- 1Department of Tuina, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
- 2Department of Radiology, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
- 3Department of Acupuncture and Rehabilitation, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
- 4Department of Andrology, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
- 5First Clinical Medical College, Nanjing University of Chinese Medicine, Nanjing, China
Background: The effectiveness of Tuina therapy has been confirmed in treating pain of patients with cervical spondylosis (CS), however, its therapeutic mechanism is still unclear. This study aimed to observe the changes of regional brain activity following Tuina therapy in patients with painful CS based on resting-state functional magnetic resonance imaging (rs-fMRI) data.
Methods: A total of 27 patients with CS and 27 healthy subjects (HCs) were enrolled in this study. All patients received Tuina therapy every 2 days for 2 weeks. The clinical manifestations of patients were evaluated by the Visual Analog Scale (VAS) and Neck Disability Index (NDI) before and after treatment. In addition, rs-fMRI data were collected and preprocessed in all patients before and after treatment, as well as HCs. HCs underwent a 1-time rs-fMRI scan, whereas CS patients underwent 2-times of rs-fMRI scan. The measure of regional homogeneity (ReHo) was calculated and compared between groups. Finally, relationships between altered brain regions and clinical characteristics were evaluated by Pearson’s correlation analysis.
Results: After Tuina therapy, VAS and NDI scores of patients decreased. Before treatment, CS patients showed higher ReHo values in the left middle temporal gyrus, left thalamus, right anterior and posterior cingulate gyrus, left inferior parietal gyrus and lower ReHo values in the right gyrus rectus when compared with HCs. After treatment, CS patients exhibited higher ReHo values in the left inferior temporal gyrus, right anterior and posterior cingulate gyrus, left inferior parietal gyrus and lower ReHo values in the right rectus gyrus when compared with HCs. CS patients after treatment demonstrated higher ReHo values in the left inferior occipital gyrus when compared with those before treatment. Positive correlations were found between ReHo values of the right rectus gyrus and VAS, NDI scores in CS patients before treatment. Differences of VAS scores between before and after treatment were negatively correlated with ReHo values of the left inferior temporal gyrus in CS patients after treatment.
Conclusion: This study demonstrated the presence of asynchronous activity in certain brain regions in CS patients, which might be associated with pain and cervical spine dysfunction. Tuina therapy might modulate asynchronous activity of abnormal brain regions, which might contribute to the effectiveness of Tuina therapy in alleviating pain and cervical spine dysfunction in CS patients.
1 Introduction
Cervical spondylosis (CS) is a condition characterized by typical symptoms such as pain, numbness in the upper extremities and limited neck movement, and its primary clinical manifestation is pain (1). CS is now considered as a significant global public health burden, which is recognized as the fourth most disabling disease worldwide with an estimated annual global prevalence of over 30% (2). In 2020, a Global Burden of Disease survey reported a global age-standardized prevalence of 3551.1 cases and an incidence of 806.6 cases of neck pain per 100,000 people, respectively (3). It is essential to understand the sources of pain and the mechanism underlying the effectiveness of treatment. Biomechanical influences, such as osteophyte formation, disk degeneration, a decrease in the sagittal diameter of the spinal canal (4), inflammatory biomarkers including CRP, IL-1β, TNF-α, IL-6, etc. (5), as well as dysfunction in pain conduction and modulation at the level of the brain and spinal cord, are commonly associated with the pain symptom in patients with CS.
The central pathophysiological mechanism of pain in the cervical spine is complex. Previous study on the central mechanism of CS showed that the neurological symptoms were more pronounced in patients with CS (6). These symptoms were found to be negatively correlated with the activity in the precuneus, posterior cingulate gyrus and positively associated with the activity in the precentral gyrus, supplementary motor areas (6). Considering the multiple influencing factors, patients with CS might experience abnormal activation in multiple regions that caused the symptoms including pain, reduced neck movement and negative emotion. Resting-state functional magnetic resonance imaging (rs-fMRI) is a noninvasive technique to evaluate spontaneous brain activity of subjects during rest. Regional homogeneity (ReHo) is a commonly used parameter for measuring the level of local synchronization of intrinsic fMRI signals by calculating Kendall’s coefficient concordance (KCC) between the time series of a given voxel and those of its neighboring voxels (7). This method is commonly used to detect brain regions with altered activation in patients with neuropsychiatric disorders, as well as patients complained of pain (8–11). However, fewer studies have explored the central pathogenesis of CS using rs-fMRI. The utilization of neuroimaging methods to investigate the mechanisms of Tuina is currently being employed in multitude spinal disorders. For instance, Chen et al. examined the function of default mode network in the brain in patients with lumbar disk herniation and discovered that Tuina could modify the patient’s pain modulation patterns to achieve pain (12). Furthermore, rs-fMRI has been employed to investigate the analgesic effects of Tuina. Animal studies had demonstrated that the impact of Tuina on neuropathic pain relievers pain behavior by promoting cortical remodeling (13).
Various non-surgical protocols have been used to treat CS, including pharmacological treatments such as non-steroidal anti-inflammatory drugs (NSAIDs), selective 5-hydroxytryptamine reuptake inhibitors (SSRIs), opioids, sedative-hypnotic drugs, as well as non-pharmacological treatments such as physiotherapy, injections, surgical interventions (14, 15). However, the application of NSAIDs is limited by their adverse effects and poor tolerance (16). Considering the positive effects and safety, clinicians often recommend complementary therapies, including Tuina, acupuncture, exercise, yoga, tai ji, Chinese herbs and sports (17). The effectiveness of Tuina in the treatment of CS have been confirmed in numerous previous studies and the symptom of neck pain can be effectively relieved by Tuina therapy by raising the neck pain threshold (18–20). Proper release maneuvers can improve the tension of the cervical musculoskeletal nerves (21–23). Tuina therapy is now commonly used as a conservative treatment for CS. Manipulation practitioners employ a range of techniques to relax the cervical muscles and joints, including kneading, pressing, lifting and pulling. This is done in order to correct imbalanced joints and to relax tense and tired muscles. A randomized controlled trial study demonstrated that a combination of different manipulative techniques significantly improved pain symptoms and mobility function in patients with vertebral artery whiplash (24). A randomized controlled trial study of chronic neck pain by Kang et al. revealed that resistance exercise combined with massage achieved superior outcomes in terms of pain, cervical mobility, and trapezius muscle tone in patients with chronic neck pain (25). However, the therapeutic efficacy of Tuina is inconclusive due to its specificity, such as different therapeutic precision and methods, sample size issues, methodological quality and limitations of existing scientific tools in clinical studies of Tuina therapy.
