- 1Jinzhou Medical University, Jinzhou, China
- 2The Second Affiliated Hospital of China Medical University, Shenyang, China
- 3Department of Nuclear Medicine, Nanchong Central Hospital, Nanchong, China
- 4Department of Neurosurgery, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, China
Background: It remains unclear about the pathogenesis of intracranial aneurysms (IAs) in the setting of autoimmune disorders (ADs). However, the underlying systemic inflammatory characteristics of ADs may affect IAs through shared inflammatory pathways. Therefore, this study was conducted to explore the relationship between ADs and IAs and assess causal effects.
Methods: In this study, 6 common ADs were included to explore their causal relationship with IAs. Besides, a bidirectional two-sample univariable Mendelian randomization (UVMR) analysis was performed. In addition, the primary analysis was performed by the inverse variance weighted (IVW) and Bayesian weighted Mendelian randomization (BWMR) method, and a series of sensitivity analyses were performed to assess the robustness of the results. Further, the data related to ADs and IAs were collected from open genome-wide association study studies (GWASs) and the Cerebrovascular Disease Knowledge Portal (CDKP) (including 11,084 cases and 311,458 controls), respectively. These analyses were conducted based on both the East Asian and European populations. Moreover, 6 ADs were subject to grouping according to connective tissue disease, inflammatory bowel disease, and thyroid disease. On that basis, a multivariate MR (MVMR1) analysis was further performed to explore the independent causal relationship between each AD and IAs, and an MVMR 2 analysis was conducted to investigate such potential confounders as smoking, alcohol consumption, and systolic blood pressure. Finally, these results were verified based on the data from another GWAS of IAs.
Results: The UVMR analysis results demonstrated that systemic lupus erythematosus (SLE) was associated with a high risk of IAs in the East Asian population (IVW OR, 1.06; 95%CI, 1.02–1.11; p = 0.0065, UVMR), which was supported by the results of BWMR (OR, 1.06; 95%CI, 1.02–1.11; p = 0.0067, BWMR), MVMR1 (OR, 1.06; 95%CI, 1.01–1.10; p = 0.015, MVMR1), MVMR2 (OR, 1.05; 95%CI, 1.00–1.11; p = 0.049, MVMR2), and sensitivity analyses. The results in the validation group also suggested a causal relationship between SLE and IAs (IVW OR, 1.04; 95% CI, 1.00–1.09; p = 0.046). The reverse MR analysis results did not reveal a causal relationship between IAs and ADs.
Conclusion: In this MR study, SLE was validated to be a risk factor for IAs in the East Asian population. Therefore, the management of IAs in patients with SLE should be highlighted to avoid stroke events.
1 Introduction
Intracranial aneurysms (IAs) are defined as locally abnormal enlargement of the lumen of cerebral arteries due to congenital defects or external factors, exhibiting a tumor-like protrusion on the artery wall, namely, cystic or band-like changes in the structure (1, 2). As reported in imaging studies using arteriography and MRI, the incidence of IAs ranges from 0.5 to 3% in the general population. In a prevalence study based on the European population, aneurysms were detected in the MRI-based screening of approximately 1.8% of adult subjects. As per a cross-sectional study in China, 7% of adults aged 35–75 years were diagnosed with aneurysms during brain magnetic resonance angiography screening (3). Although the etiology of IAs has not been defined, their initiation and growth are correlated with flow-related wall shear stress, hereditary factors, and inflammation. It has been suggested that inflammation plays a pivotal role in the occurrence of IAs. Some diseases, such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Sjögren’s syndrome (SS), known for their systemic inflammatory attributes, might affect the development of IAs through inflammatory pathways (4). Therefore, this study was conducted to explore the relationship between autoimmune disorders (AD) and IAs. These findings may contribute to improving the screening of patients with cerebral aneurysms or identifying new therapeutic targets for the medical management of IAs.
Inflammation may be a major mechanism for impairing the vascular wall and is associated with the development of the aneurysm wall. The formation of abdominal aortic aneurysms has been validated to correlate with such autoimmune diseases as rheumatoid arthritis (RA), hypothyroidism, and SLE (5–7). IAs may follow a similar pathophysiological mechanism. According to several case reports, the causes of cerebral hemorrhage and IAs in people with SLE, RA, multiple sclerosis, sarcoidosis, and Sjögren’s syndrome (SS) may be related to the underlying mechanisms of autoimmune cerebrovascular inflammation (8–13). A smaller size of aneurysms at rupture was found in patients with ADs; Further, patients with ADs had significantly smaller average ruptured aneurysms than those without ADs, according to a retrospective review including 190 patients with ruptured and unruptured cystic IAs (4). As a result, these studies indicate that ADs might wield influence on the development of IAs. However, the impact of diverse confounders (like the usage of steroid hormones in treatment and the presence of hypertension) cannot be eliminated in existing studies. Furthermore, the limited scale of these studies curtails their capacity to thoroughly dissect the causal relationships.
Mendelian randomization (MR) is a causal inference method, which can be employed to adeptly probe the influence of modifiable exposure on diseases. It leverages genetic variation to furnish compelling evidence of robust associations (14). Besides, it harnesses the merits of consolidated statistics originating from extensive genome-wide association studies (GWASs) conducted across sweeping cohorts. Overall, this method makes use of bigger sample sizes and reduces potential biases that may occur in single-sample analyses to increase the accuracy of causal estimations in two-sample MR studies, in which the data from two independent sources are employed. To further clarify the causal relationship between ADs and IAs, multivariate MR (MVMR) and reverse MR analyses were also performed in this study, which may minimize confounders and exclude reverse causality, thus contributing to more stable and reliable results.
