- 1First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2Office of Academic Affairs, Shandong University of Traditional Chinese Medicine, Jinan, China
Objectives: The incidence of vascular dementia (VaD) is steadily rising annually, significantly impacting the mental well-being and overall quality of life of the elderly, and imposing substantial economic burdens on families and society. In recent years, non-pharmacological therapies as supplementary treatments for VaD have garnered significant attention and have been extensively utilized in clinical settings. Consequently, a network meta-analysis (NMA) was conducted by us to assess the effectiveness of various non-pharmacological therapies in the management of VaD.
Design: We systematically searched seven databases from their inception up to January 2024 to identify randomized controlled trials focusing on non-pharmacological interventions for the treatment of VaD. The methodological quality and risk of bias were rigorously assessed utilizing the RoB 2.0 evaluation tool. The NMA was performed using R software and STATA 14 software, adhering to frequentist theory principles. Additionally, sensitivity analysis, meta-regression analysis, and funnel plot were conducted to assess the stability, heterogeneity, and publication bias, respectively.
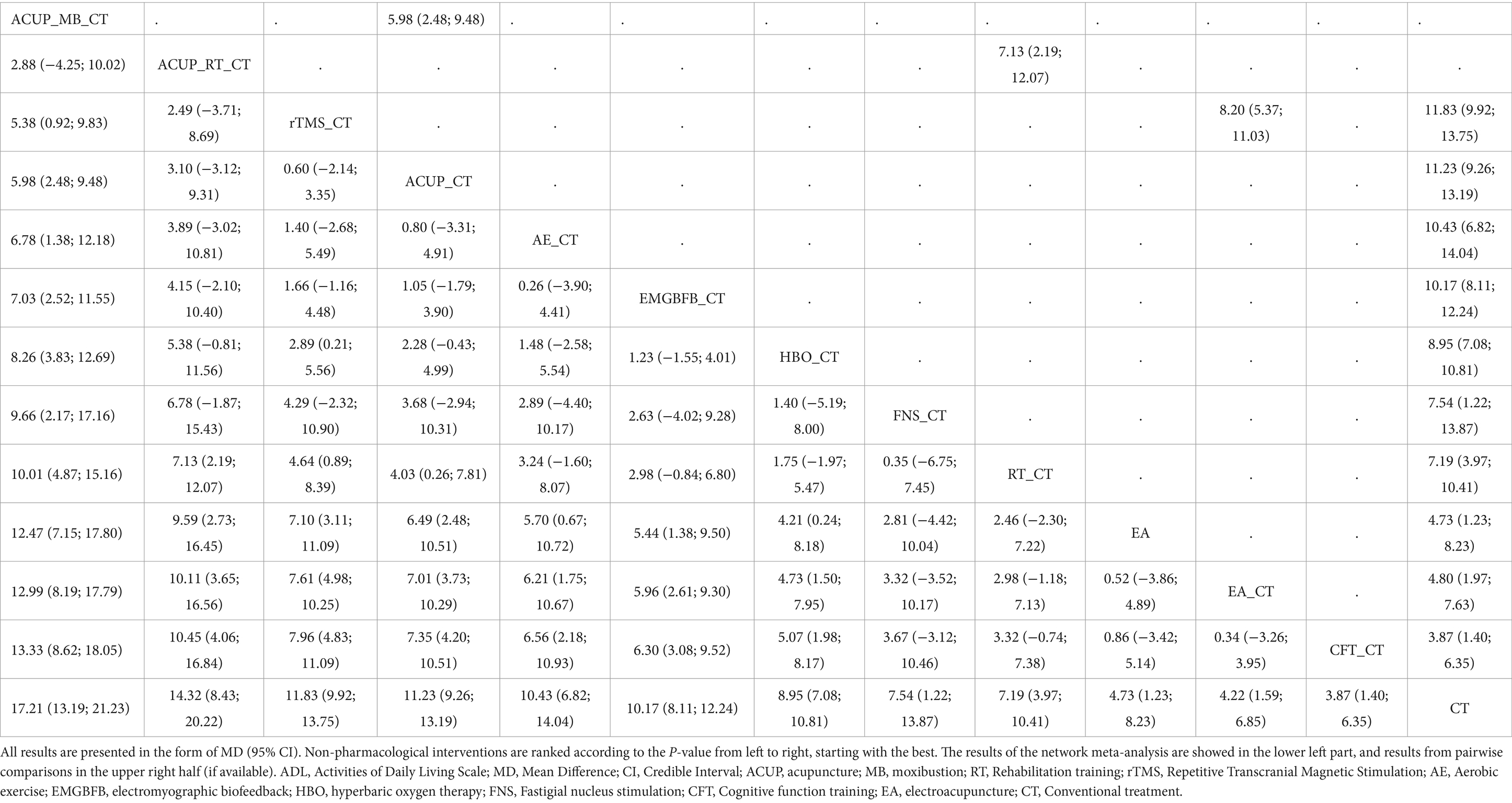
Results: The NMA included 91 eligible studies involving 7,657 patients. The NMA results indicated that in terms of improving Mini-Mental State Examination (MMSE), the following non-pharmacological interventions ranked higher based on p-value: acupuncture_moxibustion_ conventional treatment (ACUP_MB_CT) [P-score = 0.95; pooled mean difference (95% CI): 5.09 (3.82; 6.36)], fastigial nucleus stimulation_CT (FNS_CT) [0.87; 4.51 (2.59; 6.43)], ACUP_rehabilitation training_CT (ACUP_RT_CT) [0.84; 4.19 (2.77; 5.61)], repetitive transcranial magnetic stimulation_CT (rTMS_CT) [0.82; 3.98 (3.08; 4.88)], and aerobic exercise_CT (AE_CT) [0.82; 4.25 (1.86; 6.64)]. Regarding improvement in Activities of Daily Living Scale (ADL), the following non-pharmacological interventions ranked higher based on P-score: ACUP_MB_CT [0.98; 17.21 (13.19; 21.23)], ACUP_RT_CT [0.87; 14.32 (8.43; 20.22)], rTMS_CT [0.78; 11.83 (9.92; 13.75)], and ACUP_CT [0.73; 11.23 (9.26; 13.19)]. No significant adverse reactions were reported in the included studies.
Conclusion: ACUP_MB_CT may be considered the most efficacious intervention for enhancing cognitive function and daily living skills in individuals diagnosed with VaD. Furthermore, ACUP_RT_CT, rTMS_CT, FNS_CT, ACUP_CT, and AE_CT also demonstrate significant clinical utility. Non-pharmacological interventions are unlikely to significantly increase adverse reactions and has a certain degree of safety.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier [CRD42024498902].
1 Introduction
Vascular dementia (VaD) is a syndrome of severe cognitive dysfunction caused by ischemic stroke, hemorrhagic stroke, and cerebrovascular disease causing hypoperfusion in brain regions such as memory, cognition, and behavior (1). Patients with VaD also have severe impairment of financial capacity (2). Notably, vascular risk factors (3) or comorbidities such as depressive symptoms (2) also accelerate the decline in cognitive function and financial capacity, severely affecting patients’ ability to perform daily life and quality of life. VaD, being the second most prevalent form of dementia following Alzheimer’s disease, comprises 15–20% of cases in Western nations (4) and as much as 40% in Asian countries and regions (5). Owing to the escalating occurrence of cerebrovascular ailments and improved post-stroke survival rates, the prevalence of VaD continues to increase (6). Therefore, Effective interventions are critical to the healthcare enterprise, healthcare professionals, caregivers, and patients themselves.
The pathogenesis of VaD is commonly believed to involve brain vascular disease that damages the frontal, temporal, and limbic systems, ultimately leading to cognitive impairment (7, 8). Research has found that degeneration, damage, and inflammation of the central nervous system caused by cerebrovascular disease can disrupt the blood–brain barrier (9, 10), whose permeability is closely associated with cognitive function (11). Through additional research, various cellular biological mechanisms and hypotheses such as excitotoxicity, oxidative stress, neuroinflammation, and neuronal apoptosis have been progressively uncovered (12–15). The interplay among diverse complex mechanisms (16) has somewhat contributed to the challenge of managing VaD in clinical settings. Presently, there are no specialized pharmacological agents available for VaD treatment. The treatment of VaD primarily focuses on treating primary brain vascular diseases and promoting brain function recovery to delay disease progression and extend life. Numerous drugs have been subjected to randomized controlled trials to test their efficacy, including acetylcholinesterase inhibitors such as donepezil and galantamine, N-methyl-D-aspartate receptor (NMDAR) antagonists like memantine, and drugs that improve brain function. Nonetheless, a network meta-analysis (NMA) has revealed that though these medications can partially ameliorate clinical symptoms, their efficacy is largely comparable, yielding unsatisfactory long-term outcomes (17). The fact that their efficacy often entails gastrointestinal, hepatic, and renal adverse reactions poses a significant challenge (18). In recent years, non-pharmacological therapies have been widely used in the treatment of VaD due to their advantages such as simplicity, affordability, and minimal adverse effects. Therefore, the exploration of non-pharmacological therapies holds significant value.
In the past, traditional meta-analyses have indicated that non-pharmacological therapies are effective in enhancing cognitive function and activities of daily living in patients with VaD (19–21). The study conducted by You and colleagues (19) reported the beneficial effects of hyperbaric oxygen therapy for VaD; however, the limited sample size in their study might have led to an overestimation of the therapy’s efficacy. Chen et al. (20) demonstrated that acupuncture could be advantageous for VaD; however, their control group encompassed both conventional treatments and non-conventional interventions like proprietary Chinese medicines and Chinese herbal tonics. Among these studies, only the research conducted by Jiang et al. (21) incorporated comparisons of non-pharmacological interventions in subgroup analyses, albeit with only two studies included. Hence, these meta-analyses failed to provide robust evidence, primarily comparing against conventional treatments. NMAs are considered the highest level of evidence in treatment guidelines (22). However, existing network meta-analyses of non-pharmacological interventions have mainly focused on mild cognitive impairment (23) or Alzheimer’s disease (24). While there is a NMA for VaD, it primarily focuses on the aspect of acupuncture (25). Their research found that combined acupuncture therapy is superior to single intervention in improving cognitive function and activities of daily living. However, clinicians face challenges in selecting the most suitable interventions from a range of non-pharmacological therapies. Therefore, this study utilizes a NMA to comprehensively and systematically compare the impacts of different non-pharmacological therapies on enhancing cognitive function and activities of daily living in patients with VaD. This research also provides evidence-based support for clinicians in choosing treatment strategies.
2 Materials and methods
We performed a systematic review and NMA according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (26). In addition, this study has been registered with PROSPERO, under the number 42024498902.
2.1 Search strategies
We searched the data in PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang Database (Wanfang), China Science and Technology Journal Database (VIP) and Chinese Biomedical Literature Database (SinoMed) from the database’s inception through January 2024 using Medical Subject Headings (MeSH) for “vascular dementia” and “complementary therapies” search terms in Supplementary Appendix 1. In order to ensure the comprehensiveness of the study, we conducted additional searches by reviewing the reference lists of previously published systematic reviews that were identified through the Cochrane Database of Systematic Reviews (search terms: vascular dementia, complementary therapies; limits: none) and PubMed (search terms: vascular dementia, complementary therapies; limits: systematic reviews or meta-analysis). We also searched the Chinese Clinical Trial Registry and Clinicaltrials.gov for some unpublished clinical trials.
2.2 Eligibility criteria
The inclusion criteria were based on the PICOS (participants, interventions, comparators, outcomes, and study design) approach (26). Studies included in this meta-analysis must meet the following criteria and report specific experimental characteristics: (a) Participants had to meet the diagnostic criteria for VaD, including the Chinese Guidelines for the Diagnosis and Treatment of Dementia and Cognitive Impairment and the Diagnostic and Statistical Manual of Mental Disorders (DSM-V). Dementia within 3 months of stroke, sudden onset of cognitive decline or fluctuating or step-like progressive cognitive impairment. Neuropsychological, magnetic resonance imaging, and electron computed tomography scans are required for the diagnosis of VaD. Participants’ eligibility is not limited by age, gender, race, geographic region, ethnicity, or duration of illness. (b) The intervention in the study must incorporate a minimum of one non-pharmacological therapy. Detailed information about these therapies is provided in Supplementary Appendix 2. Only non-pharmacological therapies can be used as the experimental group for comparison with the control group. (c) The control group received conventional anti-dementia drug treatment and symptomatic supportive treatment. Anti-dementia drugs such as donepezil, galantamine, and memantine were used. For supportive treatment, antiplatelet agents like aspirin and clopidogrel, as well as conventional lipid-lowering drugs, hypoglycemic agents, and antihypertensive medications, were administered. In head-to-head studies, any single or combination of non-pharmacological therapies may be employed as the treatment modality. (d) The study must incorporate at least one outcome measure, such as MMSE and ADL. (e) The study design of the included articles must follow a randomized controlled trial methodology.
Exclusion criteria for this study were: (a) patients with Alzheimer’s disease or dementia caused by other factors, as well as those with various mild cognitive impairments and non-dementia vascular cognitive impairments; (b) patients who meet the diagnosis of depression or other psychiatric disorders or who have severe neurological impairments that interfere with neuropsychological assessment; (c) studies with duplicate publications or duplicate data; (d) non-RCT studies, such as meta-analyses, reviews, theoretical discussions, clinical experiences, animal experiments, etc.; (e) Unable to access the original text or extract the mean and standard deviation of the study, or unable to obtain the research data from the authors; (f) studies that did not have one primary endpoint or secondary endpoint indicator as a primary endpoint indicator.