In this study, we aimed to explore the differences in resting-state brain activity between CS patients and matched healthy controls (HCs), as well as the central mechanism underlying therapeutic effects of Tuina therapy in the treatment of CS. We hypothesized that (1) compared to HCs, CS patients might exhibit altered activity in specific brain regions that were linked to clinical symptoms of patients, and (2) after Tuina therapy, CS patients’ clinical symptoms might be improved, which might be achieved by the changes of spontaneous neural activity in these specific brain regions.
2 Participants and methods
2.1 Participants
The Ethics Committee of Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine approved this study (Ethics Approval No. 2022NL-169-02). In addition, it was registered in the China Clinical Trial Registration Center (registration number: ChiCTR2200066373), available.1 Moreover, all subjects gave their written informed consent to participate in the study. All patients were recruited from the Department of Tuina, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine from September 2022 to December 2022. CS was diagnosed based on the 11th Revision of the International Classification of Diseases (ICD-11) (26) for CS and neck pain.
The inclusion criteria were as follows: (1) meeting the diagnostic criteria for CS; (2) aged 20–55; (3) right-handedness; (4) patients could either be treatment naive or had received prior therapy with a minimum washout period of 28 days; (5) those who were willing to be treated by Tuina therapy; (6) had good compliance and signed written informed consent.
The exclusion criteria were as follows: (1) neurogenic CS with surgical indications, excluding patients with spinal cord cervical spondylosis; (2) serious organic diseases and mental diseases; (3) patients with vertebral artery-type cervical spine vertigo episodes who were unable to take care of themselves; (4) patients with broken skin or other skin diseases at the site of Tuina treatment; (5) women who were breastfeeding, pregnant, or preparing to become pregnant; (6) patients with other chronic and persistent pain who needed to take pain medication; (7) patients participating in other clinical trials; (8) patients who had a fear of previous Tuina therapy or MRI examination or who suffered from claustrophobia.
2.2 Intervention of Tuina
The physician used a thumb of one hand and the four remaining fingers of the other hand, to apply pressure at Tuina acupoints on both sides of the neck muscles, including Jingbailao (EX-HN14), Fengchi (GB20) and Wangu (GB12). Additionally, longitudinal vertical flicking techniques were used on the neck muscles in the direction of their fibers for 5–6 repetitions. The physician applied Tuina manipulation on the neck and shoulder muscles, and repeated Tuina treatment 5–6 times, and used kneading techniques on various muscle groups in the scapular region of the patient’s back. Furthermore, acupressure points such as Bingfeng (SI12), Quqiguan (SI13), Shoulder Well (GB21) and Tianzong (SI11) were pressed and held, and the patient’s comfort level was considered to determine the optimal length of the treatment. The treatment lasted about 15–20 min and was done 2–3 times per week, with a one-day break in between, for a total of 6 treatments over 2 weeks.
2.3 Assessment measures
The Visual Analog Scale (VAS) was used to evaluate the level of pain in the cervical spine area, with a score of 0 representing the lowest level of pain and a score of 10 representing the highest level of pain. The Neck Disability Index (NDI) was used to evaluate the degree of cervical spine dysfunction based on 10 aspects, including pain intensity, reading, heavy lifting, headache, work, sleep, driving, recreational activities, concentration and self-care aspects of life.
2.4 MRI data acquisition and preprocessing
MRI data were acquired using a 3.0 T GE MRI scanner. All participants were instructed to stay relaxed and awake, keep eyes closed, not think of anything particular during the entire MRI scanning procedure. T1-weighted structural data were acquired using the following parameters: repetition time (TR) = 7.7 ms; echo time (TE) = 3.1 ms; slice thickness = 1 mm; field of view (FOV) = 256 × 256 mm2; matrix = 256 × 256; number of slices = 160. All rs-MRI data were acquired using the following parameters: TR = 2000 ms; TE = 30 ms; slice thickness = 3.5 mm; FOV = 224 × 224 mm2; matrix = 80 × 80; number of slices = 33; number of volumes = 240. Based on MATLAB, MRI data preprocessing was performed using the software of Data Processing Assistant for Resting-State fMRI (DPARSF) (27) according to the steps presented in our previous study. Subjects with head-translation more than 2.0 mm or rotation more than 2.0° were excluded in this study. In order to prevent other factors from interfering with the collection of rs-fMRI data, all participants were instructed on their body and emotional state, and were asked to maintain a still head and body, close their eyes, and refrain from thinking about anything. They were also instructed to remain relaxed.
2.5 ReHo calculation
The steps of ReHo calculation were as follows: (1) linear detrending; (2) temporal band-pass filtering; (3) regress out nuisance covariates. Finally, Kendall’s coefficients of concordance (KCC) were calculated by the correlations between the time series of a voxel and those of its 26 nearest neighbor voxels in a voxel-wise manner, which were defined as ReHo values. For standardization purpose, ReHo values were transformed to zReHo values by Fisher’s r-to-z transformation. Finally, all zReHo values were smoothed. ReHo reflects the synchronization of spontaneous brain activity within a region by measuring the consistency of a given voxel and its adjacent voxels. ReHo can evaluate the activity levels of brain regions and reflect the consistency of local neuronal activity levels. An increase in ReHo represents an increase in the consistency of local brain neuronal activity, while a decrease in ReHo indicates a decrease.
2.6 Statistical analysis
In this study, between-group differences in demographic and clinical data were compared using independent two-samples t-tests (normally distributed data), non-parametric tests (non-normally distributed data) and chi-squared tests (count data) by SPSS 25.0 software. p < 0.05 was considered statistically significant. In addition, between-group differences in ReHo values were compared using t-tests by the software of Resting-State fMRI Data Analysis Toolkit (REST) (28). The methods of GRF (voxel-level p < 0.001 and cluster-level p < 0.05) and AlphaSim (p < 0.001 and minimum cluster size was set at six voxels) methods in REST software were used for the correction of multiple comparisons. Finally, relationships between altered brain regions and clinical characteristics were evaluated by Pearson’s correlation analysis. p < 0.05 was considered statistically significant.
