
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 13 June 2024
Sec. Neurorehabilitation
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1402129
Objective: There is currently a lack of evidence in evidence-based medicine regarding acupuncture treatment for experimental intracerebral hemorrhage (ICH). The aim of this study was to systematically evaluate the efficacy of acupuncture treatment for experimental ICH based on neurological function scores and brain water content (BWC).
Methods: Eight mainstream Chinese and English databases were searched. Outcome measures included neurological function scores and BWC, and subgroup analysis was conducted based on study characteristics.
Results: A total of 32 studies were included. Meta-analysis results indicated that compared to the control group, the acupuncture group showed significant reductions in mNSS (MD = −3.16, p < 0.00001), Bederson score (MD = −0.99, p < 0.00001), Longa score (MD = −0.54, p < 0.0001), and brain water content (MD = −5.39, p < 0.00001). Subgroup analysis revealed that for mNSS, the autologous blood model (MD = −3.36) yielded better results than the collagenase model (MD = −0.92, p < 0.00001), and simple fixation (MD = −3.38) or no fixation (MD = −3.39) was superior to sham acupuncture (MD = −0.92, p < 0.00001). For BWC, the autologous blood model (MD = −7.73) outperformed the collagenase model (MD = −2.76, p < 0.00001), and GV20–GB7 (MD = −7.27) was more effective than other acupuncture points (MD = −2.92, p = 0.0006).
Conclusion: Acupuncture significantly improves neurological deficits and brain edema in experimental ICH. Acupuncture at GV20 - GB7 is more effective than at other points. These findings support further studies to translate acupuncture into clinical treatment for human ICH.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023435584.
Intracerebral hemorrhage (ICH) is the subtype of stroke with the highest mortality and disability rates worldwide, with an early mortality rate of up to 40%, significantly impacting human health (1). General therapies for ICH include early airway protection, urgent reversal of coagulation disorders, control of malignant hypertension, and surgical interventions (2). Additionally, stem cells, melatonin, and other agents have shown significant improvement in pathological indicators post-ICH (3, 4). Therefore, seeking effective therapies is crucial for achieving a favorable prognosis after ICH.
Acupuncture is recognized by the World Health Organization (WHO) as an effective treatment for stroke. Studies have shown that acute head acupuncture intervention can improve Clinical Neurological Function Deficit Scale scores in ICH patients (5). Furthermore, Li et al. reported that early head acupuncture intervention can improve the level of consciousness and serum brain-derived neurotrophic factor levels in ICH patients (6). Numerous preclinical studies on ICH have demonstrated that acupuncture can improve neurological function in ICH rats by modulating inflammatory factors, cell apoptosis, autophagy, and other mechanisms, thereby alleviating pathological changes in brain tissue (7–12). Therefore, a comprehensive analysis of the effectiveness of acupuncture in treating ICH is necessary.
Meta-analysis is a systematic statistical method aimed at combining and analyzing results from multiple independent studies to obtain conclusions with greater statistical significance and reliability. Two meta-analyses have shown that acupuncture therapy can effectively alleviate myofascial pain and pain caused by gastric cancer (13, 14). This article provides a systematic evaluation of the efficacy of acupuncture intervention in experimental ICH, offering insights for preclinical ICH research and serving as a reference for the selection of clinical ICH treatment methods.
This study was conducted based on the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2020 Checklist, and detailed information can be found in Supplements 1 and 2. The study has been registered in PROSPERO with the registration number-CRD42023435584.
We use authoritative neurological function scoring scales, including the modified Neurological Severity Score (mNSS), Bederson score, and Longa score, to observe the improvement of acupuncture on neurological function in ICH. Additionally, brain water content (BWC) is used to observe the extent of improvement in brain tissue edema in ICH.
The inclusion criteria are as follows:
1. Published animal experiments on ICH in either Chinese or English;
2. Intervention group receiving acupuncture or electroacupuncture treatment, control group receiving sham acupuncture or no treatment;
3. Outcome measures include mNSS, Bederson score, Longa score, or BWC.
The exclusion criteria are as follows:
1. Unable to extract valid outcome measures;
2. Duplicate publications;
3. Unable to access the full text;
4. Interventions combined with other therapies such as moxibustion, embedded thread at acupoints, etc.;
5. Literature not peer-reviewed, such as conference papers, master’s or doctoral theses, or monographs.
Two researchers (ZW and MJ) independently screened titles and abstracts from the search records, assessing potentially eligible studies for further evaluation of full texts. In cases of missing information, authors were contacted for clarification. Conflicts were resolved through discussion between the two authors.
The databases retrieved include PubMed, EMBASE, Cochrane Library, Web of Science, Chinese National Knowledge Infrastructure (CNKI), Wanfang Data Information Site, VIP Information Database, and Chinese Biomedical Literature Database (CBM). The retrieval period extends from the establishment of the databases to June 18, 2023. The retrieval strategy combines subject terms and free-text terms, and the search query is approximately as follows: (intracerebral hemorrhage) AND (acupuncture OR acupuncture therapy OR acupuncture points OR electroacupuncture) AND (rats OR mice OR models, animal OR animal experimentation). For a detailed retrieval strategy, please refer to Supplements 3.
Two researchers (ZW and MJ) independently extracted data from the included studies and stored it in Excel. Any discrepancies between the two authors were resolved through discussion. Missing information was requested from the original authors via email. In cases of no response, this was noted in the discussion section.
The following information was extracted from the included literature:
1. First author and publication year;
2. Animal gender, species, weight, and sample size (n);
3. Type of anesthetic, injection method, and dosage;
4. Modeling method;
5. Intervention details, including selected acupoints, acupuncture methods, and timing;
6. Control measures;
7. Neurobehavioral and brain edema indicators (mean ± standard deviation).
The time point selected for extraction was day 3 after ICH. In cases of multiple duplicate articles reporting the same outcome data, we prioritized data from the article with higher research quality. If research quality was the same, we chose the more recent publication for data extraction. For experiments with multiple intervention groups, we extracted data from the group with the most favorable outcomes. If data were presented as mean ± standard error, we calculated mean ± standard deviation using the formula SD = SE × √n. When data were available only in image format, we used GetData Graph Digitizer 2.25 to extract values. Additionally, we conducted a thorough review of extracted data, excluding any unreliable data (such as data with excessively small standard deviations, given that neurofunctional scores are typically integers), to ensure accurate analysis results.”
The two researchers, ZW and MJ, independently used SYRCLE’s risk of bias tool to assess the quality of the included literature. SYRCLE, which stands for Systematic Review Center for Laboratory animal Experimentation, adapted this tool from the Cochrane risk of bias tool, and it is widely used to evaluate the methodological quality of animal experiments (15). The tool includes the following items:
1. Sequence generation;
2. Baseline characteristics;
3. Allocation concealment;
4. Random housing;
5. Blinding of animal caregivers and researchers;
6. Random outcome assessment;
7. Blinding of outcome assessors;
8. Incomplete outcome data;
9. Selective outcome reporting;
10. Other sources of bias.
The providers of the tool do not recommend evaluating a study as “high-quality” or “low-quality” based on a total score because summing scores inevitably involves assigning “weights” to specific domains in the tool, and it is difficult to prove that the assigned weights are reasonable (15). If there are disagreements during the quality assessment process, these are resolved through discussion or by involving a third researcher (WZ).