2.3 Outcome indicators
The Mini-Mental State Examination (MMSE) is primarily used to provide a comprehensive, accurate, and rapid assessment of the intellectual status and degree of cognitive impairment in patients with VaD. Additionally, the Barthel Index is utilized as the activities of daily living scale (ADL) to evaluate the patient’s ability to perform daily activities, assessing self-care and functional independence. Adverse reactions from various randomized controlled trials (RCTs), including symptoms like dizziness, headache, syncope, and hematoma, will be recorded to assess the safety of different treatments. Thus, the primary outcome measure in our study is the MMSE, with ADL as the secondary outcome measure (Supplementary Appendix 3).
2.4 Data collection
Two independent researchers (YYH and ZGH) screened potentially eligible papers by reading the titles, abstracts, and full texts of their respective articles based on the inclusion and exclusion criteria. Two researchers (YYH and ZGH) independently retrieved publication details, patient characteristics (such as the number of patients, gender distribution, and disease duration), pertinent intervention specifics (including treatment period, frequency, and time), as well as the mean and standard deviation of outcome measures. If the standard deviation (SD) was not explicitly provided, we derived it by utilizing standard errors (SE), 95% confidence intervals, quartiles, upper and lower range limits of variability, and disparities in baseline values. For image type data, GetData software was used to perform the extraction. If data remained unavailable, we would then reach out to the respective authors of the publications. If discrepancies arise, consultation with a third researcher (QYW) would be sought to reach a resolution.
2.5 Quality assessment and CINeMA
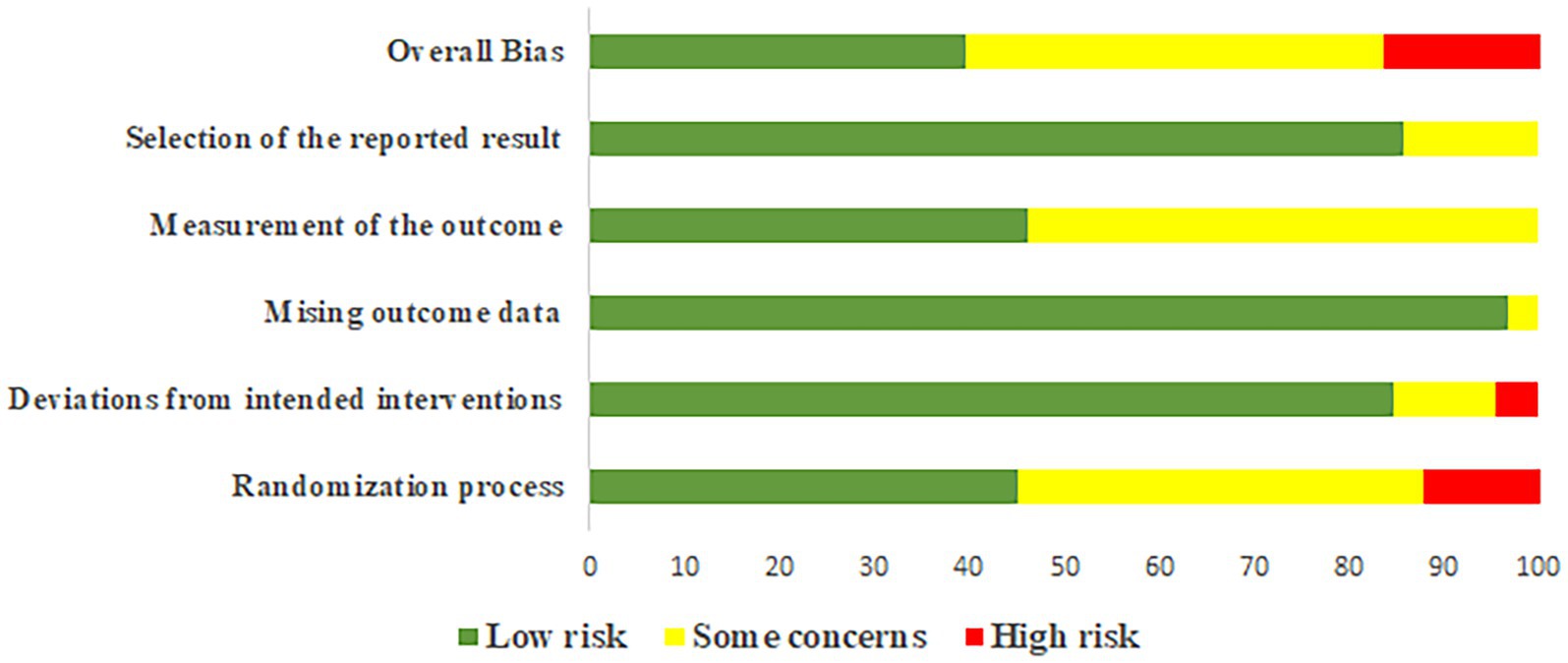
Two investigators (YYH and ZGH) referred to the Cochrane Collaboration’s recommendation of the latest Risk of Bias assessment tool 2.0 (ROB 2.0) for risk of bias assessment (27). ROB 2.0 comprises of five modules: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result. The results of each module were assessed using the modular decision pathway diagrams. Ultimately, these results were summarized to determine the overall assessment of bias, which was categorized as “Low risk,” “Some concerns,” or “High risk” based on the contents of the literature. We used the online application Confidence in Network Meta-Analysis (CINeMA) to assess the certainty of evidence for each outcome, categorizing the evidence into four levels: high, moderate, low, and very low (28). It is worth noting that interactions between different domains may influence each other. Therefore, we analyzed all six CINeMA domains collectively to prevent duplicative situations that could jeopardize the overall quality of evidence due to interconnected issues.
2.6 Data synthesis and analysis
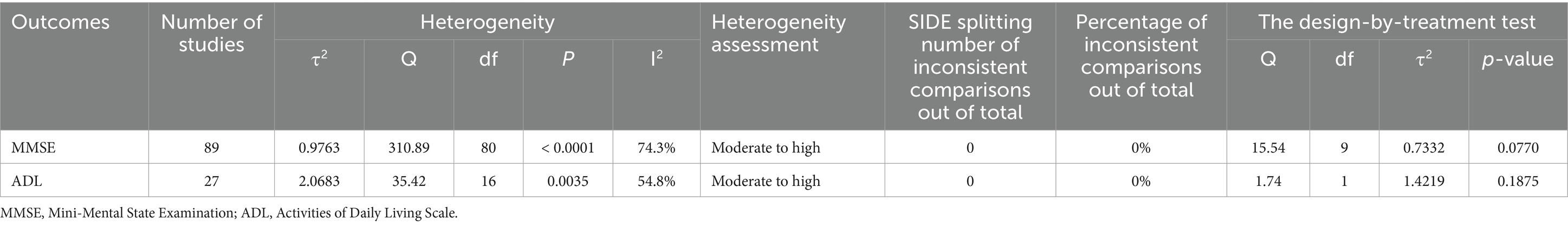
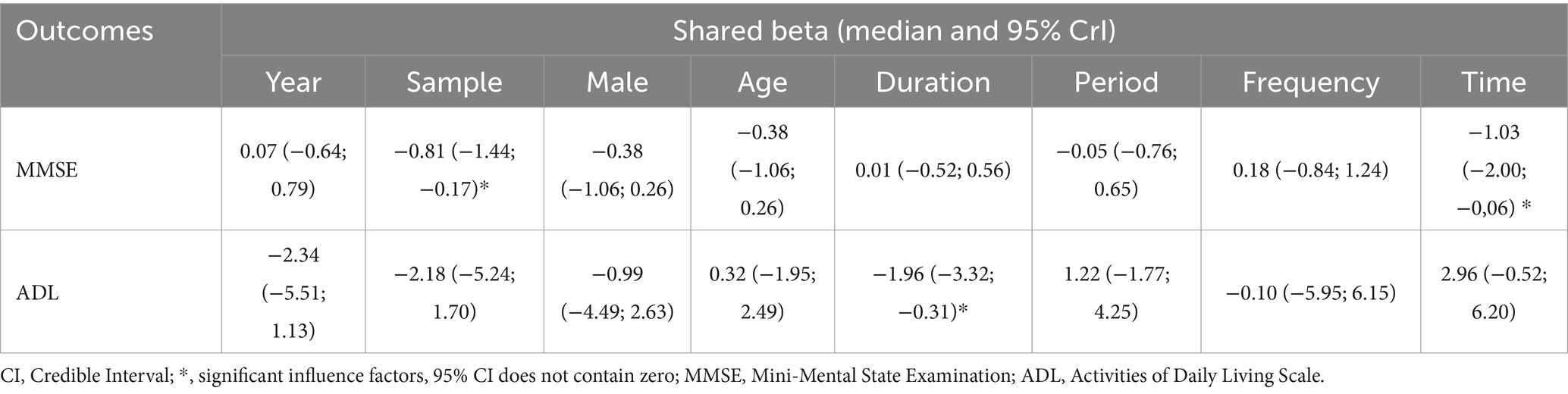
We conducted statistical analysis using R software (version 4.3.2) and Stata software (14.0) (29, 30). Within a frequentist framework, we employed the “meta” and “netmeta” packages in R for NMA. Continuous variables were represented by mean difference (MD), and their 95% confidence intervals (CI) were calculated. We utilized the “network map” command in Stata to create a network diagram. Here, node size indicated the sample size of interventions, while the thickness of edges represented the number of studies comparing two direct interventions. Furthermore, our forest plot presented MD summary values and their 95%CI for all comparisons. Additionally, the P-score in the forest plot assessed the efficacy of different non-pharmacological therapies, with higher scores denoting superior efficacy. Simultaneously, we conducted cluster analysis on two distinct outcome indicators to identify interventions with superior combined efficacy. Global heterogeneity and inconsistency were assessed utilizing the “decomp.design” function in R software. The global I2 statistic was employed to evaluate heterogeneity, where I2 values exceeding 50% signify notable heterogeneity, prompting the application of a random-effects model. Furthermore, global consistency and the Separated Indirect From Direct Evidence (SIDE) test were utilized to evaluate overall and local inconsistency (31). The R package “gemtc” was used to pinpoint sources of heterogeneity in the study, including variables like publication year, sample size, gender, age, illness duration, treatment duration, treatment frequency, and treatment timing. The stability of treatment effects across different outcome indicators in network meta-regression was evaluated by computing the mean values of covariates from the models. Studies with treatment durations outside the 4–16 weeks range and those exhibiting high bias risk were excluded, followed by a sensitivity analysis. To identify publication bias and small study effects within the included studies, comparison-adjusted funnel plots were employed.
