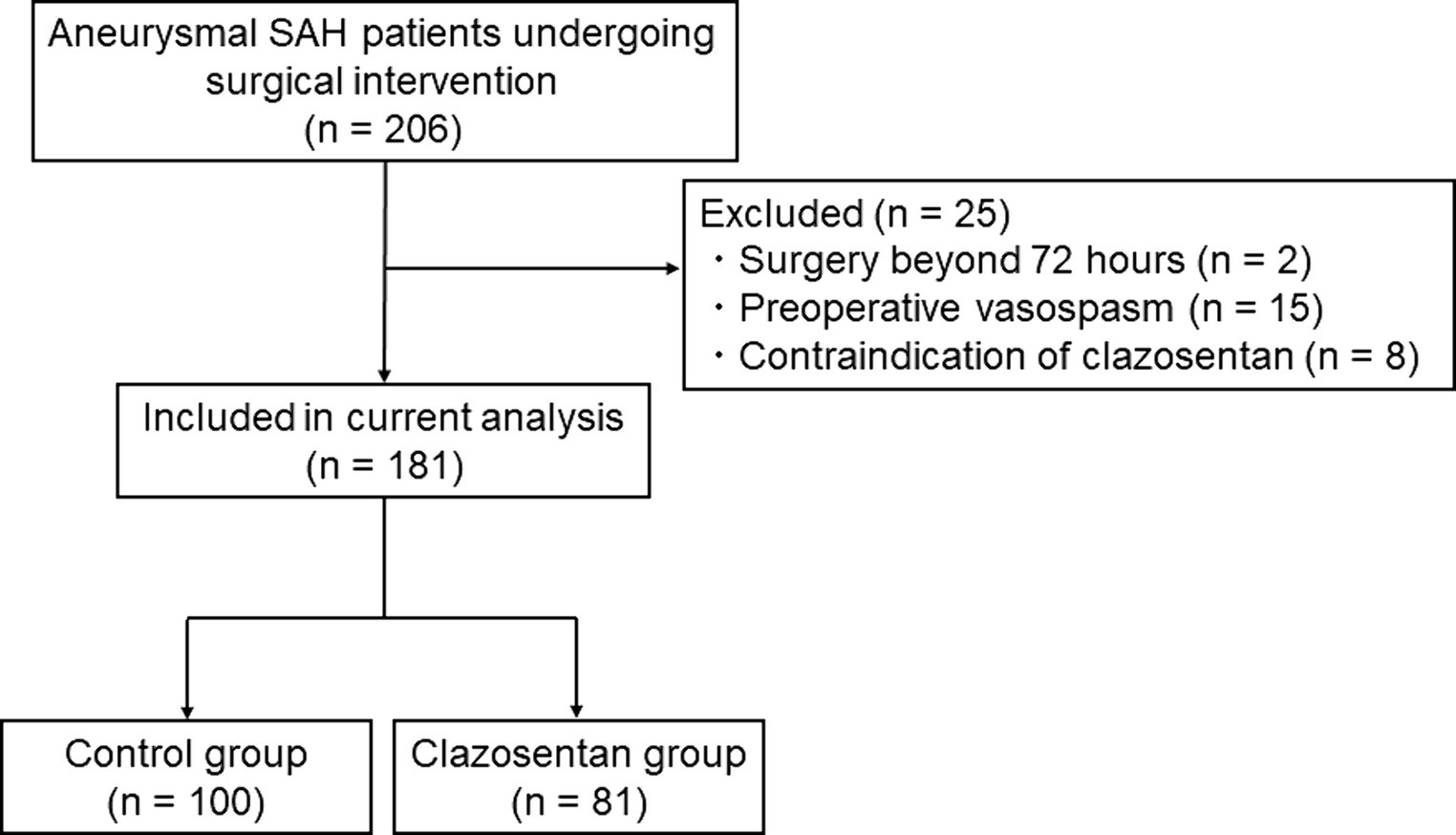
Figure 2. Flowchart showing the included and excluded patients with aneurysmal subarachnoid hemorrhage in this study (April 2021 to March 2023).