We conducted meta-analysis and subgroup analysis using Review Manager 5.3 and performed sensitivity analysis, meta-regression analysis, and publication bias assessment using Stata 14.0. Neurofunctional scores and BWC were treated as continuous variables, with Mean Difference (MD), 95% confidence intervals (CI), and p-values calculated to assess intergroup differences between intervention and control groups. Heterogeneity was evaluated using I2, with values exceeding 50% indicating high heterogeneity and prompting the use of a random-effects model; otherwise, a fixed-effects model was applied. Study results and heterogeneity assessment were presented using forest plots. Publication bias was assessed via funnel plots, and Egger’s test and the trim-and-fill method were employed for outcome measures with more than 10 studies to assess bias. Sensitivity analysis involved systematically excluding individual studies to observe their impact on the overall effect size. For outcomes with high heterogeneity, meta-regression analysis identified potential sources of heterogeneity. Subgroup analysis based on study characteristics such as animal species, ICH modeling methods, acupuncture points, and control measures was conducted for outcome measures with more than 10 studies. The Q-test based on variance analysis was used to assess subgroup differences, with a significance level of p < 0.05 considered statistically significant.
Following the PRISMA guidelines, we conducted literature screening. A total of 1,456 articles were retrieved based on the search strategy. After removing duplicates, 834 articles remained. Upon reviewing titles and abstracts, 633 articles were excluded for the following reasons: (1) Not related to intracerebral hemorrhage; (2) Interventions involving non-acupuncture or combined with other therapies; (3) Human studies; (4) In vitro experiments; (5) Reviews, conference papers, patents, or books; (7) Theses. Among the 202 articles subjected to full-text assessment, 170 were excluded due to inconsistent outcome measures (n = 154), unreliable data (n = 6), data duplication (n = 5), and inability to extract outcome measures (n = 5). The remaining 32 studies were included in our meta-analysis (Figure 1).
Among the 32 included studies (12, 16–46), 13 studies utilized Sprague–Dawley (SD) rats, 16 studies used Wistar rats, 2 studies employed guinea pigs, and 1 study utilized spontaneously hypertensive rats (SHR), all of which were male. Regarding anesthesia, 14 studies employed intraperitoneal injection of chloral hydrate, 12 studies used intraperitoneal injection of pentobarbital, and 6 studies did not specify the type of anesthetic used. In terms of modeling, 23 studies induced ICH by autologous blood injection into the caudate nucleus, while 9 studies induced caudate nucleus hemorrhage using collagenase. For the intervention group, 21 studies employed acupuncture from Baihui (GV20) to Qubin (GB7). In the control group, 16 studies underwent the same procedure for fixation but without acupuncture intervention as in the intervention group; 15 studies did not implement any measures, and 1 study used sham acupuncture as a control. Regarding outcome measures, 11 studies reported mNSS; 5 studies reported Bederson score; 6 studies reported Longa score; and 14 studies reported BWC. The assessment time ranged from 6 h to 28 days after ICH surgery, with all studies reporting outcomes on day 3. The detailed study characteristics of this research are provided in Table 1.
As shown in Table 2, among the 32 studies, for selection bias, 28 studies (87.5%) used randomized group allocation, while only one study (3.13%) reported balanced baseline characteristics between the intervention and control groups. None of the studies clearly described allocation concealment. For performance bias, 15 studies (46.88%) reported randomized housing of animals, but none mentioned whether blinding was implemented for caregivers and researchers performing acupuncture. For detection bias, three studies (9.38%) conducted randomized outcome assessments, and only one study (3.13%) reported blinding of assessors. For attrition bias, all studies reported complete study data. Regarding reporting bias, 31 studies (96.88%) did not selectively report results. For other biases, 29 studies (90.63%) were judged to have no other risks of bias.
In 11 studies, acupuncture demonstrated a significant improvement in mNSS after experimental ICH (n = 246, MD = −3.16, 95% CI [−3.66 ~ −2.66], p < 0.00001; heterogeneity Chi2 = 78.68, df = 10 (p < 0.00001), I2 = 87%; Figure 2A). Among the 5 studies examining Bederson score, acupuncture also showed a significant effect in improving outcomes after experimental ICH (n = 98, MD = −0.99, 95% CI [−1.20 ~ −0.78], p < 0.00001; heterogeneity Chi2 = 4.76, df = 4 (p = 0.31), I2 = 16%; Figure 2B). Additionally, in 6 studies, acupuncture led to a significant improvement in Longa score after experimental ICH (n = 100, MD = −0.54, 95% CI [−0.80 ~ −0.29], p < 0.0001; heterogeneity Chi2 = 9.91, df = 5 (p = 0.08), I2 = 50%; Figure 2C). For BWC, 14 studies demonstrated a significant improvement following acupuncture treatment after experimental ICH (n = 215, MD = −5.39, 95% CI [−6.90 ~ −3.89], p < 0.00001; heterogeneity Chi2 = 1180.48, df = 13 (p < 0.00001), I2 = 99%; Figure 2D). The heterogeneity in mNSS and BWC outcomes is statistically significant. Therefore, meta-regression and subgroup analysis were conducted to explore the sources of heterogeneity.
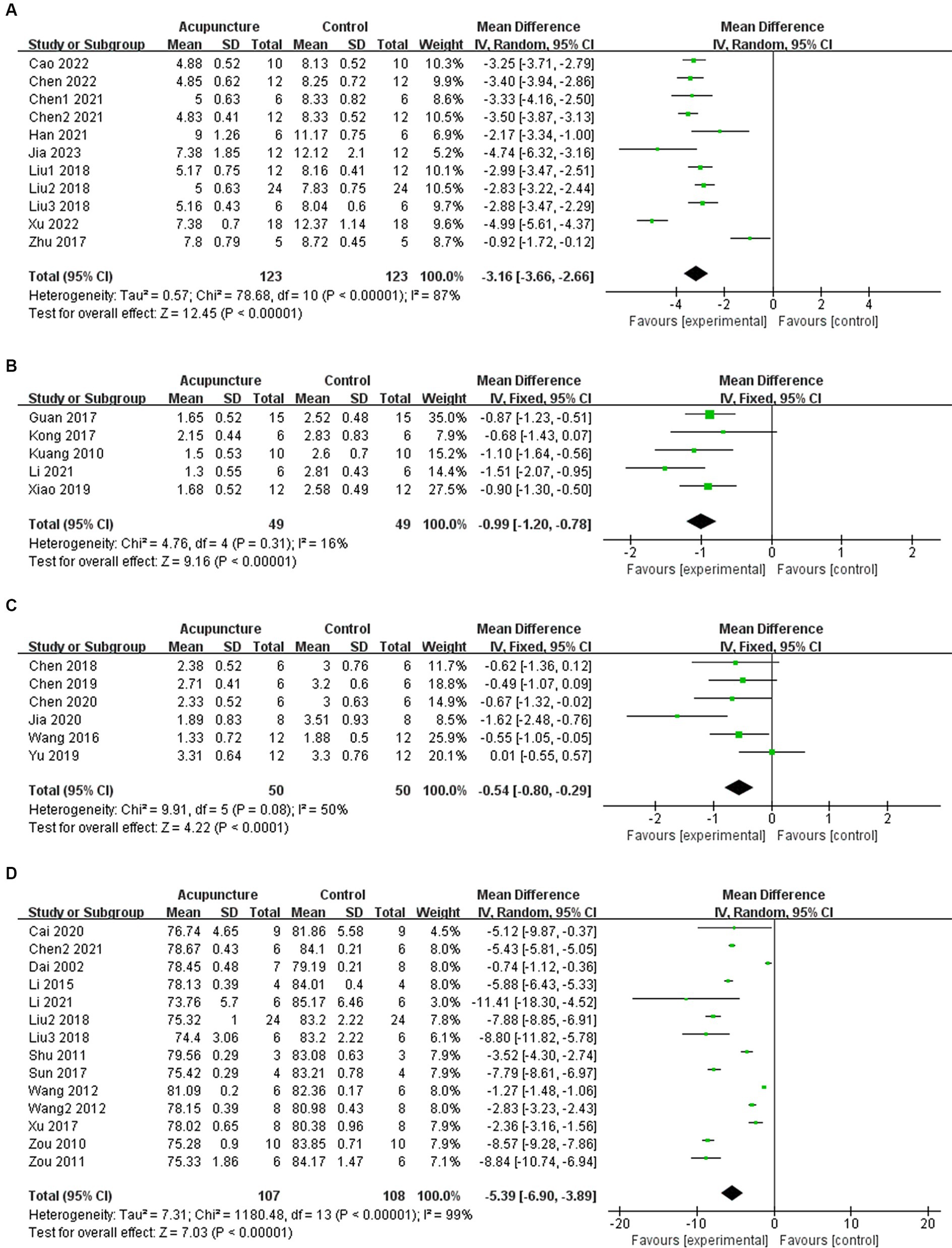
Figure 2. Summary statistics of effect sizes for (A) modified neurological severity score, (B) Bederson score, (C) Longa score, and (D) brain water content.