2 Materials and methods
2.1 Study design
Firstly, the instrumental variables (IVs) for ADs and IAs were curated based on summary statistics from both the European and East Asian populations. Besides, UVMR was utilized to estimate the causal impact of ADs on IAs. Additionally, 6 ADs were subject to grouping according to connective tissue disease, inflammatory bowel disease, and thyroid disease. Moreover, MVMR1 was conducted to discern the independent effects of each AD on IAs; MVMR2 was performed on the exposure, including alcohol consumption, smoking, and systolic blood pressure, to avoid the influence of confounders (15). To further fortify our analysis, a reverse MR was conducted to counteract the potential effects of reverse causality. The study design process is illustrated in Figure 1. Furthermore, another GWAS of IAs was selected for validation. The MR analysis should be performed based on three core assumptions: (1) there is a strong correlation between IVs and exposure; (2) there is independence between IVs and confounders; and (3) IVs can only exert an effect on outcomes through exposure.
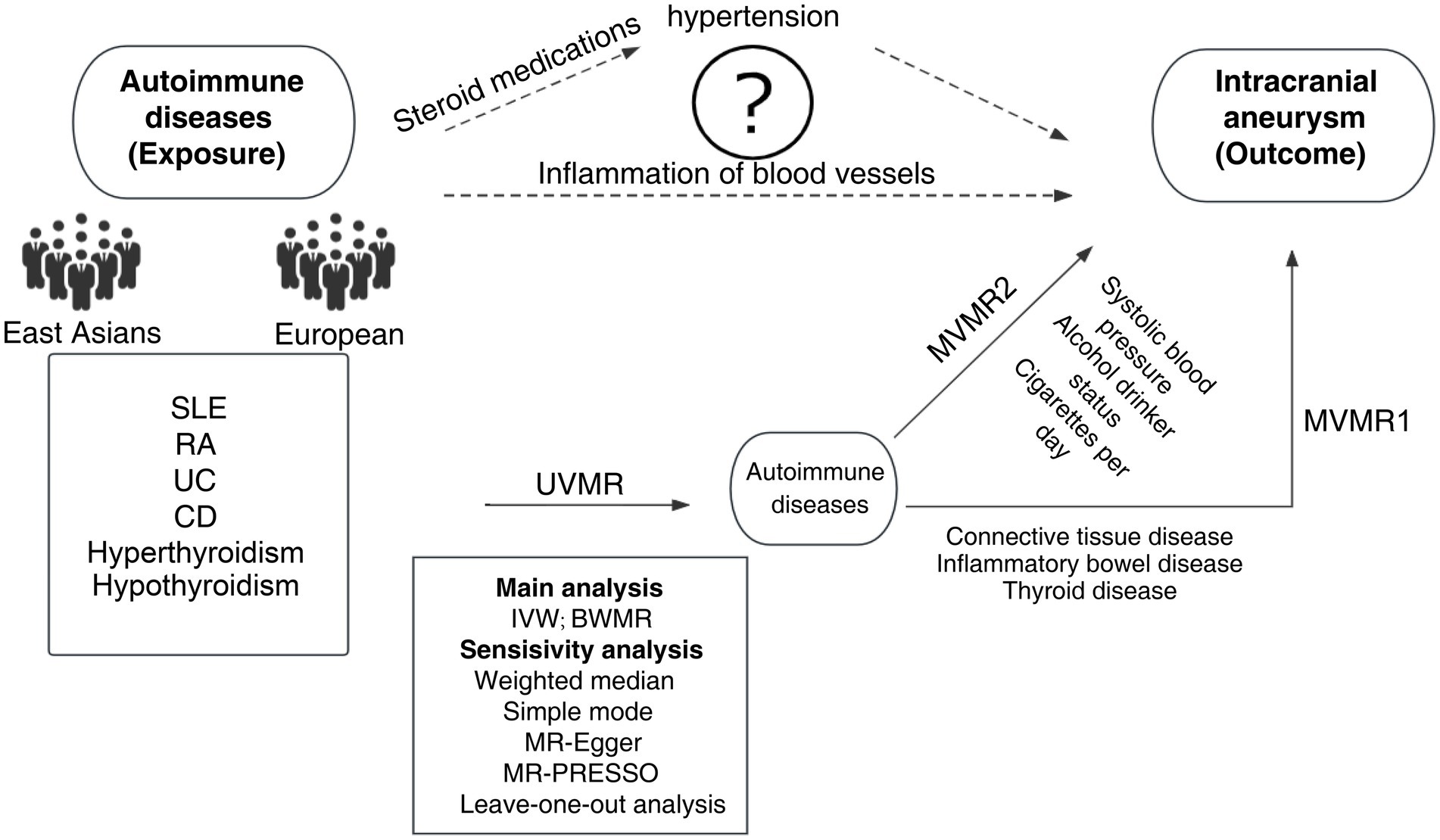
Figure 1. MR design and flowchart of this study. MR, Mendelian randomization; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; UC, ulcerative colitis; CD, Crohn’s disease; UVMR, univariable Mendelian randomization; MVMR, multivariate Mendelian randomization; BWMR, Bayesian weighted Mendelian randomization; IVW, inverse variance weighted; MR-PRESSO, MR pleiotropy residual sum and outlier test.