The postoperative outcomes are shown in Table 2. The primary efficacy endpoint reporting vasospasm-related symptomatic infarcts occurred in 6.2% (5/81) of the clazosentan group and 16.0% (16/100) of the control group, demonstrating that the incidence of vasospasm-related symptomatic infarcts was significantly lower in the clazosentan group (p = 0.032). However, there was no significant difference in the incidence of definite DCI (p = 0.19). The secondary efficacy endpoint, the incidence of moderate-to-severe angiographic vasospasm within 14 days post-aneurysmal SAH, occurred in 25.9% (21/81) of the clazosentan group and 44.0% (44/100) of the control group (p = 0.013). Interestingly, the incidence of vasospasm in the distal arteries was significantly lower in the clazosentan group (13.6% vs. 34.0%, p = 0.002), whereas no significant difference was observed in the proximal arteries (18.5% vs. 23.0%, p = 0.58). Analysis of another secondary efficacy endpoint revealed that favorable clinical outcomes at discharge (mRS <3) were significantly higher in the clazosentan group than in the control group (79.0% vs. 66.0%, p = 0.038). The need for rescue therapy was reported in 16.1% (13/ 81) in the clazosentan group and 34% (34/100) in the control group (p = 0.006).
Next, we investigated the risk factors for vasospasm-related symptomatic infarcts (Table 3). Univariate analysis showed that the administration of clazosentan was the only negative risk factor correlated with the occurrence of vasospasm-related symptomatic infarcts (p = 0.032). As shown in Table 3, after adjustment for the potential confounders of sex and age, administration of clazosentan was significantly associated with fewer vasospasm-related symptomatic infarcts (odds ratio 0.32 [0.11–0.95], p = 0.041).
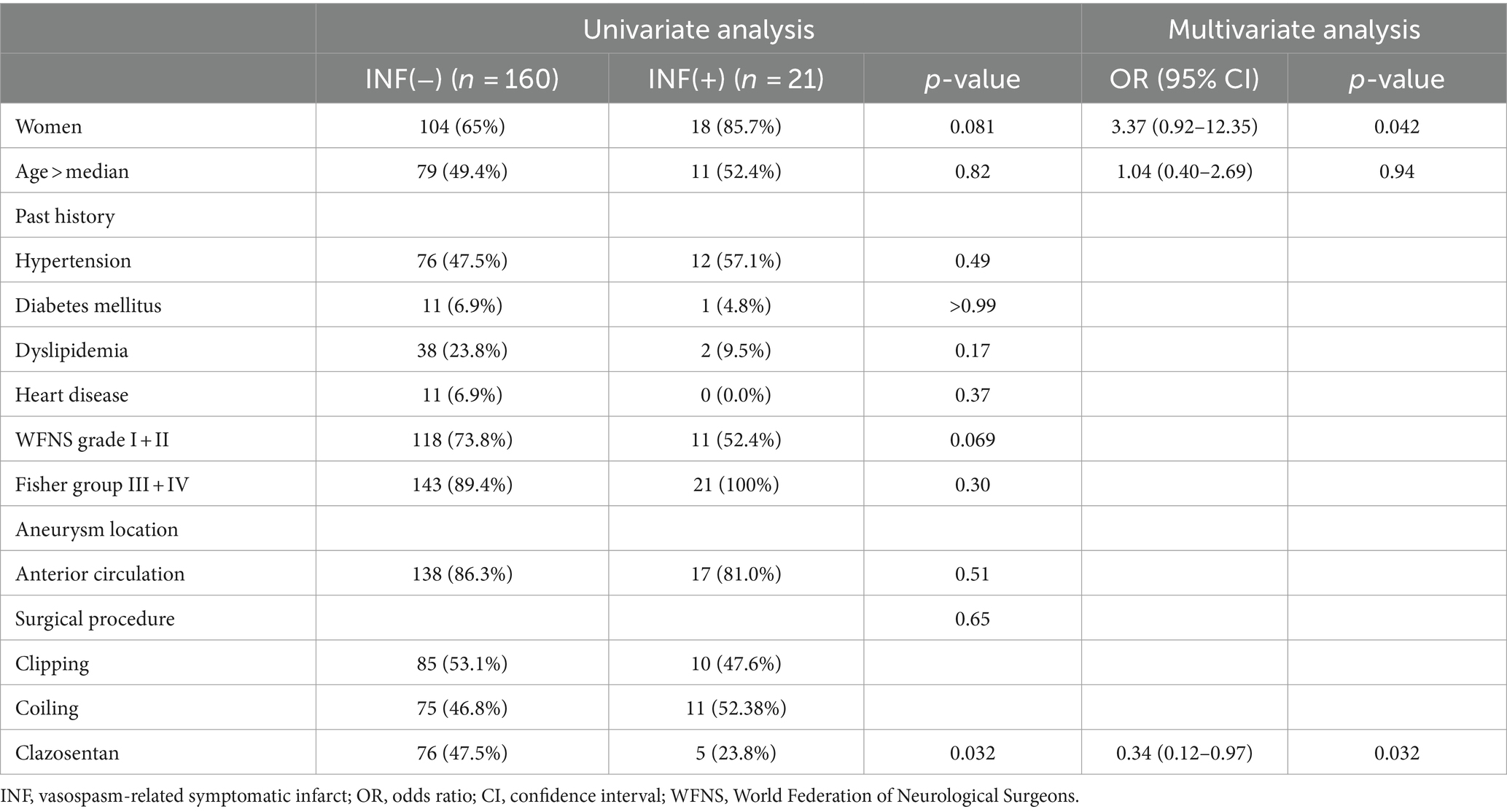
Table 3. Univariate and multivariate analysis for factors associated with vasospasm-related symptomatic infarct.
