- 1Department of Rehabilitation Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Department of Rehabilitation Medicine, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 3Key Laboratory of Rehabilitation Medicine in Sichuan Province, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Frontotemporal dementia (FTD) is a neurodegenerative disease with clinical, pathological, and genetic heterogeneity. FTD is receiving increasing attention because it is the second leading cause of early-onset dementia after Alzheimer’s disease. This study aimed to analyse the research trends and hotspots of FTD from 2000 to 2022 using bibliometrics.
Methods: Papers related to FTD from 2000 to 2020 were systematically searched through the Web of Science Core Collection (WOSCC). Citespace and Vosviewer software were used to visually analyse the retrieved data of countries/regions, institutions, journals, authors, references, and keywords. Microsoft Excel was used to generate the annual publications and growth trends.
Results: There were 10,227 papers included in the bibliometric analysis. The annual publication output on FTD has increased significantly from 2000 to 2022, with papers published in 934 academic journals and 87 countries/regions. The Journal of Alzheimer’s Disease was the most popular, with 488 papers about FTD. The most productive countries/regions, institutions, and authors are the United States (n = 4,037), the University of California San Francisco (n = 687), and Miller, Bruce L. (n = 427), respectively. The article by Katya Rascovsky and her colleagues published on Brain in 2011 was the most cocited paper, with 625 citations. The research hotspots in this field were the clinical diagnostic criteria, subdivision, and pathological mechanism of FTD, such as tau protein, chromosome 17, progranulin, TDP-43, and C9orf72.
Conclusion: The future research direction is based on biomarkers and pathological mechanisms to diagnose and differential diagnose FTD from the aspects of behavior, neuropathology, neuroimaging, and serum markers.
1 Introduction
Frontotemporal dementia (FTD) is a neurodegenerative clinical syndrome characterized by progressive changes in executive function, behavior, language, or motor function (1). The neuroanatomical features of FTD are correlated with impairment and neuronal loss in the frontal and temporal lobes and now include more extensive cortical, subcortical, brainstem, and cerebellar involvement (2). FTD is the third leading cause of late-onset dementia (age ≥ 65 years) after Alzheimer’s disease (AD) and Lewy body dementia and the second leading cause of early-onset dementia (age < 65 years) after AD (1, 3). FTD accounts for 10–20% of all dementia cases (4). One meta-analysis suggested that the prevalence of FTD ranged from 3 to 26% (5). However, the disease is easily missed and misdiagnosed, which may underestimate the true prevalence (1). Clinically, FTD can be divided into the behavioral variant of FTD (bvFTD) and primary progressive aphasia (PPA) (6). PPA includes two subtypes, namely, nonfluent variant PPA (nfvPPA) and semantic variant PPA (svPPA). In addition, FTD may co-exist with related motor neurone disease, Parkinson’s disease, corticobasal syndrome, or progressive supronuclear palsy (PSP), which may be special subtypes of FTD. PSP is characterized by vertical supranuclear ophthalmoplegia, extrapyramidal muscle rigidity, gait ataxia, and mild dementia (7). In addition to pure forms of PSP, PSP pathology can be found in patients with Parkinson’s disease and frontotemporal dementia, leading to possible misdiagnosis of PSP (8). The two main aggregated proteins in the brains of patients with FTD are tau and TDP-43, while a small number of patients aggregate in fused-in-sarcoma proteins (9). Approximately 40% of people with FTD have a family history (10), and one-third of FTD is inherited by autosomal dominant mutations in three genes: progranulin (GRN), microtubule-associated protein tau (MAPT) and chromosome 9 open reading frame 72 (C9orf72) (11). Several FTD syndromes (bvFTD, svPPA, and nfvPPA) are pathologically clinically and radiologically heterogeneous (12). With the development of neuroimaging technology, studies have revealed numerous biomarkers for various syndromes of FTD, thus narrowing the differential diagnosis and improving diagnostic accuracy (2, 12, 13). The two techniques most commonly used to evaluate FTD in clinical and research settings are structural magnetic resonance imaging (MRI) and positron emission tomography. Currently, there are no FDA-approved treatments for FTD (4). FTD treatment is limited to symptom control, and most compounds used to treat AD-type dementia are ineffective against FTD (9). Therapies targeting genetic forms of FTD have shown significant progress, and noninvasive neuroregulatory techniques have shown potential in alleviating symptoms and enhancing cognition of patients with FTD (14). FTD is of increasing concern, as it accounts for up to 10% of middle-age-onset dementia and imposes a social, financial and emotional burden on patients and caregivers (15). There has been a significant increase in publications on FTD research, including genetics, pathology, neuroimaging, and therapeutic interventions.
Bibliometric analysis is a quantitative method to explore the development trends and research hotspots of certain scientific fields (16). General reviews were studied by individuals through literature summary and extraction and thus cannot visually provide the temporal and spatial distribution of countries, institutions, authors, and journals (17). A well-organized bibliometric analysis can save researchers and clinicians time by providing a clearer overview, cutting-edge hotspots, and trends in a specific field (18). In recent years, bibliometrics has been widely used to analyse neurodegenerative diseases such as AD (19, 20), Parkinson’s disease (21), multiple sclerosis (22), and cerebral amyloid angiopathy (23). To our knowledge, only Guido et al. (24) included 1,436 articles and reviews to conduct a bibliometric analysis of FTD in 2015. FTD has attracted increasing attention, with a growing number of publications which include improvements in clinical, genetic and molecular characteristics, advances in plasma and cerebrospinal fluid biomarkers, and innovations in structural and functional imaging, providing new insights into FTD (25–31). It is necessary to update a bibliometric analysis to better show the current research status, research hotspots and development trends. This study aimed to comprehensively analyse the global research status, research hotspots and trends of FTD from 2000 to 2022 by conducting a bibliometric analysis. These findings may help quantify the characteristics of countries, institutions, journals and authors, as well as analyse citations, keywords and research trends.
2 Materials and methods
2.1 Data source
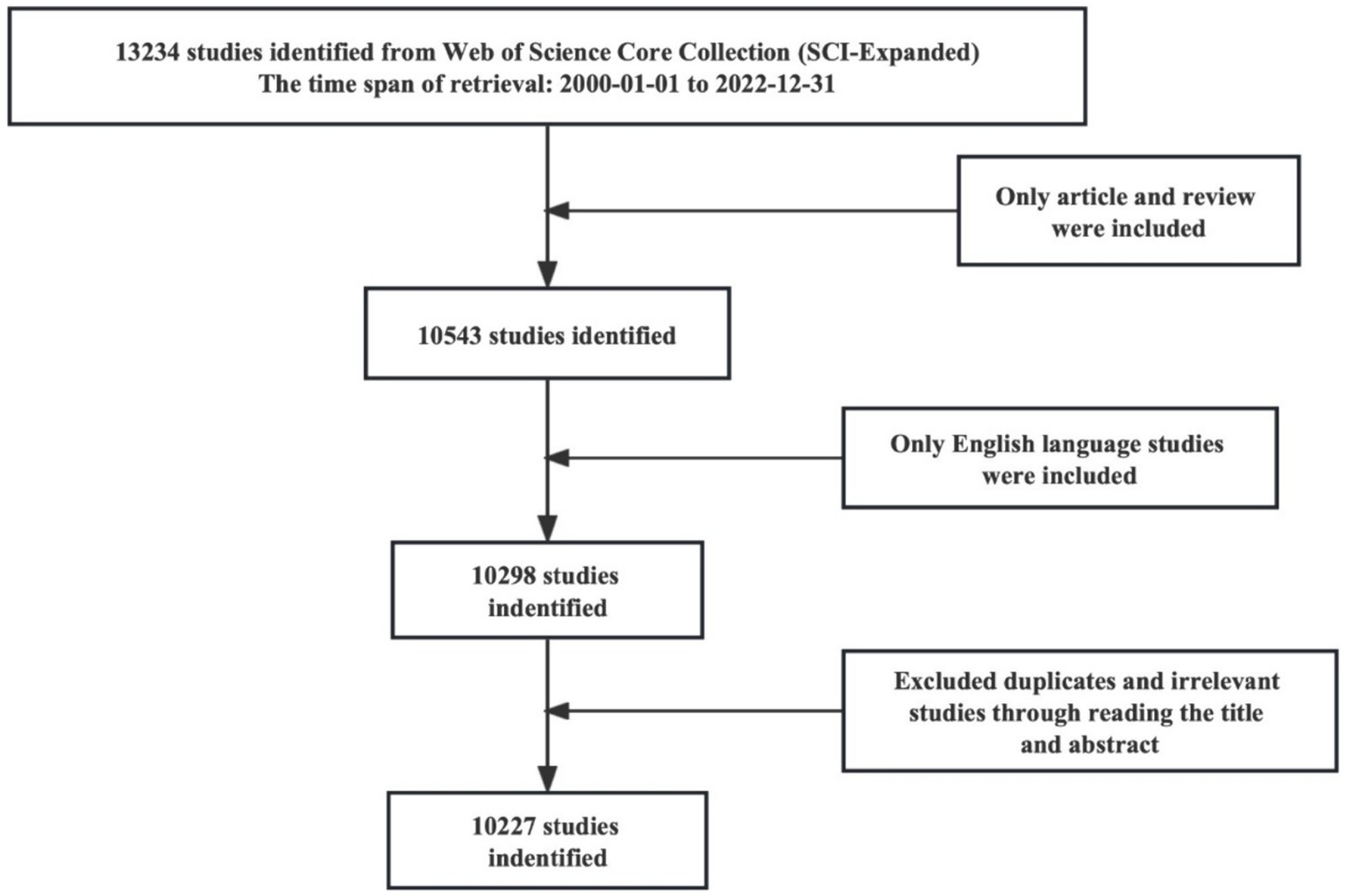
Full records of publications related to frontotemporal dementia were retrieved and downloaded from the Science Citation Index Expanded (SCI-Expanded) of the Web of Science Core Collection database (WoSCC) on July 5, 2023. The search strategy is shown in Supplementary Appendix A, and the time span was set from January 1, 2000, to December 31, 2022. The type of publication was limited to articles and review articles, and the publication language was confined to English. The retrieved data were independently screened by XXC and CH to confirm the relationship with frontotemporal dementia, and any controversy was resolved by negotiation. Duplicate articles were excluded. Out of the 13,234 articles initially identified, 10,227 were included for further bibliometric analysis. The retrieval process is shown in Figure 1.
2.2 Bibliometric analysis
The general information for each paper obtained from WoSCC included the number of publications, country, institution, author, title, abstract, keywords, publication year, reference, and citation. This study analyzed several characteristics of publications, including annual publications and growth trends, countries, institutions, journals, authors, cocited authors, cocited references and keywords. All data were downloaded in the plain text format from WoS and then imported to Citespace (version 6.2. R4, 64-bit) and Vosviewer (Version 1.6.19) for further bibliometric analysis. Microsoft Excel 2019 was used to generate the annual publications and growth trends (Figure 2). In the visual map established by Citespace and Vosviewer, each node represents an element, such as a country, institution, journal, or author. The larger the node size is, the greater the number of reflections, and the larger the link between the nodes is, the stronger the degree of collaboration (32).