3 Results
3.1 Literature screening process and basic characteristics
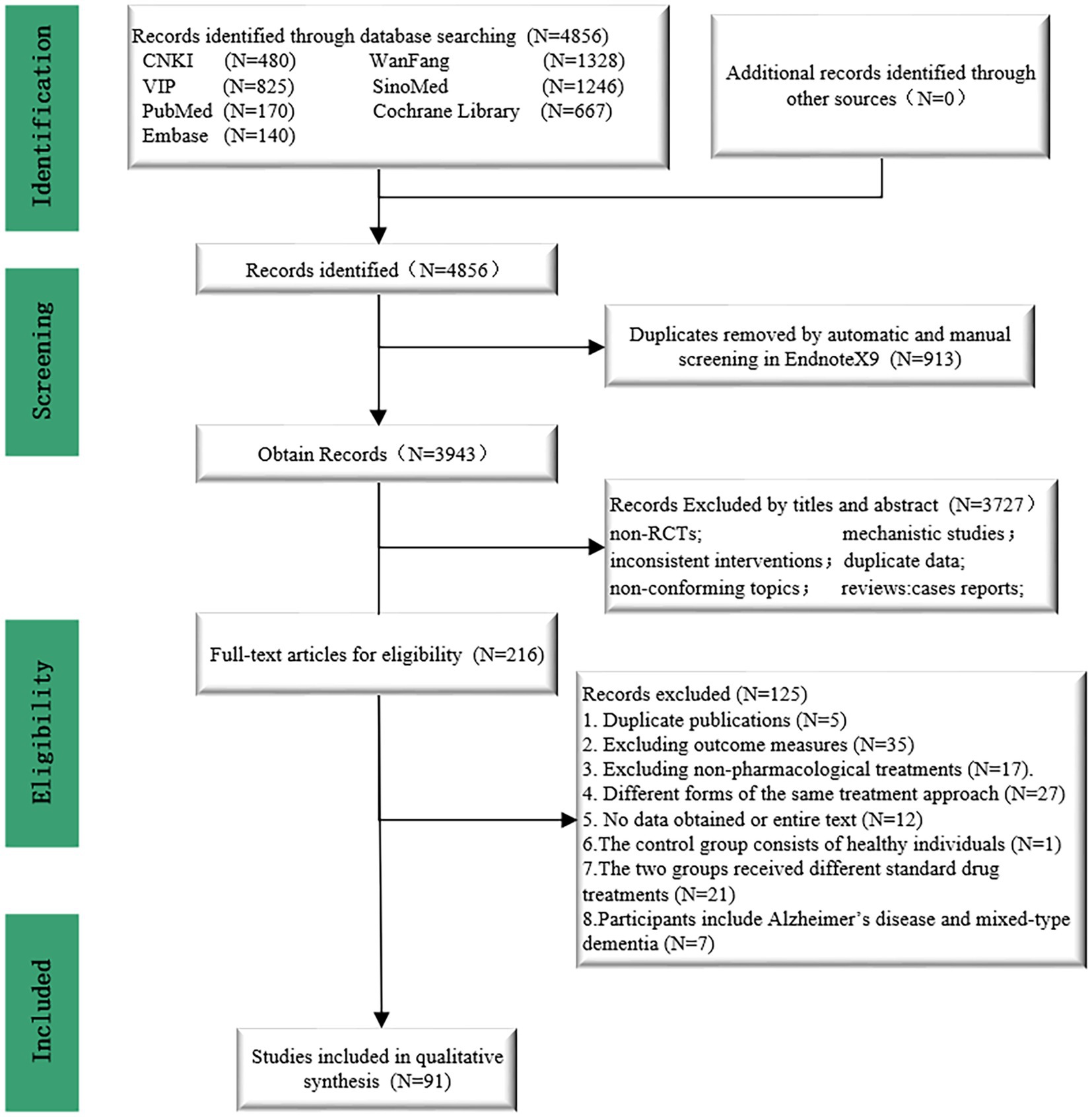
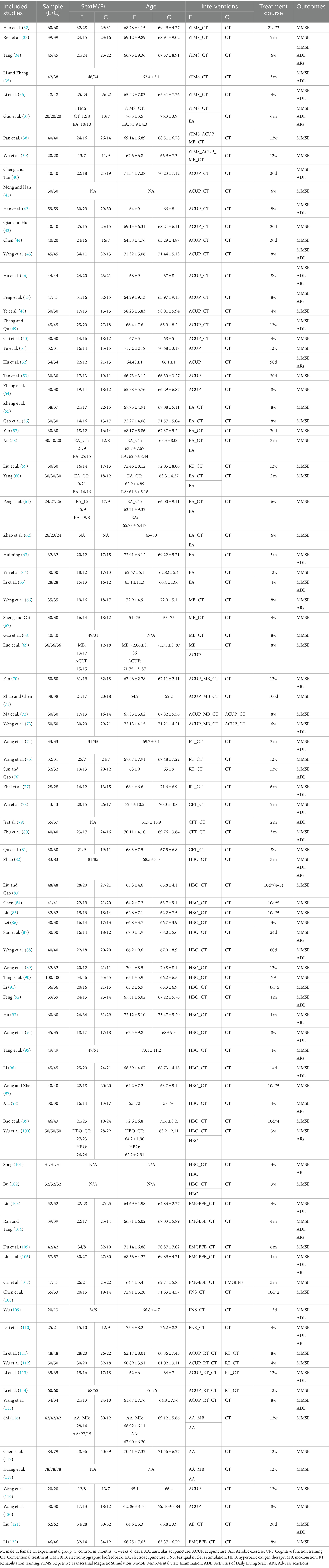
Figure 1 illustrates the specific details of the literature screening process. After searching relevant literature databases, a total of 4,856 articles were obtained. Following the removal of 913 duplicates using Endnote X9 software, 3,943 articles were excluded based on abstracts and titles, leaving 216 full-text articles. Subsequently, two researchers finalized the inclusion of 91 studies based on the established inclusion and exclusion criteria (32–122). The specific details of the literature screening process can be seen in Figure 1. Table 1 contains information about the 91 studies of RCTs published between 2005 and 2023 that met the criteria for natriuresis. The 91 studies included a total of 7,657 participants, with 4,235 (55.31%) males and 3,422 (44.69%) females, predominantly elderly individuals, with sample sizes ranging from 33 to 234 and an average disease duration of 19.07 months (SD 13.42). Among the 91 studies, 21 different treatment modalities were included (Supplementary Appendix 2), with an average treatment duration of 8.16 weeks (SD 4.53), treatment frequencies ranging from 1 to 14 times per week (average 6.4 times, SD 1.79), and treatment durations per session ranging from 16 to 80 min (average 45.1 min, SD 15.41) (Supplementary Appendix 4). Basic characteristics of the included studies such as authors, publication year, participant information (average age, gender), interventions, duration, and outcome indicators were summarized in Table 1. The detailed interventions for each study are in Supplementary Appendix 4.
There were 21 treatment modalities forming 27 direct comparisons, including auricular acupuncture (AA) vs. AA_moxibustion (AA_MB) (2 comparisons), AA vs. conventional treatment (CT) (3 comparisons), AA_MB vs. CT (2 comparisons), acupuncture (ACUP) vs. CT (7 comparisons), ACUP vs. MB (1 comparison), ACUP_CT vs. ACUP_MB_CT (2 comparisons), ACUP_CT vs. CT (11 comparisons), ACUP_MB_CT vs. CT (2 comparisons), ACUP_rehabilitation training_CT ACUP_RT_CT vs. CT (1 comparison), ACUP_RT_CT vs. RT_CT (4 comparisons), aerobic exercise_CT (AE_CT) vs. CT (1 comparison), cognitive function training_CT (CFT_CT) vs. CT (4 comparisons), electroacupuncture (EA) vs. CT (7 comparisons), EA_CT vs. CT (8 comparisons), electromyographic biofeedback_CT (EMGBFB_CT) vs. CT (5 comparisons), Fastigial nucleus stimulation_CT (FNS_CT) vs. CT (3 comparisons), hyperbaric oxygen therapy (HBO) vs. CT (3 comparisons), HBO_CT vs. CT (21 comparisons), MB vs. CT (1 comparison), MB_CT vs. CT (3 comparisons), RT_CT vs. CT (5 comparisons), repetitive transcranial magnetic stimulation_ACUP_MB_CT (rTMS_ACUP_MB_CT) vs. CT (2 comparisons), rTMS_CT vs. CT (6 comparisons), EA vs. EA_CT (4 comparisons), EA_CT vs. rTMS_CT (1 comparison), EMGBFB vs. EMGBFB_CT (1 comparison), HBO vs. HBO_CT (3 comparisons).
3.2 Bias risk assessment of involved literature
The bias risk of each study can be identified in Supplementary Appendix 5, while the summary of bias risk across all studies is depicted in Figure 2. The proportion of studies with low bias risk during the randomization process was 45.05%, deviations from intended interventions was 84.62%, missing outcome data stands at 96.70%, the measurement of outcomes was 46.15%, and the selection of reported results was 85.71%. Overall, the proportion of studies with high bias risk is 16.48%, medium bias risk was 43.96%, and low bias risk accounts for 39.56%.
3.3 Network meta-analysis
3.3.1 MMSE
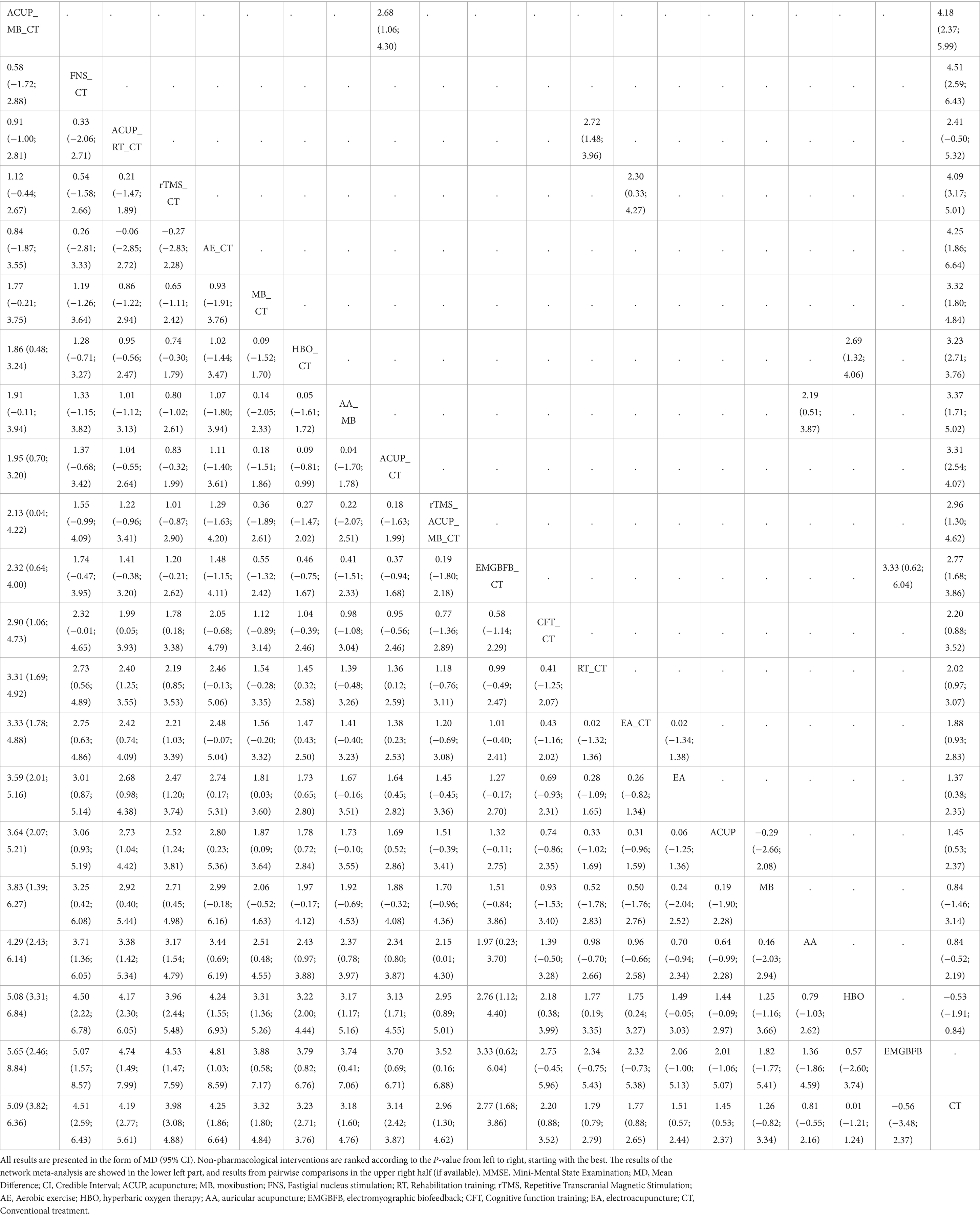
Figure 3 shows a network graph of different non-pharmacological interventions for VaD. Eighty nine studies (32–78, 80–102, 104–122) (97.80%) involving 7,413 participants (96.81%) evaluated the MMSE in the context of 21 non-pharmacological interventions, forming 7 closed loops, with the largest number of studies concentrating on HBO_CT (21 studies) (Figure 3A). Figure 4 shows the pooled MD values for different nonpharmacological interventions compared to CT and the ranking of different nonpharmacological interventions according to P-score. Sixteen non-pharmacological therapies significantly improved MMSE compared to CT, with MDs (95%CI) ranging from 5.09 (3.82; 6.36) for ACUP_MB_CT to 1.45 (0.53; 2.37) for ACUP (Figure 4A). Ranked by the degree of MMSE improvement, ACUP_MB_CT (P-score = 0.95) was defined as the best, while CT (0.07) was considered the worst (Figure 4A). Table 2 shows the results of the NMA on MMSE. NMA results indicated that ACUP_MB_CT, FNS_CT, ACUP_RT_CT, rTMS_CT, AE_CT, MB_CT, HBO_CT, AA_MB, ACUP_CT, rTMS_ACUP_MB_CT, and EMGBFB_CT showed significant significance compared to many other treatments (more than 2).
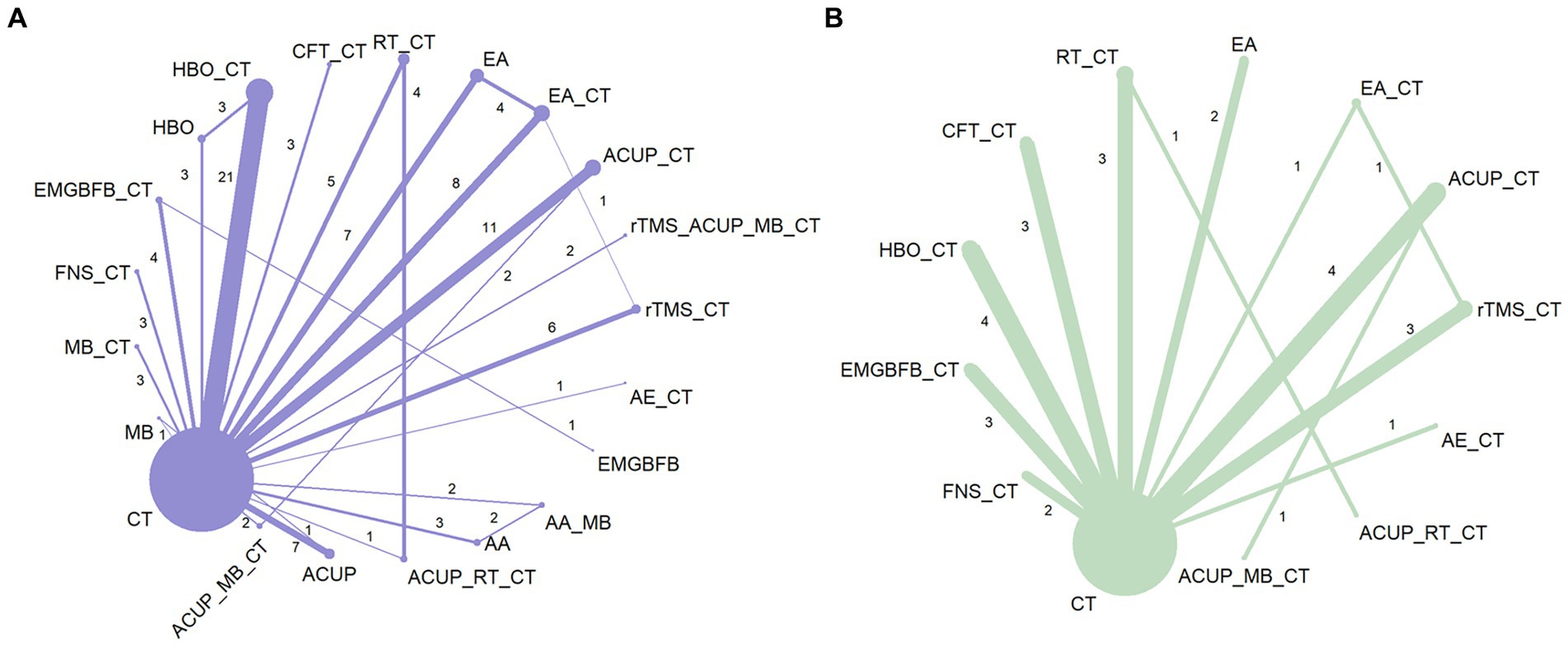
Figure 3. Network graph of network meta-analysis for main outcomes. (A) Mini-Mental State Examination (MMSE), (B) Activities of Daily Living Scale (ADL). AA, auricular acupuncture; ACUP, acupuncture; AE, Aerobic exercise; CFT, Cognitive function training; CT, Conventional treatment; EMGBFB, electromyographic biofeedback; EA, electroacupuncture; FNS, Fastigial nucleus stimulation; HBO, hyperbaric oxygen therapy; MB, moxibustion; RT, Rehabilitation training; rTMS, Repetitive Transcranial Magnetic Stimulation.