None of the patients in either group died within 12 weeks of the aneurysmal SAH. As shown in Table 2, the incidence of pulmonary edema was 19.8% (16/81) in the clazosentan group and 5.0% (5/100) in the control group, which was statistically significant (p = 0.002). Moreover, the body weight change during the vasospasm period showed a significant difference between the groups (2.5 kg vs. 1.0 kg, p < 0.001).
Next, we assessed the risk factors for pulmonary edema (Table 4). Univariate analysis demonstrated that age (p = 0.039), clazosentan administration (p = 0.002), and body weight change (p < 0.001) were significant factors associated with the incidence of pulmonary edema. As shown in Table 4, after adjustment for the potential confounders of sex and age, administration of clazosentan was significantly associated with pulmonary edema (odds ratio 4.58 [1.58–13.25], p = 0.005). None of the patients with pulmonary edema required ventilator management or experienced morbidity.
Here, we investigated the effectiveness of clazosentan in patients with aneurysmal SAH in real-world clinical practice. The major findings were as follows: (i) The incidence of vasospasm-related symptomatic infarction together with angiographic vasospasm was significantly lower in the clazosentan group than in the control group; (ii) the clazosentan protocol was associated with the better clinical outcomes at discharge compared with the conventional protocol using fasudil hydrochloride and cilostazol; and (iii) the occurrence of pulmonary edema was higher in the clazosentan group, although its complications did not affect morbidity and mortality. Based on these findings, this retrospective cohort study revealed the superiority of the clazosentan protocol over the conventional management protocol in preventing vasospasm-related ischemic events after aneurysmal SAH in the Japanese population.