The sensitivity analysis results indicate that after excluding any individual trial, there were no significant changes in the results for mNSS, Bederson, Longa, and BWC (Figures 3A–D). This indicates good stability of the meta-analysis results. For both mNSS and BWC outcomes, removing any single study did not lead to significant changes in heterogeneity, indicating that the source of heterogeneity is not attributed to any specific study.
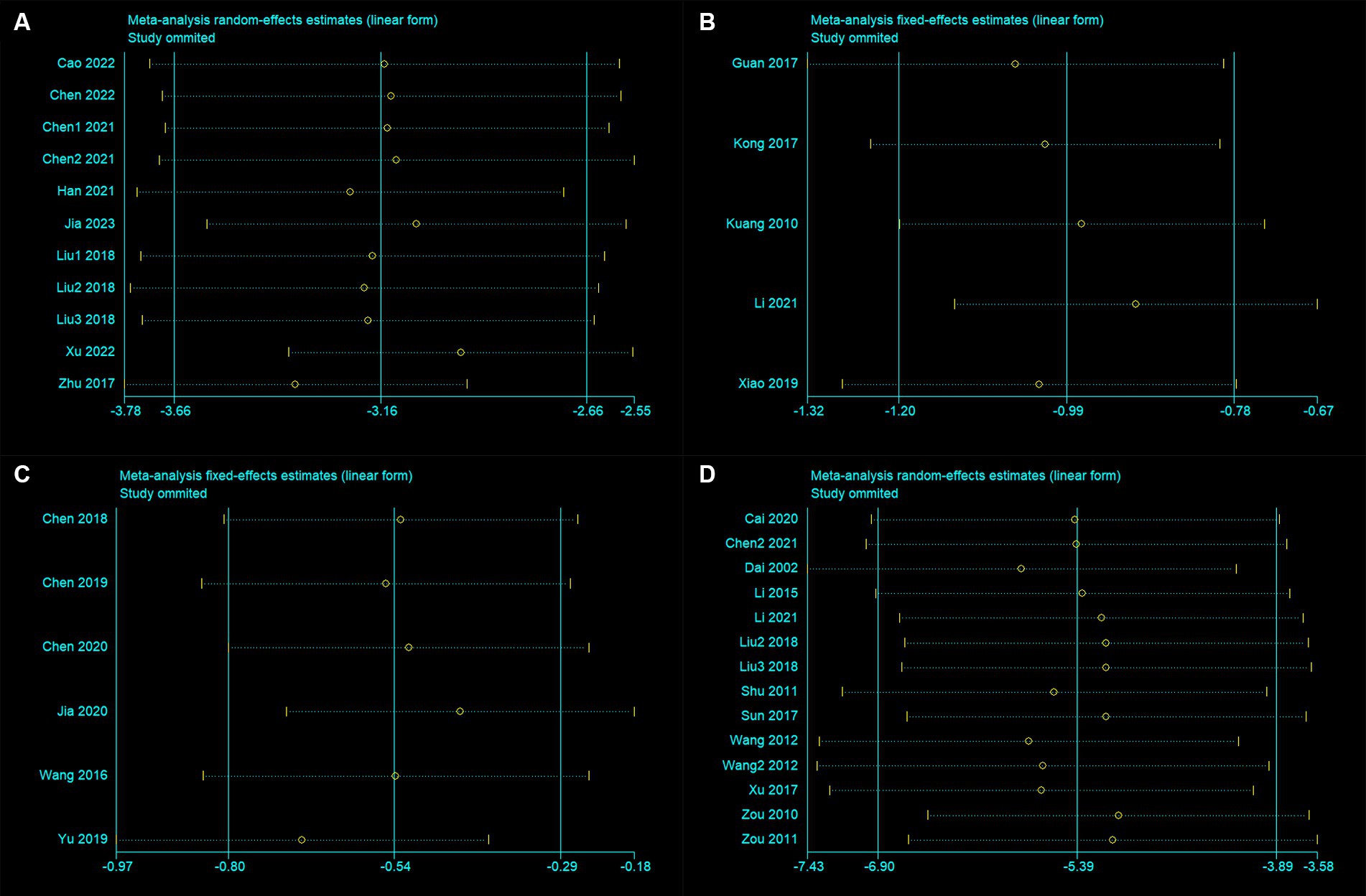
Figure 3. Sensitivity analysis for (A) modified neurological severity score, (B) Bederson score, (C) Longa score, and (D) brain water content.
Creating a funnel plot is not recommended due to the limited number of studies for both Bederson and Longa (both less than 10). Instead, Egger’s test was employed to observe potential publication bias. The funnel plot for mNSS appears to be relatively symmetrical, while the funnel plot for BWC shows some asymmetry (Figures 4A,B). Egger’s test results confirmed the absence of significant publication bias in mNSS, Bederson, and Longa outcomes, but indicated significant bias in BWC (mNSS p = 0.846, Bederson p = 0.67, Longa p = 0.12, BWC p = 0.026). For BWC, we utilized the trim-and-fill method to estimate the number of potentially missing studies and observed changes in the combined effect size estimate. The effect size estimate after correction remained consistent with the uncorrected value (MD −5.39, 95% CI [−6.90 ~ −3.89], p < 0.001), suggesting that there are no “missing” studies (Figure 4C).