2.2 GWAS summary data for ADs
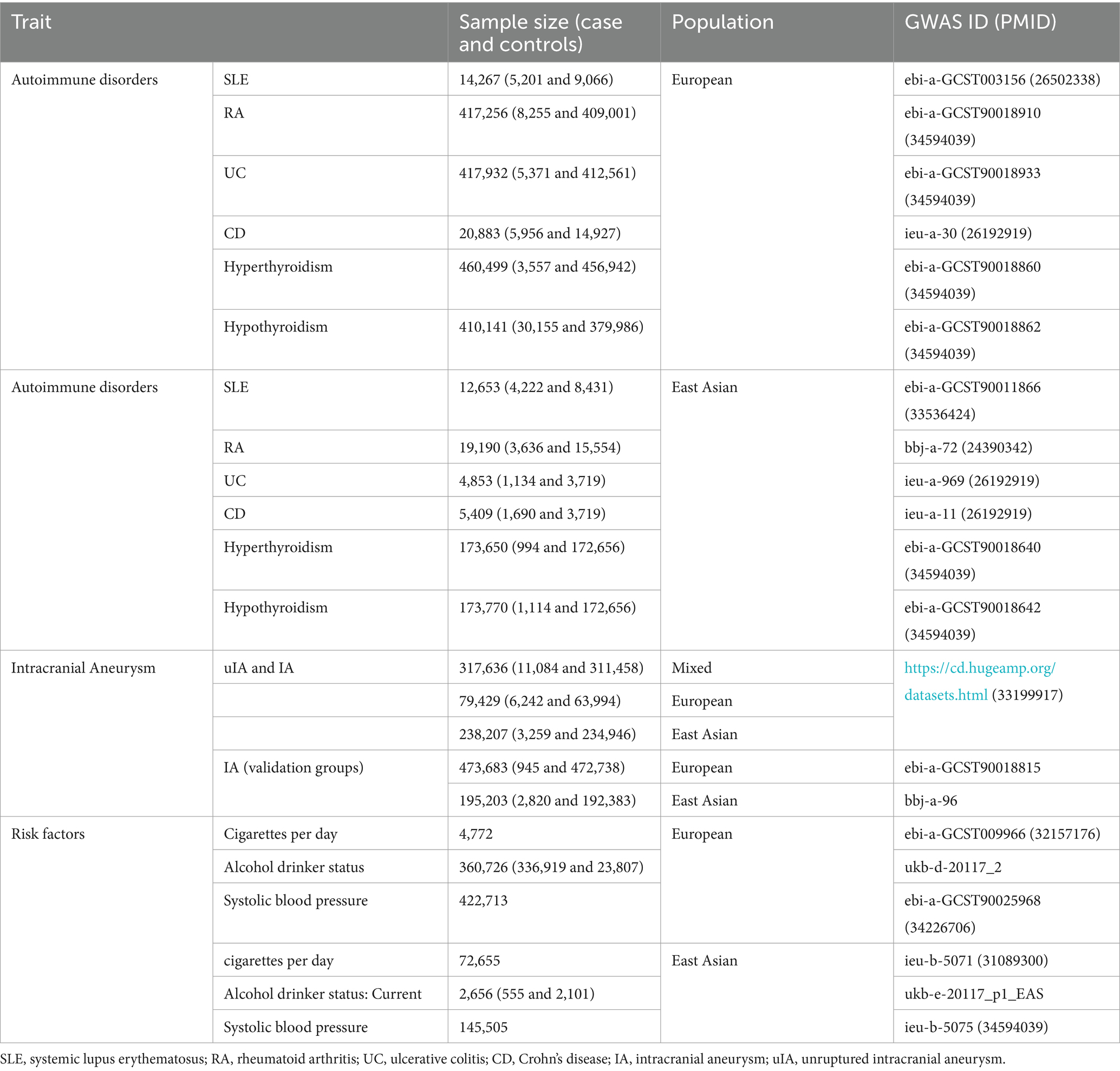
In this study, 6 common ADs, including SLE, RA, ulcerative colitis (UC), Crohn’s disease (CD), hyperthyroidism, and hypothyroidism, were included to explore the relationship between ADs and IAs. These data can be obtained from the website1 based on the GWAS ID in Table 1 or in the original GAWS article (16–19), which contained the GWAS data that were available to the public and did not require an ethical review. To acquire more significant research and dependable results, the data with the biggest sample size were also selected and then stratified based on the races in the European and East Asian populations. The details of these data are summarized in Table 1.
2.3 GWAS summary data for IAs
A GWAS including 11,084 cases and 311,458 controls of the European and East Asian populations yielded summary statistics for IAs. The summary data were collected from the CDKP (20). Among the GWAS summary data of IAs (ruptured and unruptured) (n = 6,242) against controls (n = 63,994) in the European population and those of IAs (ruptured and unruptured) (n = 3,259) against controls (n = 234,946) in the East Asian population, there were 2,662 cases and 164,009 controls from Biobank Japan (BBJ) and 597 cases and 70,939 controls from China Kadoorie Biobank (CKB).
In the validation group, the GWAS data of IAs were obtained from the OpenGWAS database, including 945 cases and 472,738 controls in the European population and 2,820 cases and 192,383 controls in the East Asian population. All details of these data are summarized in Table 1.
2.4 IV selection
Single nucleotide polymorphisms (SNPs), as IVs with a good correlation with each AD, were used in this MR study. Besides, a variety of quality control strategies were employed to filter eligible genetic IVs that conformed to the three main MR assumptions. Firstly, the SNPs associated with ADs were isolated from the entire genome (p < 5 × 10−8). For hyperthyroidism and hypothyroidism in the East Asian population, a threshold of p < 5 × 10−6 was set for more IVs. In addition, a threshold of r2 = 0.001 was used to reduce linkage imbalance and trimmed SNPs within a window size of 10,000 kb to ensure the independence of each IV (LD). Then, the pool of IVs and SNPs that were missing from the results was purged of palindromic SNPs. The same criteria were adopted in the validation group. Lastly, the F statistic for IVs was calculated to identify whether there was weak instrumental bias. Using the formula F = β2exposure/SE2 exposure (21), the F statistic was larger than 10, proving that there was no bias due to weak IVs (22).