3 Results
3.1 Publication output analysis
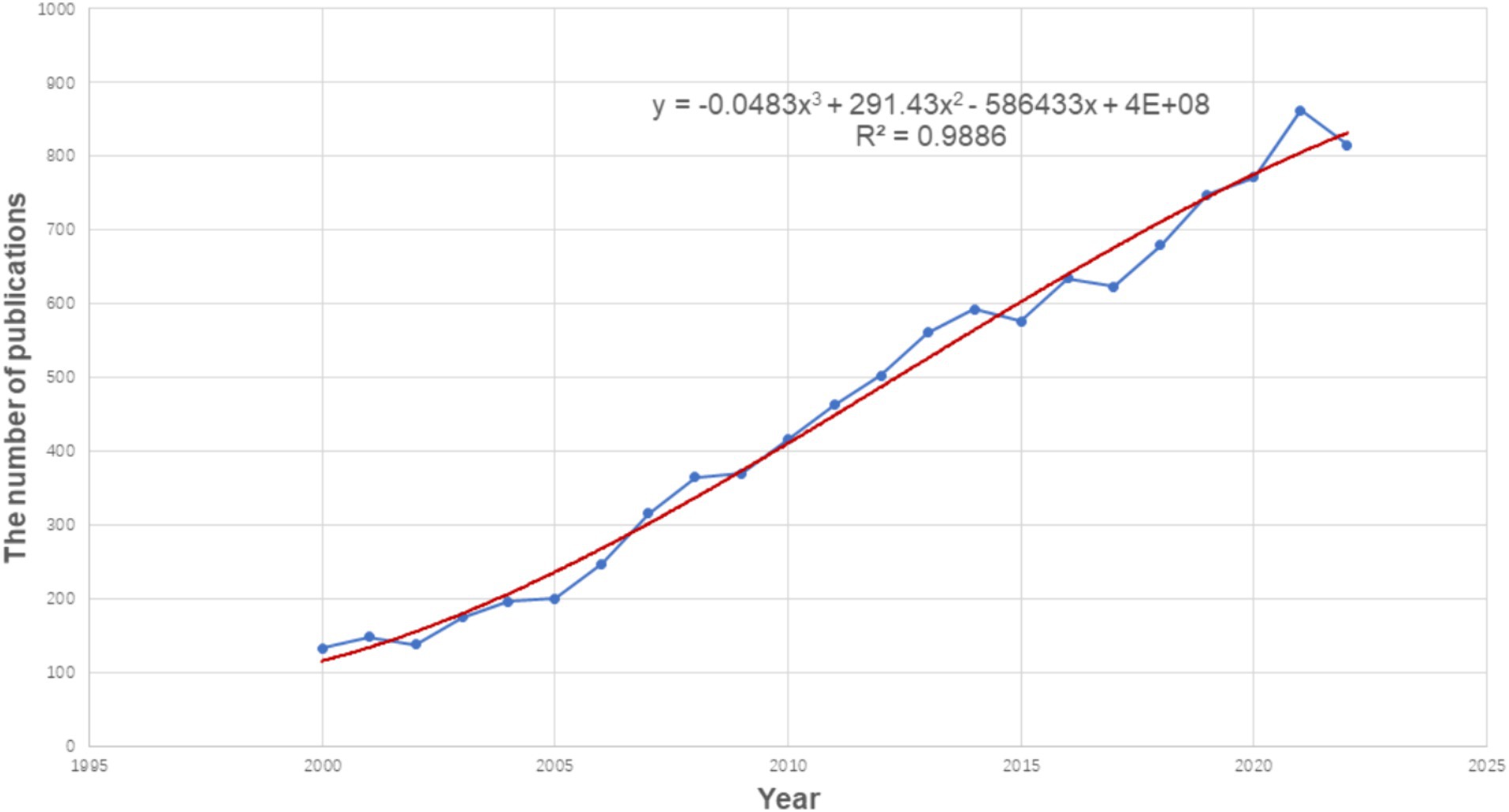
From 2000 to 2022, the annual publication output increased significantly, as shown in Figure 2, indicating that researchers are paying increasing attention to FTD each year. Since 2012, the number of annual publications has exceeded 500 for 10 consecutive years, and up to 815 by 2022. Linear regression analysis showed that the annual publication volume was positively correlated with the publication year (R2 = 0.989, p < 0.001) (Figure 2). Therefore, we speculate that the annual number of articles in 2023 is expected to reach 900. The number of articles published each year increased year by year, which shows that FTD is an active research field and has received extensive attention from scholars.
3.2 Analysis of countries/regions and institutions
Analysis of countries/regions and institutions can identify objects with high scientific productivity and influence and present their cooperation in the field. The retrieved publications came from 87 countries/regions. The top 10 countries in terms of number of publications, citations, average citations, and centrality are shown in Table 1. From 2000 to 2022, the United States produced the most publications (n = 4,037), followed by England (n = 1,872), Italy (n = 1,271), Germany (n = 906), and Canada (n = 884). The top three countries in terms of literature citations and average citations are the United States, England and Canada. Among all countries, the country with the highest centrality is England (0.20). Table 1 also shows the top 10 most productive institutions. The institution with the largest publications is the University of California San Francisco (n = 687), followed by Mayo Clinic (n = 584), University of Pennsylvania (n = 474), University College London (n = 455), and University of Cambridge (n = 363). Figure 3 illustrates the network map of collaborating countries/regions and institutions. According to the total link strength, we found that the United States has close and extensive partnerships in research with other countries, followed by England, Germany, Italy, Canada, and the Netherlands. Links between the University of California San Francisco, Mayo Clinic, University College London, University of Toronto, University of Pennsylvania, and other institutions indicate close collaboration.
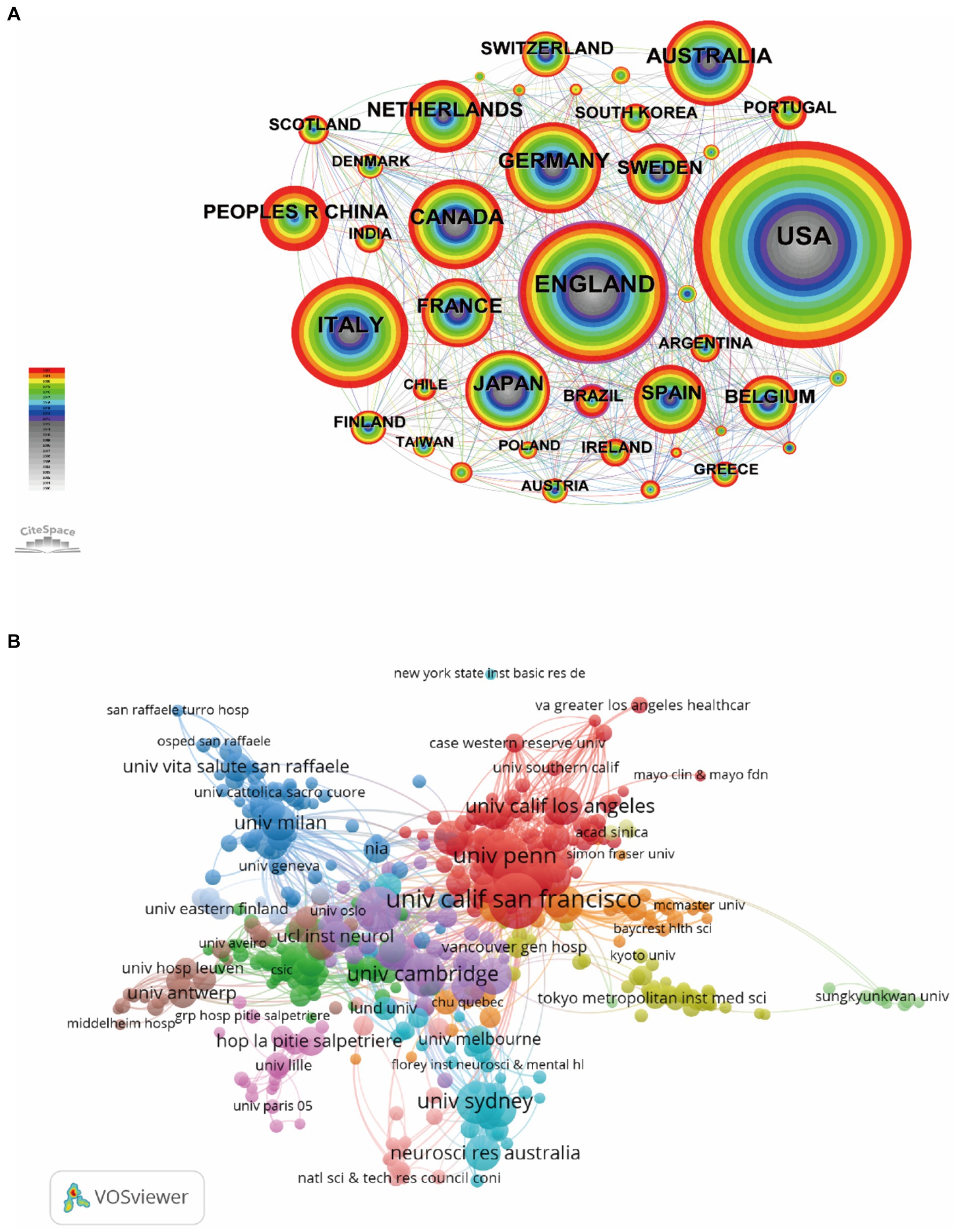
Figure 3. Network map of collaborating countries/regions (A) and institutions (B). Each node represents one country/region or institution, the size of the circle represents the number of publications, and the link strength of the connections between two nodes represents the closeness of cooperation.
3.3 Analysis of published journals
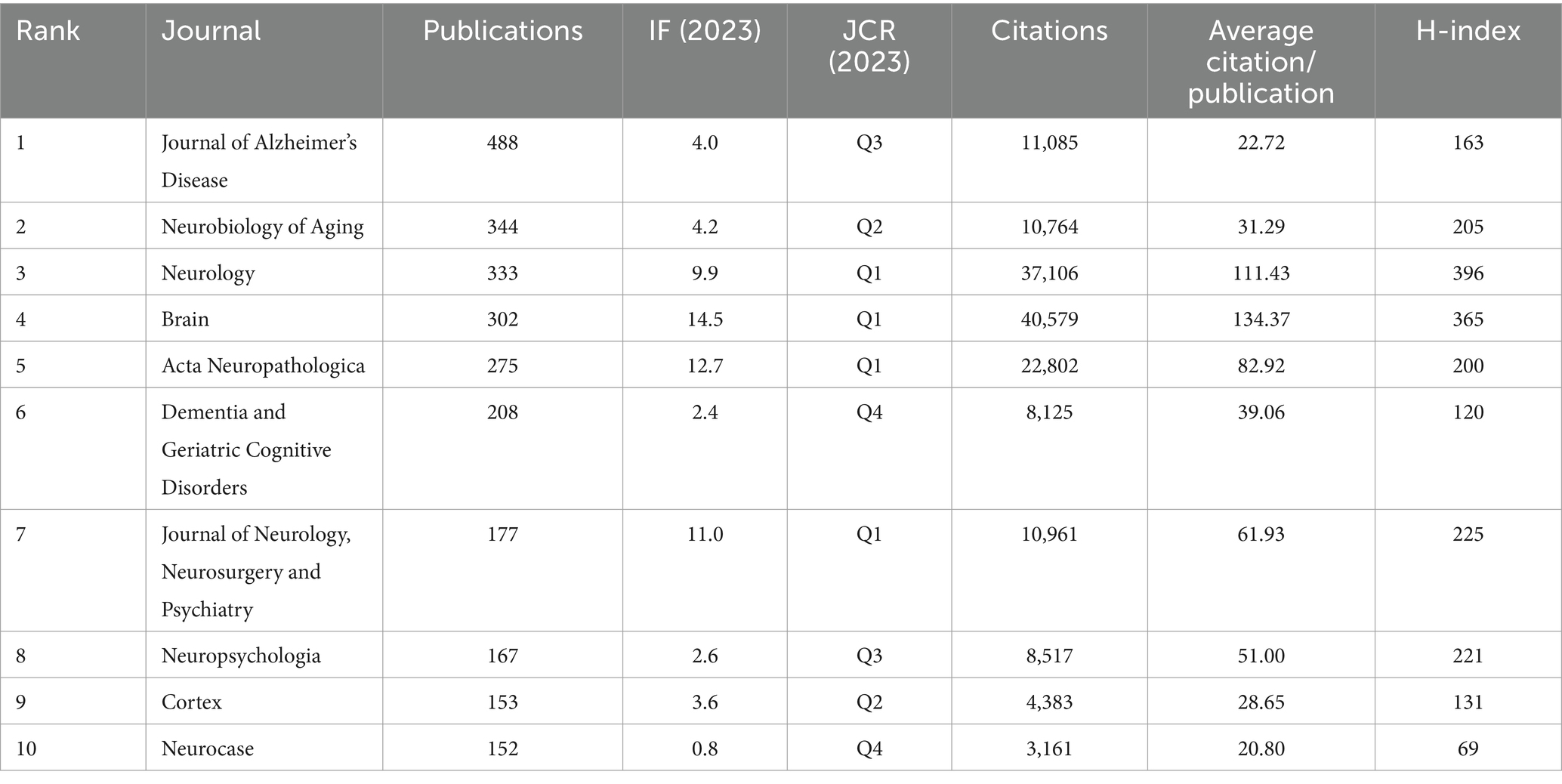
Journal analysis can provide a useful reference for publishing research results. A total of 934 different journals published articles on FTD research. Table 2 shows the top 10 journals in terms of publication volume. The Journal of Alzheimer’s Disease (n = 488, IF = 4.0) was the most productive journal for publishing FTD-related articles, followed by Neurobiology of Aging (n = 344, IF = 4.2), Neurology (n = 333, IF = 9.9), Brain (n = 302, IF = 14.5), and Acta Neuropathologica (n = 275, IF = 12.7). Among the top 10 journals, Brain not only has the greatest number of total citations (n = 40,579) but also has the highest impact factor (14.5) and average citations per paper (134.37). Its h-index is 365. It is a journal that provides researchers and clinicians with the best original contributions in the field of neurology, and its continuously published papers have become classics in the field. In the top 10 journals, Neurology has the highest H-index of 396. Journal cocitation analysis can analyse the correlation and similarity between two articles (33). The more cocitations of a journal, the greater the influence of the journal in a specific research field. As shown in Figure 4, Vosviewer identified the top three cocited journals, which were Neurology (38,202), Brain (27,717) and Acta neuropathologica (19,248).