3 Results
3.1 Comparison of demographic and clinical data
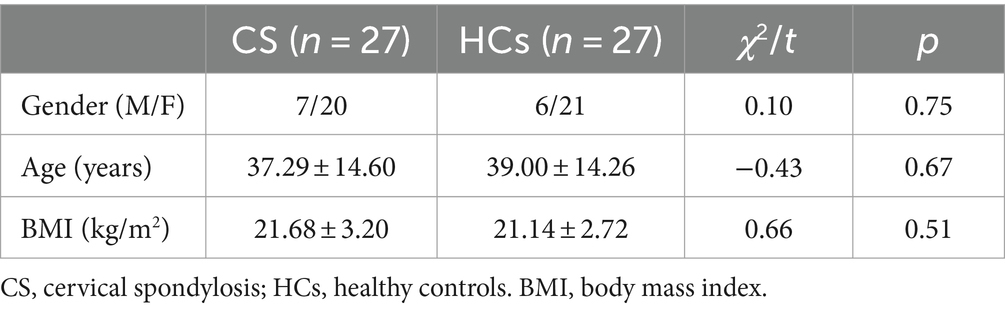
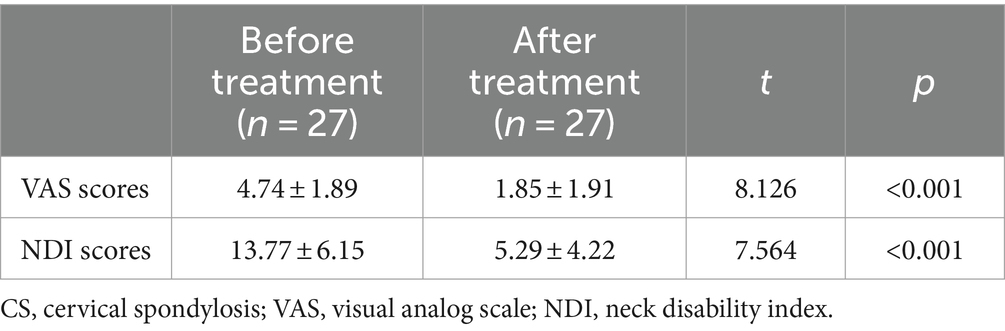
No significant differences were observed in the gender, age and BMI between CS patients and HCs (Table 1). After treatment, CS patients exhibited decreased VAS and NDI scores when compared with those before treatment (Table 2).
3.2 Comparison of ReHo values between groups
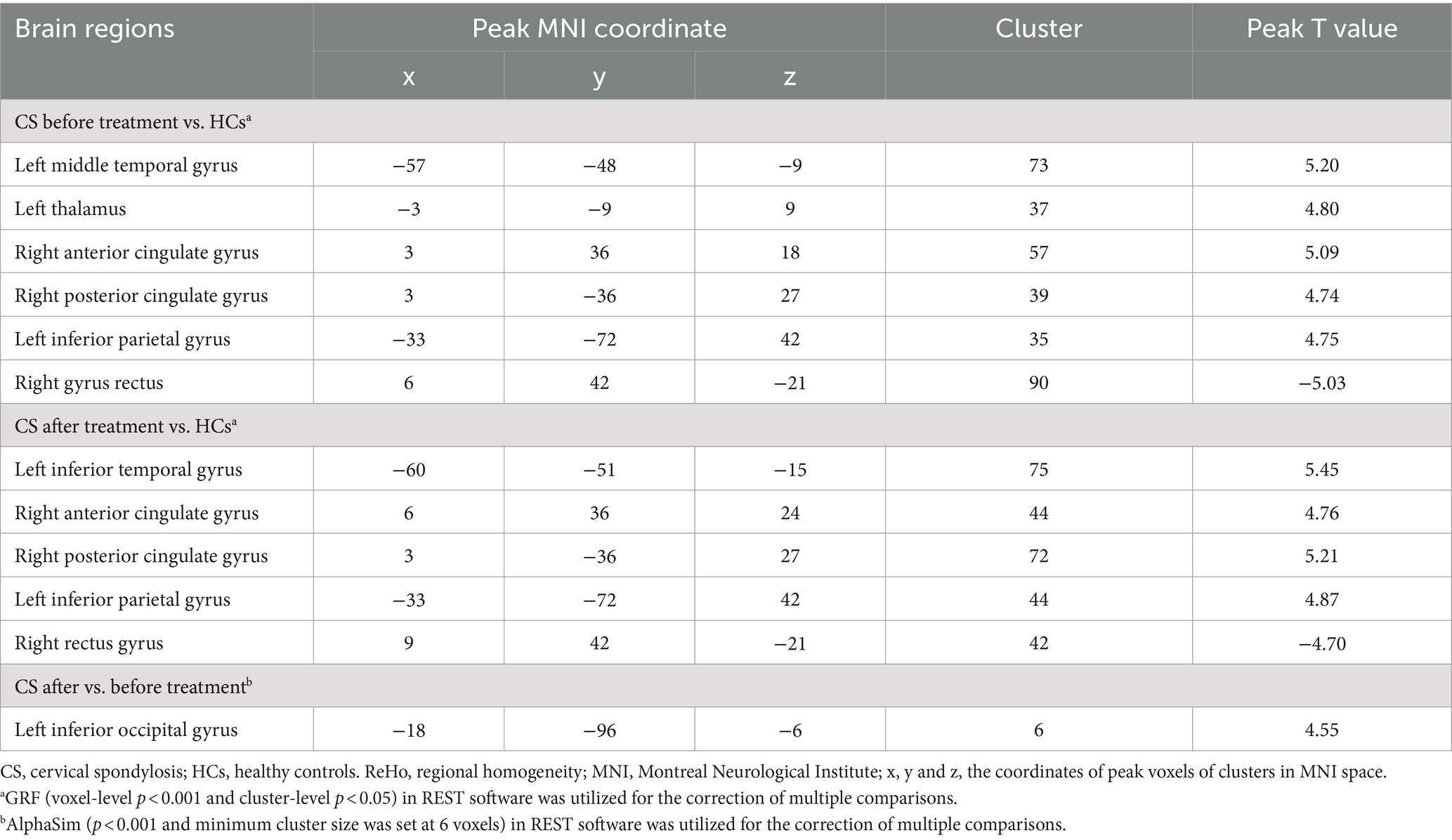
Before treatment, CS patients showed higher ReHo values in the left middle temporal gyrus, left thalamus, right anterior and posterior cingulate gyrus, left inferior parietal gyrus and lower ReHo values in the right gyrus rectus when compared with HCs (Table 3; Figure 1).