2.5 Statistical analysis
R software 4.3.1, BWMR, and TwoSampleMR were used in this study. The IVW approach is the primary focus of UVMR, and it can be applied in the absence of potential horizontal pleiotropism (23). BWMR is causally inferred by the variational expectation–maximization (VEM) algorithm, which further considers the uncertainty of weak effects. The pleiotropy has been addressed by BWMR outlier detection, and the BWMR results are also reliable (24). In this study, MVMR derived from UVMR was also performed. In regression models, some key variables and outcomes are often used to perform univariate regression first, followed by the addition of confounders to conduct multivariate regression for corrections. The same is true in MR, which can be used to correct confounders, especially when multiple SNPs overlap. Subsequently, MR-Egger, simple mode, weighted median, and weighted model techniques were added to the IVW results (25). As long as the beta values of other methods are aligned in the same direction, significant results from the IVW method are taken into consideration meaningfully even when other methods are not. After the Bonferroni correction, a p value below 0.008 (0.05/6) was considered statistically significant. The presence of horizontal pleiotropy was identified using MR-Egger intercepts (26). Level pleiotropy outliers may be found using the MR-PRESSO framework, and outlier removal can be utilized to correct IVW estimates (27). To verify whether a particular outlier variable had an impact on the effect estimates, a stay-aside analysis was carried out. Moreover, Cochran’s Q test was performed by IVW and MR-Egger analyses to assess the heterogeneity of each SNP, and heterogeneity was indicated by Q-statistics with a p-value <0.05.
3 Results
3.1 MR results in the European population
In this study, 30, 11, 9, 36, 7, and 44 SNPs were incorporated in the UVMR for SLE, RA, UC, CD, hyperthyroidism, and hypothyroidism, respectively, within the European population. The F-statistic values of these SNPs all exceeded 10, with the average F-statistic values being 86.43, 52.10, 44.93, 64.97, 58.16, and 80.13, respectively, as listed in Supplementary Table S1. The absence of weak IVs was evident. However, no causal relationship between ADs and IAs was identified in the European population, as indicated by p-values exceeding 0.05 in each case (Figure 2; Supplementary Tables S2–S4). The scatter plot and forest plot of each outcome are illustrated in Supplementary Figures S2, S3. The MVMR results also revealed no causal relationship between ADs and IAs (Figure 2). The results of all BWMR analyses are presented in Supplementary Figures S4–S9. Similarly, the reverse MR analysis failed to reveal a causal relationship between IAs and ADs (Supplementary Tables S6, S7). Moreover, multiple sensitivity analyses were also conducted, revealing horizontal pleiotropy in hypothyroidism and IAs results, heterogeneity in CD and IAs results, and outliers identified by the MR-PRESSO analysis (Supplementary Table S5). In the reverse MR analysis, the MR-Egger method indicated no horizontal pleiotropy; the Q-test demonstrated heterogeneity in the results of SLE, UC, and CD; MR-PRESSO highlighted outliers in the results of UC and CD (Supplementary Table S8). The leave-one-out analysis confirmed that individual SNPs did not drive the results, as depicted in Supplementary Figure S1.
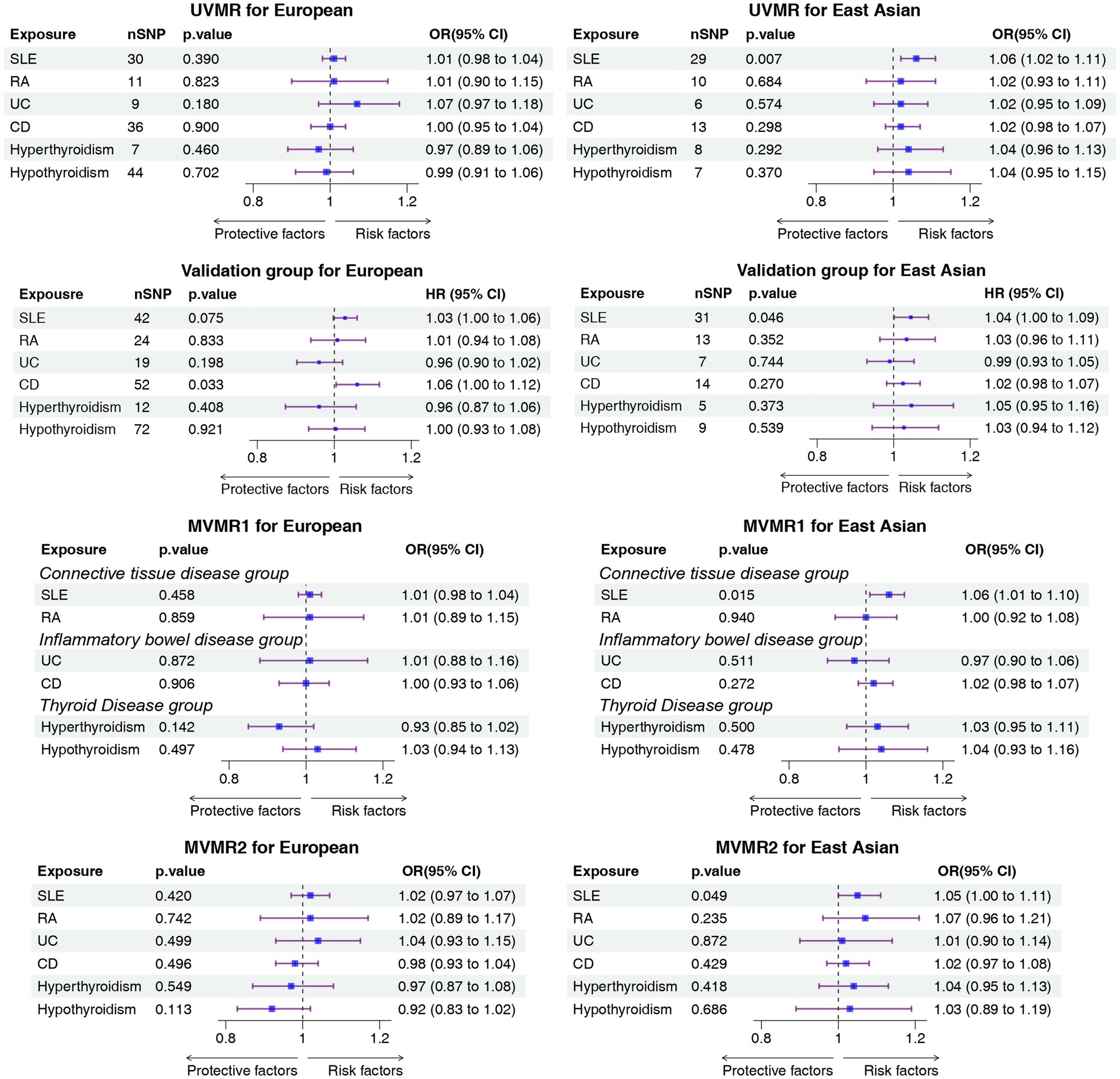
Figure 2. Forest plot of the causal association between ADs and IA. ADs, autoimmune disorders; IA, intracranial aneurysms; UVMR, univariable mendelian randomization; MVMR, multivariable mendelian randomization; OR, odds ratio; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; UC, ulcerative colitis; CD, Crohn’s disease.