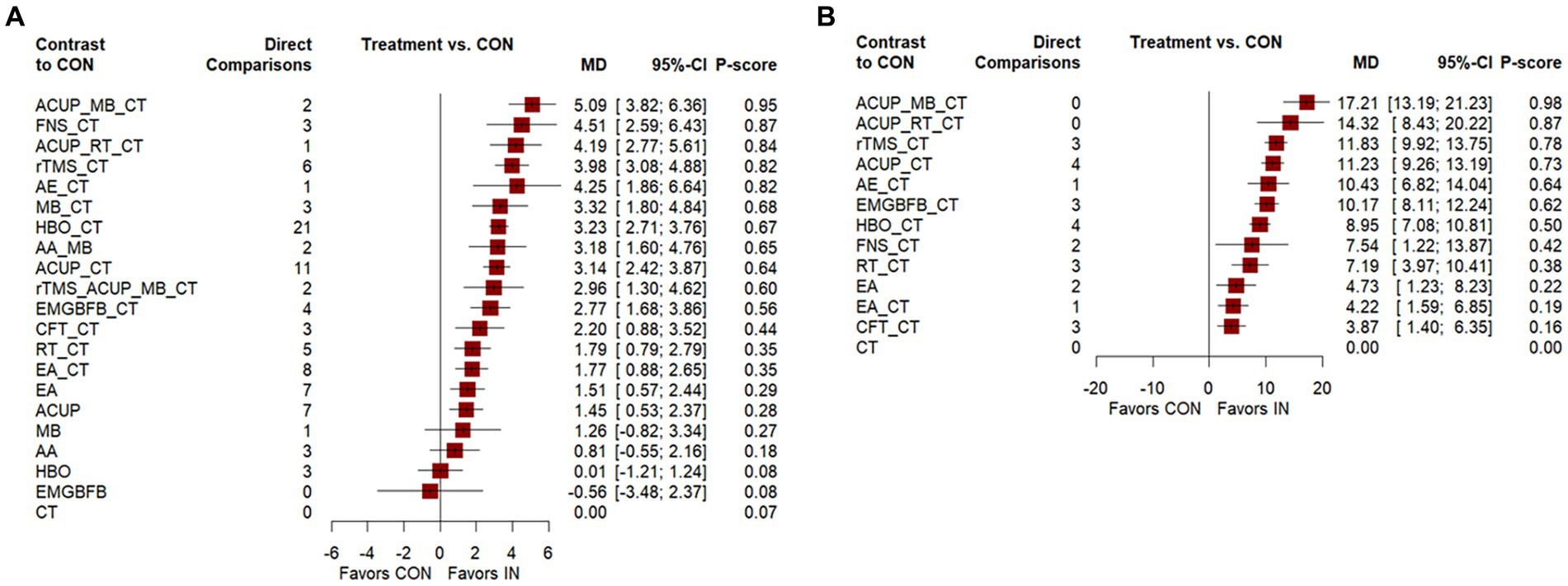
Figure 4. Forest plot of network meta-analysis for main outcomes. (A) Mini-Mental State Examination (MMSE), (B) Activities of Daily Living Scale (ADL). AA, auricular acupuncture; ACUP, acupuncture; AE, Aerobic exercise; CFT, Cognitive function training; CT, Conventional treatment; EMGBFB, electromyographic biofeedback; EA, electroacupuncture; FNS, Fastigial nucleus stimulation; HBO, hyperbaric oxygen therapy; MB, moxibustion; RT, Rehabilitation training; rTMS, Repetitive Transcranial Magnetic Stimulation.
3.3.2 ADL
Twenty seven studies (34, 36, 40, 45, 46, 49, 63, 65, 71, 74, 76–80, 88, 92, 94, 97, 103, 104, 109, 110, 113, 121) (29.67%) involving 2,105 participants (27.49%) evaluated the ADL in the context of 12 non-pharmacological therapies, forming a closed loop, with ACUP_CT and HBO_CT vs. CT (10 studies) being the most studied interventions (Figure 3B). Compared to CT, all 12 non-pharmacological therapies significantly improved ADL, with MDs (95%CI) ranging from 17.21 (13.19; 21.23) for ACUP_MB_CT to 3.87 (1.40; 6.35) for CFT_CT (Figure 4B). Ranked by the degree of ADL improvement, ACUP_MB_CT (0.98) was defined as the best, while CT was considered the worst (Figure 4B). Table 3 shows the results of the NMA on ADL. The NMA results indicated that ACUP_MB_CT, ACUP_RT_CT, rTMS_CT, ACUP_CT, AE_CT, EMGBFB_CT, and HBO_CT showed significant significance compared to many other treatments (more than 2).
3.4 Cluster analysis
Figure 5 shows the results of the cluster analysis. We conducted cluster analysis on the MMSE and ADL outcomes in this study to identify interventions that were effective for improving both outcomes. The cluster analysis of MMSE and ADL showed that ACUP_MB_CT, ACUP_RT_CT, rTMS_CT, AE_CT, and ACUP_CT were located in the upper right corner, indicating relatively better performance.
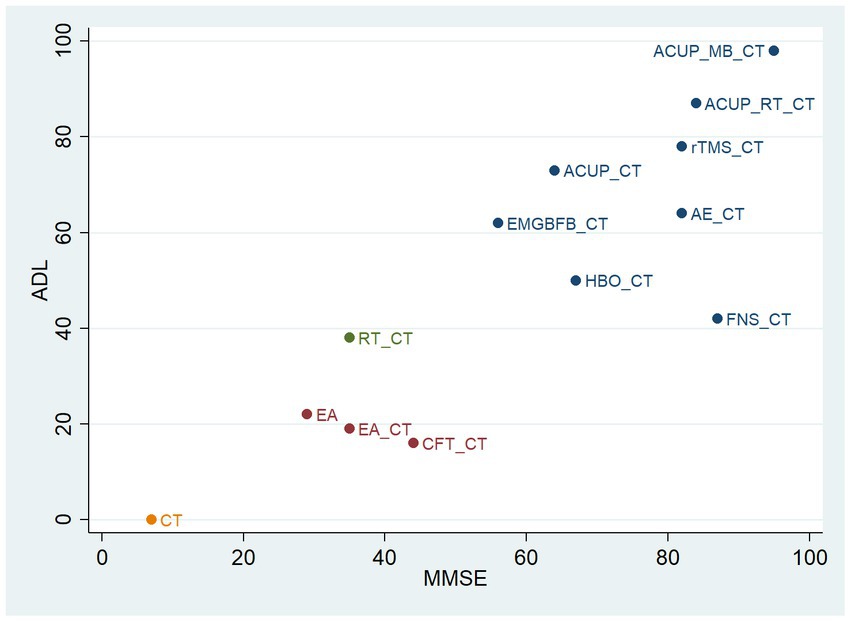
Figure 5. Cluster analysis plot of main outcomes. ACUP, acupuncture; MB, moxibustion; RT, Rehabilitation training; rTMS, Repetitive Transcranial Magnetic Stimulation; AE, Aerobic exercise; EMGBFB, electromyographic biofeedback; HBO, hyperbaric oxygen therapy; FNS, Fastigial nucleus stimulation; CFT, Cognitive function training; EA, electroacupuncture; CT, Conventional treatment; MMSE, Mini-Mental State Examination; ADL, Activities of Daily Living Scale.
3.5 Adverse reactions
Among the 18 studies (34, 37, 46, 47, 52, 53, 66, 70, 82, 87, 93, 95, 98, 100, 101, 104, 106, 110) included, adverse reactions were reported in all cases. Specifically, 14 studies (34, 37, 46, 47, 66, 70, 87, 93, 95, 98, 100, 104, 106, 110) documented various adverse reactions, primarily characterized by symptoms such as nausea, abdominal pain, and dizziness, which exhibited mild intensity and did not disrupt the treatment procedures. These adverse reactions were predominantly noted in research studies linked to rTMS_CT, ACUP_CT, MB_CT, ACUP_MB_CT, HBO_CT, EMGBFB_CT, and FNS_CT. Additional details regarding the specific adverse reactions were accessed in Supplementary Appendix 6.
3.6 The small sample effect and publication bias
The comparative adjusted funnel plot results demonstrate that the funnel plots of MMSE and ADL are generally symmetrical (Figure 6). The study findings are symmetrically distributed around the midline at the top, indicating a lower likelihood of small sample effects.
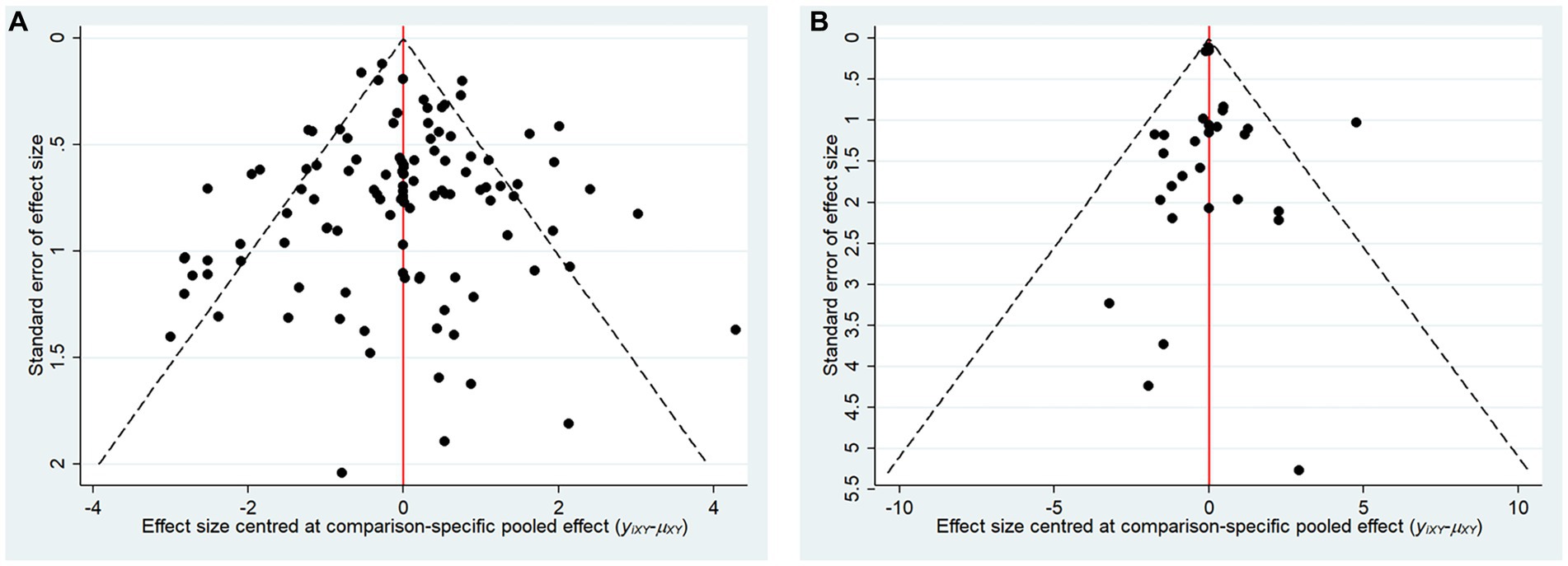
Figure 6. Comparison adjusted funnel plots for main outcomes. (A) Mini-mental state examination (MMSE), (B) Activities of Daily Living Scale (ADL).
3.7 Heterogeneity and certainty of evidence
Table 4 shows the results of the assessment of heterogeneity and inconsistency. The heterogeneity results varied from moderate to high, with a global I2 of 74.3% for MMSE and 54.8% for ADL. Moreover, none of the global inconsistencies in the outcome measures were statistically significant, and the local inconsistency assessed by the SIDE test did not demonstrate substantial disparities (Table 4; Supplementary Appendix 7). Furthermore, the level of evidence grading for each outcome measure varied from very low to high certainty, suggesting an overall low quality (Supplementary Appendix 10).