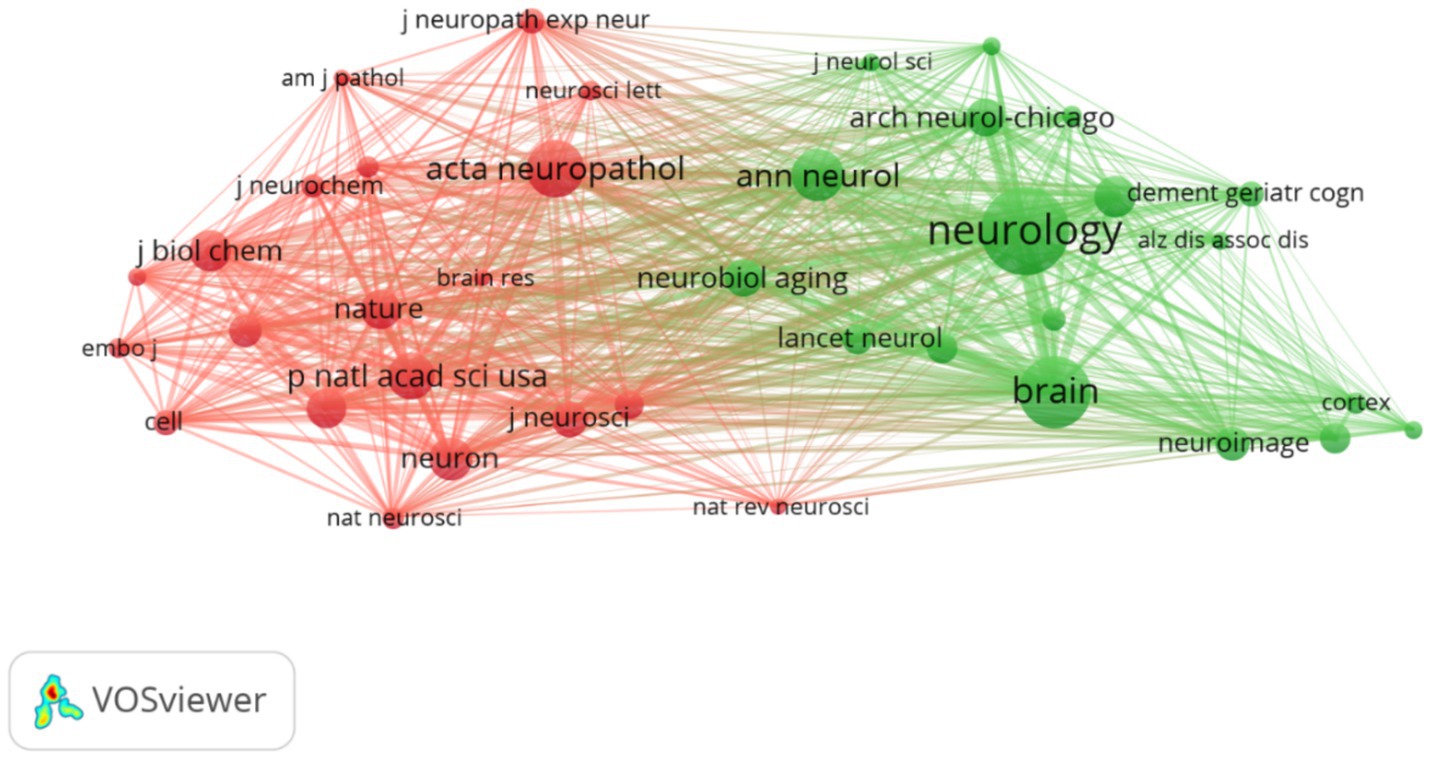
Figure 4. Network map of cocited journals. Each node represents one journal, the size of the circle represents the cocitation counts, and the link connecting the two circles represents the cocited relationship between journals.
3.4 Analysis of authors and cocited authors
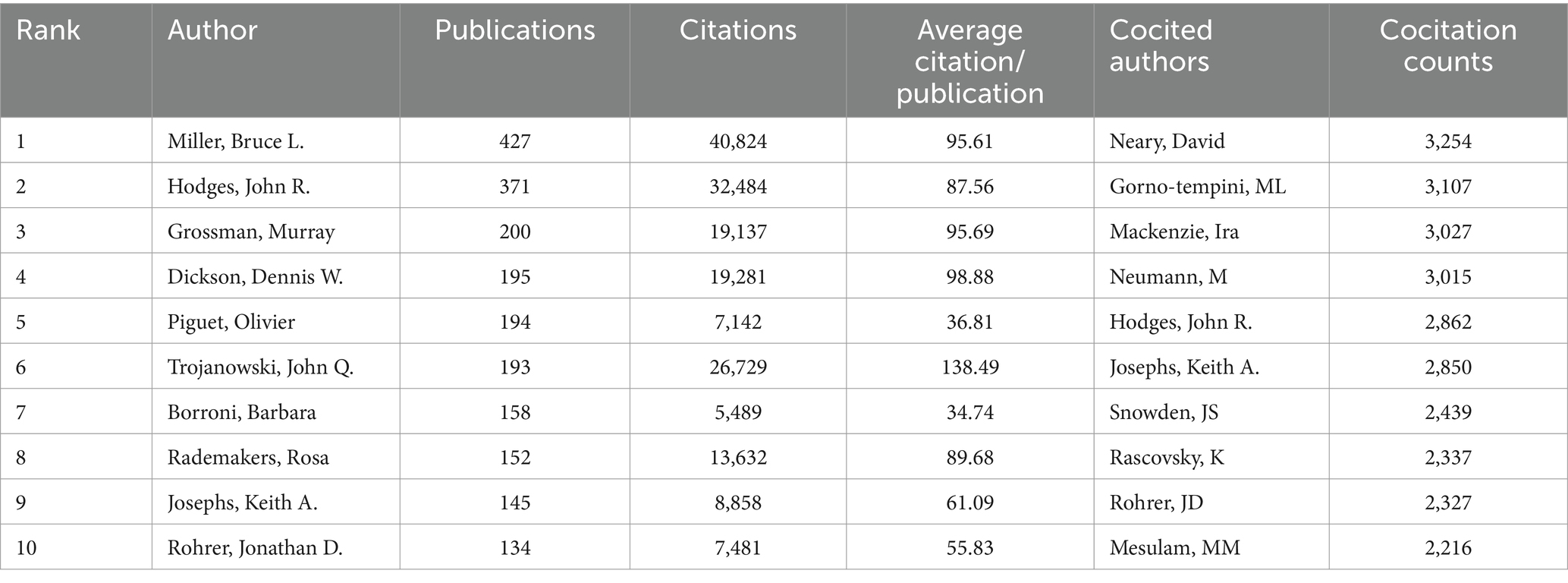
The published papers were contributed by 33,909 authors. The top 10 authors and cocited authors that contributed papers on FTD research are shown in Table 3. The most productive authors are Miller, Bruce L. (n = 427), followed by Hodges, John R. (n = 371), Grossman, Murray (n = 200), Dickson, Dennis W. (n = 195), and Piguet, Olivier (n = 194). Miller, Bruce L. (40,824 citations) had the highest number of citations among all the authors, who focused on FTD, dementia, pathology, Alzheimer’s disease and neuroscience. His body of work in FTD is particularly relevant to semantic dementia. Trojanowski, John Q. had a significantly higher number of citations per item (138.49) than the other authors. Neary, David (3,254 citations) was the most cocited author, followed by Gorno-tempini, ML (3,107 citations), Mackenzie, Ira (3,027 citations), Neumann, M (3,015 citations), and Hodges, John R. (2,862 citations). In frontotemporal lobar degeneration, Neary, David works on issues such as genetics, which are connected to tau protein and charged multivesicular body protein 2B. Figure 5 shows the network maps of authors and cocited authors.
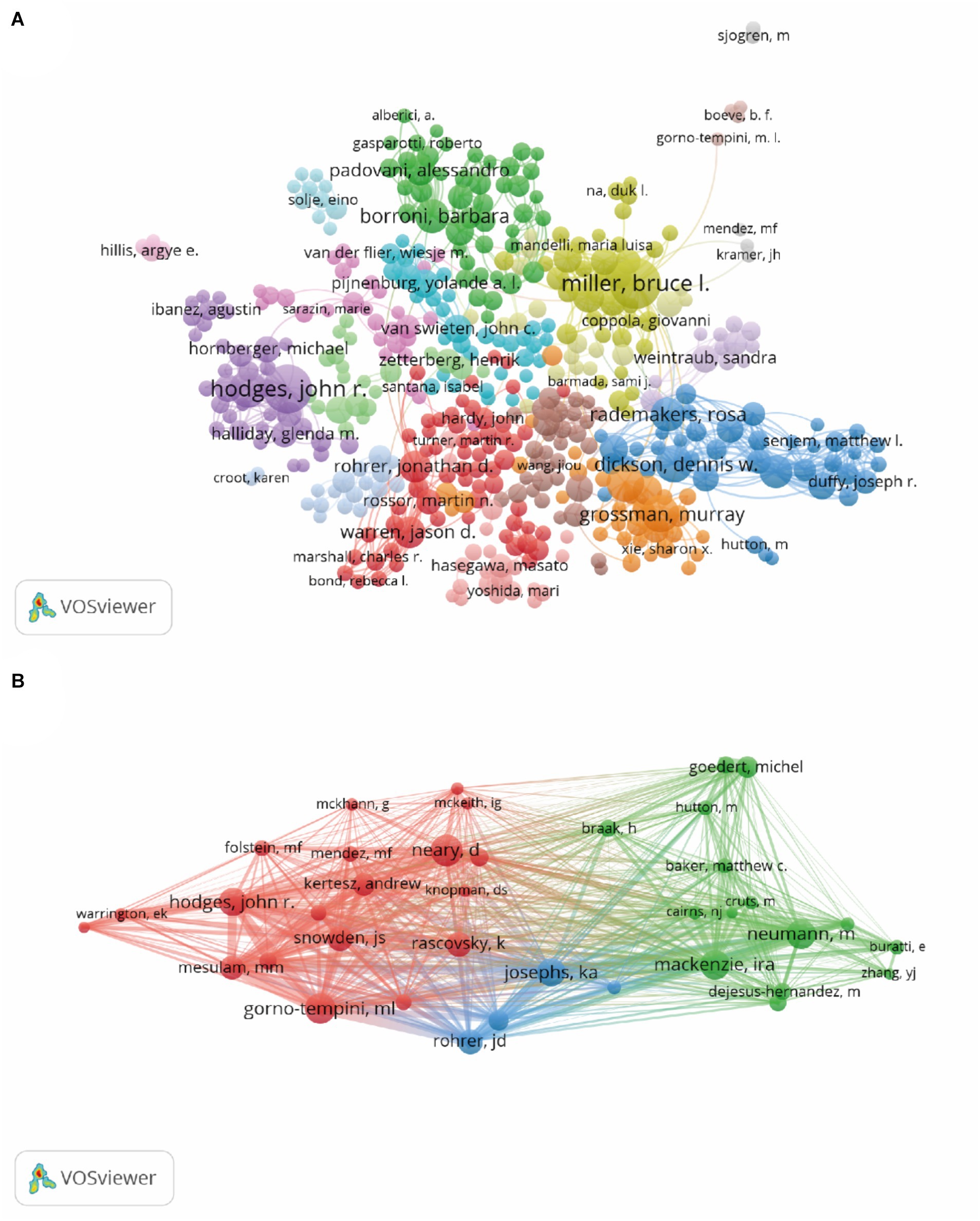
Figure 5. Network map of authors (A) and cocited authors (B). Each node represents one author, the size of the circle represents the number of publications (A) or cocitation counts (B), and the link connecting the two circles represents the co-occurrence (A) or cocitation (B) relationship between authors.