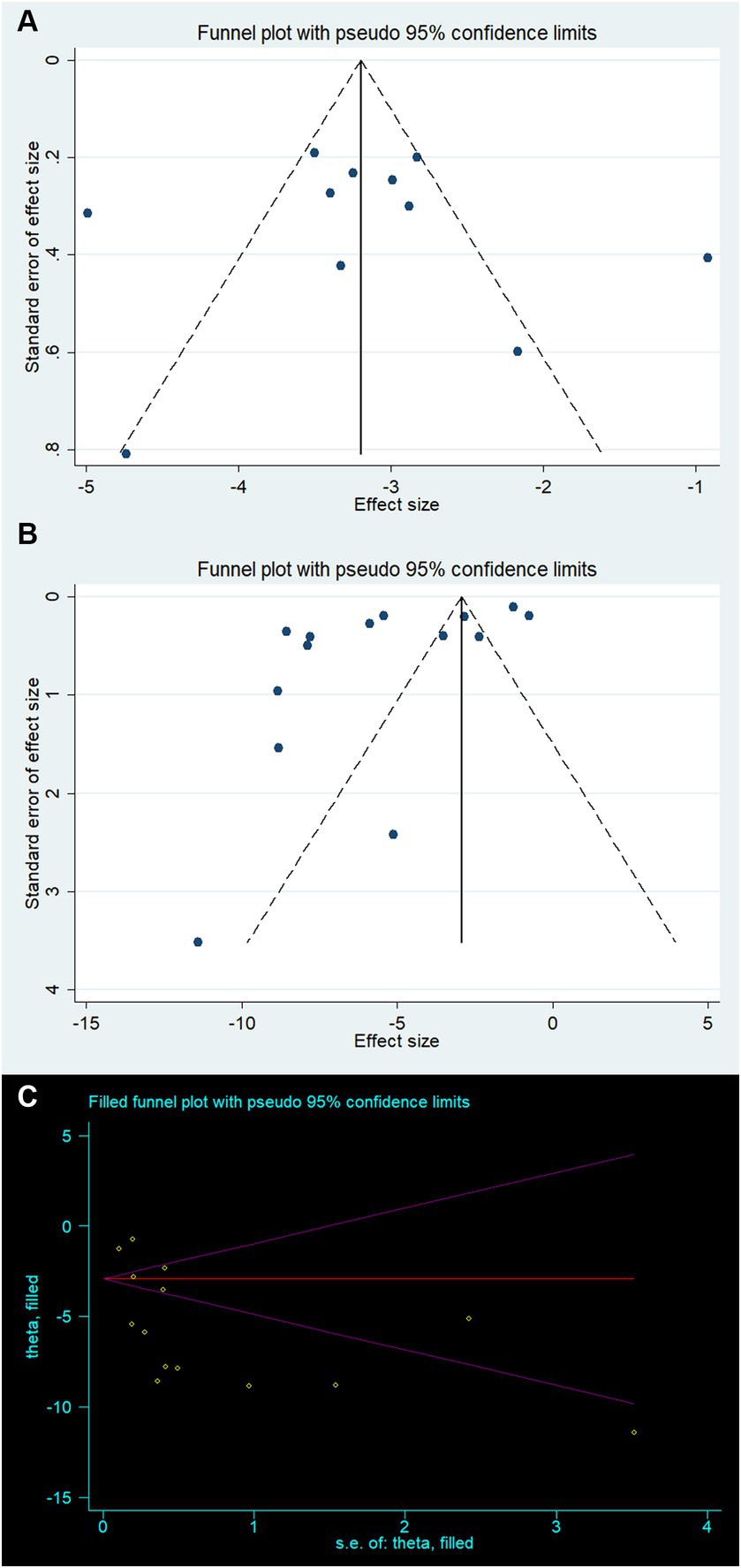
Figure 4. Publication bias for (A) modified neurological severity score, (B) brain water content, and (C) trim-and-fill method to brain water content.
Due to the observed high heterogeneity in the analysis results for mNSS and BWC (with I2 values of 87 and 99% respectively), we conducted meta-regression analysis to examine the correlation between study characteristics and intervention effects, aiming to identify the sources of this heterogeneity. The results indicate that: (1) for mNSS, the modeling method is a significant contributor to its heterogeneity (p = 0.018); (2) for BWC, acupuncture points (p = 0.005) and modeling method (p < 0.001) are significant contributors to its heterogeneity.
Subgroup analysis was conducted based on study characteristics (species, modeling method, acupuncture points, or control measures) to explore the sources of heterogeneity for mNSS and BWC. However, since some studies did not specify the name of the anesthetic used, subgroup analysis was not performed on this factor. (1) The subgroup analysis results for mNSS showed significant differences in the estimated effects among different modeling methods (Chi2 = 28.46, df = 1, p < 0.00001). The autologous blood model (MD = −3.36, 95% CI [−3.78 ~ −2.95], p < 0.00001) yielded better results compared to the collagenase model (MD = −0.92, 95% CI [−1.72 ~ −0.12], p = 0.02). There were also significant differences among different control measures (Chi2 = 33.55, df = 2, p < 0.00001). The effects estimated for simple fixation (MD = −3.38, 95% CI [−4.02 ~ −2.73], p < 0.00001) or no intervention (MD = −3.39, 95% CI [−3.66 ~ −3.12], p < 0.00001) were larger, while the effect estimated for sham acupuncture (MD = −0.92, 95% CI [−1.72 ~ −0.12], p = 0.02) was the smallest. In addition, there were no significant differences observed between different species or acupuncture points. (2) The subgroup analysis for BWC demonstrated a significant difference in the estimated effects among different acupuncture points (Chi2 = 11.77, df = 1, p = 0.0006). GV20 - GB7 (MD = −7.27, 95% CI [−9.13 ~ −5.40], p < 0.00001) showed better results compared to other acupuncture points (MD = −2.92, 95% CI [−4.56 ~ −1.28], p = 0.0005). There were also significant differences among different modeling methods (Chi2 = 24.25, df = 1, p < 0.00001). The effects estimated for the autologous blood model (MD = −7.73, 95% CI [−9.15 ~ −6.31], p < 0.00001) were larger compared to the collagenase model (MD = −2.76, 95% CI [−4.13 ~ −1.38], p < 0.0001). Additionally, there were no significant differences observed between different species or control measures. The detailed information for subgroup analysis is available in Tables 3, 4.
A previous meta-analysis reviewed studies up to 2009 and found that scalp acupuncture may improve neurological deficits in patients with acute hypertensive ICH (47). Additionally, Li et al. reviewed studies up to 2013 and demonstrated potential efficacy of GV20-based scalp acupuncture in acute ICH animal models (48). However, these studies only included literature on acupuncture points located on the head and are outdated, necessitating updated and comprehensive data to support their conclusions. A meta-analysis by Wang et al. indicated that acupuncture combined with minimally invasive surgery or basic treatment has certain efficacy for hypertensive intracerebral hemorrhage compared to minimally invasive surgery or basic treatment alone (49). Unfortunately, this analysis did not perform subgroup analyses based on study characteristics to discuss the differences between various acupuncture methods or different populations. Therefore, we screened all studies on acupuncture treatment for ICH to date and conducted subgroup analyses to explore the potential impact of different study characteristics on effect sizes. The results showed that acupuncture intervention significantly improved neurobehavioral and brain edema outcomes in experimental ICH, including mNSS (MD = −3.16, p < 0.00001), Bederson score (MD = −0.99, p < 0.00001), Longa score (MD = −0.54, p < 0.0001), and BWC (MD = −5.39, p < 0.00001). Subgroup analyses indicated that different modeling methods, acupuncture points, or control measures may influence effect sizes.
Current research indicates that acupuncture therapy can reduce inflammatory responses and improve synaptic plasticity (50, 51). Additionally, numerous studies have explored the precise mechanisms of acupuncture in treating ICH. Research has shown that acupuncture promotes microglial M2 polarization through the miR-34a-5p/Klf4 and PD-1/PD-L1 signaling pathways, resulting in anti-inflammatory effects (29, 52). Besides its anti-inflammatory properties, acupuncture enhances mitochondrial autophagy after ICH, reduces the number of apoptotic cells, and inhibits ferroptosis in neurons (7–12). Furthermore, acupuncture improves the integrity of the blood–brain barrier by downregulating the expression of caveolin-1 and matrix metalloproteinases (53). Additionally, acupuncture promotes the secretion of neurotrophic factors and angiogenesis around the hematoma following ICH (6, 54, 55). Based on these mechanisms, acupuncture exerts neuroprotective effects, thereby improving neurological recovery after ICH.