3.8 Network meta-regression and sensitivity analysis
Table 5 shows the results of the meta-regression. We identified sources of heterogeneity through meta-regression and sensitivity analysis, with a primary focus on baseline information, treatment duration, treatment frequency, and other covariates. We found that sample, duration and time were the sources of heterogeneity in this study. Additionally, we compared the adjusted results with the original outcomes through the centralization of values for various covariates according to the model. The MDs of non-pharmacological interventions types did not change significantly, and the hierarchy largely remained consistent compared to the unadjusted model (Supplementary Appendix 8). Refined sensitivity analyses, which excluded studies with high risk of bias or focused on studies with treatment duration between 4 and 16 weeks, did not significantly influence the MDs and rankings (Supplementary Appendix 9). In conclusion, the results of our study were stable.
4 Discussion
Our study included 91 studies on non-pharmacological therapies and 2 outcome indicators. We conducted a comprehensive evaluation of the effectiveness of various non-pharmacological therapies in managing VaD through NMA. We found that the majority of non-pharmacological therapies employed as complementary treatments for VaD were statistically significant. The NMA results indicated that (1) acupuncture-related therapies achieved high rankings in both MMSE and ADL assessments, including ACUP_MB_CT, ACUP_RT_CT, and ACUP_CT; (2) 16 non-pharmacological therapies significantly improved the MMSE, with ACUP_MB_CT showing the best effect, and FNS_CT, ACUP_RT_CT, rTMS_CT, AE_CT achieving similarly high p-values; (3) 12 non-pharmacological therapies significantly improved the ADL, with ACUP_MB_CT showing the best effect; (4) rTMS_CT and AE_CT also showed significant improvements in both MMSE and ADL.
In addition, our study revealed that all non-pharmacological therapies combined with conventional treatment significantly outperformed conventional treatment in improving ADL. Furthermore, in terms of enhancing MMSE, most non-pharmacological therapies combined with conventional treatment were superior to conventional treatment; however, MB, AA, HBO, and EMGBFB showed no significant difference compared to conventional treatment in this aspect. This lack of significance may be attributed to the limited number of studies incorporating MMSE or the lower baseline MMSE scores. Notably, the analysis indicated that rTMS_ACUP_MB_CT did not improve MMSE as effectively as utilizing rTMS_CT, ACUP_CT, or MB_CT alone. Given the quality, quantity, and baselines of the included RCTs, more research is necessary to validate this observation. Importantly, the findings suggest that non-pharmacological therapies did not significantly increase the incidence of adverse reactions based on the outcomes reported in the included studies.
As clinical trials and animal experiments progress, the mechanisms of non-pharmacological therapies for treating VaD are gradually being unveiled. Acupuncture-related treatments such as ACUP_MB_CT, ACUP_RT_CT, and ACUP_CT have demonstrated promising outcomes in enhancing MMSE and ADL scores. This indicates that acupuncture is a clinically valuable approach, and its synergistic effects can be enhanced when combined with RT or MB therapies. Acupuncture is a unique traditional Chinese therapy known for its multi-target, multi-faceted, and holistic approach. Recent research indicates that acupuncture holds promise in reducing peripheral inflammation and immune abnormalities by targeting inflammatory mediators like Interleukin-1 beta (IL-1β), IL-2, and Tumor Necrosis Factor-alpha (TNF-α) (123), consequently alleviating neural inflammation and ameliorating cognitive impairments (124). Furthermore, acupuncture exhibits a direct mechanism for enhancing cognitive function affected by neural inflammation through the inhibition of the microRNA-93 (miR-93)-mediated Toll-like receptor 4 (TLR4) signaling pathway (125, 126). Past investigations have underscored the pivotal role of TLR4 in mediating inflammatory responses of immune cells within the central nervous system (127), directly linking it to brain damage and neuronal death observed in cases of cerebral ischemia and stroke (128). Furthermore, according to an MRI-based imaging study, acupuncture has been shown to enhance cerebral white matter perfusion and maintain myelin integrity, subsequently safeguarding cognitive function (129). Notably, acupuncture can boost synaptic plasticity (130), acting as the biological foundation for learning and memory processes (131). According to the included studies, we found that Baihui (DU20), Shenting (DU24), Si Shencong (EX-HN1), and Zu Sanli (ST36) were the most commonly used acupoints for treating vascular dementia. A functional brain imaging study showed that adding DU20 enhanced cognitive function by enhancing the medial temporal lobe system, thalamus system, and prefrontal cortex system (132). Furthermore, acupuncture discovered by Yang et al. reduced the inhibitory effects of the 2-vessel occlusion model on hippocampal long-term potentiation, thereby protecting synaptic plasticity. Among the acupoints, DU20 and ST36 exhibited the best therapeutic effect (133). Additionally, needling DU20 and DU24 augmented the density of dendritic spines in the hippocampus of rats (134). An increase in dendritic spine density is associated with improvements in cognitive processes such as learning and memory (135). Within clinical settings, acupuncture has demonstrated promising outcomes in improving limb movement, swallowing function, and language skills (136–138), thereby playing a crucial role in rejuvenating patients’ everyday life capabilities. As a passive non-pharmacological intervention, the integration of acupuncture with active rehabilitation exercises can enhance the restoration of motor and daily life functions (139). Consequently, these findings may elucidate the favorable rankings of acupuncture-related interventions in enhancing MMSE and ADL within this NMA.
ACUP_MB_CT ranks the best according to the P-score in improving both MMSE and ADL. Moxibustion enhances neurogenesis and angiogenesis in rats by upregulating the expression of nestin, doublecortin, and CD34 in the hippocampus (140). Furthermore, It further improves cognitive function in rats with VaD by attenuating hippocampal neuronal apoptosis (141). We speculate that the combined effect of acupuncture and moxibustion makes ACUP_MB_CT the most effective in improving MMSE and ADL.
The clinical value of FNS_CT in enhancing MMSE performance is notable; however, its effectiveness in improving ADL is relatively limited. In upcoming clinical trials, the consideration of integrating other treatment modalities to address this limitation is warranted. FNS, as a non-invasive electrical stimulation therapy, plays a crucial role in enhancing cerebral blood flow and exerting neuroprotective effects by inhibiting excitotoxicity, neuroinflammation, and cell apoptosis (142). Significantly, animal experiments have demonstrated that FNS can downregulate NLRP3 mRNA and protein expression, thereby inhibiting autophagy processes and suppressing the expression of caspase 1, IL-1β, and IL-18. This leads to a reduction in neuroinflammation, neuronal apoptosis, and an improvement in cognitive function among patients (143).
One treatment method is unlikely to be the sole best approach for VaD. rTMS_CT and AE_CT also demonstrate good efficacy. This provides more treatment options for healthcare professionals and patients to choose the most suitable approach based on individual circumstances. Studies have shown that rTMS can improve the learning and memory abilities of rats with VaD by upregulating the expression of vascular endothelial growth factor, brain-derived neurotrophic factor, and the NMDAR (144). BDNF is well-known for its important role in repairing and regenerating neural cells, as well as enhancing neural function (145). Additionally, the NMDAR is closely associated with synaptic plasticity within the hippocampal cornu ammonis 1 region (146). Previous research has also found that rTMS can protect hippocampal cholinergic neurons damaged by chronic brain ischemia-hypoxia and restore the activity of the hippocampal cholinergic system (147). These may be reasons why rTMS_CT ranks highly in improving ADL and MMSE. AE regulates the expression of Beclin-1 in the hippocampus, impacting autophagy and apoptosis, and enhancing hippocampal function (148). This exercise also promotes brain blood circulation, increasing cerebral blood flow (149). Furthermore, aerobic exercise enhances metabolism, strengthens muscle training, and improves cardiovascular function, all contributing to improved daily life abilities and cognitive function (150, 151) Based on the analysis presented above and the results of NMA, it is evident that the combination of non-pharmacological therapy with conventional treatment can address VaD through diverse pathways and targets, offering valuable insights for clinicians to select more effective and suitable non-pharmacological interventions.
5 Strengths and limitations
To our knowledge, this is the first NMA comparing the effectiveness and safety of different non-pharmacological therapies for VaD. The NMA combines direct and indirect evidence to compare different interventions, thereby enhancing the evidence. It also provides a comprehensive evaluation and ranking of various interventions to identify their strengths and weaknesses. We searched 7 databases, including 3 English databases and 4 Chinese databases, to increase the breadth and diversity of studies. We assessed the overall heterogeneity and used network meta-regression to explore potential sources of heterogeneity. For inconsistency, we performed both global inconsistency tests and localized inconsistency tests using the SIDE test, which allows for clear assessment of significant differences between each node comparison. We demonstrated the stability of our results through network meta-regression and sensitivity analysis.
Our research also has certain limitations: (1) There may be certain methodological limitations. Out of the included studies, 41 (45.05%) described specific randomization methods, 37 (40.66%) only mentioned randomization, and 13 (14.29%) grouped participants based on visit order or did not mention it. Moreover, only 3 studies mentioned blinding. (2) The sample size ranged from 33 to 234, resulting in moderate to high heterogeneity in the studies. However, the funnel plot did not reveal any significant small sample effects, suggesting the possible presence of individual studies with larger sample sizes. (3) The included studies had a wide range of disease duration (approximately 1–85 months). Previous studies have found that overall, VaD worsens with time. Our meta-regression also identified disease duration as a source of heterogeneity in this study. (4) The included studies were predominantly conducted in China, which may affect the generalizability of the results. (5) Most literature reported adverse reactions descriptively, and there were safety variations among different interventions, so we only conducted descriptive analysis. (6) Our treatment methods were classified based on the descriptions in the included literature, resulting in 21 different treatment methods, which may introduce some bias to the results. For example, rTMS_ACUP_MB_CT is a combination of multiple treatment methods, while EA does not include conventional treatment. Therefore, more research is needed in the future to support our findings.