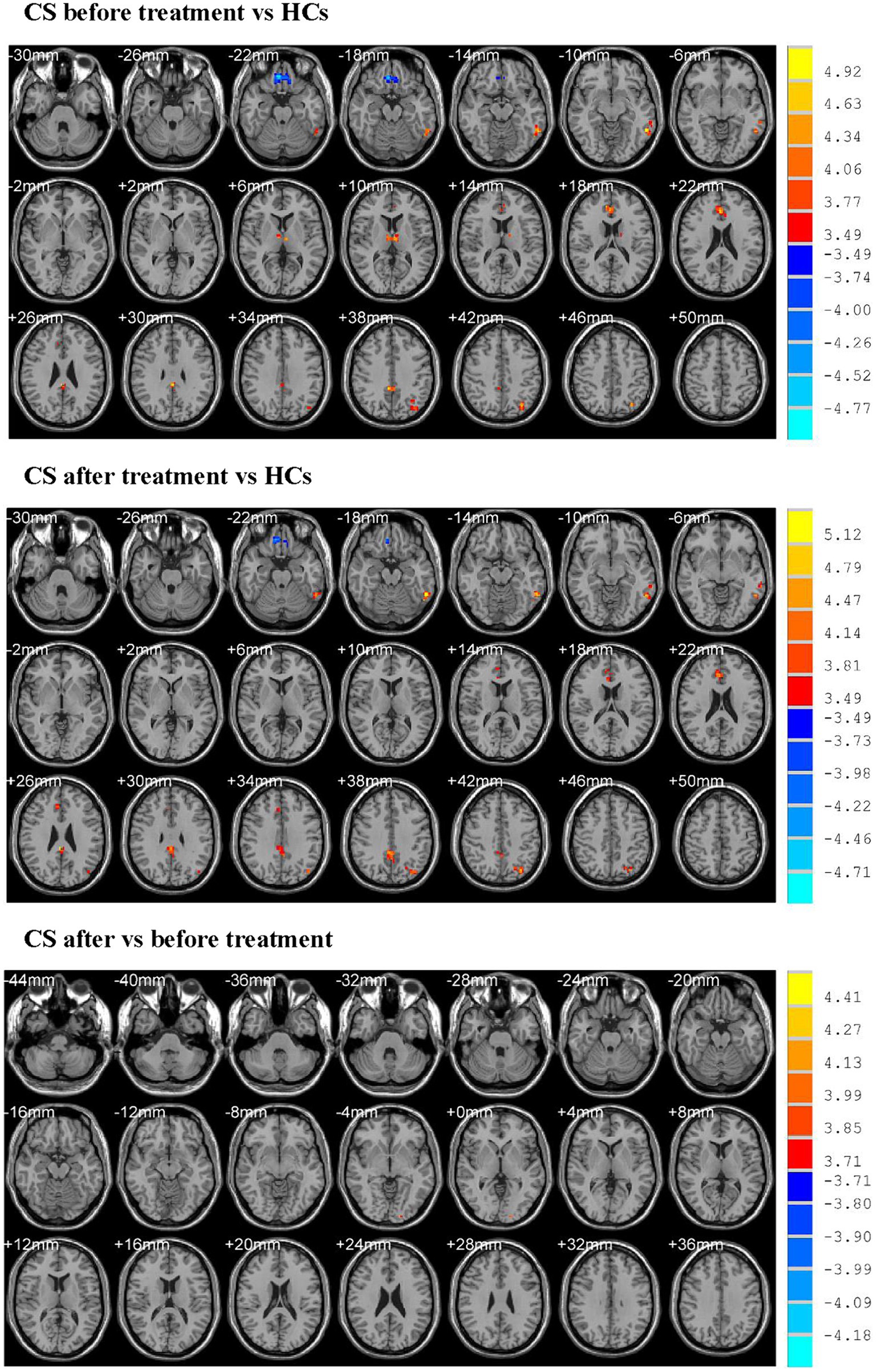
Figure 1. Showed altered ReHo values. CS, cervical spondylosis; HCs, healthy controls. ReHo, regional homogeneity.
After treatment, CS patients exhibited higher ReHo values in the left inferior temporal gyrus, right anterior and posterior cingulate gyrus, left inferior parietal gyrus and lower ReHo values in the right rectus gyrus when compared with HCs (Table 3; Figure 1).
CS patients after treatment demonstrated higher ReHo values in the left inferior occipital gyrus when compared with those before treatment (Table 3; Figure 1).
3.3 Relationships between altered brain regions and clinical characteristics
Positive correlations were found between ReHo values of the right rectus gyrus and VAS (r = 0.517, p = 0.006), NDI (r = 0.395, p = 0.041) scores in CS patients before treatment (Figure 2). Differences of VAS scores between before and after treatment were negatively correlated with ReHo values of the left inferior temporal gyrus in CS patients after treatment (r = −0.45, p = 0.032; Figure 3).
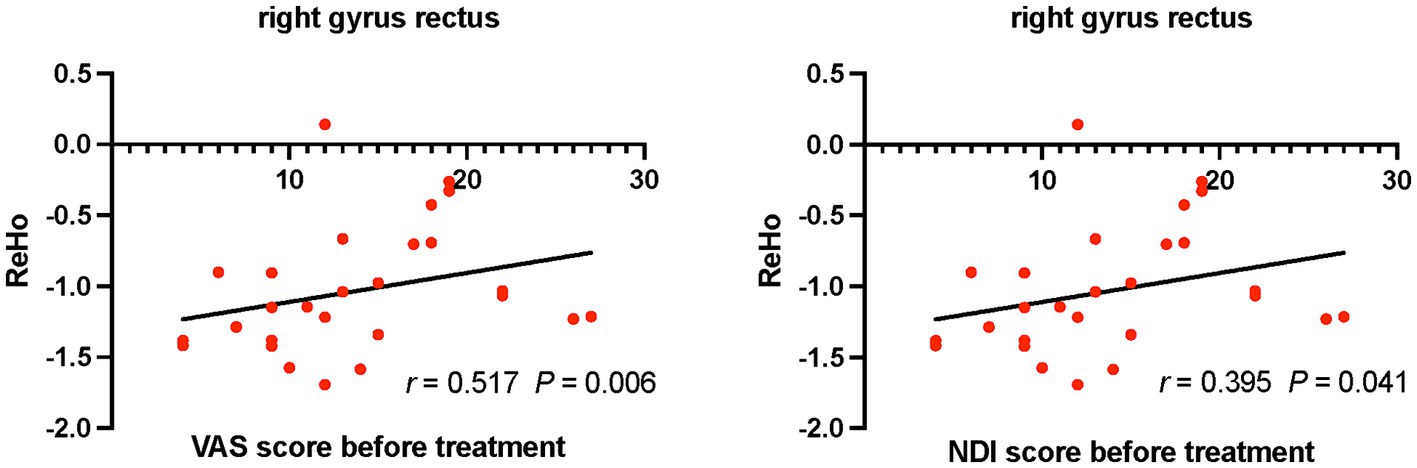
Figure 2. Relationships between VAS, NDI scores and ReHo values in CS patients before treatment. CS, cervical spondylosis. ReHo, regional homogeneity. VAS, visual analog scale; NDI, neck disability index.