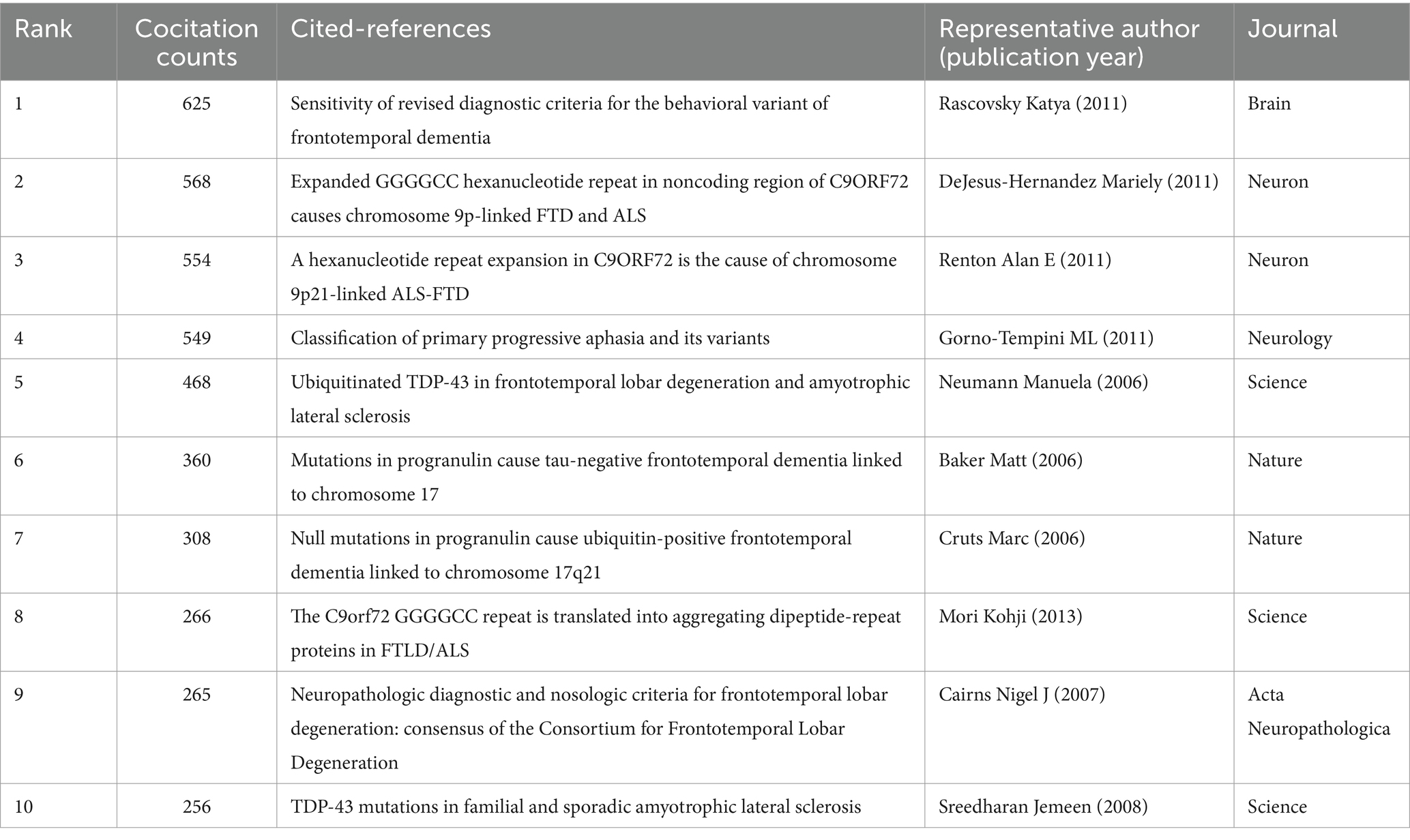
3.5 Analysis of cocited references
Highly cited articles may lay the foundation for a certain field and often have important reference value (34). Figure 6 shows the network map of cocited references, and Table 4 shows the top 10 cocited references related to frontotemporal dementia. These papers focused on the diagnostic criteria and genetic research topics of FTD. The article by Rascovsky Katya and his colleagues published on Brain in 2011 (35) was the most cocited paper, with 625 citations. The authors indicated that the proposed International Behavioral Variant FTD Criteria Consortium criteria provided a sensitive criterion for bvFTD diagnosis, allowing for early identification of the syndrome when disease-modifying therapies are expected to be most effective.
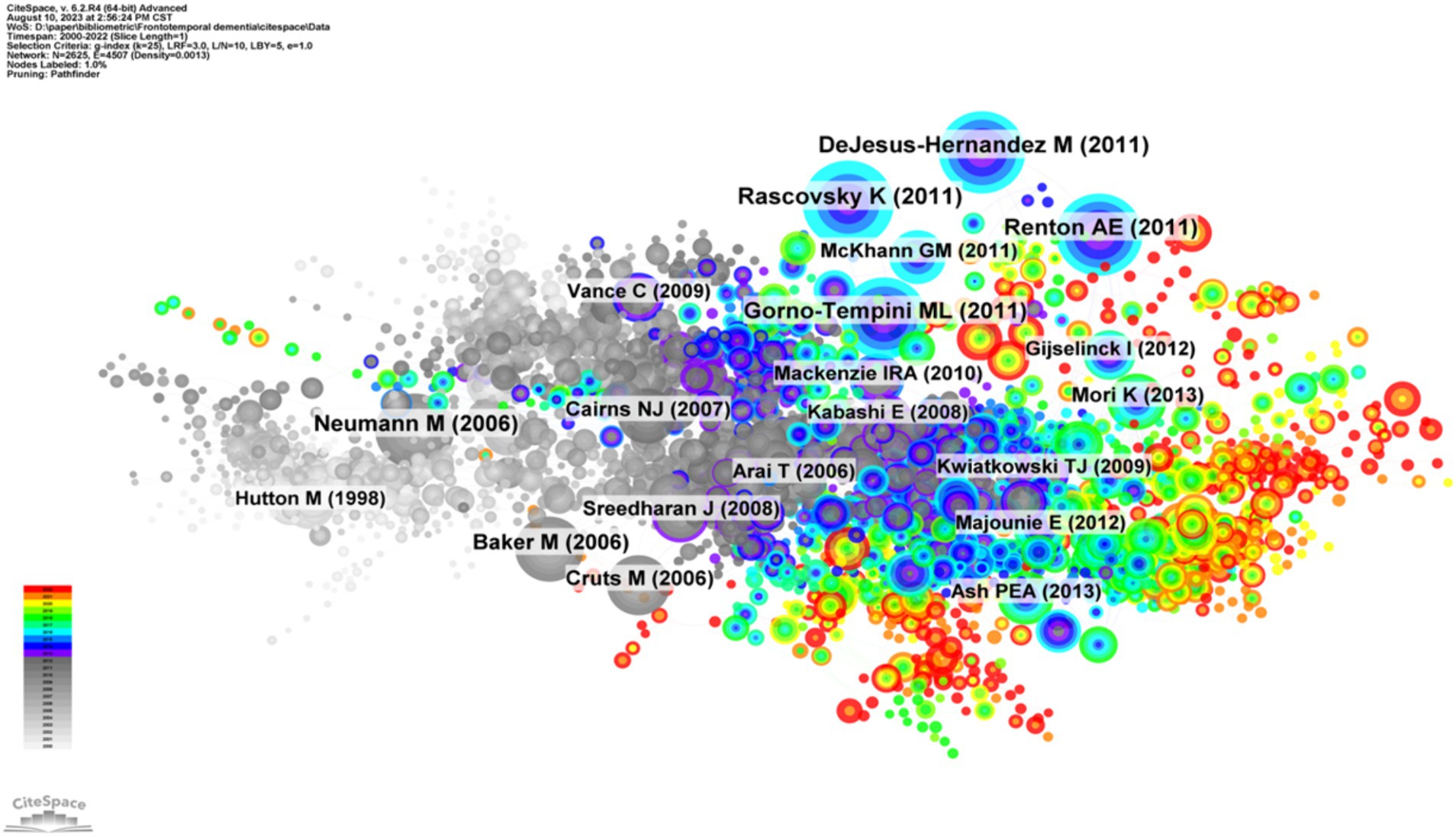
Figure 6. Visualization of the cocited references network. Each node represents one reference, the size of the circle represents the cocitation counts, and the link connecting the two circles represents the cocited relationship between references.
Clustering of cocited literature is good for finding the cutting edge in a field. The cluster analysis of these cocited references produced 16 main clusters (Figure 7A) and their timelines for each cluster label (Figure 7B). The top 10 clusters that reflect the knowledge base and research progress in FTD are #0 semantic dementia, #1 FTDP-17, #2 TDP-43, #3 C9orF72, #4 progranulin, #5 protein aggregation, #6 biomarkers, #7 FUS, #8 primary progressive aphasia, and #9 psychosis. The modular Q-value is 0.80, which is relatively high, indicating that the particularity of scientific mapping has been clearly defined in cocited clustering. In the timeline view of the 16 clusters, the earliest research directions were Cluster #0 semantic dementia, #1 FTDP-17, #4 progranulin, and #15 subcortical, with most papers published approximately 1995. Clusters #3 C9orF72, #5 protein aggregation, #6 biomarkers, #10 lysosome, and #11 positron emission tomography were the main topics of the latest publications, with most papers published approximately 2021.