3.2 MR results in the East Asian population
In this study, 29, 10, 6, 13, 8, and 7 SNPs were incorporated for SLE, RA, UC, CD, hyperthyroidism, and hypothyroidism, respectively, in the East Asian population. The F-statistic values of these SNPs all exceeded 10, with the average F-statistic values being 63.07, 67.77, 61.71, 72.63, 33.61, and 23.19, respectively (Supplementary Table S1). This confirmed the absence of weak IVs. According to the UVMR results, a statistically significant relationship between IA incidence and SLE was identified in the East Asian population (IVW OR, 1.06; 95% CI, 1.02–1.11; p = 0.0065, UVMR). This result was supported in BWMR (OR, 1.06; 95%CI, 1.02–1.11; p = 0.0067, BWMR) and MVMR1 (IVW OR, 1.06; 95% CI, 1.01–1.10; p = 0.015, MVMR1). In MVMR2, the relationship between SLE and IAs was weak but remained statistically significant (IVW OR, 1.05; 95% CI, 1.00–1.11; p = 0.049, MVMR2) (Figure 2; Supplementary Tables S2–S4). The scatter plot and forest plot of each outcome are depicted in Supplementary Figures S2, S3. The results of all BWMR analyses are presented in Supplementary Figures S10–S15. The reverse MR analysis did not reveal a causal relationship between IAs and ADs (Supplementary Tables S6, S7). Moreover, sensitivity analyses were also conducted, revealing no heterogeneity and no horizontal pleiotropy in all results, which was also supported by the MR-PRESSO results (Supplementary Tables S5, S8). Furthermore, it was also confirmed that the causal effect was not driven by a single SNP (Supplementary Figure S2).
3.3 Validation groups
In the East Asian population, the MR results suggested a causal relationship between SLE and IAs (IVW OR, 1.04; 95% CI, 1.00–1.09; p = 0.046), and this result was also supported by sensitivity analyses. In the European population, the MR results suggested a causal relationship between CD and IAs, but the MR-PRESSO results indicated the presence of pleiotropy. Relevant results are summarized in Figure 2, and detailed results are listed in the Supplementary materials.
4 Discussion
To the best of our knowledge, this is the first study to employ the MR method and GWAS summary statistics to investigate the causal relationship between ADs and the risk of intracranial aneurysms (IAs) in both the European and East Asian populations. Within the univariable Mendelian randomization (UVMR) and MVMR analyses, a heightened incidence of IAs was observed in systemic lupus erythematosus (SLE) within the East Asian population. This result was also supported in the validation group. However, there was no causal relationship identified between the other five ADs and IAs in both populations.
Currently, it remains unclear about the pathogenesis of IAs, particularly in the context of ADs. It has been validated in numerous epidemiological studies on IAs that IAs have a potential link with the occurrence and development of ADs (3). In the absence of pathological confirmation, most studies have proposed two hypotheses to explain the reason why ADs increase the risk of IAs (4, 28, 29) (Figure 1). IAs may result from inflammatory pathways, which may be potentially exacerbated by ADs. Hemodynamic stress can cause endothelial damage, and endothelial dysfunction along with vasculitis may contribute to the development of IAs (30, 31). The infiltration of inflammatory cells, including macrophages, monocytes, mast cells (32), and T lymphocytes, as well as complement activation (32–38), has been confirmed in all IAs. Berry aneurysms, or parts thereof, are reported to be autoimmune and can be attributed to inflammatory diseases in the blood vessels. This is partially supported by the identification of inflammatory infiltrates in the wall of IAs (39). A case–control study revealed an independent relationship between hypothyroidism and unruptured intracranial aneurysm (uIA). Autoimmune hypothyroidism may induce endothelial dysfunction through a chronic inflammatory mechanism mediated by inflammatory cytokines (6). IAs may be linked to the administration of steroid drugs in patients with ADs. Classic risk factors such as atherosclerosis and hypertension are complications associated with SLE patients (29, 40). Steroids and/or cytotoxic immunosuppressants are used in the treatment of SLE in the acute phase. The side effects of these agents can also lead to hypertension. Saccular aneurysms are associated with atherosclerosis and hypertension. In a Taiwanese study, the administration of relatively high average daily doses of steroids was identified as an independent risk factor for the increased risk of subarachnoid hemorrhage (SAH) in SLE patients (41). These two hypotheses are purely theoretical speculations. They may be independent or they may represent common pathways leading to the occurrence of IAs. Combined with the findings of this study, it is necessary to conduct further experimental verification.
As an autoimmune disease, SLE is more common in young females and exhibits the highest incidence in China (about 0.07%) (42). Numerous studies have found an association between SLE and IAs (29, 41, 43, 44). In a study comparing the cohorts of immune-mediated diseases with the control cohorts to calculate the SAH rate, patients with SLE exhibited the highest risk among all immune-mediated diseases (RR = 3.76, 3.08–4.55) (28). Endothelial dysfunction and vasculitis in SLE patients may contribute to the development of IAs (45, 46). In contrast to the general population, the proportion of saccular aneurysms is lower in SLE patients, while more common aneurysms such as spindle aneurysms or pseudoaneurysms are prevalent among these patients (29, 46, 47). Typically, patients experiencing SAH with vascular-negative aneurysms tend to be older and have a benign clinical course (48). Conversely, SLE patients presenting with SAH and angionegative aneurysms are usually relatively young and face a high mortality rate (29, 46, 47). The higher incidence of multiple aneurysms is another characteristic of SLE patients, with the incidence of single and multiple aneurysms in SLE patients being 69.5 and 31.6%, respectively (49).