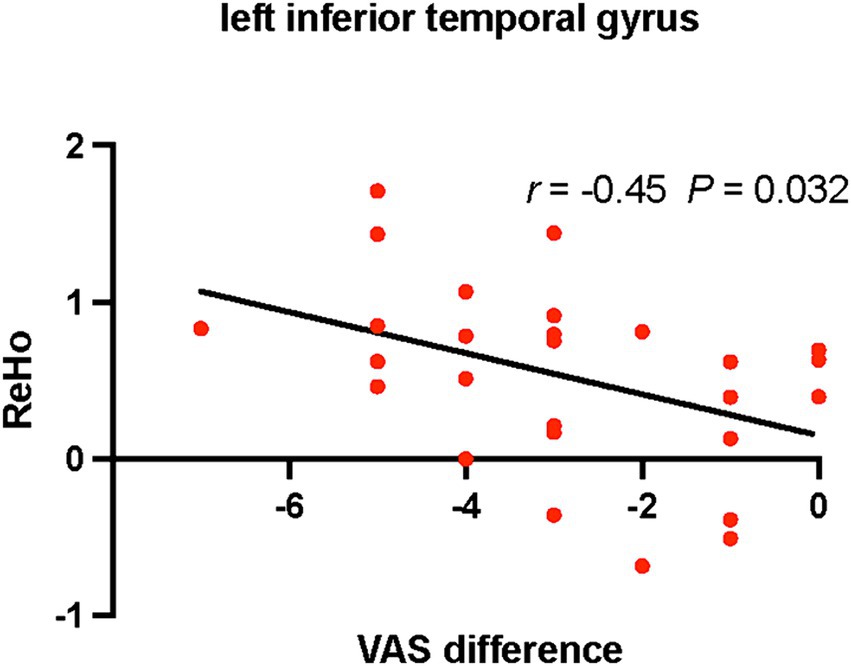
Figure 3. Relationships between differences of VAS scores and ReHo values in CS patients after treatment. CS, cervical spondylosis. ReHo, regional homogeneity. VAS, visual analog scale.
4 Discussion
To the best of our knowledge, this was the first rs-fMRI study exploring the central pathological mechanism of CS and the changes of brain activation associated with Tuina therapy in the treatment of CS. Compared with HCs, CS patients before treatment showed increased consistency of spontaneous neural activity in the left middle temporal gyrus, left thalamus, right anterior and posterior cingulate gyrus, left inferior parietal gyrus, as well as decreased consistency of neural activity in the right gyrus rectus. After treatment, CS patients exhibited higher activity in the left inferior temporal gyrus, right anterior and posterior cingulate gyrus, left inferior parietal gyrus and lower activity in the right rectus gyrus when compared with HCs. Moreover, CS patients after treatment demonstrated higher activity in the left inferior occipital gyrus when compared with those before treatment. Positive correlations were found between ReHo values of the right rectus gyrus and VAS, NDI scores in CS patients before treatment. Differences of VAS scores between before and after treatment were negatively correlated with ReHo values of the left inferior temporal gyrus in CS patients after treatment.
Previous MRI study showed that neck pain could cause reduced gray matter volume in the right middle cingulate cortex, right superior temporal gyrus, right precuneus in patients with chronic cervical spondylotic pain and these patients displayed decreased functional connectivity between the right precuneus and bilateral medial prefrontal cortex (29). Additionally, gray matter volume of the right middle cingulate cortex, right superior temporal gyrus and right precuneus, as well as resting-state functional connectivity between the right precuneus and bilateral medial prefrontal cortex, were negatively correlated with the VAS respectively (29). Another rs-fMRI study demonstrated that CS patients had altered amplitude of low-frequency fluctuation (ALFF) in the middle cingulate cortex, cerebellum and middle frontal gyrus (30). Previous studies demonstrated that pain could cause extensive functional alterations in several brain networks, particularly in the default mode network (DMN) (31, 32). The DMN is composed of several brain regions, including the medial prefrontal cortex, inferior parietal lobule, posterior cingulate gyrus/precuneus, hippocampus, angular gyrus and temporal lobe (33, 34). These regions exhibit strong neural activity during resting-state and decreased synchronized intrinsic neuronal activation during goal-oriented tasks (35, 36). Recurrent patients with chronic pain showed abnormal functional connectivity in DMN, which suggested that patients’ recurrent pain states were associated with enhanced spontaneous neural activity in the thalamus, temporal cortex, cingulate cortex (32).
Abnormal brain areas attributed to DMN, such as the thalamus, posterior cingulate gyrus, middle temporal gyrus and inferior temporal gyrus, were also identified in this study. The middle temporal gyrus is considered as a key region of DMN (37), and it is also a region involved in language and word processing, emotion and memory processing (38, 39). The middle temporal gyrus can integrate cognitional and emotional information under nociceptive stimulation and can express the degree of pain perception under the control of cortical–limbic system (40). The inferior temporal gyrus is an important region for cognition and learning (41). The left inferior parietal gyrus is a component of the frontoparietal network, and it is more specialized in the semantic encoding of retrieval of scene memories and executive processing of perceptual motions compared to the right inferior parietal gyrus (42). The anterior cingulate gyrus, which is a brain region associated with sensory and pain modulation, plays a role in receiving and storing painful emotional information. Pain triggers the activation of anterior cingulate gyrus to improve the transmission of sensory-motor neurons (42–46). Suppressing the activity in the anterior cingulate gyrus could lead to a reduction in pain and adverse emotion (45). The anterior and posterior cingulate gyrus are critical constituents of the limbic system (47). The posterior cingulate gyrus is a brain region that possesses several features, including sensory monitoring, spatial localization, internal orientation and high metabolic rate (48). Additionally, it receives nociceptive information from the thalamus, which contributes to its sensitivity. In addition, the rectus gyrus, located on the orbital surface of the frontal lobe, is a part of the limbic system and medial prefrontal brain network. This region is the main origin of downstream connections from the medial prefrontal lobe to the hypothalamus, brainstem, and it is a part of the limbic system and DMN, which plays a key role in transmitting information in the brain (47, 49).