As a conventional management protocol for aneurysmal SAH, we used fasudil hydrochloride and cilostazol, both of which are widely used in the prevention of vasospasm in Japan. Fasudil hydrochloride, a Rho-kinase inhibitor, is a potent vasodilator discovered and developed in Japan (13). Moreover, cilostazol, a selective inhibitor of phosphodiesterase 3, has also a vasodilatory effect on the cerebral arteries as well as an antiplatelet effect (14). Recent randomized phase 3 trials (6), which led to the approval of clazosentan in Japan, prohibited concomitant use of other anti-vasospasm drugs. Our study provides new evidence that clazosentan is better able to prevent vasospasm-related infarct than the conventional combination therapy with fasudil hydrochloride and cilostazol in real clinical practice.
Clazosentan is a selective endothelin receptor subtype A antagonist and endothelin-1 is a potent, long-lasting endogenous vasoconstrictor that has been implicated in the pathogenesis of vasospasm (15, 16). As in previous clinical trials, our study demonstrated that clazosentan significantly reduced angiographic vasospasm. Interestingly, in our study clazosentan prevented angiographic vasospasm not in the proximal cerebral arteries but in the distal cerebral arteries, which differs from the results of previous clinical trials which showed the anti-vasospastic effects of clazosentan both in the proximal and the distal cerebral arteries (6). This difference might be explained by the use of fasudil hydrochloride and cilostazol in the control group, which mainly targets proximal vasospasm (13, 14). In addition, clazosentan was reported to have a stronger effect on distal cerebral arteries than on proximal cerebral arteries (17). Our study has indicated the powerful anti-vasospastic effect of clazosentan over the current standard of care.
The improved clinical outcomes of clazosentan treatment were an important finding of this study. Other than the clinical trials reported by Endo et al. (6), several randomized controlled trials failed to show an improvement in clinical outcomes with clazosentan despite the reduction of angiographic vasospasm (2–5, 18). Although multifactorial causes of DCI, such as cortical spreading ischemia and microcirculatory dysfunction (19), might partly account for the negative findings (20), clazosentan is not conceptually effective for vasospasm-unrelated DCI. Moreover, the benefits in terms of improved clinical outcomes caused by the ability of clazosentan to reduce angiographic vasospasm may be offset by the adverse effects of the drug. Indeed, in our study, there was no significant decrease in DCI caused by clazosentan, despite a decrease in vasospasm-related symptomatic infarcts. Nevertheless, clazosentan improved the functional outcomes at discharge, probably because our clazosentan protocol with strict fluid management effectively prevented clazosentan-associated side effects and highlighted its anti-vasospastic effect. As we investigated the clinical outcomes only at discharge, further studies are warranted to assess the impact of clazosentan on long-term clinical outcomes.
Pulmonary edema associated with excess fluid balance is a major concern of clazosentan treatment, leading to discontinuation of clazosentan and outcome deterioration (11). The mechanism of action of clazosentan is not specific to the cerebral vessels, and could affect the cardiovascular and pulmonary systems (18). To counteract this complication, we made changes to the infusion management. When the water balance exceeded 500 ml/12 h, a reduction in fluid volume and intravenous injection of loop diuretics were considered. Using this infusion management regimen, no patients needed intensive care for pulmonary edema (i.e., ventilator management), and only two patients (2.5%) discontinued clazosentan due to clazosentan-associated side effects. Strict infusion management did not increase the risk of vasospasm or vasospasm-related infarcts. Therefore, strict infusion management combined with rescue loop diuretics may be reasonable to prevent adverse events when using clazosentan.
This study had several limitations. First, this was a retrospective, single-center study. A large, multicenter, prospective cohort trial of clazosentan versus conventional management protocols would be beneficial. Second, nimodipine was not used as a standard of care in the control group in this study because nimodipine, which has the best evidence for the treatment of DCI, has not been approved in Japan. Third, this study only presented short-term clinical outcomes. Long-term follow-up would be beneficial in the future study. Forth, the infusion management differed between the two protocols, which might have influenced the minor medical therapy-independent outcomes. Fifth, fasudil hydrochloride was used in some of the patients with probable DCI in the clazosentan group, which might have influenced the minor medical therapy outcomes.