Quality assessment results indicate that all studies reported complete data, with the vast majority having no selective reporting or other risks of bias. In terms of experimental design, most studies implemented randomized group allocation, and nearly half conducted randomized housing of animals. However, the degree of randomization in outcome assessment was low. Additionally, blinding was poorly implemented throughout the experimental process. To some extent, blinding is more challenging for acupuncture compared to other treatments, as those performing acupuncture are aware of whether they are administering the treatment (unlike medical treatments, which can use placebos that look similar). Nonetheless, randomization and blinding are essential for high-quality animal experiments (56). Future studies could address the blinding issue by employing acupuncturists who are unaware of the experimental design.
Animal studies of ICH typically involve the use of SD rats and Wistar rats, with some studies also utilizing SHR rats and guinea pigs. Kunze et al. reported that SD rats and Wistar rats show similar modified Bederson test scores at several hours or days after middle cerebral artery occlusion (57), indicating that different species exhibit similar neurological deficits post-stroke. Our study results demonstrate no significant differences in mNSS scores among SD rats, Wistar rats, and guinea pigs. Likewise, there were no significant differences in BWC among SD rats, Wistar rats, and SHR rats. This indicates that acupuncture intervention has consistent effects across different species following ICH.
The most widely used methods for inducing ICH include autologous blood injection and collagenase injection, with other methods such as microballoon inflation and spontaneous intracerebral hemorrhage. Zhao et al. reported that in SD rat models of ICH induced by collagenase or non-heparinized autologous blood, there were no statistically significant differences between the two groups in Bederson score and Longa score on 2 day, as well as BWC on 4 day postoperatively (58). However, a study on mice ICH indicated that the BWC on 3 day in the collagenase-induced ICH model was significantly higher than in the autologous blood-induced model, and the disruption of the blood–brain barrier was more severe in the collagenase group (59). Additionally, bacterial collagenase may increase inflammatory responses and have neurotoxic effects, particularly at high doses (60). Our results indicate that the improvement in mNSS and BWC after acupuncture intervention is higher in the autologous blood model compared to the collagenase model, which may be related to the additional adverse effects of collagenase on brain tissue. Intra-group heterogeneity remains significant, which may be related to the dosage of autologous blood and collagenase used. Therefore, a standardized modeling method is crucial for reducing heterogeneity and providing sufficient evidence.
Baihui (GV20) is an empirically effective acupuncture point for stroke treatment, situated 7 cun above the midpoint of the anterior hairline, at the intersection point of a line connecting the tips of both ears and the midline of the head. Qubin (GB7) is located at the intersection of the vertical line at the anterior temporal hairline and the horizontal line through the tip of the ear. In most pre-clinical studies of ICH, GV20 was consistently selected, and needling was directed toward GB7, often employing rapid twisting and stimulating techniques. Numerous studies have reported that acupuncture at GV20 - GB7 alleviates early neurological damage after experimental ICH through mechanisms such as regulating cell apoptosis, inflammation, ferroptosis, microglial polarization, and mitochondrial autophagy, thereby promoting brain function recovery (7, 11, 24, 29, 61–63). Our study indicates that compared to other acupoints, acupuncture at GV20 - GB7 has a better effect on improving BWC after ICH, but there is no significant difference in mNSS. Considering the potential mechanisms of scalp acupuncture, we argue that GV20 - GB7 is the most effective acupoint for acupuncture treatment of ICH.
In 1995, the WHO defined sham acupuncture in their “Clinical Guidelines on Acupuncture” as a form of acupuncture based on acupuncture theory but deemed inappropriate for treating the specific disease. Since then, Sham acupuncture has been widely used in clinical acupuncture randomized controlled trials. Presently, sham acupuncture encompasses superficial needling or non-penetration of acupuncture points, as well as needling or non-penetration at non-acupuncture points. However, some of these methods may not fully account for the intricate mechanisms of acupuncture, as shallow needling at or near acupuncture points might also have therapeutic effects on the disease (64). Dincer argues that categorizing all these varied measures as “sham acupuncture” controls is misleading and scientifically unacceptable (65). In 2022, the WHO’s “International Standard Terminologies on Traditional Medicine” defines sham acupuncture as a “control that simulates acupuncture needling without actual penetration, ideally producing no physiological response,” avoiding skin penetration effects. Our results indicate that for BWC, there were no significant differences observed between the control group with simple fixation, taking no action, and sham acupuncture. However, for mNSS, simple fixation and taking no action yielded better results compared to sham acupuncture. This discrepancy may stem from the small sample size in the sham acupuncture subgroup, resulting in unstable outcomes, or it may be due to the use of a skin-penetrating sham acupuncture method with the connection of an electroacupuncture device in the control group, inducing a partial “treatment effect.” Sham acupuncture primarily functions as a placebo to blind the subjects, preventing them from knowing whether they are receiving treatment. This challenge is not applicable in animal experiments. In the future, exploring a scientifically sound sham acupuncture method that can blind both the practitioner and the subjects while minimizing additional effects on the disease is essential.
Meta-regression analysis revealed that the modeling method significantly contributed to the heterogeneity in mNSS, while both acupuncture points and modeling methods were significant sources of heterogeneity for BWC. We conducted subgroup analyses based on predefined study characteristics to pinpoint sources of heterogeneity. However, persistent high heterogeneity within certain subgroups remains, possibly due to the interaction effects among different study characteristics, such as modeling methods and acupuncture points. To some extent, heterogeneity is inherent in meta-analyses of animal experiments because different experimental environments or methods can be important sources of heterogeneity. For example, the site of brain water content measurement might be the basal ganglia, the hemorrhagic hemisphere, or the whole brain. However, some articles did not report the measurement site, preventing further analysis. The random effects model can tolerate a certain degree of heterogeneity and provide a conservative estimate of effect sizes. We used this model to analyze mNSS and BWC. Additionally, the fixed effects model was applied to the Bederson and Longa scores, which had low heterogeneity. The results indicate that acupuncture can significantly improve neurological deficits and brain edema in experimental ICH. Nonetheless, future studies should focus on standardizing experimental procedures and providing detailed reports of experimental steps to enhance the stability and reproducibility of the results.
This study has undertaken significant efforts. Firstly, it has reviewed all existing literature on acupuncture treatment for experimental ICH, whereas the last similar review was conducted a decade ago. Secondly, during data extraction, we excluded studies with unreliable data (e.g., studies with unreasonably small standard deviations in neurological function scores, given that all four scales use integer scores). Additionally, due to the high heterogeneity of two indicators, we employed meta-regression and subgroup analysis to identify sources of heterogeneity and to explore the potential effects of different species, modeling methods, acupuncture points, and control measures on effect sizes. Finally, sensitivity analysis and publication bias tests were conducted to confirm the stability of the results.
This study still has some limitations. Firstly, meta-regression results indicated that the modeling method and acupuncture points were significant sources of heterogeneity. However, high heterogeneity remained within subgroups after subgroup analysis. We used a random effects model to conservatively estimate the impact of acupuncture on the mNSS and BWC indicators of experimental ICH. Despite this, the results should be interpreted with caution. Additionally, current literature provides limited information on the injection doses of autologous blood and collagenase during modeling, which directly affects the extent of brain tissue damage post-hemorrhage. Furthermore, the literature often fails to report the specific brain regions where BWC was measured, affecting the stability of BWC effect size estimates. Therefore, future research should aim to thoroughly report experimental designs to minimize heterogeneity across studies, striving for robust evidence-based results to guide the clinical translation of acupuncture treatment for ICH.