6 Conclusion
This study, which utilizes the NMA method, aims to compare the efficacy and safety of non-pharmacological therapies in conjunction with conventional treatments for VaD. In summary, following pairwise comparisons of different treatment methods and utilizing P-score ranking and cluster analysis, ACUP_MB_CT emerges as the most effective intervention for enhancing VaD, as indicated by improvements in both MMSE and ADL. Moreover, ACUP_RT_CT, rTMS_CT, ACUP_CT, FNS_CT, and AE_CT exhibit significant efficacy across various domains. As for safety, the descriptive results reveal no instances of serious adverse reactions, and it is noted that non-pharmacological therapies do not lead to a significant increase in adverse reactions, thereby indicating a certain degree of safety. It is anticipated that the outcomes of this study will assist clinicians, caregivers, and patients in making informed decisions.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
YY: Writing – review & editing, Writing – original draft, Software, Methodology, Formal analysis, Data curation, Conceptualization. YQ: Writing – review & editing, Validation, Supervision. SL: Writing – review & editing, Software, Methodology, Investigation. GZ: Writing – review & editing, Software, Investigation, Data curation, Conceptualization. YR: Writing – review & editing, Software, Resources, Project administration, Methodology. ML: Writing – review & editing, Visualization, Validation, Supervision, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Inheritance Studio of Traditional Chinese Medicine Master Zhang Canjia of State Administration of Traditional Chinese Medicine (no. [2010]59), the Chinese Medicine Science and Technology Project of Shandong Province in 2023 (M-2023031), and the Key Project of Undergraduate Teaching Reform Research in Shandong Province (Z2023292).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer CD declared a shared affiliation with the authors to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1397088/full#supplementary-material
References
1. O’brien, JT, and Thomas, A. Vascular dementia. Lancet. (2015) 386:1698–706. doi: 10.1016/S0140-6736(15)00463-8
2. Giannouli, V, and Tsolaki, M. Vascular dementia, depression, and financial capacity assessment. Alzheimer Dis Assoc Disord. (2021) 35:84–7. doi: 10.1097/WAD.0000000000000374
3. Giannouli, V, and Tsolaki, M. Liberating older adults from the bonds of vascular risk factors: what is their impact on financial capacity in amnestic mild cognitive impairment? Psychiatry Clin Neurosci. (2022) 76:246–50. doi: 10.1111/pcn.13348
4. Hébert, Ŕ, Lindsay, J, Verreault, R, Rockwood, K, Hill, G, and Dubois, MF. Vascular Dementia. Stroke. (2000) 31:1487–93. doi: 10.1161/01.str.31.7.1487
5. Yu, WX, and Wang, YJ. Epidemiology status and development trends of Asian vascular cognitive impairment. Chin Front Med. (2020) 12:1–7. doi: 10.12037/yxqy.2020.10-01
6. Pinkston, JB, Alekseeva, N, and González Toledo, E. Stroke and dementia. Neurol Res. (2009) 31:824–31. doi: 10.1179/016164109x12445505689643
7. Inoue, Y, Shue, F, Bu, G, and Kanekiyo, T. Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease. Mol Neurodegener. (2023) 18:46. doi: 10.1186/s13024-023-00640-5
8. Yang, T, Sun, Y, Lu, Z, Leak, RK, and Zhang, F. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res Rev. (2017) 34:15–29. doi: 10.1016/j.arr.2016.09.007
9. Candelario-Jalil, E, Dijkhuizen, RM, and Magnus, T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. (2022) 53:1473–86. doi: 10.1161/strokeaha.122.036946
10. Korczyn, AD . Mixed dementia--the most common cause of dementia. Ann N Y Acad Sci. (2002) 977:129–34. doi: 10.1111/j.1749-6632.2002.tb04807.x
11. Uchida, Y, Kan, H, Sakurai, K, Arai, N, Inui, S, Kobayashi, S, et al. Iron leakage owing to blood-brain barrier disruption in small vessel disease CADASIL. Neurology. (2020) 95:e1188–98. doi: 10.1212/wnl.0000000000010148
12. Wang, XX, Zhang, B, Xia, R, and Jia, QY. Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur Rev Med Pharmacol Sci. (2020) 24:9601–14. doi: 10.26355/eurrev_202009_23048
13. Hei, Y, Chen, R, Yi, X, Long, Q, Gao, D, and Liu, W. HMGB1 neutralization attenuates hippocampal neuronal death and cognitive impairment in rats with chronic cerebral Hypoperfusion via suppressing inflammatory responses and oxidative stress. Neuroscience. (2018) 383:150–9. doi: 10.1016/j.neuroscience.2018.05.010
14. Kang, YC, Zhang, L, Su, Y, Li, Y, Ren, WL, and Wei, WS. MicroRNA-26b regulates the microglial inflammatory response in hypoxia/ischemia and affects the development of vascular cognitive impairment. Front Cell Neurosci. (2018) 12:154. doi: 10.3389/fncel.2018.00154
15. Rajeev, V, Fann, DY, Dinh, QN, Kim, HA, de Silva, TM, Lai, MKP, et al. Pathophysiology of blood brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment. Theranostics. (2022) 12:1639–58. doi: 10.7150/thno.68304
16. Iadecola, C . The pathobiology of vascular dementia. Neuron. (2013) 80:844–66. doi: 10.1016/j.neuron.2013.10.008
17. Cochrane Dementia and Cognitive Improvement GroupBattle, CE, Abdul-Rahim, AH, Shenkin, SD, Hewitt, J, and Quinn, TJ. Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis. Cochrane Database Syst Rev. (2021) 2021:Cd013306. doi: 10.1002/14651858.CD013306.pub2
18. Kröger, E, Mouls, M, Wilchesky, M, Berkers, M, Carmichael, PH, van Marum, R, et al. Adverse drug reactions reported with cholinesterase inhibitors: an analysis of 16 years of individual case safety reports from VigiBase. Ann Pharmacother. (2015) 49:1197–206. doi: 10.1177/1060028015602274
19. You, Q, Li, L, Xiong, SQ, Yan, YF, Li, D, Yan, NN, et al. Meta-analysis on the efficacy and safety of hyperbaric oxygen as adjunctive therapy for vascular dementia. Front Aging Neurosci. (2019) 11:86. doi: 10.3389/fnagi.2019.00086
20. Chen, Y, Wang, H, Sun, Z, Su, X, Qin, R, Li, J, et al. Effectiveness of acupuncture for patients with vascular dementia: a systematic review and meta-analysis. Complement Ther Med. (2022) 70:102857. doi: 10.1016/j.ctim.2022.102857
21. Jiang, X, Lu, T, Dong, Y, Shi, J, Duan, M, and Zhang, X. Effectiveness and safety of moxibustion for vascular dementia: a systematic review and meta-analysis. Medicine. (2022) 101:e29804. doi: 10.1097/MD.0000000000029804
22. Leucht, S, Chaimani, A, Cipriani, AS, Davis, JM, Furukawa, TA, and Salanti, G. Network meta-analyses should be the highest level of evidence in treatment guidelines. Eur Arch Psychiatry Clin Neurosci. (2016) 266:477–80. doi: 10.1007/s00406-016-0715-4
23. Li, R, Xu, C, Zhong, P, Wang, K, Luo, YX, Xiao, L, et al. Efficacy of acupuncture and pharmacological therapies for vascular cognitive impairment with no dementia: a network meta-analysis. Front Aging Neurosci. (2023) 15:1181160. doi: 10.3389/fnagi.2023.1181160
24. Luo, G, Zhang, J, Song, Z, Wang, Y, Wang, X, Qu, H, et al. Effectiveness of non-pharmacological therapies on cognitive function in patients with dementia: a network meta-analysis of randomized controlled trials. Front Aging Neurosci. (2023) 15:1131744. doi: 10.3389/fnagi.2023.1131744
25. Wen, J, Cao, Y, Chang, S, Huang, Q, Zhang, Z, Wei, W, et al. A network meta-analysis on the improvement of cognition in patients with vascular dementia by different acupuncture therapies. Front Neurosci. (2022) 16:1053283. doi: 10.3389/fnins.2022.1053283
26. Hutton, B, Salanti, G, Caldwell, DM, Chaimani, A, Schmid, CH, Cameron, C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
27. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
28. Nikolakopoulou, A, Higgins, JPT, Papakonstantinou, T, Chaimani, A, del Giovane, C, Egger, M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. (2020) 17:e1003082. doi: 10.1371/journal.pmed.1003082
29. Shim, S, Yoon, BH, Shin, IS, and Bae, JM. Network meta-analysis: application and practice using Stata. Epidemiol Health. (2017) 39:e2017047. doi: 10.4178/epih.e2017047
30. Shim, SR, Kim, SJ, Lee, J, and Rücker, G. Network meta-analysis: application and practice using R software. Epidemiol Health. (2019) 41:e2019013. doi: 10.4178/epih.e2019013
31. Dias, S, Welton, NJ, Caldwell, DM, and Ades, AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. (2010) 29:932–44. doi: 10.1002/sim.3767
32. Hao, JW, Li, J, and Li, QQ. Observation of the therapeutic effects of ozagrel combined with rTMS on patients with vascular dementia after cerebral infarction. Intern Med. (2022) 17:21–4. doi: 10.16121/j.cnki.cn45-1347/r.2022.01.06
33. Ren, YF, Fu, SQ, Yu, M, Zhang, HT, and Zhang, SL. Clinical efficacy of edaravone combined with rTMS and its effects on serum Ang-1, MMP-9, and LPO levels in patients with vascular dementia. J Clin Psychosom Dis. (2018) 24:25–7, 62. doi: 10.3969/j.issn.1672-187X.2018.06.009
34. Yang, MJ . Clinical effects of high-frequency repetitive transcranial magnetic stimulation combined with nimodipine in the treatment of vascular dementia. Contemp Med China. (2023) 30:11–15. doi: 10.3969/j.issn.1674-4721.2023.11.005
35. Li, XY, and Zhang, T. Observations on the therapeutic efficacy of TMS combined with nimodipine and donepezil in the treatment of vascular dementia. Clin Med Res Pract. (2016) 1:56–7. doi: 10.19347/j.cnki.2096-1413.2016.23.027
36. Li, H, Cao, X, Zhong, X, and Zheng, XW. The effects of rTMS therapy on serum NSE levels and cognitive function in patients with vascular dementia. J Lab Med Clin Res. (2020) 17:647–9, 710. doi: 10.3969/j.issn.1672-9455.2020.05.022
37. Guo, L, Chen, WM, Wu, XH, Wu, BJ, Wang, XY, Ye, XP, et al. Clinical effects of repeated transcranial magnetic stimulation and electroacupuncture on the psychiatric symptoms of patients with vascular dementia. World Latest Med Inform Abstr. (2021) 21:128–9, 131. doi: 10.3969/j.issn.1671-3141.2021.76.052
38. Pan, HS, Mo, CY, and Hu, XY. The impact of acupuncture combined with TMS on cognitive function and quality of life in patients with mild vascular dementia. J Shenzhen Integr Trad Chin Western Med. (2018) 28:30–1. doi: 10.16458/j.cnki.1007-0893.2018.06.014
39. Wu, XH, Hu, XY, Chen, WM, Liu, SM, Feng, WX, Guo, L, et al. Clinical efficacy evaluation of acupuncture combined with repeated transcranial magnetic stimulation on cognitive function and sleep disorders in patients with mild vascular dementia. Chin J Mod Drug Appl. (2020) 14:185–7. doi: 10.14164/j.cnki.cn11-5581/r.2020.10.087
40. Cheng, NF, and Tan, F. The effect of the awakening and refreshing needling method on cognitive function and cerebral blood flow status in patients with vascular dementia. Guangxi J Trad Chin Med. (2021) 44:43–5. doi: 10.3969/j.issn.1003-0719.2021.03.016
41. Meng, YC, and Han, JX. Clinical observation of tonifying qi, regulating blood, supporting the root, and nurturing the primal needling method in the treatment of vascular dementia. J Acup Clin Med. (2011) 27:35–7. doi: 10.3969/j.issn.1005-0779.2011.09.016
42. Han, H, Li, X, Jiang, HN, Xu, K, and Wang, Y. Effect of early acupuncture on cognitive function in patients with vascular dementia after cerebral infarction. Zhongguo Zhen Jiu. (2021) 41:979–83. doi: 10.13703/j.0255-2930.20210117-k0008
43. Qiao, SG, and Hu, N. Clinical observation of acupuncture treatment for vascular dementia in elderly patients after cerebral infarction. Chin Trad Mod Dist Educ Trad Chin Med. (2023) 21:111–3. doi: 10.3969/j.issn.1672-2779.2023.13.040
44. Chen, YJ . Efficacy analysis of acupuncture in the treatment of vascular dementia. Chin J Prim Med Pharm. (2011) 18:2801–2802. doi: 10.3760/cma.j.issn.1008-6706.2011.20.036
45. Wang, HZ, Wang, YX, and Ni, HB. Clinical efficacy observation of acupuncture-medicine combination therapy for vascular dementia after cerebral infarction. Mod Diagn Treat. (2015) 26:57+67.
46. Hu, FX, Sun, YP, Hai, X, and Shi, YQ. Therapeutic efficacy of scalp acupuncture combined with ozacekstine on vascular dementia and its regulation on serum Livin, NOS, and brain neurotransmitters. Shanghai J Acup Moxibust. (2019) 38:607–12. doi: 10.13460/j.issn.1005-0957.2019.06.0607
47. Feng, XR, Zhang, SL, and Huang, CC. Effects of acupuncture combined with Dingkibenfen capsules on cognitive function and MRI imaging in patients with vascular dementia. J Cardiovasc Rehabil Med. (2019) 28:233–237
48. Ye, BY, Kang, JJ, Yu, ZZ, Chen, AZ, and Lin, Y. Donepezil combined with scalp acupuncture in the treatment of 30 cases of vascular dementia. Fujian J Trad Chin Med. (2016) 47:58–9. doi: 10.13260/j.cnki.jfjtcm.011199
49. Zhang, SL, and Qu, YH. Clinical study on the treatment of vascular dementia with acupuncture therapy combined with nemedipine tablets. New Trad Chin Med. (2022) 54:196–9. doi: 10.13457/j.cnki.jncm.2022.19.041
50. Cui, LL, Zhu, CQ, Wang, J, Jiang, LS, Chen, SF, et al. Clinical observation on the treatment of vascular dementia with Tongdu Tiaoshen acupuncture combined with nemedipine. Shanghai J Acup Moxibust. (2015) 34:714–6. doi: 10.13460/j.issn.1005-0957.2015.08.0714
51. Yu, T, Shi, JW, and Han, JX. Clinical observation on acupuncture treatment of mild to moderate vascular dementia. Liaoning J Trad Chin Med. (2007) 34:978–80. doi: 10.3969/j.issn.1000-1719.2007.07.070
52. Hu, JJ, Zhang, TF, Zhang, HX, Sun, GJ, Liu, YC, Liu, YP, et al. Clinical observation on the effect of scalp acupuncture on cognitive scale in patients with vascular dementia. Shanghai J Acup Moxibust. (2009) 28:15–7. doi: 10.13460/j.issn.1005-0957.2009.01.006
53. Tan, T, Ren, Z, and Tan, ZA. Clinical study on regulating mind and governing governor vessel acupuncture method in treating vascular dementia. Chin Med Guide. (2017) 23:66–8. doi: 10.13862/j.cnki.cn43-1446/r.2017.04.020
54. Zhang, R, Wang, Y, and Sun, S. Clinical observation on the treatment of vascular dementia with kidney-tonifying and brain-strengthening acupuncture. J Clin Acup Moxibust. (2010) 26:13–4. doi: 10.3969/j.issn.1005-0779.2010.01.006
55. Zheng, SH, Wu, YJ, Jiao, JK, Wei, LL, Ren, R, Cui, X, et al. Clinical randomized controlled study on Jin’s three-needle therapy for the treatment of vascular dementia. J Clin Acup Moxibust. (2011) 27:7–10. doi: 10.3969/j.issn.1005-0779.2011.03.003
56. Gao, Q, Chen, S, and Huang, Y. Effect of head acupuncture on mild vascular dementia after cerebral infarction. J Pract Trad Chin Med. (2013) 2013:890–1. doi: 10.3969/j.issn.1004-2814.2013.11.001
57. Yao, W . Clinical observation on the influence of Yizhi Tiaoshen needling method on cognitive function of patients with vascular dementia. Henan University of Traditional Chinese Medicine. (2017).
58. Xu, L . Clinical study on the treatment of senile vascular dementia with Dianzhen Sishen Cong acupoint. Guangzhou University of Chinese Medicine. (2006).
59. Liu, LJ, Xie, F, Chen, XY, Wu, DD, Zhang, Y, et al. Analysis of the efficacy and impact on cognitive function of rehabilitation therapy on elderly patients with vascular dementia. Chin J Modern Drug Appl. (2022) 16:245–7. doi: 10.14164/j.cnki.cn11-5581/r.2022.04.094
60. Yang, X . Clinical study on the treatment of vascular dementia with Dianzhen Zhisan primary needle. Guangzhou University of Chinese Medicine (2008).