A postoperative management protocol centering on clazosentan was associated with the reduced vasospasm-related symptomatic infarction and improved clinical outcomes compared to the conventional management protocol in Japanese clinical practice. These results suggest that clazosentan might be a promising treatment option for counteracting cerebral vasospasm after aneurysmal SAH.
The datasets presented in this article are not readily available because data are not publicly available due to ethical reasons. Further inquiries can be directed to the corresponding author. Requests to access the datasets should be directed to HS, c2FrYXRhQG5zZy5tZWQudG9ob2t1LmFjLmpw.
The studies involving humans were approved by Institutional Review Board of Kohnan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The requirement for informed consent was waived from the Institutional Review Board of Kohnan Hospital because the analysis used anonymous clinical data obtained after each patient agreed to the treatment and provided written consent. We used the opt-out method to obtain approval for this study using a poster approved by the Institutional Review Board.
HS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Visualization, Writing – original draft, Writing – review & editing. AK: Data curation, Writing – review & editing. HU: Data curation, Writing – review & editing. SH: Data curation, Writing – review & editing. SO: Formal analysis, Validation, Writing – review & editing. NK: Data curation, Writing – review & editing. MY: Data curation, Writing – review & editing. KN: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. TT: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. HE: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Japan Society for the Promotion of Science KAKENHI (grant number 23K08536).
The authors wish to thank Editage (www.editage.com) for the English language editing.
KN, TT, and HE declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: consigned research funds from Idorsia Pharmaceuticals Japan Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. de Oliveira, JG, Beck, J, Ulrich, C, Rathert, J, Raabe, A, and Seifert, V. Comparison between clipping and coiling on the incidence of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Neurosurg Rev. (2007) 30:22–31. doi: 10.1007/s10143-006-0045-5
2. Fujimura, M, Joo, JY, Kim, JS, Hatta, M, Yokoyama, Y, and Tominaga, T. Preventive effect of Clazosentan against cerebral vasospasm after clipping surgery for aneurysmal subarachnoid hemorrhage in Japanese and Korean patients. Cerebrovasc Dis. (2017) 44:59–67. doi: 10.1159/000475824
3. Macdonald, RL, Higashida, RT, Keller, E, Mayer, SA, Molyneux, A, Raabe, A, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. (2011) 10:618–25. doi: 10.1016/S1474-4422(11)70108-9
4. Macdonald, RL, Higashida, RT, Keller, E, Mayer, SA, Molyneux, A, Raabe, A, et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke. (2012) 43:1463–9. doi: 10.1161/STROKEAHA.111.648980
5. Macdonald, RL, Kassell, NF, Mayer, S, Ruefenacht, D, Schmiedek, P, Weidauer, S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. (2008) 39:3015–21. doi: 10.1161/STROKEAHA.108.519942
6. Endo, H, Hagihara, Y, Kimura, N, Takizawa, K, Niizuma, K, Togo, O, et al. Effects of clazosentan on cerebral vasospasm-related morbidity and all-cause mortality after aneurysmal subarachnoid hemorrhage: two randomized phase 3 trials in Japanese patients. J Neurosurg. (2022) 137:1707–17. doi: 10.3171/2022.2.JNS212914
7. Muraoka, S, Asai, T, Fukui, T, Ota, S, Shimato, S, Koketsu, N, et al. Real-world data of clazosentan in combination therapy for aneurysmal subarachnoid hemorrhage: a multicenter retrospective cohort study. Neurosurg Rev. (2023) 46:195. doi: 10.1007/s10143-023-02104-2
8. Kimura, T . Letter to the editor. Clazosentan was superior to placebo, but was the comparison appropriate? J Neurosurg. (2023) 139:299–300. doi: 10.3171/2023.1.JNS2316
9. Ishihara, H, and Suzuki, M. Japanese guidelines for the Management of Stroke 2015: overview of the chapter on subarachnoid hemorrhage. Nihon Rinsho. (2016) 74:677–80.
10. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, and Vandenbroucke, JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. (2007) 335:806–8. doi: 10.1136/bmj.39335.541782.AD
11. Vercauteren, M, Trensz, F, Pasquali, A, Cattaneo, C, Strasser, DS, Hess, P, et al. Endothelin ET(a) receptor blockade, by activating ET(B) receptors, increases vascular permeability and induces exaggerated fluid retention. J Pharmacol Exp Ther. (2017) 361:322–33. doi: 10.1124/jpet.116.234930
12. Vergouwen, MD, Vermeulen, M, van Gijn, J, Rinkel, GJE, Wijdicks, EF, Muizelaar, JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. (2010) 41:2391–5. doi: 10.1161/STROKEAHA.110.589275
13. Shibuya, M, Suzuki, Y, Sugita, K, Saito, I, Sasaki, T, Takakura, K, et al. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg. (1992) 76:571–7. doi: 10.3171/jns.1992.76.4.0571
14. Senbokuya, N, Kinouchi, H, Kanemaru, K, Ohashi, Y, Fukamachi, A, Yagi, S, et al. Effects of cilostazol on cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a multicenter prospective, randomized, open-label blinded end point trial. J Neurosurg. (2013) 118:121–30. doi: 10.3171/2012.9.JNS12492
15. Davenport, AP, Hyndman, KA, Dhaun, N, Southan, C, Kohan, DE, Pollock, JS, et al. Endothelin. Pharmacol Rev. (2016) 68:357–418. doi: 10.1124/pr.115.011833
16. Yanagisawa, M, Kurihara, H, Kimura, S, Tomobe, Y, Kobayashi, M, Mitsui, Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. (1988) 332:411–5. doi: 10.1038/332411a0
17. Higashida, RT, Bruder, N, Gupta, R, Guzman, R, Hmissi, A, Marr, A, et al. Reversal of vasospasm with Clazosentan after aneurysmal subarachnoid hemorrhage: a pilot study. World Neurosurg. (2019) 128:e639–48. doi: 10.1016/j.wneu.2019.04.222
18. Pontes, JPM, Santos, MDC, Gibram, FC, Rodrigues, NMV, Cavalcante-Neto, JF, Barros, ADM, et al. Efficacy and safety of Clazosentan after aneurysmal subarachnoid hemorrhage: an updated Meta-analysis. Neurosurgery. (2023) 93:1208–19. doi: 10.1227/neu.0000000000002601
19. Suzuki, H, Kanamaru, H, Kawakita, F, Asada, R, Fujimoto, M, and Shiba, M. Cerebrovascular pathophysiology of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Histol Histopathol. (2021) 36:143–58. doi: 10.14670/HH-18-253
Keywords: subarachnoid hemorrhage, cerebral vasospasm, delayed cerebral ischemia, delayed ischemic neurologic deficit, clazosentan, endothelin
Citation: Sakata H, Kanoke A, Uchida H, Haryu S, Omodaka S, Kimura N, Yoshida M, Niizuma K, Tominaga T and Endo H (2024) Prophylactic management of cerebral vasospasm with clazosentan in real clinical practice: a single-center retrospective cohort study. Front. Neurol. 15:1413632. doi: 10.3389/fneur.2024.1413632
Received: 07 April 2024; Accepted: 23 May 2024;
Published: 06 June 2024.
Edited by:
Masahito Kawabori, Hokkaido University, JapanReviewed by:
Fulvio Tartara, University Hospital of Parma, ItalyCopyright © 2024 Sakata, Kanoke, Uchida, Haryu, Omodaka, Kimura, Yoshida, Niizuma, Tominaga and Endo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hidenori Endo, aC1lbmRvQG5zZy5tZWQudG9ob2t1LmFjLmpw; Hiroyuki Sakata, c2FrYXRhQG5zZy5tZWQudG9ob2t1LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.