The thalamus, anterior cingulate gyrus, posterior cingulate gyrus, insula, amygdala and periaqueductal gray matter are considered as “pain matrix,” indicating that injurious nociceptive stimuli can exert feedback modulation on these regions (50–52). Following 2 weeks of Tuina therapy, CS patients exhibited higher ReHo values in the left inferior temporal gyrus, right anterior and posterior cingulate gyrus, left inferior parietal gyrus and lower ReHo values in the right rectus gyrus when compared with HCs. Increased ReHo values of the anterior cingulate gyrus after treatment suggested increased compensatory inhibition of the nociceptive neural circuit associated with the thalamic-cingulate cortex, which might lead to a reduction in pain sensation. Furthermore, compensatory spontaneous neural activity was observed in the posterior cingulate gyrus due to the reduction in pain sensation. However, the thalamus disappeared in the differences of brain regions between CS patients and HCs. The thalamus is the relay station for information rectification and transmission of injurious nociceptive stimulus in the brain, which can feedback activate the thalamus (53, 54). These findings suggested that inhibition of the nociceptive neural circuit involving the thalamus-cingulate cortex, resulting in a reduction in pain sensation. Therefore, we speculated that the thalamus was an important relay station for pain information transmission, and the cingulate cortex, temporal lobe cortex were the key regions for encoding information about injurious nociceptive transmitted upstream from the thalamus in CS patients with neck pain.
The study confirmed the ameliorative effect of Tuina therapy on pain and cervical spine dysfunction in patients with CS. The changes in the ReHo values of the right rectus gyrus and left inferior temporal gyrus, in conjunction with the improvement in symptom scores observed prior to and following Tuina therapy, provided evidences that the analgesic effect and relief of cervical spine dysfunction could be fed back into the central nervous system through brain area activity. However, the only brain area that displayed differences in ReHo values before and after treatment was the left inferior occipital gyrus. The small number of differences observed in brain areas might be due to the selection of right-handed subjects, along with a small sample size and the shorter treatment period, which were the two limitations of this study. The results of this study were partly inconsistent with previous studies, but there were some similarities and overlaps. Possible explanations for this could be that CS itself was a clinical symptom manifestation and it had several subtypes. Additionally, previous studies had smaller and mostly observational samples, which might have contributed to the differences in results. Therefore, future prospective longitudinal follow-up and multimodal neuroimaging studies with larger sample sizes were needed to further validate these findings, as well as other objective indicators including inflammatory biomarkers and additional imaging techniques like PET or EEG in patients with different subtypes of CS. Moreover, a negative correlation was found between changes in VAS scores and ReHo values in the left inferior temporal gyrus. However, the single finding of a negative correlation between VAS score changes and ReHo values was not sufficient to establish a definitive relationship. The joint evaluation of these multiple indicators (brain structure, brain electrophysiological and metabolic activity, inflammatory biomarkers) might better establish a definitive relationship.
5 Conclusion
In conclusion, CS patients showed impaired activity in the pain-related brain regions, resulting in neck pain and limited neck movement. Tuina therapy could improve the clinical conditions of pain and cervical mobility, which might be achieved by modulating asynchronous activity of abnormal brain regions. All these findings provided new insights into understanding the central mechanism underlying the therapeutic effects of Tuina in the treatment of CS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. YF: Formal analysis, Investigation, Methodology, Software, Writing – original draft, Data curation. XW: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. LS: Data curation, Investigation, Methodology, Writing – original draft. YH: Data curation, Investigation, Writing – original draft, Methodology. CL: Data curation, Investigation, Formal analysis, Software, Writing – original draft. TY: Data curation, Investigation, Conceptualization, Writing – original draft, Methodology. LC: Data curation, Investigation, Writing – original draft, Methodology. JC: Investigation, Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. MX: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work was supported by the grants of the third batch of academic experience inheritance work project for elderly traditional Chinese medicine experts in Jiangsu Province (Su Traditional Chinese Medicine Science and Education [2019] No. 8); Jiangsu Province Traditional Chinese Medicine Technology Development Plan Project (No. MS2023028); Excellent Young Doctor Training Program of Jiangsu Province Hospital of Chinese Medicine (No. 2023QB0126).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Theodore, N . Degenerative Cervical Spondylosis. N Engl J Med. (2020) 383:159–68. doi: 10.1056/NEJMra2003558
2. Kazeminasab, S, Nejadghaderi, SA, Amiri, P, Pourfathi, H, Araj-Khodaei, M, Sullman, MJM, et al. Neck pain: global epidemiology, trends and risk factors. BMC Musculoskelet Disord. (2022) 23:26. doi: 10.1186/s12891-021-04957-4