61. Peng, XH, Zhao, Y, Liu, Y, Liu, J, and Li, QL. Randomized controlled study on the effect of head electroacupuncture on cognitive function in vascular dementia. Sichuan J Trad Chin Med. (2009) 27:113–5.
62. Zhao, L, Zhang, H, Zheng, Z, and Jiao, H. Electroacupuncture on the head points for improving cognition in patients with vascular dementia. J Tradit Chin Med. (2009) 29:29–34. doi: 10.1016/s0254-6272(09)60027-3
63. Huiming, W . Clinical efficacy observation of electroacupuncture in the treatment of vascular dementia. Tianjin J Tradit Chin Med. (2007) 24:218–220. doi: 10.3969/j.issn.1672-1519.2007.03.013
64. Yin, J, Li, L, and Feng, AC. Clinical observation of electroacupuncture at head acupoints in improving symptoms of patients with vascular dementia. China J Trad Chin Med. (2011) 29:415–6. doi: 10.13193/j.archtcm.2011.02.193.yinjq.006
65. Li, Q, Li, L, Xu, Q, Min, Y, and Qu, F. Effects of electroacupuncture Zhi san acupuncture on cognitive function and behavioral abilities in patients with vascular dementia. Chin J Practi Neurol Dis. (2015) 18:1–2.
66. Wang, M, Lin, L, and Zhang, F. Therapeutic effect and mechanism of moxibustion on vascular dementia. Acup Tuina Sci. (2020) 18:47–52. doi: 10.1007/s11726-020-1156-1
67. Sheng, DD, and Cai, S. Observation on the therapeutic effect of warming and tonifying kidney moxibustion in treating vascular dementia. J Pract Trad Chin Med. (2017) 33:58–9.
68. Gao, YJ, Yang, H, Yang, YH, An, XR, Liu, Q, Xue, XW, et al. Efficacy evaluation of hui medicine moxibustion combined with nimodipine in the treatment of vascular dementia. J Trad Chin Med Ethn Med. (2018) 29:119–20. doi: 10.3969/j.issn.1008-0805.2018.01.039
69. Luo, BH, Yu, JC, Hu, YQ, Xue, LF, Liang, YJ, Tan, BN, et al. Clinical observation of Yiqi Tiaoxue, Fuben Peiyuan drug thread moxibustion in the treatment of mild to moderate vascular dementia. J Trad Chin Med Ethn Med. (2015) 26:649–51. doi: 10.3969/j.issn.1008-0805.2015.03.053
70. Fan, WQ . Observations on the therapeutic effect of integrated traditional Chinese and Western medicine in the treatment of vascular dementia. J Pract Trad Chin Med. (2019) 35:1116–1117.
71. Zhao, RX, and Chen, ZP. Clinical observation of acupuncture treatment for 76 cases of vascular dementia. Front Med Pharm. (2012) 2:335–6. doi: 10.3969/j.issn.2095-1752.2012.07.397
72. Ma, L, Ai, LW, and Feng, Y. Clinical observation of scalp acupuncture combined with moxibustion at GV14 in the treatment of vascular dementia with kidney essence deficiency type. J Acup Moxibust Clin. (2018) 34:33–6. doi: 10.3969/j.issn.1005-0779.2018.06.011
73. Wang, H, Yang, X, and Liu, YJ. Efficacy of warm needle acupuncture with Yongquan point as the main point in the treatment of vascular dementia (kidney essence deficiency type) and its influence on cognitive function, serum IL-8, TNF-α, and Livin levels in patients. Sichuan Trad Chin Med. (2021) 39:207–10.
74. Wang, XP, Chen, X, Li, Z, and Zhang, KZ. Effect of donepezil combined with rehabilitation training on cognitive function and plasma neurotransmitters in elderly patients with vascular dementia. J Stroke Nerv Dis. (2018) 35:632–4. doi: 10.19845/j.cnki.zfysjjbzz.2018.07.012
75. Wang, HJ, Xu, YT, and Wang, XL. The impact of rehabilitation training on vascular dementia. Southwest Milit Med. (2007) 2007:21–2. doi: 10.3969/j.issn.1672-7193.2007.04.012
76. Sun, PJ, and Gao, X. The effects of rehabilitation training on the intelligence and functional abilities of patients with vascular dementia. Chin J Popul Health. (2016) 28:56–7. doi: 10.3969/j.issn.1672-0369.2016.14.027
77. Zhai, ZY, An, J, Sun, M, and Feng, J. Therapeutic observation of the efficacy of rehabilitation training combined with atorvastatin in subcortical ischemic vascular dementia. Chin J Rehabil. (2015) 30:14–6. doi: 10.3870/zgkf.2015.01.004
78. Wu, XL, Shao, LC, and Ruan, SF. Clinical study of cognitive training combined with drug therapy in vascular dementia. J Shenzhen Integr Med. (2018) 28:130–1. doi: 10.16458/j.cnki.1007-0893.2018.22.058
79. Ji, J, Xie, YS, Xie, LX, and Xiao, YT. Clinical study of drug therapy and cognitive training in the treatment of vascular dementia. Gansu Med J. (2017) 36:246–8. doi: 10.15975/j.cnki.gsyy.2017.04.003
80. Zhu, KF, Chen, YH, Hong, ZP, and Deng, T. Efficacy observation of cognitive function rehabilitation training combined with drug therapy in patients with vascular dementia. Chin Med Guide. (2023) 21:69–71. doi: 10.15912/j.cnki.gocm.2023.36.044
81. Qu, YT, Huang, SS, Xiao, FN, and Lan, HL. Effects of cognitive function training on cerebral hemodynamics and hemorheology in elderly patients with mild to moderate vascular dementia. Contemp Nurse. (2019) 26:36–9.
82. Zhao, PX . Observation on the efficacy of dihydroergotoxine mesylate soft capsules combined with hyperbaric oxygen therapy in the treatment of vascular dementia. Prim Health Care Forum. (2015) 19:610–611.
83. Liu, L, and Gao, Y. Observation on the efficacy of oxiracetam combined with hyperbaric oxygen therapy in the treatment of vascular dementia. Chin J Peoples Health. (2015) 27:80–1. doi: 10.3969/j.issn.1672-0369.2015.02.041
84. Chen, SD . Observation on the therapeutic effect of oxiracetam combined with hyperbaric oxygen in the treatment of vascular dementia in 41 cases. Med Theory Pract. (2011) 24:1425–1426. doi: 10.3969/j.issn.1001-7585.2011.12.028
85. Liu, ZY . Clinical efficacy analysis of oxiracetam combined with hyperbaric oxygen therapy in the treatment of vascular dementia. China medical device. Information. (2016) 22:87–88. doi: 10.15971/j.cnki.cmdi.2016.18.043
86. Lei, XD . Clinical efficacy observation of oxiracetam combined with hyperbaric oxygen therapy in the treatment of vascular dementia. Heilong Med Sci. (2016) 39:147+149. doi: 10.3969/j.issn.1008-0104.2016.01.068
87. Sun, W, Yang, JB, Xu, JF, Zhang, Q, Zhou, Q, Meng, XL, et al. Clinical efficacy observation of Dibenzoylmethane soft capsule combined with hyperbaric oxygen therapy in the treatment of elderly vascular dementia. J Pract Cardiopul Vasc Dis. (2015) 23:62–4. doi: 10.3969/j.issn.1008-5971.2015.09.019
88. Wang, YS, Wu, ZP, Wang, P, Zhao, YC, and Tang, WG. Clinical efficacy observation of Dibenzoylmethane soft capsule combined with hyperbaric oxygen therapy in the treatment of vascular dementia. Chin Mod Doct. (2013) 51:58–60.
89. Wang, SP, Tao, Z, Ding, SJ, Cheng, JC, Yv, SB, Wang, XQ, et al. Clinical observation of hyperbaric oxygen combined with donepezil hydrochloride in the treatment of vascular dementia. Chin J Phys Med Rehabil. (2009) 31:478–80. doi: 10.3760/cma.j.issn.0254-1424.2009.07.016
90. Tang, LC, Zheng, ZY, Li, XH, Liang, YH, and Fang, HB. The influence of hyperbaric oxygen on cognition in patients with vascular dementia. Chin J Med Guide. (2013) 11:92. doi: 10.15912/j.cnki.gocm.2013.13.564
91. Li, W . Efficacy analysis of hyperbaric oxygen combined with olanzapine in the treatment of 36 cases of vascular dementia. Chin J Med Sci. (2013) 3:63–64.
92. Feng, YF . Effect of hyperbaric oxygen combined with Piracetam tablets and Dibenzoyl soft capsules in the treatment of elderly patients with vascular dementia. Henan Med Res. (2020) 29:2782–3. doi: 10.3969/j.issn.1004-437X.2020.15.039
93. Hu, Q . Efficacy of hyperbaric oxygen combined with donepezil and Memantine in the treatment of elderly patients with vascular dementia. J Chronic Dis. (2023) 24:75–7. doi: 10.16440/j.Cnki.1674-8166.2023.01.20
94. Wang, YS, Wang, P, Wu, ZP, and Zhao, YC. Observation on the efficacy of hyperbaric oxygen combined with donepezil in the treatment of vascular dementia. Health Res. (2014) 2014:397–9. doi: 10.3969/j.issn.1674-6449.2014.04.013
95. Yang, L, Liu, Q, Han, XZ, and Wang, S. Effects of hyperbaric oxygen combined with Huperzine a on cognitive function and levels of serum hypoxia-inducible factor-1α and vascular endothelial growth factor in elderly patients with vascular dementia. Chin J Chron Dis Prevent Control. (2018) 26:676–9. doi: 10.16386/j.cjpccd.issn.1004-6194.2018.09.010
96. Li, ZC . Analysis of the efficacy of hyperbaric oxygen combined with donepezil hydrochloride and Memantine in the treatment of elderly patients with vascular dementia. Capit Food Med. (2020) 27:53. doi: 10.3969/j.issn.1005-8257.2020.08.040
97. Wang, SL, and Zhai, LH. Observation on the efficacy of hyperbaric oxygen combined with Ozatine in the treatment of 40 cases of vascular dementia. Chin Med Guide. (2011) 9:312–3. doi: 10.15912/j.cnki.gocm.2011.32.324
98. Xia, XY . Observation on the efficacy of hyperbaric oxygen therapy for vascular dementia. Asia Pacif Trad Med. (2012) 8:84–5. doi: 10.3969/j.issn.1673-2197.2012.01.041
99. Bao, ZY, Zhong, XB, Kang, P, Deng, BG, and Yuan, HQ. Observation on the efficacy of hyperbaric oxygen therapy in patients with vascular dementia. J Gannan Med Univ. (2007) 2007:532–3. doi: 10.3969/j.issn.1001-5779.2007.04.018
100. Wu, Y, Liang, Y, and Huang, DD. Observation on the efficacy of Ozatine combined with hyperbaric oxygen therapy in the treatment of vascular dementia. Chin J Pract Neurol Dis. (2010) 13:13–4. doi: 10.3969/j.issn.1673-5110.2010.07.006
101. Song, YH . Observation on the efficacy of Ozatine combined with hyperbaric oxygen therapy in the treatment of vascular dementia: a study of 63 cases. Dajia Health. (2012) 6:5–7.
102. Bu, GW . Clinical efficacy observation of combined application of Ozatine and hyperbaric oxygen in 96 cases of vascular dementia patients. Psychologist. (2012) 2012:80. doi: 10.3969/j.issn1007-8231.2012.10.082
103. Liu, YQ . Observation on the efficacy of Ozatine combined with electromyography biofeedback therapy in the treatment of vascular dementia. J Mathem Med. (2020) 33:754–5. doi: 10.3969/j.issn.1004-4337.2020.05.057
104. Ran, Q, and Yang, GD. Observation on the efficacy of Dingbenqai soft capsules combined with electromyography biofeedback therapy in the treatment of vascular dementia. J Clin Psychosom Dis. (2018) 24:42–44+75. doi: 10.3969/j.issn.1672-187X.2018.04.013
105. Du, HQ, Liu, SD, Gao, J, and Kong, YM. Efficacy of electromyography biofeedback therapy on vascular dementia and its impact on NSE expression. Ningxia Med J. (2015) 37:519–21. doi: 10.13621/j.1001-5949.2015.06.0519
106. Liu, YL, Guo, YJ, Meng, WJ, and Kong, YM. Effects of electromyography biofeedback therapy combined with Ozatine on peripheral blood heme oxygenase-1, soluble apoptosis factor levels, and cognitive function in elderly patients with vascular dementia. Chin J Phys Dev. (2022) 45:559–563. doi: 10.3760/cma.j.cn115455-20201118-01620
107. Cai, R, Xie, YX, and Feng, L. Impact of Dingbenqai soft capsules combined with electromyography biofeedback on MMSE scores and changes in serum MMP-9, LPO levels in patients with vascular dementia. J Med Forum. (2018) 39:41–44.
108. Chen, SS, He, Y, and Yuan, PH. Effects of electrical stimulation of the cerebellar dentate nucleus on cognitive function and hemorheology in patients with vascular dementia. Shaanxi Med J. (2013) 42:731–732+734. doi: 10.3969/j.issn.1000-7377.2013.06.043
109. Wu, BJ, Zhang, YM, Yue, W, et al. (2003). Recent efficacy of electrical stimulation of the cerebellum in the treatment of vascular dementia. China Rehabilitation. (2005) 20:301–301. doi: 10.3870/j.issn.1001-2001.2005.05.018
110. Dai, JW, Cao, Y, and Hu, ZB. Clinical observation of donepezil combined with electrical stimulation of the cerebellar dentate nucleus in the treatment of vascular dementia. Chin J Clin Med. (2008) 2:52–53.