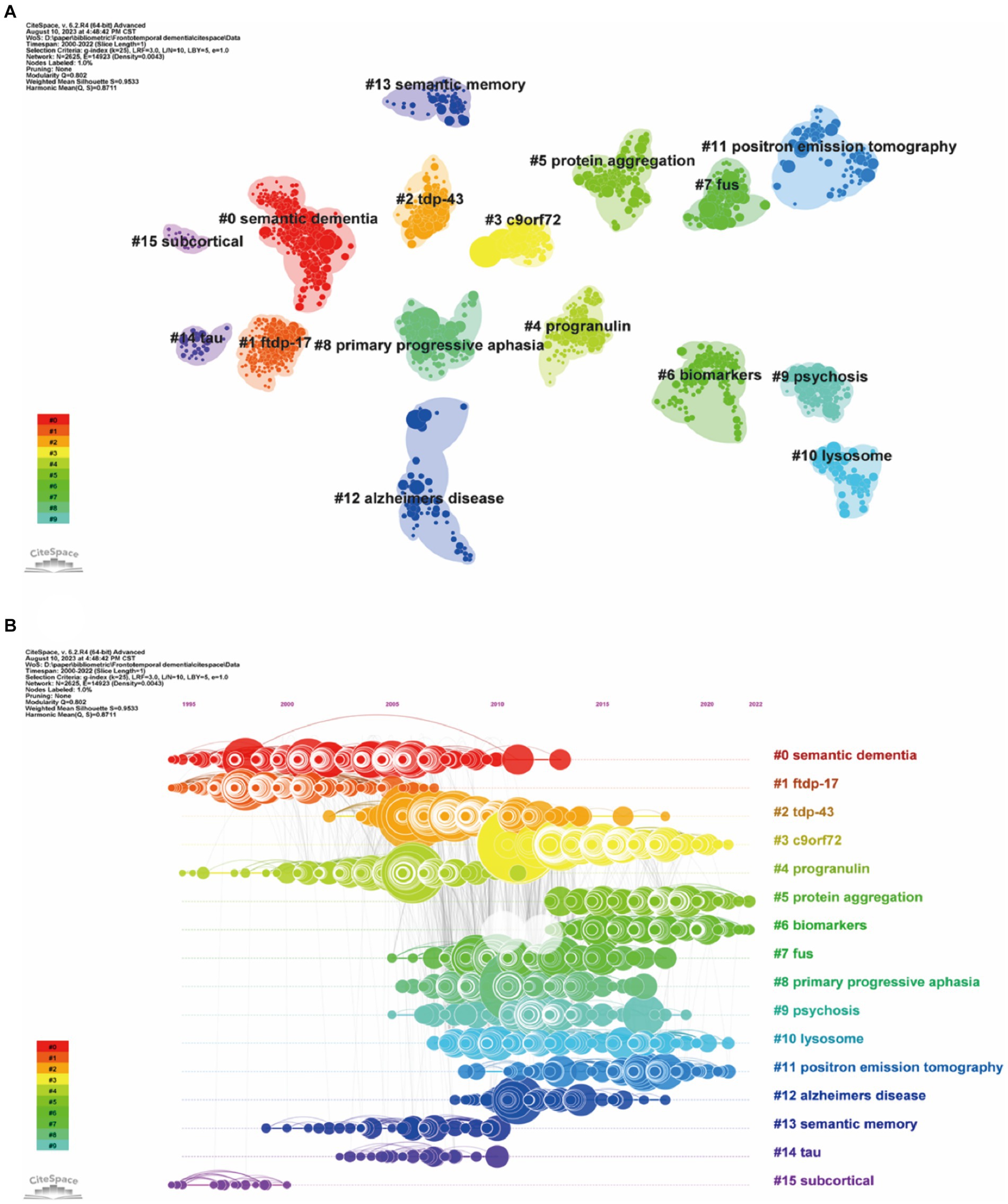
Figure 7. Clustered network map of cocited references (A) and their timelines for each cluster label (B). Each node represent one co-cited article, and nodes are organized in different clusters gathered into a network or timeline of cocitation; the node size reflects the cocited counts, and the link indicate the cocited relationship; cluster labels were extracted from keywords.
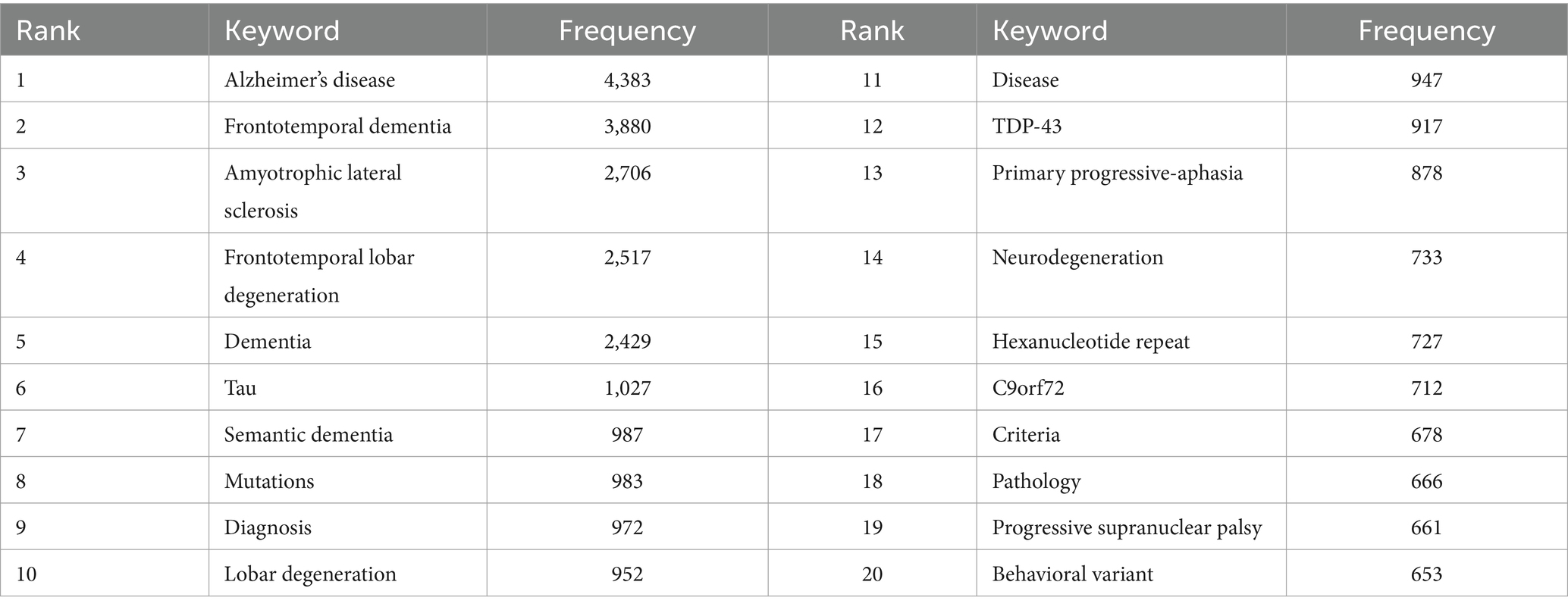
3.6 Analysis of co-occurrence keywords and burst keywords
Keyword co-occurrence analysis was used to identify research trends and hotspots in the FTD field. As shown in Table 5, the top 3 high-frequency co-occurrence keywords were “Alzheimer’s disease,” “frontotemporal dementia,” and “amyotrophic lateral sclerosis,” followed by “frontotemporal lobar degeneration,” “dementia,” “tau,” “semantic dementia,” “mutations,” “diagnosis,” and “lobar degeneration.” The co-occurrence keywords network (Figure 8A) and the clustered network map of keywords (Figure 8B) were shown. In this study, keywords were divided into 4 clusters: #0 Amyotrophic lateral sclerosis, #1 semantic dementia, #2 frontotemporal dementia, and #3 tau. When the modularity of the cluster graph is greater than 0.3, the clustering structure is significant, and when the weighted mean silhouette reaches 0.7, the clustering result is convincing. since the modularity was 0.414 and the weighted mean silhouette was 0.77 in this study, the results of the keywords cluster graph can be considered reasonable and convincing. In FTD from 2000 To 2022, the research frontiers were amyotrophic lateral sclerosis, PPA, and tau pathology.
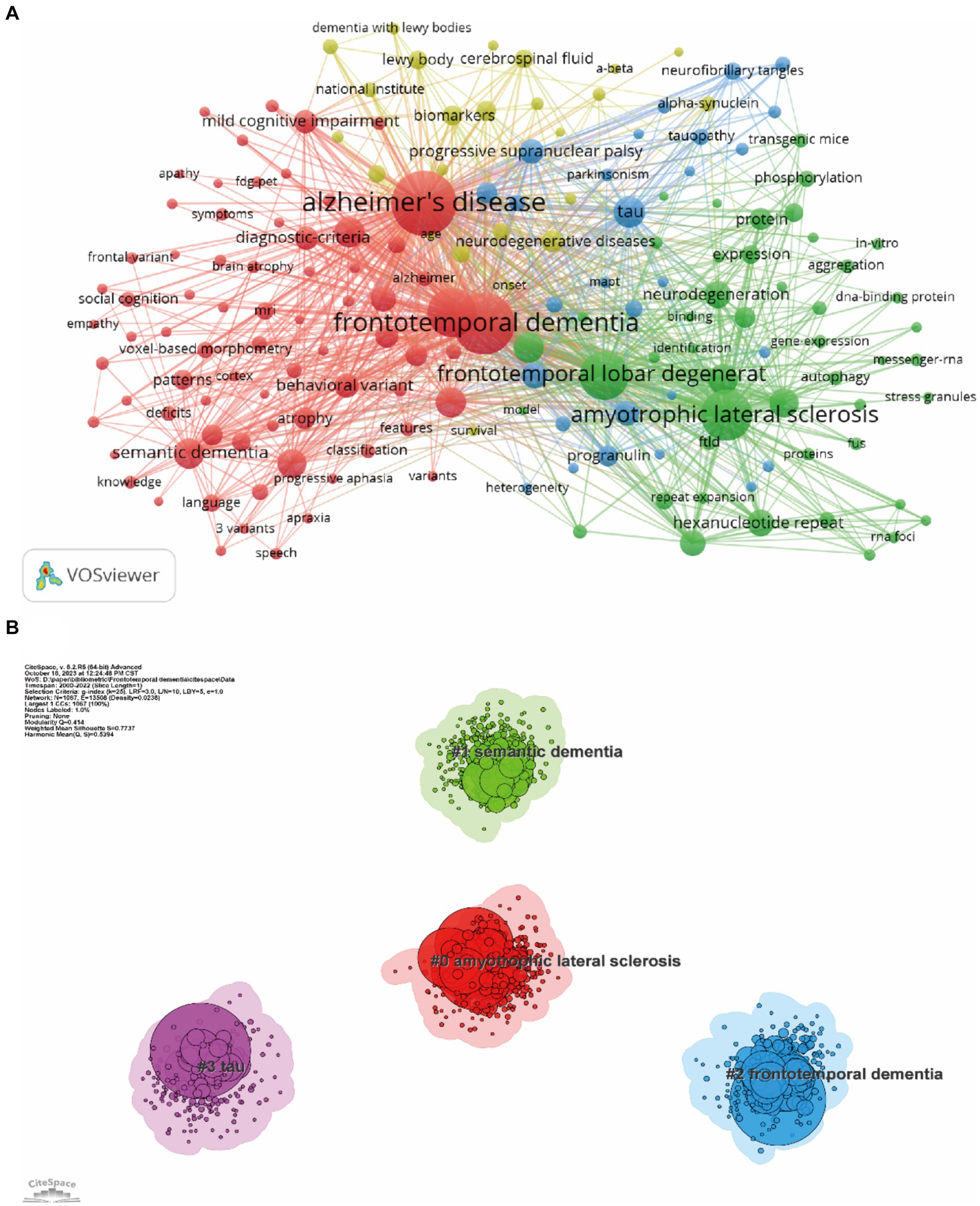
Figure 8. Co-occurrence keywords network (A) and the clustered network map of keywords (B). Each node represent one keyword, the size of the circle represents the co-occurrence frequencies, and the link represents the co-occurrence relationship between the two keywords (A). Nodes are organized in different clusters gathered into a network of cocitation, and cluster labels were extracted from keywords (B).