Acupuncture significantly improves neurological deficits and brain edema in experimental ICH. Acupuncture at GV20 - GB7 is more effective than at other points. These findings support further studies to translate acupuncture into clinical treatment for human ICH.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
ZW: Conceptualization, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. MJ: Formal analysis, Software, Validation, Visualization, Writing – original draft. TW: Methodology, Writing – review & editing. BZ: Validation, Writing – review & editing. HD: Writing – review & editing. YD: Validation, Writing – review & editing. JY: Writing – review & editing. WZ: Funding acquisition, Project administration, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant number 81774416).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1402129/full#supplementary-material
ICH, intracerebral hemorrhage; mNSS, modified neurological severity score; BWC, brain water content; EA, electroacupuncture; SD, Sprague–Dawley; SHR, spontaneously hypertensive rats; MD, mean difference; CI, confidence intervals.
1. Pinho, J, Costa, AS, Araujo, JM, Amorim, JM, and Ferreira, C. Intracerebral hemorrhage outcome: a comprehensive update. J Neurol Sci. (2019) 398:54–66. doi: 10.1016/j.jns.2019.01.013
2. Dastur, CK, and Yu, W. Current management of spontaneous intracerebral haemorrhage. Stroke Vasc Neurol. (2017) 2:21–9. doi: 10.1136/svn-2016-000047
3. Li, C, Qin, H, Zeng, L, Hu, Z, and Chen, C. Efficacy of stem cell therapy in animal models of intracerebral hemorrhage: an updated meta-analysis. Stem Cell Res Ther. (2022) 13:452. doi: 10.1186/s13287-022-03158-7
4. Zeng, L, Zhu, Y, Hu, X, Qin, H, Tang, J, Hu, Z, et al. Efficacy of melatonin in animal models of intracerebral hemorrhage: a systematic review and meta-analysis. Aging (Albany NY). (2021) 13:3010–30. doi: 10.18632/aging.202457
5. Wang, HQ, Bao, CL, Jiao, ZH, and Dong, GR. Efficacy and safety of penetration acupuncture on head for acute intracerebral hemorrhage: a randomized controlled study. Medicine (Baltimore). (2016) 95:e5562. doi: 10.1097/MD.0000000000005562
6. Li, L, Wang, X, Guo, J, Chen, Y, and Wang, Z. Effect of acupuncture in the acute phase of intracerebral hemorrhage on the prognosis and serum BDNF: a randomized controlled trial. Front Neurosci. (2023) 17:1167620. doi: 10.3389/fnins.2023.1167620
7. Kong, Y, Li, S, Zhang, M, Xu, W, Chen, Q, Zheng, L, et al. Acupuncture ameliorates neuronal cell death, inflammation, and Ferroptosis and downregulated miR-23a-3p after intracerebral hemorrhage in rats. J Mol Neurosci. (2021) 71:1863–75. doi: 10.1007/s12031-020-01770-x
8. Li, MY, Dai, XH, Yu, XP, Zou, W, Teng, W, Liu, P, et al. Scalp acupuncture protects against neuronal Ferroptosis by Activating the p62-Keap1-Nrf2 pathway in rat models of intracranial Haemorrhage. J Mol Neurosci. (2022) 72:82–96. doi: 10.1007/s12031-021-01890-y
9. Liu, H, Zhang, B, Li, XW, du, J, Feng, PP, Cheng, C, et al. Acupuncture inhibits mammalian target of rapamycin, promotes autophagy and attenuates neurological deficits in a rat model of hemorrhagic stroke. Acupunct Med. (2022) 40:59–67. doi: 10.1177/09645284211028873
10. Liu, P, Yu, X, Dai, X, Zou, W, Yu, X, Niu, M, et al. Scalp acupuncture attenuates brain damage after intracerebral hemorrhage through enhanced Mitophagy and reduced apoptosis in rats. Front Aging Neurosci. (2021) 13:718631. doi: 10.3389/fnagi.2021.718631
11. Guan, R, Li, Z, Dai, X, Zou, W, Yu, X, Liu, H, et al. Electroacupuncture at GV20-GB7 regulates mitophagy to protect against neurological deficits following intracerebral hemorrhage via inhibition of apoptosis. Mol Med Rep. (2021) 24:492. doi: 10.3892/mmr.2021.12131
12. Zhu, Y, Deng, L, Tang, H, Gao, X, Wang, Y, Guo, K, et al. Electroacupuncture improves neurobehavioral function and brain injury in rat model of intracerebral hemorrhage. Brain Res Bull. (2017) 131:123–32. doi: 10.1016/j.brainresbull.2017.04.003
13. Xiong, J, Zhou, X, Luo, X, Gong, X, Jiang, L, Luo, Q, et al. Acupuncture therapy on myofascial pain syndrome: a systematic review and meta-analysis. Front Neurol. (2024) 15:1374542. doi: 10.3389/fneur.2024.1374542
14. Zhou, X, Zhang, J, Jiang, L, Zhang, S, Gu, Y, Tang, J, et al. Therapeutic efficacy of acupuncture point stimulation for stomach cancer pain: a systematic review and meta-analysis. Front Neurol. (2024) 15:1334657. doi: 10.3389/fneur.2024.1334657
15. Hooijmans, CR, Rovers, MM, de Vries, RB, Leenaars, M, Ritskes-Hoitinga, M, and Langendam, MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
16. Cao, HT, Yu, XP, Dai, XH, Teng, W, Kuang, BL, Yu, WW, et al. Effect of acupuncture on M1 type microglia in rats with acute ICH. J Clin Acupunct Moxibustion. (2022) 38:55–60. doi: 10.19917/j.cnki.1005-0779.022055
17. Chen, Q, Song, W, Tang, Y, Tang, Y, Kang, Y, and Zhu, L. Electroacupuncture reduces cerebral hemorrhage injury in rats by improving cerebral Iron metabolism. Mediat Inflamm. (2022) 2022:1–10. doi: 10.1155/2022/6943438
18. Chen, QX, Kong, Y, Yu, TT, Zhang, Y, Liu, P, and Zhang, X. Effect of acupuncture on the expression of Heme oxygenase 1 and inflammatory factors in rats with intracerebral hemorrhage. Rehabil Med. (2021) 31:408–14. doi: 10.3724/SP.J.1329.2021.05009
19. Chen, QX, Kong, Y, Yu, TT, Zhang, Y, Liu, P, Zhang, X, et al. Effect of needling GV20-through-GB7 on hematoma and expressions of CD36 and HO-1 in rats with acute ICH. J Clin Acupunct Moxibustion. (2021) 37:59–63. doi: 10.19917/j.cnki.1005-0779.021163
20. Han, JW, Yang, JW, Chen, LL, and Du, YH. Effect of acupuncture on expressions of NGB and Cyt-C in brain tissues around hematoma of cerebral hemorrhage rats. Liaoning. J Tradit Chin Med. (2021) 48:236–365. doi: 10.13192/j.issn.1000-1719.2021.07.063