111. Li, J, Zhang, L, Xin, GL, Wang, L, and Guan, Y. Clinical efficacy of acupuncture Kangfaneurotransmitter regulation method in treating patients with vascular dementia and its impact on cognitive function and quality of life. World J Integr Trad Western Med. (2023) 18:377–82. doi: 10.13935/j.cnki.sjzx.230230
112. Wu, FY, Wang, LN, Sun, YN, and Lai, SD. Effects of the acupuncture Tonifying mind and enhancing intelligence method combined with rehabilitation training on patients with vascular dementia. Chin J Minhe Med. (2022) 34:65–7. doi: 10.3969/j.issn.1672-0369.2022.04.021
113. Li, WM, Liu, Q, Hong, J, Hu, ML, and Li, F. Effects of the temporal three-needle therapy on mild to moderate vascular dementia and its impact on serum hemorheology, ApoE, and AchE. J Qiqihar Med Coll. (2023) 44:441–5. doi: 10.3969/j.issn.1002-1256.2023.05.010
114. Li, WM, Li, F, Liang, DD, Hu, ML, and Li, F. Clinical observation of temporal three-needle combined with rehabilitation training in the treatment of vascular dementia. J Yunnan Univ Trad Chin Med. (2017) 40:66–8. doi: 10.19288/j.cnki.issn.1000-2723.2017.03.016
115. Wang, HY, Zhang, LM, Cao, Y, Huang, JL, and Wu, JJ. Clinical observation of acupuncture combined with rehabilitation training in the treatment of vascular dementia. J Hubei J Trad Chin Med. (2009) 31:11–2. doi: 10.3969/j.issn.1000-0704.2009.07.005
116. Shi, GR . Clinical observation of auricular acupressure combined with moxibustion in the treatment of vascular dementia. Guangzhou University of Chinese Medicine (2011).
117. Chen, Q, Huang, HM, Xu, YJ, Lu, RL, Zhou, XH, and Zhou, C. Controlled study of auricular point taping and pressing therapy for treatment of vascular dementia. Zhongguo Zhen Jiu. (2009) 29:95–7. doi: 10.13703/j.0255-2930.2009.02.004
118. Kuang, WC, Lu, YJ, Huang, F, Yang, HT, Lu, YQ, Lai, YQ, et al. Clinical observation of acupuncture combined with moxibustion in the treatment of vascular dementia in 78 cases. J Jilin Trad Chin Med. (2012) 32:406–8. doi: 10.13463/j.cnki.jlzyy.2012.04.027
119. Wang, XY, Han, LP, Zhao, F, and Tang, N. Clinical observation of acupuncture treatment in vascular dementia. J Pract Trad Chin Med. (2019) 35:1153–4.
120. Wang, LJ, Chu, HR, and Li, F. Clinical observation of Bianjing Cijing combined with Tiansan needle in the treatment of vascular dementia. J Trad Chin Med Clin Pharmacol. (2014) 26:221–2. doi: 10.16448/j.cjtcm.2014.03.027
121. Liu, SH . Observation on the therapeutic effect of drug intervention combined with exercise rehabilitation in the treatment of vascular dementia. Chinese journal of continuing. Med Educ. (2016) 8:174–175. doi: 10.3969/j.issn.1674-9308.2016.24.113
122. Li, R . Efficacy evaluation of Diphenylthiodimethylsulfonium bromide combined with electromyographic biofeedback in the treatment of vascular dementia. Chin Folk Ther. (2017) 25:86–8. doi: 10.3969/j.issn.1007-5798.2017.10.067
123. Pan, P, Ma, Z, Zhang, Z, Ling, Z, Wang, Y, Liu, Q, et al. Acupuncture can regulate the peripheral immune cell Spectrum and inflammatory environment of the vascular dementia rat, and improve the cognitive dysfunction of the rats. Front Aging Neurosci. (2021) 13:706834. doi: 10.3389/fnagi.2021.706834
124. Dadsetan, S, Balzano, T, Forteza, J, Cabrera-Pastor, A, Taoro-Gonzalez, L, Hernandez-Rabaza, V, et al. Reducing peripheral inflammation with infliximab reduces Neuroinflammation and improves cognition in rats with hepatic encephalopathy. Front Mol Neurosci. (2016) 9:106. doi: 10.3389/fnmol.2016.00106
125. Yang, R, Yang, J, and Huang, L. Acupuncture attenuates cognitive impairments in vascular dementia through inhibiting miR-143-3p. Acta Biochim Pol. (2022) 69:805–10. doi: 10.18388/abp.2020_6132
126. Wang, L, Yang, JW, Lin, LT, Huang, J, Wang, XR, Su, XT, et al. Acupuncture attenuates inflammation in microglia of vascular dementia rats by inhibiting miR-93-mediated TLR4/MyD88/NF-κB signaling pathway. Oxidative Med Cell Longev. (2020) 2020:8253904. doi: 10.1155/2020/8253904
127. Hua, F, Ma, J, Ha, T, Kelley, JL, Kao, RL, Schweitzer, JB, et al. Differential roles of TLR2 and TLR4 in acute focal cerebral ischemia/reperfusion injury in mice. Brain Res. (2009) 1262:100–8. doi: 10.1016/j.brainres.2009.01.018
128. Wang, YC, Lin, S, and Yang, QW. Toll-like receptors in cerebral ischemic inflammatory injury. J Neuroinflammation. (2011) 8:134. doi: 10.1186/1742-2094-8-134
129. Ma, SM, Wang, L, Su, XT, Yang, NN, Huang, J, Lin, LL, et al. Acupuncture improves white matter perfusion and integrity in rat model of vascular dementia: an MRI-based imaging study. Front Aging Neurosci. (2020) 12:582904. doi: 10.3389/fnagi.2020.582904
130. Kan, B, Dong, Z, Tang, Z, Zhao, L, and Li, Z. Acupuncture improves synaptic plasticity of SAMP8 mice through the RhoA/ROCK pathway. Curr Alzheimer Res. (2023) 20:420–30. doi: 10.2174/1567205020666230828095826
131. Gui, J, Liu, J, Han, Z, Yang, X, Ding, R, Yang, J, et al. The dysfunctionality of hippocampal synapses may be directly related to PM-induced impairments in spatial learning and memory in juvenile rats. Ecotoxicol Environ Saf. (2023) 254:114729. doi: 10.1016/j.ecoenv.2023.114729
132. Huang, Y, Lai, XS, and Tang, AW. Comparative study of the specificities of needling acupoints DU20, DU26, and HT7 in intervening vascular dementia in different areas in the brain on the basis of scale assessment and cerebral functional imaging. Chin J Integr Med. (2007) 13:103–8. doi: 10.1007/s11655-007-0103-z
133. Ye, Y, Li, H, Yang, JW, Wang, XR, Shi, GX, Yan, CQ, et al. Acupuncture attenuated vascular dementia-induced hippocampal long-term potentiation impairments via activation of D1/D5 receptors. Stroke. (2017) 48:1044–51. doi: 10.1161/STROKEAHA.116.014696
134. Lin, R, Wu, Y, Tao, J, Chen, B, Chen, JX, Zhao, CK, et al. Electroacupuncture improves cognitive function through rho GTPases and enhances dendritic spine plasticity in rats with cerebral ischemia-reperfusion. Mol Med Rep. (2016) 13:2655–60. doi: 10.3892/mmr.2016.4870
135. Walker, CK, and Herskowitz, JH. Dendritic spines: mediators of cognitive resilience in aging and Alzheimer’s disease. Neuroscientist. (2021) 27:487–505. doi: 10.1177/1073858420945964
136. Li, B, Deng, S, Zhuo, B, Sang, B, Chen, J, Zhang, M, et al. Effect of acupuncture vs sham acupuncture on patients with Poststroke motor aphasia: a randomized clinical trial. JAMA Netw Open. (2024) 7:e2352580. doi: 10.1001/jamanetworkopen.2023.52580
137. Wu, B, Ding, Y, Peng, M, Wang, X, Li, Y, and Cheng, X. Influence of acupuncture and other clinical factors on the recovery of limb motor function in patients after stroke: a retrospective study. J Multidiscip Healthc. (2023) 16:463–74. doi: 10.2147/jmdh.S398202
138. Ma, Y, Guo, H, Geng, L, Jia, Z, Li, G, Shen, W, et al. Effect of quick acupuncture combined with rehabilitation therapy on improving motor and swallowing function in patients with stroke. Clin Rehabil. (2024) 38:793–801. doi: 10.1177/02692155241228694
139. Yu, Z, Yang, X, Qin, F, Ma, T, Zhang, J, Leng, X, et al. Effects of acupuncture synchronized rehabilitation therapy on upper limb motor and sensory function after stroke: a study protocol for a single-center, 2 × 2 factorial design, randomized controlled trial. Front Neurol. (2023) 14:1162168. doi: 10.3389/fneur.2023.1162168
140. Weilan, Q, Yan, Y, Xuemei, L, Wei, S, Xiaohan, L, Qingke, M, et al. Effect of medicated thread moxibustion on apoptosis of hippocampal neurons in rat models of chronic cerebral ischemic vascular dementia. Cell Mol Biol (Noisy-le-Grand). (2018) 64:107–12. doi: 10.14715/cmb/2018.64.13.20
141. Yang, K, Song, XG, Ruan, JR, Cai, SC, Zhu, CF, Qin, XF, et al. Effect of moxibustion on cognitive function and proteins related to apoptosis of hippocampal neurons in rats with vascular dementia. Zhongguo Zhen Jiu. (2021) 41:1371–8. doi: 10.13703/j.0255-2930.20210124-k0006
142. Wang, J, Dong, WW, Zhang, WH, Zheng, J, and Wang, X. Electrical stimulation of cerebellar fastigial nucleus: mechanism of neuroprotection and prospects for clinical application against cerebral ischemia. CNS Neurosci Ther. (2014) 20:710–6. doi: 10.1111/cns.12288
143. Xia, D, Sui, R, Min, L, Zhang, L, and Zhang, Z. Fastigial nucleus stimulation ameliorates cognitive impairment via modulating autophagy and inflammasomes activation in a rat model of vascular dementia. J Cell Biochem. (2019) 120:5108–17. doi: 10.1002/jcb.27787
144. Zhang, N, Xing, M, Wang, Y, Tao, H, and Cheng, Y. Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF-NMDAR pathways in a rat model of vascular dementia. Neuroscience. (2015) 311:284–91. doi: 10.1016/j.neuroscience.2015.10.038
145. Allen, SJ, Watson, JJ, Shoemark, DK, Barua, NU, and Patel, NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. (2013) 138:155–75. doi: 10.1016/j.pharmthera.2013.01.004
146. Wang, F, Geng, X, Tao, HY, and Cheng, Y. The restoration after repetitive transcranial magnetic stimulation treatment on cognitive ability of vascular dementia rats and its impacts on synaptic plasticity in hippocampal CA1 area. J Mol Neurosci. (2010) 41:145–55. doi: 10.1007/s12031-009-9311-7
147. Zhang, XQ, Li, L, Huo, JT, Cheng, M, and Li, LH. Effects of repetitive transcranial magnetic stimulation on cognitive function and cholinergic activity in the rat hippocampus after vascular dementia. Neural Regen Res. (2018) 13:1384–9. doi: 10.4103/1673-5374.235251
148. Gao, L, Liu, F, and Liu, R. The mechanism of aerobic exercise regulating the PI3K/Akt-mTOR signaling pathway intervenes in hippocampal neuronal apoptosis in vascular dementia rats. Int J Environ Res Public Health. (2023) 20:1893. doi: 10.3390/ijerph20031893
149. Tomoto, T, Pasha, E, Sugawara, J, Tarumi, T, Chiles, C, Curtis, B, et al. Effects of 1-year aerobic exercise training on cerebral blood flow and arterial stiffness in amnestic mild cognitive impairment. FASEB J. (2020) 34:1. doi: 10.1096/fasebj.2020.34.s1.02052
150. Smiley-Oyen, AL, Lowry, KA, Francois, SJ, Kohut, ML, and Ekkekakis, P. Exercise, fitness, and neurocognitive function in older adults: the “selective improvement” and “cardiovascular fitness” hypotheses. Ann Behav Med. (2008) 36:280–91. doi: 10.1007/s12160-008-9064-5
Keywords: vascular dementia, non-pharmacological interventions, complementary and alternative therapies, cognitive function, network meta-analysis
Citation: Yi Y, Qu Y, Lv S, Zhang G, Rong Y and Li M (2024) Comparative efficacy and safety of non-pharmacological interventions as adjunctive treatment for vascular dementia: a systematic review and network meta-analysis. Front. Neurol. 15:1397088. doi: 10.3389/fneur.2024.1397088
Edited by:
Ioannis Liampas, University of Thessaly, GreeceReviewed by:
Antonio Guaita, Fondazione Golgi Cenci, ItalyChengda Dong, Shandong University of Traditional Chinese Medicine, China
Jolanta Dorszewska, Poznan University of Medical Sciences, Poland
Vaitsa Giannouli, Aristotle University of Thessaloniki, Greece
Copyright © 2024 Yi, Qu, Lv, Zhang, Rong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Li, bGltaW5nMTIwN0AxMjYuY29t
 Yunhao Yi
Yunhao Yi Yiwei Qu
Yiwei Qu Shimeng Lv
Shimeng Lv Guangheng Zhang
Guangheng Zhang Yuanhang Rong
Yuanhang Rong Ming Li2*
Ming Li2*