The mechanisms by which SLE leads to IAs have not been defined. Most scholars attribute IAs to the inflammation of intracranial arteries or artery walls caused by SLE, but the specific molecular mechanisms have not been sufficiently clarified, which requires further basic experimental research. In large-scale studies on SLE in North America and Europe, the incidence of clinically defined SAH in patients with central nervous system involvement is about 0.3% (50–53). A Japanese study showed that the prevalence of SLE in the Japanese population and the prevalence of SHA in SLE patients were 1.28 and 3.9%, respectively, which were higher than those in Western countries (53). In this study, a correlation between SLE and IAs was only found in the East Asian population. However, the reasons and mechanisms for this difference between the two populations are currently unknown. It may be attributed to the differences in the data itself or inherent racial differences, which should be explored in further research.
In contrast to previous observational studies, several critical advantages augment the credibility and robustness of the conclusions in this study. Firstly, to the best of our knowledge, this study represents the first attempt to explore the causal relationship between ADs and IAs through a two-sample MR analysis. This innovative approach enabled us to thoroughly and rigorously examine the causal effects of ADs on IAs. Additionally, a range of sophisticated MR techniques, encompassing bidirectional MR analysis, MVMR analysis, and MR-PRESSP methods, were used in this study. The bidirectional MR analysis allowed the scrutinization of the potential influence of reverse causality, while MVMR further enabled an exploration of intricate interactions between ADs and IAs. The MR-PRESSO method effectively avoided potential abnormalities. Furthermore, a series of stringent sensitivity analyses, including checks for heterogeneity and pleiotropy, were conducted to substantiate the reliability of our results. Finally, our results were also validated in the validation group to make the results more reliable.
Nevertheless, there are still several inherent limitations in this study. Firstly, we conducted relevant explorations based on the East Asian and European populations, not global populations, and hence caution is needed when applying the results to other populations. Secondly, the results of our study differed in both populations. Therefore, further research is needed to identify the significance of this difference. Thirdly, the aneurysm GWAS data including both uIA and ruptured IAs were collected in this study, but data limitations cannot be avoided. Fourthly, although causal effects can be derived by MR methods, the limitations of this study are still obvious due to a lack of prospective studies. Finally, only 6 ADs were included in this study, and there may be other ADs associated with IAs, which may be attributed to a lack of data on autoimmune disease-associated GWASs in the East Asian population. After the data that were pleiotropic and unreliable in results were excluded, these 6 autoimmune diseases were incorporated into this study, thus ensuring the consistency in the studies in both populations.
5 Conclusion
In conclusion, the results of this study suggested that SLE was associated with a higher risk of IAs in the East Asian population. This underscored the pivotal clinical importance for healthcare practitioners to promptly recognize and diagnose IA patients within the framework of SLE. Therefore, strengthening cerebrovascular disease management in individuals with SLE becomes imperative, which may contribute to mitigating the morbidity and mortality associated with aneurysmal SAH in this patient population.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
CT: Conceptualization, Data curation, Software, Visualization, Writing – original draft, Writing – review & editing. RR: Methodology, Writing – original draft. BP: Conceptualization, Investigation, Writing – original draft. MX: Project administration, Validation, Writing – original draft. JH: Data curation, Resources, Writing – review & editing. ZX: Formal analysis, Supervision, Writing – original draft. ZZ: Data curation, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1412114/full#supplementary-material
Footnotes
References
1. Chalouhi, N, Ali, MS, Starke, RM, Jabbour, PM, Tjoumakaris, SI, Gonzalez, LF, et al. Cigarette smoke and inflammation: role in cerebral aneurysm formation and rupture. Mediat Inflamm. (2012) 2012:271582:1–12. doi: 10.1155/2012/271582
2. Keedy, A. An overview of intracranial aneurysms. McGill J Med. (2006) 9:141–6. doi: 10.26443/mjm.v9i2.672
3. Brown, RD Jr, and Broderick, JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol. (2014) 13:393–404. doi: 10.1016/s1474-4422(14)70015-8
4. Matur, AV, Yamani, AS, Robinson, MW, Smith, MS, Shirani, P, Grossman, AW, et al. Association between underlying autoimmune disease and small aneurysm size at rupture. J Neurosurg. (2023) 138:701–8. doi: 10.3171/2022.5.Jns22750