Burst keyword detection can identify emerging concepts that have gained significant relevant attention over a period of time. Figure 9 shows the top 25 keywords with the strongest bursts over the past two decades. Picks disease ranked first with the highest burst strength (102.90) followed by corticobasal degeneration (55.11) presenile dementia (45.83) and frontal lobe degeneration (44.70). This indicates that the early understanding of the cerebral cortex characteristics of FTD is in constant progress. From 2000 to 2009 the keywords “tau” “chromosome 17” and “FTDP-17” appeared indicating that the research during this period was related to mutations in the tau gene on chromosome 17. Furthermore phase separation has been the most active burst keyword since 2018 which partly reflects future research trends that may be related to the phase separation of proteins and RNA
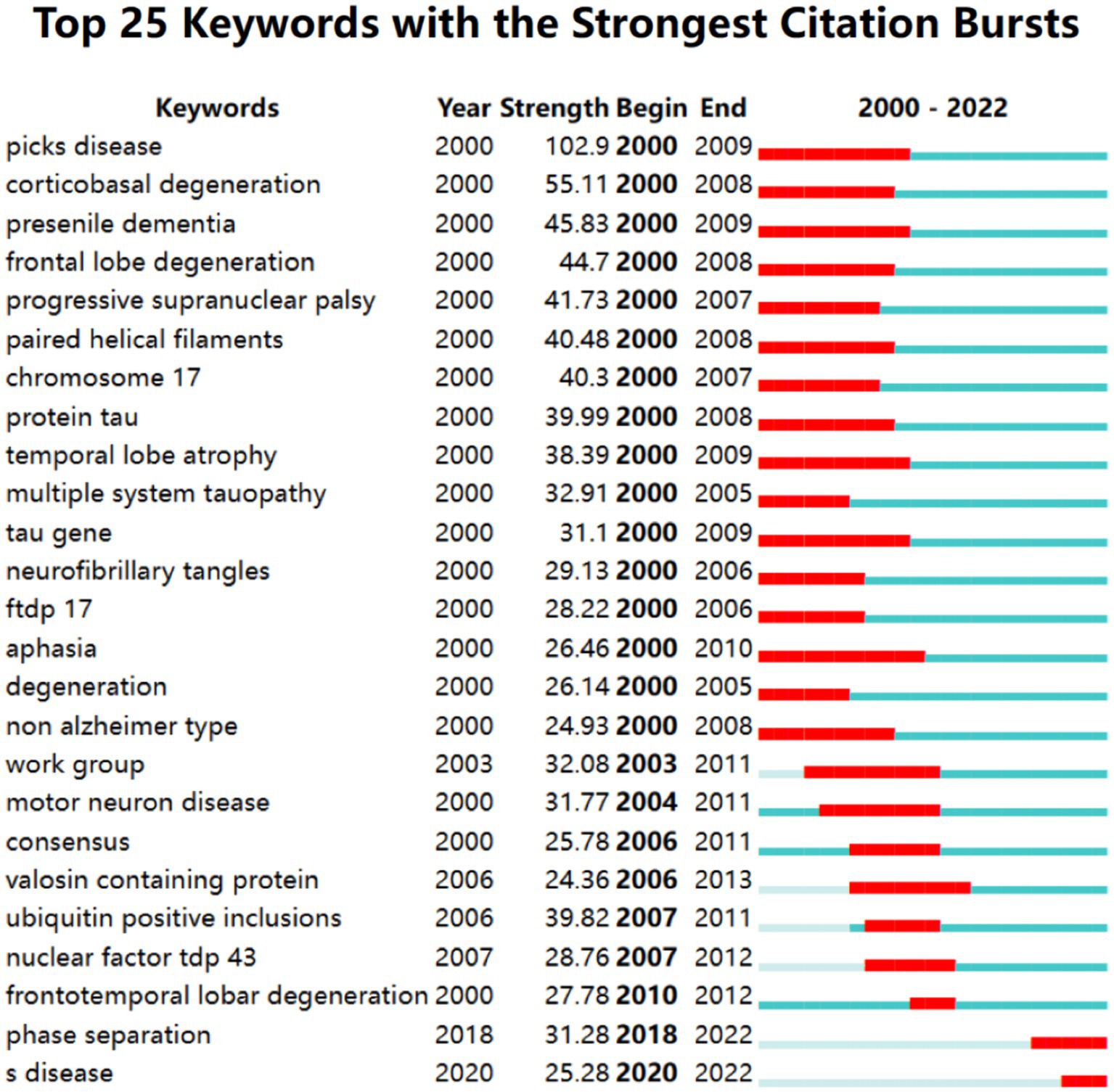
Figure 9. Keywords with the strongest citation bursts in publications on frontotemporal dementia between 2000 and 2022.
4 Discussion
4.1 General information
Our research found that the annual publications on FTD have increased over the past two decades. The number of published papers in 2022 is 1.76 times that of 2011 and 6.08 times that of 2000. We estimate that the annual number of publications in 2023 is expected to reach 900. FTD is attracting increasing attention and interest from researchers, which may be related to the increased incidence of neurodegenerative diseases caused by population aging (36). Of the top 10 countries/regions contributing to FTD research, the United States, England, and Italy lead the way, accounting for 70%. The top five most productive institutions are California San Francisco, Mayo Clinic, University of Pennsylvania, University College London, and University of Cambridge, which are all from the United States and England. Nodes with high centrality (≥0.10) imply “bridge” effects in the global cooperation network for these countries/regions (37). Among all countries, only England (0.20) and Brazil (0.12) have centrality above 0.1, suggesting that England is likely to maintain a dominant position in FTD research, while Brazil plays an important role in global collaboration. The top three most productive journals are the Journal of Alzheimer’s Disease, Neurobiology of Aging, and Neurology, accounting for 11.3%. This indicates that the researchers focused on neuroscience and geriatrics. Neurosciences, neurology, psychiatry, genetics heredity, and behavioral sciences are the main categories in WOS. In terms of author contributions, Miller, Bruce L. ranks first in publishing papers on FTD (n = 427), who focuses on semantic dementia, pathology, Alzheimer’s disease and neuroscience. The 10 most cited papers, mainly related to the diagnostic criteria of FTD, C9orF72, TDP-43, and chromosome 17, were published between 2006 and 2013, which may be a period of high-quality development in the field of FTD research.
4.2 Research hotspots and trends in FTD research
Keyword co-occurrence can present Hot topics In an academic field, and keyword bursts indicate emerging trends and potentially valuable research directions to some extent (38). Reference clustering and timelines Can characterize emerging topics in the discipline (16, 39). Based on this, we summarized the research hotspots and trends in FTD research.
4.2.1 Research hotspots
The validation of clinical diagnostic criteria and the subdivision of FTD is one of the research hotspots. The red cluster (Figure 8A) mainly includes the clinical manifestation of FTD according to keywords, such as mild cognitive impairment, semantic dementia, behavior variant, progressive aphasia, and brain atrophy. Typical presentation of FTD includes behavioral and speech variant, forming bvFTD and PPA, respectively. At present, the authoritative diagnostic criteria for bvFTD and PPA still refer to the two consensuses published in 2011 (35, 40). “Possible” bvFTD can be diagnosed by three of six typical manifestations, including disinhibition, apathy, loss of sympathy or empathy, perseverative or compulsive behaviors, hyperorality, and dysexecutive syndrome (35). nfv-PPA was defined as slow, grammatically incorrect language output and difficulty understanding sentences with complex grammar (40). Semantic dementia is characterized by impaired comprehension of naming and individual words, as well as deficits in recognition of object information. Nevertheless, the overlap of symptoms with other syndromes and the occurrence of complications such as other neurodegenerative diseases (Parkinson’s disease or other motor neuron diseases) in the various stages of FTD can still lead to difficulties in the diagnosis and differential diagnosis of FTD (41–43). Subsequently, studies have confirmed the sensitivity and accuracy of these diagnosis criteria (44, 45). For example, the sensitivity of the bvFTD criteria is as high as 85–90% (44). However, with the increasing understanding of FTD, some researchers have proposed that a large proportion of bvFTD and PPA cases are not classified and that the diagnostic criteria are inadequate (46, 47), suggesting that further research to revise the diagnostic criteria and add classifications of FTD are needed. “Alzheimer’s disease,” “Frontotemporal dementia,” “Amyotrophic lateral sclerosis,” “Dementia,” “Semantic dementia,” “Primary progressive-aphasia” are the diseases or syndromes that appear frequently in keywords. The diagnosis and differential diagnosis of FTD from AD, other types of dementia, and ALS have always been the main research content and an important clinical focus (48, 49) because these diseases have similar clinical manifestations and pathological and genetic examinations.
The pathological mechanism of FTD is another important research hotspot. The top 20 keywords included those related to the FTD pathomechanism, such as “Tau,” “Mutations,” “Lobar degeneration,” “Neurodegeneration,” “TDP-43,” “Hexanucleotide repeat,” and “C9orf72.” It has been reported that hyperphosphorylation of MAPT causes FTD (50), presenting tau neurofibrillary inclusion pathology (51). In 2006, Baker et al. (52) demonstrated that mutations in GRN located on chromosome 17q21.31 would cause FTD without mutations in MAPT. Another breakthrough in the same year was that TDP-43 was identified as a major component of sporadic and familial cases of frontotemporal lobar degeneration (FTLD) with ubiquitin-positive, tau-negative inclusions (FTLD-U) and amyotrophic lateral sclerosis (ALS) (53). In 2007, the diagnostic criteria for FTLD were established based on existing criteria and included a new molecular pathology, TDP-43 protein disease, which was considered the most common histological finding in FTLD (54). DeJesus-Hernandez et al. (55) published an article in Neuron in 2011 reporting amplification of the noncoding GGGGCC hexanucleotide repeat of gene C9orF72, which is strongly associated with disease in large relatives of FTD/ALS. This suggested that repeated amplification of C9orF72 was the main cause of FTD and ALS. Additionally, Renton et al. (56) published an article in Neuron in 2011 demonstrating that a massive hexanucleotide repeat expansion within C9orF72 is the cause of chromosome 9p21-linked ALS, FTD and ALS-FTD in 2011. In 2013, Mori et al. (57) found that the C9orf72 GGGGCC repeat is translated into aggregated dipeptide repeat proteins in FTLD/ALS, which are presumably generated by non-ATG-initiated translation from the expanded GGGGCC repeat in three reading frames. These results have made outstanding contributions to the research of FTD and have been published in high-quality journals such as Nature (IF = 64.8), Science (IF = 56.9), and Neuron (IF = 16.2). Further research was being conducted based on the above mechanisms. In addition, “tau” remains the focus of research. As the highest content of microtubule-associated protein, tau protein can bind to the formed microtubules, maintain microtubule stability, and participate in a variety of cellular functions (58). Neurodegenerative diseases associated with abnormal phosphorylation and mutation of tau protein are known as tauopathies, such as frontotemporal lobe degeneration (59). The mechanism of FTD based on pathological changes in tau protein is still unclear. In recent years, researchers have found that it might associate with mitochondrial protein (60), extracellular protein (61), and glutamate signaling (62). The study of the underlying pathogenesis mediated by tau is useful for the targeted blocking treatment of FTD. At present, there is no specific drug for FTD, and the current drug treatment is mainly used to alleviate the symptoms of patients (26). Nonpharmacological treatments have limited benefits to older patients with dementia (63, 64). Therefore, researchers have spent much time studying the pathogenesis of FTD, including pathophysiology, genetics, and biomarkers, in an attempt to explore targeted therapies to treat FTD (65). To date, drugs targeting pathological tau (66) and GRN (67) have been developed.