3. Safiri, S, Kolahi, AA, Hoy, D, Buchbinder, R, Mansournia, MA, Bettampadi, D, et al. Global, regional, and national burden of neck pain in the general population, 1990-2017: systematic analysis of the global burden of disease study 2017. BMJ. (2020) 368:m791. doi: 10.1136/bmj.m791
4. Shedid, D, and Benzel, EC. Cervical spondylosis anatomy: pathophysiology and biomechanics. Neurosurgery. (2007) 60:S1–7. doi: 10.1227/01.Neu.0000215430.86569.C4
5. Carp, SJ, Barbe, MF, Winter, KA, Amin, M, and Barr, AE. Inflammatory biomarkers increase with severity of upper-extremity overuse disorders. Clin Sci (Lond). (2007) 112:305–14. doi: 10.1042/cs20060050
6. Woodworth, DC, Holly, LT, Salamon, N, and Ellingson, BM. Resting-state functional magnetic resonance imaging connectivity of the brain is associated with altered sensorimotor function in patients with cervical spondylosis. World Neurosurg. (2018) 119:e740–9. doi: 10.1016/j.wneu.2018.07.257
7. Zang, Y, Jiang, T, Lu, Y, He, Y, and Tian, L. Regional homogeneity approach to fMRI data analysis. NeuroImage. (2004) 22:394–400. doi: 10.1016/j.neuroimage.2003.12.030
8. Qiu, X, Xu, W, Zhang, R, Yan, W, Ma, W, Xie, S, et al. Regional homogeneity brain alterations in schizophrenia: an activation likelihood estimation Meta-analysis. Psychiatry Investig. (2021) 18:709–17. doi: 10.30773/pi.2021.0062
9. Wang, Q, Wang, C, Deng, Q, Zhan, L, Tang, Y, Li, H, et al. Alterations of regional spontaneous brain activities in anxiety disorders: a meta-analysis. J Affect Disord. (2022) 296:233–40. doi: 10.1016/j.jad.2021.09.062
10. Pan, P, Zhan, H, Xia, M, Zhang, Y, Guan, D, and Xu, Y. Aberrant regional homogeneity in Parkinson's disease: a voxel-wise meta-analysis of resting-state functional magnetic resonance imaging studies. Neurosci Biobehav Rev. (2017) 72:223–31. doi: 10.1016/j.neubiorev.2016.11.018
11. Zhang, SS, Wu, W, Liu, ZP, Huang, GZ, Guo, SG, and Yang, JM. Altered regional homogeneity in experimentally induced low back pain: a resting-state fMRI study. J Neuroeng Rehabil. (2014) 11:115. doi: 10.1186/1743-0003-11-115
12. Chen, XM, Wen, Y, Chen, S, Jin, X, Liu, C, Wang, W, et al. Traditional Chinese manual therapy (Tuina) reshape the function of default mode network in patients with lumbar disc herniation. Front Neurosci. (2023) 17:1125677. doi: 10.3389/fnins.2023.1125677
13. Wu, Z, Guo, G, Zhang, Y, Li, Y, He, T, Zhu, Q, et al. Resting-state functional magnetic resonance imaging reveals brain remodeling after Tuina therapy in neuropathic pain model. Front Mol Neurosci. (2023) 16:1231374. doi: 10.3389/fnmol.2023.1231374
14. Sanders, SH, Harden, RN, and Vicente, PJ. Evidence-based clinical practice guidelines for interdisciplinary rehabilitation of chronic nonmalignant pain syndrome patients. Pain Pract. (2005) 5:303–15. doi: 10.1111/j.1533-2500.2005.00033.x
15. Seo, BK, Lee, JH, Kim, PK, Baek, YH, Jo, DJ, and Lee, S. Bee venom acupuncture, NSAIDs or combined treatment for chronic neck pain: study protocol for a randomized, assessor-blind trial. Trials. (2014) 15:132. doi: 10.1186/1745-6215-15-132
16. Bindu, S, Mazumder, S, and Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. (2020) 180:114147. doi: 10.1016/j.bcp.2020.114147
17. Plastaras, CT, Schran, S, Kim, N, Sorosky, S, Darr, D, Chen, MS, et al. Complementary and alternative treatment for neck pain: chiropractic, acupuncture, TENS, massage, yoga, tai chi, and Feldenkrais. Phys Med Rehabil Clin N Am. (2011) 22:521–37. doi: 10.1016/j.pmr.2011.02.011
18. Lin, JH, Shen, T, Chung, RC, and Chiu, TT. The effectiveness of Long's manipulation on patients with chronic mechanical neck pain: a randomized controlled trial. Man Ther. (2013) 18:308–15. doi: 10.1016/j.math.2012.11.005
19. Dunning, JR, Cleland, JA, Waldrop, MA, Arnot, C, Young, I, Turner, M, et al. Upper cervical and upper thoracic thrust manipulation versus nonthrust mobilization in patients with mechanical neck pain: a multicenter randomized clinical trial. J Orthop Sports Phys Ther. (2012) 42:5–18. doi: 10.2519/jospt.2012.3894
20. Grayson, JE, Barton, T, Cabot, PJ, and Souvlis, T. Spinal manual therapy produces rapid onset analgesia in a rodent model. Man Ther. (2012) 17:292–7. doi: 10.1016/j.math.2012.02.004
21. Pickar, JG . Neurophysiological effects of spinal manipulation. Spine J. (2002) 2:357–71. doi: 10.1016/s1529-9430(02)00400-x
22. Haik, MN, Alburquerque-Sendín, F, and Camargo, PR. Short-term effects of thoracic spine manipulation on shoulder impingement syndrome: a randomized controlled trial. Arch Phys Med Rehabil. (2017) 98:1594–605. doi: 10.1016/j.apmr.2017.02.003
23. Sillevis, R, Cleland, J, Hellman, M, and Beekhuizen, K. Immediate effects of a thoracic spine thrust manipulation on the autonomic nervous system: a randomized clinical trial. J Man Manip Ther. (2010) 18:181–90. doi: 10.1179/106698110x12804993427126
24. Ding, Q, Yan, M, Zhou, J, Yang, L, Guo, J, Wang, J, et al. Clinical effects of innovative tuina manipulations on treating cervical spondylosis of vertebral artery type and changes in cerebral blood flow. J Tradit Chin Med. (2012) 32:388–92. doi: 10.1016/S0254-6272(13)60043-6
25. Kang, T, and Kim, B. Cervical and scapula-focused resistance exercise program versus trapezius massage in patients with chronic neck pain: a randomized controlled trial. Medicine (Baltimore). (2022) 101:e30887. doi: 10.1097/md.0000000000030887
27. Chao-Gan, Y, and Yu-Feng, Z. DPARSF: a MATLAB toolbox for "pipeline" data analysis of resting-state fMRI. Front Syst Neurosci. (2010) 4:13. doi: 10.3389/fnsys.2010.00013