5. Pujades-Rodriguez, M, Duyx, B, Thomas, SL, Stogiannis, D, Rahman, A, Smeeth, L, et al. Rheumatoid arthritis and incidence of twelve initial presentations of cardiovascular disease: a population record-linkage cohort study in England. PLoS One. (2016) 11:e0151245. doi: 10.1371/journal.pone.0151245
6. Kurata, A, Kawakami, T, Sato, J, Sakamoto, A, Muramatsu, T, and Nakabayashi, K. Aortic aneurysms in systemic lupus erythematosus: a Meta-analysis of 35 cases in the literature and two different pathogeneses. Cardiovasc Pathol. (2011) 20:e1–7. doi: 10.1016/j.carpath.2010.01.003
7. Savage, C, Deanfield, JE, and Jung, RT. Aortic aneurysm in a patient with long-standing hypothyroidism. Postgrad Med J. (1982) 58:706–7. doi: 10.1136/pgmj.58.685.706
8. Hayashi, K, Morofuji, Y, Suyama, K, and Nagata, I. Recurrence of subarachnoid hemorrhage due to the rupture of cerebral aneurysms in a patient with Sjögren's syndrome. Case Report. Neurol Med Chir (Tokyo). (2010) 50:658–61. doi: 10.2176/nmc.50.658
9. Komatsu, T, Mitsumura, H, Yuki, I, and Iguchi, Y. Primary Sjögren's syndrome presenting with multiple aneurysmal dilatation of cerebral arteries and causing repetitive intracranial hemorrhage. J Neurol Sci. (2016) 365:124–5. doi: 10.1016/j.jns.2016.04.035
10. O'Dwyer, JP, Al-Moyeed, BA, Farrell, MA, Pidgeon, CN, Collins, DR, Fahy, A, et al. Neurosarcoidosis-related intracranial Haemorrhage: three new cases and a systematic review of the literature. Eur J Neurol. (2013) 20:71–8. doi: 10.1111/j.1468-1331.2012.03783.x
11. Walker, M, Young, CC, Levitt, MR, and Saal-Zapata, G. Multiple sclerosis in patients with intracranial aneurysms: coincidence or correlation? Clin Neurol Neurosurg. (2020) 195:105864. doi: 10.1016/j.clineuro.2020.105864
12. Uekawa, K, Kaku, Y, Amadatsu, T, Matsuzaki, H, Ohmori, Y, Kawano, T, et al. Intracranial and Extracranial multiple arterial dissecting aneurysms in rheumatoid arthritis: a case report. Interv Neuroradiol. (2021) 27:212–8. doi: 10.1177/1591019920965359
13. Jang, DI, Lee, AH, Shin, HY, Song, HR, Park, JH, Kang, TB, et al. The role of tumor necrosis factor alpha (Tnf-Α) in autoimmune disease and current Tnf-Α inhibitors in therapeutics. Int J Mol Sci. (2021) 22:2719. doi: 10.3390/ijms22052719
14. Davey Smith, G, and Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
15. Zhou, W, Cai, J, Li, Z, and Lin, Y. Association of Atopic Dermatitis with autoimmune diseases: a bidirectional and multivariable two-sample Mendelian randomization study. Front Immunol. (2023) 14:1132719. doi: 10.3389/fimmu.2023.1132719
16. Bentham, J, Morris, DL, Graham, DSC, Pinder, CL, Tombleson, P, Behrens, TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. (2015) 47:1457–64. doi: 10.1038/ng.3434
17. Sakaue, S, Kanai, M, Tanigawa, Y, Karjalainen, J, Kurki, M, Koshiba, S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. (2021) 53:1415–24. doi: 10.1038/s41588-021-00931-x
18. Liu, JZ, van Sommeren, S, Huang, H, Ng, SC, Alberts, R, Takahashi, A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. (2015) 47:979–86. doi: 10.1038/ng.3359
19. Okada, Y, Wu, D, Trynka, G, Raj, T, Terao, C, Ikari, K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. (2014) 506:376–81. doi: 10.1038/nature12873
20. Bakker, MK, van der Spek, RAA, van Rheenen, W, Morel, S, Bourcier, R, Hostettler, IC, et al. Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat Genet. (2020) 52:1303–13. doi: 10.1038/s41588-020-00725-7
21. Pierce, BL, Ahsan, H, and Vanderweele, TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
22. Pierce, BL, and Burgess, S. Efficient Design for Mendelian Randomization Studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. (2013) 178:1177–84. doi: 10.1093/aje/kwt084
23. Bowden, J, Davey Smith, G, and Burgess, S. Mendelian randomization with invalid instruments: effect estimation and Bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
24. Zhao, J, Ming, J, Hu, X, Chen, G, Liu, J, and Yang, C. Bayesian weighted Mendelian randomization for causal inference based on summary statistics. Bioinformatics. (2020) 36:1501–8. doi: 10.1093/bioinformatics/btz749
25. Burgess, S, Bowden, J, Fall, T, Ingelsson, E, and Thompson, SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. (2017) 28:30–42. doi: 10.1097/ede.0000000000000559
26. Bowden, J, Del Greco, MF, Minelli, C, Davey Smith, G, Sheehan, N, and Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. (2017) 36:1783–802. doi: 10.1002/sim.7221
27. Verbanck, M, Chen, CY, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
28. Ramagopalan, SV, Pakpoor, J, Seminog, O, Goldacre, R, Graham, L, and Goldacre, MJ. Risk of subarachnoid Haemorrhage in people admitted to hospital with selected immune-mediated diseases: record-linkage studies. BMC Neurol. (2013) 13:176. doi: 10.1186/1471-2377-13-176
29. Tsukamoto, E, Tanei, T, Senda, J, Kato, T, Naito, T, Ishii, K, et al. Subarachnoid hemorrhage after ischemic stroke associated with systemic lupus erythematosus and antiphospholipid syndrome. World Neurosurg. (2020) 136:248–52. doi: 10.1016/j.wneu.2020.01.030
30. Frösen, J, Cebral, J, Robertson, AM, and Aoki, T. Flow-induced, inflammation-mediated Arterial Wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg Focus. (2019) 47:E21. doi: 10.3171/2019.5.Focus19234