21. Jia, KP, Liang, Q, Liu, GP, GF, CAI, and Wu, JL. Effect of cross electro-nape-acupuncture on cAMP-PKA pathway in medullary tissue of cerebral hemorrhage bulbar injury model Guinea pigs. J Tradit Chin Med. (2023) 64:1157–64. doi: 10.13288/j.11-2166/r.2023.11.014
22. Liu, XY, Zou, W, and Yu, XP. Effect of point - to - point scalp acupuncture on SYK Expressionin rats with cerebral hemorrhage. J Clin Acupunct Moxibustion. (2018) 34:52–6. doi: 10.3969/j.issn.1005-0779.2018.02.015
23. Liu, XY, Zou, W, and Yu, XP. Effect of needling GV20-to-GB7 on BWC and lL-1β mRNA in lCH rats. Inform Tradit. Chin Med. (2018) 35:79–82. doi: 10.19656/j.cnki.1002-2406.180058
24. Liu, XY, Dai, XH, Zou, W, Yu, XP, Teng, W, Wang, Y, et al. Acupuncture through Baihui (DU20) to Qubin (GB7) mitigates neurological impairment after intracerebral hemorrhage. Neural Regen Res. (2018) 13:1425–32. doi: 10.4103/1673-5374.235298
25. Xu, WT, Xue, YM, Wang, C, Kong, Y, and Zou, W. Mechanism study of needling DU20 through GB7 in inhibiting neurological function injury in rats with ICH by regulating EGFR/STAT3 pathway. J Clin Acupunct Moxibustion. (2022) 38:70–5. doi: 10.19917/j.cnki.1005-0779.022137
26. Guan, RQ, Zou, W, Yu, XP, Sun, XW, Dai, XH, Teng, W, et al. Expression of CD62p in acute cerebral hemorrhage rats intervened by Acupunture of Baihui-to-Qubin. Acta Chin Med Pharmacol. (2017) 45:74–8. doi: 10.19664/j.cnki.1002-2392.2017.04.022
27. Kong, Y, Duan, HC, Liu, Y, Wang, C, and Tan, Y. Study of penetration acupuncture on p-STAT3 of cerebral hemorrhage Rats' brain tissues after JNKPathway inhibition. J Emerg Tradit Chin. (2017) 26:1895–8. doi: 10.3969/j.issn.1004-745X.2017.11.005
28. Kuang, HY, Zou, W, Yu, XP, Sun, XW, Zhao, JH, Teng, W, et al. Effects of Baihui-Qubin scalp-acupuncture on expressions of neuron specific enolase in acute Intercerebral hemorrhage. Acta Chin Med Pharmacol. (2010) 38:76–9. doi: 10.19664/j.cnki.1002-2392.2010.05.028
29. Li, D, Zhao, Y, Bai, P, Li, Y, Wan, S, Zhu, X, et al. Baihui (DU20)-penetrating-Qubin (GB7) acupuncture regulates microglia polarization through miR-34a-5p/Klf4 signaling in intracerebral hemorrhage rats. Exp Anim. (2021) 70:469–78. doi: 10.1538/expanim.21-0034
30. Xiao, Y, Wu, K, Gao, X, Feng, Y, and Liu, B. Effects of activating blood circulation to dissipate blood stasis on the expression of HMGB1 and PKA/CREB in rats with cerebral haemorrhage. Acta med Mediterr. (2019) 35:1211–7. doi: 10.19193/0393-6384_2019_3_185
31. Chen, QX, Zhu, LW, Sun, ZR, Zhang, Y, Dai, XH, and Yu, WW. Effects of acupuncture on the expressions of IL-10and TGF-beta protein in brain tissue of the rats with acute cerebral hemorrhage. World J Integr Trad Western Med. (2018) 13:1481–4. doi: 10.13935/j.cnki.sjzx.181101
32. Chen, QX, Zhu, LW, Pang, XM, Zhang, Y, Sun, ZR, and Liu, P. Effect of acupuncture on the expression of TLR4/MyD88 signaling pathway key factors in rats with acute intracerebral hemorrhage. World J Integr Trad Western Med. (2019) 14:815–18,25. doi: 10.13935/j.cnki.sjzx.190618
33. Chen, QX, Zhu, LW, Pang, XM, Zhang, Y, Sun, ZR, Liu, XY, et al. Effect of acupuncture negative regulation of key factors of TLR4/TRIF signaling pathway on expression of inflammatory response in rats with cerebral hemorrhage. World J Integr Trad Western Med. (2020) 15:283–7. doi: 10.13935/j.cnki.sjzx.200218
34. Jia, KP, Pei, SY, Wang, H, Wang, X, and GF, CAI. Safety research on treatment of cough reflex disorder after cerebral hemorrhage with crossing nape electroacupuncture in guinea pig. Acupunct Res. (2020) 45:954–60. doi: 10.13702/j.1000-0607.200279
35. Wang, Y. Effect of electroacupuncture on the expression of protease-activated receptor-1 in rats with acute cerebral hemorrhage. Chin J Integr med. Cardiovasc Dis. (2016) 14:367–70. doi: 10.3969/j.issn.1672-1349.2016.04.010
36. Yu, XP, Kuang, BL, Dai, XH, Zou, W, Teng, W, Yu, WW, et al. Effect of acupuncture on ICH rats and its influence to GSK-3βExpression. J Clin Acupunct Moxibustion. (2019) 2:61–4. doi: 10.3969/j.issn.1005-0779.2019.02.018
37. Cai, G-F, Sun, Z-R, Zhuang, Z, Zhou, H-C, Gao, S, Liu, K, et al. Cross electro-nape-acupuncture ameliorates cerebral hemorrhage-induced brain damage by inhibiting necroptosis. World J Clin Cases. (2020) 8:1848–58. doi: 10.12998/wjcc.v8.i10.1848
38. Dai, GZ, Chen, YL, and Gu, FL. Effect of electro-acupuncture on pathology, water content in brain tissue and neurologic function defect scoring in rat model of idiopathic cerebral hemorrhage. Chin J Integr Tradit West Med. (2002) 2:133–5. doi: 10.3321/j.issn:1003-5370.2002.02.016
39. Li, XY, Shi, S, Li, DT, and Bao, R. Experimental study on scalp point-to-point for water content of brain and expression of AQP-4 in rats with cerebral hemorrhage. J Clin Acupunct Moxibustion. (2015) 5:70–2. doi: 10.3969/j.issn.1005-0779.2015.05.025
40. Shu, S, Yang, LK, You, YL, Qian, X, Hu, T, and Zhou, S. Effects of Electroacupuncture at Shuigou (CV26) on dynamic cerebral edema and blood brain barrier in hypertensive intracerebral hemorrhage rats. Acad J Shang Univ Tradit. Chin Med. (2011) 25:69–71. doi: 10.16306/j.1008-861x.2011.02.019
41. Sun, J, Zhou, Q, Huang, FF, Shen, J, and Zhou, H. Protective effect of acupuncture on brain injury in acute stage of intracerebral hemorrhage in rats and its mechanism. J Yunnan Univ Chin Med. (2017) 40:14–7. doi: 10.19288/j.cnki.issn.1000-2723.2017.06.004
42. Wang, XY, Zhang, Y, and Xu, RH. Effect of Electroacupuncture on brain edema and MMP-9 dynamic changes in Rat's brain after cerebral hemorrhage. J Hubei Univ Chin Med. (2012) 14:12–4. doi: 10.3969/j.issn.1008-987x.2012.05.04
43. Wang, GF, Yang, C, and Cui, H. The research of acupuncture combing with Ditan decoction to neuroethology and cerebral edema of rat on the acute cerebral hemorrhage. J Emerg Tradit Chin. (2012) 21:1769–71. doi: 10.3969/j.issn.1004-745X.2012.11.027