4.2.2 Emerging trends
It is worth noting that “Phase separation” might be a hotspot starting in 2018 (Figure 9). Protein phase separation (PPS) is widespread in cells and drives a variety of important biological functions (68). PPS at the wrong time or place can create blockages or clumps of molecules associated with neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease (69). This may be an underlying mechanism of FTD. Although much progress has been made in the pathology of FTD, the neuropathology of FTD is heterogeneous (70), and patients with FTD may differ significantly in age of onset, manifestations, and course of disease even with the same mutation (71). Therefore, based on the above biomarkers and pathological changes, researchers have started to diagnose and differentially diagnose FTD from many aspects, such as behavioral manifestations, neuropathology, neuroimaging, and serum markers (72). Focusing on neuropsychological measures of social cognition is of great importance for the early diagnosis of FTD through clinical manifestations (73). Van der Ende et al. (74) measured NPTX2 concentrations in the cerebrospinal fluid of 260 carriers with pathogenic mutations in GRN, C9orf72 or MAPT and some noncarrier individuals and found that NPTX2 might be a meaningful biomarker for the diagnosis of FTD. Zhu et al. (75) found that the binding of plasma glial fibrillary acidic protein and plasma neurofilament light chain could distinguish FTD from AD. Oeckl et al. (76) supported the differential diagnosis of FTD by serum glial fibrillary acidic protein. The pathological changes in the frontotemporal lobe in patients with FTD revealed by multimodal MRI may have certain reference value for the diagnosis and prognosis of FTD (77). Nguyen et al. (78) proposed a novel MRI index called the frontotemporal dementia index, which might help in the differential diagnosis between AD and FTD. In addition, a large-sample longitudinal study on cognitive characteristics and biomarkers of FTD has been reported (79), which has important implications for fully understanding the progression of FTD and diagnosing FTD at different stages. Therefore, the future direction may be biomarker-based cerebrospinal fluid, peripheral blood, and imaging tests and then build predictive models to accurately predict the specific pathological biochemical types of FTD individuals.
4.3 Strength and limitations
To our knowledge, this study is the first bibliometric analysis to evaluate the hotspots and cutting edges of FTD-related publications over the past two decades. This study included 10,227 publications on FTD and comprehensively analyzed the number of publications, citations, H-index, collaboration between countries/regions and institutions, cocitations of journals/authors/references, and co-occurrence keywords and burst keywords.
However, there are some limitations in this study. First, we only searched the literature in the SCI-E of the WoSCC database because the articles in WoSCC are the most commonly used data source in bibliometrics and can represent the majority of the information to some extent (40). Second, only English language papers were included, which may ignore relevant studies published in other languages. Third, some recent high-quality publications may not receive enough attention because of low citation frequency, while older articles accumulate more citations. This may diminish the importance of the recently published article. Therefore, readers should be aware that all of this can lead to bias in our results. Finally, bibliometric software cannot distinguish between abbreviations of terms with different names, which can lead to bias in statistical results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XC: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. YC: Formal analysis, Validation, Writing – original draft, Writing – review & editing. BN: Investigation, Resources, Supervision, Writing – review & editing. CH: Conceptualization, Formal analysis, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the General Program of Natural Science Foundation of Sichuan Provincial Department of Science and Technology (grant no. 2024NSFSC0564) and Sichuan Provincial Health Commission Universal Application Project (grant no. 20PJ018).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1399600/full#supplementary-material
References
1. Bang, J, Spina, S, and Miller, BL. Frontotemporal dementia. Lancet. (2015) 386:1672–82. doi: 10.1016/S0140-6736(15)00461-4
2. Swift, IJ, Sogorb-Esteve, A, Heller, C, Synofzik, M, Otto, M, Graff, C, et al. Fluid biomarkers in frontotemporal dementia: past, present and future. J Neurol Neurosurg Psychiatry. (2021) 92:204–15. doi: 10.1136/jnnp-2020-323520
3. Hodges, JR, Davies, R, Xuereb, J, Kril, J, and Halliday, G. Survival in frontotemporal dementia. Neurology. (2003) 61:349–54. doi: 10.1212/01.WNL.0000078928.20107.52
4. Olney, NT, Spina, S, and Miller, BL. Frontotemporal dementia. Neurol Clin. (2017) 35:339–74. doi: 10.1016/j.ncl.2017.01.008
5. Vieira, RT, Caixeta, L, Machado, S, Silva, AC, Nardi, AE, Arias-Carrión, O, et al. Epidemiology of early-onset dementia: a review of the literature. Clin Pract Epidemiol Ment Health. (2013) 9:88–95. doi: 10.2174/1745017901309010088
6. Boeve, BF, Boxer, AL, Kumfor, F, Pijnenburg, Y, and Rohrer, JD. Advances and controversies in frontotemporal dementia: diagnosis, biomarkers, and therapeutic considerations. Lancet Neurol. (2022) 21:258–72. doi: 10.1016/S1474-4422(21)00341-0
7. Steele, JC, Richardson, JC, and Olszewski, J. Progressive supranuclear palsy. A heterogeneous degeneration involving the BRAIN STEM, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol. (1964) 10:333–59. doi: 10.1001/archneur.1964.00460160003001
8. Coughlin, DG, and Litvan, I. Progressive supranuclear palsy: advances in diagnosis and management. Parkinsonism Relat Disord. (2020) 73:105–16. doi: 10.1016/j.parkreldis.2020.04.014
9. Magrath Guimet, N, Zapata-Restrepo, LM, and Miller, BL. Advances in treatment of frontotemporal dementia. J Neuropsychiatry Clin Neurosci. (2022) 34:316–27. doi: 10.1176/appi.neuropsych.21060166
10. Rohrer, JD, Guerreiro, R, Vandrovcova, J, Uphill, J, Reiman, D, Beck, J, et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology. (2009) 73:1451–6. doi: 10.1212/WNL.0b013e3181bf997a
11. Greaves, CV, and Rohrer, JD. An update on genetic frontotemporal dementia. J Neurol. (2019) 266:2075–86. doi: 10.1007/s00415-019-09363-4
12. Peet, BT, Spina, S, Mundada, N, and La Joie, R. Neuroimaging in frontotemporal dementia: heterogeneity and relationships with underlying neuropathology. Neurotherapeutics. (2021) 18:728–52. doi: 10.1007/s13311-021-01101-x
13. Puppala, GK, Gorthi, SP, Chandran, V, and Gundabolu, G. Frontotemporal dementia - current concepts. Neurol India. (2021) 69:1144–52. doi: 10.4103/0028-3886.329593
14. Benussi, A, and Borroni, B. Advances in the treatment and management of frontotemporal dementia. Expert Rev Neurother. (2023) 23:621–39. doi: 10.1080/14737175.2023.2228491
15. Antonioni, A, Raho, EM, Lopriore, P, Pace, AP, Latino, RR, Assogna, M, et al. Frontotemporal dementia, where do we stand? A narrative review. Int J Mol Sci. (2023) 24:11732. doi: 10.3390/ijms241411732
16. Chen, C, and Song, M. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One. (2019) 14:e0223994. doi: 10.1371/journal.pone.0223994
17. Zhang, GF, Gong, WX, Xu, ZY, and Guo, Y. Alzheimer’s disease and epilepsy: the top 100 cited papers. Front Aging Neurosci. (2022) 14:926982. doi: 10.3389/fnagi.2022.926982
18. Donthu, N, Kumar, S, Mukherjee, D, Pandey, N, and Lim, WM. How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res. (2021) 133:285–96. doi: 10.1016/j.jbusres.2021.04.070
19. Serrano-Pozo, A, Aldridge, GM, and Zhang, Q. Four decades of research in Alzheimer’s disease (1975-2014): a bibliometric and Scientometric analysis. J Alzheimers Dis. (2017) 59:763–83. doi: 10.3233/JAD-170184
20. Xiu, R, Sun, Q, Li, B, and Wang, Y. Mapping research trends and hotspots in the link between Alzheimer’s disease and gut microbes over the past decade: a bibliometric analysis. Nutrients. (2023) 15:3203. doi: 10.3390/nu15143203
21. Yang, C, Wang, X, Tang, X, Wang, R, and Bao, X. Stem-cell research of Parkinson disease: bibliometric analysis of research productivity from 1999 to 2018. World Neurosurg. (2020) 134:e405–11. doi: 10.1016/j.wneu.2019.10.087
22. Aleixandre-Benavent, R, Alonso-Arroyo, A, González de Dios, J, Vidal Infer, A, González Muñoz, M, and Sempere, ÁP. Bibliometric profile of the global scientific research on multiple sclerosis (2003–2012). Mult Scler. (2015) 21:235–45. doi: 10.1177/1352458514540357
23. Charidimou, A, Fox, Z, Werring, DJ, and Song, M. Mapping the landscape of cerebral amyloid angiopathy research: an informetric analysis perspective. J Neurol Neurosurg Psychiatry. (2016) 87:252–9. doi: 10.1136/jnnp-2015-310690
24. Guido, D, Morandi, G, Palluzzi, F, and Borroni, B. Telling the story of frontotemporal dementia by bibliometric analysis. J Alzheimers Dis. (2015) 48:703–9. doi: 10.3233/JAD-150275
25. Häkkinen, S, Chu, SA, and Lee, SE. Neuroimaging in genetic frontotemporal dementia and amyotrophic lateral sclerosis. Neurobiol Dis. (2020) 145:105063. doi: 10.1016/j.nbd.2020.105063
26. Khoury, R, Liu, Y, Sheheryar, Q, and Grossberg, GT. Pharmacotherapy for frontotemporal dementia. CNS Drugs. (2021) 35:425–38. doi: 10.1007/s40263-021-00813-0
27. Seelaar, H, and Van Swieten, JC. In vivo PET imaging of neuroinflammation in familial frontotemporal dementia. J Neurol Neurosurg Psychiatry. (2021) 92:231. doi: 10.1136/jnnp-2020-324348
28. Boland, S, Swarup, S, Ambaw, YA, Malia, PC, Richards, RC, Fischer, AW, et al. Deficiency of the frontotemporal dementia gene GRN results in gangliosidosis. Nat Commun. (2022) 13:5924. doi: 10.1038/s41467-022-33500-9
29. Katzeff, JS, Bright, F, Phan, K, Kril, JJ, Ittner, LM, Kassiou, M, et al. Biomarker discovery and development for frontotemporal dementia and amyotrophic lateral sclerosis. Brain. (2022) 145:1598–609. doi: 10.1093/brain/awac077
30. Wang, J, Wang, B, and Zhou, T. The advance on frontotemporal dementia (FTD)’s neuropathology and molecular genetics. Mediat Inflamm. (2022) 2022:1–6. doi: 10.1155/2022/5003902
31. Tampi, RR. Treatments for frontotemporal dementia (FTD): a network meta-analysis. Am J Geriatr Psychiatry. (2023) 31:1074–6. doi: 10.1016/j.jagp.2023.07.005
32. Xiao, L, Huo, X, Wang, Y, Li, W, Li, M, Wang, C, et al. A bibliometric analysis of global research status and trends in neuromodulation techniques in the treatment of autism spectrum disorder. BMC Psychiatry. (2023) 23:183. doi: 10.1186/s12888-023-04666-3