31. Turjman, AS, Turjman, F, and Edelman, ER. Role of fluid dynamics and inflammation in intracranial aneurysm formation. Circulation. (2014) 129:373–82. doi: 10.1161/circulationaha.113.001444
32. Ishibashi, R, Aoki, T, Nishimura, M, Hashimoto, N, and Miyamoto, S. Contribution of mast cells to cerebral aneurysm formation. Curr Neurovasc Res. (2010) 7:113–24. doi: 10.2174/156720210791184916
33. Krex, D, Schackert, HK, and Schackert, G. Genesis of cerebral aneurysms--an update. Acta Neurochir. (2001) 143:429–48. doi: 10.1007/s007010170072
34. Chalouhi, N, Ali, MS, Jabbour, PM, Tjoumakaris, SI, Gonzalez, LF, Rosenwasser, RH, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. (2012) 32:1659–76. doi: 10.1038/jcbfm.2012.84
35. Frösen, J, Tulamo, R, Paetau, A, Laaksamo, E, Korja, M, Laakso, A, et al. Saccular intracranial aneurysm: pathology and mechanisms. Acta Neuropathol. (2012) 123:773–86. doi: 10.1007/s00401-011-0939-3
36. Chyatte, D, Bruno, G, Desai, S, and Todor, DR. Inflammation and intracranial aneurysms. Neurosurgery. (1999) 45:1137–46. doi: 10.1097/00006123-199911000-00024
37. Frösen, J, Piippo, A, Paetau, A, Kangasniemi, M, Niemelä, M, Hernesniemi, J, et al. Remodeling of saccular cerebral artery Aneurysm Wall is associated with rupture: histological analysis of 24 Unruptured and 42 ruptured cases. Stroke. (2004) 35:2287–93. doi: 10.1161/01.STR.0000140636.30204.da
38. Tulamo, R, Frösen, J, Junnikkala, S, Paetau, A, Kangasniemi, M, Peláez, J, et al. Complement system becomes activated by the classical pathway in intracranial aneurysm walls. Lab Investig. (2010) 90:168–79. doi: 10.1038/labinvest.2009.133
39. Tulamo, R, Frösen, J, Hernesniemi, J, and Niemelä, M. Inflammatory changes in the Aneurysm Wall: a review. J Neurointerv Surg. (2018) 10:i58–67. doi: 10.1136/jnis.2009.002055.rep
40. Tang, SC, Lee, CF, Lee, CW, and Jeng, JS. Systemic lupus erythematosus flare up manifestation as cerebral and spinal subarachnoid hemorrhage. Lupus. (2011) 20:1211–3. doi: 10.1177/0961203311399305
41. Chang, YS, Liu, CJ, Chen, WS, Lai, CC, Wang, SH, Chen, TJ, et al. Increased risk of subarachnoid hemorrhage in patients with systemic lupus erythematosus: a Nationwide population-based study. Arthritis Care Res (Hoboken). (2013) 65:601–6. doi: 10.1002/acr.21846
42. Kiriakidou, M, and Ching, CL. Systemic lupus erythematosus. Ann Intern Med. (2020) 172:ITC81–96. doi: 10.7326/aitc202006020
43. Baizabal Carvallo, JF, Cantú Brito, C, Estañol, B, and García Ramos, GS. Subarachnoid hemorrhage as a complication of systemic lupus erythematosus. Cerebrovasc Dis. (2007) 24:301–4. doi: 10.1159/000105684
44. Shaban, A, and Leira, EC. Neurological complications in patients with systemic lupus erythematosus. Curr Neurol Neurosci Rep. (2019) 19:97. doi: 10.1007/s11910-019-1012-1
45. Fayyaz, A, Igoe, A, Kurien, BT, Danda, D, James, JA, Stafford, HA, et al. Haematological manifestations of lupus. Lupus Sci Med. (2015) 2:e000078. doi: 10.1136/lupus-2014-000078
46. Feigin, VL, Rinkel, GJ, Lawes, CM, Algra, A, Bennett, DA, van Gijn, J, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. (2005) 36:2773–80. doi: 10.1161/01.STR.0000190838.02954.e8
47. Torné, R, Rodríguez-Hernández, A, Bernard, T, Arikan Abelló, F, Vilalta Castan, J, and Sahuquillo, J. Subarachnoid hemorrhage in systemic lupus erythematosus: systematic review and report of three cases. Clin Neurol Neurosurg. (2015) 128:17–24. doi: 10.1016/j.clineuro.2014.10.018
48. van Gijn, J, Kerr, RS, and Rinkel, GJ. Subarachnoid Haemorrhage. Lancet. (2007) 369:306–18. doi: 10.1016/s0140-6736(07)60153-6
49. Owada, T, Takahashi, K, and Kita, Y. Subarachnoid hemorrhage in systemic lupus erythematosus in Japan: two case reports and a review of the literature. Mod Rheumatol. (2009) 19:573–80. doi: 10.3109/s10165-009-0206-9
50. Kaell, AT, Shetty, M, Lee, BC, and Lockshin, MD. The diversity of neurologic events in systemic lupus erythematosus. Prospective clinical and computed tomographic classification of 82 events in 71 patients. Arch Neurol. (1986) 43:273–6. doi: 10.1001/archneur.1986.00520030063016
51. Futrell, N, and Millikan, C. Frequency, etiology, and prevention of stroke in patients with systemic lupus erythematosus. Stroke. (1989) 20:583–91. doi: 10.1161/01.str.20.5.583
52. Sibley, JT, Olszynski, WP, Decoteau, WE, and Sundaram, MB. The incidence and prognosis of central nervous system disease in systemic lupus erythematosus. J Rheumatol. (1992) 19:47–52.
Keywords: autoimmune disorders, intracranial aneurysms, Mendelian randomization, SNPs, multivariable Mendelian randomization, causal relationship
Citation: Tang C, Ruan R, Pan B, Xu M, Huang J, Xiong Z and Zhang Z (2024) The relationship between autoimmune disorders and intracranial aneurysms in East Asian and European populations: a bidirectional and multivariable two-sample Mendelian randomization study. Front. Neurol. 15:1412114. doi: 10.3389/fneur.2024.1412114
Edited by:
Stefano Maria Priola, Health Sciences North, CanadaReviewed by:
Abhijith Matur, University of Cincinnati, United StatesLinshuoshuo Lyu, Vanderbilt University Medical Center, United States
Copyright © 2024 Tang, Ruan, Pan, Xu, Huang, Xiong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenxing Zhang, emhlbnhpbmcxOTc0QDE2My5jb20=
 Chao Tang
Chao Tang Rongcheng Ruan1
Rongcheng Ruan1