44. Xu, CL, Wang, F, and Dong, HS. Effect of music Electroacupuncture on Perifocal ferric ions and neuronal apoptosis percentage and the brain water content in rats with acute cerebral hemorrhage. Shanghai J Acupunct Moxibustion. (2017) 36:840–5. doi: 10.13460/j.issn.1005-0957.2017.07.0840
45. Zou, W, Kuang, HY, Yu, XP, Sun, XW, Zhao, JH, Teng, W, et al. Effects of BaiHui-quBin scalp-aupuncture on brain edema and expressions of matrix Metalloproteinase-9 in acute Intercerebral hemorrhage. Inform Tradit. Chin Med. (2010) 27:81–4. doi: 10.3969/j.issn.1002-2406.2010.05.034
46. Zou, W, Wang, L, Li, D, Sun, XW, and Kong, Y. The effect of acupuncture on the expression of NF-κBp65 in the brain tissue around hematoma caused by intracerebral hemorrhage and contents of brain water in Wistar rats. Inform Tradit. Chin Med. (2011) 28:88–91. doi: 10.19656/j.cnki.1002-2406.2011.04.043
47. Zheng, GQ, Zhao, ZM, Wang, Y, Gu, Y, Li, Y, Chen, XM, et al. Meta-analysis of scalp acupuncture for acute hypertensive intracerebral hemorrhage. J Altern Complement Med. (2011) 17:293–9. doi: 10.1089/acm.2010.0156
48. Li, HQ, Li, JH, Liu, AJ, Ye, MY, and Zheng, GQ. GV20-based acupuncture for animal models of acute intracerebral haemorrhage: a preclinical systematic review and meta-analysis. Acupunct Med. (2014) 32:495–502. doi: 10.1136/acupmed-2014-010546
49. Wang, M, Jia, M, Zhang, XY, du, WQ, Jiao, WW, Chen, Q, et al. Systematic review and Meta-analysis of efficacy and safety of acupuncture therapy on hypertensive intracerebral hemorrhage. Zhongguo Zhong Yao Za Zhi. (2021) 46:4644–53. doi: 10.19540/j.cnki.cjcmm.20210429.501
50. Tu, W, Chen, X, Wu, Q, Ying, X, He, R, Lou, X, et al. Acupoint application inhibits nerve growth factor and attenuates allergic inflammation in allergic rhinitis model rats. J Inflamm (Lond). (2020) 17:4. doi: 10.1186/s12950-020-0236-9
51. Zhou, K, Wu, Q, Yue, J, Yu, X, Ying, X, Chen, X, et al. Electroacupuncture suppresses spinal nerve ligation-induced neuropathic pain via regulation of synaptic plasticity through upregulation of basic fibroblast growth factor expression. Acupunct Med. (2022) 40:379–88. doi: 10.1177/09645284211066499
52. Liu, Y, Zheng, S, Zhang, X, Guo, W, du, R, Yuan, H, et al. Electro-nape-acupuncture regulates the differentiation of microglia through PD-1/PD-L1 reducing secondary brain injury in acute phase intracerebral hemorrhage rats. Brain Behav. (2023) 13:e3229. doi: 10.1002/brb3.3229
53. Li, HQ, Li, Y, Chen, ZX, Zhang, XG, Zheng, XW, Yang, WT, et al. Electroacupuncture exerts neuroprotection through Caveolin-1 mediated molecular pathway in intracerebral hemorrhage of rats. Neural Plast. (2016) 2016:7308261. doi: 10.1155/2016/7308261
54. Luo, JK, Zhou, HJ, Wu, J, Tang, T, and Liang, QH. Electroacupuncture at Zusanli (ST36) accelerates intracerebral hemorrhage-induced angiogenesis in rats. Chin J Integr Med. (2013) 19:367–73. doi: 10.1007/s11655-013-1458-y
55. Zhou, HJ, Tang, T, Zhong, JH, Luo, JK, Cui, HJ, Zhang, QM, et al. Electroacupuncture improves recovery after hemorrhagic brain injury by inducing the expression of angiopoietin-1 and -2 in rats. BMC Complement Altern Med. (2014) 14:127. doi: 10.1186/1472-6882-14-127
56. Landis, SC, Amara, SG, Asadullah, K, Austin, CP, Blumenstein, R, Bradley, EW, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. (2012) 490:187–91. doi: 10.1038/nature11556
57. Kunze, A, Zierath, D, Drogomiretskiy, O, and Becker, K. Variation in behavioral deficits and patterns of recovery after stroke among different rat strains. Transl Stroke Res. (2014) 5:569–76. doi: 10.1007/s12975-014-0337-y
58. Zhao, XY, and Zhang, XL. Establishment of composite experimental animal model of brain edema after cerebral hemorrhagic. Ann. Palliat Med. (2021) 10:362–71. doi: 10.21037/apm-20-2308
59. Jia, P, He, J, Li, Z, Wang, J, Jia, L, Hao, R, et al. Profiling of blood-brain barrier disruption in mouse intracerebral hemorrhage models: collagenase injection vs. autologous arterial whole blood infusion. Front Cell Neurosci. (2021) 15:699736. doi: 10.3389/fncel.2021.699736
60. CL, ML, Silasi, G, Auriat, AM, and Colbourne, F. Rodent models of intracerebral hemorrhage. Stroke. (2010) 41:S95–8. doi: 10.1161/STROKEAHA.110.594457
61. Zhang, B, Dai, XH, Yu, XP, Zou, W, Teng, W, Sun, XW, et al. Baihui (DU20)-penetrating-Qubin (GB7) acupuncture inhibits apoptosis in the perihemorrhagic penumbra. Neural Regen Res. (2018) 13:1602–8. doi: 10.4103/1673-5374.237123
62. Li, D, Chen, QX, Zou, W, Sun, XW, Yu, XP, Dai, XH, et al. Acupuncture promotes functional recovery after cerebral hemorrhage by upregulating neurotrophic factor expression. Neural Regen Res. (2020) 15:1510–7. doi: 10.4103/1673-5374.257532
63. Zou, W, Chen, QX, Sun, XW, Chi, QB, Kuang, HY, Yu, XP, et al. Acupuncture inhibits Notch1 and Hes1 protein expression in the basal ganglia of rats with cerebral hemorrhage. Neural Regen Res. (2015) 10:457–62. doi: 10.4103/1673-5374.153696
64. Zhang, LL, Chu, Q, Wang, S, Lai, H, and Xie, BB. Is sham acupuncture as effective as traditional Chinese acupuncture? It's too early to say. Chin J Integr Med. (2016) 22:483–9. doi: 10.1007/s11655-016-2458-5
Keywords: intracerebral hemorrhage, acupuncture, meta-analysis, neurological function score, brain water content
Citation: Wu Z, Jiao M, Wang T, Zhang B, Dong H, Du Y, Yao J and Zou W (2024) Efficacy of acupuncture in experimental intracerebral hemorrhage: a systematic review and meta-analysis. Front. Neurol. 15:1402129. doi: 10.3389/fneur.2024.1402129
Received: 18 March 2024; Accepted: 31 May 2024;
Published: 13 June 2024.
Edited by:
Hao Chi, Southwest Medical University, ChinaReviewed by:
Xuancheng Zhou, Southwest Medical University, ChinaCopyright © 2024 Wu, Jiao, Wang, Zhang, Dong, Du, Yao and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zou, a3Vhbmd6b3UxOTY1QGZveG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.