33. Yang, LJ, Han, LX, and Liu, NX. A new approach to journal co-citation matrix construction based on the number of co-cited articles in journals. Scientometrics. (2019) 120:507–17. doi: 10.1007/s11192-019-03141-9
34. Cheng, K, Guo, Q, Shen, Z, Yang, W, Zhou, Y, Sun, Z, et al. Frontiers of ferroptosis research: an analysis from the top 100 most influential articles in the field. Front Oncol. (2022) 12:948389. doi: 10.3389/fonc.2022.948389
35. Rascovsky, K, Hodges, JR, Knopman, D, Mendez, MF, Kramer, JH, Neuhaus, J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. (2011) 134:2456–77. doi: 10.1093/brain/awr179
36. Young, JJ, Lavakumar, M, Tampi, D, Balachandran, S, and Tampi, RR. Frontotemporal dementia: latest evidence and clinical implications. Ther Adv Psychopharmacol. (2018) 8:33–48. doi: 10.1177/2045125317739818
37. Zhang, J, Song, L, Jia, J, Tian, W, Lai, R, Zhang, Z, et al. Knowledge mapping of necroptosis from 2012 to 2021: a bibliometric analysis. Front Immunol. (2022) 13:917155. doi: 10.3389/fimmu.2022.917155
38. Xiao, F, Li, C, Sun, J, and Zhang, L. Knowledge domain and emerging trends in organic photovoltaic technology: a Scientometric review based on CiteSpace analysis. Front Chem. (2017) 5:67. doi: 10.3389/fchem.2017.00067
39. Ma, L, Ma, J, Teng, M, and Li, Y. Visual analysis of colorectal Cancer immunotherapy: a bibliometric analysis from 2012 to 2021. Front Immunol. (2022) 13:843106. doi: 10.3389/fimmu.2022.843106
40. Gorno-Tempini, ML, Hillis, AE, Weintraub, S, Kertesz, A, Mendez, M, Cappa, SF, et al. Classification of primary progressive aphasia and its variants. Neurology. (2011) 76:1006–14. doi: 10.1212/WNL.0b013e31821103e6
41. Chare, L, Hodges, JR, Leyton, CE, McGinley, C, Tan, RH, Kril, JJ, et al. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry. (2014) 85:865–70. doi: 10.1136/jnnp-2013-306948
42. Woollacott, IO, and Rohrer, JD. The clinical spectrum of sporadic and familial forms of frontotemporal dementia. J Neurochem. (2016) 138:6–31. doi: 10.1111/jnc.13654
43. Moore, KM, Nicholas, J, Grossman, M, McMillan, CT, Irwin, DJ, Massimo, L, et al. Age at symptom onset and death and disease duration in genetic frontotemporal dementia: an international retrospective cohort study. Lancet Neurol. (2020) 19:145–56. doi: 10.1016/S1474-4422(19)30394-1
44. Harris, JM, Gall, C, Thompson, JC, Richardson, AM, Neary, D, du Plessis, D, et al. Sensitivity and specificity of FTDC criteria for behavioral variant frontotemporal dementia. Neurology. (2013) 80:1881–7. doi: 10.1212/WNL.0b013e318292a342
45. Bisenius, S, Neumann, J, and Schroeter, ML. Validating new diagnostic imaging criteria for primary progressive aphasia via anatomical likelihood estimation meta-analyses. Eur J Neurol. (2016) 23:704–12. doi: 10.1111/ene.12902
46. Wicklund, MR, Duffy, JR, Strand, EA, Machulda, MM, Whitwell, JL, and Josephs, KA. Quantitative application of the primary progressive aphasia consensus criteria. Neurology. (2014) 82:1119–26. doi: 10.1212/WNL.0000000000000261
47. Barker, MS, Gottesman, RT, Manoochehri, M, Chapman, S, Appleby, BS, Brushaber, D, et al. Proposed research criteria for prodromal behavioural variant frontotemporal dementia. Brain. (2022) 145:1079–97. doi: 10.1093/brain/awab365
48. Musa, G, Slachevsky, A, Muñoz-Neira, C, Méndez-Orellana, C, Villagra, R, González-Billault, C, et al. Alzheimer’s disease or behavioral variant frontotemporal dementia? Review of key points toward an accurate clinical and neuropsychological diagnosis. J Alzheimers Dis. (2020) 73:833–48. doi: 10.3233/JAD-190924
49. Martinez, B, and Peplow, PV. MicroRNA biomarkers in frontotemporal dementia and to distinguish from Alzheimer’s disease and amyotrophic lateral sclerosis. Neural Regen Res. (2022) 17:1412–22. doi: 10.4103/1673-5374.330591
50. Hutton, M. Missense and splice site mutations in tau associated with FTDP-17: multiple pathogenic mechanisms. Neurology. (2001) 56:S21–5. doi: 10.1212/WNL.56.suppl_4.S21
51. Ingram, EM, and Spillantini, MG. Tau gene mutations: dissecting the pathogenesis of FTDP-17. Trends Mol Med. (2002) 8:555–62. doi: 10.1016/S1471-4914(02)02440-1
52. Baker, M, Mackenzie, IR, Pickering-Brown, SM, Gass, J, Rademakers, R, Lindholm, C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. (2006) 442:916–9. doi: 10.1038/nature05016
53. Neumann, M, Sampathu, DM, Kwong, LK, Truax, AC, Micsenyi, MC, Chou, TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. (2006) 314:130–3. doi: 10.1126/science.1134108
54. Cairns, NJ, Bigio, EH, Mackenzie, IR, Neumann, M, Lee, VM, Hatanpaa, KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol. (2007) 114:5–22. doi: 10.1007/s00401-007-0237-2
55. DeJesus-Hernandez, M, Mackenzie, IR, Boeve, BF, Boxer, AL, Baker, M, Rutherford, NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. (2011) 72:245–56. doi: 10.1016/j.neuron.2011.09.011
56. Renton, AE, Majounie, E, Waite, A, Simón-Sánchez, J, Rollinson, S, Gibbs, JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. (2011) 72:257–68. doi: 10.1016/j.neuron.2011.09.010
57. Mori, K, Weng, SM, Arzberger, T, May, S, Rentzsch, K, Kremmer, E, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. (2013) 339:1335–8. doi: 10.1126/science.1232927
58. Wang, Y, and Mandelkow, E. Tau in physiology and pathology. Nat Rev Neurosci. (2016) 17:5–21. doi: 10.1038/nrn.2015.1
59. Liang, SY, Wang, ZT, Tan, L, and Yu, JT. Tau toxicity in neurodegeneration. Mol Neurobiol. (2022) 59:3617–34. doi: 10.1007/s12035-022-02809-3
60. Tracy, TE, Madero-Pérez, J, Swaney, DL, Chang, TS, Moritz, M, Konrad, C, et al. Tau interactome maps synaptic and mitochondrial processes associated with neurodegeneration. Cell. (2022) 185:712–28.e14. doi: 10.1016/j.cell.2021.12.041
61. Yuste-Checa, P, Trinkaus, VA, Riera-Tur, I, Imamoglu, R, Schaller, TF, Wang, H, et al. The extracellular chaperone clusterin enhances tau aggregate seeding in a cellular model. Nat Commun. (2021) 12:4863. doi: 10.1038/s41467-021-25060-1
62. Bowles, KR, Silva, MC, Whitney, K, Bertucci, T, Berlind, JE, Lai, JD, et al. ELAVL4, splicing, and glutamatergic dysfunction precede neuron loss in MAPT mutation cerebral organoids. Cell. (2021) 184:4547–63.e17. doi: 10.1016/j.cell.2021.07.003
63. Tsen, C, Andreatto, CAA, Aily, JB, Pelicioni, PHS, Neto, DB, Mattiello, SM, et al. Effects of telehealth on functional capacity, mental health and quality of life among older people with dementia: LAPESI telehealth protocol for a randomized controlled trial. Physiother Res Int. (2023) 28:e1981. doi: 10.1002/pri.1981
64. Zucchella, C, Sinforiani, E, Tamburin, S, Federico, A, Mantovani, E, Bernini, S, et al. The multidisciplinary approach to Alzheimer’s disease and dementia: a narrative review of non-pharmacological treatment. Front Neurol. (2018) 9:1058. doi: 10.3389/fneur.2018.01058
65. Tsai, RM, and Boxer, AL. Therapy and clinical trials in frontotemporal dementia: past, present, and future. J Neurochem. (2016) 138:211–21. doi: 10.1111/jnc.13640
66. Panza, F, Lozupone, M, Seripa, D, Daniele, A, Watling, M, Giannelli, G, et al. Development of disease-modifying drugs for frontotemporal dementia spectrum disorders. Nat Rev Neurol. (2020) 16:213–28. doi: 10.1038/s41582-020-0330-x
67. Rhinn, H, Tatton, N, McCaughey, S, Kurnellas, M, and Rosenthal, A. Progranulin as a therapeutic target in neurodegenerative diseases. Trends Pharmacol Sci. (2022) 43:641–52. doi: 10.1016/j.tips.2021.11.015
68. Lim, CM, González Díaz, A, Fuxreiter, M, Pun, FW, Zhavoronkov, A, and Vendruscolo, M. Multiomic prediction of therapeutic targets for human diseases associated with protein phase separation. Proc Natl Acad Sci USA. (2023) 120:e2300215120. doi: 10.1073/pnas.2300215120
69. Wegmann, S, Eftekharzadeh, B, Tepper, K, Zoltowska, KM, Bennett, RE, Dujardin, S, et al. Tau protein liquid-liquid phase separation can initiate tau aggregation. EMBO J. (2018) 37:e98049. doi: 10.15252/embj.201798049
70. Mackenzie, IR, and Neumann, M. Molecular neuropathology of frontotemporal dementia: insights into disease mechanisms from postmortem studies. J Neurochem. (2016) 138:54–70. doi: 10.1111/jnc.13588
71. Benussi, A, Padovani, A, and Borroni, B. Phenotypic heterogeneity of monogenic frontotemporal dementia. Front Aging Neurosci. (2015) 7:171. doi: 10.3389/fnagi.2015.00171
72. Ducharme, S, Dols, A, Laforce, R, Devenney, E, Kumfor, F, van den Stock, J, et al. Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain. (2020) 143:1632–50. doi: 10.1093/brain/awaa018
73. Bertoux, M, Delavest, M, de Souza, LC, Funkiewiez, A, Lépine, JP, Fossati, P, et al. Social cognition and emotional assessment differentiates frontotemporal dementia from depression. J Neurol Neurosurg Psychiatry. (2012) 83:411–6. doi: 10.1136/jnnp-2011-301849
74. van der Ende, EL, Xiao, M, Xu, D, Poos, JM, Panman, JL, Jiskoot, LC, et al. Neuronal pentraxin 2: a synapse-derived CSF biomarker in genetic frontotemporal dementia. J Neurol Neurosurg Psychiatry. (2020) 91:612–21. doi: 10.1136/jnnp-2019-322493
75. Zhu, N, Santos-Santos, M, Illán-Gala, I, Montal, V, Estellés, T, Barroeta, I, et al. Plasma glial fibrillary acidic protein and neurofilament light chain for the diagnostic and prognostic evaluation of frontotemporal dementia. Transl Neurodegen. (2021) 10:50. doi: 10.1186/s40035-021-00275-w
76. Oeckl, P, Anderl-Straub, S, Von Arnim, CAF, Baldeiras, I, Diehl-Schmid, J, Grimmer, T, et al. Serum GFAP differentiates Alzheimer’s disease from frontotemporal dementia and predicts MCI-to-dementia conversion. J Neurol Neurosurg Psychiatry. (2022) 93:659–67. doi: 10.1136/jnnp-2021-328547
77. Jiskoot, LC, Panman, JL, Meeter, LH, Dopper, EGP, Donker Kaat, L, Franzen, S, et al. Longitudinal multimodal MRI as prognostic and diagnostic biomarker in presymptomatic familial frontotemporal dementia. Brain. (2019) 142:193–208. doi: 10.1093/brain/awy288
78. Nguyen, HD, Clément, M, Planche, V, Mansencal, B, and Coupé, P. Deep grading for MRI-based differential diagnosis of Alzheimer’s disease and frontotemporal dementia. Artif Intell Med. (2023) 144:102636. doi: 10.1016/j.artmed.2023.102636
Keywords: bibliometric analysis, frontotemporal dementia, Alzheimer’s disease, Citespace, Vosviewer
Citation: Chen X, Chen Y, Ni B and Huang C (2024) Research trends and hotspots for frontotemporal dementia from 2000 to 2022: a bibliometric analysis. Front. Neurol. 15:1399600. doi: 10.3389/fneur.2024.1399600
Edited by:
Charbel Moussa, Georgetown University, United StatesReviewed by:
Philippe Léon Louis Poindron, NeuroSys, FranceCinzia Coppola, Università della Campania Luigi Vanvitelli, Italy
Copyright © 2024 Chen, Chen, Ni and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Huang, Y2hlbmdodWFuZ19zY3VAMTYzLmNvbQ==
†Present address: Cheng Huang, West China Hospital, Sichuan University, Chengdu, Seichuan, China
 Xinxin Chen1,2,3
Xinxin Chen1,2,3 Yin Chen
Yin Chen Cheng Huang
Cheng Huang