28. Song, XW, Dong, ZY, Long, XY, Li, SF, Zuo, XN, Zhu, CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. (2011) 6:e25031. doi: 10.1371/journal.pone.0025031
29. Yang, Q, Xu, H, Zhang, M, Wang, Y, and Li, D. Volumetric and functional connectivity alterations in patients with chronic cervical spondylotic pain. Neuroradiology. (2020) 62:995–1001. doi: 10.1007/s00234-020-02413-z
30. Bai, L, Zhang, L, Chen, Y, Li, Y, Ma, D, Li, W, et al. Middle cingulate cortex function contributes to response to non-steroidal anti-inflammatory drug in cervical spondylosis patients: a preliminary resting-state fMRI study. Neuroradiology. (2022) 64:1401–10. doi: 10.1007/s00234-022-02964-3
31. Jones, SA, Morales, AM, Holley, AL, Wilson, AC, and Nagel, BJ. Default mode network connectivity is related to pain frequency and intensity in adolescents. NeuroImage Clinical. (2020) 27:102326. doi: 10.1016/j.nicl.2020.102326
32. Alshelh, Z, Marciszewski, KK, Akhter, R, di Pietro, F, Mills, EP, Vickers, ER, et al. Disruption of default mode network dynamics in acute and chronic pain states. NeuroImage Clin. (2018) 17:222–31. doi: 10.1016/j.nicl.2017.10.019
33. Menon, V . 20 years of the default mode network: a review and synthesis. Neuron. (2023) 111:2469–87. doi: 10.1016/j.neuron.2023.04.023
34. Raichle, ME . The brain's default mode network. Annu Rev Neurosci. (2015) 38:433–47. doi: 10.1146/annurev-neuro-071013-014030
35. Borserio, BJ, Sharpley, CF, Bitsika, V, Sarmukadam, K, Fourie, PJ, and Agnew, LL. Default mode network activity in depression subtypes. Rev Neurosci. (2021) 32:597–613. doi: 10.1515/revneuro-2020-0132
36. Zhou, HX, Chen, X, Shen, YQ, Li, L, Chen, NX, Zhu, ZC, et al. Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. NeuroImage. (2020) 206:116287. doi: 10.1016/j.neuroimage.2019.116287
37. Lu, F, Yang, W, Wei, D, Sun, J, Zhang, Q, and Qiu, J. Superior frontal gyrus and middle temporal gyrus connectivity mediates the relationship between neuroticism and thought suppression. Brain Imaging Behav. (2022) 16:1400–9. doi: 10.1007/s11682-021-00599-1
38. Fox, KC, Spreng, RN, Ellamil, M, Andrews-Hanna, JR, and Christoff, K. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. NeuroImage. (2015) 111:611–21. doi: 10.1016/j.neuroimage.2015.02.039
39. Tulving, E . Episodic memory: from mind to brain. Rev Neurol (Paris). (2004) 160:S9–S23. doi: 10.1016/s0035-3787(04)70940-6
40. Zhao, R, Su, Q, Song, Y, Yang, Q, Wang, S, Zhang, J, et al. Brain-activation-based individual identification reveals individually unique activation patterns elicited by pain and touch. NeuroImage. (2022) 260:119436. doi: 10.1016/j.neuroimage.2022.119436
41. Rolls, ET, Cheng, W, and Du, J. Functional connectivity of the right inferior frontal gyrus and orbitofrontal cortex in depression. Soc Cogn Affect Neurosci. (2020) 15:75–86. doi: 10.1093/scan/nsaa014
42. Apkarian, AV, Bushnell, MC, Treede, RD, and Zubieta, JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. (2005) 9:463–84. doi: 10.1016/j.ejpain.2004.11.001
43. Chen, T, Taniguchi, W, Chen, QY, Tozaki-Saitoh, H, Song, Q, Liu, RH, et al. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat Commun. (2018) 9:1886. doi: 10.1038/s41467-018-04309-2
44. Zhuo, M . A synaptic model for pain: long-term potentiation in the anterior cingulate cortex. Mol Cell. (2007) 23:259–71. doi: 10.1016/S1016-8478(23)10716-3
45. Zhuo, M . Cortical excitation and chronic pain. Trends Neurosci. (2008) 31:199–207. doi: 10.1016/j.tins.2008.01.003
46. Vogt, BA . Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. (2005) 6:533–44. doi: 10.1038/nrn1704
47. Vogt, BA . Cingulate cortex in the three limbic subsystems. Handb Clin Neurol. (2019) 166:39–51. doi: 10.1016/b978-0-444-64196-0.00003-0
48. Leech, R, and Sharp, DJ. The role of the posterior cingulate cortex in cognition and disease. Brain J Neurol. (2014) 137:12–32. doi: 10.1093/brain/awt162
49. Ongür, D, and Price, JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. (2000) 10:206–19. doi: 10.1093/cercor/10.3.206
50. Matsuo, Y, Kurata, J, Sekiguchi, M, Yoshida, K, Nikaido, T, and Konno, SI. Correction to: attenuation of cortical activity triggering descending pain inhibition in chronic low back pain patients: a functional magnetic resonance imaging study. J Anesth. (2018) 32:311–2. doi: 10.1007/s00540-018-2455-2
51. Peirs, C, and Seal, RP. Neural circuits for pain: recent advances and current views. Science. (2016) 354:578–84. doi: 10.1126/science.aaf8933
52. Coghill, RC, Sang, CN, Maisog, JM, and Iadarola, MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. (1999) 82:1934–43. doi: 10.1152/jn.1999.82.4.1934
53. Casey, KL, Minoshima, S, Morrow, TJ, and Koeppe, RA. Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. J Neurophysiol. (1996) 76:571–81. doi: 10.1152/jn.1996.76.1.571
Keywords: cervical spondylosis, pain, Tuina therapy, resting-state functional magnetic resonance imaging, regional homogeneity
Citation: Song S, Fang Y, Wan X, Shen L, Hu Y, Lu C, Yue T, Chen L, Chen J and Xue M (2024) Changes of regional brain activity following Tuina therapy for patients with painful cervical spondylosis: a resting-state fMRI study. Front. Neurol. 15:1399487. doi: 10.3389/fneur.2024.1399487
Edited by:
Xiaofei Hu, Army Medical University, ChinaReviewed by:
Qingguang Zhu, Shanghai University of Traditional Chinese Medicine, ChinaJing Fan, Cleveland Clinic, United States
Copyright © 2024 Song, Fang, Wan, Shen, Hu, Lu, Yue, Chen, Chen and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhuai Chen, amlhbmh1YWljaGVuQDEyNi5jb21t; Mingxin Xue, anNodGNteG14QHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
 Shilong Song1†
Shilong Song1† Jianhuai Chen
Jianhuai Chen